Abstract

Polycyclic aromatic hydrocarbons (PAHs) with six and seven rings were synthesized via directed metalation and cross-coupling of chrysenyl N,N-diethyl carboxamides with o-tolyl and methylnaphthalenyl derivatives. In the presence of competing ortho sites, the site selectivity in iodination of chrysenyl amides by directed ortho metalation (DoM) was influenced by the lithium base. The catalyst ligand bite angle was presumably important in the cross-coupling of sterically hindered bulky PAHs. Subsequent directed remote metalation of biaryls under standard conditions and at elevated temperatures afforded various fused six- and seven-ring PAHs, all in good yields and with fluorescent properties.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) with extended π-conjugation find their applications in catalysis,1,2 nonlinear optics,3 circularly polarized luminescence,4 organic electronic materials,5 and optoelectronic devices.6−9 PAHs are used as the synthetic building blocks for carbon-rich materials such as fullerenes, nanographenes, and nanotubes.10,11 The intriguing photophysical properties of spiral PAHs have led to development of methods for the synthesis of novel, helically twisted PAHs12 and C3-symmetric spiro-annulated molecules.13 The discovery of superconductivity in metal-doped PAHs14 and an increasing variety of material properties in PAH adducts15,16 has revived the purpose of preparing large or small PAHs which are/are not sterically congested. The material properties of PAHs are often controlled by frontier molecular orbitals that are influenced by the modes of ring fusion, geometry, dopant,17 and functionalities in their periphery.18−21 Synthesis of the aromatic core in nonlinear and nonplanar directions may lead to interesting new packing arrangements and different electronic properties.22,23 Therefore, exploration of new approaches to synthesize these organic molecules in high purity and large scale is deemed important.
Apart from the general methods reported for the preparation of PAHs,24,25 representative examples of the recent approaches include cross-couplings followed by metal-catalyzed intramolecular cyclizations,26−29 C–H activation,30,31 oxidative alkene arylation of o-aryl styrenes,32,33 photochemical cyclizations,34−37 directional synthesis using transient directing groups,38 π-annulation reactions,39 APEX (annulative π-extension) reactions,40 from cycloaddition reactions of aryne precursors,41−43 alkyne [2 + 2 + 2] cycloisomerizations,44−46 and Diels–Alder reactions47,48 among other protocols.49−52
The combinations of directed metalation and cross-couplings are well explored as a versatile and efficient route for the synthesis of phenanthrenes (Scheme 1).53−55 Directed ortho metalation (DoM)56 has proved to be an efficient method for the prefunctionalization of substrates required for cross-coupling, while the Suzuki–Miyaura cross-coupling has been the preferred reaction to make biaryls. The biaryls thus obtained can be cyclized by metalating the remote position through directed remote metalation (DreM) to form various aromatic structures, for example, the phenanthrene natural product gymnopusin.57,58
Scheme 1. Synthesis of Phenanthrenes by Directed Metalation and Cross-Coupling Strategies.
However, examples of DreM with moieties larger than phenyl in the biaryl substrates are scarce. One example of remote metalation of N,N-diethyl-2-(3-methylnaphthalen-2-yl)benzamide exists for the synthesis of tetraphene (benzo[a]anthracene, Scheme 2a), while DreM on 2-methylnaphthalen-1-yl failed (Scheme 2b).59 An attempt to make chrysene from 1-methylnaphthalen-2-yl benzamide gave a fluorenone instead (Scheme 2c).60 Fluorenones are usually formed only in the absence of an ortho (peri)-methyl group in the biaryls.58 These intriguing results in the presence of three regioisomers of the methylnaphthalenyl moiety inspired us to do a systematic exploration of the DreM reaction on methylnaphthalenyl-containing biaryl derivatives to make larger PAHs. Additionally, the detailed mechanism of DreM explaining the driving force behind the kinetic complex-induced proximity effect (CIPE) pathway and the thermodynamic pathway based on acid strength of protons is still unclear.58,61
Scheme 2. DreM Resulting in Different Products Depending on the Connecting Position of the Naphthyl Group. Examples (a,b) by Fu, et al.(59) and Example (c) by Lorentzen, et al.(63).
Herein, we report the synthesis of six- and seven-ring PAHs using directed metalation (DoM and DreM) and Suzuki–Miyaura cross-coupling of chrysenyl carboxamides with o-tolyl and the three regioisomers of methylnapthalenes. The bulky and sterically hindered PAH substrates pose a challenge for the cross-coupling reaction; for instance, the catalysts reported for ortho-substituted anthracene derivatives are not commercially available,62 and simple catalysts59 give modest yields. Thus, new reaction conditions had to be found without compromising the yields.
2. Results and Discussion
The required N,N-diethyl chrysenecarboxamides (1a and 1b) were prepared from the corresponding chrysenecarboxylic acids.64 To prefunctionalize the substrates for cross-coupling, N,N-diethylchrysene-1-carboxamide (1a) and N,N-diethylchrysene-3-carboxamide (1b) were subjected to s-BuLi/TMEDA-mediated DoM protocol. However, DoM and subsequent electrophilic quench with Br2 and B(OCH3)3 in separate experiments were unsuccessful on both 1a and 1b. This was unanticipated as similar bromination on chrysenes with the N,N-diethylcarbamate directing group was reported in good yields.60 Thereafter, I2 was used as an electrophile; besides, iodo substrates are more reactive in the Suzuki–Miyaura cross-coupling. N,N-diethyl-2-iodochrysene-1-carboxamide (2a) was obtained from 1a in excellent yield using s-BuLi/TMEDA and 1 M I2 in THF (Scheme 3a). Under identical conditions, however, 1b afforded N,N-diethyl-2-iodochrysene-3-carboxamide (2b) in poor yield (30%) because of the formation of a complex mixture of unexpected side products, including traces of C-4 iodination. An in situ quench (ISQ) reaction of 1b with s-BuLi/TMEDA and TMSCl also resulted in poor yield of the silylated product 2c and traces of the C-4 substituted product (Scheme 3c), indicating competitive side reactions in the metalation of 1b. Thereupon, a weaker and sterically bulkier LiTMP base gave the desired iodo product 2b regioselectively in good yield (Scheme 3b). It is worth mentioning, in this context, that our previous DoM study on chrysene-3-yl N,N-diethyl-O-carbamate with s-BuLi/TMEDA afforded only the C2-iodination product regioselectively in excellent yield.60 In DoM reactions, s-BuLi (pKa = 51) and LiTMP (pKa = 37.3)65 are the most commonly used bases.66 In the present work, they were chosen over LDA (pKa = 35.7)67 based on their base strength, for the previous studies reported pKa > 37.2 for ortho C–H bonds in monosubstituted benzenes with strong DMGs.65
Scheme 3. Electrophilic Substitution of N,N-Diethylchrysenecarboxamides by DoM.
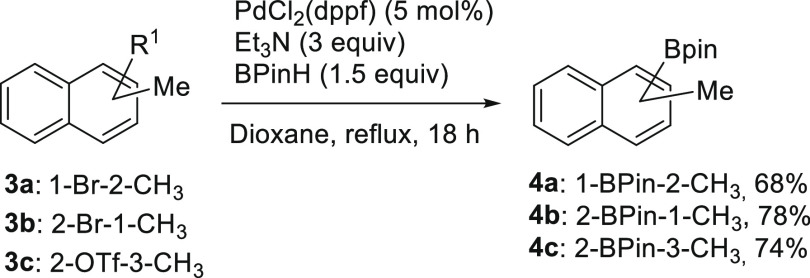
With the iodo-coupling partners 2a and 2b in hand, focus was on the preparation of the three regioisomers of methylnaphthalenyl boronates. For the synthesis of boronate-coupling partners, 1-bromo-2-methylnaphthalene (3a), 2-bromo-1-methylnaphthalene (3b), and 3-methylnaphthalen-2-yl trifluoromethanesulfonate (3c) were prepared according to literature procedures (Supporting Information, Scheme S1).27,68−70 Initially, the bromo-methylnaphthalenes were all converted to boronic acids (S6–S8) by lithium–halogen exchange reactions and used further without purification (Supporting Information, Scheme S2).71 However, considering the ease of handling and purification of boronic esters, compounds 3a–3c were subjected to Miyaura borylation using a reported procedure,72 to synthesize the pinacol esters 4a–4c in good yields, as shown in Scheme 4.
Scheme 4. Preparation of Methylnaphthalenyl Boron Pinacolate Cross-Coupling Partners.
Initial cross-coupling experiments conducted on 2a–2b with commercially available o-tolyl boronic acid, in the presence of PdCl2(dppf) and Na2CO3 in DME and H2O, afforded the cross-coupled product in 90 and 77% yields, respectively (Table 1).63 Unfortunately, these conditions failed for both methylnaphthalenyl boronic acid and the boron pinacolate (BPin) analogues 4b–4c (Supporting Information, Table S1). In most of these experiments, the dehalogenated side product was observed, inferring a slower transmetalation step going from o-tolylboronates to methylnapthalenylboronates. Several cross-coupling conditions using available catalysts, bases, and solvents were attempted in search of suitable reaction conditions to cross-couple these ortho-substituted bulky substrates (Supporting Information, Tables S1 and S2).
Table 1. Products from the Suzuki–Miyaura Cross-Coupling Reactions.
2a/2b (1 equiv), boronate (1.5 equiv), PdCl2(dppf) (5 mol %), Na2CO3 (3 equiv). DME/H2O, 90 °C.
2a/2b (1 equiv), boronate (1.5 equiv), PdCl2(dppe) (5 mol %), Cs2CO3 (3 equiv). DMF, 4 Å MS, 120 °C.
Same conditions as b, but in toluene at 110 °C.
Contemplating the observations from these unsuccessful experiments made us question the relative influence of electronic and steric factors. The ligand bite angle (β), described in Figure 1, has been considered important for Suzuki–Miyaura cross-coupling of sterically demanding substrates.62,73 These substrates required a wide bite-angled trans-spanning ligand to allow trans conformation at the metal center. However, the Pd center must undergo a trans to cis isomerization before the reductive elimination. Excessively large bite angles can deform the catalyst.74 On the other hand, extremely small bite angles, as in PdCl2(dppm) (Figure 1), might be unsuitable for an efficient reductive elimination because of reduced electron density at the metal center.75 Presuming that a ligand with a narrow bite angle could be effective, leading directly to a cis conformation at the metal center, we chose the PdCl2(dppe) catalyst as a compromise for further experiments.
Figure 1.
Ligand bite angle (β°) for selected catalysts: PdCl2(dppf),76 PdCl2(dppp),76,77 PdCl2(dppe),77,78 and PdCl2(dppm)78
Fortunately, the chosen catalyst PdCl2(dppe) was suitable for cross-coupling of all our bulky PAH substrates (Table 1). The use of BPins and anhydrous reaction conditions (dry solvent and molecular sieves) were helpful to avoid deboronation, which is usually observed in such cross-coupling reactions. Both Cs2CO3 (preferable) and KOtBu (in tBuOH as the solvent) can be used for the reaction. The experiments were initially conducted in toluene to avoid hydrodehalogenation usually observed in polar protic solvents. The yields obtained were acceptable with exception of 5f (10%). The addition of the Ag2O additive or changing the base to K3PO4 did not improve the yield of 5f, but changing the solvent to DMF increased the yield significantly. The yields of remaining cross-coupling experiments were also improved; therefore, PdCl2(dppe) with Cs2CO3 in DMF with 4 Å molecular sieves is the preferable condition to cross-couple the methylnaphthalenyl BPin derivatives with 2a-2b in good to excellent yields (Table 1).
Thereon, DreM of biaryls 5a–5h was studied to synthesize larger six- and seven-ring PAHs. Compounds 5a, 5d, 5e, and 5h were cyclized neatly using the regular DreM conditions of excess LDA in THF at 0 °C (Table 2). As phenols are sometimes prone to oxidation, forming quinones, the DreM reaction products were protected by one-pot addition of 1 M TBDMSCl to the reaction mixture at RT. The remaining biaryls failed to cyclize under regular DreM conditions. However, a slight increase in temperature to 40 °C after the addition of LDA afforded 6b and 6f in good to moderate yields. To avoid unwanted reactivity of the Li base with THF at elevated temperatures79 and facilitate the addition of LDA at 40 °C, these experiments were conducted in benzene.80 Direct addition of LDA at 40 °C afforded slightly higher yields (Table 2). Unfortunately, compounds 5c and 5g did not form products by increasing the temperature. Ultimately, biaryl 5g was exposed to LDA in refluxing benzene that led to partial decomposition of the substrate, but no trace of the desired product.
Table 2. PAHs Synthesized from DreM on Cross-Coupled Products 6a–6i.
LDA (3 equiv), THF, 0 °C, 30 min, then RT, 1 h, TBDMSCl (3.1 equiv), RT, 17 h.
LDA (3.5 equiv), 40 °C, benzene, 1 h, TBDMSCl (3.5 equiv), 40 °C, 14 h.
LDA (3.5 equiv), 0 °C, benzene, 40 °C, 1 h, TBDMSCl (3.5 equiv), RT, 17 h.
In these DreM experiments, reactivity was determined by the regioisomeric methylnaphthalenyl groups. The 3-methylnaphthalen-2-yl group (5d, 5h → 6d, 6h) reacted readily at 0 °C, while the 2-methylnaphthalen-1-yl group (5b, 5f → 6b, 6f) reacted at 40 °C. The 1-methylnaphthalen-2-yl group (5c and 5g) failed to cyclize at all. Besides, attached to a simple benzamide (5j), the 1-methylnaphthalen-2-yl group formed a fluorenone.63 Although all of these biaryls display some atropisomerism, there is no indication that this variation in reactivity occurs from rotational barriers.63
The mechanism of DreM on biarylic benzamides, as proposed by Snieckus and Mortier,81 involves the first metalation at the ortho position of the benzamide (DoM-site) at low temperatures. At temperatures above −30 °C, ortho metalation is expected to rapidly equilibrate toward metalation of the DreM sites on the other aryl moiety (remote and lateral positions). This is then followed by a cyclization reaction with the amide group. A few quench experiments were performed to gain more insight into this reactivity. By this proposed mechanism, our ISQ experiments trap the metalation site after equilibration. Compound 5d underwent ISQ with TMSCl at 0 °C, resulting in bis-silylation of the methyl group (7, Scheme 5). Deuterium quench experiments and ISQ experiments with TMSCl on compounds 5c and 5g were unsuccessful. A stronger base such as n-BuLi decomposed the starting material. Apparently, 5c and 5g lack a favorable deprotonation site, and stronger bases deprotonate indiscriminately, decomposing the starting material.
Scheme 5. ISQ Experiments on Selected Substrates.
ISQ of 5j (Scheme 5) gave a silylated product (8) and a second TLC spot with a complex NMR spectrum, explaining the moderate yield of product 8. The exact position of the silyl group in ISQ product 8 could not be determined from its overlapping spectrum of atropdiastereomers, but an HMBC 2D-NMR experiment of the product displayed a correlation between the amide carbonyl and ortho-H on benzamide, excluding silylation ortho to the amide (DoM position). The integral of the methyl group (3H) and lack of an HMBC correlation between the methyl-C and TMS-H ruled out silylation on the methyl group. Increased rotational barriers, resulting in sharp signals of two atropisomers, make it reasonable to assume silylation in 3′ position, as drawn in product 8 (Scheme 5). Apparently, 5j is deprotonated on the ring instead of on the methyl group during the DreM reaction, explaining the formation of fluorenone (Scheme 2c).60
The successful synthesis of 6b and 6f prompted us to reinvestigate the DreM of 5i (Scheme 2b).59 The yield of 5i (Scheme 6) was improved from 25 to 78% by changing the cross-coupling reaction conditions of 4a and 2d (Supporting Information, Scheme S3). This catalyst also increased the yield of 5j to quantitative. Following DreM reaction of biaryl 5i did indeed give the cyclized product at 40 °C for a combined yield of 88%. However, direct derivatization of the free phenol with TBDMSCl was less efficient, giving unprotected 6ib as the major product.
Scheme 6. Reinvestigated Synthesis of 6ib.
To further increase the scope of this methodology, we attempted to cross-couple two chrysenes toward the synthesis of a fused nine-ring PAH. Already, at the Miyaura borylation step on 2-bromo-3-methylchrysene, solubility became an issue, and poor yield (23%) of the BPin analogue was afforded with a solvent change. The cross-coupled product obtained in traces was practically insoluble, and further experiments were abandoned. Strategic substitutions to increase solubility will be necessary in order to make even larger PAH systems.
2.1. Fluorescence Measurements of Products 6
The final PAHs displayed a bluish fluorescence in UV light. Fluorescence spectra are dependent on the molecular shape, rigidness, and planarity;82 although of similar size, the DreM products (Table 2) display a variety of geometrical arrangements of the benzene rings. Although most of the molecules should be planar, the carbo-[4]helicene end of 6b and 6f will be twisted out of plane. Torsion angles within [4]helicenes and carbo-[4]helicene substructures are reported from 23 to 33°, depending on substitution patterns.29,83−86 Absorption, fluorescence, and excitation spectra were measured from 1 × 10–6 M sample solutions in CHCl3. The normalized spectra are given in Figure 2, while λmax and Stokes shifts are given in Table 3. CHCl3 was chosen as the solvent because of solubility issues. Although CHCl3 has significant absorption below 250 nm, it should not affect the fluorescence spectra. The lower part of the absorption spectra is affected, but the main absorption is clearly visible.
Figure 2.
Plots showing UV–vis absorption and fluorescence spectra of PAHs dissolved in CHCl3 with normalized intensity on the y-axis and wavelengths in nanometer on the x-axis.
Table 3. Stokes Shift of the Synthesized PAHs.
| compound | λabs (nm) | λexc (nm) | λemis (nm) | Stokes shift (nm) | Stokes shift (cm–1) |
|---|---|---|---|---|---|
| 6a | 298 | 297 | 396 | 98 | 102,041 |
| 6b | 317 | 317 | 416 | 99 | 101,010 |
| 6d | 318 | 316 | 434 | 116 | 86,207 |
| 6e | 311 | 311 | 412 | 101 | 99,010 |
| 6f | 330 | 329 | 428 | 98 | 102,041 |
| 6h | 334 | 333 | 445 | 111 | 90,090 |
| 6ia | 290 | 289 | 392 | 102 | 98,039 |
Although acenes have regular strong bathochromic shifts of about 100 nm per ring, the effect is much smaller for phenacenes.82,87,88 We observed a small red shift in the absorption spectra for each benzene ring added and an additional 11–16 nm for a terminal anthracene moiety in the molecule. The emission spectra also showed similar perturbations between the molecules, but with a slightly stronger bathochromic shift when the anthracene moiety was present at the end of the molecule. [6]Phenacene derivative 6a had a 98 nm Stokes shift. Chang et al. measured the Stokes shift of [6]phenacene to 90 nm, while some analogues with substituents on the terminal ring had 90–95 nm Stokes shift.89 Despite the variety in shape, our compounds gave similar spectra with 98–102 nm Stokes shift. Only compounds 6d and 6h, with a terminal anthracene moiety, deviate with 116 and 111 nm Stokes shift. The expected nonplanarity of 6b and 6f gave no visible impact on the spectra. It should be noted that a photophysical study of [6]phenacene by laser excitation in a glass matrix at 77 K found some weak absorption bands close to the fluorescence peak and calculated the Stokes shift to 4 nm.90
Benzo[c]phenanthrene (3,4-benzophenanthrene) is reported to have a quantum yield of 0.12 and a similar fluorescence spectrum to 6ia.87 In our experiments at 1 × 10–6 M solution, 6ib had a fluorescence too weak to be measured, and 6ia had much lower signal strength than the other compounds. Although quantum yields were not measured, 6a–6h must have good quantum yields to give this difference in signal strength.
3. Conclusions
In this first study to expand directed metalation and cross-coupling strategies to the synthesis of medium-sized PAHs, we can observe several effects of the larger PAH systems. Although most of the extra bulk of the PAHs should point away from the catalyst in the cross-couplings, these molecules still have more steric hindrance than the well-explored biphenyls used in phenanthrene synthesis. Rather than a further increase in the catalyst’s ligand bite angle, apparently a smaller bite angle was beneficial in these systems. Although DreM is usually performed at 0 °C (with a slow increase to room temperature during the experiment), we found that some configurations needed 40 °C to react. This demonstrated that the DreM reaction is not limited to flat PAHs but can also generate twisted out-of-plane PAHs. In situ quench experiments on selected biaryls reveal that the 1-methylnaphthalen-2-yl substituent fails to get deprotonated at the methyl group. Further studies will be needed to determine if this is due to an unfavorable conformation blocking the directing effect of the amide group or a pKa effect of the naphthalenyl group.
4. Experimental Information
4.1. General Information
All the reactions were conducted under an inert N2 atmosphere, in oven-dried glassware. The anhydrous solvents THF, DMF, toluene, and benzene were purchased commercially and used as supplied. All other solvents were dried over molecular sieves before use. BuLi (molar solution in cyclohexane) was titrated for accurate concentration. N,N,N′,N′-Tetramethylethylenediamine (TMEDA) and di-isopropyl amine (DIIPA) were distilled before use and stored over KOH. 4,4,5,5-Tetramethyl-1,3,2-dioxaborolane (pinBH) was purchased commercially and used as it is. Anhydrous TBDMSCl was purchased as 1 M solution in THF and used as it is. The synthesized compounds were purified using silica gel 40–63 μm. Routine TLC analysis was carried out on silica gel-coated aluminum sheets that were purchased from Merck KGaA. Plates were viewed with a 254 nm ultraviolet lamp. 1H NMR spectra were obtained on a 400 MHz Bruker AVANCE III spectrometer. 13C NMR spectra were obtained at 100 MHz. All NMR spectra were processed using Topspin NMR software. All chemical shift values are reported in parts per million (ppm) relative to the solvent signal and were determined in CDCl3 + TMS (CDCl3 at 7.26 ppm, 1H NMR and 77.2 ppm, 13C NMR) or C2D2Cl4 (5.91 ppm, 1H NMR and 73.78 ppm, 13C NMR) or DMSO-d6 (2.50 ppm, 1H NMR and 39.52 ppm, 13C NMR) with coupling constant (J) values reported in Hertz. The notation of signals is proton: δ chemical shift in ppm (multiplicity, J value(s), number of protons). Carbon: δ chemical shift in ppm (number of carbons). If assignment is ambiguous, for example, in the case of overlapping signals, a range of shifts is reported as the multiplet. Peaks due to solvent impurities in the region of 0–5 ppm (1H NMR)/0–40 ppm (13C NMR) are left unassigned. The 1H NMR spectrum of EtOAc solvent impurities is also included. High-resolution mass spectra (HRMS) were obtained using either the positive and/or negative electrospray ionization (ESI) technique or time-of-flight (TOF) mass detection. IR spectra were recorded on an Agilent Carey 600 FTIR spectrometer using KBr pellets. Melting points of recrystallized samples were recorded on a Stuart Scientific melting point apparatus SMP3 and are uncorrected. UV–visible absorption spectra of recrystallized samples were measured on a VWR UV-1600PC spectrophotometer. Fluorescence emission and excitation spectra of recrystallized samples were measured on an F-7000 FL spectrophotometer.
4.2. General Procedures
The following general procedures A–D cover the important reactions applied and discussed in the main article. Any deviation from the general procedure, reference to applied procedures, and detailed amounts of reagents and yields are given for each compound together with the characterization of compounds.
4.2.1. General Procedure A for DoM
In an inert dry N2 atmosphere, s-BuLi (1.5 equiv, 1.13 M in cyclohexane) was added to compound 1 (1 equiv) in anhydrous THF at −78 °C. The reaction mixture was stirred for 30 min before an electrophile (1.5 equiv) was added to it at −78 °C. The reaction mixture was allowed to reach RT in 15 h. After completion of the reaction, the mixture was quenched with satd. aq. NH4Cl (50 mL). The crude product was extracted into EtOAc (3 × 50 mL). The combined organic layer was washed with brine (50 mL), dried over anhydrous Na2SO4, and evaporated in vacuo. The crude product 2 was purified using column chromatography (EtOAc in heptane).
4.2.2. General Procedure B for Borylation of Methylnapthalenes
An oven-dried round bottom flask was fitted with a condenser and purged with N2 to maintain an inert atmosphere. Starting material 3 (1 equiv) and PdCl2(dppf) (5 mol %) were fed into the reaction flask and stirred in anhydrous dioxane at RT for 10 min. After the addition of triethyl amine (3 equiv) and pinacol borane (1.5 equiv), the reaction mixture was refluxed using a heating mantle for 17 h. At the end, the reaction mixture was brought to RT and filtered through a pad of Celite. To the reaction mixture was added water (50 mL), and the crude product was extracted into EtOAc (3 × 50 mL). The combined organic layer was washed with brine (50 mL), dried over anhydrous Na2SO4, and evaporated in vacuo. The crude product was purified by flash chromatography to afford product 4 (initially eluted with pure heptane to remove the starting material, and then, silica was deactivated with 5% Et3N in heptane to elute the product).
4.2.3. General Procedure C for Cross-Coupling
An oven-dried round bottom flask was fitted with a condenser and purged with N2 to maintain an inert atmosphere. A mixture of N,N-diethyl-2-iodochrysene carboxamide 2a/2b (1 equiv) and PdCl2(dppe) (5 mol %) was stirred in anhydrous DMF at RT for 10 min. Methylnaphthalenyl BPin (4a–4c) was added followed by the addition of Cs2CO3 (3 equiv). The reaction mixture was refluxed at 120 °C using a heating mantle for 24 h. After completion of the reaction, it was cooled down to RT and filtered through a pad of Celite to remove Pd black. To the reaction mixture was added water (10 mL), and the crude product was extracted into EtOAc (3 × 10 mL). The combined organic layer was washed with brine (10 mL), dried over anhydrous Na2SO4, and evaporated in vacuo. The crude product was purified using column chromatography (EtOAc in heptane).
4.2.4. General Procedure D for DreM
An oven-dried round bottom flask was purged with N2 before unsymmetrical biaryl 5 (1 equiv) was dissolved in anhydrous THF. The solution was brought to 0 °C, and a freshly prepared LDA (3.0 equiv of n-BuLi was added to 3.1 equiv of diisopropyl amine in anhydrous THF stirred at 0 °C for 15 min) was added dropwise to it. The reaction mixture was stirred at 0 °C for 30 min before allowing it to reach RT where again it was stirred for 1 h. At this point, 1 M TBDMSCl (3.1 equiv) was slowly added, and the mixture was stirred at RT for 17 h. After completion of the reaction, it was quenched with satd. aq NH4Cl (10 mL). The product was extracted into EtOAc (3 × 10 mL). The combined organic layer was washed with brine (10 mL), dried over anhydrous Na2SO4, and evaporated in vacuo. The crude product was purified using flash column chromatography (EtOAc or DCM in heptane).
4.2.5. General Procedure E for DreM at 40 °C
An oven-dried round bottom flask was purged with N2 before unsymmetrical biaryl 5 (1 equiv) was dissolved in anhydrous benzene. The solution was brought to 40 °C using a heating mantle, and freshly prepared LDA (3.5 equiv of n-BuLi was added to 3.6 equiv of diisopropyl amine in anhydrous benzene stirred at 0 °C for 15 min) was added dropwise to it. The reaction mixture was stirred at 40 °C for 1 h. Then, 1 M TBDMSCl (3.5 equiv) was slowly added, and the mixture was stirred at 40 °C for 14 h. After completion of the reaction, the reaction mixture was brought to RT and quenched with satd. aq. NH4Cl (10 mL). The product was extracted into EtOAc (3 × 10 mL). The combined organic layer was washed with brine (10 mL), dried over anhydrous Na2SO4, and evaporated in vacuo. The crude product was purified using column chromatography (EtOAc in heptane).
4.2.6. General Procedure for UV–Visible and Fluorescence Spectroscopic Measurements
For end products 6a–6ia, stock solutions of 10–3 M CHCl3 were prepared, which were half-diluted in the same solvent to 10–6 M. These solutions were used to record the maximum absorption wavelength by scanning the sample from 200 to 900 nm.
Using the same 10–6 M solutions, the emission spectra of all the samples were recorded at maximum absorption wavelength. The excitation spectra were then measured using maximum emission wavelength. All the data from UV–visible absorption and fluorescence spectroscopy were plotted in normalized graphs to calculate the Stoke’s shift.
4.3. Characterization of Compounds
4.3.1. N,N-Diethylchrysene-1-carboxamide (1a)
Chrysene-1-carboxylic acid (0.732 g, 2.69 mmol), SOCl2 (0.59 mL, 8.06 mmol), and a drop of DMF as the catalyst were heated to reflux in toluene (15 mL) for 16 h. After cooling down to RT, the solvent was evaporated in vacuo to yield crude chrysene-1-carbonyl chloride as a yellow solid that was subsequently used in the next step.
Diethyl amine (0.84 mL, 8.06 mmol) was added slowly to a solution of chrysene-1-carbonyl chloride (2.69 mmol) in THF (60 mL) at 0 °C. The reaction mixture was refluxed in an oil bath for 16 h. After cooling down to RT, the reaction mixture was quenched with 1 M HCl (60 mL) and extracted with Et2O (3 × 60 mL). The combined organic layer was washed with satd aq NaHCO3 (60 mL) and brine (60 mL), dried over anhydrous Na2SO4, and evaporated in vacuo. The crude product was purified using column chromatography (20% EtOAc in heptane) to obtain 1a (0.84 g, 99%) as an off-white solid.
mp 156.5–158.3 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.82 (d, J = 8.8 Hz, 1H), 8.78 (d, J = 8.2 Hz, 1H), 8.77 (d, J = 9.2 Hz, 1H), 8.72 (d, J = 9.1 Hz, 1H), 8.04 (d, J = 9.1 Hz, 1H), 8.02–7.98 (m, 2H), 7.75–7.70 (m, 2H), 7.68–7.64 (m, 1H), 7.55 (dd, J = 1.0, 7.0 Hz, 1H), 3.96–3.88 (br m, 1H), 3.64–3.56 (br m, 1H), 3.19–3.11 (br m, 2H), 1.43 (t, J = 7.1 Hz, 3H), 1.02 (t, J = 7.1 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 170.5, 135.9, 132.3, 130.9, 130.5, 128.7, 128.4 (2C), 128.3, 127.9, 127.0, 126.7, 126.2, 123.9, 123.8, 123.77, 123.3, 122.4, 121.2, 43.3, 39.2, 14.4, 13.2.
FTIR (KBr, cm–1): 2965 (w), 1619 (vs), 1594 (s), 1465 (s), 1287 (s), 1101 (s), 774 (s).
HRMS (ESI/TOF) m/z: calcd for C23H22ON, 328.1696 [M + H]+; found, 328.1698.
4.3.2. N,N-Diethylchrysene-3-carboxamide (1b)
Chrysene-3-carboxylic acid (1.68 g, 6.12 mmol), SOCl2 (3.25 mL, 18.6 mmol), and a drop of DMF as the catalyst were heated to reflux in toluene (20 mL) for 16 h. After cooling down to RT, the solvent was evaporated in vacuo to yield crude chrysene-3-carbonyl chloride as a yellow solid that was subsequently used in the next step.
Diethyl amine (1.93 mL, 18.6 mmol) was added slowly to the solution of chrysene-3-carbonyl chloride (6.18 mmol) in THF (60 mL) at 0 °C. The reaction mixture was refluxed in an oil bath for 16 h. After cooling down to RT, the reaction mixture was quenched with 1 M HCl (100 mL) and extracted with Et2O (3 × 100 mL). The combined organic layer was washed with satd aq NaHCO3 (100 mL) and brine (100 mL), dried over anhydrous Na2SO4, and evaporated in vacuo. The crude product was purified using column chromatography (20% EtOAc in heptane) to obtain 1b (2.03 g, quant) as an off-white solid.
mp 156.5–157.7 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.82 (s, 1H), 8.71 (d, J = 9.0 Hz, 1H), 8.69 (d, J = 9.2 Hz, 1H), 8.67 (d, J = 8.9 Hz, 1H), 7.99 (d, J = 8.5 Hz, 1H), 7.97 (d, J = 6.9 Hz, 1H), 7.97 (s, 1H), 7.95 (d, J = 7.0 Hz, 1H), 7.70–7.60 (m, 2H), 7.63 (dd, J = 1.2, 8.1 Hz, 1H), 3.66 (br s, 2H), 3.33 (br s, 2H), 1.35 (br s, 3H), 1.16 (br s, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 171.6, 135.3, 132.3, 132.2, 130.4, 130.2, 128.7, 128.6 (2C), 128.2, 127.7, 126.9, 126.8, 126.6, 124.2, 123.1, 122.2, 121.4, 121.0, 43.5, 39.5, 14.3, 13.1.
FTIR (KBr, cm–1): 2975 (w), 1623 (vs), 1425 (s), 1283 (s), 1094 (s), 828 (s), 762 (s).
HRMS (ESI/TOF) m/z: calcd for C23H22ON, 328.1696 [M + H]+; found, 328.1702.
4.3.3. N,N-Diethyl-2-iodochrysene-1-carboxamide (2a)
Following general procedure A, compound 1a (873 mg, 2.67 mmol) in anhydrous THF (35 mL) was treated with s-BuLi (3.55 mL, 4.01 mmol, 1.13 M in cyclohexane) and TMEDA (0.60 mL, 4.01 mmol) for 30 min before adding I2 (4 mL, 4.01 mmol, 1 M in anhydrous THF) at −78 °C and then warmed to RT over 8 h. Standard workup (eluent: 20% EtOAc in heptane) afforded product 2a as an off-white solid (1.16 g, 96%).
mp 238.6–241.0 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.73 (d, J = 8.2 Hz, 1H), 8.72 (d, J = 9.3 Hz, 1H), 8.61 (d, J = 9.1 Hz, 1H), 8.45 (d, J = 8.9 Hz, 1H), 8.03 (d, J = 8.9 Hz, 1H), 8.01 (d, J = 9.1 Hz, 1H), 7.99 (dd, J = 1.1, 9.1 Hz, 1H), 7.91 (d, J = 9.3 Hz, 1H), 7.74–7.69 (m, 1H), 7.67–7.63 (m, 1H), 4.03–3.95 (m, 1H), 3.66–3.95 (m, 1H), 3.19–3.14 (m, 2H) 1.46 (t, J = 7.1 Hz, 3H), 1.01 (t, J = 7.2 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 169.7, 140.9, 136.2, 132.5, 130.4, 130.3, 129.9, 128.7, 128.4, 128.3, 128.1, 127.2, 127.0, 125.0, 124.1, 123.3, 123.25, 120.8, 91.8, 43.2, 39.2, 14.1, 12.7.
FTIR (KBr, cm–1): 2963 (w), 1616 (vs), 1436 (m), 1274 (s), 1099 (m), 811 (s), 752 (s).
HRMS (ESI/TOF) m/z: calcd for C23H21ONI, 454.0662 [M + H]+; found, 454.0662.
4.3.4. N,N-Diethyl-2-iodochrysene-3-carboxamide (2b)
Following general procedure A, compound 1b (500 mg, 1.53 mmol) in anhydrous THF (15 mL) was treated with freshly prepared LiTMP (4.58 mmol in anhydrous THF) for 1.5 h before adding I2 (5.35 mL, 5.35 mmol, 1 M in anhydrous THF) at −78 °C and then warmed to RT over 16 h. Standard workup (eluent: 20% EtOAc in heptane) afforded the product 2b as an off-white solid (0.58 g, 84%).
mp 224.7–226.5 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.71–8.67 (m, 2H), 8.58 (s, 1H), 8.56 (d, J = 9.2 Hz, 1H), 8.43 (s, 1H), 7.99–7.95 (m, 2H), 7.82 (d, J = 9.1 Hz, 1H), 7.71–7.67 (m, 1H), 7.66–7.62 (m, 1H), 4.00–3.97 (br m, 1H), 3.42–3.39 (br m, 1H), 3.25–3.17 (br m, 2H) 1.40 (t, J = 7.1 Hz, 3H), 1.10 (t, J = 7.1 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 170.4, 140.2, 139.0, 133.3, 132.4, 130.4, 129.9, 128.8, 128.7, 128.2, 127.9, 127.1, 126.9, 125.6, 123.2, 123.0, 121.5, 120.6, 90.2, 43.1, 39.3, 14.1, 12.7.
FTIR (KBr, cm–1): 2965 (w), 1635 (vs), 1423 (s), 1279 (s), 1106 (m), 823 (s), 812 (s), 756 (s).
HRMS (ESI/TOF) m/z: calcd for C23H21ONI, 454.0662 [M + H]+; found, 454.0666.
4.3.5. N,N-Diethyl-2-(trimethylsilyl)chrysene-3-carboxamide (2c)
Following general procedure A, compound 1a (108 mg, 0.33 mmol) and TMSCl (0.06 mL, 0.50 mmol) in anhydrous THF (4 mL) were treated with s-BuLi (0.44 mL, 0.50 mmol, 1.13 M in cyclohexane) and TMEDA (0.07 mL, 0.50 mmol) for 30 min at −78 °C and then warmed to RT over 7 h. Standard workup with EtOAc (3 × 10 mL) and column chromatography (15–20% EtOAc in heptane) afforded the product 2c (73 mg, 55%) as an off-white solid.
mp 166.9–169.0 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.78 (d, J = 8.3 Hz, 1H), 8.75 (d, J = 9.1 Hz, 1H), 8.65 (d, J = 9.1 Hz, 1H), 8.58 (s, 1H), 8.23 (s, 1H), 8.02 (d, J = 8.9 Hz, 2H), 8.00 (dd, J = 1.0, 7.0 Hz, 1H), 7.74–7.70 (m, 1H), 7.67–7.63 (m, 1H), 3.70 (q, J = 7.0 Hz, 2H), 3.27 (q, J = 7.0 Hz, 2H), 1.40 (t, J = 7.0 Hz, 3H), 1.16 (t, J = 7.0 Hz, 3H), 0.43 (s, 1H).
13C{1H} NMR (100 MHz, CDCl3): δ = 172.9, 140.4, 136.6, 135.8, 132.4, 131.4, 130.6, 130.1, 129.0, 128.7, 128.0, 127.8, 127.3, 127.0, 126.7, 123.3, 122.0, 121.0, 120.1, 43.8, 39.4, 14.2, 13.1, 0.1.
FTIR (KBr, cm–1): 2966 (w), 1633 (vs), 1457 (m), 1283 (s), 1243 (m), 1112 (m), 861 (s), 839 (vs), 749 (s).
HRMS (ESI/TOF) m/z: calcd for C26H30ONSi, 400.2091 [M + H]+; found, 400.2093.
4.3.6. N,N-Diethyl-2-iodobenzamide (2d)
Following general procedure A, benzamide (386 mg, 2.18 mmol) in anhydrous THF (3 mL) was added slowly to a solution of s-BuLi (2.90 mL, 3.27 mmol, 1.13 M in cyclohexane) and TMEDA (0.49 mL, 3.27 mmol) in anhydrous THF (2 mL) and stirred for 30 min before adding I2 (3.27 mL, 1 M in anhydrous THF) at −78 °C. The reaction mixture was warmed to RT over 6 h. Standard workup with EtOAc (3 × 10 mL) and column chromatography (20% EtOAc in heptane) afforded the product 2d (0.46 g, 68%) as a yellow oil.
Characterization data were in accordance with the literature.91
4.3.7. 4,4,5,5-Tetramethyl-2-(2-methylnaphthalen-1-yl)-1,3,2-dioxaborolane (4a)
Following general procedure B, 1-bromo-2-methyl naphthalene69 (3a, 2.08 g, 9.05 mmol) was stirred with PdCl2(dppf) (0.37 g, 5 mol %) for 10 min in dioxane (36 mL), pinacol borane (1.97 mL, 13.6 mmol) and Et3N (3.78 mL, 27.2 mmol) were added at RT, and heated to reflux. After completion of the reaction, workup afforded 4a (1.67 g, 68%) as an off-white solid. A second experiment using 3.12 g of 3a afforded 4a in 65% yield.
mp 92.4–94.5 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.11 (d, J = 8.5 Hz, 1H), 7.76 (dd, J = 1.3, 7.4 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.46–7.42 (m, 1H), 7.40–7.36 (m, 1H), 7.28 (d, J = 8.5 Hz, 1H), 2.62 (s, 3H), 1.49 (s, 12H).
13C{1H} NMR (100 MHz, CDCl3): δ = 141.5, 136.8, 131.5, 129.7, 128.6, 128.3 (2C), 127.6, 126.1, 124.7, 84.1 (2C), 25.2 (3C), 25.1, 22.8. The NMR data are in accordance with literature values.92−94
FTIR (KBr, cm–1): 2975 (w), 1507 (m), 1467 (m), 1303 (m), 1258 (m), 1144 (m), 1132 (m), 857 (vs), 843 (vs), 816 (vs), 742 (vs).
HRMS (ESI/TOF) m/z: calcd for C17H22O2B, 269.1713 [M + H]+; found, 269.1707.
4.3.8. 4,4,5,5-Tetramethyl-2-(1-methylnaphthalen-2-yl)-1,3,2-dioxaborolane (4b)
Following general procedure B, 2-bromo-1-methyl naphthalene69 (3b, 2.97 g, 13.4 mmol) was stirred with PdCl2(dppf) (0.49 g, 5 mol %) for 10 min in dioxane (40 mL), pinacol borane (2.93 mL, 20.2 mmol) and Et3N (5.62 mL, 40.3 mmol) were added at RT, and heated to reflux. After completion of the reaction, workup afforded 4b (2.82 g, 78%) as an off-white solid. A second experiment using 2 g of 3b gave product 4b in 77% yield.
mp 83.2–84.1 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.83–8.80 (m, 1H), 8.04–8.01 (m, 1H), 8.00 (d, J = 7.0 Hz, 1H), 7.57–7.50 (m, 2H), 7.34 (dd, J = 0.8, 7.0 Hz, 1H), 2.72 (s, 3H), 1.43 (s, 12H).
13C{1H} NMR (100 MHz, CDCl3): δ = 138.3, 137.2, 135.8, 132.6, 129.2, 126.2 (3C), 125.5, 124.4, 83.8 (2C), 25.1 (4C), 20.1.
FTIR (KBr, cm–1): 2979 (w), 1344 (w), 1291 (m), 1145 (m), 1114 (m), 858 (s), 849 (s), 760 (vs).
HRMS (ESI/TOF) m/z: calcd for C17H22O2B, 269.1713 [M + H]+; found, 269.1707.
4.3.9. 4,4,5,5-Tetramethyl-2-(3-methylnaphthalen-2-yl)-1,3,2-dioxaborolane (4c)
Following general procedure B, 2-methylnaphthalen-3-yl trifluoromethanesulfonate27 (3c, 1.00 g, 3.45 mmol) was stirred with PdCl2(dppf) (141 mg, 5 mol %) for 10 min in dioxane (15 mL), pinacol borane (0.60 mL, 4.13 mmol) and Et3N (1.5 mL, 10.4 mmol) were added at RT, and heated to reflux. After completion of the reaction, workup afforded 4c (0.68 g, 74%) as an off-white solid. A second experiment using 2.02 g of 3c afforded product 4c in 66% yield.
mp 61.3–62.8 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.35 (s, 1H), 7.85 (d, J = 8.1 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.60 (s, 1H), 7.50–7.45 (m, 1H), 7.42–7.38 (m, 1H), 2.70 (s, 3H), 1.41 (s, 12H).
13C{1H} NMR (100 MHz, CDCl3): δ = 140.5, 137.5 (2C), 135.1, 131.2, 128.4, 127.3, 127.1, 127.0, 125.0, 83.7 (2C), 25.1 (4C), 22.8. The NMR data are in accordance with literature values.95
FTIR (KBr, cm–1): 2976 (w), 1350 (s), 1329 (s), 1147 (s), 1135 (s), 1031 (m), 960 (m), 856 (m), 754 (m), 750 (m).
HRMS (ESI/TOF) m/z: calcd for C17H22O2B, 269.1713 [M + H]+; found, 269.1706.
4.3.10. N,N-Diethyl-2-(o-tolyl)chrysene-1-carboxamide (5a)
Compound 2a (110 mg, 0.24 mmol), PdCl2(dppf) (9 mg, 5 mol %), o-tolyl boronic acid (40 mg, 0.29 mmol), and Na2CO3 (77 mg, 0.73 mmol) were all added in sequence to DME (5 mL) and then H2O (2 mL). The reaction mixture was stirred at 85 °C for 17 h. After completion of the reaction, workup following standard procedure B (eluent: 20% EtOAc in heptane) afforded the cross-coupled product 5a (91 mg, 90%) as a brown solid (major/minor rotamers = 56:44).
mp 205.2–210.3 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.85–8.73 (br m, 8H), 8.05–8.00 (br m, 6H), 7.75–7.71 (m, 2H), 7.68–7.56 (br m, 5H), 7.30–7.18 (br m, 7H), 3.93 (br, 2H), 3.25–2.78 (br, 6H), 2.31 (s, 3H), 2.26 (s, 3H, minor), 0.93–0.77 (12H).
13C{1H} NMR (100 MHz, CDCl3): δ = 169.2, 169.1, 139.9, 138.6, 138.1, 136.7, 135.4, 135.2, 134.9, 134.6, 132.3, 131.5, 130.5 (2C), 130.2, 129.9, 129.2, 129.1, 128.7 (2C), 128.3, 128.2, 128.0 (2C), 127.9, 127.0 (2C), 126.7 (2C), 125.8, 124.7, 123.5 (2C), 123.3, 122.8, 122.4 (2C), 121.2 (2C), 42.9, 42.4, 38.0, 37.8, 20.6, 20.5, 14.0 (2C), 12.1, 11.8.
FTIR (KBr, cm–1): 2976 (w), 2929 (w), 1622 (s), 1438 (m), 1271 (s), 1220 (m), 1099 (m), 796 (s), 761 (vs).
HRMS (ESI/TOF) m/z: calcd for C30H28ON, 418.2165 [M + H]+; found, 418.2167.
4.3.11. N,N-Diethyl-2-(2-methylnaphthalen-1-yl)chrysene-1-carboxamide (5b)
According to general procedure C, 2a (104 mg, 0.23 mmol), PdCl2(dppe) (7 mg, 5 mol %), BPin 4a (123 mg, 0.46 mmol), and Cs2CO3 (224 mg, 0.69 mmol) were all added in sequence to anhydrous DMF (3 mL) containing 4 Å molecular sieves. After completion of the reaction, standard workup (eluent: 15% EtOAc in heptane) afforded the cross-coupled product 5b (71 mg, 66%) as a brown solid (major/minor rotamers = 83:17).
mp 91.3–94.8 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.94 (d, J = 8.5 Hz, 1H), 8.81 (d, J = 9.0 Hz, 2H), 8.80 (d, J = 9.1 Hz, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.06 (d, J = 9.2 Hz, 1H), 8.04–8.02 (m, 1H), 7.89–7.87 (m, 1H), 7.83 (d, J = 8.4 Hz, 1H), 7.76–7.72 (m, 1H), 7.70 (d, J = 8.6 Hz, 1H), 7.69–7.68 (m, 1H), 7.66–7.63 (m, 1H, minor), 7.48 (d, J = 8.4 Hz, 1H), 7.42–7.39 (m, 2H), 7.31–7.27 (m, 1H), 3.79–3.74 (m, 1H), 3.21–3.16 (m, 1H), 2.84–2.79 (m, 2H), 2.43 (s, 3H), 2.34 (s, 3H, minor), 0.92 (app t, J = 7.0 Hz, 3H, minor), 0.71 (t, J = 7.1 Hz, 3H), 0.39 (t, J = 7.1 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3, major isomer): δ = 168.7, 136.4, 135.9, 135.0, 134.7, 132.6, 132.4, 131.8, 130.5, 130.1, 129.3, 129.1, 128.7, 128.4, 128.3, 128.2, 127.9, 127.89, 127.8, 127.0, 126.7, 126.0, 125.4, 124.6, 124.5, 123.4, 123.3, 122.4, 121.2, 42.9, 37.3, 21.5, 13.9, 11.4.
FTIR (KBr, cm–1): 2925 (w), 1630 (s), 1439 (m), 1273 (m), 1097 (m), 812 (s), 752 (s).
HRMS (ESI/TOF) m/z: calcd for C34H30ON, 468.2322 [M + H]+; found, 468.2322.
4.3.12. N,N-Diethyl-2-(1-methylnaphthalen-2-yl)chrysene-1-carboxamide (5c)
According to general procedure C, 2a (94 mg, 0.21 mmol), PdCl2(dppe) (6 mg, 5 mol %), BPin 4b (111 mg, 0.42 mmol), and Cs2CO3 (205 mg, 0.63 mmol) were all added in sequence to anhydrous DMF (3 mL) containing 4 Å molecular sieves. After completion of the reaction, standard workup (eluent: 15% EtOAc in heptane) afforded the cross-coupled product 5c (97 mg, quant) as a red solid (major/minor rotamers = 77:13).
mp 194.5–198.1 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.88 (d, J = 7.9 Hz, 1H, minor), 8.86 (d, J = 8.6 Hz, 1H), 8.81 (app dd, J = 3.2, 9.6 Hz, 2H), 8.77 (d, J = 9.2 Hz, 1H), 8.13–8.10 (m, 2H), 8.05 (d, J = 9.1 Hz, 1H), 8.02 (br d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.3 Hz, 1H), 7.80 (d, J = 8.6 Hz, 1H), 7.78 (d, J = 7.00 Hz, 1H), 7.76–7.72 (m, 1H, major, 1H minor), 7.69 (d, J = 8.6 Hz, 1H, minor), 7.69–7.65 (m, 1H, major, 1H, minor), 7.58–7.52 (m, 1H, major, 1H, minor), 7.47–7.42 (m, 2H, major, 2H, minor), 7.36 (dd, J = 0.6, 7.2 Hz, 1H, minor), 7.29 (d, J = 7.1 Hz, 1H, minor), 3.81–3.74 (m, 1H, major, 1H, minor), 3.36–3.27 (m, 1H, minor), 3.13–3.00 (m, 2H), 2.96–2.88 (m, 2H, minor), 2.78 (s, 3H, major), 2.77 (s, 3H, minor), 2.50–2.41 (m, 1H), 0.93 (t, J = 7.1 Hz, 3H, minor), 0.71 (t, 3H, J = 7.1 Hz, 3H), 0.54 (t, J = 7.1 Hz, 3H), 0.52 (t, J = 7.1 Hz, 1H, minor).
13C{1H} NMR (100 MHz, CDCl3): δ = 169.2 (major), 168.8 (minor), 136.6, 135.7, 135.6, 135.4, 134.8, 134.6, 134.5, 132.9, 132.6, 132.5, 132.4, 131.9, 130.5, 130.2, 130.1, 130.06, 129.6, 129.3, 128.7, 128.67 (2C), 128.5, 128.4, 128.3, 128.27, 127.9, 126.9 (2C), 126.7, 126.3, 126.2, 126.1, 125.7, 125.6, 125.5, 125.2, 124.8, 124.76, 124.7, 123.7, 123.4, 123.3, 122.7, 122.5, 121.2 (2C), 43.0 (minor), 42.7 (major), 38.0 (major), 37.7 (minor), 19.7 (2C), 14.1 (minor), 13.8 (major), 12.0 (major), 11.7 (minor).
FTIR (KBr, cm–1): 2973 (w), 2930 (w), 1626 (s), 1481 (m), 1274 (s), 1097 (m), 806 (vs), 760 (vs).
HRMS (ESI/TOF) m/z: calcd for C34H30ON, 468.2322 [M + H]+; found, 468.2324.
4.3.13. N,N-Diethyl-2-(3-methylnaphthalen-2-yl)chrysene-1-carboxamide (5d)
According to general procedure C, 2a (104 mg, 0.23 mmol), PdCl2(dppe) (7 mg, 5 mol %), BPin 4c (123 mg, 0.46 mmol), and Cs2CO3 (224 mg, 0.69 mmol) were all added in sequence to anhydrous DMF (3 mL) containing 4 Å molecular sieves. After completion of the reaction, standard workup (eluent: 15% EtOAc in heptane) afforded cross-coupled product 5d (87 mg, 81%) as an off-white solid (major/minor rotamers = 51:49). A second experiment using 1.16 g of 2a gave 5d in 58% yield.
mp 208.7–212.4 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.87–8.72 (m, 8H), 8.19 (s, 1H), 8.13–8.01 (m, 6H), 7.94–7.92 (br d, J = 7.5 Hz, 1H), 7.85–7.62 (m, 12H), 7.52–7.45 (m, 4H), 3.81 (br s, 2H), 3.42–3.33 (br m, 1H), 3.17–3.01 (br m, 4H), 2.79–2.72 (br m, 1H), 2.47 (s, 3H, major), 2.44 (s, 3H, minor), 0.98 (br m, 3H), 0.76 (br t, J = 6.6 Hz, 3H), 0.65–0.57 (br m, 6H).
13C{1H} NMR (100 MHz, CDCl3): δ = 169.1 (major), 169.0 (minor), 139.2, 136.9, 136.5, 135.0, 134.8, 133.5, 133.3, 133.1, 132.3 (2C), 131.8, 131.3, 130.6, 130.5, 130.0, 129.3, 129.0, 128.7 (2C), 128.5, 128.4, 128.2, 127.9, 127.85, 127.3, 127.0, 126.9, 126.7, 126.3, 126.0, 125.6, 125.4, 124.8, 124.5, 123.4, 123.3 (2C), 122.8, 122.5, 121.2, 42.9, 42.6, 38.2, 37.7, 21.1, 14.0 (2C), 11.9, 11.7.
FTIR (KBr, cm–1): 2969 (w), 2927 (w), 1628 (vs), 1469 (m), 1422 (m), 1276 (s), 1097 (m), 798 (s), 744 (vs).
HRMS (ESI/TOF) m/z: calcd for C34H30ON, 468.2322 [M + H]+; found, 468.2325.
4.3.14. N,N-Diethyl-2-(o-tolyl)chrysene-3-carboxamide (5e)
Compound 2b (65 mg, 0.14 mmol), PdCl2(dppf) (6 mg, 5 mol %), o-tolyl boronic acid (24 mg, 0.17 mmol), and Na2CO3 (46 mg, 0.43 mmol) were all added in sequence to DME (2 mL) and then H2O (1 mL). The reaction mixture was stirred at 85 °C for 17 h. After completion of the reaction, workup according to standard procedure B (eluent: 20% EtOAc in heptane) afforded the cross-coupled product 5e (46 mg, 77%) as an off-white solid with a mixture of rotamers.
mp 195.4–199.0 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.80 (s, 1H), 8.79 (d, J = 8.3 Hz, 1H), 8.78 (d, J = 9.1 Hz, 1H), 8.72 (d, J = 9.1 Hz, 1H), 8.04 (d, J = 9.1 Hz, 1H), 8.02 (app d, J = 1.3, 8.0 Hz, 1H), 8.00 (d, J = 8.9 Hz, 1H), 7.89 (s, 1H), 7.75–7.71 (m, 1H), 7.68–7.64 (m, 1H), 7.31–7.24 (br, 4H), 3.89 (br s, 1H), 3.19–2.90 (br, 3H), 2.33 (br s, 3H), 0.95 (br s, 3H), 0.77 (br s, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 170.4, 132.4, 130.6, 130.3, 129.6, 128.8 (2C), 128.6, 128.2, 127.94, 127.9 (2C), 127.0 (2C), 126.9 (2C), 126.7, 123.3 (2C), 122.4, 121.2, 42.6 (2C), 38.2 (2C), 20.5, 13.9 (2C), 11.9 (2C).
FTIR (KBr, cm–1): 2969 (w), 2930 (w), 1637 (vs), 1470 (s), 1433 (s), 1281 (s), 1096 (s), 1081 (m), 822 (m), 813 (s), 753 (vs).
HRMS (ESI/TOF) m/z: calcd for C30H28ON, 418.2165 [M + H]+; found, 418.2167.
4.3.15. N,N-Diethyl-2-(2-methylnaphthalen-1-yl)chrysene-3-carboxamide (5f)
According to general procedure C, 2b (106 mg, 0.22 mmol), PdCl2(dppe) (7 mg, 5 mol %), BPin 4a (118 mg, 0.44 mmol), and Cs2CO3 (216 mg, 0.66 mmol) were all added in sequence to anhydrous DMF (3 mL) containing 4 Å molecular sieves. After completion of the reaction, standard workup (eluent: 20% EtOAc in heptane) afforded the cross-coupled product 5f (48 mg, 46%) as a pale brown solid with a mixture of rotamers.
mp 237.4–240.8 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.87 (s, 1H), 8.82 (d, J = 8.9 Hz, 2H), 8.75 (d, J = 9.1 Hz, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.04 (d, J = 8.5 Hz, 2H), 7.99 (s, 1H), 7.86 (d, J = 8.2 Hz, 1H), 7.83 (d, J = 8.5 Hz, 1H), 7.77–7.73 (m, 1H), 7.70–7.66 (m, 1H), 7.47 (br d, J = 8.4 Hz, 2H), 7.42–7.38 (m, 1H), 7.33–7.29 (br m, 1H), 3.65–2.49 (br m, 4H), 2.41 (s, 3H), 0.94 (s, 3H), 0.36 (br t, J = 6.9 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 169.8, 137.0, 135.3, 135.0, 132.8, 132.4, 132.1, 131.9, 130.9, 130.6, 129.6, 128.8, 128.7, 128.2, 128.0, 127.8, 127.1, 127.0, 126.7, 125.5, 124.6, 123.3, 122.4, 121.1, 43.1, 37.9, 21.4, 14.0, 11.4.
FTIR (KBr, cm–1): 2969 (w), 2929 (w), 1629 (vs), 1467 (m), 1425 (s), 1282 (s), 1067 (m), 818 (s), 749 (s).
HRMS (ESI/TOF) m/z: calcd for C34H30ON, 468.2322 [M + H]+; found, 468.2322.
4.3.16. N,N-Diethyl-2-(1-methylnaphthalen-2-yl)chrysene-3-carboxamide (5g)
According to general procedure C, 2b (107 mg, 0.24 mmol), PdCl2(dppe) (7 mg, 5 mol %), BPin 4b (127 mg, 0.47 mmol), and Cs2CO3 (231 mg, 0.71 mmol) were all added in sequence to anhydrous DMF (3 mL) containing 4 Å molecular sieves. After completion of the reaction, standard workup (eluent: 20% EtOAc in heptane) afforded the cross-coupled product 5g (103 mg, 93%) as a brown solid with a mixture of rotamers.
mp 228.0–230.0 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 8.92 (s, 1H), 8.77 (app dd, J = 2.6, 9.1 Hz, 3H), 8.16–7.82 (br m, 3H), 8.05 (br d, J = 9.1 Hz, 1H), 8.02 (br d, J = 7.9 Hz, 1H), 8.00 (br d, J = 9.1 Hz, 1H), 7.74–7.64 (br m, 3H), 7.59–7.55 (br m, 1H), 7.49–7.27 (br m, 3H), 3.76 (br s, 1H), 3.31–3.16 (br, 1H), 2.89–2.46 (br, 2H), 2.79 (s, 3H), 1.01–0.37 (br m, 6H).
13C{1H} NMR (100 MHz, CDCl3): δ = 170.3, 137.1, 136.3, 135.7, 134.9, 134.4, 132.9, 132.5, 132.4, 131.8, 131.5, 131.3, 130.5, 130.2, 129.6, 129.1, 128.7, 128.6, 128.1, 127.9, 127.0, 126.9, 126.6, 126.2, 126.0, 125.7, 125.5, 124.8, 124.2, 123.8, 123.2, 122.4, 121.9, 121.5, 121.2, 42.8, 38.1, 19.7, 13.6, 11.8.
FTIR (KBr, cm–1): 2975 (w), 1618 (m), 1426 (m), 1275 (m), 767 (s), 748 (vs).
HRMS (ESI/TOF) m/z: calcd for C34H30ON, 468.2322 [M + H]+; found, 468.2324.
4.3.17. N,N-Diethyl-2-(3-methylnaphthalen-2-yl)chrysene-3-carboxamide (5h)
According to general procedure C, 2b (83 mg, 0.18 mmol), PdCl2(dppe) (5 mg, 5 mol %), BPin 4c (98 mg, 0.37 mmol), and Cs2CO3 (179 mg, 0.55 mmol) were all added in sequence to anhydrous DMF (3 mL) containing 4 Å molecular sieves. After completion of the reaction, standard workup (eluent: 20% EtOAc in heptane) afforded the cross-coupled product 5h (69 mg, 81%) as a pale brown solid with a mixture of rotamers.
mp 225.4–230.8 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3, major isomer): δ = 8.86 (br s, 1H), 8.78 (d, J = 8.3 Hz, 1H), 8.77 (d, J = 9.2 Hz, 1H), 8.75 (d, J = 9.1 Hz, 1H), 8.05 (d, J = 9.0 Hz, 1H), 8.03–7.99 (m, 2H), 7.97 (s, 1H), 7.83 (br d, J = 7.8 Hz, 2H), 7.79 (s, 1H), 7.75–7.71 (m, 1H), 7.68–7.65 (m, 1H), 7.52–7.44 (m, 2H), 3.77–2.92 (br m, 4H), 2.50 (s, 3H), 0.96 (br s, 3H), 0.57 (t, J = 7.1 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 170.3, 136.3, 133.3, 132.4, 131.8, 131.5, 130.5, 130.0, 129.6, 128.7 (2C), 128.6, 128.1, 127.9 (2C), 127.0 (2C), 126.9 (2C), 126.7, 126.2, 125.5, 123.2, 122.4, 121.1, 42.7, 38.2, 21.1, 13.9, 11.8.
FTIR (KBr, cm–1): 2974 (w), 1634 (s), 1624 (s), 1424 (s), 1285 (m), 1094 (m), 1068 (m), 889 (s), 811 (s), 748 (vs).
HRMS (ESI/TOF) m/z: calcd for C34H30ON, 468.2322 [M + H]+; found, 468.2324.
4.3.18. N,N-Diethyl-2-(2-methylnaphthalen-1-yl)benzamide (5i)
According to general procedure C, 2d (145 mg, 0.48 mmol), Pd2(dba)3 (22 mg, 5 mol %), SPhos (39 mg, 20 mol %), BPin 4a (154 mg, 0.57 mmol), and Cs2CO3 (468 mg, 1.44 mmol) were all added in sequence to anhydrous DMF (4 mL) containing 4 Å molecular sieves. After completion of the reaction (17 h), standard workup (eluent: 20% EtOAc in heptane) afforded the cross-coupled product 5i (118 mg, 78%) as a red solid of a mixture of rotamers. A second experiment using 1.32 g of 2d gave 5i in 60% yield.
Characterization data were in accordance with the literature.23
mp 137.8–138.8 °C (DCM).
1H NMR (400 MHz, CDCl3): δ = 7.80 (d, J = 7.5 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.54–7.43 (m, 3H), 7.39–7.28 (m, 5H), 3.47–2.60 (br, 4H), 2.31 (s, 3H), 0.86 (br s, 3H), 0.27 (t, J = 6.9 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3): δ = 169.6, 138.1, 137.1, 135.3, 132.6, 131.8, 131.3, 128.7 (2C), 127.9, 127.5 (2C), 126.4, 125.4, 124.6, 42.9, 37.7, 21.3, 13.9, 11.4.
FTIR (KBr, cm–1): 2972 (m), 1627 (vs), 1459 (vs), 1432 (vs), 1290 (vs), 1081 (s), 826 (vs), 783 (vs), 767 (vs).
HRMS (ESI/TOF) m/z: calcd for C22H23ONNa, 340.1672 [M + Na]+; found, 340.1675.
4.3.19. N,N-Diethyl-2-(1-methylnaphthalen-2-yl)benzamide (5j)
According to general procedure C, 2d (1.33 g, 4.39 mmol), Pd2(dba)3 (200 mg, 5 mol %), SPhos (360 mg, 20 mol %), BPin 4b (1.42 g, 5.28 mmol), and Cs2CO3 (4.30 g, 13.2 mmol) were all added in sequence to anhydrous DMF (40 mL) containing 4 Å molecular sieves. After completion of the reaction (17 h), standard workup (eluent: 20% EtOAc in heptane) afforded the cross-coupled product 5j (1.389 g, quant) as a pale red oil as a mixture of rotamers (solidified slowly to a red solid).
Characterization data were in accordance with the literature.25
mp 89.3–91.7 °C (DCM).
HRMS (ESI/TOF) m/z: calcd for C22H23ONNa, 340.1672 [M + H]+; found, 340.1675.
4.3.20. (Benzo[c]picen-7-yloxy)(tert-butyl)dimethylsilane (6a)
Compound 5a (94 mg, 0.23 mmol) in anhydrous THF (3 mL) was added to freshly prepared LDA (0.56 mmol, 0.56 M in anhydrous THF) at 0 °C and stirred for 30 min. The reaction mixture was then stirred at RT for 1 h; TBDMSCl (0.56 mL, 0.56 mmol, 1 M in THF) was added and left to react at RT for 17 h and subsequently quenched with satd aq NH4Cl solution (10 mL). The product had poor solubility and was hence extracted with toluene (3 × 10 mL). The combined organic layer was washed with brine (10 mL), dried over anhydrous MgSO4, and evaporated to dryness under reduced pressure. The crude product was washed with acetone to obtain the pure TBDMS-protected product 6a (70 mg, 63%) as an off-white solid.
mp 261.0–263.0 °C (acetone).
1H NMR (400 MHz, C2D2Cl4): δ = 9.99 (d, J = 9.6 Hz. 1H), 8.97 (d, J = 9.4, 1H), 8.89 (d, J = 9.5 Hz, 1H), 8.84 (d, J = 8.3 Hz, 1H), 8.79 (d, J = 9.5 Hz, 1H), 8.77 (d, J = 10.0 Hz, 1H), 8.71–8.69 (m, 1H), 8.00–7.96 (m, 2H), 7.81–7.79 (m, 1H), 7.72–7.68 (m, 1H), 7.64–7.60 (m, 1H), 7.57–7.53 (m, 2H), 7.35 (s, 1H), 1.08 (s, 9H), 0.28 (s, 6H).
13C{1H} NMR (100 MHz, C2D2Cl4): δ = 152.1, 132.5, 131.8, 130.8, 130.0, 129.4, 129.1, 128.5, 127.9, 127.7, 127.65, 127.1, 126.9, 126.7, 126.67 (2C), 126.4, 124.7, 124.0, 123.3, 123.1, 122.6, 121.8, 121.7, 120.1, 114.7, 26.1 (3C), 18.6, −3.8 (2C).
FTIR (KBr, cm–1): 2957 (w), 2927 (w), 1615 (w), 1441 (m), 1284 (m), 1252 (m), 1104 (s), 837 (vs), 762 (vs).
UV–vis (CHCl3): λmax = 298 nm.
Fluorescence (CHCl3): λex = 297 nm; λem = 396 nm.
HRMS (ESI/TOF) m/z: calcd for C32H30OSi, 458.2060 [M]+; found, 458.2059.
4.3.21. tert-Butyl(dibenzo[a,m]picen-17-yloxy)dimethylsilane (6b)
Following general procedure E, LDA (0.53 mmol, 0.53 M in anhydrous C6H6) was added to solution of 5b (71 mg, 0.15 mmol) in anhydrous C6H6 (2 mL) at 40 °C. The reaction mixture was then stirred for 2 h before adding TBDMSCl (0.53 mL, 0.53 mmol, 1 M in THF) at 40 °C and left to react for 16 h at the same temperature. Standard workup (eluent: 5% EtOAc in heptane) afforded the TBDMS-protected product 6b (66 mg, 85%) as a red solid.
mp 236.6–238.2 °C (DCM).
1H NMR (400 MHz, CDCl3): δ = 10.01 (d, J = 9.5 Hz, 1H), 9.20 (d, J = 9.4 Hz, 1H), 9.03 (d, J = 8.4 Hz, 1H), 8.92 (d, J = 8.3 Hz, 1H), 8.88 (d, J = 9.5 Hz, 1H), 8.87 (d, J = 9.6 Hz, 1H), 8.83 (d, J = 9.2 Hz, 1H), 8.02 (app d, J = 9.0 Hz, 3H), 7.90 (app d, J = 8.6 Hz, 1H), 7.78–7.60 (m, 5H), 7.48 (s, 1H), 1.20 (s, 9H), 0.40 (s, 6H).
13C{1H} NMR (100 MHz, CDCl3): δ = 152.6, 132.9, 132.1, 131.6, 131.5, 130.5, 130.3, 129.1, 129.0, 128.7, 128.6, 128.4, 128.1, 128.1, 127.9, 127.8, 127.4, 127.2, 126.7, 126.6, 126.3, 125.8, 125.4, 124.9, 123.4, 123.0, 122.0, 121.3, 120.0, 115.4, 26.4 (3C), 18.9, −3.5 (2C).
FTIR (KBr, cm–1): 2926 (w), 2856 (w), 1596 (s), 1532 (m), 1424 (s), 1361 (s), 1259 (vs), 1199 (s), 1105 (vs), 1061 (s), 838 (vs), 781 (vs).
UV–vis (CHCl3): λmax = 317 nm.
Fluorescence (CHCl3): λex = 317 nm; λem = 416 nm.
HRMS (ESI/TOF) m/z: calcd for C36H33OSi, 509.2295 [M + H]+; found, 509.2281.
4.3.22. tert-Butyl(dibenzo[b,m]picen-7-yloxy)dimethylsilane (6d)
Following general procedure D, LDA (0.46 mmol, 0.46 M in anhydrous THF) was added to solution of 5d (71 mg, 0.15 mmol) in anhydrous THF (2 mL). After reaction with TBDMSCl (0.47 mL, 0.47 mmol, 1 M in THF), standard workup (eluent: 30% DCM in heptane) afforded the TBDMS-protected product 6d (77 mg, quant) as a brown solid.
mp 310.4–312.7 °C (DCM).
1H NMR (400 MHz, C2D2Cl4): δ = 9.95 (d, J = 9.7 Hz, 1H), 9.21 (s, 1H), 9.08 (d, J = 9.5, 1H), 9.02 (d, J = 9.3 Hz, 1H), 8.84 (d, J = 8.4 Hz, 1H), 8.80 (d, J = 9.3 Hz, 1H), 8.76 (d, J = 9.8 Hz, 1H), 8.27 (s, 1H), 8.11 (d, J = 7.5 Hz, 1H), 8.01–7.97 (m, 3H), 7.72–7.68 (m, 1H), 7.64–7.61 (m, 1H), 7.52–7.45 (m, 2H), 7.40 (s, 1H), 1.08 (s, 9H), 0.31 (s, 6H).
13C{1H} NMR (100 MHz, C2D2Cl4): δ = 151.9, 132.1, 131.8, 131.0, 130.9, 130.6, 129.94, 129.9, 129.1, 128.6, 128.5, 127.9, 127.8, 127.7, 127.1, 127.08, 126.8, 126.7, 126.1, 124.9, 124.8, 123.8, 123.3, 122.9, 122.2, 122.0, 121.7, 120.2, 113.8, 99.4, 26.1 (3C), 18.6, −3.8 (2C).
FTIR (KBr, cm–1): 2928 (w), 2857 (w), 1617 (s), 1440 (s), 1261 (vs), 1220 (vs), 1167 (s), 1107 (s), 878 (s), 849 (vs), 814 (s), 780 (s).
UV–vis (CHCl3): λmax = 318 nm.
Fluorescence (CHCl3): λex = 316 nm; λem = 434 nm.
HRMS (ESI/TOF) m/z: calcd for C36H33OSi, 509.2295 [M + H]+; found, 509.2286.
4.3.23. tert-Butyl(dibenzo[c,k]tetraphen-13-yloxy)dimethylsilane (6e)
According to general procedure D, 5e (46 mg, 0.11 mmol) in anhydrous THF (2 mL) was added to LDA (0.28 mmol, 0.28 M in anhydrous THF) at 0 °C and stirred for 30 min. After reaction with TBDMSCl (0.28 mL, 0.28 mmol, 1 M in THF), standard workup (eluent: 10% EtOAc in heptane) afforded the protected product 6e (34 mg, 68%) as a brown solid.
mp 227.4–229.1 °C (EtOAc).
1H NMR (400 MHz, CDCl3): δ = 9.73 (s, 1H), 9.19 (s, 1H), 8.89 (d, J = 9.0 Hz, 1H), 8.79 (d, J = 9.3 Hz, 1H), 8.78 (d, J = 8.12 Hz, 1H), 8.72 (d, J = 9.2 Hz, 1H), 8.20 (d, J = 9.2 Hz, 1H), 8.10 (d, J = 9.0 Hz, 1H), 8.03 (dd, J = 0.7, 8.0 Hz, 1H), 7.78–7.76 (m, 1H), 7.75–7.70 (m, 1H), 7.67–7.63 (m, 1H), 7.62–7.56 (m, 2H), 7.09 (s, 1H), 1.30 (s, 9H), 0.45 (s, 6H).
13C{1H} NMR (100 MHz, CDCl3): δ = 150.1, 133.2, 132.5, 131.0, 130.8, 130.4, 129.1, 128.7, 128.68, 128.2, 128.0, 127.9, 127.8, 127.4, 127.36, 127.2, 126.9, 126.5, 124.9, 123.3, 122.9, 122.3, 121.8, 121.4, 117.9, 110.8, 26.3 (3C), 18.8, −3.9 (2C).
FTIR (KBr, cm–1): 2956 (w), 2926 (w), 1613 (s), 1553 (s), 1455 (vs), 1311 (vs), 1252 (s), 1190 (s), 1104 (vs), 890 (s), 835 (vs), 812 (vs), 780 (vs), 750 (vs).
UV–vis (CHCl3): λmax = 311 nm.
Fluorescence (CHCl3): λex = 412 nm; λem = 311 nm.
HRMS (ESI/TOF) m/z: calcd for C32H31OSi, 459.2139 [M + H]+; found 459.2133.
4.3.24. (Benzo[a]naphtho[2,1-k]tetraphen-15-yloxy)(tert-butyl)dimethylsilane (6f)
According to general procedure E, 5f (26 mg, 0.06 mmol) in anhydrous C6H6 (1 mL), LDA (0.2 mmol. 0.2 M in anhydrous C6H6), and TBDMSCl (0.2 mL, 0.2 mmol, 1 M in THF) gave, after completed reaction and standard workup (eluent: 5% EtOAc in heptane), the TBDMS-protected product 6f (14 mg, 50%) as a red solid along with traces of the unprotected product.
mp 220.1–223.5 °C (cyclohexane + DCM).
1H NMR (400 MHz, CDCl3): δ = 9.85 (s, 1H), 9.71 (s, 1H), 9.28 (d, J = 8.5 Hz, 1H), 8.94 (d, J = 9.1 Hz, 1H), 8.82 (d, J = 8.3 Hz, 1H), 8.77 (d, J = 9.2 Hz, 1H), 8.26 (d, J = 9.2 Hz, 1H), 8.14 (d, J = 8.9 Hz, 1H), 8.05 (d, J = 8.0 Hz, 2H), 7.94 (d, J = 8.4 Hz, 1H), 7.79–7.73 (m, 3H), 7.69–7.60 (m, 2H), 7.20 (s, 1H), 1.31 (s, 9H), 0.48 (s, 6H).
13C{1H} NMR (100 MHz, CDCl3): δ = 150.5, 132.8, 132.6, 132.0, 130.8, 130.78, 130.7, 130.3, 128.9, 128.8 (2C), 128.5, 128.4, 128.3, 128.2, 128.1, 127.9, 127.8, 127.2, 126.9, 126.8, 126.5 (2C), 125.1, 123.4, 122.8, 121.8, 121.5, 117.6, 111.5, 26.3 (3C), 18.8, −3.9 (2C).
FTIR (KBr, cm–1): 2953 (w), 2925 (w), 1596 (s), 1422 (m), 1257 (s), 1104 (s), 849 (vs), 750 (s).
UV–vis (CHCl3): λmax = 330 nm.
Fluorescence (CHCl3): λex = 329 nm; λem = 428 nm.
HRMS (ESI/TOF) m/z: calcd for C36H33OSi, 509.2295 [M + H]+; found, 509.2289.
4.3.25. tert-Butyldimethyl(naphtho[1,2-c]pentaphen-8-yloxy)silane (6h)
Following general procedure D, LDA (0.51 mmol, 0.51 M in anhydrous THF), 5h (80 mg, 0.17 mmol) in anhydrous THF (3 mL), and TBDMSCl (0.56 mL, 0.56 mmol, 1 M in THF) gave, after completed reaction and standard workup (eluent: 30% DCM in heptane), the protected product 6h (48 mg, 54%) as a brown solid.
mp 246.3–248.1 °C (EtOAc).
1H NMR (400 MHz, CDCl3): δ = 9.63 (s, 1H), 9.27 (s, 1H), 9.20 (s, 1H), 8.85 (d, J = 9.0 Hz, 1H), 8.79 (d, J = 8.40 Hz, 1H), 8.76 (d, J = 9.2 Hz, 1H), 8.24 (d, J = 9.1 Hz, 1H), 8.14 (s, 1H), 8.14–8.12 (m, 1H), 8.08 (d, J = 9.0 Hz, 1H), 8.01 (dd, J = 0.9, 8.0 Hz, 1H), 7.99–7.97 (m, 1H), 7.75–7.70 (m, 1H), 7.67–7.63 (m, 1H), 7.57–7.50 (m, 2H), 7.09 (s, 1H), 1.30 (s, 9H), 0.47 (s, 6H).
13C{1H} NMR (100 MHz, CDCl3): δ = 149.9, 132.8, 132.5, 131.8, 131.2, 131.1, 130.8, 130.5, 129.4, 128.7, 128.63, 128.6, 128.5, 128.4, 127.8, 127.8, 127.3, 126.9, 126.7, 126.5, 126.1, 125.1, 124.7, 123.3, 122.6, 121.9, 121.89, 121.3, 117.9, 110.4, 26.3 (3C), 18.8, −3.9 (2C).
FTIR (KBr, cm–1): 2955 (w), 2927 (w), 1620 (vs), 1447 (m), 1341 (m), 1252 (s), 1208 (s), 1098 (vs), 888 (vs), 744 (vs).
UV–vis (CHCl3): λmax = 334 nm.
Fluorescence (CHCl3): λex = 333 nm; λem = 445 nm.
HRMS (ESI/TOF) m/z: calcd for C36H33OSi, 509.2295 [M + H]+; found, 509.2288.
4.3.26. (Benzo[c]phenanthren-5-yloxy)(tert-butyl)dimethylsilane (6ia)
Freshly prepared LDA (0.72 mmol, 0.72 M in anhydrous C6H6) was added to solution of 5i (65 mg, 0.21 mmol) in anhydrous C6H6 (2 mL) at 0 °C and stirred for 30 min. The reaction mixture was then stirred at 40 °C for 1 h before adding TBDMSCl (0.72 mL, 0.72 mmol, 1 M in THF) at RT and left to react for 17 h. Workup similar to standard procedure C (gradient elution 5%–15% EtOAc in heptane) afforded the TBDMS-protected product 6ia (16 mg, 22%) as a red oil and unprotected product 6ib (33 mg, 66%) as a red solid.
1H NMR (400 MHz, CDCl3, 6ia): δ = 9.12 (d, J = 8.4 Hz, 1H), 9.05 (d, J = 8.5 Hz, 1H), 8.45 (dd, J = 1.3, 8.0 Hz, 1H), 7.99 (dd, J = 1.3, 8.0 Hz, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.73–7.70 (m, 1H), 7.71 (d, J = 8.5 Hz, 1H), 7.69–7.63 (m, 3H), 7.59–7.55 (m, 1H), 7.18 (s, 1H), 1.16 (s, 9H), 0.38 (s, 6H).
13C{1H} NMR (100 MHz, CDCl3): δ = 150.3, 132.7, 131.9, 130.5, 129.3, 128.7, 128.0, 127.7, 127.5, 126.5, 126.47, 126.3, 125.6, 125.1, 123.1, 122.9, 111.7, 26.1, 18.7, −4.0.
FTIR (KBr, cm–1): 2956 (w), 2928 (w), 1599 (m), 1254 (m), 1093 (m), 855 (m), 833 (m).
UV–vis (CHCl3): λmax = 290 nm.
Fluorescence (CHCl3): λex = 289 nm; λem = 392 nm.
HRMS (ESI/TOF) m/z: calcd for C24H27OSi, 359.1826 [M + H]+; found, 359.1825.
4.3.27. Benzo[c]phenanthren-5-ol (6ib)
Characterization data were in accordance with the literature.96
mp 100.6–103.5 °C (EtOAc).
1H NMR (400 MHz, CDCl3): δ = 9.14 (d, J = 8.5 Hz, 1H), 9.05 (d, J = 8.5 Hz, 1H), 8.45 (d, J = 7.8 Hz, 1H), 7.99 (dd, J = 1.2, 7.9 Hz, 1H), 7.84 (d, J = 8.4 Hz, 1H), 7.73–7.63 (m, 4H), 7.59–7.55 (m, 1H), 7.09 (br s, 1H), 5.71 (br s, 1H).
13C{1H} NMR (100 MHz, CDCl3): δ = 150.1, 132.6, 131.8, 130.5, 128.7, 128.0, 127.9, 127.4, 126.8, 126.4, 126.1, 126.0, 125.7, 125.0, 122.7, 122.3, 107.4.
FTIR (KBr, cm–1): 3388 (w), 1697 (s), 1674 (s), 1586 (s), 1426 (m), 1276 (m), 1224 (m), 753 (s).
HRMS (ESI) m/z: calcd for C18H11O2, 259.0765 [M – H + O]−; found, 259.0764.
4.3.28. 2-(3-(bis(Trimethylsilyl)methyl)naphthalen-2-yl)-N,N-diethylchrysene-1-carboxamide (7)
Similar to general procedure D, 5d (96 mg, 0.21 mmol) and TMSCl (0.08 mL, 0.64 mmol) in anhydrous THF (5 mL) were treated with LDA (0.62 mmol, 0.62 M in anhydrous THF) for 1.5 h at 0 °C and warmed to RT over 17 h. Standard workup (gradient elution 5%–15% EtOAc in heptane) afforded the disilylated product 7 (77 mg, 61%) as an off-white solid.
mp 223.0–226.5 °C (EtOAc).
1H NMR (400 MHz, CDCl3): δ = 8.84 (d, J = 8.7 Hz, 1H), 8.81 (d, J = 6.7 Hz, 1H), 8.79 (d, J = 8.6 Hz, 2H), 8.08 (d, J = 9.1 Hz, 1H), 8.07 (d, J = 9.3 Hz, 1H), 8.03 (dd, J = 1.0, 7.9 Hz, 1H), 7.81 (d, J = 8.1 Hz, 1H), 7.77–7.66 (m, 4H), 7.64 (s, 1H), 7.55 (s, 1H), 7.50–7.45 (m, 1H), 7.41–7.37 (m, 1H), 3.78–3.69 (m, 1H), 3.47–3.38 (m, 1H), 3.31–3.14 (m, 2H), 2.11 (s, 1H), 1.00–0.94 (m, 6H), 0.13 (s, 9H), 0.10 (s, 9H).
13C{1H} NMR (100 MHz, CDCl3): δ = 168.6, 141.5, 138.9, 136.0, 135.4, 133.2, 132.4, 130.8, 130.5, 129.9, 129.7, 128.7, 128.65, 128.6, 128.4, 128.2, 127.9, 127.4, 127.1, 127.0, 126.98, 126.8, 126.0, 125.0, 124.6, 123.3, 122.3, 122.25, 121.3, 42.6, 37.1, 24.0, 13.8, 12.5, 1.4 (3C), 1.2 (3C).
FTIR (KBr, cm–1): 2951 (w), 2896 (w), 1638 (vs), 1438 (s), 1247 (vs), 1095 (m), 839 (vs), 746 (vs).
HRMS (ESI) m/z: calcd for C40H46ONSi2, 612.3112 [M + H]+; found, 612.3119.
4.3.29. N,N-Diethyl-2-(1-methyl-3-(trimethylsilyl)naphthalen-2-yl)benzamide (8)
Similar to the general procedure D, 5j (123 mg, 0.39 mmol) and TMSCl (0.15 mL, 1.20 mmol) in anhydrous THF (3 mL) were treated with freshly prepared LDA (1.16 mmol, 1.16 M in anhydrous THF) at 0 °C for 1.5 h and then stirred at RT for 16 h. Standard workup (eluent: 30% EtOAc in heptane) afforded product 8 (70 mg, 46%) as a brown amorphous solid.
1H NMR (400 MHz, CDCl3, major rotamer): δ = 8.05 (d, J = 8.4 Hz, 1H), 7.88 (d, J = 8.5 Hz, 1H), 7.73–7.68 (m, 1H), 7.67–7.65 (m, 1H), 7.55–7.53 (m, 1H), 7.53–7.49 (m, 1H), 7.43–7.41 (m, 1H), 7.42 (s, 1H), 7.33–7.30 (m, 1H), 3.71–3.61 (m, 1H), 2.98–2.89 (m, 1H), 2.72 (s, 3H), 2.67–2.58 (m, 1H), 2.42–2.33 (m, 1H), 0.55 (t, J = 7.1 Hz, 3H), 0.36–0.32 (m, 3H), 0.33 (s, 9H).
13C{1H} NMR (100 MHz, CDCl3, major rotamer): δ = 170.6, 142.9, 138.8, 136.0, 135.2, 134.2, 134.1, 133.0, 132.5, 131.8, 128.6, 126.8, 126.3, 126.2, 125.7, 125.4, 124.8, 43.0, 37.9, 19.7, 12.9, 11.9, 0.3 (3C).
FTIR (KBr, cm–1): 2957 (m), 2935 (m), 1633 (vs), 1479 (vs), 1460 (vs), 1440 (vs), 1288 (vs), 1243 (vs), 1138 (s), 1112 (s), 1094 (s), 990 (s), 854 (vs).
HRMS (ESI/TOF) m/z: calcd for C25H32ONSi, 390.2248 [M + H]+; found, 390.2251.
Acknowledgments
The authors gratefully acknowledge Hiwot M. Tiruye at the University of Stavanger for the syntheses of S4 and S6. We would also like to thank Ing. Jostein A. Johansen at the University of Tromsø for providing HRMS analysis of our compounds.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c01097.
Overview of applied literature procedures for starting materials, unsuccessful cross-coupling experiments, additional cross-coupling experiments to make 5i and 5j, and NMR spectra of the compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kumar R.; Gleißner E. H.; Tiu E. G. V.; Yamakoshi Y. C70 as a Photocatalyst for Oxidation of Secondary Benzylamines to Imines. Org. Lett. 2016, 18, 184–187. 10.1021/acs.orglett.5b03194. [DOI] [PubMed] [Google Scholar]
- Karras M.; Dąbrowski M.; Pohl R.; Rybáček J.; Vacek J.; Bednárová L.; Grela K.; Starý I.; Stará I. G.; Schmidt B. Helicenes as Chirality-Inducing Groups in Transition-Metal Catalysis: The First Helically Chiral Olefin Metathesis Catalyst. Chem.—Eur. J. 2018, 24, 10994–10998. 10.1002/chem.201802786. [DOI] [PubMed] [Google Scholar]
- Planells M.; Pizzotti M.; Nichol G. S.; Tessore F.; Robertson N. Effect of torsional twist on 2nd order non-linear optical activity of anthracene and pyrene tricyanofuran derivatives. Phys. Chem. Chem. Phys. 2014, 16, 23404–23411. 10.1039/c4cp03509g. [DOI] [PubMed] [Google Scholar]
- Takaishi K.; Iwachido K.; Takehana R.; Uchiyama M.; Ema T. Evolving Fluorophores into Circularly Polarized Luminophores with a Chiral Naphthalene Tetramer: Proposal of Excimer Chirality Rule for Circularly Polarized Luminescence. J. Am. Chem. Soc. 2019, 141, 6185–6190. 10.1021/jacs.9b02582. [DOI] [PubMed] [Google Scholar]
- Ball M.; Zhong Y.; Wu Y.; Schenck C.; Ng F.; Steigerwald M.; Xiao S.; Nuckolls C. Contorted Polycyclic Aromatics. Acc. Chem. Res. 2015, 48, 267–276. 10.1021/ar500355d. [DOI] [PubMed] [Google Scholar]
- Bonaccorso F.; Sun Z.; Hasan T.; Ferrari A. C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611. 10.1038/nphoton.2010.186. [DOI] [Google Scholar]
- Duong H. M.; Bendikov M.; Steiger D.; Zhang Q.; Sonmez G.; Yamada J.; Wudl F. Efficient Synthesis of a Novel, Twisted and Stable, Electroluminescent “Twistacene”. Org. Lett. 2003, 5, 4433–4436. 10.1021/ol035751v. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Ho M.-T.; Tao Y.-T. Unsymmetrically Extended Polyfused Aromatics Embedding Coronene and Perylene Frameworks: Syntheses and Properties. Org. Lett. 2016, 18, 200–203. 10.1021/acs.orglett.5b03291. [DOI] [PubMed] [Google Scholar]
- Shi L.; Liu Z.; Dong G.; Duan L.; Qiu Y.; Jia J.; Guo W.; Zhao D.; Cui D.; Tao X. Synthesis, Structure, Properties, and Application of a Carbazole-Based Diaza[7]helicene in a Deep-Blue-Emitting OLED. Chem.—Eur. J. 2012, 18, 8092–8099. 10.1002/chem.201200068. [DOI] [PubMed] [Google Scholar]
- Kato K.; Segawa Y.; Scott L. T.; Itami K. Synthesis, Properties, and Packing Structures of Corannulene-Based π-Systems Containing Heptagons. Chem.—Asian J. 2015, 10, 1635–1639. 10.1002/asia.201500560. [DOI] [PubMed] [Google Scholar]
- Stępień M.; Gońka E.; Żyła M.; Sprutta N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. 10.1021/acs.chemrev.6b00076. [DOI] [PubMed] [Google Scholar]
- Itami K.; Yano Y.; Ito H.; Segawa Y. Helically Twisted Tetracene: Synthesis, Crystal Structure, and Photophysical Properties of Hexabenzo[a,c,fg,j,l,op]tetracene. Synlett 2016, 27, 2081–2084. 10.1055/s-0035-1561455. [DOI] [Google Scholar]
- Kotha S.; Ali R.; Panguluri N. R.; Datta A.; Kannaujiya K. K. Synthesis and photophysical properties of star-shaped blue green emitting π-conjugated spirotruxenes. Tetrahedron Lett. 2018, 59, 4080–4085. 10.1016/j.tetlet.2018.10.005. [DOI] [Google Scholar]
- Xue M.; Cao T.; Wang D.; Wu Y.; Yang H.; Dong X.; He J.; Li F.; Chen G. F. Superconductivity above 30 K in alkali-metal-doped hydrocarbon. Sci. Rep. 2012, 2, 389. 10.1038/srep00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Chen S.; Lam J. W. Y.; Lu P.; Zhong Y.; Wong K. S.; Kwok H. S.; Tang B. Z. Creation of highly efficient solid emitter by decorating pyrene core with AIE-active tetraphenylethene peripheries. Chem. Commun. 2010, 46, 2221–2223. 10.1039/b921451h. [DOI] [PubMed] [Google Scholar]
- Ingale S. A.; Seela F. A Ratiometric Fluorescent On–Off Zn2+ Chemosensor Based on a Tripropargylamine Pyrene Azide Click Adduct. J. Org. Chem. 2012, 77, 9352–9356. 10.1021/jo3014319. [DOI] [PubMed] [Google Scholar]
- Crossley D. L.; Kahan R. J.; Endres S.; Warner A. J.; Smith R. A.; Cid J.; Dunsford J. J.; Jones J. E.; Vitorica-Yrezabal I.; Ingleson M. J. A modular route to boron doped PAHs by combining borylative cyclisation and electrophilic C–H borylation. Chem. Sci. 2017, 8, 7969–7977. 10.1039/c7sc02793a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.; Ye Q.; Chi C.; Wu J. Low band gap polycyclic hydrocarbons: from closed-shell near infrared dyes and semiconductors to open-shell radicals. Chem. Soc. Rev. 2012, 41, 7857–7889. 10.1039/c2cs35211g. [DOI] [PubMed] [Google Scholar]
- Omer K. M.; Ku S.-Y.; Wong K.-T.; Bard A. J. Efficient and Stable Blue Electrogenerated Chemiluminescence of Fluorene-Substituted Aromatic Hydrocarbons. Angew. Chem., Int. Ed. 2009, 48, 9300–9303. 10.1002/anie.200904156. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Cao Y.; Colella N. S.; Liang Y.; Brédas J.-L.; Houk K. N.; Briseno A. L. Unconventional, Chemically Stable, and Soluble Two-Dimensional Angular Polycyclic Aromatic Hydrocarbons: From Molecular Design to Device Applications. Acc. Chem. Res. 2015, 48, 500–509. 10.1021/ar500278w. [DOI] [PubMed] [Google Scholar]
- Xia H.; Liu D.; Xu X.; Miao Q. Ambipolar organic semiconductors from electron-accepting cyclopenta-fused anthracene. Chem. Commun. 2013, 49, 4301–4303. 10.1039/c2cc34992b. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Hanifi D.; Alvarez S.; Antonio F.; Pun A.; Klivansky L. M.; Hexemer A.; Ma B.; Liu Y. Charge Transport Anisotropy in n-Type Disk-Shaped Triphenylene-Tris(aroyleneimidazole)s. Org. Lett. 2011, 13, 6528–6531. 10.1021/ol202814y. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Huang D.-C.; Venkateswarlu S.; Tao Y.-T. Nonlinear Polyfused Aromatics with Extended π-Conjugation from Phenanthrotriphenylene, Tetracene, and Pentacene: Syntheses, Crystal Packings, and Properties. J. Org. Chem. 2018, 83, 11614–11622. 10.1021/acs.joc.8b01582. [DOI] [PubMed] [Google Scholar]
- Dorel R.; Echavarren A. M. Strategies for the Synthesis of Higher Acenes. Eur. J. Org. Chem. 2017, 14–24. 10.1002/ejoc.201601129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.; Ge H.; Liu S. H.; Yin J. Arynes in the synthesis of polycyclic aromatic hydrocarbons. RSC Adv. 2013, 3, 22727–22738. 10.1039/c3ra43804j. [DOI] [Google Scholar]
- Mori H.; Chen X.-c.; Chang N.-h.; Hamao S.; Kubozono Y.; Nakajima K.; Nishihara Y. Synthesis of Methoxy-Substituted Picenes: Substitution Position Effect on Their Electronic and Single-Crystal Structures. J. Org. Chem. 2014, 79, 4973–4983. 10.1021/jo500543h. [DOI] [PubMed] [Google Scholar]
- Dorel R.; McGonigal P. R.; Echavarren A. M. Hydroacenes Made Easy by Gold(I) Catalysis. Angew. Chem., Int. Ed. 2016, 55, 11120–11123. 10.1002/anie.201604952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamane V.; Hannen P.; Fürstner A. Synthesis of Phenanthrenes and Polycyclic Heteroarenes by Transition-Metal Catalyzed Cycloisomerization Reactions. Chem.—Eur. J. 2004, 10, 4556–4575. 10.1002/chem.200400220. [DOI] [PubMed] [Google Scholar]
- Thomson P. F.; Parrish D.; Pradhan P.; Lakshman M. K. Modular, Metal-Catalyzed Cycloisomerization Approach to Angularly Fused Polycyclic Aromatic Hydrocarbons and Their Oxidized Derivatives. J. Org. Chem. 2015, 80, 7435–7446. 10.1021/acs.joc.5b00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N.-h.; Chen X.-c.; Nonobe H.; Okuda Y.; Mori H.; Nakajima K.; Nishihara Y. Synthesis of Substituted Picenes through Pd-Catalyzed Cross-Coupling Reaction/Annulation Sequences and Their Physicochemical Properties. Org. Lett. 2013, 15, 3558–3561. 10.1021/ol401375n. [DOI] [PubMed] [Google Scholar]
- Ozaki K.; Kawasumi K.; Shibata M.; Ito H.; Itami K. One-shot K-region-selective annulative π-extension for nanographene synthesis and functionalization. Nat. Commun. 2015, 6, 6251. 10.1038/ncomms7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Britt L. H.; Murphy G. K. Oxidative, Iodoarene-Catalyzed Intramolecular Alkene Arylation for the Synthesis of Polycyclic Aromatic Hydrocarbons. Chem.—Eur. J. 2018, 24, 17002–17005. 10.1002/chem.201804786. [DOI] [PubMed] [Google Scholar]
- Murai M.; Hosokawa N.; Roy D.; Takai K. Bismuth-Catalyzed Synthesis of Polycyclic Aromatic Hydrocarbons (PAHs) with a Phenanthrene Backbone via Cyclization and Aromatization of 2-(2-Arylphenyl)vinyl Ethers. Org. Lett. 2014, 16, 4134–4137. 10.1021/ol5018273. [DOI] [PubMed] [Google Scholar]
- Protti S.; Artioli G. A.; Capitani F.; Marini C.; Dore P.; Postorino P. Preparation of (substituted) picenes via solar light-induced Mallory photocyclization. RSC Adv. 2015, 5, 27470–27475. 10.1039/c5ra02855h. [DOI] [Google Scholar]
- Guijarro A.; Carrera M.; de la Viuda M. 3,3′,5,5′-Tetra-tert-butyl-4,4′-diphenoquinone (DPQ)-Air as a New Organic Photocatalytic System: Use in the Oxidative Photocyclization of Stilbenes to Phenacenes. Synlett 2016, 27, 2783–2787. 10.1055/s-0036-1588598. [DOI] [Google Scholar]
- Mallory F. B.; Mallory C. W.. Photocyclization of Stilbenes and Related Molecules. In Organic Reactions; Wiley, 2005; Vol. 30. [Google Scholar]
- Rajeshkumar V.; Courté M.; Fichou D.; Stuparu M. C. Synthesis and Properties of Large Polycyclic Aromatic Hydrocarbons with Planar and Non-Planar Structural Motifs. Eur. J. Org. Chem. 2016, 6010–6014. 10.1002/ejoc.201601236. [DOI] [Google Scholar]
- Tang M.; Yu Q.; Wang Z.; Zhang C.; Sun B.; Yi Y.; Zhang F.-L. Synthesis of Polycyclic Aromatic Hydrocarbons (PAHs) via a Transient Directing Group. Org. Lett. 2018, 20, 7620–7623. 10.1021/acs.orglett.8b03359. [DOI] [PubMed] [Google Scholar]
- Fu W. C.; Wang Z.; Chan W. T. K.; Lin Z.; Kwong F. Y. Regioselective Synthesis of Polycyclic and Heptagon-embedded Aromatic Compounds through a Versatile π-Extension of Aryl Halides. Angew. Chem., Int. Ed. 2017, 56, 7166–7170. 10.1002/anie.201703551. [DOI] [PubMed] [Google Scholar]
- Peng Q.; Zhang W.; Zhao K.; Du Y.; Feng C.; Wang B. Q.; Fang D. M.; Chen X. Z.; Ni H. L.; Xiang S. K. Amide-Directed Bay-Region Two-Step Annulative π-Extension (APEX) of Biphenyls and Terphenyls with Diaryliodonium Salts: Efficient Access to Polycyclic Aromatic Hydrocarbons. Adv. Synth. Catal. 2020, 362, 206–212. 10.1002/adsc.201901010. [DOI] [Google Scholar]
- Pozo I.; Cobas A.; Peña D.; Guitián E.; Pérez D. 1,7-Naphthodiyne: a new platform for the synthesis of novel, sterically congested PAHs. Chem. Commun. 2016, 52, 5534–5537. 10.1039/c6cc01214k. [DOI] [PubMed] [Google Scholar]
- Pérez D.; Peña D.; Guitián E. Aryne Cycloaddition Reactions in the Synthesis of Large Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2013, 5981–6013. 10.1002/ejoc.201300470. [DOI] [Google Scholar]
- Romero C.; Peña D.; Pérez D.; Guitián E. Palladium-Catalyzed [2 + 2 + 2] Cycloadditions of 3,4-Didehydrophenanthrene and 1,2-Didehydrotriphenylene. J. Org. Chem. 2008, 73, 7996–8000. 10.1021/jo8013947. [DOI] [PubMed] [Google Scholar]
- Stará I. G.; Starý I. Helically Chiral Aromatics: The Synthesis of Helicenes by [2 + 2 + 2] Cycloisomerization of π-Electron Systems. Acc. Chem. Res. 2020, 53, 144–158. 10.1021/acs.accounts.9b00364. [DOI] [PubMed] [Google Scholar]
- Buchta M.; Rybáček J.; Jančařík A.; Kudale A. A.; Buděšínský M.; Chocholoušová J. V.; Vacek J.; Bednárová L.; Císařová I.; Bodwell G. J.; Starý I.; Stará I. G. Chimerical Pyrene-Based [7]Helicenes as Twisted Polycondensed Aromatics. Chem.—Eur. J. 2015, 21, 8910–8917. 10.1002/chem.201500826. [DOI] [PubMed] [Google Scholar]
- Teplý F.; Stará I. G.; Starý I.; Kollárovič A.; Šaman D.; Rulíšek L.; Fiedler P. Synthesis of [5]-, [6]-, and [7]Helicene via Ni(0)- or Co(I)-Catalyzed Isomerization of Aromatic cis,cis-Dienetriynes. J. Am. Chem. Soc. 2002, 124, 9175–9180. 10.1021/ja0259584. [DOI] [PubMed] [Google Scholar]
- Kitamura C.Synthesis of Substituted Oligoacenes via Diels–Alder Reactions and Substituent Effects on Molecular Structure, Packing Arrangement, and Solid-State Optical Properties. In Methods and Applications of Cycloaddition Reactions in Organic Syntheses; Nishiwaki N., Ed.; Wiley, 2014; pp 407–428. [Google Scholar]
- Mannes P. Z.; Onyango E. O.; Gribble G. W. Triple Benzannulation of Naphthalene via a 1,3,6-Naphthotriyne Synthetic Equivalent. Synthesis of Dibenz[a,c]anthracene. J. Org. Chem. 2015, 80, 11189–11192. 10.1021/acs.joc.5b01972. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Xu Z.; Si W.; Oniwa K.; Bao M.; Yamamoto Y.; Jin T. Synthesis of extended polycyclic aromatic hydrocarbons by oxidative tandem spirocyclization and 1,2-aryl migration. Nat. Commun. 2017, 8, 15073. 10.1038/ncomms15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao I.; Shimizu M.; Hiyama T. 9-Stannafluorenes: 1,4-Dimetal Equivalents for Aromatic Annulation by Double Cross-Coupling. Angew. Chem., Int. Ed. 2009, 48, 7573–7576. 10.1002/anie.200903779. [DOI] [PubMed] [Google Scholar]
- Bonifacio M. C.; Robertson C. R.; Jung J.-Y.; King B. T. Polycyclic Aromatic Hydrocarbons by Ring-Closing Metathesis. J. Org. Chem. 2005, 70, 8522–8526. 10.1021/jo051418o. [DOI] [PubMed] [Google Scholar]
- McAtee C. C.; Riehl P. S.; Schindler C. S. Polycyclic Aromatic Hydrocarbons via Iron(III)-Catalyzed Carbonyl–Olefin Metathesis. J. Am. Chem. Soc. 2017, 139, 2960–2963. 10.1021/jacs.7b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K. B.; Rantanen T.; Dörfler T.; Snieckus V. Directed Metalation–Suzuki–Miyaura Cross-Coupling Strategies: Regioselective Synthesis of Hydroxylated 1-Methyl-phenanthrenes. J. Org. Chem. 2015, 80, 9410–9424. 10.1021/acs.joc.5b01300. [DOI] [PubMed] [Google Scholar]
- Böhme T.; Lorentzen M.; Jørgensen K. B. Regiospecific Synthesis of Dimethylphenanthrenes. Polycyclic Aromat. Compd. 2017, 37, 106–113. 10.1080/10406638.2016.1179651. [DOI] [Google Scholar]
- Snieckus V. M.Transformation at Arenes. In Handbook of C–H Transformations; Dyker P. G., Ed.; Wiley, 2005; pp 106–118. [Google Scholar]
- Board J.; Cosman J. L.; Rantanen T.; Singh S. P.; Snieckus V. The Directed ortho Metallation-Cross-Coupling Fusion: Development and Application in Synthesis. Platin. Met. Rev. 2013, 57, 234–258. 10.1595/147106713x672311. [DOI] [Google Scholar]
- Wang X.; Snieckus V. Synthetic strategies based on aromatic metalation - cross coupling links. regiospecific synthesis of the phenanthrene natural product gymnopusin. Tetrahedron Lett. 1991, 32, 4879–4882. 10.1016/s0040-4039(00)93485-3. [DOI] [Google Scholar]
- Tilly D.; Magolan J.; Mortier J. Directed Remote Aromatic Metalations: Mechanisms and Driving Forces. Chem.—Eur. J. 2012, 18, 3804–3820. 10.1002/chem.201103920. [DOI] [PubMed] [Google Scholar]
- Fu J.-m.; Snieckus V. The directed ortho metalation - palladium catalyzed cross coupling connection. A general regiospecific route to 9-phenanthrols and phenanthrenes. Exploratory further metalation. Can. J. Chem. 2000, 78, 905–919. 10.1139/v00-055. [DOI] [Google Scholar]
- Kancherla S.; Lorentzen M.; Snieckus V.; Jørgensen K. B. Directed ortho-Metalation and Anionic ortho-Fries Rearrangement of Polycyclic Aromatic O-Carbamates: Regioselective Synthesis of Substituted Chrysenes. J. Org. Chem. 2018, 83, 3590–3598. 10.1021/acs.joc.7b03210. [DOI] [PubMed] [Google Scholar]
- Whisler M. C.; MacNeil S.; Snieckus V.; Beak P. Beyond Thermodynamic Acidity: A Perspective on the Complex-Induced Proximity Effect (CIPE) in Deprotonation Reactions. Angew. Chem., Int. Ed. 2004, 43, 2206–2225. 10.1002/anie.200300590. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Li C.; Senanayake C. H.; Tang W. An Efficient Method for Sterically Demanding Suzuki–Miyaura Coupling Reactions. Chem.—Eur. J. 2013, 19, 2261–2265. 10.1002/chem.201203898. [DOI] [PubMed] [Google Scholar]
- Lorentzen M.; Kalvet I.; Sauriol F.; Rantanen T.; Jørgensen K. B.; Snieckus V. Atropisomerism in Tertiary Biaryl 2-Amides: A Study of Ar–CO and Ar–Ar′ Rotational Barriers. J. Org. Chem. 2017, 82, 7300–7308. 10.1021/acs.joc.7b00890. [DOI] [PubMed] [Google Scholar]
- Manuscript in preparation. Chrysenecarboxylic acids were prepared photochemically as esters by the Mallory reaction:; a Jørgensen K. B. Photochemical Oxidative Cyclisation of Stilbenes and Stilbenoids—The Mallory-Reaction. Molecules 2010, 15, 4334–4358. 10.3390/molecules15064334. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kancherla S.Regioselective Syntheses and Functionalizations of Polycyclic Aromatic Hydrocarbons: Directed Metalation and C–H Activation. Ph.D. Thesis UiS, no. 484, University of Stavanger, 2019. [Google Scholar]
- Fraser R. R.; Bresse M.; Mansour T. S. Ortho lithiation of monosubstituted benzenes: a quantitative determination of pKa values in tetrahydrofuran. J. Am. Chem. Soc. 1983, 105, 7790–7791. 10.1021/ja00364a078. [DOI] [Google Scholar]
- Snieckus V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 1990, 90, 879–933. 10.1021/cr00104a001. [DOI] [Google Scholar]
- Fraser R. R.; Baignée A.; Bresse M.; Hata K. Lithium dialkylamides. 13C parameters and slow proton transfer. Tetrahedron Lett. 1982, 23, 4195–4198. 10.1016/s0040-4039(00)88702-x. [DOI] [Google Scholar]
- Grimmer N. E.; Coville N. J.; de Koning C. B.; Cook L. M. Zirconium bis-indenyl compounds. Synthesis and X-ray crystallography study of 1- and 2-substituted bis(R-indenyl)zirconium dichloride metallocenes. J. Organomet. Chem. 2000, 616, 112–127. 10.1016/s0022-328x(00)00570-2. [DOI] [Google Scholar]
- Podgoršek A.; Stavber S.; Zupan M.; Iskra J. Environmentally benign electrophilic and radical bromination “on water”: H2O2–HBr system versus N-bromosuccinimide. Tetrahedron 2009, 65, 4429–4439. 10.1016/j.tet.2009.03.034. [DOI] [Google Scholar]
- Motomura T.; Hideko N.; Michinori S.; Masahiro M.; Yoshihiko I. Synthesis and Structural Analysis of Oligo(naphthalene-2,3-diyl)s. Bull. Chem. Soc. Jpn. 2005, 78, 142–146. 10.1246/bcsj.78.142. [DOI] [Google Scholar]
- Pathak R.; Nhlapo J. M.; Govender S.; Michael J. P.; van Otterlo W. A. L.; de Koning C. B. A concise synthesis of novel naphtho[a]carbazoles and benzo[c]carbazoles. Tetrahedron 2006, 62, 2820–2830. 10.1016/j.tet.2006.01.012. [DOI] [Google Scholar]
- Murata M.; Oyama T.; Watanabe S.; Masuda Y. Palladium-Catalyzed Borylation of Aryl Halides or Triflates with Dialkoxyborane: A Novel and Facile Synthetic Route to Arylboronates. J. Org. Chem. 2000, 65, 164–168. 10.1021/jo991337q. [DOI] [PubMed] [Google Scholar]
- van Zeist W.-J.; Visser R.; Bickelhaupt F. M. The Steric Nature of the Bite Angle. Chem.—Eur. J. 2009, 15, 6112–6115. 10.1002/chem.200900367. [DOI] [PubMed] [Google Scholar]
- van Zeist W.-J.; Bickelhaupt F. M. Steric nature of the bite angle. A closer and a broader look. Dalton Trans. 2011, 40, 3028–3038. 10.1039/c0dt01550d. [DOI] [PubMed] [Google Scholar]
- Dierkes P.; van Leeuwen P. W. N. M. The bite angle makes the difference: a practical ligand parameter for diphosphine ligands. J. Chem. Soc., Dalton Trans. 1999, 1519–1530. 10.1039/a807799a. [DOI] [Google Scholar]
- Hayashi T.; Konishi M.; Kobori Y.; Kumada M.; Higuchi T.; Hirotsu K. Dichloro[1,1′-bis(diphenylphosphino)ferrocene]palladium(II): an effective catalyst for cross-coupling of secondary and primary alkyl Grignard and alkylzinc reagents with organic halides. J. Am. Chem. Soc. 1984, 106, 158–163. 10.1021/ja00313a032. [DOI] [Google Scholar]
- Birkholz M.-N.; Freixa Z.; van Leeuwen P. W. N. M. Bite angle effects of diphosphines in C–C and C–X bond forming cross coupling reactions. Chem. Soc. Rev. 2009, 38, 1099–1118. 10.1039/b806211k. [DOI] [PubMed] [Google Scholar]
- Steffen W. L.; Palenik G. J. Crystal and molecular structures of dichloro[bis(diphenylphosphino)methane]palladium(II), dichloro[bis(diphenylphosphino)ethane]palladium(II), and dichloro[1,3-bis(diphenylphosphino)propane]palladium(II). Inorg. Chem. 1976, 15, 2432–2439. 10.1021/ic50164a025. [DOI] [Google Scholar]
- Clayden J.; Yasin S. A. Pathways for decomposition of THF by organolithiums: the role of HMPA. New J. Chem. 2002, 26, 191–192. 10.1039/b109604d. [DOI] [Google Scholar]
- Castanet A.-S.; Tilly D.; Véron J.-B.; Samanta S. S.; De A.; Ganguly T.; Mortier J. Combined directed ortho-metalation, remote metalation, and Stille cross-coupling strategies. Concise stereoselective synthesis of polysubstituted 9H-fluoren-9-ones. Tetrahedron 2008, 64, 3331–3336. 10.1016/j.tet.2008.01.122. [DOI] [Google Scholar]
- Tilly D.; Fu J.-m.; Zhao B.-p.; Alessi M.; Castanet A.-S.; Snieckus V.; Mortier J. On the Mechanism of the Directed ortho and Remote Metalation Reactions of N,N-Dialkylbiphenyl 2-carboxamides. Org. Lett. 2010, 12, 68–71. 10.1021/ol902268h. [DOI] [PubMed] [Google Scholar]
- Nijegorodov N.; Zvolinsky V.; Luhanga P. V. C. Photonics and photochemical stability of aromatic molecules, family related in π-structure but different in planarity, rigidity and molecule symmetry. J. Photochem. Photobiol., A 2008, 196, 219–226. 10.1016/j.jphotochem.2007.12.028. [DOI] [Google Scholar]
- Biet T.; Martin K.; Hankache J.; Hellou N.; Hauser A.; Bürgi T.; Vanthuyne N.; Aharon T.; Caricato M.; Crassous J.; Avarvari N. Triggering Emission with the Helical Turn in Thiadiazole-Helicenes. Chem.—Eur. J. 2017, 23, 437–446. 10.1002/chem.201604471. [DOI] [PubMed] [Google Scholar]
- Katz A.; Carrell H. L.; Glusker J. P. Dibenzo[a,l]pyrene (dibenzo[def,p]chrysene): fjord-region distortions. Carcinogenesis 1998, 19, 1641–1648. 10.1093/carcin/19.9.1641. [DOI] [PubMed] [Google Scholar]
- Lakshman M. K.; Kole P. L.; Chaturvedi S.; Saugier J. H.; Yeh H. J. C.; Glusker J. P.; Carrell H. L.; Katz A. K.; Afshar C. E.; Dashwood W.-M.; Kenniston G.; Baird W. M. Methyl Group-Induced Helicity in 1,4-Dimethylbenzo[c]phenanthrene and Its Metabolites: Synthesis, Physical, and Biological Properties. J. Am. Chem. Soc. 2000, 122, 12629–12636. 10.1021/ja002072w. [DOI] [Google Scholar]
- Banerjee S.; Sinha S.; Pradhan P.; Caruso A.; Liebowitz D.; Parrish D.; Rossi M.; Zajc B. Regiospecifically Fluorinated Polycyclic Aromatic Hydrocarbons via Julia–Kocienski Olefination and Oxidative Photocyclization. Effect of Fluorine Atom Substitution on Molecular Shape. J. Org. Chem. 2016, 81, 3983–3993. 10.1021/acs.joc.5b02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Lin T.; Pan X.; Wang W. Graphene-like Molecules Based on Tetraphenylethene Oligomers: Synthesis, Characterization, and Applications. Chem. Mater. 2014, 26, 4221–4229. 10.1021/cm501590w. [DOI] [Google Scholar]
- Sun Z.; Wu J. Higher Order Acenes and Fused Acenes with Near-infrared Absorption and Emission. Aust. J. Chem. 2011, 64, 519–528. 10.1071/ch11037. [DOI] [Google Scholar]
- Chang N.-h.; Mori H.; Chen X.-c.; Okuda Y.; Okamoto T.; Nishihara Y. Synthesis of Substituted [6]Phenacenes through Suzuki–Miyaura Coupling of Polyhalobenzene with Alkenylboronates and Sequential Intramolecular Cyclization via C–H Bond Activation. Chem. Lett. 2013, 42, 1257–1259. 10.1246/cl.130584. [DOI] [Google Scholar]
- Okamoto H.; Yamaji M.; Gohda S.; Sato K.; Sugino H.; Satake K. Photochemical synthesis and electronic spectra of fulminene ([6]phenacene). Res. Chem. Intermed. 2013, 39, 147–159. 10.1007/s11164-012-0639-1. [DOI] [Google Scholar]
- Snégaroff K.; L’Helgoual’ch J.-M.; Bentabed-Ababsa G.; Nguyen T. T.; Chevallier F.; Yonehara M.; Uchiyama M.; Derdour A.; Mongin F. Deprotonative Metalation of Functionalized Aromatics Using Mixed Lithium–Cadmium, Lithium–Indium, and Lithium–Zinc Species. Chem.—Eur. J. 2009, 15, 10280–10290. 10.1002/chem.200901432. [DOI] [PubMed] [Google Scholar]
- Bedoya J. R. H. S.Pyridyldiamido transition metal complexes, production and use thereof. WO 2012134615 A1, 2012.
- Wang Y.; Lin Z.; Fan H.; Peng X. Photoinduced DNA Interstrand Cross-Link Formation by Naphthalene Boronates via a Carbocation. Chem.—Eur. J. 2016, 22, 10382–10386. 10.1002/chem.201601504. [DOI] [PubMed] [Google Scholar]
- Chen H.; Xu L.-X.; Yan L.-J.; Liu X.-F.; Xu D.-D.; Yu X.-C.; Fan J.-X.; Wu Q.-A.; Luo S.-P. Mononuclear Copper(I) complexes based on phenanthroline derivatives P^N^N^P tetradentate ligands: Syntheses, crystal structure, photochemical properties. Dyes Pigm. 2020, 173, 108000. 10.1016/j.dyepig.2019.108000. [DOI] [Google Scholar]
- Haketa T.; Ito H.; Kudo Y.; Kawamura M.; Takahashi R.. Preparation of aromatic amine compounds for organic electroluminescent elements and electronic apparatus. WO 2016056640 A1, 2016.
- Huang Y.; Chan G. H.; Chiba S. Amide-Directed C–H Sodiation by a Sodium Hydride/Iodide Composite. Angew. Chem., Int. Ed. 2017, 56, 6544–6547. 10.1002/anie.201702512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.