Abstract
INTRODUCTION:
Liver cirrhosis and its complication — hepatocellular carcinoma (HCC) — have been associated with increased exhaled limonene. It is currently unclear whether this increase is more strongly associated with the presence of HCC or with the severity of liver dysfunction.
METHODS:
We compared the exhaled breath of 40 controls, 32 cirrhotic patients, and 12 cirrhotic patients with HCC using the Breath Biopsy platform. Breath samples were analyzed by thermal desorption–gas chromatography–mass spectrometry. Limonene levels were compared between the groups and correlated to bilirubin, albumin, prothrombin time international normalized ratio, and alanine aminotransferase.
RESULTS:
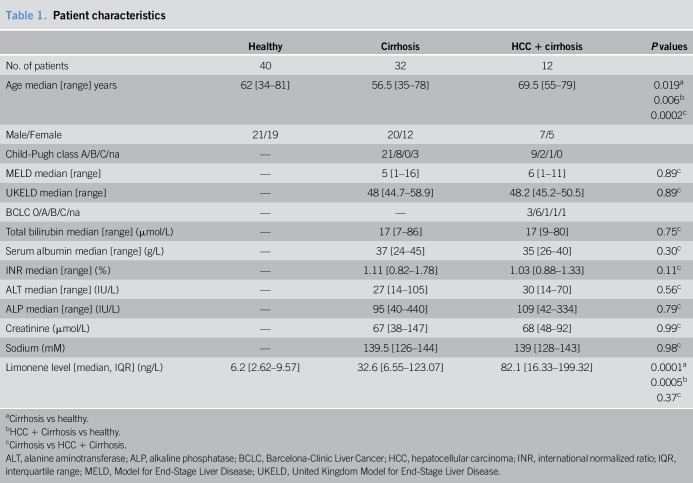
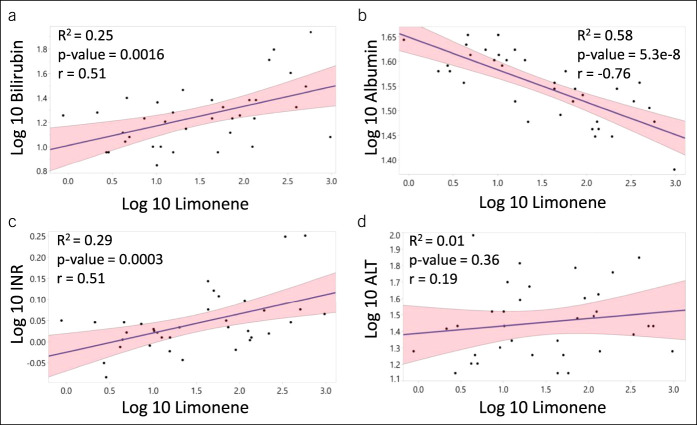
Breath limonene concentration was significantly elevated in subjects with cirrhosis-induced HCC (M: 82.1 ng/L, interquartile range [IQR]: 16.33–199.32 ng/L) and cirrhosis (M: 32.6 ng/L, IQR: 6.55–123.07 ng/L) compared with controls (M: 6.2 ng/L, IQR: 2.62–9.57 ng/L) (P value = 0.0005 and 0.0001, respectively) with no significant difference between 2 diseased groups (P value = 0.37). Levels of exhaled limonene correlated with serum bilirubin (R2 = 0.25, P value = 0.0016, r = 0.51), albumin (R2 = 0.58, P value = 5.3e-8, r = −0.76), and international normalized ratio (R2 = 0.29, P value = 0.0003, r = 0.51), but not with alanine aminotransferase (R2 = 0.01, P value = 0.36, r = 0.19).
DISCUSSION:
Exhaled limonene levels are primarily affected by the presence of cirrhosis through reduced liver functional capacity, as indicated by limonene correlation with blood metrics of impaired hepatic clearance and protein synthesis capacity, without further alterations observed in subjects with HCC. This suggests that exhaled limonene is a potential non-invasive marker of liver metabolic capacity (see Visual abstract, Supplementary Digital Content 1, http://links.lww.com/CTG/A388).
INTRODUCTION
Cirrhosis is the end-stage condition of necroinflammation and fibrogenesis of the liver induced by chronic hepatic injury (1). Disease progression is often asymptomatic with 50% of the cases diagnosed at advanced stages when episodes of liver decompensation occur (2). Cirrhosis is the main risk factor predisposing to hepatocellular carcinoma (HCC) (3). HCC ranks as the third most common cancer-related death worldwide (4,5). Only patients diagnosed at the earliest stages can benefit from curative treatments and expect a 5-year survival of 50%–80% (3,6). Unfortunately, in the Western countries, as few as 30% of the cases are detected at early stages despite the establishment of semiannual surveillance protocols for the at-risk populations (7). These data indicate a pressing need for more efficient screening tests for the early detection of chronic hepatic injury and HCC.
Analysis of volatile organic compounds (VOCs) in exhaled breath represents an emerging diagnostic approach with the potential to develop safe, non-invasive tests for the early detection of cancer (8,9). Exhaled VOCs are low molecular weight compounds that can be endogenous, resulting from physiological or pathological metabolic processes (10), or exogenous (EVOCs), resulting from dietary or environmental exposure. Dietary EVOCs are absorbed and reach breath through blood circulation, diffusing into the lungs via gaseous exchanges between blood and air (8) and can be used to assess metabolic functions in vivo.
Limonene is an EVOC taken-up through the consumption of plant-derived foods, especially citrus in the form of fruits, flavored foods, and beverages (11). It is rapidly absorbed and metabolized in the liver by the enzymes CYP2C9 and CYP2C19 presumably to transcarveol and perillyl alcohol, respectively (12). High concentrations of limonene have been found in the breath of cirrhotic patients because of reduced hepatic clearance (13–18). This reduction is attributed to the downregulation of CYP2C9 and CYP2C19, which, in turn, increases the half-life of limonene in the bloodstream, leading to higher abundance in the breath (13–18). O'Hara et al. (17) found a moderate increase of breath limonene in patients affected by HCC with underlying cirrhosis. Furthermore, proteomic profiling of resected early-stage liver primary tumors and comparison with paired non-tumor liver tissues showed a general downregulation of the CYP 450 system, including CYP2C9 and CYP2C19, in the cancer tissues (19).
Given these findings, the effects of patients having HCC in addition to cirrhosis on limonene concentrations in breath need further investigation. Understanding this in more detail is crucial to shed light on the utility of a limonene breath test. We therefore investigated, in cirrhotic patients, the impact of disease severity, measured as Child-Pugh (CP) class, on exhaled limonene levels and ascertained whether the presence of HCC, in cirrhotic patients with matching CP class, independently modulates exhaled limonene.
METHODS
Study design and subjects
This study is a cross-sectional case-control study, part of Owlstone Medical's PAN-study (NCT03756597). This study was approved by the ethics committee of the East of England—Cambridge East Research Ethics Committee (REC reference: 18/EE/0041. IRAS ID: 237560), and all participants provided written informed consent.
Subjects younger than 30 years of age were recruited from the clinical research facility at Addenbrooke's Hospital (Cambridge) or through the Cambridge BioResource. Cirrhotic patients had an established diagnosis according to EASL and AASLD guidelines (20,21). Disease severity was classified using the CP, the United Kingdom Model for End-Stage Liver Disease, and the Model for End-Stage Liver Disease scoring systems (22,23). Per protocol, only patients with CP A or B were eligible for this study, regardless of disease etiology. Subjects with HCC were identified through semiannual surveillance imaging of cirrhotic risk population and confirmed by either cytology/histology or cross-sectional radiology, as defined in the European Association for the Study of the Liver HCC guidelines (21) and classified according to the Barcelona-Clinic Liver Cancer staging system (24). No patients with HCC were receiving any anticancer treatment at the time of sampling.
Control subjects had no known liver disease and were excluded if they were under investigation or had a history of malignancy in the past 2 years.
Breath sampling
Breath collection was performed in a single room at the Addenbrooke's Hospital (Cambridge, UK) for all the subjects involved in the study. Breath samples were collected by adsorption onto 1/4″ × 3 1/2″ inert-coated stainless-steel tubes with Tenax TA/carbograph 5TD adsorbent material (Markes International, Llantrisant, UK) through a ReCIVA Breath Sampler (Owlstone Medical, Cambridge, UK) (25). Before sampling, the tubes were conditioned in a TC-20 (Markes International) by a N2 flow at 20 psi and 320 °C for 4 hours. Before sampling, the system was allowed to calibrate and adjust to the breathing pattern of the subject. Approximately 1.5 L of breath was sampled per tube at 225 mL/min. Ambient contamination was minimized by using the CASPER Portable Air Supply (Owlstone Medical) (25). The tubes were stored at a temperature of 4–8 °C for no more than 4 weeks before limonene was analyzed.
No dietary restrictions were applied to any participant. To generate a crude estimate of limonene intake, an unvalidated food questionnaire was administered to all cirrhotic subjects to classify them into daily and non-daily consumers of limonene-containing foods.
Limonene measurements
The tubes containing breath samples were purged in a TD-100 (Markes International). Samples were first desorbed at 210 °C and focused onto a cold trap U-T12ME-2S, Material/Emission, C4-C32 (Markes international) at 20 °C. Cryofocused analytes were then desorbed at 300 °C for 3 minutes and purged by helium into the GC column (TraceGOLD TG-624SilMS; Thermo Fisher Scientific, Waltham, MA), with a sample flow split ratio of 10:1 and temperature gradient steps of 40 °C for 1 minute, 270 °C with a rate of 10 °C/min, and 300 °C with a rate of 30 °C/min for 5 minutes. Mass detection was performed using a Q Exactive GC Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Fisher Scientific) scanning from m/z 30 to m/z 450 with a resolving power of 60,000. Electron ionization voltage was set at 70 V. The ion source and heater temperatures were, respectively, fixed at 230 and 250 °C.
Limonene identification and peak integration were performed with the Chromeleon Chromatography Data System (Thermo Fisher Scientific) by injecting a limonene standard (62118; Sigma Aldrich, Gillingham, UK). Total ion chromatograms were subjected to a NIST library search and the product ion signals with m/z values of 67, 79, 93, 107, 121, and 136, and an elution time of 11 minutes was assigned to limonene. The product ion at m/z 67 was used as a quantifier by comparison with a limonene standard curve (5–1,500 ng on-tube, Supplementary Digital Content 1, http://links.lww.com/CTG/A387). Limonene on breath corresponding to peaks below 5 ng have been extrapolated. No baseline correction was applied to the chromatograms. Limonene values were normalized to the exact volume of collected breath as recorded by the ReCIVA Breath Sampler system. Equipment and site blanks were also analyzed to estimate potential contaminations.
Data analysis
The data were analyzed by using JMP version 15 (SAS Institute, Cary, NC, 1989–2019). Limonene concentration is expressed as ng/L of breath and reported as median and interquartile range (IQR). The other parameters are reported as median, minimum, and maximum. Distribution of breath limonene concentration for each group was assessed using a Shapiro-Wilk test and found to be non-normally distributed, so non-parametric tests were used for intergroup comparisons. A Mann-Whitney U test was used to test for significant changes between the groups. Limonene and other parameters were Log10 transformed to obtain normal distribution. Least square regression was used to correlate breath limonene levels with blood metrics, Model for End-Stage Liver Disease, and United Kingdom Model for End-Stage Liver Disease scoring systems. A P value lower than 0.05 was regarded as statistically significant. A limonene receiver operating characteristic plot was built to estimate diagnostic accuracy. The cutoff point was selected as the concentration of limonene corresponding to the Youden index (highest [sensitivity − (1 − specificity)]).
RESULTS
Subject characteristics
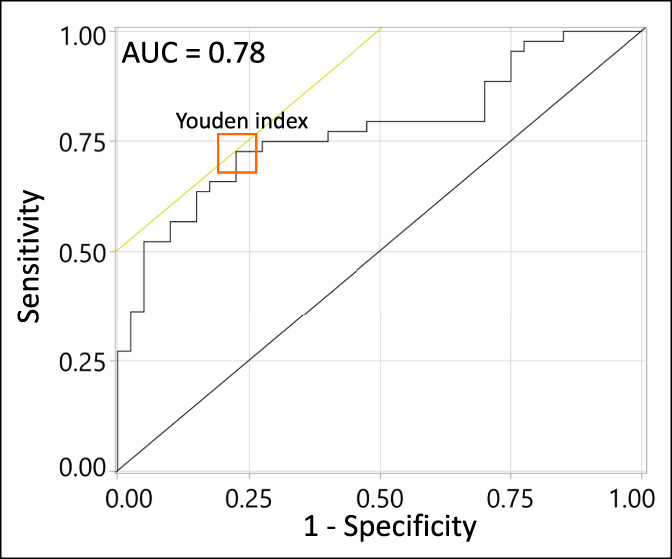
Breath samples were collected from 40 cirrhosis- and HCC-free controls (m: age 62 [34–81] years, m/f/[21/19]), 32 patients with cirrhosis (m: 56.5 [35–78] years, m/f [20/12]), and 12 patients with cirrhosis complicated by HCC (m: 69.5 [55–79] years, m/f [7/5]). Study subject details are provided in Table 1. The following observations can be made. The unbalanced sex ratio within each group reflects the higher prevalence of HCC in male patients (26). Across all subjects, the exhaled limonene levels were not affected by sex (P value = 0.308), as previously described (17,27). Significant differences in age were observed between the study groups (P values: 0.019, 0.006, and 0.0002, respectively, for cirrhosis vs healthy, HCC+cirrhosis vs healthy, and cirrhosis vs HCC+cirrhosis). No significant differences were observed for blood metrics between the study groups (Table 1).
Table 1.
Patient characteristics
| Healthy | Cirrhosis | HCC + cirrhosis | P values | |
| No. of patients | 40 | 32 | 12 | |
| Age median [range] years | 62 [34–81] | 56.5 [35–78] | 69.5 [55–79] | 0.019a 0.006b 0.0002c |
| Male/Female | 21/19 | 20/12 | 7/5 | |
| Child-Pugh class A/B/C/na | — | 21/8/0/3 | 9/2/1/0 | |
| MELD median [range] | — | 5 [1–16] | 6 [1–11] | 0.89c |
| UKELD median [range] | — | 48 [44.7–58.9] | 48.2 [45.2–50.5] | 0.89c |
| BCLC 0/A/B/C/na | — | — | 3/6/1/1/1 | |
| Total bilirubin median [range] (µmol/L) | — | 17 [7–86] | 17 [9–80] | 0.75c |
| Serum albumin median [range] (g/L) | — | 37 [24–45] | 35 [26–40] | 0.30c |
| INR median [range] (%) | — | 1.11 [0.82–1.78] | 1.03 [0.88–1.33] | 0.11c |
| ALT median [range] (IU/L) | — | 27 [14–105] | 30 [14–70] | 0.56c |
| ALP median [range] (IU/L) | — | 95 [40–440] | 109 [42–334] | 0.79c |
| Creatinine (µmol/L) | — | 67 [38–147] | 68 [48–92] | 0.99c |
| Sodium (mM) | — | 139.5 [126–144] | 139 [128–143] | 0.98c |
| Limonene level [median, IQR] (ng/L) | 6.2 [2.62–9.57] | 32.6 [6.55–123.07] | 82.1 [16.33–199.32] | 0.0001a 0.0005b 0.37c |
Cirrhosis vs healthy.
HCC + Cirrhosis vs healthy.
Cirrhosis vs HCC + Cirrhosis.
ALT, alanine aminotransferase; ALP, alkaline phosphatase; BCLC, Barcelona-Clinic Liver Cancer; HCC, hepatocellular carcinoma; INR, international normalized ratio; IQR, interquartile range; MELD, Model for End-Stage Liver Disease; UKELD, United Kingdom Model for End-Stage Liver Disease.
Exhaled limonene levels
Median exhaled limonene levels in patients with cirrhosis (m: 32.6 ng/L, IQR: 6.55–123.07 ng/L) and patients with cirrhosis complicated by HCC (m: 82.1 ng/L, IQR: 16.33–199.32 ng/L) were significantly higher than those of the controls (m: 6.22 ng/L, IQR: 2.62–9.57 ng/L) at P values of 0.0001 and 0.0005, respectively. The presence of HCC in study subjects with cirrhosis did not influence the exhaled limonene levels (P value = 0.37) (Figure 1 and Supplementary Digital Content 2, http://links.lww.com/CTG/A387). Therefore, all subjects with cirrhosis and cirrhosis complicated by HCC were gathered in one group for all the subsequent analysis, increasing the statistical power of those analyses.
Figure 1.
Breath limonene levels are elevated in patients with cirrhosis compared with control subjects. Box plot showing breath limonene concentration in healthy volunteers, patients with cirrhosis, and patients who progressed to HCC. HCC, hepatocellular carcinoma.
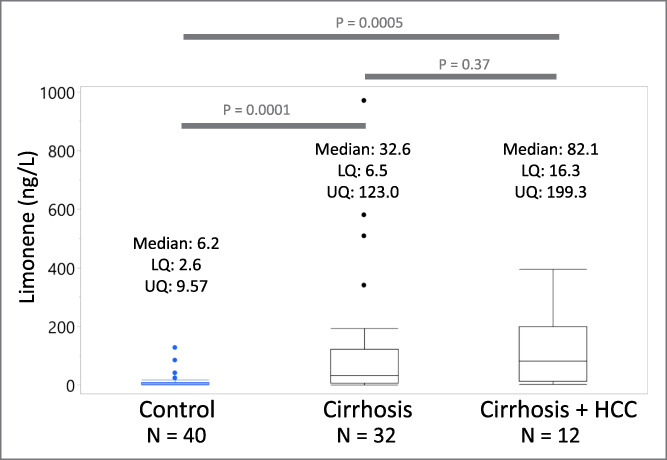
An exploratory receiver operating characteristic analysis, detailed in Figure 2, resulted in an area under the curve of 0.78 for the prediction of cirrhosis. At a threshold of 10.2 ng/L, an optimal overall accuracy was achieved (Youden index) with a sensitivity of 0.73 and 1-specificity of 0.23.
Figure 2.
Performance of breath limonene as a classifier for liver cirrhosis. Receiver operating characteristic (ROC) plot obtained using breath limonene levels of control individuals against that of patients with cirrhosis and cirrhosis complicated by HCC. The orange square indicates the Youden index. HCC, hepatocellular carcinoma.
Association with disease severity
Stratification of subjects by CP score (23) shows that exhaled limonene levels in class B (median: 159.9 ng/L, IQR: 35.1–400.5 ng/L) are significantly higher compared with those in class A (median: 12.6 ng/L, IQR: 4.6–58.0 ng/L) (P value = 0.0117) (Figure 3). Consistent with this result, a least square regression shows that breath limonene correlates with bilirubin (R2 = 0.25, P value = 0.0016, r = 0.51) (Figure 4a), albumin (R2 = 0.58, P value = 5.3e-8, r = −0.76) (Figure 4b), and international normalized ratio (INR) (R2 = 0.29, P value = 0.0003, r = 0.51) (Figure 4c). However, limonene does not correlate with alanine aminotransferase (ALT) (R2 = 0.01, P value = 0.36, r = 0.19), a biomarker of liver damage (Figure 4d).
Figure 3.
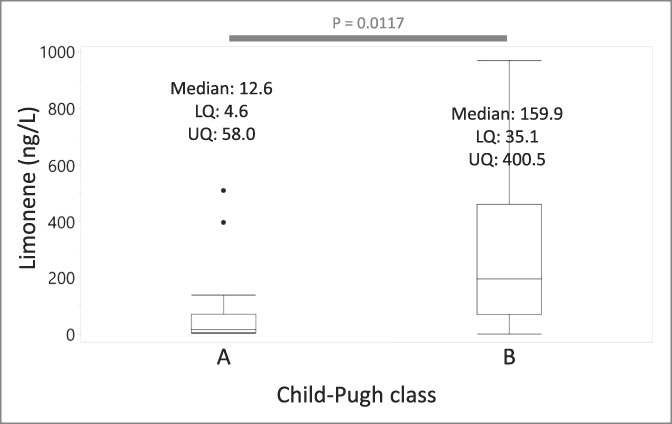
Breath limonene levels associate with the severity of cirrhosis. Box plot showing the breath limonene concentration of patients stratified by the severity of cirrhosis determined by Child-Pugh classification. Three patients taking anticoagulants drugs were excluded.
Figure 4.
Correlation of breath limonene levels with blood metrics related to liver function. Least square regression analysis of Log10 transformed limonene against (a) bilirubin, (b) albumin, (c) international normalized ratio (INR), and (d) alkaline phosphatase. ALT, alanine aminotransferase.
Impact of exposure and medications
The amount of ingested limonene depends on the dietary intake. Classification of the disease population in daily and non-daily citrus product consumers shows that patients ingesting citrus products on a daily basis have higher breath limonene (m: 131.9 ng/L, IQR: 10.7–367.9 ng/L) compared with non-daily consumers (m: 15.4 ng/L, IQR: 4.9–43.5 ng/L) (P value = 0.0093) (see Supplementary Digital Content 3, http://links.lww.com/CTG/A387).
Drugs interacting with CYP2C9 and CYP2C19 may modulate their activity (28,29) and affect limonene clearance. Therefore, we recorded medications taken by the patients within 1 week before breath sampling. Patients taking CYP2C9/19 substrates showed significantly increased breath limonene concentration compared with those not taking these medications (M: 74.0 and 10.2 ng/L, respectively) (P value = 0.0053). On the contrary, patients taking CYP2C9/19 inhibitors showed no significant difference compared with patients not taking those drugs (P value = 0.25) (M: 50.7 and 11.2 ng/L, respectively). Antacids, antibiotics, and lactulose also showed no effects on breath limonene levels (P value = 0.41) (see Supplementary Digital Content 4, http://links.lww.com/CTG/A387).
Detailed examination of the impact of comorbidities such as diabetes, portal hypertension, and increased body mass index on the exhaled limonene levels revealed no differences (data not shown).
DISCUSSION
In this pilot study, we have established that exhaled limonene levels are elevated in subjects with liver cirrhosis, regardless of the presence of HCC. Our data point toward the impact that cirrhosis has on liver function, being the primary driver for the elevation of breath limonene levels. These observations are in line with previous observations suggesting that limonene is primarily metabolized by the CYP450 enzymatic system (12). This implies that an exhaled limonene test could be a non-invasive way to evaluate liver functional capacity.
Our results confirm and expand on previous findings by O'Hara et al. (17) who demonstrated that limonene levels are elevated in subjects with HCC and cirrhosis and a study by Fernandez Del Rio et al. (14) which showed that restoring hepatic function by liver transplant brings breath limonene levels to baseline in the following weeks. Similarly, Sinha et al. (30) found high levels of limonene in the breath of patients with non-alcoholic steatohepatitis (NASH) cirrhosis, compared with those of patients with non-alcoholic fatty liver disease, further supporting our conclusion that cirrhosis is the primary driver for the elevation of breath limonene levels.
In this study, we have significantly expanded on this concept by showing that limonene correlates with established blood metrics of liver function, but not with ALT, a biomarker of liver damage. In agreement with our results, Sinha et al. (30) also mentioned a negative correlation between breath limonene and serum albumin. A difference can be observed with a study by Friedman et al. (15) that showed a correlation between lung air limonene and prothrombin time, but not with serum bilirubin and albumin. This discrepancy with our results may be explained by different approaches used to compare limonene with blood metrics. Here, we performed a linear regression analysis instead of stratifying the patients based on their breath limonene levels.
A strong aspect of our study is the diligent analysis of exhaled limonene levels. First, we ensured that the inhaled breath was purified air, isolated from any possible environmental limonene contamination. Second, the volume of sampled breath was standardized and closely monitored. Finally, extensive analytical validation was performed to assure that the sampled levels of limonene could be reliably quantified. Taken together, this gives us a high degree of confidence in our ability to provide absolute quantitation of limonene on breath. The detailed clinical characterization of cases furthermore adds to the strength of this study allowing us to evaluate the impact of disease severity and medication on exhaled limonene.
A limitation of the study is the unstandardized dietary intake of limonene. On one hand, this represents clinical reality; on the other hand, it could explain the relatively high number of false negatives based on a threshold of 10.2 ng/L exhaled limonene (27%). We tried to correct for this difference by administering a questionnaire attempting to quantify limonene intake. Importantly, this questionnaire was not validated and can therefore only provide a qualitative measure of limonene intake. A difference was observed between study subjects with low and high limonene intakes. Interestingly, 17 patients who consumed citrus products less then daily showed breath limonene >10.2 ng/L. The likely cause of these elevated levels is that, with reduced liver function, unmetabolized limonene is stored in fat tissue (31) and hence persists in the body for a long time (14). These observations undoubtedly indicate that standardizing limonene exposure would be vital to increasing performance of a limonene breath test and extend this approach to earlier stages of liver diseases such as NASH.
Our study supports the notion that the differences between control and diseased subjects observed in this study reflect differences in the activity of the CYP450 enzyme system. It is well established that chronic hepatic conditions result in downregulation of the CYP450 system (32,33), reduction of liver functional units (34), reduced portal blood flow, and impaired liver extraction capacity (34–36). The exact molecular mechanism for this is still unclear (32), but interleukin 6 (IL-6), a proinflammatory cytokine, may play an important role in this process. Increased levels of this cytokine were found in patients with liver conditions predisposing to HCC (37–41), including hepatic cirrhosis (34). Furthermore, IL-6 treatment of human primary hepatocytes downregulates the expression of the CYP450 system, including CYP2C9 and CYP2C19 (42), the limonene metabolizing enzymes (12). Consistent with these in vitro data, clearance of CYP450-metabolized drugs correlates inversely with plasma IL-6 levels in patients with various types of cancer (43). The reduction of functional liver units is a consequence of replacement of parenchymal tissue with fibrotic tissue that also alters the vascular architecture (34). These modifications lead to progressive sinusoidal capillarization, endothelial cells defenestration, and deposition of basement membrane components within the perisinusoidal space (34). Taken together, these alterations compromise the effective extraction capacity of the liver (44), including its ability to extract limonene.
Given the lipophilicity of limonene, an adipose tissue compartment model may best describe breath limonene pharmacokinetics (2,14,31). In a condition of reduced liver function, normal environmental exposure can overcome hepatic clearance, resulting in limonene accumulation in the fat. Ultimately, this results in prolonged elevated levels of limonene in the blood and therefore the breath. In healthy individuals, the hepatic metabolic capacity to biotransform limonene is sufficient to prevent the accumulation of limonene in the adipose tissue, resulting in rapidly decreasing breath levels after exposure.
An important mechanistic observation in this study is the fact that exhaled limonene levels correlate positively with bilirubin and INR, negatively with albumin, and do not correlate with ALT. We can speculate that this underpins the relation between exhaled limonene and the functional capacity of the liver. Bilirubin is removed by the liver through conjugation, and hence, increased levels of this substance may indicate an impaired ability to do so. Normal INR and albumin values depend on the protein synthesis capacity of the liver that is impaired in cirrhosis. Finally, ALT is released during the lysis of liver cells without it directly reflecting a functional liver parameter. Taken together, this evidence points in the direction that exhaled limonene is a non-invasive way of evaluating liver metabolic capacity by providing a proxy measurement of CYP2C9 and CYP2C19 metabolism.
Given the observed increase in liver disease, particularly NASH, there is an ever more pressing need for non-invasive tools that can easily detect liver impairment. A breath test is an attractive proposition from the perspective of being a noninvasive test that would be easily provided at the point of care. This study suggests that a breath test can complement currently available blood tests and provide clinicians with additional tools to guide their clinical decision-making. Further validation of a breath test for limonene in an intention to diagnose population in combination with blood and imaging tests could provide a valuable means of monitoring the increased number of patients.
In summary, this study has found clearly elevated levels of limonene in the breath of subjects with liver cirrhosis. This seems to be driven primarily by functional impairment of the liver, underpinning earlier observations that downregulation of the CYP450 system occurring in cirrhosis may be the underlying mechanism. Further evaluation of a limonene breath test will help elucidate its potential to aid clinical decision-making in subjects with hepatic diseases.
CONFLICTS OF INTEREST
Guarantor of the article: Marc P. van der Schee, MD, PhD.
Specific author contributions: G.F., I.O., and R.S. contributed equally to this work. M.P.v.d.S., I.O., M.A., O.G., C.A.M., M.A., M.H., R.C.F., V.K.S., and B.B.: conceived and designed the study. R.S., M.C.: developed the analytical methodology to meet requirements for quantitation of breath samples. A.K.T., A.d.S.: coordinated the clinical trial. I.D.-B., G.K., A.M.L.: recruited patients and collected breath. G.F.: analyzed data and drafted the article. M.W.: provided support for statistical analysis. All authors contributed to the interpretation of data, critical revision of the manuscript for important intellectual content, and approved the final manuscript.
Financial support: None to report.
Potential competing interests: G.F., I.O., R.S., M.C., A.K.T., M.W., O.G., A.d.S., M.A., B.B., M.P.v.d.S.: are employees of Owlstone Medical.
Study Highlights.
WHAT IS KNOWN
✓ Diagnosis of liver dysfunction remains challenging because of the lack of reliable biomarkers.
✓ Breath limonene concentrations are generally found to be higher in patients with cirrhosis compared with healthy controls.
WHAT IS NEW HERE
✓ Limonene has been independently confirmed as a breath biomarker associated with cirrhosis. However, onset of HCC over underlying cirrhosis has no effect on the limonene concentrations in the breath.
✓ Elevated breath limonene is associated with the severity of liver dysfunction and correlates with biomarkers reflecting disease severity, hepatic clearance, and protein synthesis capacity, but not with a biomarker that reflects liver damage.
TRANSLATIONAL IMPACT
✓ Exhaled limonene represents a potential biomarker for the non-invasive detection of chronic liver diseases and associated impairments of hepatic function.
ACKNOWLEDGMENT
We wish to acknowledge the selfless contributions of all subjects who partook in this study, Rebecca C. Fitzgerald, and the support of Cancer Research UK (CRUK).
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A387 and http://links.lww.com/CTG/A388
REFERENCES
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61. [DOI] [PubMed] [Google Scholar]
- 2.Holzhutter HG, Wuensch T, Gajowski R, et al. A novel variant of the (13)C-methacetin liver function breath test that eliminates the confounding effect of individual differences in systemic CO2 kinetics. Arch Toxicol 2020;94:401–15. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–14. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M. Japan's successful model of nationwide hepatocellular carcinoma surveillance highlighting the urgent need for global surveillance. Liver Cancer 2012;1:141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaude E, Nakhleh MK, Patassini S, et al. Targeted breath analysis: Exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J Breath Res 2019;13:032001. [DOI] [PubMed] [Google Scholar]
- 9.Solga SF, Risby TH. Issues and Challenges in Human Breath Research: Perspectives from Our Experience. Elsevier B.V.: Amsterdam, 2013, pp 19–24. [Google Scholar]
- 10.van der Schee MP, Paff T, Brinkman P, et al. Breathomics in lung disease. Chest 2015;147:224–31. [DOI] [PubMed] [Google Scholar]
- 11.Kim YW, Kim MJ, Chung BY, et al. Safety evaluation and risk assessment of d-Limonene. J Toxicol Environ Health B Crit Rev 2013;16:17–38. [DOI] [PubMed] [Google Scholar]
- 12.Miyazawa M, Shindo M, Shimada T. Metabolism of (+)- and (-)-limonenes to respective carveols and perillyl alcohols by CYP2C9 and CYP2C19 in human liver microsomes. Drug Metab Dispos 2002;30:602–7. [DOI] [PubMed] [Google Scholar]
- 13.Dadamio J, Van den Velde S, Laleman W, et al. Breath biomarkers of liver cirrhosis. J Chromatogr B Analyt Technol Biomed Life Sci 2012;905:17–22. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez Del Rio R, O'Hara ME, Holt A, et al. Volatile biomarkers in breath associated with liver cirrhosis—comparisons of pre- and post-liver transplant breath samples. EBioMedicine 2015;2:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman MI, Preti G, Deems RO, et al. Limonene in expired lung air of patients with liver disease. Dig Dis Sci 1994;39:1672–6. [DOI] [PubMed] [Google Scholar]
- 16.Morisco F, Aprea E, Lembo V, et al. Rapid “breath-print” of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS One 2013;8:e59658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Hara ME, Fernández Del Río R, Holt A, et al. Limonene in exhaled breath is elevated in hepatic encephalopathy. J Breath Res 2016;10:046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pijls KE, Smolinska A, Jonkers DM, et al. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci Rep 2016;6:19903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Sun A, Zhao Y, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 2019;567:257–61. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver. Electronic address easloffice@easloffice.eu, European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 22.Cholongitas E, Germani G, Burroughs AK. Prioritization for liver transplantation. Nat Rev Gastroenterol Hepatol 2010;7:659–68. [DOI] [PubMed] [Google Scholar]
- 23.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 24.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markar SR, Brodie B, Chin ST, et al. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br J Surg 2018;105:1493–500. [DOI] [PubMed] [Google Scholar]
- 26.Yang D, Hanna DL, Usher J, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: A surveillance, epidemiology, and end results analysis. Cancer 2014;120:3707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak J, Preti G. Volatile disease biomarkers in breath: A critique. Curr Pharm Biotechnol 2011;12:1067–74. [DOI] [PubMed] [Google Scholar]
- 28.Frye RF, Zgheib NK, Matzke GR, et al. Liver disease selectively modulates cytochrome P450—mediated metabolism. Clin Pharmacol Ther 2006;80:235–45. [DOI] [PubMed] [Google Scholar]
- 29.Branch RA, James JA, Read AE. The clearance of antipyrine and indocyanine green in normal subjects and in patients with chronic lever disease. Clin Pharmacol Ther 1976;20:81–9. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R, Lockman KA, Homer NZM, et al. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Reports 2020:100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller JA, Hakim IA, Chew W, et al. Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content. Nutr Cancer 2010;62:783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietrich CG, Götze O, Geier A. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J Gastroenterol 2016;22:72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elbekai RH, Korashy HM, El-Kadi AO. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab 2004;5:157–67. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013;144:512–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J Hepatol 2016;8:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George J, Murray M, Byth K, et al. Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology 1995;21:120–8. [PubMed] [Google Scholar]
- 37.Haukeland JW, Damås JK, Konopski Z, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006;44:1167–74. [DOI] [PubMed] [Google Scholar]
- 38.Heinz D, Peters M, Prange R, et al. Possible role of human interleukin-6 and soluble interleukin-6 receptor in hepatitis B virus infection. J Viral Hepat 2001;8:186–93. [DOI] [PubMed] [Google Scholar]
- 39.Kugelmas M, Hill DB, Vivian B, et al. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology 2003;38:413–9. [DOI] [PubMed] [Google Scholar]
- 40.Wieckowska A, Papouchado BG, Li Z, et al. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008;103:1372–9. [DOI] [PubMed] [Google Scholar]
- 41.Zhang F, Yao S, Zhang M, et al. Roles of circulating soluble interleukin (IL)-6 receptor and IL-6 receptor expression on CD4+ T cells in patients with chronic hepatitis B. Int J Infect Dis 2011;15:e267–71. [DOI] [PubMed] [Google Scholar]
- 42.Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos 2007;35:1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br J Cancer 2002;87:277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straub AC, Clark KA, Ross MA, et al. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J Clin Invest 2008;118:3980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]