Abstract
Objectives:
Current approaches to systemic antithrombotic therapy in support of extracorporeal membrane oxygenation are limited and are hampered by both thrombotic and hemorrhagic complications. An alternative approach is needed.
Design:
Inhibition of coagulation factor XI/activated factor XI is an appealing pathway for antithrombotic support of extracorporeal membrane oxygenation. Selective inhibition of the contact pathway of coagulation could reduce bleeding risk, and because factor XI is linked with the inflammatory and complement systems, it can also be viewed as a biologically plausible target for the prevention of abnormal thrombosis during extracorporeal membrane oxygenation.
Conclusions:
We introduce initial information on EP-7041, a parenteral, potent, and selective, small-molecule activated factor XIa inhibitor with pharmacodynamic and pharmacokinetic characteristics that appear well suited for use in a critical care environment.
Keywords: anticoagulation, antithrombotic therapy, extracorporeal membrane oxygenation, factor XI, hemorrhage
The use of extracorporeal membrane oxygenation (ECMO) has greatly expanded over the past 20 years and is expected to continue to rise, perhaps supported by increased use during the coronavirus disease 2019 pandemic (1). This marked increase has been attributed to a number of factors, including technological advances, more aggressive interventional care in acute cardiovascular deterioration, and growth and acceptance of cardiovascular/cardiopulmonary bridge technologies (2).
The overall impact of ECMO treatment on mortality and other clinical outcomes in many of these populations is still unclear, owing to a lack of broad data collections among constituent cohorts. It is known, however, that there are frequent complications with the use of ECMO, resulting from vascular injury with cannula placement (3), coagulopathy with both thromboembolic and hemorrhagic sequelae (4), lung injury despite lung rest (5), stroke (6), infection (7), and heparin-induced thrombocytopenia (HIT) (8), among others. It is challenging to titrate systemic antithrombotic therapy to prevent clotting within the circuit, without increasing the frequency and/or severity of bleeding complications in the patient. Limited high-quality data to guide antithrombotic management in patients on ECMO result in marked practice variability among centers (9), as well as recognition of a need for alternative antithrombotic strategies (10).
CLOT PREVENTION AND HEMOSTASIS IN ECMO
Unfractionated heparin (UFH) remains the standard antithrombotic agent for ECMO support, but with that therapy, bleeding remains the most common complication of ECMO (11). Antithrombotic therapy of some type is nonetheless generally required for ECMO, as contact activation of both coagulation and platelets, as well as shear forces inside the circuit, predispose to clotting.
The impact of the extracorporeal circuit itself on hemostasis is potentially significant. Hemolysis associated with abnormal hemodynamics within the circuit may contribute to systemic dysregulation of hemostasis, whereas heparin coating on circuit surfaces may further predispose patients to heparin sensitivity and HIT without lowering thrombosis risk (12). The systemic inflammatory response syndrome (SIRS), recognized to occur in ECMO, along with contact between the patient’s blood and the circuit, activates the coagulation cascade, leading to effects on fibrinolysis, thrombin formation, and platelet function (13). A recent study showed that bleeding events occurred in nearly two thirds of UFH-facilitated ECMO episodes and correlated to hospital mortality (14).
Antithrombotic strategies currently employed in ECMO include (15) the following: UFH, the advantages of which include familiarity and ease of administration, ready ability to measure effect (activated partial thromboplastin time [aPTT], activated clotting time), and ready reversibility; disadvantages include activation of PF4 antibodies and HIT, heparin “resistance,” and unreliability of laboratory measures of anticoagulation. A second option is low-molecular-weight heparin (LMWH): advantages include ease of administration, lower risk of HIT than with UFH; although not as readily monitored as UFH, anti-Factor XIa activity can be assessed rapidly in many larger centers; LMWH is not as readily reversed as UFH. Direct thrombin inhibitors (bivalirudin and argatroban) comprise a third option: advantages include efficacy independent of antithrombin levels, reliable dose-response, utility in known HIT, and ability to monitor; disadvantages include the lack of a reversal agent, lower activity in areas of stasis, and (for bivalirudin) a ceiling effect on aPTT and (for argatroban) potential interference with international normalized ratio measurement. Finally, no anticoagulation: at times, and usually for brief periods, anticoagulation may be withheld while on ECMO, although this can prove to be quite risky due to possibility for significant thrombosis to follow.
IS FACTOR XI/XIA A REASONABLE ANTITHROMBOSIS TARGET IN ECMO?
A summary of recent studies suggests that in addition to systemic anticoagulation, an acquired von Willebrand syndrome, platelet dysfunction, and activation of fibrinolysis likely contribute to the increased hemorrhage rates seen in ECMO. Selective inhibition of the contact (intrinsic) pathway of coagulation, specifically at the levels of factor XI (FXI) or factor XII (FXII), has potential applicability to the ECMO setting. The contact system consists of FXI, FXII, prekallikrein, and high-molecular-weight kininogen. FXI is an appealing target in ECMO owing to its obligatory role in contact activation of thrombin production and its significant contributory role in tissue factor-initiated thrombin generation. Activated FXI (FXIa) contributes to coagulation by promoting thrombin-mediated fibrin generation; FXI can be reciprocally activated by thrombin. In addition, because FXI is linked with the inflammatory and complement systems (as in SIRS), FXI can be seen as a biologically plausible target for the prevention of thrombosis in ECMO (16).
The most significant potential benefit of inhibiting FXI for ECMO, however, lies in its lack of contribution to normal hemostasis. Humans with congenital FXI deficiency (hemophilia C) may have a mild bleeding diathesis, which may become more clinically significant only after serious injury (surgery, trauma). Clinically, native FXI deficiency is most often found in the Ashkenazi Jewish population; those afflicted show significantly reduced rates of venous and arterial thromboembolisms compared with those with normal FXI levels (17). It has therefore been suggested that in vivo hemostasis is primarily dependent on the extrinsic pathway of coagulation, with the intrinsic (including contact) pathway providing primarily amplification of this process, which appears nonessential for preventing clinical bleeding (18).
In light of these issues, inhibition of FXI/FXIa becomes an attractive potential alternative to current anticoagulant strategies for ECMO support. The mechanistic strategies for FXI inhibition being studied to date include antisense oligonucleotides that reduce hepatic synthesis of FXI, monoclonal antibodies that block FXI activation or FXIa activity, aptamers that bind FXI/FXIa, and small molecules that block the active site of FXIa or cause allosteric modulation. Only one agent is being studied in the ECMO milieu: EP-7041, an IV small molecule FXIa antagonist now in active development (Fig. 1). Phase 1 human studies showed EP-7041 to be safe and well-tolerated (19). Indeed, in vivo studies with EP-7041 have demonstrated the ability of the molecule to inhibit thrombosis while minimizing the risk of unwanted bleeding.
Figure 1.
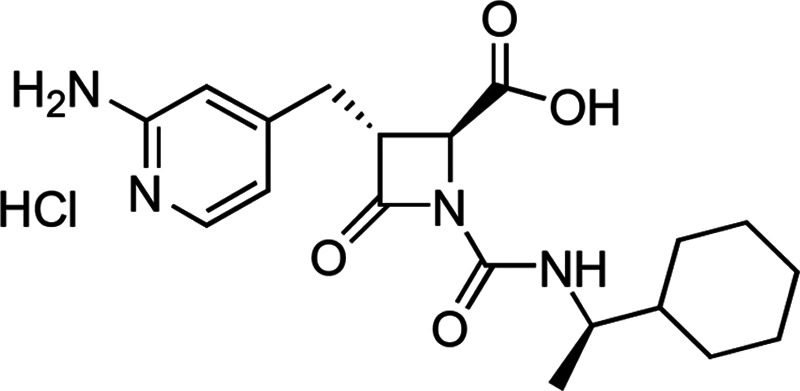
(2S,3R)-3-([2-Aminopyridin-4-yl]methyl)-1-([{1R}-1-cyclohexylethyl] carbamoyl)-4-oxoazetidine-2-carboxylic acid hydrochloride. The chemical formula of the hydrochloride (HCl) salt of EP-7041 is C19H27ClN4O4.
Safety considerations are quite attractive for the ECMO model: EP-7041 is highly selective for the target, inhibiting human FXIa with an IC50 of 7.1 nM, whereas the inhibition of human factor Xa, thrombin, and trypsin were significantly higher at 14,000 nM, greater than 20,000 nM and 4,800 nM, respectively (unpublished data). This lack of off-target effects supports a very broad therapeutic index compared with other anticoagulants. Although several anti-FXIa antibodies and antisense oligonucleotides have achieved similar specificity and selectivity (20), no small-molecule inhibitor displaying these critical characteristics has been described in the literature to date (18).
Second, EP-7041 has a very short half-life compared with antibody- and antisense-derived molecules, as well as to small molecules in development for oral administration (18). In humans, EP-7041 has a terminal elimination half-life of approximately 45 minutes following a single IV bolus, with a low volume of distribution (≅ 350 mL/kg), with a shorter pharmacodynamic (per aPTT) half-life; in healthy volunteers, at a dose of 0.6 mg/kg/hr, the aPTT decreased from 1.7 × baseline just prior to the end of a 5-day continuous infusion to just 1.2 × baseline within 1 hour of terminating the infusion (Fig. 2). Although pharmacologic blockade does not necessarily mirror native factor deficiency, these pharmacodynamic variables in the context of the clinical experience with inherited FXI deficiency do offer some reassurance that a specific reversal agent may not be required for the short-lived antithrombotic effect of EP-7041.
Figure 2.
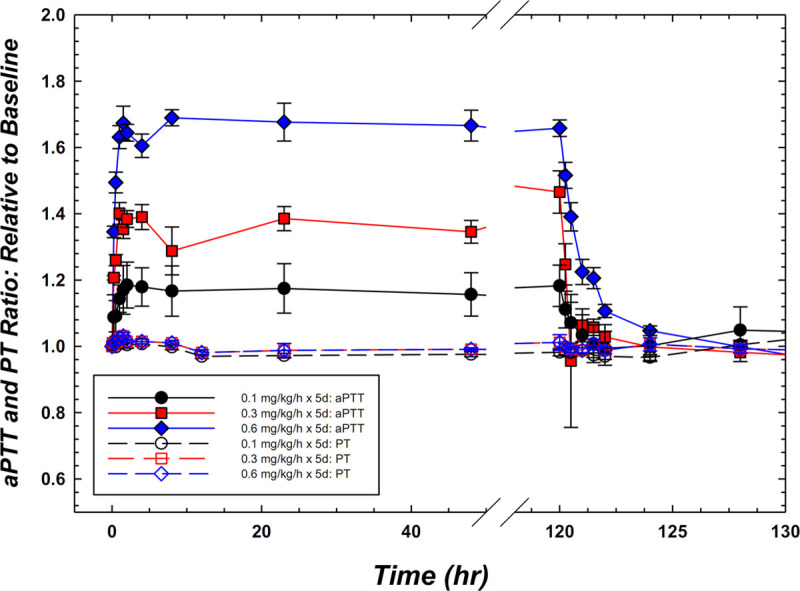
Pharmacodynamic activated partial thromboplastin time (aPTT) response to EP-7041 in humans following continuous IV administration for 5 d. At 0.6 mg/kg/hr, the mean increase in aPTT was 1.7 compared with baseline, importantly with no off-target change in prothrombin time (PT). In addition, the results highlight the rapid onset, prompt clearance, and predictable dose-related elevations in the pharmacodynamic marker, aPTT.
Third, both hepatic (primary) and renal (secondary) mechanisms contribute to clearance of EP-7041 (unpublished data). Multiple clearance pathways, combined with a broad therapeutic index, generally provide for a better safety profile than those associated with the single-path clearance of the anticoagulants currently employed in clinical practice. In addition, because EP-7041 acts directly at the target active site, it produces a predictably consistent antithrombotic effect (as measured by the aPTT), regardless of patient fluid volume status or cofactor depletion. Furthermore, as a molecule structurally unrelated to heparins, there is no risk of HIT or HIT-related thrombosis. Thus, the pharmacodynamic and pharmacokinetic characteristics of EP-7041—IV administration, small volume of distribution, rapid onset of action, stable, predictable effect on coagulation as measured by the aPTT, short pharmacodynamic half-life, and apparent lack of dependence on renal clearance mechanisms—appear well-suited for use in a critical care environment.
Finally, by preferentially inhibiting thrombosis rather than normal hemostasis, EP-7041 holds the promise of preventing circuit and vascular thromboembolism with substantially less inhibition of local hemostasis, potentially enabling safer use of ECMO or other invasive vascular intervention (surgery, ventricular assist device placement, etc.) in critically ill patients.
In the mesenteric arterial puncture model in the rat, there was no bleeding liability of EP-7041 at dose levels up to 30-fold greater than those needed to afford statistically significant efficacy in a venous thrombosis/clot model in the same species. Similar bleeding times were observed in EP-7041–treated animals (0.3, 3, and 10 mg/kg IV) versus those treated with vehicle. The minimum efficacious dose for EP-7041 in an established rat model (20) of thrombosis is 0.3 mg/kg. In comparison, heparin at its efficacious dose of 25 U/kg + 50 U/kg/hr increased bleeding time by 32% versus vehicle-treated animals (245 ± 27 vs 186 ± 23 s; p = 0.056) and was shown to markedly increase bleeding time at just twice its efficacious dose (386 ± 60 s; p = 0.0021) (Fig. 3).
Figure 3.
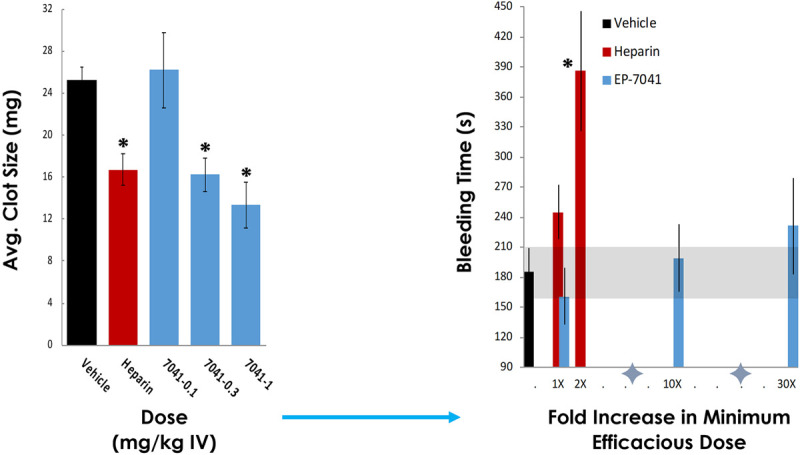
Preclinical efficacy and safety of EP-7041 versus unfractionated heparin (UFH) in standard ferric chloride thrombosis (A) and mesenteric artery bleeding (B) models in the rat. Results showed an equivalent effect of EP-7041 and UFH on average (Avg.) clot size, but unlike UFH, that demonstrated an increase in bleeding times at its minimum efficacious dose (dose titrated to human target (international normalized ratio [INR] 2-3; 25 U/Kg + 50 U/Kg/hr) and marked bleeding at just twice this dose), EP-7041 was without an appreciable increase in bleeding, even at dose levels 30-fold greater than those that afforded efficacy (*p < 0.05; Unpublished data).
Study of EP-7041 in the ECMO setting is ongoing. We have developed a canine model of veno-venous ECMO to compare the efficacy of EP-7041 with UFH. Doses were targeted to maintain aPTT in the heparin arm between 1.5 × –2.0 × baseline (mean 1.6×). Over the course of a 2.5-hour ECMO run, pressure proximal to the membrane oxygenator (MO) increased in both heparin- and EP-7041–treated animals, although pressure increases tended to be transient. However, bleeding volume was at least five times greater in heparin-anticoagulated animals than with the highest dose of EP-7041 tested (aPTT = 2.3 ± 0.1 × baseline). A small group of vehicle controls (saline) were also studied. All vehicle-treated animals failed to complete the ECMO run, fully occluding the MO within 20–50 minutes (mean, 39 min) (21). These studies support the notion that FXIa inhibition may be an effective alternative to heparin anticoagulation during veno-venous ECMO. After successful completion of this model, we plan to evaluate EP-7041 as an antithrombotic management strategy for ECMO in patients.
SUMMARY
Selective inhibition of FXI/FXIa is an appealing strategy for antithrombotic therapy during ECMO. The current standard of care for therapy is UFH despite the common thrombotic and hemorrhagic complications that accompany its use. Inhibition of FXI/FXIa, such as with EP-7041, offers a potentially novel approach to reducing the imbalance between procoagulant and anticoagulant factors in ECMO, and thorough research in this setting is warranted.
Footnotes
Dr. Pollack is a scientific consultant to eXIthera Pharmaceuticals, Inc. Drs. Kurz and Hayward are employed by eXIthera Pharmaceuticals, Inc.
REFERENCES
- 1.Kowalewski M, Fina D, Słomka A, et al. COVID-19 and ECMO: The interplay between coagulation and inflammation—a narrative review. Crit Care. 2020; 24:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerke AK, Tang F, Cavanaugh JE, et al. Increased trend in extracorporeal membrane oxygenation use by adults in the United States since 2007. BMC Res Notes. 2015; 8:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augusto R, Silva MP, Campos J, et al. Rev Port Cir Cardiotorac Vasc. 2019; 26:45–50 [PubMed] [Google Scholar]
- 4.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017; 63:60–67 [DOI] [PubMed] [Google Scholar]
- 5.ProQuest. C58 Critical Care Case Reports: Notable Causes and Complications in Acute Respiratory Failure: Weaning Mechanical Ventilation During Vv-ECMO: The Successful Use of Aprv. 2017. Available at: https://search.proquest.com/openview/5f4e290ce9247bd777c5b76297767ead/1?pq-origsite=gscholar&cbl=40575. Accessed March 12, 2020
- 6.Xie A, Lo P, Yan TD, et al. Neurologic complications of extracorporeal membrane oxygenation: A review. J Cardiothorac Vasc Anesth. 2017; 31:1836–1846 [DOI] [PubMed] [Google Scholar]
- 7.ProQuest. C54 Critical Care Case Reports: SEPSIS: Scedosporium Apiospermum Infection: Lethality and Complications in the Process of Treatment With Extracorporeal Membrane Oxygenation (ECMO). 2016. Available at: https://search.proquest.com/openview/24d1ed4ffbd1ba018ca3f7d6ce3e8344/1?pq-origsite=gscholar&cbl=40575. Accessed March 12, 2020
- 8.Koerner MM, Harper MD, Gordon CK, et al. Adult cardiac veno-arterial extracorporeal life support (VA-ECMO): Prevention and management of acute complications. Ann Cardiothorac Surg. 2019; 8:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chlebowski MM, Baltagi S, Carlson M, et al. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020; 24:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong JJ-M, Lam JCM, Mok YH, et al. Anticoagulation in extracorporeal membrane oxygenation. J Emerg Crit Care Med. 2018; 2 [Google Scholar]
- 11.Lotz C, Streiber N, Roewer N, et al. Therapeutic interventions and risk factors of bleeding during extracorporeal membrane oxygenation. ASAIO J. 2017; 63:624–630 [DOI] [PubMed] [Google Scholar]
- 12.Warkentin TE. Heparin-coated. intravascular devices and heparin-induced thrombocytopenia. Heparin-Induced Thrombocytopenia. 2013. Fifth, Boca Raton, FL: CRC Press; 573–590 [Google Scholar]
- 13.Thangappan K, Cavarocchi NC, Baram M, et al. Systemic inflammatory response syndrome (SIRS) after extracorporeal membrane oxygenation (ECMO): Incidence, risks and survivals. Heart Lung. 2016; 45:449–453 [DOI] [PubMed] [Google Scholar]
- 14.Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016; 6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juffermans NP, van Stijn I, Dupuis H, et al. Neth J Crit Care. 2018; 26:1 [Google Scholar]
- 16.Silasi R, Keshari RS, Lupu C, et al. Inhibition of contact-mediated activation of factor XI protects baboons against S aureus-induced organ damage and death. Blood Adv. 2019; 3:658–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preis M, Hirsch J, Kotler A, et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017; 129:1210–1215 [DOI] [PubMed] [Google Scholar]
- 18.DeLoughery EP, Olson SR, Puy C, et al. The safety and efficacy of novel agents targeting factors XI and XII in early phase human trials. Semin Thromb Hemost. 2019; 45:502–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward NJ, Goldberg DI, Morrel EM, et al. Abstract 13747: Phase 1a/1b Study of EP-7041: A novel, potent, selective, small molecule FXIa inhibitor. Circulation. 2017; 136Suppl_1A13747 [Google Scholar]
- 20.Schumacher WA, Seiler SE, Steinbacher TE, et al. Antithrombotic and hemostatic effects of a small molecule factor XIa inhibitor in rats. Eur J Pharmacol. 2007; 570:167–174 [DOI] [PubMed] [Google Scholar]
- 21.Kurz M, Pollack C, Jr, Connors J, et al. Anticoagulation with the Novel, Small-molecule Factor XIa (fXIa) Antagonist, EP-7041, Prevents Oxygenator Clotting but Conserves Hemostasis in a Canine Extracorporeal Circulation (ECMO) Model [abstract]. Res Pract Thromb Haemost. 2020; 4 (Suppl 1) Available at: https://abstracts.isth.org/abstract/anticoagulation-with-the-novel-small-molecule-factor-xa-fxia-antagonist-ep-7041-prevents-oxygenator-clotting-but-conserves-hemostasis-in-a-canine-extracorporeal-circulation-ecmo-model/. Accessed August 21, 2020 [Google Scholar]


