Abstract
Despite broad application of magnetic nanoparticles in biomedicine and electronics, only a few in vivo studies on biocompatibility are available. In this study, toxicity of magnetic metal oxide nanoparticles on the respiratory system was examined in vivo by single intratracheal instillation in mice. Bronchoalveolar lavage fluid (BALF) samples were collected for proteome analyses by LC–MS/MS, testing Fe3O4 nanoparticles doped with increasing amounts of cobalt (Fe3O4, CoFe2O4 with an iron to cobalt ratio 5:1, 3:1, 1:3, Co3O4) at two doses (54 μg, 162 μg per animal) and two time points (day 1 and 3 days postinstillation). In discovery phase, in-depth proteome profiling of a few representative samples allowed for comprehensive pathway analyses. Clustering of the 681 differentially expressed proteins (FDR < 0.05) revealed general as well as metal oxide specific responses with an overall strong induction of innate immunity and activation of the complement system. The highest expression increase could be found for a cluster of 39 proteins, which displayed strong dose-dependency to iron oxide and can be attributed to neutrophil extracellular trap (NET) formation. In-depth proteome analysis expanded the knowledge of in vivo NET formation. During screening, all BALF samples of the study (n = 166) were measured label-free as single-injections after a short gradient (21 min) LC separation using the Evosep One system, validating the findings from the discovery and defining protein signatures which enable discrimination of lung inflammation. We demonstrate a proteomics-based toxicity screening with high sample throughput easily transferrable to other nanoparticle types. Data are available via ProteomeXchange with identifier PXD016148.
Keywords: quantitative proteomics, LC−MS/MS, bronchioalveolar lavage fluid, magnetic metal oxide nanoparticles, iron cobalt oxide nanoparticles, neutrophil extracellular trap formation, NETosis
The field of nanotechnology is constantly expanding, and fast, reliable screening techniques for biocompatibility of nanomaterials are needed to ensure human safety. Nanomaterials are particulate substances with at least one dimension less than 100 nm. Due to their properties arising from their composition, surface, and size, their application spectrum is broad, ranging from biomedicine, electronics, health care, food, cosmetics, textiles, and others.1−3 Single parameter in vitro assays, e.g., for extracellular lactate dehydrogenase (LDH), reactive oxygen species (ROS), inflammation, and DNA strand breaks are well established tests to assess cytotoxicity and genotoxicity of chemicals including nanomaterials.4 Although reliable, they are lacking comprehensive depth to deal with the individual complexity of cellular responses to the immense amount of newly generated materials. In order to improve biocompatibility of engineered nanoparticles, it is also imperative to better understand the mechanisms of action of adverse health effects in vivo.5 With recent advances in liquid chromatography and mass spectrometry, we propose a robust analysis platform for biocompatibility testing of nanomaterials which allows sensitive and comprehensive measurements of cellular responses with high sample throughput. As a proof of principle, we tested simultaneously different types of magnetic nanoparticles at different concentrations and exposure times. Magnetic nanoparticles have a wide range of applications due to their superparamagnetic property and size and are of great interest in biomedicine (e.g., magnetic resonance imaging (MRI), molecular imaging, magnetic particle imaging, targeted tumor therapy, drug delivery).6 Among them, iron oxide nanoparticles are the most used and studied magnetic nanoparticles due to their high biocompatibility and ease of synthesis. The iron oxide Fe3O4 (magnetite) is one of the most magnetic minerals, with Fe3O4 nanoparticles or crystals naturally occurring in bacteria or in the brain of migratory animals navigating in the Earth’s magnetic field.7 Iron oxide nanoparticles change properties including band gap and consequently redox activity when doped with other metals such as cobalt. It has been shown that cobalt ferrite (CoFe2O4) nanoparticles display increased magnetic properties and chemical stability,8 making them superior contrast agents in MRI. Cobalt nanoparticles are widely used in the technology sector for magnetic sensors, magnetic memory, and recently also for wireless communication. Although cobalt-based nanoparticles have been connected with increased cytotoxicity, genotoxicity, oxidative stress, and tumor formation, parallel testing of Co, CoO, and Co3O4 nanoparticles showed the lowest cytotoxicity and no DNA damage response for Co3O4.9 Similarly, testing of Co3O4 nanoparticles in vitro in cell lines representing lung, liver, nervous, and gastrointestinal systems showed only mild cytotoxic or genotoxic effects only observable at high concentrations (>100 μg/mL).10 Daily dietary cobalt intake has been estimated at 5–50 μg/day, with a very small fraction coming from vitamin B12 (Cobalamin). Danish occupational exposure limit for cobalt is 0.05 mg/m3. System-wide omics analyses are becoming more popular in nanosafety research investigating the effects of nanomaterials on cell lines (reviewed in ref (11)); however, only a few in vivo studies exist to date, in large part as a result of the time, cost, and need for ethical approval. From these system-wide characterizations, the most commonly observed changes include oxidative stress, metabolism, cytoskeleton, and cell cycle. Inhalation of nanoparticles is the most likely nonintentional occupational exposure type. From classical approaches, it is well-known that inhalation of nanomaterials can induce immune and complement system activation and increases the risk to develop chronic lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), or lung cancer as well as cardiovascular disease. Although a few omics studies have been conducted on nanomaterial toxicity after inhalation12−17 or pulmonary exposure,18−22 information on magnetic or iron oxide nanoparticles is missing and a robust high-throughput screening method with associated bioinformatics workflow allowing widespread utilization and broad generalization of the approaches has been lacking.
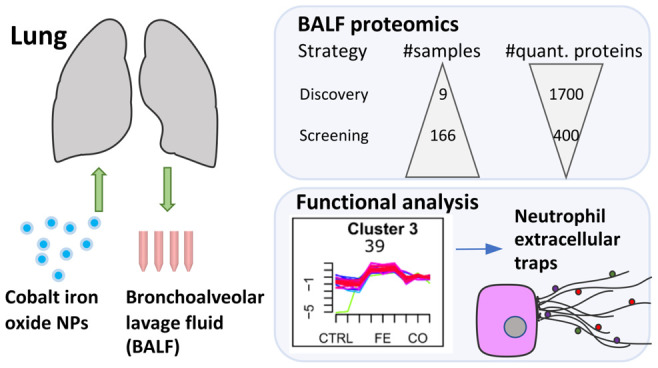
Here, we studied the effects of magnetic iron oxide (Fe3O4) nanoparticles doped with cobalt oxide (Co3O4) at different ratios after intratracheal instillation in mice using label-free quantitative proteomics. Carbon nanoparticles (carbon black, Printex 90) were included as inflammation-inducing reference material,22−27 which is a standard in nanotoxicology and environmental research mimicking air pollution from combustion, diesel engines, and tire wear emissions. Carbon black has an annual industrial production of 10 million tons, most of which is used in tires; the rest can be found in paints, rubber, and plastics. The concentrations used for instillation correspond to exposure for 3–8 working days at the occupational exposure limit according to the Occupational Safety and Health Administration. Our analysis pipeline consists of high-resolution mass spectrometry connected to the recently introduced Evosep One LC system, specifically developed for clinical proteomics, which enables robust and fast measurements by a combination of low-pressure sample flow with in-loop gradient storage and minimal overhead time.28 This setup enables the analysis of >60 samples per day and allows for large-scale comprehensive nanobio and mechanistic nanotoxicology studies for the elucidation of molecular initiating events and resulting adverse outcome pathways. After performance evaluation of the Evosep One LC system, two proteomic strategies were followed, with a discovery phase including few samples (<10) for a classic in-depth label-based proteomic profiling and a screening phase including many samples (>100) for validation of the platform.
Results and Discussion
Experimental Design
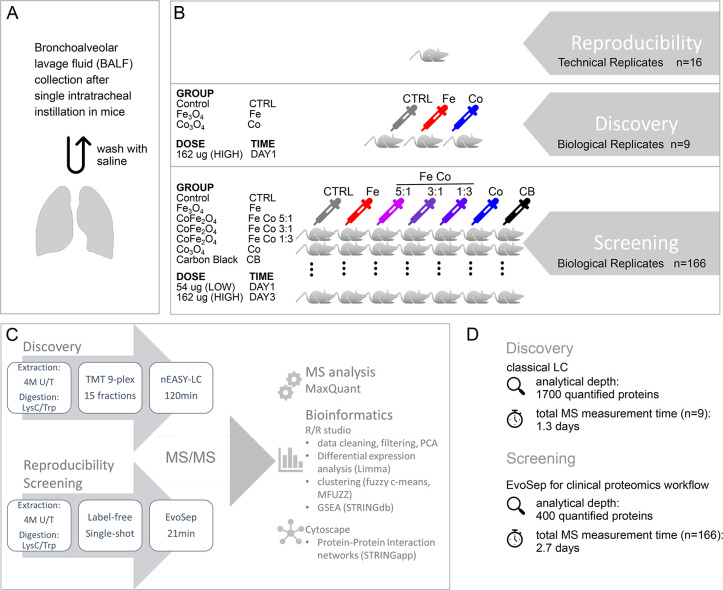
The goal of this study was to investigate magnetic iron cobalt oxide nanoparticles (cobalt doped Fe3O4 nanoparticles with increasing amounts of cobalt) after pulmonary exposure while in parallel presenting a proteomics platform that is easily transferable to large-scale nanotoxicology screening as part of an integrated assessment and testing approach for regulation of nanoparticles. BALF is a proximal biofluid that can be used to monitor airway inflammation and toxic responses in the lung. It is routinely sampled for differential lung diagnostics and has been discussed as a source for early detection of lung cancer. In order to assess effects of metal oxide nanoparticles upon inhalation, bronchoalveolar lavage fluid from mice dosed by single intratracheal instillation was collected and subjected to classical biocompatibility assays as well as proteome analysis (Figure 1A). Magnetic oxide nanoparticles with iron and cobalt oxide (Fe3-xCoxO4) at different ratios (1:0, 5:1, 3:1, 1:3, 0:1) were tested at two concentrations (54 μg, 162 μg per animal) and two time points after instillation (day 1, day 3). Time points for proteome analysis (day 1 and day 3 post instillation) were chosen based on previous studies which showed that exposure to various metal oxides induce pulmonary acute phase response, which peaks at day 1 or day 3.29,30 As a positive control carbon black (CB) nanomaterial known to induce lung inflammation31 was included for both time points but only at the high dosage (162 μg per animal).
Figure 1.
Experimental design and proteome analysis workflow. (A) Mice were treated by single intratracheal instillation with either 0 μg (vehicle control, CTRL), 54 μg (low dose, LOW), or 162 μg (high dose, HIGH) of nanoparticles per animal. Bronchoalveolar lavage fluid (BALF) was obtained by two washes with saline 1 day or 3 days postinstillation. (B) Reproducibility: To evaluate reproducibility of the Evosep One LC system technical replicates (n = 16) of a single BALF sample were measured as 21 min gradients. Discovery: In-depth BALF proteome profiling was performed on selected samples from day 1 postinstillation with high dose nanoparticle treatment (162 μg/animal). Samples of iron oxide (Fe3O4) and cobalt oxide (Co2O3) nanoparticle treatment were compared to controls with three biological replicates per group. Screening: Within the whole study the effects of six different types of nanoparticles (Fe3O4, CoFe2O4 (Fe Co 5:1), CoFe2O4 (Fe Co 3:1), CoFe2O4 (Fe Co 1:3), Co3O4, and carbon black) were tested at two doses (LOW, HIGH; except for carbon black (HIGH only)) and two time points (1 day and 3 days postinstillation). In total, BALF samples of 166 animals were measured. (C) Schematic of proteome analysis workflow. To obtain maximal proteome coverage, BALF samples of the discovery phase were prefractionated (high pH reverse phase, 15 fractions) and run as 120 min gradients with classic nanoLC system setup. Samples were TMT labeled and run together as 9plex. BALF samples of the whole study (n = 166, Screening) as well as technical replicates (Reproducibility) were run label-free as single-shot injections on 21 min gradients using the Evosep One LC system. MS raw files were analyzed by MaxQuant. Downstream bioinformatic analyses were performed within the R environment as indicated with protein–protein interaction network visualization in Cytoscape. (D) Summary of analytical depth and measurement time for Discovery and Screening phase.
Proteomics experiments were divided into three parts, test of reproducibility, discovery, and screening phase (Figure 1B,C). The reproducibility of the recently introduced Evosep One LC system was evaluated by measuring of technical replicates (n = 16). During the discovery phase, selected representative samples with 3 biological replicates per group (total n = 9) including pure iron oxide nanoparticles, pure cobalt oxide nanoparticles, and vehicle controls were subjected to in-depth proteome profiling by extensive prefractionation and including isobaric tandem mass tag (TMT) labeling following a classical LC–MS/MS setup. This step allowed us to identify affected pathways and generate hypotheses regarding mechanisms of the effects of nanoparticles. During screening, all samples of the study were measured label-free as single-shot injections separated on short gradients of 21 min using the robust, high-throughput Evosep One LC system. This step allowed a fast screening of the five different types of magnetic metal oxide nanoparticles on BALF, at two concentrations and two time points together with their representative controls (total n = 166). All screening measurements were completed in only 2.7 days. An overview of the LC–MS/MS setup and analytical pipeline for all three proteomic parts is depicted in Figure 1D.
Synthesis and Characterization of Nanoparticles
The nanoparticles used in this study provided by Promethan Particles were synthesized by continuous hydrothermal synthesis starting from metal salts as precursors with iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O) and cobalt(II)acetate tetrahydrate (Co(C2H3O2)2·4H2O) for iron and cobalt oxide, respectively. The salts were used at different ratios to produce nanoparticles with iron to cobalt composition of 5:1, 3:1, and 1:3. Synthesis and characterization of the nanoparticles used have been described in detail.32 Nanoparticles were provided as liquid dispersions, safe for the end user to prevent accidental inhalation. All metal oxide nanoparticles used had a primary particle diameter below 40 nm based on transmission electron microscopy (TEM) imaging (Figure S1A) and a zeta potential between −4 and 24 mV in deionized water. Since the nanoparticles were uncoated, in deionized water they formed agglomerates with sizes of 103 nm for Co oxide and up to 1635 nm for Fe Co 3:1 based on dynamic light scattering (DLS) analysis. Average surface area by Brunauer–Emmett–Teller (BET) analysis was 50 m2/g except for Fe Co 5:1 (100 m2/g) and Fe Co 3:1 (132 m2/g) (Figure S1B). Carbon black or Printex90 has been characterized previously by BET with an average size of 14 nm and a surface area of 300 m2/g.33
Classic Nanoparticle Compatibility Tests: Pulmonary Inflammation, Cytotoxicity, and Genotoxicity
To evaluate the extent of pulmonary inflammation induced by the nanoparticles, the cellular content of BALF was assessed. Differential cell counting was performed for neutrophils, macrophages, lymphocytes, eosinophils, and epithelial cells (Figure S2A, Table S1). Normal cell counts for BALF include >85% alveolar macrophages, 10–15% lymphocytes, ≤3% neutrophils, ≤1% eosinophils, and ≤5% epithelial cells.34 For all treatment groups, there was a strong neutrophil influx, except for low dose pure iron oxide nanoparticles which reached base level 3 days after exposure. On the contrary, macrophages showed pulmonary evasion upon nanoparticle treatment, with similar iron dose dependency as neutrophils. Lymphocyte influx was visible after 3 days of instillation with a mild increase for all treatment groups at high dose. Eosinophils showed a prominent late response with a clear correlation with increasing cobalt content in the nanoparticles and were also increased after carbon black instillation. Epithelial cells were slightly affected in a cobalt responsive manner, with normalization back to baseline levels after 3 days of instillation. Similar to the neutrophil influx profile, the acute phase response gene Saa3 was strongly increased at day 1 after instillation, displaying dose dependency and iron inducibility (Figure S2A). Cytotoxicity was evaluated by measuring extracellular lactate dehydrogenase (LDH) and total protein content (Figure S2B). Elevated LDH was observed 3 days after instillation for high-dose nanoparticle exposure with cobalt-dose dependency as for carbon black. Total protein content in BALF was elevated with dose dependency for both time points with the highest values for pure cobalt oxide nanoparticles. As a measure for genotoxicity, DNA strand breaks were evaluated by the comet assay35−37 in both BALF and lung as well as liver, the main target organ for nanoparticle accumulation after translocation (Figure S2C). Increased levels of DNA strand breaks were observed for BAL cells from mice exposed to pure iron oxide nanoparticles and for mice exposed to Fe2CoO4 nanoparticles with 1:3 and 3:1 iron to cobalt ratios. The cytotoxicity observed for the cobalt-group could have interfered with an inflammatory response.
To summarize, according to classical toxicity testing, all the tested nanoparticles induced dose dependent acute inflammatory and acute phase responses whereas particles with high iron content induced DNA strand breaks in BAL cells. There was a clear iron dose response for the extent of pulmonary neutrophil influx and macrophage evasion. This observation has to the best of our knowledge not been reported before. However, there is a link of siderophore binding proteins in neutrophils for bacterial iron starvation and pathogen secreted iron-binding siderophores.38 In a recent study, it was shown that bacterial siderophores not just bind iron to support their growth but also to steal iron from neutrophils to hijack their anti-inflammatory response by preventing reactive oxygen species (ROS) generation.39 Macrophages, known to play a crucial role in iron uptake, storage, and recycling with specific regard to erythrocyte phagocytosis40 contain a repertoire of iron-binding or transporting proteins. Similar as for neutrophils, iron content has been suggested to be important for ROS generation in macrophages.41 Cobalt dose dependency could be observed for eosinophil influx and an increase in epithelial cells, the latter as a sign for increased cytotoxicity with higher cobalt content. Cobalt has been linked to the induction of occupational hard metal-induced asthma with strong pulmonary eosinophilic inflammation.42 Further, it has been shown that after instillation with cobalt nanoparticles, the amount of solubilized cobalt ions correlate with eosinophil numbers,43 which supports our observation of cobalt oxide dose dependent pulmonary eosinophil influx. The best tolerated were pure iron oxide nanoparticles at lower concentration, consistent with their known biocompatibility. On the other hand, pure iron oxide nanoparticles induced DNA strand breaks, indicating genotoxicity.
Proteomics
Reproducibility of Evosep One
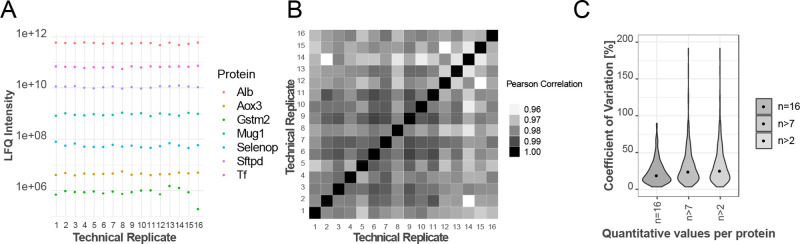
Requirements for clinical proteomics include high sample throughput, accuracy, robustness, simple sample preparation, and deep proteome coverage while dealing with the high dynamic range in biological matrixes spanning up to 11 orders of magnitude for serum and plasma or 10 orders for bronchoalveolar lavage fluid. Deep proteome coverage is traditionally achieved by reducing sample complexity by peptide separation into multiple fractions and/or using long liquid chromatography gradients which increases measurement time and reduces sample throughput. While measurement time can be reduced by incorporating labeling strategies (e.g., isobaric chemical labeling TMT) which allows multiplexing currently of up to 16 samples, this adds additional steps to the sample preparation. The dynamic range issue inherent to biological samples can be addressed by depletion of a few highly abundant proteins such as albumin but again increases sample processing and therefore variability. Recent advances in liquid chromatography and high-resolution mass spectrometry rekindled the interest in clinical proteomics. Robustness and reproducibility of the Evosep One LC system was evaluated by repeated measurements (n = 16) of one representative BALF sample using the 21 min gradient method (Figure 2), including intra-assay variability. The representative BALF sample was distributed in 16 wells to perform in parallel individual protein extraction, digestion, and peptide loading on EvoSep tips. On average, 450 proteins were quantified with protein measurements covering 6 orders of magnitude (Figure S3A). Label free quantitation (LFQ) intensities of six selected proteins spanning the whole dynamic range are shown, demonstrating high reproducibility. High correlation between technical replicates was observed with an average Pearson correlation coefficient of 0.98, ranging from 0.958 to 0.988 for pairwise comparisons of protein intensities. Likewise, the median coefficient of variation (CV) was 19% based on 203 quantified proteins (no missing values, n = 16). Allowing increasing numbers of missing values, the CVs increase to a maximum of 25.3% based on 474 proteins (n > 2) (Figure 2, Figure S3B). In clinical diagnostic assays, a CV < 20 is considered acceptable. Applying this filter, 53% of the proteins with no missing values passed. The analytical performance of the EvoSep One system presented here is in the expected range and similar to what has been previously reported.28 Thus, the validation step has demonstrated that the presented workflow has acceptable inter- and intra-assay reproducibility.
Figure 2.
High reproducibility of the Evosep One LC system. (A) Reproducibility of LFQ intensities for selected proteins covering 6 orders of magnitude. (B) Matrix of Pearson Correlation coefficients for 16 technical replicates. (C) Distribution of coefficients of variation (CVs) based on proteins with n = 16, n > 7, and n > 2 quantitative measurements within 16 technical replicates. Median CVs (annotated with black dots) were 19%, 24.1%, and 25.3% corresponding to 203, 350, and 474 quantified proteins, respectively.
Discovery Phase
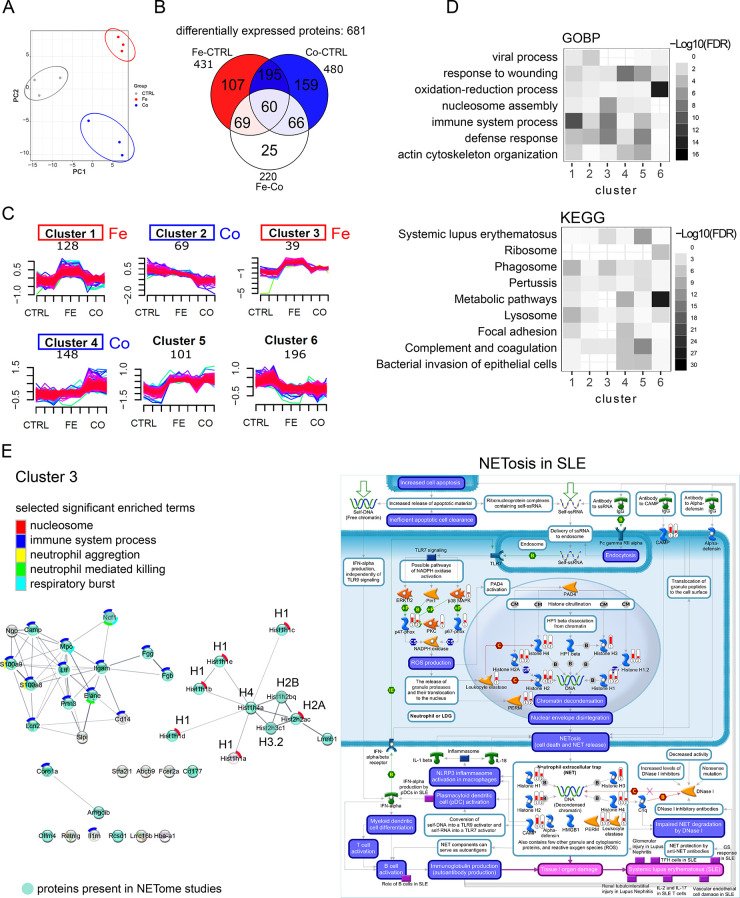
The effects of iron and cobalt oxide nanoparticles on the proteome content of BALF were profiled in depth achieved by extensive prefractionation. In combination with chemical isobaric TMT labeling which allowed multiplexing of the 9 samples in one experiment, accurate protein quantification was ensured. In-depth profiling of BALF led to the complete quantification of 1766 proteins (Figure S4A). Principal component analysis (PCA) displays the expected clustering of replicates and separation between experimental groups (control (CTRL), iron oxide nanoparticles (Fe3O4 NP; abbreviated as Fe), cobalt oxide nanoparticles (Co3O4 NP; abbreviated as Co)) (Figure 3A). Differential expression analysis was performed, and 681 proteins were found to be significant (FDR < 0.05) (Table S2). Protein expression distribution between experimental conditions is shown (Figure 3B) with 431 proteins differentially expressed for Fe vs CTRL, 480 proteins for Co vs CTRL, and 220 for Fe vs Co. Differentially expressed proteins were clustered by applying unsupervised fuzzy-c means algorithm (Table S3) with the number of protein members per cluster annotated (Figure 3C). Protein content changes in BALF can be described by a general increase (cluster 5) or decrease (cluster 6) in response to metal oxide nanoparticles. Metal oxide nanoparticles specific changes are represented by clusters 1–4, with an increase to Fe3O4 nanoparticles (clusters 1 and 3) or Co3O4 nanoparticles (cluster 4) and a mild decrease to Co3O4 nanoparticles (cluster 2). The most prominent expression differences can be observed for the iron oxide responsive proteins of cluster 3. Gene set enrichment analyses for all deregulated proteins (Figure S4B) show a high content of extracellular secreted proteins, highly associated with extracellular vesicles, exosomes, and blood microparticles as well as focal adhesion, cytoskeleton, extracellular matrix, and nucleosome. Functionally, proteins with, e.g., endopeptidase inhibitor activity and oxidoreductases were highly enriched. In accordance with sample treatment, iron ion binding has been found significant only for iron oxide nanoparticles. Among the top GO biological process terms or KEGG pathways enriched were complement and coagulation cascades and metabolic pathways with a considerable response to cobalt oxide, whereas immune system and response to metal ion or bacterium appeared to be more prominent in response to iron oxide. Similarly enriched were proteins associated with phagosome, lysosome, bacterial invasion of epithelial cells, and systemic lupus erythematosus. Those results are in accordance with findings in previous proteomics or transcriptomics studies; however, the iron oxide and cobalt oxide dependent shift for enriched functions has not been reported. Performing enrichment separately for the six clusters (Figure 3D) confirms the stronger immune response for iron oxide nanoparticles, whereas cobalt oxide is more associated with wound healing and complement activation. General downregulation is associated with metabolic enzymes with a high content of proteins involved in oxidation–reduction. Based on MetaCore enrichment (Figures S5–S10, lower panels), both iron oxide response clusters (1 and 3) are associated strongly with neutrophil mediated immunity (Figures S5 and S7), which is in accordance with the iron dose dependency observed for the pulmonary neutrophil influx (Figure S2A). Proteins from cluster 2 showing moderate downregulation with cobalt oxide response are enriched for iron import and epithelial progenitor cell differentiation as well as metabolism of xenobiotics by cytochrome 450 (Cyp2f2, Gstm1, Gsto1, Adh5). Proteins of cobalt oxide response cluster 4 (upregulation) are associated with wound healing including cytoskeleton remodeling, complement activation, and fibrosis development. More differential enrichment analysis of proteins from cluster 6 (general downregulation) revealed enzymes connected to glutathione metabolism, NRF2 regulated oxidative stress, protein folding, as well as carboxylic and oxoacid metabolic process (Figure S10). Downregulation of proteins of clusters 2 and 6 might be related to the observed pulmonary macrophage evasion; however, the iron dose response expected from differential cell counting was not observed. Among the most strongly enriched proteins in BALF samples upon single instillation with metal oxide nanoparticles were histone proteins, identified with 14 isoforms covering core histones (H2A, H2B, H3, H4) and linker histones (H1) distributed in clusters 3 (intense iron oxide dose-dependent upregulation, 10 histone isoforms), 4 (cobalt oxide dose-dependent upregulation, 1 histone isoform), and 5 (general upregulation, 3 histone isoforms).
Figure 3.
Deep BALF proteome profiling: discovery phase. (A) Principal component analysis, (B) Venn diagram of differentially expressed proteins (FDR < 0.05) for three comparisons: Fe vs CTRL, Co vs CTRL, Fe vs Co. (C) Fuzzy c-means clustering of differentially expressed proteins (FDR < 0.05). Number of protein members per cluster are indicated. Clusters of proteins with specific response for iron oxide or cobalt oxide are color coded. Iron oxide, red; cobalt oxide, blue. (D) Tile plots depict enrichment analysis for proteins of each cluster for selected terms of Gene Ontology Biological Processes (GOBP) and KEGG pathways (KEGG). (E) Left panel: protein–protein interaction network of cluster 3 with strong response to iron oxide. Members of this cluster are classical NETosis signature proteins (Elane, MPO, Prtn3, histones). Proteins represented as turquoise nodes were also identified in LC–MS/MS studies as NETosis associated proteins.52−57 Significant enriched terms are annotated and sorted from top (most significant) to bottom (less significant) including nucleosome (red), immune system process (blue), neutrophil aggregation (yellow), neutrophil mediated killing (green), and respiratory burst (turquoise). Histone isoforms are annotated with their associated histone family (H1, H2A, H2B, H3.2, H4). Right panel: “NETosis in SLE” pathway (highly enriched for cluster 3, FDR 10–17) map generated by MetaCore analysis with overlaid protein expression of Fe vs CTRL (1) and Co vs CTRL (2) comparisons. Red expression bars indicate up-regulation of significant proteins (FDR < 0.05). Detailed pathway legend can be found on the MetaCore Web site: https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf.
Cluster 3 is of particular interest as it displays the strongest changes in expression with enhanced specificity for iron oxide nanoparticles and shows in addition pronounced enrichment for nucleosome assembly among clusters (Figure 3D). A functional protein–protein interaction network (Figure 3E, left panel) of the 38 members of cluster 3 consists of histones and immune response and neutrophil-associated proteins, with myeloperoxidase (Mpo), neutrophil elastase (Elane, NE), and proteinase 3 (Prtn3) among them indicative of neutrophil extracellular trap (NET) formation. NET formation or NETosis is a defense mechanism where neutrophils externalize a DNA mesh decorated with anti-inflammatory proteins such as endopeptidases to capture and defend against pathogens.44 “NETosis in systemic lupus erythematosus (SLE)” as the most enriched pathway with MetaCore analysis (FDR 10–17) further confirms this observation (Figure S7). Graphic representation of this pathway with overlaid expression shows a 30% coverage with 10 out of 31 proteins identified (Figure 3E, right panel). Cluster 3 “NETosis in SLE” proteins are histone H1, histone H1.2, histone H2A, histone H2, histone H4, Mpo (abbreviated as PERM on MetaCore pathway map), Camp, and Ncf1 with Elane (abbreviated as Leukocyte elastase on MetaCore pathway map), and histone H3 is only significant for exposure to iron oxide nanoparticles. NETosis proteins Mapk14, PKC, and Ncf2 clustered together with intermediate iron oxide response cluster 1. This indicates that additional potential candidates for NETosis can be found in clusters 1 and 3. Two types of NETosis can be distinguished, phagocyte NADPH oxidase (Nox2) dependent and independent, which are studied in vitro using the protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA) and the calcium ionophore A23187 as classical stimulants, respectively. The PKC activator PMA leads to cytosolic ROS generation by Mpo perforating the nuclear membrane while Elane translocates to the nucleus and initiates chromatin decondensation and extortion. The calcium ionophore A23187 induces the production of mitochondrial ROS and the activation of the SK3 channel,45 which leads to PAD4 mediated hypercitrullination of mostly histones. NETosis is an important defense mechanism of neutrophils against pathogens; chronic NET formation, however, has been associated with the induction of autoimmune diseases (rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE)) based on production of PAD4-mediated citrullinated autoantigens (mostly histone H3)46 as well as chronic lung diseases and pulmonary cancer metastasis.47 Persistent inhalation of irritants like in tobacco lead to chronic NET formation,48 while NETs have been associated with awakening dormant cancer cells linking it to lung cancer induction.49 Inducers of NETosis are bacteria and viruses but also crystals, immune complexes, and chemical changes. The association of nanoparticles and NETosis induction is a very young field and has been observed for polystyrene, nanodiamonds,50 graphene oxide, Au and Ag nanoparticles (reviewed in ref (51)) and is not induced by particles larger than 1000 nm.50 Depending on the stimulating agent different mechanisms inducing NETosis have been discussed, which might influence the nature of the NETs. In an effort to elucidate NET associated proteins, several groups applied LC–MS/MS.52−57 Comparing our data (clustered 681 DEGs, Figure S11A) to the comprehensive studies on proteins associated with NETs (Figure S11B), we further validated our clustering results. Compared to Lim and colleagues54 (164 NET proteins), 64 proteins were found in common, with a large proportion of these being cluster 3 proteins. Comparing to the most comprehensive NETproteome study (791 NET proteins),52 136 proteins were common with a high percentage of proteins for clusters 1 and 3. Similar observations were made in two other studies,53,56 with one study distinguishing between the NET proteome of healthy donors and autoimmune patients (RA, SLE). The distribution of the overlapping proteins indicates the importance of clusters 1 and 3. Interestingly, there was an increased overlap among NETs of autoimmune patients and the cobalt responsive cluster 4. Besides neutrophils, other immune cells can also form extracellular traps (ETs) as has been observed for macrophages (METs) and eosinophils (EETs),58 explaining the distribution of NET proteins over the clusters. In the case of cobalt dependent cluster 4 together with differential BALF cellular content, this could indicate EET formation. NETs are also known to induce alternative complement pathways,59 which goes together with the strong enrichment observed for cluster 5 proteins. Considering all NET proteome studies, 213 out of 681 significant deregulated proteins were found to be in common with a similar over-representation of proteins of clusters 1 (49%) and 3 (63%) as already seen for single study comparisons (Figure S11C, Table S4). Comparing our data to the 4 comprehensive NET proteome studies, then 25 proteins are in common and can be regarded as core proteins among NET associated proteins (Figure S11D, Table S5). A total of 24 of 38 proteins of cluster 3 (63%) (Figure S7, Figure S11C) are validated NET proteins with lactotransferrin (Ltf) and lipocalin 2 (Lcn2) as iron binding proteins. Among the 14 additional potential NET associated proteins were the iron-binding proteins hemoglobin alpha and beta. Other iron-binding proteins with significant changes include ferritin (cluster 1). Among the histones, 7 out of 10 are known to be citrullinated, indicating NOX2-independent NETosis. In the referenced NET proteome studies nonphysiological inducing agents were used in vitro, while our study adds knowledge from in vivo NET formation from physiological “real” agents. In the study by Chapman,53 NET associated proteins were quantified for both NETosis types (PMA, A23187) side by side. Signature proteins based on relative expression intensity for each type are H3 histones, annexins, and azurocidin for NOX2-dependent and LCN2, H1 histones, CRISP3, MMP8, and MMP9 among others for NOX2-independent NETosis. Based on H1 histones, which are members of cluster 3 in our study and are absent or poorly expressed in PMA-induced NETosis, we can conclude that pulmonary metal oxide nanoparticle exposure leads to predominantly NOX2-independent NETosis.
Screening Phase
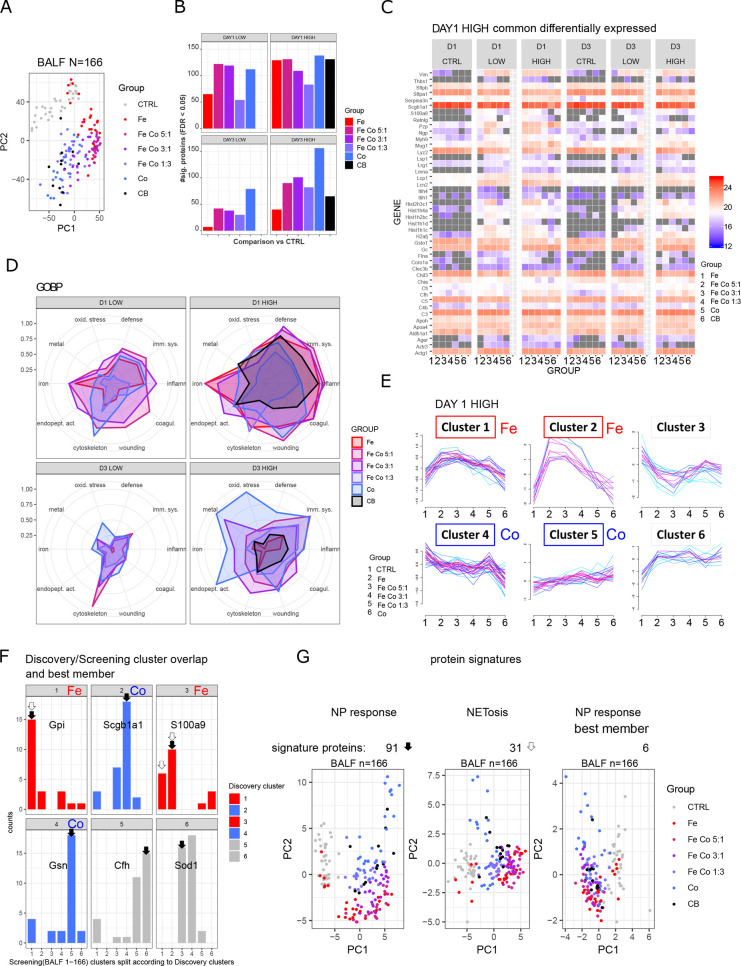
From the discovery phase in-depth profiling, both general and specific changes in the BALF proteome to specific metal oxide nanoparticles were observed. For validation purposes as well as supporting the transfer of nanomaterial testing to a broader clinical/environmental setup, we integrated into our proteomics workflow the Evosep One LC system, which allows robust and accurate LC–MS/MS measurements with high sample throughput (60 samples per day with 21 min gradients). Different types of metal oxide nanoparticles (Fe3O4, CoFe2O4 (Fe Co 5:1), CoFe2O4 (Fe Co 3:1), CoFe2O4 (Fe Co 1:3), Co3O4) were tested at two doses and two time points (4 conditions: day1 low dose, D1 LOW; day 1 high dose, D1 HIGH; day 3 low dose, D3 LOW; day 3 high dose, D3 HIGH) after a single pulmonary instillation. In total, BALF proteomes of 166 mice were measured by LC–MS/MS in only 2.7 days. Robust measurements were achieved for all samples (Figure S12A) with an average of 400 quantified proteins per sample (Figure S12B). PCA (Figure 4A) clearly sets apart vehicle controls (2% serum instillation) from nanoparticle-treated animals. While the nanoparticle treatment groups are not completely separated, the color gradient of the samples suggests dose-dependency of the response to the metal oxide used with BALF samples from iron oxide nanoparticle instillation furthest away from cobalt oxide and cobalt iron oxide in between following decreasing iron cobalt composition. The inflammation control (carbon black, Printex90) clusters with cobalt oxide nanoparticles, which highlights the increased cytotoxicity of cobalt. Performing PCA on sample subsets splitting for time point and dose, iron oxide nanoparticle response is temporary with BALF samples from the low dose and a later time point cluster together with the controls (Figure S12C). This observation shows that pure iron oxide nanoparticles are, as expected, the most biocompatible of the nanoparticles tested. Differential expression analysis was performed for each treatment group and condition with comparisons made against pooled controls for each time point (FDR < 0.05) (Figure 4B, Figure S12D, Table S6) In total, 330 proteins were differentially expressed, with 159 already found during the discovery phase. Most changes were observed for the high dose at day 1 postinstillation with 44 significantly different proteins in common for all iron and cobalt oxide nanoparticle treatments (Figure 4C). Among those were histones (6 isoforms), Lcn2, Ngp, and S100a9, already identified as strong responders to iron oxide nanoparticles (cluster 3). Lipocalin 2 (Lcn2) regulates iron homeostasis and is released by, e.g., neutrophils to bind and neutralize bacterial siderophores as part of an antimicrobial defense mechanism by preventing iron uptake by bacteria.60 Lipocalins can be found on the surface of mucosal surfaces such as lung but also intracellularly and in blood circulation. Lipocalin 2 has been found to be elevated in the serum of patients with COPD and asthma. The strongest expression changes were found for neutrophil extracellular trap (NET) associated proteins from the discovery cluster 3 which displayed high iron dose dependency, confirming our findings from the discovery phase. Histone coverage included 5 H1 linker histone isoforms (Figure S13A) and 4 core histone isoforms covering H2B, H3, and H4 (Figure S13B). Extracellular histones as part of NETs are bactericidal but have also been shown to be highly cytotoxic and contribute largely to tissue injury. Among the linker histones, the most pronounced was Hist1h1c (H1C), able to regulate IRF3 mediated IFNβ signaling.61
Figure 4.
Fast screening of BALF proteome by highly reproducible Evosep LC system, screening phase. (A) Principal component analysis of BALF samples of all animals (n = 166). (B) Numbers of differentially expressed proteins (FDR < 0.05) per treatment group. All comparisons were made against pooled controls per time point. (C) Common proteins which show differential expression upon metal oxide nanoparticles treatment (high concentration) at day one postinstillation. (D) Normalized enrichment for 10 selected GOBP terms for comparisons among the four experimental conditions (D1 LOW, D1 HIGH, D3 LOW, D3 HIGH). The maximal −log10(FDR) value per enrichment term over all conditions was set to 1. Abbreviations of enriched terms: defense (defense response), imm. sys. (immune system process), inflamm. (inflammatory response), coagul. (blood coagulation), wounding (response to wounding), cytoskeleton (actin cytoskeleton organization), endopet. act. (regulation of endopeptidase activity), iron (iron ion homeostasis), metal (response to metal ion), oxid. stress (response to oxidative stress). (E) Fuzzy c-means clustering of differentially expressed proteins (FDR < 0.05) from day 1 postinstillation with 162 μg nanoparticles/animal (DAY 1 HIGH). Clusters of proteins with specific response for iron oxide or cobalt oxide are annotated (red, iron oxide; blue, cobalt oxide). (F) Overlapping significant differentially expressed proteins from the discovery and screening phases are shown. Distribution of cluster assignment is depicted. Protein members of clusters with best matching pattern were selected (black arrow), representing a signature of 91 proteins (NP response). Based on the highest combined membership values, one protein per cluster was selected (annotated gene name), representing a signature of six proteins (NP response best member). Based on the iron oxide response pattern (white arrow), 31 proteins were selected, representing a NETosis signature. (G) Principal component analyses of the whole data set filtered for the three protein signatures: NP response, NETosis, and NP response best member with 91, 31, and 6 proteins, respectively, resemble grouping of total BALF screening.
Common proteins from other conditions (low dose day 1, high dose day 3) are shown in Figure S12E. The most unique proteins were found following exposure to pure cobalt oxide nanoparticles for all conditions (Figure S12D). Comparing all samples, 45 proteins were found to be unique to cobalt and were enriched in Nrf2-mediated antioxidant stress response and detoxification including, e.g., Sod2, Sod3, Gpx1, and Txn1. As already observed by PCA, the response to pure iron oxide nanoparticles according to the BALF proteome changes is transient, especially at low dose. Enrichment analysis (Figure 4D, Figure S14) gave similar results as for the discovery in-depth profiling. Protein changes were associated with the immune system, inflammation, complement and blood coagulation, response to wounding and actin cytoskeleton remodeling. In summary, it becomes apparent that cobalt oxide containing nanoparticles induce a delayed response while pure iron oxide nanoparticles are cleared most efficiently.
Clustering of significantly different proteins from the day 1 high dose conditions (Figure 4E) confirmed observations made during the discovery phase. Similar clusters were found, and by including nanoparticles with an incremental increase of cobalt oxide composition (Fe 1:0, Fe Co 5:1, Fe Co 3:1, Fe Co 1:3, Co 0:1), the dose-dependency of the metal oxide nanoparticle type could be followed. Comparing the screening cluster assignment to the discovery clusters and visualizing the overlap between the two strategies (discovery, screening) shows that both results are in accordance (Figure 4F, left panel). By selecting proteins which were assigned to the same cluster type (black arrow, Figure 4F, left panel), we defined a protein signature of 91 from the screening data and called them NP response. Of those, selecting the best protein of each cluster, defined by the highest combined membership score, a six-protein signature was generated (Gpi, Scgb1a1, S100a9, Gsn, Cfh, Sod1). A NETosis signature consisting of 31 proteins based on overlapping proteins with positive iron oxide nanoparticle response (discovery clusters 1 and 3, screening clusters 1 and 2) was also defined (white arrow, Figure 4F, left panel). All three protein signatures allowed the discrimination of controls versus nanoparticle treated samples (Figure 4F, right panel) similarly as profiling around 400 proteins from the whole data set (Figure 4A) with treatment dependency was visible. In order to test the discriminatory power of these signature proteins (Table S7), they were applied to a comprehensive BALF data set covering the time course of bleomycin induced reversible lung fibrosis,62 which can be seen after 3–21 days of treatment, while from 28 days onward, the healing process was initiated and the samples cluster with the untreated samples. The 6-protein signature was not sufficient to separate the meaningful samples. Applying the NETosis signature, two clear groups emerged, separating controls with the very early stage from the medium time point, whereas other stages were distributed in both groups. Applying our protein signature of 91 proteins on this data set and filtering for proteins with no missing values, we further reduced our signature to 24 proteins and were able to separate the experimental groups in the same way as for the whole data set based on 400 quantified proteins (Figure S15). With this example, it was demonstrated that the proposed nanotoxicology platform produces not only meaningful results but is also a powerful tool for signature or biomarker discovery.
Conclusions
A nanotoxicology proteomics screening platform transferable to all kinds of nanomaterials is presented here. As proof of principle, cobalt ferrite (CoFe2O4) magnetic nanoparticles were selected with iron and cobalt content at different ratios, which were intratracheal instilled in mice and bronchoalveolar lavage collected. The Evosep One LC system28 combined with high-resolution mass spectrometry and short gradient peptide separation ensured robust and fast measurements with high-sample throughputs desirable in diagnostics. The 21 min/60 samples a day protocol allowed average quantification of 400 proteins per BALF sample. The performance of the Evosep One LC system was good allowing fast and robust measurements with a median CV of 19%. In line with previous studies, immune system and complement activation were dominating responses from pulmonary nanoparticle exposure. Based on differential expression analysis, iron oxide nanoparticles were better tolerated with faster clearance than any of the Co doped variants. Most prominent changes in the BALF proteome with strong iron dose response could be attributed to NETosis or NET formation. NET associated proteins quantified during the discovery phase by deep proteome profiling were validated by comparison to other NET proteome studies, and potential NET candidates were added. While most studies on NETosis are limited to the cell culture environment using nonphysiological inducing agents, knowledge to NETosis formation in vivo in response to real life stimuli was added. The combination of discovery and screening generated data protein signatures can be applied to detect lung injury or NETosis.
Methods
Animal Experiments
Female C57BL/6J BomTac mice were obtained from Taconic Europe (Ejby, Denmark). Mice were allocated arbitrarily to experimental groups and were acclimatized for 1 week before the start of the experiments. All mice were housed with up to 6 animals per cage in controlled environmental conditions; temperature (21 ± 1 °C), humidity (50% ± 10%) and 12 h light/dark period. Mice had access to food and water ad libitum. All mice were dosed by a single intratracheal instillation at 8–9 weeks of age and average weight of 20 g as previously described.63 Metal oxide nanoparticles (iron oxide doped with cobalt oxide at different ratios; Fe3O4 (magnetite), CoFe2O4 (cobalt ferrite, Fe Co 5:1), CoFe2O4 (Fe Co 3:1), CoFe2O4 (Fe Co 1:3), Co3O4), and reference material (carbon black, Printex 90) were instilled one material at a time at doses of 54 μg and 162 μg/mouse in 2% murine serum. Three mice were included for each material as the vehicle control group (instillation with 0 μg/mouse in 2% murine serum), which were combined for statistical analysis for each exposure time point. Six mice per dose group were included for each time point (day 1 and day 3). Mice were euthanized at 1 day and 3 days postexposure. All procedures complied with the EC Directive 86/609/EEC and Danish law regulating experiments with animals (The Danish Ministry of Justice, Animal Experiments Inspectorate, permission 2006/561-1123).
Intratracheal Instillation
For animal instillations, the nanomaterial dispersions were diluted in 2% murine serum in 0.45 μm Milli-Q filtered Nanopure water and sonicated for 16 min using a 400 W Branson Sonifier S-450D (Branson Ultrasonics Corp., Danbury, CT) mounted with a disruptor horn and operated at 10% amplitude as described.64 The dispersions were continuously cooled by ice/water. Dilutions were prepared directly after sonication and were further sonicated for 2 min after resuspending. Size distribution was immediately measured using DLS. Nanoparticle dispersions were administered in a 50 μL volume.
Nanoparticle Characterization
Synthesis
Magnetic iron oxide, cobalt oxide, and cobalt-doped iron oxide nanoparticles were produced at Promethean Particles Ltd. (Nottingham, U.K.) by continuous hydrothermal synthesis starting from metal salts as precursors, with iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, Sigma-Aldrich) and cobalt(II)acetate tetrahydrate (Co(C2H3O2)2·4H2O, Sigma-Aldrich) for iron and cobalt oxide, respectively. For Co doping, the salts were used at different ratios to produce nanoparticles with 5:1, 3:1, and 1:3 Fe Co compositions. Synthesis and characterization of the particles were described in detail.32 Nanoparticles were distributed as aqueous solutions.
Determination of Particle Elemental Composition
Samples were analyzed on a PerkinElmer Optima 8000 (PerkinElmer, Shelton, CT) by inductively coupled plasma-optical emission spectrometry (ICP-OES) in radial mode to determine the relative abundance of each element in the nanoparticle solutions. Triplicates of each solution were digested in 24% ICP grade hydrochloric acid for 24 h. These samples were subsequently diluted 10 000-fold in 2% nitric acid prior to ICP-OES analysis. The OES torch was aligned using 1 ppm manganese at wavelength 257.610 nm and the iron and cobalt measurements were taken at 238.204 and 228.616 nm, respectively.
DLS and Zeta Potential Characterization
Samples were diluted 1 in 5000 using deionized water (pH 7.2, 18.2 mΩ cm) prior to measurement at 25 °C on a Malvern Zetasizer (nanoZS) (Malvern Panalytical, Malvern, U.K.). Samples were allowed to equilibrate for 2 min prior to analysis with each sample analyzed in triplicates. These data were collected and averaged to determine the hydrodynamic diameter, polydispersity index (PDI), zeta potential, and electrophoretic mobility.
Transmission Electron Microscopy
TEM analysis was performed using a JEOL 1400EX 80 kV system. TEM grids were prepared by a dropcasting method, whereby a 10 μL drop of the nanoparticle suspension was deposited on a 300 mesh carbon-coated copper TEM grid (Agar Scientific, U.K.). The drop was left for approximately 2 h to allow the nanoparticles to adhere to the carbon membrane, and grids were rinsed with ultrapure water to remove excess water to avoid agglomeration. Particle diameter measurements were conducted using the Gatan Digital Micrograph software by measuring at least 100 particles for each composition.
Bronchoalveolar Lavage Fluid Preparation
Lungs were flushed twice with 1 mL of saline using a BAL cannula (BD InsyteTM, 20 GA, 1.1 mm × 48 mm, 5 mL/min) placed in the trachea. BALF recovery was 1.5 mL per animal. Cellular content was removed by centrifugation (400g, 10 min, 4 °C), and aliquots of supernatants were stored at −80 °C until analysis. Samples were only frozen and thawed once and were never refrozen.
Cellular Content of Bronchoalveolar Lavage Fluid
BAL cell numbers were determined as previously described.31 The total number of BAL cells was determined in a NucleoCounter (NC-200) to obtain total BAL cell counts. Differential cell counts were determined by differential cell counting. A volume of 40 μL of cell suspension (differential count) was collected on CYTOSPIN slides (1000 rpm, 4 min). After centrifugation, the cells were stained using the May Grünwald and Giemsa. Cell differentiation was performed counting 200 cells per slide.
Cytotoxicity Testing: Total Protein Content and LDH Assay
Total protein content in BAL fluid was measured by a Pierce BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer’s protocol. Samples and standards were assayed in duplicate in 96-well plates and incubated for 30 min at 37 °C. Absorbance was measured at 550 nm on a Victor2 1420 multilabel counter (Wallac, PerkinElmer). Protein concentrations of all acellular BAL fluid samples were calculated based on the standard curve of known albumin concentrations.
Cytotoxicity was determined using the LDH Cytotoxicity Detection Kit (Roche). Volumes of 100 μL of BAL fluid were assayed in 96-well plates in duplicate. A volume of 100 μL of LDH reaction mixture was added to each well, and the plates were incubated at room temperature for 30 min, protected from the light. Absorbance was measured at 492 nm with a reference wavelength of 630 nm.
Saa3 mRNA Levels
Total RNA was isolated from the left lung and the lateral lobe of the liver (6–23 mg) using a Maxwell 16 LEV simply RNA Tissue Kit (Promega) according to the manufacturer’s instructions. Isolated RNA was stored at −80 °C until further analysis. Saa3 mRNA levels in the lungs and Saa1 in the liver were assessed as markers of pulmonary and hepatic acute phase responses. cDNA synthesis was prepared with Taqman Reverse Transcription Reagent Kit (Applied Biosystems) according to the manufacturer’s protocol as previously described.25 The relative gene expressions of target genes Saa1 and Saa3 were determined by RT-qPCR on ViiA 7 (Applied Biosystems) and calculated by comparative method 2–ΔCT65 using 18S levels for normalization. The nucleotide sequence of Saa3 primers and the probe were forward, 5′-GCC TGG GCT GCT AAA GTC AT-3′; reverse, 5′-TGC TCC ATG TCC CGT GAA C-3′ and probe, 50 FAM-TCT GAA CAG CCT CTC TGG CAT CGC T-TAMRA-3′ and Saa1 (Mm00656927_g1). Target and reference genes were all run in triplicates in 384-well reaction plates (Thermo Fisher) including RT controls and negative controls without synthesized cDNA. Day to day variation of the plate control was less than 25%.
Genotoxicity Testing: Comet Assay
The comet assay was performed as previously described.35 DNA strand break levels were determined on frozen BAL cell suspensions, lung and liver tissue (3 mm × 3 mm piece of median lobe). Organ samples were snap frozen in NUNC cryo-tubes directly after dissection and kept at −80 °C until analysis. Frozen BAL cells preserved in DMSO were thawed quickly at 37 °C while frozen tissues were homogenized in Merchant’s medium by a steel mesh 0.2 mm. Cells were suspended in 0.7% agarose final concentration at 37 °C. Cells were embedded on 20-well Trevigen CometSlides (30 μL per well). Cooled slides were placed in the lysis buffer overnight at 4 °C. The next day, slides were rinsed in electrophoresis buffer and alkaline treated for 40 min. Electrophoresis was run with 5% circulation (70 mL/min) for 25 min at an applied voltage of 38 V (1.15 V/cm electrophoresis chamber) and a measured current of 700 mA. Slides were neutralized (2 × 5 min), fixed in ethanol for 5 min, and on a warm plate at 45 °C for 15 min. Cells were stained in 20 mL/slide bath with TE buffered SYBRGreen fluorescent stain for 15 min, dried at 37 °C for 10 min, a UV-filter and coverslip were applied, and DNA damage was analyzed by the IMSTAR Pathfinder system. The results are presented as average %TDNA value and tail length for all cells scored on each Trevigen CometSlide well. The day-to-day variation and electrophoresis efficiency was validated by including on each slide A549 epithelial lung cells exposed to PBS or 60 μM H2O2, used as negative and positive controls as described.35
Proteomics Sample Preparation
BALF protein concentration was determined by the Pierce BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer’s protocol. All samples were brought to the same protein concentration (0.1 μg/μL) by dilution with saline. Protein extraction was performed using 4 M urea/thiourea. After reduction with dithiothreitol and alkylation with iodoacetamide, proteins were digested at room temperature with Lys-C for 4 h and trypsin overnight at a 1:50 enzyme to protein ratio. For in-depth BALF profiling (discovery phase), samples from three experimental groups (day 1 high dose; CTRL, Fe3O4, and Co3O4 nanoparticles) were selected. A total of 9 samples with 3 biological replicates per group were TMT-labeled (TMT10plex minus TMT10-131, Thermo Scientific) according to the manufacturer’s protocol and were run as 9plex. Combined TMT-labeled peptides with 5 μg per channel were desalted on R2/R3 resin in StageTip format. Unlabeled peptides for reproducibility evaluation and screening were loaded on Evosep tips in 0.5% formic acid in water after C18 activation with 100% acetonitrile and equilibration.
High pH Reversed Phase Prefractionation
For in-depth BALF profiling, 45 μg of 9plex TMT labeled peptides were separated into 16 fractions by high pH reversed phase prefractionation using an UltiMate 3000 LC system (Thermo Scientific) equipped with an Acquity UPLC CSH C18 column with 300 μm × 100 mm, 1.7 μm (Waters). Peptides were eluted over a gradient from 4% to 48% solvent B over 60 min (solvent A, 20 mM ammonium formate, adjusted to pH 9.6 by NH4OH; solvent B, 80% acetonitrile, 20% 20 mM ammonium formate, adjusted to pH 9.6 by NH4OH) and pooled according to the UV spectrum. A reference BALF library composed of representative samples of the study (pooled mix of 40 μg) was fractionated label-free into 33 fractions and run together with the BALF screening samples to increase protein identification from the match between run functionality in MaxQuant analysis.
Liquid Chromatography and Mass Spectrometry
LC–MS/MS analysis for TMT-labeled BALF samples (discovery, in-depth profiling) were carried out using an EASY-nLC 1000 system connected to a Q Exactive HF mass spectrometer (ThermoFisher Scientific). Peptides were separated using a 136 min gradient on a 15 cm column with 3 μm Reprosil-Pur C18 beads (Dr. Maisch, Ammerbuch, Germany). For LC separation, the aqueous solvent A contained 0.1% (v/v) formic acid in water and the organic mobile phase 0.1% (v/v) formic acid in 95% (v/v) acetonitrile. The flow rate of the LC gradient was 250 nL/min for all steps, starting from 1% solvent B, increasing to 7% over 3 min, to 25% over 110 min, to 45% over 10 min, to 100% over 5 min, and a column wash with 100% solvent B for 8 min. MS1 spectra were acquired in positive ionization mode data dependently using a top10 method. MS1 spectra were recorded at a resolution of 60 000 in a mass range from m/z 300–1650, with an automated gain control (AGC) target of 1 × 106 ions and a maximum injection time of 25 ms in the profile mode. MS2 spectra were recorded at a resolution of 60 000 in a mass range from m/z 200–2000 in profile mode, with an AGC target of 5 × 104 ions, maximum injection time of 22 ms, isolation window m/z 1.2, normalized collision energy of 34, including charge states 2–4, dynamic exclusion of 15 s, and first fixed mass at m/z 110.
LC–MS/MS analysis for label-free BALF samples (reproducibility evaluation, screening including a reference library) was carried out using an Evosep One LC system connected to a Tribrid Fusion Lumos mass spectrometer (ThermoFisher Scientific). Peptides were separated using a 21 min gradient on a 7 cm column with 3 μm Reprosil-Pur C18 beads (Dr. Maisch, Ammerbuch, Germany) using the default parameters defined by Evosep One. MS full spectra were acquired in positive ionization mode data dependently using a top5 method. MS spectra were recorded at a resolution of 60 000 in a mass range from m/z 300–1650, with an AGC target of 1 × 106 ions and a maximum injection time of 25 ms in profile mode. MSn spectra were recorded at a resolution of 15 000 in the mass range of m/z 200–2000 in centroid mode, with an AGC target of 5 × 104 ions, a maximum injection time of 22 ms, an isolation window of m/z 1.5, and a normalized collision energy of 27.
Mass Spectrometry Data Analysis
MS raw files were analyzed with MaxQuant software (versions 1.5.7 and 1.6.166,67), and generated peak lists were searched against the murine UniProt FASTA reference proteome database downloaded in May 2018 (52 528 entries). The FDR was set to 1% for both protein and peptide identification. A maximum of two missed cleavages were allowed with a minimal peptide length of seven amino acids. Cysteine carbamidomethylation was set as fixed modification, methionine oxidation, and N-terminal acetylation were set as variable modifications. Label-free BALF samples for reproducibility evaluation and screening were analyzed allowing for match between runs with matching time window of 0.7 min and alignment time window of 3 min. For TMT-labeled BALF samples (discovery), the TMT10plex was selected as an isobaric label, excluding TMT10-131. For protein quantification, a minimal ratio count of one was allowed.
Statistics and Bioinformatics Analysis
The animal study was carried out on several different calendar dates. Vehicle controls were included on all calendar dates. Vehicle controls were subsequently pooled for each postexposure day (day 1 and day 3). BAL data, Saa3 mRNA levels, and genotoxicity were analyzed using two-way ANOVA with individual particle and dose level set as fixed category variables. Log-transformation was used in some cases. In the case of statistically significant interaction effects across the fixed variables, one-way ANOVA was performed with the dose as a fixed categorical variable. If statistically significant main effects were observed, pairwise comparison was performed using Dunnett’s test or Tukey’s post hoc test.
Statistics and bioinformatic analyses for proteomics were performed in the R environment. Differential expression analysis was performed with the limma package68 using the Bayes moderate t test. Vehicle controls included for each nanoparticle exposure were pooled per exposure time point (day 1, day 3). Proteins with FDR < 0.05 were considered significant and were clustered with fuzzy c-means algorithm using the Mfuzz package.69 Plotting of the expression patterns was based on a minimal membership value of 0.7. The Pearson correlation coefficients were calculated for proteins with a minimal 3 out of 16 quantitative measurements. Gene set enrichment analysis was carried out with the STRINGdb package70 for the categories gene ontology biological process, gene ontology molecular function, gene ontology cellular compartment, and KEGG pathways. Protein protein interaction networks were generated with the stringApp v1.4.071 within Cytoscape v3.7.1.72
Data Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium73via the PRIDE74 partner repository with the data set identifier PXD016148. Submitted were MS raw files, search parameter files (mqpar.xml), MaxQuant software versions, UniProt database FASTA file, and MaxQuant search output (proteinGroups.txt) for both Discovery and Screening.
Acknowledgments
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreements 646603 and 720952 (ACEnano)), the FP7 project NanoMILE (Grant Agreements 310451 and 320451), and the Danish Centre for Nanosafety 2. Proteomics and mass spectrometry research at University of Southern Denmark (SDU) are supported by generous grants to the VILLUM Center for Bioanalytical Sciences (VILLUM Foundation Grant No. 7292) and PRO-MS: Danish National Mass Spectrometry Platform for Functional Proteomics (Grant No. 5072-00007B).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.9b08818.
Nanoparticle characterization, classic nanoparticle compatibility testing, protein–protein interaction networks of clustered significant proteins, GO term and KEGG enrichment analysis, histone expression, and protein signature testing (PDF)
Table S1, BALF differential cell counts (XLSX)
Table S2, discovery phase: differentially expressed proteins (XLSX)
Table S3, discovery phase: cluster information on differentially expressed proteins (XLSX)
Table S4, discovery phase: overlap of clustered differentially expressed proteins with NETproteome studies (XLSX)
Table S5, discovery phase: overlap of differentially expressed proteins with NETproteome studies (XLSX)
Table S6, screening phase: differentially expressed proteins (XLSX)
Table S7, screening phase: protein signatures (XLSX)
The authors declare no competing financial interest.
Supplementary Material
References
- Pelaz B.; Alexiou C.; Alvarez-Puebla R. A.; Alves F.; Andrews A. M.; Ashraf S.; Balogh L. P.; Ballerini L.; Bestetti A.; Brendel C.; Bosi S.; Carril M.; Chan W. C. W.; Chen C.; Chen X.; Chen X.; Cheng Z.; Cui D.; Du J.; Dullin C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehemann K.; Schneider S. W.; Luger T. A.; Godin B.; Ferrari M.; Fuchs H. Nanomedicine – Challenge and Perspectives. Angew. Chem., Int. Ed. 2009, 48, 872–897. 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir A.; Westerhoff P.; Fabricius L.; Hristovski K.; von Goetz N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Sharma N.; Maitra S. S. In Vitro and In Vivo Toxicity Assessment of Nanoparticles. Int. Nano Lett. 2017, 7, 243–256. 10.1007/s40089-017-0221-3. [DOI] [Google Scholar]
- Stone V.; Pozzi-Mucelli S.; Tran L.; Aschberger K.; Sabella S.; Vogel U.; Poland C.; Balharry D.; Fernandes T.; Gottardo S.; Hankin S.; Hartl M. G.; Hartmann N.; Hristozov D.; Hund-Rinke K.; Johnston H.; Marcomini A.; Panzer O.; Roncato D.; Saber A. T.; et al. ITS-NANO - Prioritising Nanosafety Research to Develop a Stakeholder Driven Intelligent Testing Strategy. Part. Fibre Toxicol. 2014, 11, 9. 10.1186/1743-8977-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte J. W. M. Superparamagnetic Iron Oxides As MPI Tracers: A Primer and Review of Early Applications. Adv. Drug Delivery Rev. 2019, 138, 293–301. 10.1016/j.addr.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H. Long-Distance Navigation and Magnetoreception in Migratory Animals. Nature 2018, 558, 50. 10.1038/s41586-018-0176-1. [DOI] [PubMed] [Google Scholar]
- Sytnyk M.; Kirchschlager R.; Bodnarchuk M. I.; Primetzhofer D.; Kriegner D.; Enser H.; Stangl J.; Bauer P.; Voith M.; Hassel A. W.; Krumeich F.; Ludwig F.; Meingast A.; Kothleitner G.; Kovalenko M. V.; Heiss W. Tuning the Magnetic Properties of Metal Oxide Nanocrystal Heterostructures by Cation Exchange. Nano Lett. 2013, 13, 586–593. 10.1021/nl304115r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini F.; Hedberg Y.; McCarrick S.; Hedberg J.; Derr R.; Hendriks G.; Odnevall Wallinder I.; Karlsson H. L. Mechanistic Insight into Reactivity and (Geno)Toxicity of Well-Characterized Nanoparticles of Cobalt Metal and Oxides. Nanotoxicology 2018, 12, 602–620. 10.1080/17435390.2018.1470694. [DOI] [PubMed] [Google Scholar]
- Abudayyak M.; Gurkaynak T. A.; Özhan G. In Vitro Evaluation of Cobalt Oxide Nanoparticle-Induced Toxicity. Toxicol. Ind. Health 2017, 33, 646–654. 10.1177/0748233717706633. [DOI] [PubMed] [Google Scholar]
- Fröhlich E. Role of Omics Techniques in the Toxicity Testing of Nanoparticles. J. Nanobiotechnol. 2017, 15, 84. 10.1186/s12951-017-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K.-J.; Chuang K.-J.; Chen J.-K.; Hua H.-E.; Shen Y.-L.; Liao W.-N.; Lee C.-H.; Chen K.-Y.; Lee K.-Y.; Hsiao T.-C.; Pan C.-H.; Ho K.-F.; Chuang H.-C. Investigation into the Pulmonary Inflammopathology of Exposure to Nickel Oxide Nanoparticles in Mice. Nanomedicine (N. Y., NY, U. S.) 2018, 14, 2329–2339. 10.1016/j.nano.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Chézeau L.; Kohlstaedt L. A.; Le Faou A.; Cosnier F.; Rihn B.; Gaté L. Proteomic Analysis of Bronchoalveolar Lavage Fluid in Rat Exposed to TiO2 Nanostructured Aerosol by Inhalation. J. Proteomics 2019, 207, 103451. 10.1016/j.jprot.2019.103451. [DOI] [PubMed] [Google Scholar]
- Dailey L. A.; Hernández-Prieto R.; Casas-Ferreira A. M.; Jones M.-C.; Riffo-Vasquez Y.; Rodríguez-Gonzalo E.; Spina D.; Jones S. A.; Smith N. W.; Forbes B.; Page C.; Legido-Quigley C. Adenosine Monophosphate Is Elevated in the Bronchoalveolar Lavage Fluid of Mice with Acute Respiratory Toxicity Induced by Nanoparticles with High Surface Hydrophobicity. Nanotoxicology 2015, 9, 106–115. 10.3109/17435390.2014.894150. [DOI] [PubMed] [Google Scholar]
- Ganguly K.; Upadhyay S.; Irmler M.; Takenaka S.; Pukelsheim K.; Beckers J.; De Angelis M. H.; Hamelmann E.; Stoeger T.; Schulz H. Impaired Resolution of Inflammatory Response in the Lungs of JF1/Msf Mice Following Carbon Nanoparticle Instillation. Respir. Res. 2011, 12, 94. 10.1186/1465-9921-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halappanavar S.; Jackson P.; Williams A.; Jensen K. A.; Hougaard K. S.; Vogel U.; Yauk C. L.; Wallin H. Pulmonary Response to Surface-Coated Nanotitanium Dioxide Particles Includes Induction of Acute Phase Response Genes, Inflammatory Cascades, and Changes in MicroRNAs: A Toxicogenomic Study. Environ. Mol. Mutagen. 2011, 52, 425–439. 10.1002/em.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H.; Wang T.-Y.; Hong J.-H.; Cheng T.-J.; Lin C.-Y. NMR-Based Metabolomics to Determine Acute Inhalation Effects of Nano- and Fine-Sized ZnO Particles in the Rat Lung. Nanotoxicology 2016, 10, 924–934. 10.3109/17435390.2016.1144825. [DOI] [PubMed] [Google Scholar]
- Bourdon J. A.; Saber A. T.; Jacobsen N. R.; Jensen K. A.; Madsen A. M.; Lamson J. S.; Wallin H.; Møller P.; Loft S.; Yauk C. L.; Vogel U. B. Carbon Black Nanoparticle Instillation Induces Sustained Inflammation and Genotoxicity in Mouse Lung and Liver. Part. Fibre Toxicol. 2012, 9, 5. 10.1186/1743-8977-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halappanavar S.; Saber A. T.; Decan N.; Jensen K. A.; Wu D.; Jacobsen N. R.; Guo C.; Rogowski J.; Koponen I. K.; Levin M.; Madsen A. M.; Atluri R.; Snitka V.; Birkedal R. K.; Rickerby D.; Williams A.; Wallin H.; Yauk C. L.; Vogel U. Transcriptional Profiling Identifies Physicochemical Properties of Nanomaterials That Are Determinants of the In Vivo Pulmonary Response. Environ. Mol. Mutagen. 2015, 56, 245–264. 10.1002/em.21936. [DOI] [PubMed] [Google Scholar]
- Halappanavar S.; Rahman L.; Nikota J.; Poulsen S. S.; Ding Y.; Jackson P.; Wallin H.; Schmid O.; Vogel U.; Williams A. Ranking of Nanomaterial Potency to Induce Pathway Perturbations Associated with Lung Responses. NanoImpact 2019, 14, 100158. 10.1016/j.impact.2019.100158. [DOI] [Google Scholar]
- Husain M.; Wu D.; Saber A. T.; Decan N.; Jacobsen N. R.; Williams A.; Yauk C. L.; Wallin H.; Vogel U.; Halappanavar S. Intratracheally Instilled Titanium Dioxide Nanoparticles Translocate to Heart and Liver and Activate Complement Cascade in the Heart of C57BL/6 Mice. Nanotoxicology 2015, 9, 1013–1022. 10.3109/17435390.2014.996192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen S. S.; Saber A. T.; Williams A.; Andersen O.; Købler C.; Atluri R.; Pozzebon M. E.; Mucelli S. P.; Simion M.; Rickerby D.; Mortensen A.; Jackson P.; Kyjovska Z. O.; Mølhave K.; Jacobsen N. R.; Jensen K. A.; Yauk C. L.; Wallin H.; Halappanavar S.; Vogel U. MWCNTs of Different Physicochemical Properties Cause Similar Inflammatory Responses, but Differences in Transcriptional and Histological Markers of Fibrosis in Mouse Lungs. Toxicol. Appl. Pharmacol. 2015, 284, 16–32. 10.1016/j.taap.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Bendtsen K. M.; Brostrøm A.; Koivisto A. J.; Koponen I.; Berthing T.; Bertram N.; Kling K. I.; Dal Maso M.; Kangasniemi O.; Poikkimäki M.; Loeschner K.; Clausen P. A.; Wolff H.; Jensen K. A.; Saber A. T.; Vogel U. Airport Emission Particles: Exposure Characterization and Toxicity Following Intratracheal Instillation in Mice. Part. Fibre Toxicol. 2019, 16, 23. 10.1186/s12989-019-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyjovska Z. O.; Jacobsen N. R.; Saber A. T.; Bengtson S.; Jackson P.; Wallin H.; Vogel U. DNA Strand Breaks, Acute Phase Response and Inflammation Following Pulmonary Exposure by Instillation to the Diesel Exhaust Particle NIST1650b in Mice. Mutagenesis 2015, 30, 499–507. 10.1093/mutage/gev009. [DOI] [PubMed] [Google Scholar]
- Poulsen S. S.; Jackson P.; Kling K.; Knudsen K. B.; Skaug V.; Kyjovska Z. O.; Thomsen B. L.; Clausen P. A.; Atluri R.; Berthing T.; Bengtson S.; Wolff H.; Jensen K. A.; Wallin H.; Vogel U. Multi-Walled Carbon Nanotube Physicochemical Properties Predict Pulmonary Inflammation and Genotoxicity. Nanotoxicology 2016, 10, 1263–1275. 10.1080/17435390.2016.1202351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber A. T.; Jensen K. A.; Jacobsen N. R.; Birkedal R.; Mikkelsen L.; Møller P.; Loft S.; Wallin H.; Vogel U. Inflammatory and Genotoxic Effects of Nanoparticles Designed for Inclusion in Paints and Lacquers. Nanotoxicology 2012, 6, 453–471. 10.3109/17435390.2011.587900. [DOI] [PubMed] [Google Scholar]
- Wallin H.; Kyjovska Z. O.; Poulsen S. S.; Jacobsen N. R.; Saber A. T.; Bengtson S.; Jackson P.; Vogel U. Surface Modification Does Not Influence the Genotoxic and Inflammatory Effects of TiO2 Nanoparticles after Pulmonary Exposure by Instillation in Mice. Mutagenesis 2017, 32, 47–57. 10.1093/mutage/gew046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache N.; Geyer P. E.; Bekker-Jensen D. B.; Hoerning O.; Falkenby L.; Treit P. V.; Doll S.; Paron I.; Müller J. B.; Meier F.; Olsen J. V.; Vorm O.; Mann M. A Novel LC System Embeds Analytes in Pre-Formed Gradients for Rapid, Ultra-Robust Proteomics. Mol. Cell. Proteomics 2018, 17, 2284–2296. 10.1074/mcp.TIR118.000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber A. T.; Jacobsen N. R.; Jackson P.; Poulsen S. S.; Kyjovska Z. O.; Halappanavar S.; Yauk C. L.; Wallin H.; Vogel U. Particle-Induced Pulmonary Acute Phase Response May Be the Causal Link between Particle Inhalation and Cardiovascular Disease. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 517–531. 10.1002/wnan.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup N.; Rahmani F.; Jacobsen N. R.; Saber A. T.; Jackson P.; Bengtson S.; Williams A.; Wallin H.; Halappanavar S.; Vogel U. Acute Phase Response and Inflammation Following Pulmonary Exposure to Low Doses of Zinc Oxide Nanoparticles in Mice. Nanotoxicology 2019, 13, 1275–1292. 10.1080/17435390.2019.1654004. [DOI] [PubMed] [Google Scholar]
- Kyjovska Z. O.; Jacobsen N. R.; Saber A. T.; Bengtson S.; Jackson P.; Wallin H.; Vogel U. DNA Damage Following Pulmonary Exposure by Instillation to Low Doses of Carbon Black (Printex 90) Nanoparticles in Mice. Environ. Mol. Mutagen. 2015, 56, 41–49. 10.1002/em.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Römer I.; Tang S. V. Y.; Valsami-Jones E.; Palmer R. E. Crystallinity Depends on Choice of Iron Salt Precursor in the Continuous Hydrothermal Synthesis of Fe–Co Oxide Nanoparticles. RSC Adv. 2017, 7, 37436–37440. 10.1039/C7RA06647C. [DOI] [Google Scholar]
- Modrzynska J.; Berthing T.; Ravn-Haren G.; Jacobsen N. R.; Weydahl I. K.; Loeschner K.; Mortensen A.; Saber A. T.; Vogel U. Primary Genotoxicity in the Liver Following Pulmonary Exposure to Carbon Black Nanoparticles in Mice. Part. Fibre Toxicol. 2018, 15, 2. 10.1186/s12989-017-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. C.; Raghu G.; Baughman R. P.; Brown K. K.; Costabel U.; du Bois R. M.; Drent M.; Haslam P. L.; Kim D. S.; Nagai S.; Rottoli P.; Saltini C.; Selman M.; Strange C.; Wood B. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- Jackson P.; Pedersen L. M.; Kyjovska Z. O.; Jacobsen N. R.; Saber A. T.; Hougaard K. S.; Vogel U.; Wallin H. Validation of Freezing Tissues and Cells for Analysis of DNA Strand Break Levels by Comet Assay. Mutagenesis 2013, 28, 699–707. 10.1093/mutage/get049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P. The Comet Assay: Ready for 30 More Years. Mutagenesis 2018, 33, 1–7. 10.1093/mutage/gex046. [DOI] [PubMed] [Google Scholar]
- Singh N. P.; McCoy M. T.; Tice R. R.; Schneider E. L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Tabei Y.; Fukui H.; Nishioka A.; Hagiwara Y.; Sato K.; Yoneda T.; Koyama T.; Horie M. Effect of Iron Overload from Multi Walled Carbon Nanotubes on Neutrophil-Like Differentiated HL-60 Cells. Sci. Rep. 2019, 9, 2244. 10.1038/s41598-019-38598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P.; Yeoh B. S.; Olvera R. A.; Xiao X.; Singh V.; Awasthi D.; Subramanian B. C.; Chen Q.; Dikshit M.; Wang Y.; Parent C. A.; Vijay-Kumar M. Bacterial Siderophores Hijack Neutrophil Functions. J. Immunol. 2017, 198, 4293–4303. 10.4049/jimmunol.1700261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Macrophages and Iron Metabolism. Microbiol. Spectrum 2016, 10.1128/microbiolspec.MCHD-0037-2016. [DOI] [PubMed] [Google Scholar]
- Lim D.; Kim K. S.; Jeong J.-H.; Marques O.; Kim H.-J.; Song M.; Lee T.-H.; Kim J. I.; Choi H.-S.; Min J.-J.; Bumann D.; Muckenthaler M. U.; Choy H. E. The Hepcidin-Ferroportin Axis Controls the Iron Content of Salmonella -Containing Vacuoles in Macrophages. Nat. Commun. 2018, 9, 2091. 10.1038/s41467-018-04446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni A. Bronchoalveolar Lavage in the Diagnosis of Hard Metal Disease. Sci. Total Environ. 1994, 150, 69–76. 10.1016/0048-9697(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Jeong J.; Han Y.; Poland C. A.; Cho W.-S. Response-Metrics for Acute Lung Inflammation Pattern by Cobalt-Based Nanoparticles. Part. Fibre Toxicol. 2015, 12, 13. 10.1186/s12989-015-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V.; Reichard U.; Goosmann C.; Fauler B.; Uhlemann Y.; Weiss D. S.; Weinrauch Y.; Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Douda D. N.; Khan M. A.; Grasemann H.; Palaniyar N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 2817–2822. 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolska M. J.; Mahajan A.; Knopf J.; Hahn J.; Boeltz S.; Munoz L.; Bilyy R.; Herrmann M. Autoimmune, Rheumatic, Chronic Inflammatory Diseases: Neutrophil Extracellular Traps on Parade. Autoimmunity 2018, 51, 281–287. 10.1080/08916934.2018.1519804. [DOI] [PubMed] [Google Scholar]
- Inoue M.; Nakashima R.; Enomoto M.; Koike Y.; Zhao X.; Yip K.; Huang S. H.; Waldron J. N.; Ikura M.; Liu F.-F.; Bratman S. V. Plasma Redox Imbalance Caused by Albumin Oxidation Promotes Lung-Predominant NETosis and Pulmonary Cancer Metastasis. Nat. Commun. 2018, 9, 5116. 10.1038/s41467-018-07550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S.-L.; Zhang H.; Tang Q.; Bai J.; He Z.-Y.; Zhang J.-Q.; Li M.-H.; Deng J.-M.; Liu G.-N.; Zhong X.-N. Neutrophil Extracellular Traps Induced by Cigarette Smoke Activate Plasmacytoid Dendritic Cells. Thorax 2017, 72, 1084–1093. 10.1136/thoraxjnl-2016-209887. [DOI] [PubMed] [Google Scholar]
- Albrengues J.; Shields M. A.; Ng D.; Park C. G.; Ambrico A.; Poindexter M. E.; Upadhyay P.; Uyeminami D. L.; Pommier A.; Küttner V.; Bružas E.; Maiorino L.; Bautista C.; Carmona E. M.; Gimotty P. A.; Fearon D. T.; Chang K.; Lyons S. K.; Pinkerton K. E.; Trotman L. C.; et al. Neutrophil Extracellular Traps Produced during Inflammation Awaken Dormant Cancer Cells in Mice. Science 2018, 361, eaao4227 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz L. E.; Bilyy R.; Biermann M. H. C.; Kienhöfer D.; Maueröder C.; Hahn J.; Brauner J. M.; Weidner D.; Chen J.; Scharin-Mehlmann M.; Janko C.; Friedrich R. P.; Mielenz D.; Dumych T.; Lootsik M. D.; Schauer C.; Schett G.; Hoffmann M.; Zhao Y.; Herrmann M. Nanoparticles Size-Dependently Initiate Self-Limiting NETosis-Driven Inflammation. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E5856–E5865. 10.1073/pnas.1602230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Marion T. N.; Liu Y.; Zhang L.; Cao X.; Hu H.; Zhao Y.; Herrmann M. Nanomaterial Exposure Induced Neutrophil Extracellular Traps: A New Target in Inflammation and Innate Immunity. J. Immunol. Res. 2019, 2019, 3560180. 10.1155/2019/3560180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschi M.; Petretto A.; Santucci L.; Vaglio A.; Pratesi F.; Migliorini P.; Bertelli R.; Lavarello C.; Bartolucci M.; Candiano G.; Prunotto M.; Ghiggeri G. M. Neutrophil Extracellular Traps Protein Composition Is Specific for Patients with Lupus Nephritis and Includes Methyl-Oxidized Αenolase (Methionine Sulfoxide 93). Sci. Rep. 2019, 9, 7934. 10.1038/s41598-019-44379-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. A.; Lyon M.; Simpson D.; Mason D.; Beynon R. J.; Moots R. J.; Wright H. L. Caught in a Trap? Proteomic Analysis of Neutrophil Extracellular Traps in Rheumatoid Arthritis and Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 423. 10.3389/fimmu.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. H.; Adav S. S.; Sze S. K.; Choong Y. K.; Saravanan R.; Schmidtchen A. Thrombin and Plasmin Alter the Proteome of Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 1554. 10.3389/fimmu.2018.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue A. J.; Jin Y.; Knudsen G. M.; Perera N. C.; Jenne D. E.; Murphy J. E.; Craik C. S.; Hermiston T. W. Global Substrate Profiling of Proteases in Human Neutrophil Extracellular Traps Reveals Consensus Motif Predominantly Contributed by Elastase. PLoS One 2013, 8, e75141 10.1371/journal.pone.0075141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petretto A.; Bruschi M.; Pratesi F.; Croia C.; Candiano G.; Ghiggeri G.; Migliorini P. Neutrophil Extracellular Traps (NET) Induced by Different Stimuli: A Comparative Proteomic Analysis. PLoS One 2019, 14, e0218946 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C. F.; Ermert D.; Schmid M.; Abu-Abed U.; Goosmann C.; Nacken W.; Brinkmann V.; Jungblut P. R.; Zychlinsky A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida Albicans. PLoS Pathog. 2009, 5, e1000639 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C.; Leppkes M.; Muñoz L. E.; Schley G.; Schett G.; Herrmann M. Extracellular DNA Traps in Inflammation, Injury and Healing. Nat. Rev. Nephrol. 2019, 15, 559–575. 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- Wang H.; Wang C.; Zhao M.-H.; Chen M. Neutrophil Extracellular Traps Can Activate Alternative Complement Pathways. Clin. Exp. Immunol. 2015, 181, 518–527. 10.1111/cei.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X.; Yeoh B. S.; Vijay-Kumar M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. 10.1146/annurev-nutr-071816-064559. [DOI] [PubMed] [Google Scholar]
- Liu X.; Yang C.; Hu Y.; Lei E.; Lin X.; Zhao L.; Zou Z.; Zhang A.; Zhou H.; Chen H.; Qian P.; Jin M. HIST1H1C Regulates Interferon-β and Inhibits Influenza Virus Replication by Interacting with IRF3. Front. Immunol. 2017, 8, 350. 10.3389/fimmu.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller H. B.; Fernandez I. E.; Burgstaller G.; Schaab C.; Scheltema R. A.; Schwarzmayr T.; Strom T. M.; Eickelberg O.; Mann M. Time- and Compartment-resolved Proteome Profiling of the Extracellular Niche in Lung Injury and Repair. Mol. Syst. Biol. 2015, 11, 819. 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.; Lund S. P.; Kristiansen G.; Andersen O.; Vogel U.; Wallin H.; Hougaard K. S. An Experimental Protocol for Maternal Pulmonary Exposure in Developmental Toxicology. Basic Clin. Pharmacol. Toxicol. 2011, 108, 202–207. 10.1111/j.1742-7843.2010.00644.x. [DOI] [PubMed] [Google Scholar]
- Hadrup N.; Bengtson S.; Jacobsen N. R.; Jackson P.; Nocun M.; Saber A. T.; Jensen K. A.; Wallin H.; Vogel U. Influence of Dispersion Medium on Nanomaterial-Induced Pulmonary Inflammation and DNA Strand Breaks: Investigation of Carbon Black, Carbon Nanotubes and Three Titanium Dioxide Nanoparticles. Mutagenesis 2017, 32, 581–597. 10.1093/mutage/gex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Cox J.; Mann M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Cox J.; Hein M. Y.; Luber C. A.; Paron I.; Nagaraj N.; Mann M. Accurate Proteome-Wide Label-Free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteomics 2014, 13, 2513–2526. 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. E.; Phipson B.; Wu D.; Hu Y.; Law C. W.; Shi W.; Smyth G. K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L.; Futschik E. Mfuzz; A Software Package for Soft Clustering of Microarray Data. Bioinformation 2007, 2, 5–7. 10.6026/97320630002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A.; Szklarczyk D.; Frankild S.; Kuhn M.; Simonovic M.; Roth A.; Lin J.; Minguez P.; Bork P.; von Mering C.; Jensen L. J. STRING v9.1: Protein-Protein Interaction Networks, with Increased Coverage and Integration. Nucleic Acids Res. 2012, 41, D808–D815. 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheva N. T.; Morris J. H.; Gorodkin J.; Jensen L. J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P.; Markiel A.; Ozier O.; Baliga N. S.; Wang J. T.; Ramage D.; Amin N.; Schwikowski B.; Ideker T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch E. W.; Csordas A.; Sun Z.; Jarnuczak A.; Perez-Riverol Y.; Ternent T.; Campbell D. S.; Bernal-Llinares M.; Okuda S.; Kawano S.; Moritz R. L.; Carver J. J.; Wang M.; Ishihama Y.; Bandeira N.; Hermjakob H.; Vizcaíno J. A. The ProteomeXchange Consortium in 2017: Supporting the Cultural Change in Proteomics Public Data Deposition. Nucleic Acids Res. 2017, 45, D1100–D1106. 10.1093/nar/gkw936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y.; Csordas A.; Bai J.; Bernal-Llinares M.; Hewapathirana S.; Kundu D. J.; Inuganti A.; Griss J.; Mayer G.; Eisenacher M.; Pérez E.; Uszkoreit J.; Pfeuffer J.; Sachsenberg T.; Yılmaz Ş.; Tiwary S.; Cox J.; Audain E.; Walzer M.; Jarnuczak A. F.; et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442–D450. 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium73via the PRIDE74 partner repository with the data set identifier PXD016148. Submitted were MS raw files, search parameter files (mqpar.xml), MaxQuant software versions, UniProt database FASTA file, and MaxQuant search output (proteinGroups.txt) for both Discovery and Screening.