Abstract
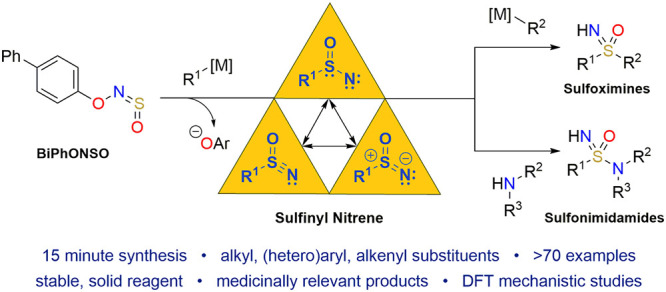
Sulfoximines and sulfonimidamides are promising compounds for medicinal and agrochemistry. As monoaza analogues of sulfones and sulfonamides, respectively, they combine good physicochemical properties, high stability, and the ability to build complexity from a three-dimensional core. However, a lack of quick and efficient methods to prepare these compounds has hindered their uptake in molecule discovery programmes. Herein, we describe a unified, one-pot approach to both sulfoximines and sulfonimidamides, which exploits the high electrophilicity of sulfinyl nitrenes. We generate these rare reactive intermediates from a novel sulfinylhydroxylamine (R–O–N=S=O) reagent through an N–O bond fragmentation process. Combining sulfinyl nitrenes with carbon and nitrogen nucleophiles enables the synthesis of sulfoximines and sulfonimidamides in a reaction time of just 15 min. Alkyl, (hetero)aryl, and alkenyl organometallic reagents can all be used as the first or second component in the reaction, while primary and secondary amines, and anilines, all react with high efficiency as the second nucleophile. The tolerance of the reaction to steric and electronic factors has allowed for the synthesis of the most diverse set of sulfoximines and sulfonimidamides yet described. Experimental and computational investigations support the intermediacy of sulfinyl nitrenes, with nitrene formation proceeding via a transient triplet intermediate before reaching a planar singlet species.
1. Introduction
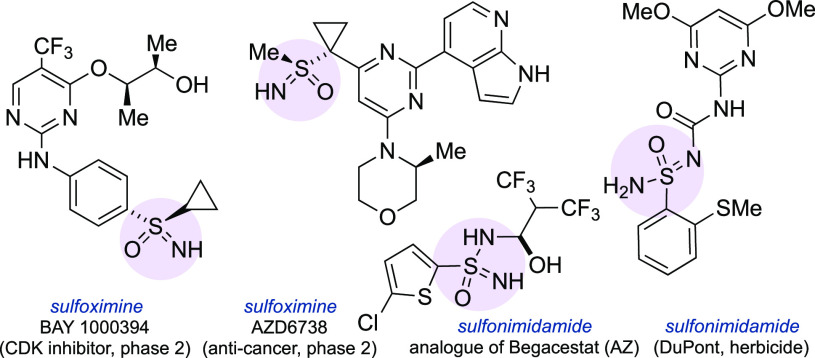
Sulfur(VI) compounds have played an outsized role in the development of medicines. Sulfones and sulfonamides in particular are key components of numerous drugs as well as agrochemicals.1 Replacing one of the oxygen atoms in these molecules with a nitrogen atom gives sulfoximines2 and sulfonimidamides.3 These compounds have recently gained traction in medicinal chemistry, first as isosteric replacements for their oxygenated analogues,4 and more recently due to increasing recognition of their potent mix of physicochemical properties. Being inherently chiral at sulfur, polar, and three-dimensional, with good aqueous solubility and high chemical and metabolic stability,5 sulfoximines and sulfonimidamides represent an invaluable addition to the medicinal chemist’s toolbox (Figure 1).6 However, although sulfonimidamides are increasingly common in pharmaceutical patents,7 and while sulfoximines appear in several clinical candidates8 and have been incorporated into a marketed agrochemical,9 they arguably still lack widespread recognition and are not yet routinely used in drug discovery. A significant reason for this is the inability to prepare these molecules quickly, ideally in one step, from widely available precursors. Considerable advances have been made in recent years in the synthesis of sulfoximines and sulfonimidamides by the Bolm and Bull groups, as well as others, which have simplified the imination of thioethers,10 sulfoxides,11 and sulfenamides.12 Sulfonimidates13 and sulfinamides14 are also established as useful intermediates toward these targets. However, these methods share the fundamental limitation of ultimately needing thiol starting materials, which can be unpleasant to use due to their odor, oxidize to form disulfides in air, and are not widely commercially available. Consequently, the preparation of sulfoximines and sulfonimidamides often requires a multistep synthetic campaign featuring multiple oxidations, and in the case of sulfonimidamides typically involving moisture-sensitive intermediates such as sulfinyl chlorides.5,15,16
Figure 1.
Bioactive aza-S(VI)-derivatives.
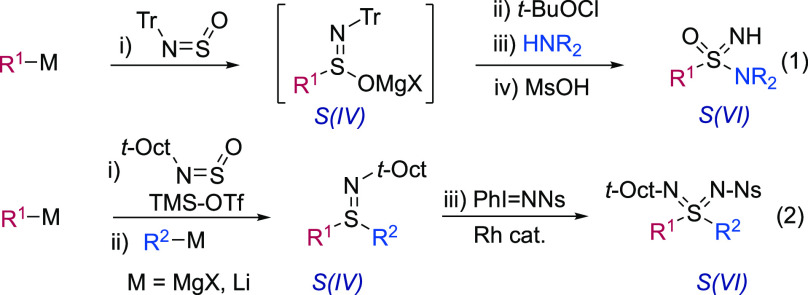
A synthetic approach starting from common nucleophiles and a central sulfur-containing electrophilic reagent would allow for more convenient access to a variety of sulfur(VI) derivatives. One embodiment of such a strategy is the recently reported SuFEx chemistry17 that uses iminosulfur oxidifluorides (RN=S(O)F2) as the electrophiles, in combination with a variety of heteroatom and carbon nucleophiles.18 In these examples, the key iminosulfur oxidifluorides are prepared from SOF4 gas, which is in turn prepared from gaseous SF4. In an alternative approach, we reported a one-pot, multistep, synthesis of sulfonimidamides from the sulfinylamine reagent N-sulfinyltritylamine (TrNSO), organometallic reagents, and amines, albeit with the requirement of an intermediate oxidative step (Scheme 1, eq 1).19,20 The scope of this reaction proved to be broad, and it has now found use in pharmaceutical companies,21 leading to TrNSO being commercially available. More recently we described a second sulfinylamine reagent, this time featuring a tert-alkyl substituent (t-Oct-NSO), and used this reagent to prepare sulfilimines, en route to sulfondiimines.22 As with the TrNSO chemistry, this route to sulfondiimines again required a S(IV) to S(VI) oxidation using an external oxidant (Scheme 1, eq 2). Neither of these sulfinylamine reagents allowed the direct synthesis of sulfoximines from an electrophilic S(VI)-intermediate. This is a common shortcoming of many sulfur(VI) electrophiles including sulfonimidoyl chlorides,15 fluorides,23 and esters,24 which tend to undergo reduction, or show low reactivity, when combined with carbon nucleophiles. In particular, sulfonimidoyl fluorides must be combined with highly basic organolithium reagents, as they provide a mixture of reduction and substitution with less reactive Grignard reagents.25 Meanwhile, sulfonimidate esters generally require an excess (2 or more equivalents) of the organomagnesium reagent to be used.13b
Scheme 1. Sulfinylamine Reagents in the Synthesis of Sulfonimidamides and Sulfondiimines, Both Featuring S(IV) to S(VI) Oxidations.
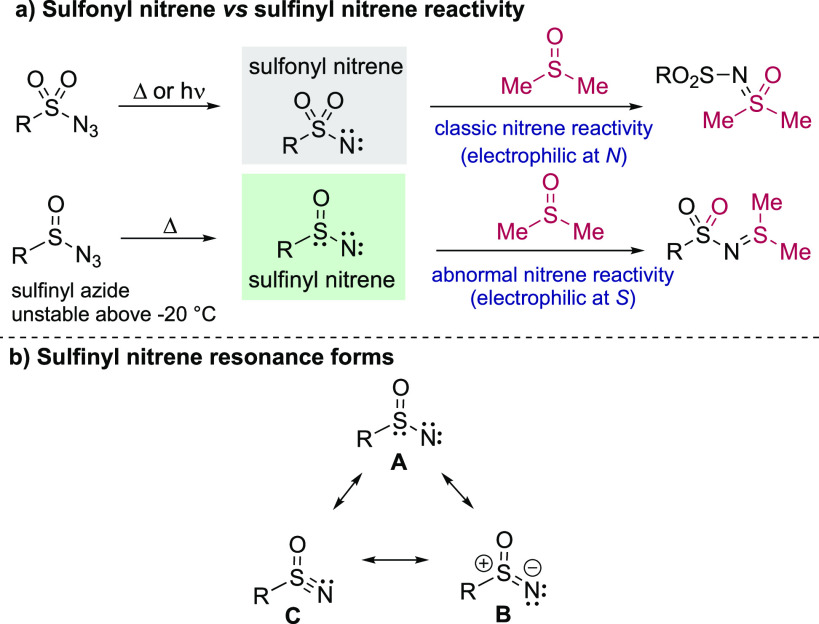
In our search for a general solution to this problem we were intrigued by the anomalous properties of little-known sulfinyl nitrenes. While sulfonyl nitrenes are among the most widely employed in modern nitrene chemistry, it is striking that the related species, sulfinyl nitrenes, which contain just one S=O bond instead of two, are almost completely absent from the literature26 since their initial report over 40 years ago by Maricich.27 Sulfonyl nitrenes undergo classic C–H insertion and aziridination reactions via the nitrogen atom. In contrast, the known reactions of sulfinyl nitrenes proceed by electrophilic attack at sulfur. For example, sulfonyl nitrenes react with sulfoxides to give sulfoximines via attack of the sulfur lone pair of the sulfoxide onto the nitrogen atom of the nitrene. Sulfinyl nitrenes, however, react with sulfoxides by attack of the sulfoxide oxygen at the sulfur atom of this nitrene, and after rearrangement provide N-sulfonyl sulfilimines (Scheme 2a).27b This stark divergence in reactivity can be rationalized by considering the resonance structures of sulfinyl nitrenes (A–B–C, Scheme 2b); the zwitterionic species B, bearing a formal positive charge on sulfur and negative charge on nitrogen, gives a representative picture of sulfinyl nitrene reactivity. Because of these anomalous properties, we speculated that these species had the potential to transform the synthesis of sulfoximines and sulfonimidamides. Nevertheless, sulfinyl nitrenes remain underexplored and have received only minimal attention.28 This is mainly due to their known precursors, sulfinyl azides, being noted to be unstable above −20 °C and exploding upon warming to room temperature, as well as delivering irreproducible results. tert-Butyl sulfinyl azide has been generated and decomposed in situ in the presence of water to provide the corresponding sulfonamide.26 However, attempts to employ amine or thiol nucleophiles with sulfinyl azides resulted only in substitution of the azide group.29 Given these challenges associated with the use of sulfinyl azides, we postulated that if we could identify a safe and convenient method to generate sulfinyl nitrenes, then new routes to a range of S(VI) derivatives should be possible. Herein, we report such a system and show that by exploiting sulfinyl nitrene intermediates we can achieve efficient routes to both sulfoximines and sulfonimidamides.
Scheme 2. (a) Sulfonyl Nitrene versus Sulfinyl Nitrene Reactivity; (b) Sulfinyl Nitrene Resonance Forms.
2. Results and Discussion
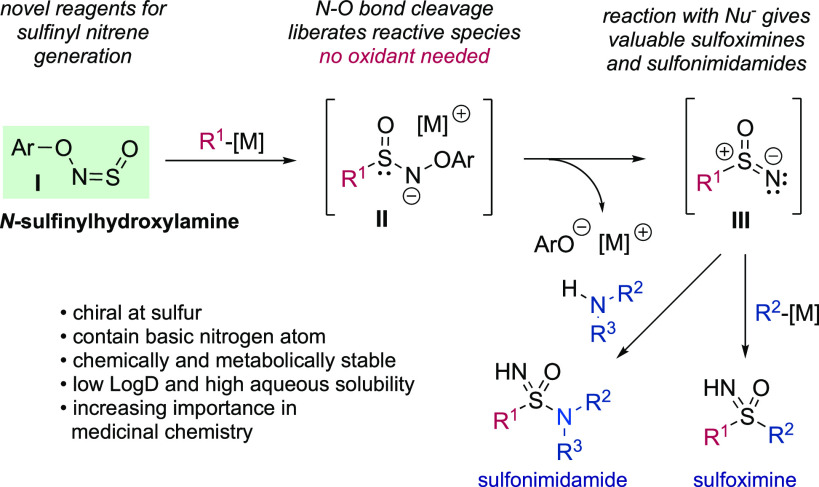
We envisioned that a sulfinylamine reagent bearing an appropriate leaving group on nitrogen would, when reacted with a carbon nucleophile, give convenient access to sulfinyl nitrenes. Our reaction design therefore centered on the use of a novel sulfinylhydroxylamine19,22,30 (I, Scheme 3). Upon combination with a carbon-centered organometallic reagent to form the negatively charged sulfinamide intermediate II, we hypothesized that the loss of a phenoxide anion by cleavage of the weak N–O bond would be favorable to give the neutral sulfinyl nitrene species III. Upon addition of a second nucleophile such as a further organometallic reagent, or an amine, sulfoximines and sulfonimidamides would be obtained. Importantly, no external oxidant would be needed to achieve the S(VI) oxidation state. Although seemingly straightforward, this reactivity has never previously been achieved.27b This is likely due to difficulties associated with the generation of sulfinyl nitrenes. As noted, sulfinyl azides react with amines via direct substitution to give sulfinamides with loss of HN3 before nitrene formation can occur.29 Sulfinyl nitrenes and the related sulfinylnitrenium cation could be formed in the presence of, and reacted with, simple alcohols;27a however, yields were low and the only sulfur-containing products isolated from this reaction were primary sulfonamides.31 Our challenge, therefore, was to develop the first method of generating sulfinyl nitrenes that would allow synthetically useful reactions with common nucleophiles.
Scheme 3. Reaction Design.
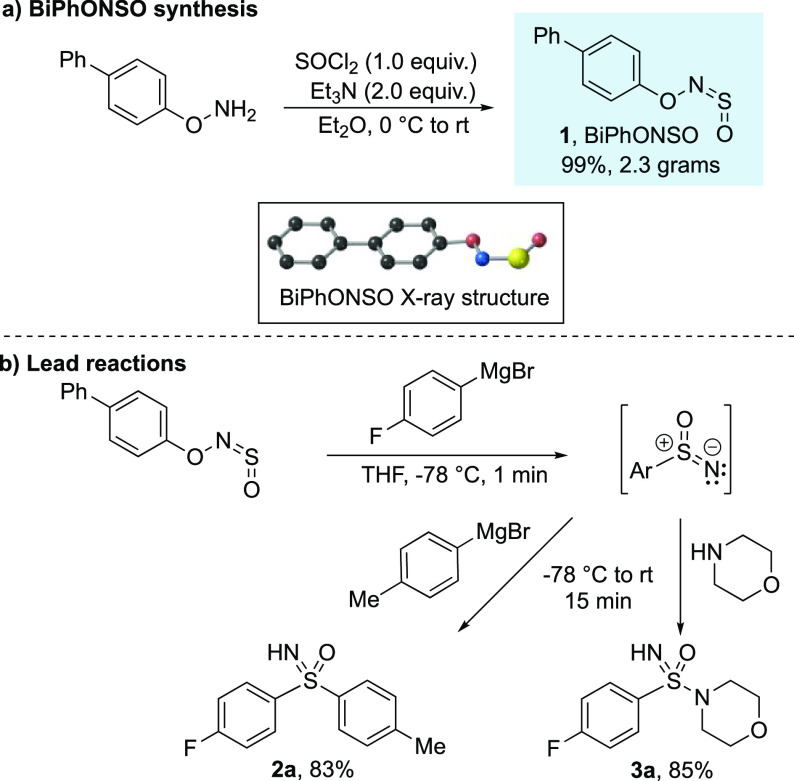
We targeted sulfinylamines30 derived from O-arylhydroxylamines, reasoning that the presence of the aromatic ring would weaken the N–O bond and promote the bond cleavage central to our reaction design. After some experimentation, we settled on sulfinylhydroxylamine 1 (BiPhONSO), a solid reagent that combines good stability with excellent reactivity and can be prepared from biphenylhydroxylamine efficiently on multigram scale (Scheme 4).32 Pleasingly, we found that sequential addition of a Grignard reagent and an amine to BiPhONSO at −78 °C for 1 min, followed by warming to room temperature, delivered sulfonimidamide 3a in 85% yield in accordance with our plan. Crucially, this approach could also be applied to sulfoximines; addition of a second Grignard reagent in place of an amine provided sulfoximine 2a in 83% yield. The novel sulfinylamine reagent, BiPhONSO, therefore enables the synthesis of sulfoximines or sulfonimidamides by simple consecutive addition of widely available organometallic reagents and amines in a reaction time of just 15 min.
Scheme 4. BiPhONSO and Initial Reactions.
With the optimized conditions in hand, we set out to determine the scope of sulfoximines that could be prepared (Table 1a). A broad range of aryl Grignard reagents could be used (2a–j). The impressive tolerance of the reaction to steric hindrance was shown by the synthesis of a rare ortho,ortho′-disubstituted sulfoximine (2h). An organolithium reagent containing an SMe group was also incorporated to obtain a sulfoximine containing mixed-valence sulfur atoms (2i), which could not be prepared using traditional oxidative methods. Likewise, the unprotected basic tertiary amine featured in compound 2j would be susceptible to oxidation. Medicinally relevant basic N-heterocycles such as pyridines and pyrimidines could be incorporated (2k–n). Alkyl nucleophiles were also competent in the reaction (2o–w). tert-Butylmagnesium chloride could even be used as the initial nucleophile to afford the highly sterically hindered S-tert-butyl sulfoximine 2v in 53% yield; such products have previously been prepared by three sequential deprotonations/methylations of an S-methyl sulfoximine.33 Oxidatively sensitive allyl and vinyl groups were compatible (2w,y). S,S-Dialkyl sulfoximines containing methyl, cyclopropyl, and cyclohexyl groups could also be prepared (2z–ab).
Table 1. (a) Scope of Sulfoximine Synthesis; (b) Four-Component Sulfoximine Synthesis.
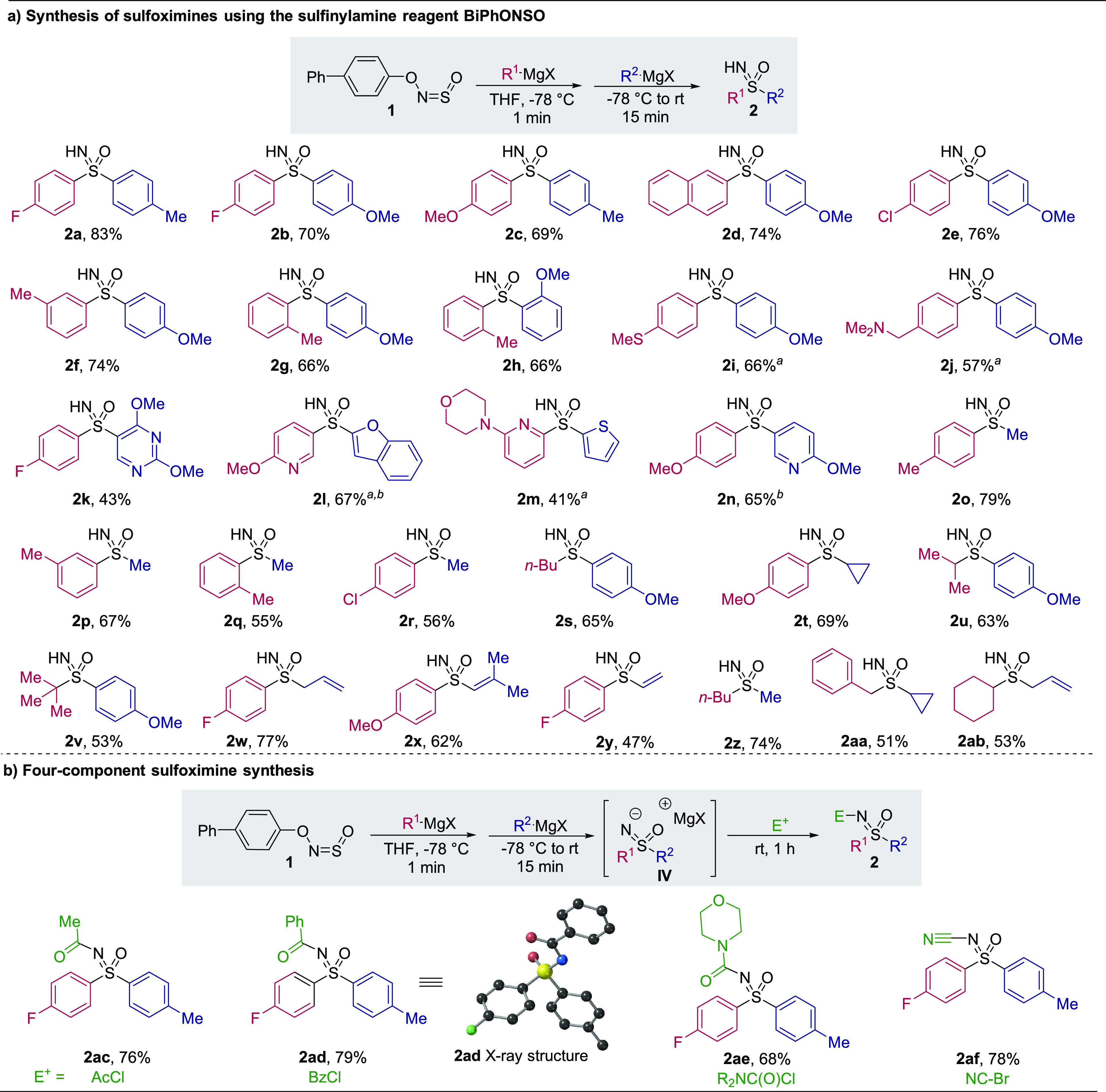
Organolithium reagent used as 1st organometallic reagent.
Organolithium reagent used as 2nd organometallic reagent.
The initial product in these syntheses is a sulfoximine anion, and we were able to exploit this by introducing an electrophilic trap as a fourth reaction component (Table 1b). For example, addition of an acid chloride delivered acetyl- and benzoyl-substituted products in high yields (2ac,ad). Urea- and cyano-containing sulfoximines could also be prepared using a carbamoyl chloride and cyanogen bromide as the electrophile, respectively (2ae,af). An N-cyanosulfoximine is incorporated into the marketed insecticide Sulfoxaflor.9
We next explored the scope of sulfonimidamides that could be prepared, keeping 4-fluorophenylmagnesium bromide constant as the organometallic component and varying the amine nucleophile (Table 2). The parent 5-, 6-, and 7-membered cyclic amines all reacted in high yields (3b–d). Cyclic amines bearing an electrophilic ester, acid-sensitive ketal, and oxidatively sensitive thioether functional groups were tolerated (3e–g). Bulky noncyclic secondary amines such as dibenzylamine and N-cyclohexylmethylamine worked well (3i,j), although a steric limit was reached with diisopropylamine (3k). Primary amines and anilines proved to be competent nucleophiles (3l–p). Functionalizations of the antidepressant Amoxapine and amines derived from the antihistamine Loratadine and the antiplatelet medication Clopidogrel were possible (3q–s). Amines containing acidic protons, such as alcohols and carbamates, could be used provided that para-toluenesulfonic acid was added prior to the amine (3t,u). We then varied the organometallic component, using morpholine or 1-Cbz-piperazine as the amine. Sterically and electronically varied aryl organometallic reagents afforded products in high yields (3v–aa). Importantly for medicinal chemists, a range of heteroaryl organometallic reagents performed well, allowing the synthesis of 2- and 3-pyridyl and pyrimidyl sulfonimidamides (3ab–ae). Five-membered heterocycles such as thiophene and benzofuran were incorporated in high yields (3af,ag), as was highly base-sensitive 4-isoxazole31 (3ah), demonstrating the mildness of the reaction conditions. As with the sulfoximines, alkyl Grignard reagents gave good yields of products (3ai–am). The first ever syntheses of S-vinyl and S-allyl sulfonimidamides were also accomplished (3an,ap). The reliance of previous methods on strongly oxidative conditions and sensitive sulfinyl chloride intermediates may explain why alkenyl substituents were not well-tolerated in the earlier week.
Table 2. Scope of Sulfonimidamide Synthesis Exploiting the BiPhONSO Reagent.
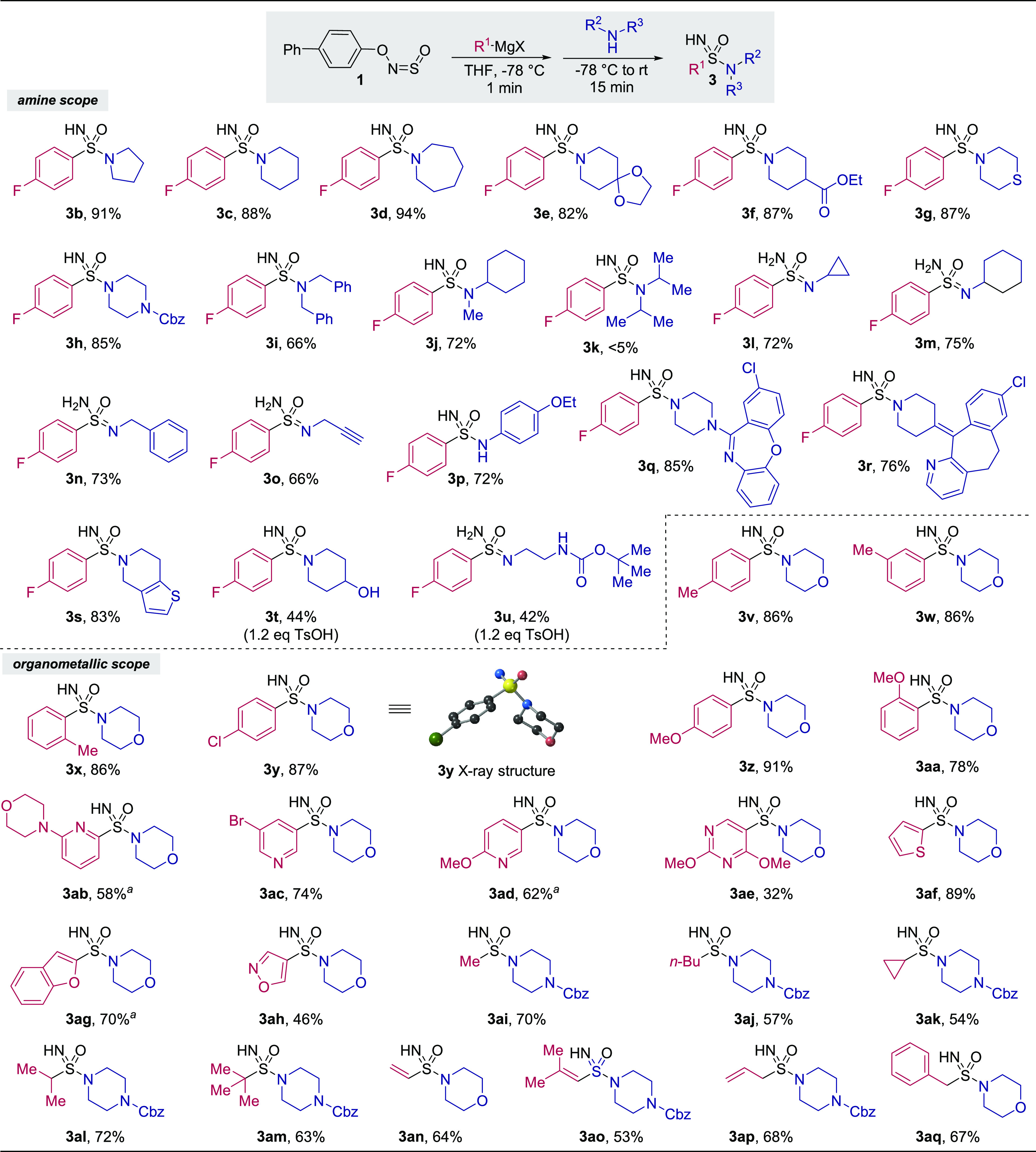
Organolithium reagent used in place of Grignard reagent.
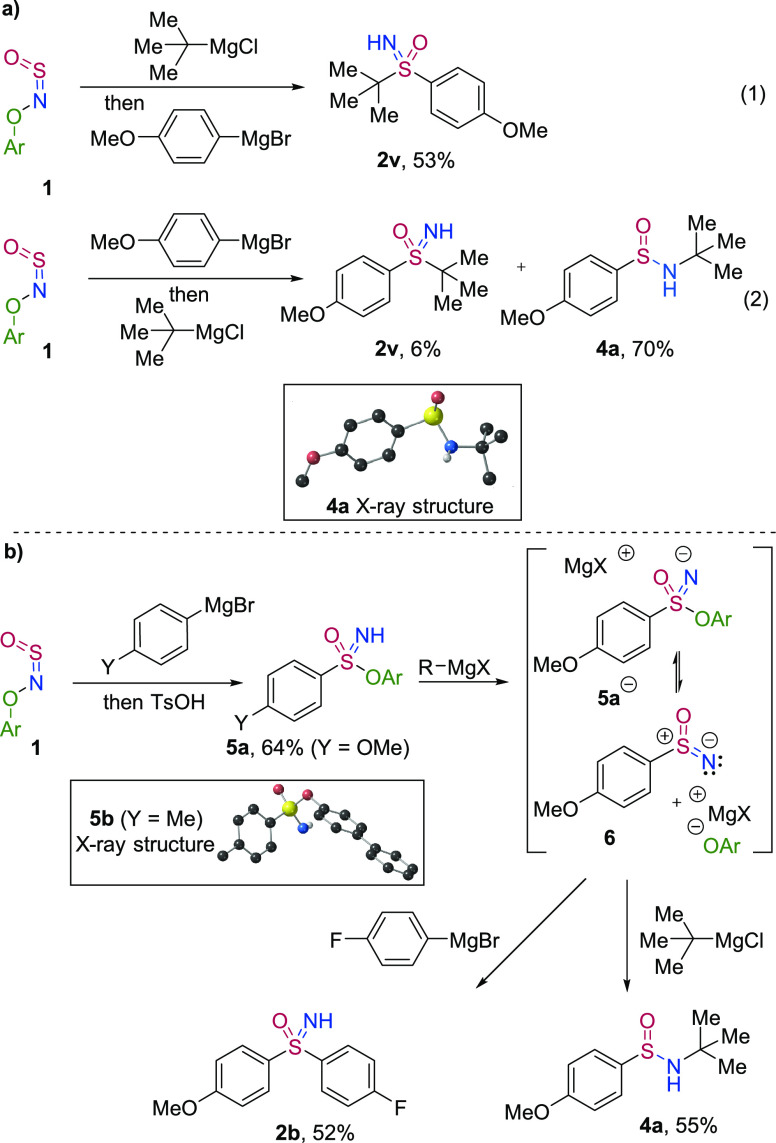
Intriguingly, the formation of S-tert-butylsulfoximine 2v was dependent on the order of addition of the Grignard reagents. When tert-butylmagnesium chloride was added first and 4-methoxyphenylmagnesium bromide second, sulfoximine 2v was isolated in 53% yield (Scheme 5a, eq 1). However, when the order was reversed, N-tert-butylsulfinamide 4a was the major product in 70% yield and the sulfoximine was present in just 6% yield (eq 2). These results provide strong evidence of a nitrene intermediate; while it is highly improbable that a sulfonimidate ester anion would react with a Grignard reagent on nitrogen, nitrenes are renowned for their electrophilicity at nitrogen. In this case, if the incoming C-nucleophile is sufficiently hindered, attack at the less accessible sulfinyl nitrene sulfur atom could become unfavorable, and formation of the sulfinamide by attack on nitrogen would dominate. We were able to isolate sulfonimidate ester 5a by addition of para-toluenesulfonic acid at −78 °C following addition of the initial Grignard reagent (Scheme 5b). Reacting sulfonimidate ester 5a with 2 equiv of tert-butylmagnesium chloride resulted also in the formation of sulfinamide 4a. Reaction of 5a with two equivalents of the aryl Grignard reagent provided sulfoximine 2b in 52% yield. Taken together, this suggests that deprotonation of the ester 5a leads to nitrene (6) formation by expulsion of the phenoxide anion. Considering this, it is likely that the anionic sulfonimidate ester 5a– is formed quickly by recombination of the nitrene (6) and the phenoxide leaving group following N–O bond fission, and can act as a source of the nitrene. The related sulfonimidate ester 5b could also be isolated and yielded X-ray quality crystals; the resultant structure is shown. Experiments using both TEMPO and 1,1-diphenylethylene as radical scavengers in reactions to prepare sulfoximines 2a and 2v, sulfonimidamide 3a, and sulfinamide 4a, all showed only minimal effect of the additives, suggesting that radical intermediates are likely not involved (see Supporting Information for details).
Scheme 5. Mechanistic Observations.
To gain more insight into the mechanism of this reaction, a computational investigation, employing density functional theory (DFT) methods, was undertaken.28 The addition of the Grignard reagent CH3MgCl to 1 was investigated, using PhONSO as a model system for sulfinylhydroxylamine 1. Figure 2a shows a pathway using a dimeric Grignard species; however, the possibility of an alternative monomeric pathway was also investigated and provided comparable results (see Supporting Information).34 The reaction is initiated by formation of the Grignard-substrate complex, D1, which undergoes an irreversible methyl addition through D-TS2–3 with a barrier of 10.5 kcal mol–1 forming intermediate D3. Subsequently, the anionic sulfinamide component in D3 undergoes intersystem crossing (ISC), from its singlet to a triplet state, forming D4. The barrier of ISC was estimated by locating the minimum energy crossing point (MECP) between the singlet and triplet potential energy surface obtaining D-MECP1. D-MECP1 was found to be 19.0 kcal mol–1 higher in energy relative to D3, having an elongated N–O bond (2.20 Å vs 1.52 Å in D3) while retaining a pyramidal geometry at sulfur. Rotation of the nitrene moiety in D4 to position the nitrogen away from the phenoxide leads to D4′, which then interconverts to the more stable singlet sulfinyl nitrene D5 via D-MECP2 (see Section 2.6 in the Supporting Information). In D5, reorientation of the phenoxide ligand to a bridging position leads to intermediate D5′, a sulfinyl nitrene with a near planar sulfur geometry and a shortened S–N bond of 1.48 Å compared to 1.61 Å in D3. Overall, these steps indicate that the generation of the sulfinyl nitrene intermediate occurs via a stepwise procedure, with the first ISC involving N–O bond fragmentation to a transient triplet intermediate, followed by the second ISC involving the pyramidal sulfinyl nitrene converting to a planar species.
Figure 2.
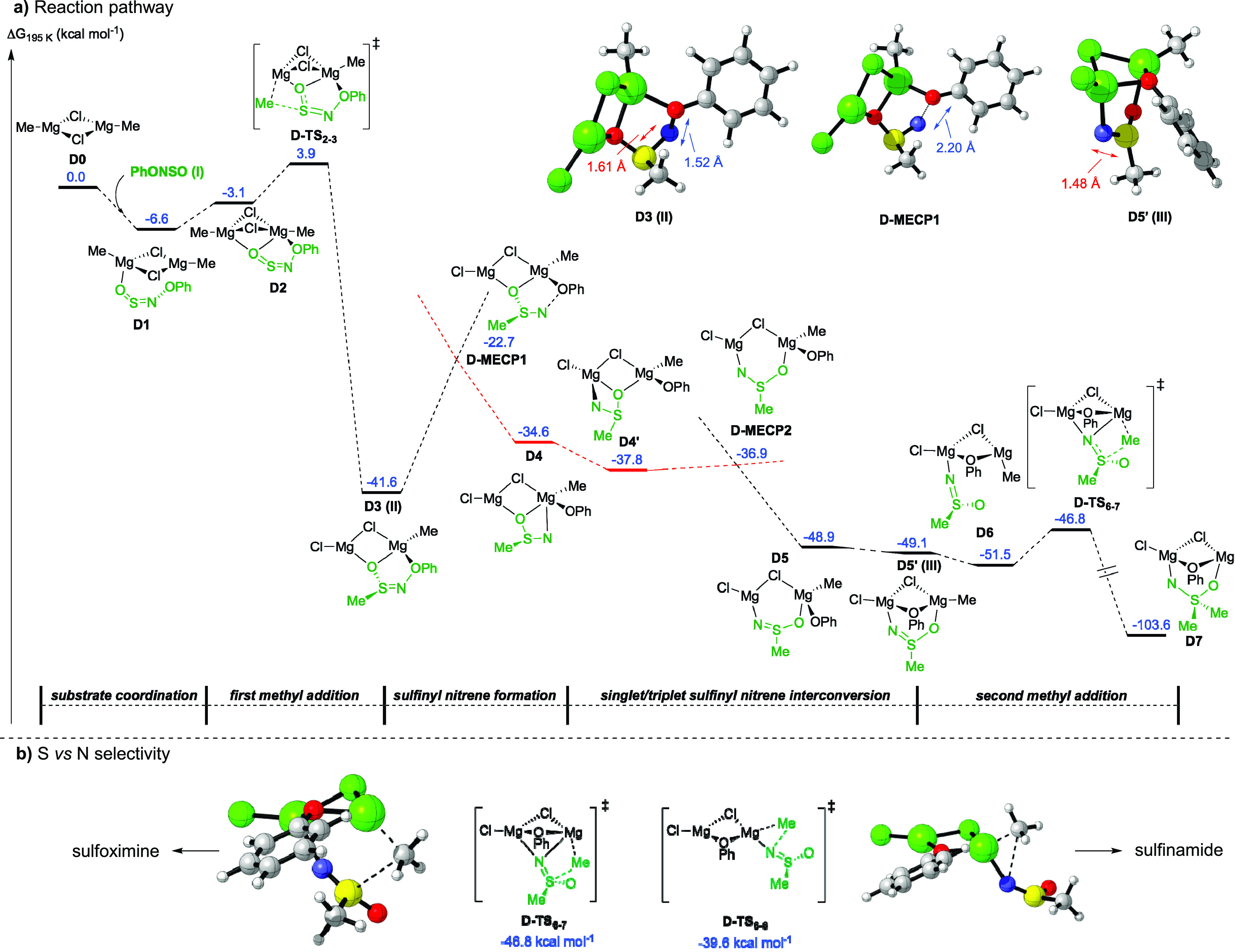
Computational study: (a) Reaction pathway; (b) S vs N selectivity. Energies at the SMD(THF)-ωB97X-D/6-311++g(d,p)//SMD(THF)-B3LYP-BJD3/6-31g(d) level of theory.
The structural characteristics of the sulfinyl nitrene component in D5′ suggest a high degree of electrophilicity at the sulfur center. Indeed, addition of a second methyl group to form sulfoximine D7 takes place via a low activation barrier (D-TS6-7 = 4.7 kcal mol−1). Furthermore, the mechanistic experiments indicate the possibility of sulfinamide formation from the nitrene, and hence, this selectivity was also examined (Figure 2b). Comparison of the energies of D-TS6–7 and D-TS6–8 shows the transition state for methyl addition to sulfur (D-TS6–7) to be 7.2 kcal mol–1 more favorable than addition to nitrogen (D-TS6–8), which qualitatively provides the same selectivity as observed experimentally for the majority of examples. Therefore, these results provide a theoretical basis for the reactivity of the sulfinyl nitrene, namely its ability to readily accept a nucleophile at the sulfur center. It is worth noting that in the case of a phenoxide nucleophile, a sulfonimidate ester anion, analogous to 5a–, would be generated reversibly (see Supporting Information, Figure S7). This supports our earlier assertion that this intermediate may act as a nitrene reservoir. Overall, this analysis suggests that formation of sulfoximines (2, D7 in Figure 2a) from sulfinylhydroxylamine (1) is energetically favorable, involving the generation of a key singlet sulfinyl nitrene intermediate with an electrophilic sulfur center.
3. Conclusion
These results show that sulfinyl nitrenes are available from novel sulfinylhydroxylamine reagents and demonstrate for the first time that they can be combined with carbon- and nitrogen-nucleophiles to provide a diverse range of sulfoximines and sulfonimidamides. We anticipate that this chemistry will transform the preparation of these compound classes, providing a new technology for medicinal and agrochemists to incorporate polar, three-dimensional scaffolds into bioactive molecules. More fundamentally, the ability to conveniently and selectively generate a hitherto virtually unexplored reactive intermediate will catalyze the development of unforeseen chemistry and molecules.
Acknowledgments
This work was supported by the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) and the EPSRC (EP/S03658X/1). Antoine de Gombert and Richard Cooper (both University of Oxford) are thanked for X-ray structure analysis, and the authors acknowledge use of the University of Oxford Advanced Research Computing (ARC) facility in carrying out this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c06986.
Experimental procedures and supporting characterization data and spectra; computational procedures, including Cartesian coordinates (PDF)
Crystallographic data for compound 4a (CIF)
Crystallographic data for compound 2ad (CIF)
Crystallographic data for compound BiPhONSO 1 (CIF)
Crystallographic data for compound 3y (CIF)
Crystallographic data for compound 5b (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Scott K. A.; Njardarson J. T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top Curr. Chem. (Cham) 2018, 376, 5. 10.1007/s41061-018-0184-5. [DOI] [PubMed] [Google Scholar]; b Feng M.; Tang B.; Liang S. H.; Jiang X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggelin M.; Zur C. Sulfoximines: Structures, Properties and Synthetic Applications. Synthesis 2000, 2000, 1–64. 10.1055/s-2000-6217. [DOI] [Google Scholar]
- Nandi G. C.; Arvidsson P. I. Sulfonimidamides: Synthesis and Applications in Preparative Organic Chemistry. Adv. Synth. Catal. 2018, 360, 2976–3001. 10.1002/adsc.201800273. [DOI] [Google Scholar]
- Sehgelmeble F.; Janson J.; Ray C.; Rosqvist S.; Gustavsson S.; Nilsson L. I.; Minidis A.; Holenz J.; Rotticci D.; Lundkvist J.; Arvidsson P. I. Sulfonimidamides as Sulfonamides Bioisosteres: Rational Evaluation through Synthetic, in Vitro, and in Vivo Studies with γ-Secretase Inhibitors. ChemMedChem 2012, 7, 396–399. 10.1002/cmdc.201200014. [DOI] [PubMed] [Google Scholar]
- Frings M.; Bolm C.; Blum A.; Gnamm C. Sulfoximines from a Medicinal Chemist’s Perspective: Physicochemical and in vitro Parameters Relevant for Drug Discovery. Eur. J. Med. Chem. 2017, 126, 225–245. 10.1016/j.ejmech.2016.09.091. [DOI] [PubMed] [Google Scholar]
- a Lücking U. Sulfoximines: A Neglected Opportunity in Medicinal Chemistry. Angew. Chem., Int. Ed. 2013, 52, 9399–9408. 10.1002/anie.201302209. [DOI] [PubMed] [Google Scholar]; b Chinthakindi P. K.; Naicker T.; Thota N.; Govender T.; Kruger H. G.; Arvidsson P. I. Sulfonimidamides in Medicinal and Agricultural Chemistry. Angew. Chem., Int. Ed. 2017, 56, 4100–4109. 10.1002/anie.201610456. [DOI] [PubMed] [Google Scholar]
- a Miller D.; Thom S.; St-Galley S.; Shannon J.; Leeson P.. Novel Compounds. Patent WO2019/068772A1, 2019.; b Biftu T.; Khan T. A.. Treating Diabetes with Dipeptidyl Peptidase-IV Inhibitors. Patent WO2014018355A1, 2014.
- a Lücking U.; Scholz A.; Lienau P.; Siemeister G.; Kosemund D.; Bohlmann R.; Briem H.; Terebesi I.; Meyer K.; Prelle K.; Denner K.; Bömer U.; Schäfer M.; Eis K.; Valencia R.; Ince S.; Nussbaum F. v.; Mumberg D.; Ziegelbauer K.; Klebl B.; Choidas A.; Nussbaumer P.; Baumann M.; Schultz-Fademrecht C.; Rühter G.; Eickhoff J.; Brands M. Identification of Atuveciclib (BAY 1143572), the First Highly Selective, Clinical PTEFb/CDK9 Inhibitor for the Treatment of Cancer. ChemMedChem 2017, 12, 1776–1793. 10.1002/cmdc.201700447. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Foote K. M.; Nissink J. W. M.; McGuire T.; Turner P.; Guichard S.; Yates J. W. T.; Lau A.; Blades K.; Heathcote D.; Odedra R.; Wilkinson G.; Wilson Z.; Wood C. M.; Jewsbury P. J. Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J. Med. Chem. 2018, 61, 9889–9907. 10.1021/acs.jmedchem.8b01187. [DOI] [PubMed] [Google Scholar]; c Vendetti F. P.; Lau A.; Schamus S.; Conrads T. P.; O’Connor M. J.; Bakkenist C. J. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget 2015, 6, 44289–44305. 10.18632/oncotarget.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Nishimura N.; Norman M. H.; Liu L.; Yang K. C.; Ashton K. S.; Bartberger M. D.; Chmait S.; Chen J.; Cupples R.; Fotsch C.; Helmering J.; Jordan S. R.; Kunz R. K.; Pennington L. D.; Poon S. F.; Siegmund A.; Sivits G.; Lloyd D. J.; Hale C.; St Jean D. J. Jr. Small molecule disruptors of the glucokinase-glucokinase regulatory protein interaction: 3. Structure-activity relationships within the aryl carbinol region of the N-arylsulfonamido-N’-arylpiperazine series. J. Med. Chem. 2014, 57, 3094–3116. 10.1021/jm5000497. [DOI] [PubMed] [Google Scholar]; e Lücking U.; Jautelat R.; Krüger M.; Brumby T.; Lienau P.; Schäfer M.; Briem H.; Schulze J.; Hillisch A.; Reichel A.; Wengner A. M.; Siemeister G. The lab oddity prevails: Discovery of Pan-CDK inhibitor (R)-S-Cyclopropyl-S-(4-{[4-{[(1R,2R)-2-hydroxy-1-methylpropyl]oxy}-5-(trifluoromethyl)pyrimidin-2-yl]amino}phenyl)sulfoximide (BAY1000394) for the treatment of cancer. ChemMedChem 2013, 8, 1067–1085. 10.1002/cmdc.201300096. [DOI] [PubMed] [Google Scholar]
- Zhu Y. M.; Loso M. R.; Watson G. B.; Sparks T. C.; Rogers R. B.; Huang J. X.; Gerwick B. C.; Babcock J. M.; Kelley D.; Hegde V. B.; Nugent B. M.; Renga J. M.; Denholm I.; Gorman K.; DeBoer G. J.; Hasler J.; Meade T.; Thomas J. D. Discovery and Characterization of Sulfoxaflor, a Novel Insecticide Targeting Sap-Feeding Pests. J. Agric. Food Chem. 2011, 59, 2950–2957. 10.1021/jf102765x. [DOI] [PubMed] [Google Scholar]
- a Verbelen B.; Siemes E.; Ehnbom A.; Rauber C.; Rissanen K.; Wöll D.; Bolm C. From One-Pot NH-Sulfoximidations of Thiophene Derivatives to Dithienylethene-Type Photoswitches. Org. Lett. 2019, 21, 4293–4297. 10.1021/acs.orglett.9b01475. [DOI] [PubMed] [Google Scholar]; b Chaabouni S.; Lohier J. F.; Barthelemy A. L.; Glachet T.; Anselmi E.; Dagousset G.; Diter P.; Pégot B.; Magnier E.; Reboul V. One-Pot Synthesis of Aryl- and Alkyl S-Perfluoroalkylated NH-Sulfoximines from Sulfides. Chem. - Eur. J. 2018, 24, 17006–17010. 10.1002/chem.201805055. [DOI] [PubMed] [Google Scholar]; c Tota A.; Zenzola M.; Chawner S. J.; St John-Campbell S.; Carlucci C.; Romanazzi G.; Degennaro L.; Bull J. A.; Luisi R. Synthesis of NH-sulfoximines from sulfides by chemoselective one-pot N- and O-transfers. Chem. Commun. 2017, 53, 348–351. 10.1039/C6CC08891K. [DOI] [PubMed] [Google Scholar]; d Lohier J.-F.; Glachet T.; Marzag H.; Gaumont A.-C.; Reboul V. Mechanistic investigation of the NH-sulfoximination of sulfide. Evidence for λ6-sulfanenitrile intermediates. Chem. Commun. 2017, 53, 2064–2067. 10.1039/C6CC09940H. [DOI] [PubMed] [Google Scholar]; e Bizet V.; Hendriks C. M. M.; Bolm C. Sulfur imidations: access to sulfimides and sulfoximines. Chem. Soc. Rev. 2015, 44, 3378–3390. 10.1039/C5CS00208G. [DOI] [PubMed] [Google Scholar]
- a Yu H.; Li Z.; Bolm C. Iron(II)-Catalyzed Direct Synthesis of NH Sulfoximines from Sulfoxides. Angew. Chem., Int. Ed. 2018, 57, 324–327. 10.1002/anie.201710498. [DOI] [PubMed] [Google Scholar]; b Zenzola M.; Doran R.; Degennaro L.; Luisi R.; Bull J. A. Transfer of Electrophilic NH Using Convenient Sources of Ammonia: Direct Synthesis of NH Sulfoximines from Sulfoxides. Angew. Chem., Int. Ed. 2016, 55, 7203–7207. 10.1002/anie.201602320. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Okamura H.; Bolm C. Rhodium-Catalyzed Imination of Sulfoxides and Sulfides: Efficient Preparation of N-Unsubstituted Sulfoximines and Sulfilimines. Org. Lett. 2004, 6, 1305–1307. 10.1021/ol049715n. [DOI] [PubMed] [Google Scholar]
- Briggs E. L.; Tota A.; Colella M.; Degennaro L.; Luisi R.; Bull J. A. Synthesis of Sulfonimidamides from Sulfenamides via an Alkoxy-amino-λ6-sulfanenitrile Intermediate. Angew. Chem., Int. Ed. 2019, 58, 14303–14310. 10.1002/anie.201906001. [DOI] [PubMed] [Google Scholar]
- a Mendonça Matos P.; Lewis W.; Argent S. P.; Moore J. C.; Stockman R. A. General Method for the Asymmetric Synthesis of N-H Sulfoximines via C-S Bond Formation. Org. Lett. 2020, 22, 2776–2780. 10.1021/acs.orglett.0c00761. [DOI] [PubMed] [Google Scholar]; b Mendonça Matos P.; Lewis W.; Moore J. C.; Stockman R. A. Sulfonimidates: Useful Synthetic Intermediates for Sulfoximine Synthesis via C-S Bond Formation. Org. Lett. 2018, 20, 3674–3677. 10.1021/acs.orglett.8b01473. [DOI] [PubMed] [Google Scholar]; c Wright M.; Martínez-Lamenca C.; Leenaerts J. E.; Brennan P. E.; Trabanco A. A.; Oehlrich D. Bench-Stable Transfer Reagent Facilitates the Generation of Trifluoromethyl-sulfonimidamides. J. Org. Chem. 2018, 83, 9510–9516. 10.1021/acs.joc.8b01244. [DOI] [PubMed] [Google Scholar]
- a Greed S.; Briggs E. L.; Idiris F. I. M.; White A. J. P.; Lücking U.; Bull J. A. Synthesis of Highly Enantioenriched Sulfonimidoyl Fluorides and Sulfonimidamides by Stereospecific SuFEx Reaction. Chem. - Eur. J. 2020, 10.1002/chem.202002265. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cividino P.; Verrier C.; Philouze C.; Carret S.; Poisson J. F. Accessing Enantiopure Endocyclic Sulfoximines Through Catalytic Cycloisomerization of Oxygenated Propargyl-Sulfinamides. Adv. Synth. Catal. 2019, 361, 1236–1240. 10.1002/adsc.201801408. [DOI] [Google Scholar]; c Aota Y.; Kano T.; Maruoka K. Asymmetric Synthesis of Chiral Sulfoximines through the S-Alkylation of Sulfinamides. Angew. Chem., Int. Ed. 2019, 58, 17661–17665. 10.1002/anie.201911021. [DOI] [PubMed] [Google Scholar]; d Aota Y.; Maeda Y.; Kano T.; Maruoka K. Efficient Synthesis of Cyclic Sulfoximines from N-Propargylsulfinamides through Sulfur-Carbon Bond Formation. Chem. - Eur. J. 2019, 25, 15755–15758. 10.1002/chem.201904501. [DOI] [PubMed] [Google Scholar]; e Aota Y.; Kano T.; Maruoka K. Asymmetric Synthesis of Chiral Sulfoximines via the S-Arylation of Sulfinamides. J. Am. Chem. Soc. 2019, 141, 19263–19268. 10.1021/jacs.9b11298. [DOI] [PubMed] [Google Scholar]; f Richards-Taylor C. S.; Martínez-Lamenca C.; Leenaerts J. E.; Trabanco A. A.; Oehlrich D. The Synthesis of Trifluoromethyl-sulfonimidamides from Sulfinamides. J. Org. Chem. 2017, 82, 9898–9904. 10.1021/acs.joc.7b01628. [DOI] [PubMed] [Google Scholar]; g Izzo F.; Schäfer M.; Stockman R.; Lücking U. A New, Practical One-Pot Synthesis of Unprotected Sulfonimidamides by Transfer of Electrophilic NH to Sulfinamides. Chem. - Eur. J. 2017, 23, 15189–15193. 10.1002/chem.201703272. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Moragas T.; Liffey R. M.; Regentová D.; Ward J. P.; Dutton J.; Lewis W.; Churcher I.; Walton L.; Souto J. A.; Stockman R. A. Sigmatropic Rearrangement of Vinyl Aziridines: Expedient Synthesis of Cyclic Sulfoximines from Chiral Sulfinimines. Angew. Chem., Int. Ed. 2016, 55, 10047–10051. 10.1002/anie.201604188. [DOI] [PubMed] [Google Scholar]; i Ye W.; Zhang L.; Ni C.; Rong J.; Hu J. Stereoselective [3 + 2] cycloaddition of N-tert-butanesulfinyl imines to arynes facilitated by a removable PhSO2CF2 group: synthesis and transformation of cyclic sulfoximines. Chem. Commun. 2014, 50, 10596–10599. 10.1039/C4CC05042H. [DOI] [PubMed] [Google Scholar]
- Johnson C. R.; Jonsson E. U.; Bacon C. C. Preparation and reactions of sulfonimidoyl chlorides. J. Org. Chem. 1979, 44, 2055–2061. 10.1021/jo01327a001. [DOI] [Google Scholar]
- For alternative approaches, see:; a Izzo F.; Schäfer M.; Lienau P.; Ganzer U.; Stockman R.; Lücking U. Exploration of Novel Chemical Space: Synthesis and in vitro Evaluation of N-Functionalized Tertiary Sulfonimidamides. Chem. - Eur. J. 2018, 24, 9295–9304. 10.1002/chem.201801557. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dong S.; Frings M.; Cheng H.; Wen J.; Zhang D.; Raabe G.; Bolm C. Organocatalytic Kinetic Resolution of Sulfoximines. J. Am. Chem. Soc. 2016, 138, 2166–2169. 10.1021/jacs.6b00143. [DOI] [PubMed] [Google Scholar]; c Chen Y.; Gibson J. A convenient synthetic route to sulfonimidamides from sulfonamides. RSC Adv. 2015, 5, 4171–4174. 10.1039/C4RA14056G. [DOI] [Google Scholar]; d Goldberg F. W.; Kettle J. G.; Xiong J.; Lin D. General synthetic strategies towards N-alkyl sulfoximine building blocks for medicinal chemistry and the use of dimethylsulfoximine as a versatile precursor. Tetrahedron 2014, 70, 6613–6622. 10.1016/j.tet.2014.06.120. [DOI] [Google Scholar]; e Funes Maldonado M.; Sehgelmeble F.; Bjarnemark F.; Svensson M.; Åhman J.; Arvidsson P. I. Synthesis and arylation of unprotected sulfonimidamides. Tetrahedron 2012, 68, 7456–7462. 10.1016/j.tet.2012.06.072. [DOI] [Google Scholar]
- a Mortenson D. E.; Brighty G. J.; Plate L.; Bare G.; Chen W.; Li S.; Wang H.; Cravatt B. F.; Forli S.; Powers E. T.; Sharpless K. B.; Wilson I. A.; Kelly J. W. ″Inverse Drug Discovery″ Strategy To Identify Proteins That Are Targeted by Latent Electrophiles As Exemplified by Aryl Fluorosulfates. J. Am. Chem. Soc. 2018, 140, 200–210. 10.1021/jacs.7b08366. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dong J.; Krasnova L.; Finn M. G.; Sharpless K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem., Int. Ed. 2014, 53, 9430–9448. 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]
- a Kitamura S.; Zheng Q.; Woehl J. L.; Solania A.; Chen E.; Dillon N.; Hull M. V.; Kotaniguchi M.; Cappiello J. R.; Kitamura S.; Nizet V.; Sharpless K. B.; Wolan D. W. Sulfur(VI) Fluoride Exchange (SuFEx)-Enabled High-Throughput Medicinal Chemistry. J. Am. Chem. Soc. 2020, 142, 10899–10904. 10.1021/jacs.9b13652. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Smedley C. J.; Zheng Q.; Gao B.; Li S.; Molino A.; Duivenvoorden H. M.; Parker B. S.; Wilson D. J. D.; Sharpless K. B.; Moses J. E. Bifluoride Ion Mediated SuFEx Trifluoromethylation of Sulfonyl Fluorides and Iminosulfur Oxydifluorides. Angew. Chem., Int. Ed. 2019, 58, 4552–4556. 10.1002/anie.201813761. [DOI] [PubMed] [Google Scholar]; c Liu F.; Wang H.; Li S.; Bare G. A. L.; Chen X.; Wang C.; Moses J. E.; Wu P.; Sharpless K. B. Biocompatible SuFEx Click Chemistry: Thionyl Tetrafluoride (SOF4)-Derived Connective Hubs for Bioconjugation to DNA and Proteins. Angew. Chem., Int. Ed. 2019, 58, 8029–8033. 10.1002/anie.201902489. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Gao B.; Li S.; Wu P.; Moses J. E.; Sharpless K. B. SuFEx Chemistry of Thionyl Tetrafluoride (SOF4) with Organolithium Nucleophiles: Synthesis of Sulfonimidoyl Fluorides, Sulfoximines, Sulfonimidamides, and Sulfonimidates. Angew. Chem., Int. Ed. 2018, 57, 1939–1943. 10.1002/anie.201712145. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li S.; Wu P.; Moses J. E.; Sharpless K. B. Multidimensional SuFEx Click Chemistry: Sequential Sulfur(VI) Fluoride Exchange Connections of Diverse Modules Launched From An SOF4 Hub. Angew. Chem., Int. Ed. 2017, 56, 2903–2908. 10.1002/anie.201611048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. Q.; Hall A.; Willis M. C. One-Pot, Three-Component Sulfonimidamide Synthesis Exploiting the Sulfinylamine Reagent N-Sulfinyltritylamine, TrNSO. Angew. Chem., Int. Ed. 2017, 56, 14937–14941. 10.1002/anie.201708590. [DOI] [PubMed] [Google Scholar]
- For the use of TrNSO in a radical approach to sulfonimidamides, see:; Bremerich M.; Conrads C. M.; Langletz T.; Bolm C. Additions to N-Sulfinylamines as an Approach for the Metal-free Synthesis of Sulfonimidamides: O-Benzotriazolyl Sulfonimidates as Activated Intermediates. Angew. Chem., Int. Ed. 2019, 58, 19014–19020. 10.1002/anie.201911075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride C.; Trzoss L. L.; Boloor A.; Sokolo-VA N.; Pastor R. M.; Staben S. T.; Stivala C.; Volgraf M.; Bronner S. M.. Sulfonimidamide compounds as inhibitors of interleukin-1 activity. Patent WO2020018975A1, 2020.
- Zhang Z. X.; Davies T. Q.; Willis M. C. Modular Sulfondiimine Synthesis Using a Stable Sulfinylamine Reagent. J. Am. Chem. Soc. 2019, 141, 13022–13027. 10.1021/jacs.9b06831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. R.; Bis K. G.; Cantillo J. H.; Meanwell N. A.; Reinhard M. F. D.; Zeller J. R.; Vonk G. P. Preparation and Reactions of Sulfonimodyl Fluorides. J. Org. Chem. 1983, 48, 1–3. 10.1021/jo00149a001. [DOI] [Google Scholar]
- Johnson C. R.; Jonsson E. U.; Wambsgans A. Nucleophilic-Substitution at Sulfur in Sulfonimodyl Compounds - Synthesis of Sulfoximines. J. Org. Chem. 1979, 44, 2061–2065. 10.1021/jo01327a002. [DOI] [Google Scholar]
- For an example showing sulfonimidoyl fluorides reacting with organolithiums to prepare sulfoximines, see ref 18d.
- Gontcharov A. V.; Liu H.; Sharpless K. B. tert-Butylsulfonamide. A New Nitrogen Source for Catalytic Aminohydroxylation and Aziridination of Olefins. Org. Lett. 1999, 1, 783–786. 10.1021/ol990761a. [DOI] [PubMed] [Google Scholar]
- a Maricich T. J.; Madhusoodanan S.; Kapfer C. A. Convergence of Mechanisms Between Sulfinyl azide and N-Alkoxysulfinamide Reactions. Tetrahedron Lett. 1977, 18, 983–986. 10.1016/S0040-4039(01)92808-4. [DOI] [Google Scholar]; b Maricich T. J.; Hoffman V. L. Chemistry of Benzenesulfinyl Azides - Reactions with sulfoxides. J. Am. Chem. Soc. 1974, 96, 7770–7781. 10.1021/ja00832a026. [DOI] [Google Scholar]; c Maricich T. J. Benzenesulfinyl azide and 1,3,5-Triphenyl-1,3,5,2,4,6-Tri thiatriazine 1,3,5-Trioxide. J. Am. Chem. Soc. 1968, 90, 7179. 10.1021/ja01027a082. [DOI] [Google Scholar]
- Wu Z.; Li D.; Li H.; Zhu B.; Sun H.; Francisco J. S.; Zeng X. Gas-Phase Generation and Decomposition of a Sulfinylnitrene into the Iminyl Radical OSN. Angew. Chem., Int. Ed. 2016, 55, 1507–1510. 10.1002/anie.201510105. [DOI] [PubMed] [Google Scholar]
- Maricich T. J.; Angeletakis C. N. Reaction of benzenesulfinyl azide with thiols and amines. Preparation of thiosulfinates and sulfinamides. J. Org. Chem. 1984, 49, 1931–1934. 10.1021/jo00185a017. [DOI] [Google Scholar]
- Kresze G.; Maschke A.; Albrecht R.; Bederke K.; Patzschke H. P.; Smalla H.; Trede A. Organic N-Sulfinyl Compounds. Angew. Chem., Int. Ed. Engl. 1962, 1, 89–98. 10.1002/anie.196200891. [DOI] [Google Scholar]
- Maricich T. J.; Jourdenais R. A.; Albright T. A. N-Alkoxybenzenesulfinamides. Evidence for an alkylation reaction. J. Am. Chem. Soc. 1973, 95, 5831–5832. 10.1021/ja00798a101. [DOI] [Google Scholar]
- Appropriate safety precautions should be considered when using hydroxylamine derivatives. See the Supporting Information for DSC data for BiPhONSO. For examples of the process scale use of hydroxylamine-derived reagents, see:; a Boyles D. C.; Curran T. T.; Parlett; Davis M.; Mauro F. Electrophilic N-Amination of Two Quinazoline-2,4-diones Using Substituted (Nitrophenyl)hydroxylamines. Org. Process Res. Dev. 2002, 6, 230–233. 10.1021/op010239f. [DOI] [Google Scholar]; b Shi Z.; Kiau S.; Lobben P.; Hynes J.; Wu H.; Parlanti L.; Discordia R.; Doubleday W. W.; Leftheris K.; Dyckman A. J.; Wrobleski S. T.; Dambalas K.; Tummala S.; Leung S.; Lo E. Development of a Practical Synthesis of a p38 Kinase Inhibitor via a Safe and Robust Amination. Org. Process Res. Dev. 2012, 16, 1618–1625. 10.1021/op300181r. [DOI] [Google Scholar]
- Levacher V.; Eriksen B. L.; Begtrup M.; Dupas G.; Quéguiner G.; Duflos J.; Bourguignon J. The tert-butyl sulfoximine group as an effective ortho-director of lithiation: Ortho-metallated S-(tert-butyl)-S-phenylsulfoximines. Tetrahedron Lett. 1999, 40, 1665–1668. 10.1016/S0040-4039(99)00060-X. [DOI] [Google Scholar]
- Peltzer R. M.; Gauss J.; Eisenstein O.; Cascella M. The Grignard Reaction - Unraveling a Chemical Puzzle. J. Am. Chem. Soc. 2020, 142, 2984–2994. 10.1021/jacs.9b11829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.