Abstract
Background
Falls are the leading cause of injuries among older adults, and trips and slips are major contributors to falls.
Objective
The authors sought to compare the effectiveness of adding a component of surface perturbation training to usual gait/balance training for reducing falls and fall-related injury in high-risk older adults referred to physical therapy.
Design
This was a multi-center, pragmatic, randomized, comparative effectiveness trial.
Setting
Treatment took place within 8 outpatient physical therapy clinics.
Patients
This study included 506 patients 65+ years of age at high fall risk referred for gait/balance training.
Intervention
This trial evaluated surface perturbation treadmill training integrated into usual multimodal exercise-based balance training at the therapist’s discretion versus usual multimodal exercise-based balance training alone.
Measurements
Falls and injurious falls were assessed with a prospective daily fall diary, which was reviewed via telephone interview every 3 months for 1 year.
A total of 211/253 (83%) patients randomized to perturbation training and 210/253 (83%) randomized to usual treatment provided data at 3-month follow-up. At 3 months, the perturbation training group had a significantly reduced chance of fall-related injury (5.7% versus 13.3%; relative risk 0.43) but no significant reduction in the risk of any fall (28% versus 37%, relative risk 0.78) compared with usual treatment. Time to first injurious fall showed reduced hazard in the first 3 months but no significant reduction when viewed over the entire first year.
Limitations
The limitations of this trial included lack of blinding and variable application of interventions across patients based on pragmatic study design.
Conclusion
The addition of some surface perturbation training to usual physical therapy significantly reduced injurious falls up to 3 months posttreatment. Further study is warranted to determine the optimal frequency, dose, progression, and duration of surface perturbation aimed at training postural responses for this population.
In the United States, each year an estimated one-third of older adults fall.1 In 2014, approximately 27,000 older adults in the United States died because of falls, 2.8 million were treated in emergency departments for fall-related injuries, and approximately 800,000 were hospitalized.2 The estimated number of fall-related injuries and hospitalizations has grown 2% and 4% per year, respectively.3 In the United States, direct medical costs for fall-related injuries in 2015 were an estimated $32 billion;4 total costs were over $111 billion per year.5
Trips and slips are major contributors to falls.6 Modifiable risk factors for falls include muscle weakness, gait and balance problems, poor vision, psychoactive medications, and home hazards.7 Systematic reviews have found that multimodal exercise programs are effective for fall prevention;8,9 evidence-based recommendations call for tailored progressive exercise providing a high level of challenge to balance, mobility training, and lower extremity strength training.9,10 Recently, the recognized importance of task-specific training targeting balance recovery mechanisms and postural responses has led to interest in perturbation-based training for falls reduction among older adults.11–13 Biomechanical studies have suggested that this training improves reactions to postural perturbations in the laboratory, reduces the risk of falling following simulated trips and slips, and can be retained over an extended period.12,14–20 Systematic reviews suggest efficacy for perturbation-based training in reducing falls.11,13 However, these studies were highly variable; many were small (<30 perturbation participants),19,21–25 focused specifically on Parkinson’s disease,22,25,26 investigated only short-term outcomes (<1 month),22,26 or found improvements only in falls designated as “trip-related” rather than all falls.27 Thus, the broad applicability of perturbation training to fall-prevention physical therapy remains unclear.
ActiveStep, a commercially available surface perturbation training device, utilizes a motorized treadmill, safety harness, and customized software to deliver surface perturbations (forward or backward displacements of the treadmill belt) during standing and walking, enabling individuals to safely and repeatedly practice in-place (body sway) and change-in-base of support (stepping) postural responses when unexpectedly perturbed (similar to standing on a bus or train during a sudden start or stop). A pilot study showed that the ActiveStep could be incorporated into a multimodal physical therapist intervention in clinical practice without any increase in the number or length of sessions; patients were generally accepting of randomization between the surface perturbation and usual treatment, and standard measures of fall risk were reduced equally in the 2 groups, suggesting no decrement in the effect of usual treatment by substitution of some of the therapy time for perturbation training.24 A 60% reduction in injurious falls at 3 months in the perturbation training group, although not statistically significant in that small pilot study, formed the premise for the current large pragmatic trial.24 The objective of the current study was to assess the comparative effectiveness of adding access to surface perturbation training to usual multimodal exercise-based balance training for reducing the risk of falls and fall-related injuries in a clinical population of high-risk older adults referred for physical therapy.
Methods
Trial Design
This study was a prospective, multi-center, highly pragmatic, randomized, comparative-effectiveness trial. The call for more pragmatic clinical trials arose out of concerns that many traditional randomized trials did not adequately inform practice because they were optimized to determine efficacy under idealized circumstances with relatively small sample sizes, extremely detailed and inflexible protocols, and highly selected participants, leading to potential overestimation of benefits and underestimation of harms.28 Pragmatic trials are typically designed to determine the effects of an intervention under the usual conditions in which it will be applied, may be better suited to studying complex interventions consisting of several interacting components and involving the skills and experience of health care professionals to deliver them, and can more directly inform a clinical or policy decision, providing more direct evidence for adoption of an intervention into real-world practice.29
Patients completing informed consent were randomly assigned by research assistants at each site in a 1-to-1 ratio using permuted blocks stratified by site and implemented using an online trial management software package to perturbation training or usual treatment. The randomization procedures ensured allocation concealment prior to treatment assignment, but blinding was not possible for the patients or clinicians. Treatment took place within routine clinical care at 8 physical therapist outpatient practices in the northeast United States with established gait/balance training programs. Sites included physical therapist practices based at academic, VA, and community hospitals as well as freestanding facilities. All the involved therapists were experienced in providing multi-modal, exercise-based gait and balance training but were given no additional formal training for the trial. The number and content of therapy sessions, within the confines of the randomized assignments, as well as the duration of treatment were at the discretion of the therapist. Essentially, the study addresses the pragmatic question of whether giving physical therapists access to surface perturbation treadmill training as part of a comprehensive gait/balance training program improves patient outcomes.
Following the treatment phase, study participants received a fall diary, which was prospectively completed and reviewed with study staff who were blinded to treatment arm during a telephone interview every 3 months for 1 year. The co-primary outcome measures for the study were the proportion of patients reporting a fall and the proportion reporting an injurious fall at each follow-up period.
Study Oversight
The trial protocol was approved by local institutional review boards at each site and registered at ClinicalTrials.gov (NCT01006967). All authors approved the submission of the manuscript for publication. Some sites already owned the ActiveStep machines for clinical use, 1 was purchased by the study, and 2 were leased by the study from the manufacturer for use during the trial.
Procedures
The usual treatment group received the usual gait/balance intervention provided to patients at their clinics. Following initial physical therapist evaluation, patients received an individualized multimodal program designed to address their specific impairments, balance deficits, and functional limitations. The typical course of treatment was 2 to 3 sessions per week for 4 to 6 weeks but varied based on individual patient need; typical session duration was 45 minutes. All participants received some common interventions including strengthening and flexibility exercises, static and dynamic balance exercises, mobility training, patient education, and home exercise programs. Home exercises were typically recommended to be done 4 to 5 times/wk or on days the patient did not attend physical therapist clinic. The home program was progressed throughout the course of care and recommended to continue after completion of the in-clinic physical therapy.
The strength and flexibility exercises addressed patient-specific deficits. Common exercises included gastrocnemius/soleus stretching, heel/toe raises, chair sit-to-stand, and resistive exercises for hip abduction and extension and knee extension. Balance exercises were designed to improve postural alignment, use of the senses for postural orientation and stability, use of anticipatory postural adjustments, integration of various sensory and motor strategies for functional balance and mobility, and the development of coordinated postural movement strategies in different environments and tasks. Mobility training focused on improving stability during a variety of walking tasks on various surfaces.
Surface Perturbation Treadmill Training
Each surface perturbation training session consisted of sequential postural disturbances delivered when standing or walking while harnessed on the ActiveStep. The ActiveStep provides 5 levels of perturbations that vary from slower, lower magnitude surface displacements (level 1) to larger and faster surface displacements (level 5). The magnitude of perturbation is defined by 4 parameters: peak velocity, elapsed time to peak velocity, elapsed time during which the peak velocity is maintained, and time required to decelerate the treadmill belt to zero velocity. In general, the level of the perturbations during training sessions progressed from less to more challenging based on the patient’s performance.
Perturbations typically occurred in both forward (trip) and backward (slip) directions, with occasional use of sideways perturbations in individual cases. Patients were either instructed to “try to keep your balance without taking a step” or “try to keep your balance and please take a step if you need to” to train either in-place or change-in-base postural responses. Additionally, perturbations could occur either from stance or while walking on the treadmill; during walking perturbations, patients were instructed to try to maintain or recover balance as best they could and keep walking. The specific conditions and sequence of perturbations were based on the therapist’s assessment of the patient’s needs and their performance on previous trials. At the start of the trial, therapists not already using the device in practice were given an introduction to the device and its use by the manufacturer; no other specific trainings, manuals, or protocols for integrating the device into the treatment program were provided. When used, perturbation training typically lasted for about 15 minutes of the treatment session; other components were adjusted to keep overall session times similar between groups. In the pilot study to test implementation feasibility, the average overall session duration was 44 minutes in the perturbation training group and 43 minutes in the usual treatment group.24 Treatment session length was not monitored during this large pragmatic trial.
Measures
Two main outcome measures were the proportion of participants with a fall and the proportion with a fall-related injury at each 3-month follow-up period out to 1 year. We used definitions from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System.30 Specifically, “By a fall, we mean when a person unintentionally comes to rest on the ground or another lower level” and “By an injury, we mean the fall caused you to limit your regular activities for at least a day or to go see a doctor.” To assist with recall, participants received a diary to prospectively record falls.
Clinical measures collected at the beginning and end of treatment included:
The Timed Up & Go Test (TUG), which measures the time it takes to rise from a seated position, walk 3 m, turn around, walk back to the chair, and sit down31
The Berg Balance Measure (BBS), which consists of 14 functional balance tasks32
The Dynamic Gait Index (DGI), which tests the ability to adapt gait under various conditions33
The Activities-specific Balance Confidence (ABC) Scale, which assesses self-reported confidence in being able to perform activities without losing balance or becoming unsteady34
These instruments are commonly used to assess gait and balance and predict fall risk, and our approach complied with current recommendations to use multiple instruments given the limited predictive value of any single measure.35,36 These measures were obtained by the therapists as part of routine clinical practice and served to identify high-risk patients at baseline for inclusion in the trial, assess similarity between the 2 groups in fall risk at the end of treatment, and assess whether the addition of perturbation-based treatment may have interfered with the effectiveness of the other aspects of the multi-modal treatment. Comorbidities, health history, and medication usage were obtained by chart review and patient self-report.
Study Population
This pragmatic trial purposefully included a diverse clinical population of elders at risk for falls. All patients aged 65 years or older referred for gait/balance physical therapy at participating sites were considered for the study. Patients underwent a baseline fall risk assessment and comprehensive examination by the therapist to determine study eligibility and collect baseline data. To be eligible, participants needed one of the following fall risk factors: fall in last year TUG > 13.5 seconds; or DGI ≤ 19/24; or BBS < 50/56; or ABC < 67%. For patients with Parkinson’s disease, the thresholds differed (TUG ≥ 8 seconds, DGI ≤ 22/24, or BBS < 54/56) due to the lower sensitivity of these instruments for predicting falls in this population.37 Patients with a primary problem related to positional vertigo or those who were not candidates for either treatment due to severe physical limitations were excluded.
Statistical Analysis
All analyses were intent to treat and performed using SAS (SAS Institute Inc., Cary, NC, USA). The primary outcome in the initial protocol in 2009 was the difference in fallers at 2 years. Based on the results of the pilot trial, the primary outcomes and analysis plans were changed in 2013 as described below. The trial registration on ClinicalTrials.gov was found not to have accurately reflected the revised protocol from 2013 and was updated July 2018. Per the final trial protocol, the primary questions of interest were the differences between treatment groups in the occurrence of falls and the occurrence of fall-related injuries after completion of the clinic-based treatment program.38 First, the proportion of patients in each group experiencing a fall and those experiencing an injurious fall at each time point were compared, allowing for direct comparison with the 3-month outcomes of the pilot study. The relative risk of falls between the 2 groups controlling for baseline fall risk assessment scores and site was assessed using generalized linear mixed models (PROC GLIMMIX). Second, survival analysis was used to evaluate for group differences in the likelihood of a posttreatment injurious fall over 1 year. Hazard ratio profile likelihood confidence limits were used to generate robust standard errors of the estimate.39 We also looked at the occurrence of all falls from treatment completion to 1 year follow-up using a zero-inflated negative binomial mixed regression model; the model regressed fall counts on the 2 treatment groups and 4 time intervals (0–3 months, 4–6 months, 6–9 months, and 9–12 months) as fixed effects, and actual fall dates within each interval and the model intercept were specified as random effects.40 For all comparisons, adjusted analyses were performed to account for characteristics associated with loss to follow-up.
Changes from baseline to the end of treatment for the continuous secondary outcomes (TUG, BBS, DGI, and ABC) were analyzed using repeated-measures analysis of variance. Treatment groups were compared based on score differences between baseline and follow-up adjusted for baseline values to evaluate for a treatment-by-time interaction.
Based on availability of data in the literature, initial sample size was calculated on reduction in fallers rather than injurious falls. Using a Cox proportional hazard model with treatment comparisons based on 2-sided testing with Type I error rate set at .05, the form of the survival curve assumed to be exponential, and hazard ratio estimated at 1.33 for standard treatment, a total sample size of around 600 was estimated to achieve a power of .80, and the initial target enrollment was 1000. With the shift in primary outcome to include fall with injury based on the results of the pilot study and slower than expected recruitment, a subsequent bootstrapped sample size calculation was performed in 2013 using simulated data from the first 194 enrollees. This analysis, which did not involve interim comparisons of outcomes in the 2 treatment groups, found that 400 participants should be sufficient to achieve 84% power to assess a difference in injurious falls. The final target enrollment for the trial was 500 to maintain as much power as possible to assess fall risk within an attainable sample size.
Role of the Funding Source
The funding source had no role in the design, data analysis, or interpretation of the results. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Results
Patients
Study enrollment, which began April 2010 and completed June 2014, and follow-up, which completed July 2015, is summarized in Figure 1. Of 1507 patients screened, 898 (60%) were eligible. Of these, 291 did not want to participate in a research study, 55 had a strong preference for 1 of the 2 treatments, and 91 reported other reasons for not participating; 506 (56%) were randomized: 253 to perturbation training and 253 to usual treatment. Eight patients in each group withdrew prior to treatment with 2 deaths prior to treatment in the perturbation training group, highlighting the frailty of this population.
Figure 1.
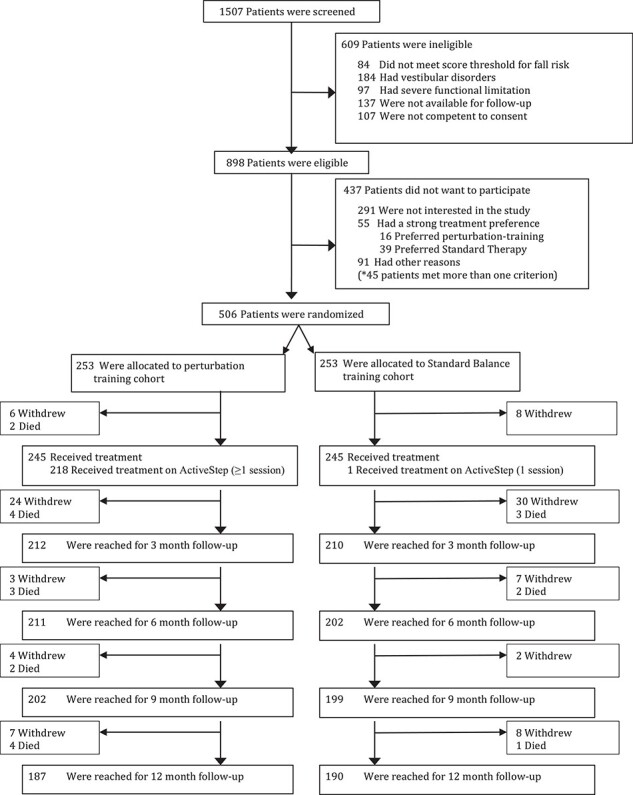
CONSORT diagram.
In the perturbation training group, 222 (88%) received at least 1 treadmill session; 1 patient in the usual treatment group received 1 treadmill session by mistake. The average number of treatment sessions was 10.4 (median 9; min 1; max 80; 1st–3rd quartiles: 6–13) and 10.4 (median 9; min 1; max 50; 1st–3rd quartiles: 6–13) in the perturbation training and usual treatment groups, respectively. In the perturbation training group, the average number of sessions utilizing the treadmill was 4.7 (SD 3.3; range 1–25).
Three-month follow-up was achieved on 212/245 (87%) of the treated patients in the perturbation training group and 210/245 (86%) in the usual treatment group. A total of 9/506 randomized patients (1.78%) died before the 3-month follow-up, again highlighting the frailty of this population. At 1 year, 187/245 (76%) and 190/245 (78%) provided follow-up in each group, respectively.
Baseline characteristics are summarized in Table 1. The average age was 78 years, about one-half were women, and over two-thirds had experienced a fall in the prior year, over one-half in the prior 3 months.
Table 1.
Characteristics of the Participants at Baselinea
| Characteristic |
Perturbation-Training (n = 253) |
Standard Treatment (n = 253) |
|---|---|---|
| Age, mean (min–max) | 78 (65–96) | 78 (65–95) |
| Female sex, no. (%) | 119 (47) | 119 (47) |
| Fall past 3 mos, no. (%) | 136 (54) | 130 (51) |
| Fall past year, no. (%) | 179 (71) | 169 (67) |
| Baseline TUG, mean ± SD | 14.4 sec ± 5.3 | 14.9 sec ± 7.0 |
| Baseline BBS, mean ± SD | 42.7 ± 7.0 | 42.5 ± 7.9 |
| Baseline DGI, mean ± SD | 16.3 ± 6.1 | 15.9 ± 4.1 |
| Baseline ABC, mean ± SD | 62.0 ± 18 | 61.0 ± 19.7 |
| Hip osteoarthritis, no. (%) | 62 (25) | 60 (24) |
| Knee osteoarthritis, no. (%) | 98 (39) | 97 (38) |
| Parkinson Disease, no. (%) | 34 (13) | 32 (13) |
| Prior stroke, no. (%) | 36 (14) | 38 (15) |
ABC = Activities-specific Balance Confidence Scale; BBS = Berg Balance Measure; DGI = Dynamic Gait Index; TUG = Timed Up & Go Test.
Primary Outcomes
At 3-month follow-up, 12/212 (5.7%) in the perturbation training group had an injurious fall compared with 28/210 (13.3%) in the usual treatment group (Tab. 2). The relative risk of injurious fall at 3 months was 0.43 (95% confidence interval [CI] = 0.22–0.82, P < .01); in the adjusted model the relative risk of injurious fall at 3 months was 0.38 (95% CI = 0.21–0.75, P < .003) (Fig. 2a). Of these injuries, 8/12 in the perturbation training group and 14/28 in the usual treatment group went to the hospital for evaluation and treatment; 1/12 in the perturbation training group and 7/28 in the usual treatment group were hospitalized for their injuries. In the adjusted survival analysis of time to first injurious fall, there was separation of the curves in the first 3 months (hazard ratio = 0.51; 95% CI = 0.26–0.99) but no significant difference when viewed over the entire first year (hazard ratio = 0.78; 95% CI 0.47–1.30) (Fig. 3).
Table 2.
Crude Counts of Subjects Experiencing Falls and Injurious Falls at Each Follow-Up
| Participants Experiencing a Fall No. (%) | Participants With an Injurious Fall No. (%) | |||
|---|---|---|---|---|
| Follow-up Period | Perturbation Training | Usual Treatment | Perturbation Training | Usual Treatment |
| 3 months | 60 (28.3%) | 77 (36.7%) | 12 (5.7%) | 28 (13.3%) |
| 6 months | 61 (28.9%) | 63 (31.2%) | 17 (8.1%) | 12 (5.9%) |
| 9 months | 59 (29.2%) | 76 (38.2%) | 20 (9.9%) | 23 (11.6%) |
| 12 months | 60 (32.1%) | 65 (34.0%) | 16 (8.6%) | 22 (11.5%) |
Figure 2.
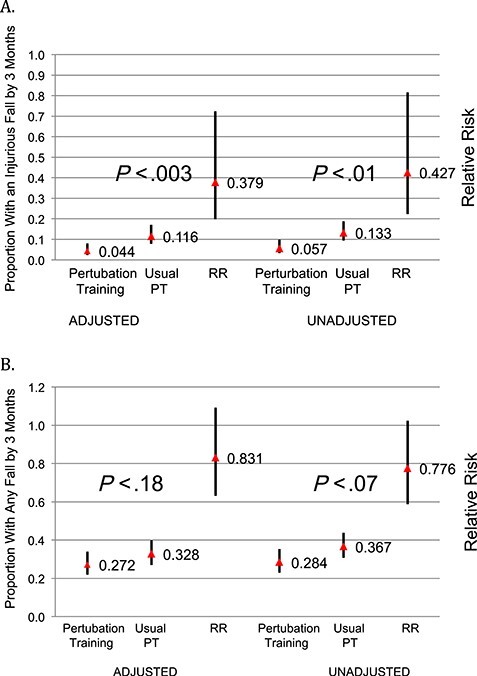
Proportion of participants with (a) an injurious fall at 3 months; (b) any fall at 3 months.
Figure 3.
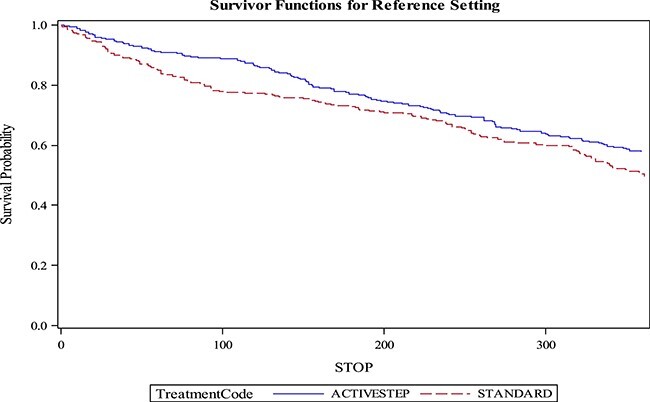
Survival analysis of time to first injurious fall.
At 3-month follow-up, 60/212 (28.3%) of the perturbation training group reported a fall compared with 77/210 (36.7%) of the usual treatment group (Tab. 2), with a relative risk of any fall at 3 months of 0.78 (95% CI = 0.59–1.03, P = .07); in the adjusted model, the relative risk of any fall at 3 months was 0.83 (95% CI = 0.63–1.09, P = .18) (Fig. 2b). In the zero-inflated negative binomial mixed regression model over 1 year, the relative risk of any fall in the perturbation training group was 0.82 (95% CI = 0.67–1.00, P = .054).
Secondary Outcomes
Measures of balance, mobility, and balance confidence before and after treatment showed significant improvements in both treatment groups but no significant differences between groups (Tab. 3). No unexpected serious adverse events were identified in either group during the study.
Table 3.
Comparison of Change in Scores Between Baseline and End of Treatment Within the Treatment Groupsa
| Perturbation Training | Usual Treatment | Difference in Changes Between Treatment Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | No. | Mean Change | SD | P value | No. | Mean Change | SD | P value | Delta (95% CI) | P value |
| ABC | 177 | 11.1 | 16.1 | <.0001 | 168 | 12.1 | 17.3 | <.0001 | −1.0 (−4.5 to 2.6) | .58 |
| BBS | 175 | 6.0 | 5.2 | <.0001 | 180 | 5.8 | 5.2 | <.0001 | 0.2 (−0.9 to 1.3) | .76 |
| DGI | 172 | 3.3 | 5.7 | <.0001 | 177 | 3.78 | 3.4 | <.0001 | −0.5 (−1.5 to 0.5) | .33 |
| TUG | 149 | −2.0 secs | 3.2 | <.0001 | 153 | −2.9 secs | 3.9 | <.0001 | 0.2 (−0.6 to 1.0) | .59 |
ABC = Activities-specific Balance Confidence Scale; BBS = Berg Balance Measure; DGI = Dynamic Gait Index; TUG = Timed Up & Go Test.
Discussion
This highly pragmatic, comparative effectiveness trial evaluated the addition of access to surface perturbation treadmill training as part of multi-modal physical therapy for preventing falls and fall-related injuries in high-risk older adults. The results show a significant reduction in fall-related injuries at 3 months but no significant reduction in falls between groups or in fall-related injuries at 1 year.
These findings are consistent with a growing body of evidence on the effectiveness of perturbation-based balance training. A recent (2015) meta-analysis of perturbation-based balance training found a relative risk for falls of 0.71 (95% CI = 0.52–0.96) relative to a variety of different control groups.11 Promising results have been seen with multiple different perturbation paradigms, including moveable floor platforms, ground surface compliance changes, and treadmill belt accelerations. Salient features of the current trial include use of customized treadmill accelerations of various magnitudes and in different directions during standing and walking tasks to provide unexpected perturbations, multiple training sessions, assessing falls in daily life posttreatment, and studying a high-risk clinical population referred for balance training to reduce fall risk.
The effect in the perturbation training group seemed to wear off at 3 months, which is consistent with studies of the longevity of other exercise interventions to prevent falls.9,41 Prior studies of perturbation training in healthy older adults suggest possible retention over longer time periods. One previous study of 162 community-dwelling women that used surface perturbation training in five 1-hour sessions over 2 weeks found that compared with no intervention, the perturbation training group had significantly reduced trip-related falls, although no significant difference in falls that were judged to be non-trip–related and avoidable at 1 year.27 Similarly, Pai et al studied 109 community-dwelling older adults with a single session of 24 laboratory-induced slips versus a control group of 103 experiencing only a single slip and found a significant 50% reduction in falls at 1 year.42 Our results suggest that perturbation training effects may not be this long lasting. A brief “booster” session of perturbation training after 3 months has been shown to help participants retain improvements in reactive balance control,11,42 and a similar intervention could be an important topic for future studies.
The specific reduction in fall-related injuries found in this study is an interesting finding and is nearly identical to the results of the pilot study for this trial (n = 59), which showed 7.7% of the perturbation training and 18.2% of the standard treatment groups had an injurious fall at 3 months (RR 0.42).24 Potential explanations for a differential effect on falls with injury compared with total falls includes preferential reduction in major versus minor falls or partial recovery after an unexpected perturbation potentially turning a fall that might otherwise result in injury into a more controlled fall without injury. Notably, Rosenblatt et al found no increase in trip-related stumbles in association with the decrease in trip-related falls after perturbation training, which may argue against the partial recovery as a potential explanation. These issues remain speculative and require additional study in future studies.
The reduction in injurious falls in the perturbation training group occurred despite no differences in the standard clinical fall risk and balance assessments (TUG, BBS, DGI) between groups. It is worth noting that although these measures assess performance on balance and adaptive gait tasks, none of them assess the ability to recover from a perturbation and therefore it may not surprising that they did not differ between treatment groups. Moreover, the fact that they did not differ suggests that the traditional measures of fall risk were similar between the groups both at baseline and the end of treatment and that the addition of some perturbation training to the multimodal exercise intervention did not adversely affect these standard measures of program effectiveness.
Limitations
A lack of blinding, although common in pragmatic trials, remains a potential limitation for this study; patients knew whether they received the perturbation training when they self-reported falls and the end-of-treatment gait and balance measures were collected by the treating therapist. It is possible that part of the effect seen in the perturbation training group could be nonspecific effects related to expectations of the newer treatment. However, if our results were biased by the patients’ knowledge of receiving the perturbation training, we would have expected to see this in self-reported measures like the ABC scale as well.
The rate of follow-up was acceptable at early time points but was limited by the frailty and advanced age of the cohort being studied. Also, because the perturbation training treatment protocols were individualized based on the needs of the patient and the judgment of the therapist, there may be variability across sites; however, the usual treatment was also individualized and reflects the reality of clinical practice in keeping with the pragmatic design of the trial. The usual treatment group demonstrated significant improvements from baseline to end of treatment in standard clinical fall-risk measures; the reduction in fall-related injuries seen in the perturbation training group were over and above what was achieved with usual exercise-based treatment rather than no treatment.
Another limitation is the lack of any measures of targeted balance recovery responses between groups, which would help confirm the mechanism of any effect. However, the biomechanics of perturbation training has been well studied,12,14–20 and our focus was on the pragmatic outcomes of falls and injurious falls.
Implications
Integration of some surface perturbation training into usual gait/balance training in high-risk older adults may substantially reduce the risk of fall-related injuries. The pragmatic nature of the study, in which physical therapists were given flexibility in how the surface perturbation training was incorporated into the patients’ treatment, suggests that the results are highly generalizable to current clinical practice. Whether greater reduction in injurious falls or a significant reduction in falls might be achieved with a more intensive or standardized perturbation training protocol is unknown, but the results from this study suggest further study is warranted. Substantial future research will be required to assess optimal protocols for the type, magnitude, direction, frequency, and volume of perturbation-based training as well as their effects in different patient subsets.
Author Contributions
Concept/idea/research design: J.D. Lurie, D. Pidgeon, K.F. Spratt
Writing: J.D. Lurie, K.M. Gill-Body, K.F. Spratt, C.M. McDonough
Data collection: J.D. Lurie, A.B. Zagaria, L. Ellis, K.M. Gill-Body, C. Burke, K. Armbrust, S. Cass
Data analysis: J.D. Lurie, K.F. Spratt
Project management: J.D. Lurie, A.B. Zagaria, K.M. Gill-Body
Fund procurement: J.D. Lurie
Providing participants: L. Ellis, D. Pidgeon, K.M. Gill-Body, C. Burke, S. Cass
Providing facilities/equipment: L. Ellis, D. Pidgeon, K.M. Gill-Body, C. Burke, S. Cass
Providing institutional liaisons: K.M. Gill-Body, K. Armbrust
Consultation (including review of manuscript before submitting): A.B. Zagaria, D. Pidgeon, K.M. Gill-Body, C. Burke, S. Cass, K.F. Spratt
Ethics Approval
The trial protocol was approved by local institutional review boards at each site.
Funding
This project was supported by the Agency for Healthcare Research and Quality (grant no. R01HS018459). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Clinical Trial Registration
The trial is registered at ClinicalTrials.gov (NCT01006967).
Disclosures and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no financial conflicts of interest. Dr Lurie has a professional relationship with the chief executive officer of Simbex, maker of the ActiveStep device tested in this trial, serving as a co-principal investigator and co-investigator on several federal grants (grant nos. R24 HD065703, P2CHD086841, P50FD004907).
Preliminary findings were initially presented at the 68th Annual Scientific Meeting of the Gerontological Society of America, Orlando, Florida, November 8–22, 2015.
Contributor Information
Jon D Lurie, Dartmouth-Hitchcock Medical Center, One Medical Center Dr, Lebanon, NH 03781 (USA), and Geisel School of Medicine at Dartmouth, Hanover, New Hampshire.
Alexandra B Zagaria, Geisel School of Medicine at Dartmouth.
Lisa Ellis, Elliot Hospital Senior Health Center Rehabilitation, Manchester, New Hampshire.
Dawna Pidgeon, Dartmouth-Hitchcock Medical Center.
Kathleen M Gill-Body, Newton-Wellesley Hospital, Newton, Massachusetts. Dr Gill-Body is a board-certified clinical specialist in neurologic physical therapy.
Christina Burke, South Shore Neurologic Associates, Patchogue, New York. Dr Burke is a board-certified clinical specialist in neurologic physical therapy.
Kurt Armbrust, White River Junction Veterans Administration Hospital, White River Junction, Vermont.
Sharil Cass, Farnum Rehabilitation Center, Keene, New Hampshire.
Kevin F Spratt, Geisel School of Medicine at Dartmouth.
Christine M McDonough, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1. Vieira ER, Palmer RC, Chaves PHM. Prevention of falls in older people living in the community. BMJ. 2016;353:i1419. [DOI] [PubMed] [Google Scholar]
- 2. Bergen G. Falls and fall injuries among adults aged ≥65 years — United States, 2014. MMWR Morb Mortal Wkly Rep. https://www.cdc.gov/mmwr/volumes/65/wr/mm6537a2.htm. Accessed January 22, 2020. [DOI] [PubMed]
- 3. Orces CH, Alamgir H. Trends in fall-related injuries among older adults treated in emergency departments in the USA. Inj Prev. 2014;20:421–423. [DOI] [PubMed] [Google Scholar]
- 4. Burns ER, Stevens JA, Lee R. The direct costs of fatal and non-fatal falls among older adults - United States. J Safety Res. 2016;58:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verma SK, Willetts JL, Corns HL, Marucci-Wellman HR, Lombardi DA, Courtney TK. Falls and fall-related injuries among community-dwelling adults in the United States. PLoS ONE 2016;11. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4792421/. Accessed January 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosen T, Mack KA, Noonan RK. Slipping and tripping: fall injuries in adults associated with rugs and carpets. J Inj Violence Res. 2013;5:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. [DOI] [PubMed] [Google Scholar]
- 8. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1: CD012424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750–1758. [DOI] [PubMed] [Google Scholar]
- 10. Avin KG, Hanke TA, Kirk-Sanchez N, et al. Management of falls in community-dwelling older adults: clinical guidance statement from the academy of geriatric physical therapy of the American Physical Therapy Association. Phys Ther. 2015;95:815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mansfield A, Wong JS, Bryce J, Knorr S, Patterson KK. Does perturbation-based balance training prevent falls? Systematic review and meta-analysis of preliminary randomized controlled trials. Phys Ther. 2015;95:700–709. [DOI] [PubMed] [Google Scholar]
- 12. McCrum C, Gerards MHG, Karamanidis K, Zijlstra W, Meijer K. A systematic review of gait perturbation paradigms for improving reactive stepping responses and falls risk among healthy older adults. Eur Rev Aging Phys Act Off J Eur Group Res Elder Phys Act. 2017;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerards MHG, McCrum C, Mansfield A, Meijer K. Perturbation-based balance training for falls reduction among older adults: current evidence and implications for clinical practice. Geriatr Gerontol Int. 2017;17:2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhatt T, Yang F, Pai Y-C. Learning to resist gait-slip falls: long-term retention in community-dwelling older adults. Arch Phys Med Rehabil. 2012;93:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bieryla KA, Madigan ML. Proof of concept for perturbation-based balance training in older adults at a high risk for falls. Arch Phys Med Rehabil. 2011;92:841–843. [DOI] [PubMed] [Google Scholar]
- 16. Bieryla KA, Madigan ML, Nussbaum MA. Practicing recovery from a simulated trip improves recovery kinematics after an actual trip. Gait Posture. 2007;26:208–213. [DOI] [PubMed] [Google Scholar]
- 17. Grabiner MD, Bareither ML, Gatts S, Marone J, Troy KL. Task-specific training reduces trip-related fall risk in women. Med Sci Sports Exerc. 2012;44:2410–2414. [DOI] [PubMed] [Google Scholar]
- 18. Grabiner MD, Donovan S, Bareither ML, et al. Trunk kinematics and fall risk of older adults: translating biomechanical results to the clinic. J Electromyogr Kinesiol. 2008;18:197–204. [DOI] [PubMed] [Google Scholar]
- 19. Mansfield A, Peters AL, Liu BA, Maki BE. Effect of a perturbation-based balance training program on compensatory stepping and grasping reactions in older adults: a randomized controlled trial. Phys Ther. 2010;90:476–491. [DOI] [PubMed] [Google Scholar]
- 20. Pai Y-C, Yang F, Bhatt T, Wang E. Learning from laboratory-induced falling: long-term motor retention among older adults. Age Dordr Neth. 2014;36:9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimada H, Obuchi S, Furuna T, Suzuki T. New intervention program for preventing falls among frail elderly people: the effects of perturbed walking exercise using a bilateral separated treadmill. Am J Phys Med Rehabil. 2004;83:493–499. [DOI] [PubMed] [Google Scholar]
- 22. Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation. 2005;20:183–190. [PubMed] [Google Scholar]
- 23. Maki BE, Cheng KC-C, Mansfield A, et al. Preventing falls in older adults: new interventions to promote more effective change-in-support balance reactions. J Electromyogr Kinesiol Off J Int Soc Electrophysiol Kinesiol. 2008;18:243–254. [DOI] [PubMed] [Google Scholar]
- 24. Lurie JD, Zagaria AB, Pidgeon DM, Forman JL, Spratt KF. Pilot comparative effectiveness study of surface perturbation treadmill training to prevent falls in older adults. BMC Geriatr. 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen X, Mak MKY. Technology-assisted balance and gait training reduces falls in patients with Parkinson’s disease: a randomized controlled trial with 12-month follow-up. Neurorehabil Neural Repair. 2015;29:103–111. [DOI] [PubMed] [Google Scholar]
- 26. Smania N, Corato E, Tinazzi M, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010;24:826–834. [DOI] [PubMed] [Google Scholar]
- 27. Rosenblatt NJ, Marone J, Grabiner MD. Preventing trip-related falls by community-dwelling adults: a prospective study. J Am Geriatr Soc. 2013;61:1629–1631. [DOI] [PubMed] [Google Scholar]
- 28. Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454–463. [DOI] [PubMed] [Google Scholar]
- 29. Lurie JD, Morgan TS. Pros and cons of pragmatic clinical trials. J Comp Eff Res. 2013;2:53–58. [DOI] [PubMed] [Google Scholar]
- 30. CDC - BRFSS - Questionnaires . 2018. Cited Mar 7, 2018. https://www.cdc.gov/brfss/questionnaires/index.htm. Accessed January 22, 2020.
- 31. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 32. Berg K, Wood-Dauphine S, Williams JI, Gayton D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother Can; 2009. http://www.utpjournals.press/doi/abs/10.3138/ptc.41.6.304. Accessed January 22, 2020. [Google Scholar]
- 33. Shumway-Cook A, Woollacott MH. Motor control : theory and practical applications. Baltimore, MD: Williams & Wilkins; 1995. https://trove.nla.gov.au/work/30708647. Accessed January 22, 2020.
- 34. Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. [DOI] [PubMed] [Google Scholar]
- 35. Park S-H. Tools for assessing fall risk in the elderly: a systematic review and meta-analysis. Aging Clin Exp Res. 2018;30:1–16. [DOI] [PubMed] [Google Scholar]
- 36. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society . Summary of the updated American Geriatrics Society/British geriatrics society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–157. [DOI] [PubMed] [Google Scholar]
- 37. Dibble LE, Christensen J, Ballard DJ, Foreman KB. Diagnosis of fall risk in Parkinson disease: an analysis of individual and collective clinical balance test interpretation. Phys Ther. 2008;88:323–332. [DOI] [PubMed] [Google Scholar]
- 38. Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. [DOI] [PubMed] [Google Scholar]
- 39. Venzon DJ, Moolgavkar SH. A method for computing profile-likelihood-based confidence intervals. J R Stat Soc Ser C Appl Stat. 1988;37:87–94. [Google Scholar]
- 40. Yau KKW, Wang K, Lee AH. Zero-inflated negative binomial mixed regression modeling of over-dispersed count data with extra zeros. Biom J. 2003;45:437–452. [Google Scholar]
- 41. Vogler CM, Menant JC, Sherrington C, Ogle SJ, Lord SR. Evidence of detraining after 12-week home-based exercise programs designed to reduce fall-risk factors in older people recently discharged from hospital. Arch Phys Med Rehabil. 2012;93:1685–1691. [DOI] [PubMed] [Google Scholar]
- 42. Pai Y-C, Bhatt T, Yang F, Wang E. Perturbation training can reduce community-dwelling older adults’ annual fall risk: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69:1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]


