Supplemental Digital Content is available in the text.
Keywords: cell therapy, coronavirus disease 2019, cytokine release syndrome, mechanical ventilation, respiratory distress syndrome
Abstract
Objectives:
To determine whether placental cell therapy PLacental eXpanded (PLX)-PAD (Pluristem Therapeutics, Haifa, Israel) may be beneficial to treating critically ill patients suffering from acute respiratory distress syndrome due to coronavirus disease 2019.
Design:
Retrospective case report of critically ill coronavirus disease 2019 patients treated with PLacental eXpanded (PLX)-PAD from March 26, 2020, to April 4, 2020, with follow-up through May 2, 2020.
Setting:
Four hospitals in Israel (Rambam Health Care Campus, Bnai Zion Medical Center, and Samson Assuta Ashdod University Hospital), and Holy Name Medical Center in New Jersey.
Patients:
Eight critically ill patients on invasive mechanical ventilation, suffering from acute respiratory distress syndrome due to coronavirus disease 2019.
Interventions:
Intramuscular injection of PLacental eXpanded (PLX)-PAD (300 × 106 cells) given as one to two treatments.
Measurements and Main Results:
Mortality, time to discharge, and changes in blood and respiratory variables were monitored during hospitalization to day 17 posttreatment. Of the eight patients treated (median age 55 yr, seven males and one female), five were discharged, two remained hospitalized, and one died. By day 3 postinjection, mean C-reactive protein fell 45% (240.3–131.3 mg/L; p = 0.0019) and fell to 77% by day 5 (56.0 mg/L; p < 0.0001). Pao2/Fio2 improved in 5:8 patients after 24-hour posttreatment, with similar effects 48-hour posttreatment. A decrease in positive end-expiratory pressure and increase in pH were statistically significant between days 0 and 14 (p = 0.0032 and p = 0.00072, respectively). A decrease in hemoglobin was statistically significant for days 0–5 and 0–14 (p = 0.015 and p = 0.0028, respectively), whereas for creatinine, it was statistically significant between days 0 and 14 (p = 0.032).
Conclusions:
Improvement in several variables such as C-reactive protein, positive end-expiratory pressure, and Pao2/Fio2 was observed following PLacental eXpanded (PLX)-PAD treatment, suggesting possible therapeutic effect. However, interpretation of the data is limited due to the small sample size, use of concomitant investigational therapies, and the uncontrolled study design. The efficacy of PLacental eXpanded (PLX)-PAD in coronavirus disease 2019 should be further evaluated in a controlled clinical trial.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to spread globally despite unprecedented social isolation and restrictions. As of June 4, 2020, the World Health Organization reported more than 6.2 million confirmed cases of COVID-19 with over 380,000 confirmed deaths. To date, despite limited evidence of efficacy in early-stage patients receiving antiviral agents, no treatments that have definitively been shown to be effective exist; nevertheless, a multipronged approach to mitigate transmission, morbidity, and mortality is ongoing worldwide (1).
In most patients, SARS-CoV-2 infections are either asymptomatic or present as cases resembling the seasonal flu or as a mild form of pneumonia. A syndrome of dysregulated and systemic immune overactivation, described as a cytokine storm or hyperinflammatory syndrome (2, 3), can develop in severely affected patients. The cytokine storm, associated with excessive production of proinflammatory cytokines and considered to be one of the major causes of vascular hyperpermeability that worsens the symptoms of acute respiratory distress syndrome, may lead to multisystem organ failure and mortality (4–7). Acute respiratory distress syndrome (ARDS), a clinical phenomenon marked by development of bilateral infiltrates and hypoxemia, defined as a worsening of the ratio of Pao2/Fio2 (P/F) (8), develops in the majority of patients with severe disease. The majority of COVID-19 patients suffering from ARDS require invasive mechanical ventilation. These patients tend to remain ventilator-dependent for 10–14 days, and up to 80% of those patients ultimately succumb to the disease (6, 9).
Current primary treatment protocols call for supportive care and supplemental oxygen with invasive mechanical ventilatory support when needed. Preliminary studies suggested that immune-modulatory or immune-suppressive treatments such as hydroxychloroquine, and interleukin (IL)-6 and IL-1 antagonists commonly used in rheumatology might be considered as treatment choices for COVID-19, particularly in severe cases (10). However, further studies on these and other treatment modalities are still underway and current data are limited. In addition, according to the Randomized Evaluation of COVid-19 thERapY trial, the use of dexamethasone was shown to reduce deaths by one-third in ventilated patients and by one-fifth in other patients receiving oxygen only. Finally, the use of convalescent plasma in these patients has shown promising results and is currently being evaluated in more than 100 clinical studies (https://clinicaltrials.gov). In a pilot study of 10 severe COVID-19 patients, transfusion of one dose of convalescent plasma was well tolerated and improved clinical symptoms and paraclinical criteria within 3 days (11).
Mesenchymal stromal cell (MSC) therapeutics are candidates for tackling the broad spectrum of COVID-19 symptoms due to their multifactorial mode-of-action (12), and are now being tested in more than 30 active clinical trials (12–14). MSC therapies have shown promising results in the treatment of ARDS and sepsis, but efficacy data are limited (15–17).
PLacental eXpanded (PLX)-PAD (PLX-PAD) (Pluristem Therapeutics, Haifa, Israel) contains placenta-derived MSC-like cells that have regenerative and immunomodulatory properties (18–26). Although PLX-PAD cells exhibit membrane markers typical of classical MSCs, they have a minimal ability to differentiate in vitro into cells of the mesodermal lineage and are, thus, termed MSC-like cells. PLX-PAD reduces the production of the proinflammatory cytokines tumor necrosis factor-α (TNF-α), interferon-γ, and IL-17A, and induces the secretion of the anti-inflammatory cytokines IL-10 and IL-1Ra. PLX-PAD also increases T-regulatory cells, decreases T cell proliferation, and shifts the macrophage population to the M2 phenotype (19, 24). In addition, PLX treatment was found to reduce lung fibrosis in a murine model (unpublished data). The cells present immune-modulatory properties versus lymphoid (T cells) and myeloid (antigen-presenting cell) cells (19), both reported to be involved in COVID-19 complications (12). PLX-PAD cells have been safely administered to hundreds of patients in clinical studies for peripheral artery disease and muscle injuries, demonstrating a very high safety profile (18, 20). Data indicate that PLX-PAD is efficacious in conditions requiring immunomodulatory and regenerative therapies, such as critical limb ischemia and postarthroplasty muscle recovery (18, 20, 21, 24).
Herein, we report the outcome of treating eight patients suffering from respiratory failure and ARDS due to COVID-19 with human placenta-derived mesenchymal-like stromal cells (PLX-PAD).
MATERIALS AND METHODS
Ethics Statement
Patients were treated in Israel under emergency/compassionate use and in the United States under single patient investigational new drug for compassionate or emergency use. The institutional review board of all medical centers approved the study and waived the need for informed consent for this retrospective study.
Test Compound
PLX-PAD is an allogeneic ex vivo placental-expanded adherent stromal cell product whose manufacturing procedure and characterization have been previously described (18). Briefly, the cells are derived from a full-term human placenta following a cesarean section and expanded using plastic adherence on tissue culture dishes, followed by 3D growth on carriers in a bioreactor. PLX-PAD is aseptically transferred to cryogenic vials at a concentration of 20 × 106 cells/mL in a mixture containing 10% dimethyl sulfoxide, 5% human albumin, and Plasma-Lyte (Baxter Healthcare, Toongabbie, NSW, Australia). Storage takes place in gas-phase liquid nitrogen at temperatures lower than −150°C. The required amount of PLX-PAD is thawed in a heated water bath (37°C) immediately prior to injection. Expression levels of angiotensin-converting enzyme 2 (ACE2) (Novus Biologicals, LLC, Centennial, CO) and type II transmembrane serine protease (TMPRSS2) (LS BIO, Seattle, WA) in PLX-PAD cells were determined by flow cytometry on three different batches of PLX-PAD according to manufacturer instructions.
PLX-PAD is an investigational product in the clinical development stage and is not authorized for sale in any country.
Treatment Procedure
Patients were treated with PLX-PAD in Israel and the United States under compassionate/emergency use frameworks that were approved by the Israeli Ministry of Health or the U.S. Food and Drug Administration, respectively. The patients were treated in the following hospitals: Rambam Health Care Campus (Haifa, Israel), Bnai Zion Medical Center (Haifa, Israel), Samson Assuta Ashdod University Hospital (Ashdod, Israel), and Holy Name Medical Center (Teaneck, NJ). PLX-PAD cells were supplied by Pluristem at no cost. Each treatment consisted of 300 million cells administered via 15 intramuscular injections (1 mL each). Injections were divided among the triceps, biceps, vastus laterals, and medialis muscles. Five of the eight patients described in this report received one treatment (300 million cells), and three received two treatments (300 million cells each) at an interval of 8 or 11 days according to physician discretion. Each patient was followed up until death or discharge from the hospital.
Clinical Data Collection
Information about the treated patients was obtained retrospectively from the hospital electronic medical records and included the following: demographics, laboratory data, hospitalization period, other treatments data, clinical data, medical history, and ventilation variables.
Data Analysis
All data analyses were conducted in Version 3.6.1 of R (R Foundation for Statistical Computing, Vienna, Austria). Using the lmerTest package, baseline-adjusted repeated measure linear models were fit with restricted maximum likelihood, and t tests were computed using Satterthwaite method. Excluded were time points in which only a single subject contributed data before running the repeated measures models.
RESULTS
Eight critically ill patients suffering from ARDS due to COVID-19 were treated between March 26, 2020, and April 4, 2020.
General Characterization of the Patients
The general characterization of the patients is described in Table 1. A total of eight patients (seven males and one female) were treated with PLX-PAD. Five received one dose of PLX-PAD (300 million cells) and three received two doses of PLX-PAD (2 × 300 million for a total of 600 million cells). All patients were confirmed for SARS-CoV-2 infection by real-time reverse transcriptase-polymerase chain reaction. The median age of the patients was 55 years (range, 22–79 yr); five out of the eight patients were at higher risk for severe illness from COVID-19 due to underlying medical conditions. The most common comorbidities were hypertension (four patients) and diabetes (four patients), with three patients suffering from both. Seven patients had body mass index above 25, and none were active smokers.
Table 1.
General Characteristics of Severe Acute Respiratory Syndrome Coronavirus 2 Infected Patients Who Received PLacental eXpanded-PAD Treatment
| Characteristic | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Subject 7 | Subject 8 |
|---|---|---|---|---|---|---|---|---|
| Site | Rambam | Bnai Zion | Rambam | Assuta | Assuta | Assuta | Bnai Zion | Holy Name |
| Age | 71 | 79 | 56 | 54 | 53 | 22 | 65 | 49 |
| Sex | Male | Male | Male | Male | Male | Male | Female | Male |
| Body mass index | > 30 | > 30 | 29.5 | 24.4 | 30 | 30.5 | > 30 | 27.8 |
| Active smoker | No | No | No | No | No | No | No | No |
| Diabetes | Yes | No | Yes | No | Yes | No | Yes | No |
| Hypertension | Yes | Yes | Yes | No | No | No | Yes | No |
| Chronic obstructive pulmonary disease | No | No | No | No | No | No | No | No |
| Ischemic heart disease | No | No | No | No | No | No | No | No |
| Number of PLacental eXpanded-PAD treatments | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 1 |
| Other investigational drugs | Hydroxychloroquine, lopinavir | Hydroxychloroquine, remdesivir | Hydroxychloroquine, lopinavir | Hydroxychloroquine, anti-IL-6 | Hydroxychloroquine, anti-IL-6 | Hydroxychloroquine | Hydroxychloroquine, lopinavir, anti-IL-6 | Hydroxychloroquine, remdesivir |
| Steroids | Yes | Yes | No | No | No | Yes | No | Yes |
| Number of hospital days | 26 | 69 | 22 | NA | 27 | 48 | NA | 56 |
| Days intubated before treatment | 5 | 14 | 1 | 10 | 10 | 2 | 2 | 22 |
| Days intubated after treatment | 20 | 16 | 7 | Ongoing | 14 | NA | 35 | 11 |
| Status | Died | Discharged | Discharged | In Hospital | Discharged | Discharged | In Hospital | Discharged |
anti-IL-6 = anti-interleukin 6, NA = not available.
Prior to PLX-PAD treatment, all patients received hydroxychloroquine, three received lopinavir/ritonavir, and two received remdesivir. In addition, three patients received IL-6 inhibitors and four steroids. Two patients required extracorporeal membrane oxygenation (ECMO). Patient number 6 required resuscitation due to a massive pulmonary embolism, which occurred 4 days after PLX-PAD treatment and was placed on ECMO until full recovery. Patient number 4 was electively placed on ECMO due to severe nonresolving ARDS and hypoxia. The average length of hospital stay was 41 days for the six patients who were discharged (with follow-up ending on May 2, 2020). Five patients were intubated for at least 5 days prior to PLX-PAD treatment, with one patient (patient number 8) being intubated for 22 days prior to PLX-PAD treatment. As of June 24, 2020, five patients had been discharged, two remain hospitalized, and one died.
Blood C-Reactive Protein Levels Fall Following Administration of PLX-PAD
All patients had elevated levels of C-reactive protein (CRP) that ranged from 82 to 394 mg/L at the time of treatment, with four having CRP levels higher than 300 mg/L. Following PLX-PAD treatment, CRP levels decreased in all eight patients starting as early as 24-hour post-treatment and continued to decrease throughout the study follow-up period (Fig. 1A). By day 3, the mean level had fallen 45% from 240.3 to 131.3 mg/L (p = 0.0019), and by day 5, it had fallen by 77% to 56.0 mg/L (p < 0.0001). By either day 14 or 15 posttreatment (sample collection date differed among the patients), six patients (data for patients number 4 and 6 are not available) had CRP levels equal to or less than 91 mg/L. At 2- and 6-day posttreatment, CRP levels reduced significantly toward normal ranges in patients number 6 (11 mg/L) and 7 (9.3 mg/L). The drop in blood CRP following injection of PLX-PAD either on day 0 (data from all subjects are shown) or, if the subject had a second injection, on either day 8 or 11 (day 11 for subject number 1 and day 8 for subjects number 3 and 7) is shown in Figure 1B. These data generally show that the higher the level of blood CRP, the more dramatic its reduction following treatment.
Figure 1.
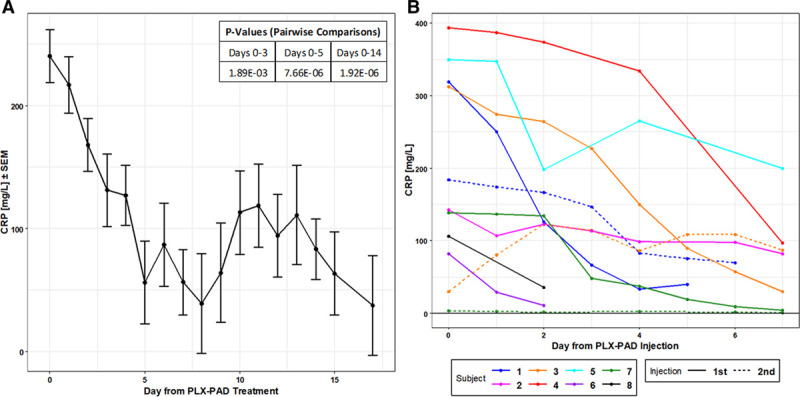
Changes in blood C-reactive protein (CRP) levels. A, Baseline-adjusted least square means of CRP levels following PLacental eXpanded (PLX)-PAD treatment (day 0) until day 17. Pairwise comparisons were performed from the differences in least square means. The table in the upper left shows the statistically significant p values between day 0 and days 3, 5, and 14. B, CRP levels by subject following either the first or the second PLX-PAD administration. For patient number 1, the second injection was on day 11, and for patients number 3 and 7, it was on day 8.
Blood Measurements
Numerous blood and respiratory variables were obtained on the subjects from day 0 (prior to PLX-PAD treatment) to day 17 and changes observed in hemoglobin, creatinine, WBCs, absolute neutrophil count, and platelet are presented in Supplemental Figure 1 (http://links.lww.com/CCX/A297). The decrease in creatinine was statistically significant between days 0 and 14 (p = 0.032), a 53% drop from 1.875 to 0.883 mg/dL.
Respiratory Variables Following Administration of PLX-PAD
P/F data before and after PLX-PAD treatment are shown in Table 2. In five patients, the ratio improved 24-hour posttreatment. The effect was still present in five patients 48-hour postinjection.
Table 2.
Pao2/Fio2 Ratio of Severe Acute Respiratory Syndrome Coronavirus 2 Infected Patients Before and After Treatment With PLacental eXpanded-PAD
| Patient No. | Before Treatment | 24-hr Post Treatment | 48-hr Post Treatment |
|---|---|---|---|
| 1 | 160 | 229 | 170 |
| 2 | 140 | 172.5 | 177.5 |
| 3 | 143 | 151 | 217 |
| 4 | 149 | 107 | 92 |
| 5 | 106 | 145 | 197 |
| 6 | 173 | 205 | 151 |
| 7 | 172 | 93 | 95 |
| 8 | 342.5 | Not available | 425 |
Improvement in Chest Radiographs Following Administration of PLX-PAD
Chest radiographs were obtained from six patients. In patients’ number 1 and 3, the radiographs demonstrated some resolution and improvement in interstitial opacities. Figure 2 provides two radiographs from patient number 3. Some improvement was also seen in patients’ number 5, 6, and 8. No improvement was seen in patient number 4. Data prior to PLX-PAD treatment were not available for patients’ number 2 and 7.
Figure 2.
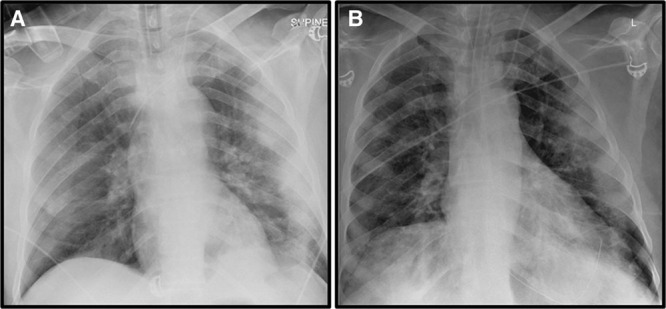
Chest radiographs of patient number 1. A chest radiograph (anteroposterior view) of the chest prior to PLacental eXpanded (PLX)-PAD treatment (A) shows diffuse opacities. Follow-up chest radiograph obtained approximately 24-hr post-PLX-PAD treatment (B) shows improvement in these findings. L = left.
Figure 3 shows that the decrease in positive end-expiratory pressure and increase in pH were both statistically significant between days 0 and 14 (p = 0.0032 and p = 0.00072, respectively), whereas the changes observed for Fio2 and Pco2 were not statistically significant. Furthermore, the increase in oxygen saturation was statistically significant by day 5 (p = 0.050).
Figure 3.
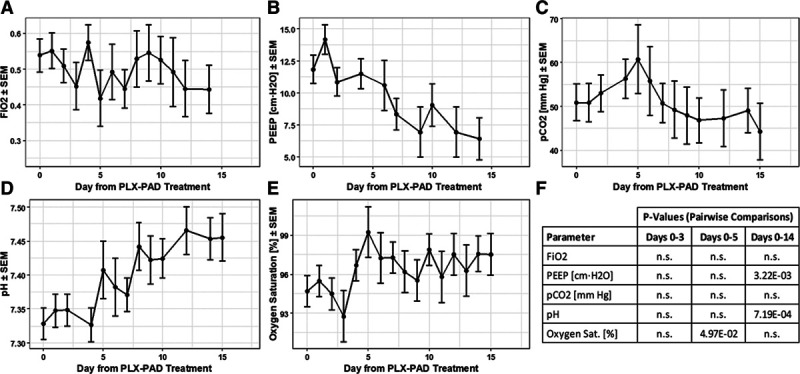
Changes in respiratory variables. Baseline-adjusted least square means of (A) Fio2, (B) positive end-expiratory pressure (PEEP), (C) Pco2, (D) pH, and (E) oxygen saturation. Statistically significant p values between day 0 and days 3, 5, and 14 are shown (F). n.s. = not significant.
Preliminary Safety Outcome
No related adverse events attributed to PLX-PAD treatment were reported.
PLX-PAD Cells Do Not Express the SARS-CoV-2 Receptor ACE2 or the Serine Protease TMPRSS2
Analysis of ACE2 and TMPRSS2 expression in PLX-PAD cells was done by flow cytometry. As shown in Supplemental Figure 2 (http://links.lww.com/CCX/A298), PLX-PAD did not express either ACE2 or TMPRSS2. Considering the necessity of both ACE2 and TMPRSS2 for SARS-CoV-2 infection (27), our results suggest that PLX-PAD cells are unlikely to become an additional target for SARS-CoV-2 within infected subjects.
DISCUSSION
In this case series, eight patients critically ill due to COVID-19 were treated with PLX-PAD, a placenta-derived mesenchymal-like cell therapy.
Several variables showed improvement in posttreatment, specifically, CRP, creatinine levels, P/F, and radiologic findings as shown in chest radiographs.
MSC-based treatment has been proposed as a suitable therapeutic approach due to their beneficial immunomodulatory and regenerative properties (14). MSCs have been studied as a promising candidate to treat certain inflammatory conditions and immunologic diseases based on their well-characterized immunomodulatory effects. The immunomodulatory activities are thought to include the following: 1) inhibition of the proliferation and function of T cells, B cells, dendritic cells, and natural killer cells; 2) monocyte polarization to anti-inflammatory M2 macrophages; and 3) production of IL-10 and decreased production of TNF-α and IL-12 (28, 29). Indeed, PLX-PAD, a MSC-like product, has been shown to have such immunomodulatory properties (19). In addition, MSCs are known for their powerful antifibrotic effects and may alleviate lung fibrosis (30, 31).
Two recent studies from China (32, 33) have evaluated MSC treatment for COVID-19 pneumonia. Both studies reveal remarkable reversal of symptoms even in severe-critical conditions.
Similar to other reports on MSCs (32), PLX-PAD is negative for both ACE2 and TMPRSS2; therefore, it can be safely used in COVID-19 patients without being a further target for SARS-CoV-2.
PLX-PAD was administered via intramuscular injection. The most frequently anticipated form of cell product delivery in ARDS and COVID-19 is the IV infusion of MSCs with the primary aim of targeting the lungs (12, 34). It is not yet clear if the IV route of administration is a safe and effective route of cell delivery in COVID-19, considering that MSC products express variable levels of a highly procoagulant tissue factor (CD142) (35). Numerous clinical reports indicate that many of the critically ill COVID-19 patients with poor prognosis are in a systemic procoagulant state at high risk of disseminated intravascular coagulation (36–41), thromboembolism, and thrombotic multiple organ failure, leading causes of death in these patients. This would make IV applications of MSCs a contraindication in COVID-19. Intramuscular injection of PLX-PAD was chosen because of longer in vivo survival of the cells, improved functionality, and a lack of hemocompatibility issues (18, 34, 42–45). Mode of delivery has a significant impact on the therapeutic activity of MSCs (45). Intramuscular delivery potentiates the dwell time of MSCs due to the favorable in vivo milieu (34, 42, 44). The highly vascularized muscle tissue serves as a physiologic environment able to supply the therapeutic cells with oxygen and nutrients, and to safeguard their prolonged survival, while also supporting their prolonged secretion of beneficial paracrine/endocrine mediators.
Anemia may be part of the pathophysiology of the COVID-19 disease, causing a multiple organ dysfunction syndrome in these severe patients. Indeed, several studies reported that severe COVID-19 patients tend to present decreased hemoglobin levels. In a systematic review and meta-analysis of data from 14,044 COVID-19 patients, severe cases had lower pooled mean hemoglobin (weighted mean difference [WMD], −4.21) compared with moderate cases (46). This was also reported in a different meta-analysis of four individual studies where hemoglobin values were essentially reduced in COVID-19 patients with severe disease, compared with those with milder forms, yielding a WMD of –7.1 g/L (47). Therefore, the decrease observed in the patients may reflect the expected disease course.
Mortality rates of patients suffering from COVID-19 ARDS who required mechanical ventilation are high and up to 80% of those patients ultimately succumb to the disease (6, 9).
The majority of COVID-19 patients suffering from ARDS require invasive mechanical ventilation. These patients tend to remain ventilator-dependent for 10–14 days.
Treating ARDS with agents having immunomodulation, anti-inflammatory, and regenerative properties can potentially assist with patient recovery and reduce death rate. PLX-PAD treatment may explain the low mortality rate described in our case series.
Mitigating the severe acute respiratory infection associated with COVID-19 as the most dangerous manifestation of this disease should be helpful for treating and reducing the death rate. Available data show that levels of serum high-sensitivity CRP were markedly higher in severe cases than in moderate cases of COVID-19, suggesting an increased level of systemic inflammation in such cases (48).
Here, we show that following PLX-PAD treatments, blood CRP decreased dramatically in all patients. The results highlight the possibility that PLX-PAD may have contributed to the reduction in the inflammatory state of the patients leading to an improvement in hypoxia, as demonstrated by an improvement in P/F, improved kidney function (reflected in normalization of creatinine levels), and improvement of opacities in chest radiographs. Reduction of systemic inflammation can also explain the improved creatinine levels. It has been suggested that acute kidney injury in COVID-19 can result from intrarenal inflammation and direct cytokine damage (49). Both the anti-inflammatory and immunomodulatory properties of PLX-PAD may explain reductions in kidney injury leading to reductions in creatinine levels.
This study has several limitations. First, this is a small case series with no controls. Second, it is unclear if these patients would have improved without administration of PLX-PAD, although the changes in chest imaging and P/F are encouraging findings. Third, all patients were treated with multiple other agents (including antiviral medications), and it is not possible to determine whether the improvement observed was related to therapies other than PLX-PAD, even though no such intervention was proven to affect significantly the disease severity. Fourth, PLX-PAD was administered 1–22 days after the initiation of mechanical ventilation. Whether a different timing of administration would have been associated with different outcomes cannot be determined.
CONCLUSIONS
In this preliminary uncontrolled case series of eight critically ill patients with COVID-19 and ARDS, administration of PLX-PAD, a placenta-derived cell therapy, was followed by an overall improvement in the clinical status of most patients. The limited sample size and study design preclude a definitive statement about the treatment effectiveness. These observations are to be further evaluated in future controlled clinical trials.
Supplementary Material
Footnotes
Drs. Barkama, Mayo, and Paz contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The investigational product (PLacental eXpanded [PLX]-PAD) was supplied by Pluristem at no cost.
Drs. Ofir, Shani, Sheleg, Allen, Shaked Nitzan, Tsarfaty, and Gilad are Pluristem employees and shareholders. Dr. Vadasz is a Pluristem consultant and Drs. Kachel, Reinke, and Volk are shareholders. Drs. Barkama, Mayo, Paz, Solopov, Mann, Appel, Birch, Zalts, and Raz Pasteur are medical doctors at the medical centers.
REFERENCES
- 1.Ingraham NE, Tignanelli CJ. Fact versus science fiction: Fighting coronavirus disease 2019 requires the wisdom to know the difference. Crit Care Explor. 2020; 2:e0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cascella M, Rajnik M, Cuomo A, et al. Features evaluation, and treatment of coronavirus (COVID-19). 2020, Treasure Island, FL: StatPearls Publishing; In: StatPearls [PubMed] [Google Scholar]
- 3.Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395:1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46:846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020; 20:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017; 377:562–572 [DOI] [PubMed] [Google Scholar]
- 9.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. COVID-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tufan A, Avanoğlu Güler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020; 50:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020; 117:9490–9496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moll G, Hoogduijn MJ, Ankrum JA. Editorial: Safety, efficacy and mechanisms of action of mesenchymal stem cell therapies. Front Immunol. 2020; 11:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoury M, Cuenca J, Cruz FF, et al. Current status of cell-based therapies for respiratory virus infections: Applicability to COVID-19. Eur Respir J. 2020; 55:2000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Rev Rep. 2020; 16:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CL, Soeder Y, Dahlke MH. Concise review: Mesenchymal stromal cell-based approaches for the treatment of acute respiratory distress and sepsis syndromes. Stem Cells Transl Med. 2017; 6:1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med. 2017; 196:266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz FF, Rocco PRM. Cell therapy for acute respiratory distress syndrome patients: The START study. J Thorac Dis. 2019; 11:S1329–S1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler T, Perka C, von Roth P, et al. Immunomodulatory placental-expanded, mesenchymal stromal cells improve muscle function following hip arthroplasty. J Cachexia Sarcopenia Muscle. 2018; 9:880–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papait A, Vertua E, Magatti M, et al. Mesenchymal stromal cells from fetal and maternal placenta possess key similarities and differences: Potential implications for their applications in regenerative medicine. Cells. 2020; 9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norgren L, Weiss N, Nikol S, et al. PLX-PAD cell treatment of critical limb ischaemia: Rationale and design of the PACE trial. Eur J Vasc Endovasc Surg. 2019; 57:538–545 [DOI] [PubMed] [Google Scholar]
- 21.Zahavi-Goldstein E, Blumenfeld M, Fuchs-Telem D, et al. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy. 2017; 19:1438–1446 [DOI] [PubMed] [Google Scholar]
- 22.Van Linthout S, Hamdani N, Miteva K, et al. Placenta-derived adherent stromal cells improve diabetes mellitus-associated left ventricular diastolic performance. Stem Cells Transl Med. 2017; 6:2135–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee P, Chiasson VL, Pinzur L, et al. Human placenta-derived stromal cells decrease inflammation, placental injury and blood pressure in hypertensive pregnant mice. Clin Sci (Lond). 2016; 130:513–523 [DOI] [PubMed] [Google Scholar]
- 24.Roy R, Brodarac A, Kukucka M, et al. Cardioprotection by placenta-derived stromal cells in a murine myocardial infarction model. J Surg Res. 2013; 185:70–83 [DOI] [PubMed] [Google Scholar]
- 25.Prather WR, Toren A, Meiron M, et al. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemia. Cytotherapy. 2009; 11:427–434 [DOI] [PubMed] [Google Scholar]
- 26.Consentius C, Akyüz L, Schmidt-Lucke JA, et al. Mesenchymal stromal cells prevent allostimulation in vivo and control checkpoints of Th1 priming: Migration of human DC to lymph nodes and NK cell activation. Stem Cells. 2015; 33:3087–3099 [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181:271–280 e278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen Med. 2019; 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow L, Johnson V, Impastato R, et al. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl Med. 2020; 9:235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni K, Liu M, Zheng J, et al. PD-1/PD-L1 Pathway mediates the alleviation of pulmonary fibrosis by human mesenchymal stem cells in humanized mice. Am J Respir Cell Mol Biol. 2018; 58:684–695 [DOI] [PubMed] [Google Scholar]
- 31.Tzouvelekis A, Toonkel R, Karampitsakos T, et al. Mesenchymal stem cells for the treatment of idiopathic pulmonary fibrosis. Front Med (Lausanne). 2018; 5:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020; 11:216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang B, Junhui C, Li T, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. ChinaXiv. 2020; 99:e21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan H, Olson SD, Kumar A, et al. Mesenchymal stromal cell therapeutic delivery: Translational challenges to clinical application. Front Immunol. 2019; 10:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrissey JH. Tissue factor: A key molecule in hemostatic and nonhemostatic systems. Int J Hematol. 2004; 79:103–108 [DOI] [PubMed] [Google Scholar]
- 36.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020; 18:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020; 7:e362–e363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020; 382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spyropoulos AC, Ageno W, Barnathan ES. Hospital-based use of thromboprophylaxis in patients with COVID-19. Lancet. 2020; 395:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braid LR, Wood CA, Wiese DM, et al. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy. 2018; 20:232–244 [DOI] [PubMed] [Google Scholar]
- 43.Giri J, Galipeau J. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 2020; 4:1987–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qazi TH, Duda GN, Ort MJ, et al. Cell therapy to improve regeneration of skeletal muscle injuries. J Cachexia Sarcopenia Muscle. 2019; 10:501–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giri J, Das R, Nylen E, et al. CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Rep. 2020; 30:1923–1934.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taneri PE, Gómez Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID-19: A systematic review and meta-analysis. Eur J Epidemiol. 2020 Aug 20. [online [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020; 42:116–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kermali M, Khalsa RK, Pillai K, et al. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sci. 2020; 254:117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020; 16:308–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


