Abstract
Purpose:
neoMONARCH assessed the biological effects of abemaciclib in combination with anastrozole in the neoadjuvant setting.
Patients and Methods:
Postmenopausal women with stage I–IIIB HR+/HER2− breast cancer were randomized to a 2-week lead-in of abemaciclib, anastrozole, or abemaciclib plus anastrozole followed by 14 weeks of the combination. The primary objective evaluated change in Ki67 from baseline to 2 weeks of treatment. Additional objectives included clinical, radiologic, and pathologic responses, safety, as well as gene expression changes related to cell proliferation and immune response.
Results:
Abemaciclib, alone or in combination with anastrozole, achieved a significant decrease in Ki67 expression and led to potent cell-cycle arrest after 2 weeks of treatment compared with anastrozole alone. More patients in the abemaciclib-containing arms versus anastrozole alone achieved complete cell-cycle arrest (58%/68% vs. 14%, P < 0.001). At the end of treatment, following 2 weeks lead-in and 14 weeks of combination therapy, 46% of intent-to-treat patients achieved a radiologic response, with pathologic complete response observed in 4%. The most common all-grade adverse events were diarrhea (62%), constipation (44%), and nausea (42%). Abemaciclib, anastrozole, and the combination inhibited cell-cycle processes and estrogen signaling; however, combination therapy resulted in increased cytokine signaling and adaptive immune response indicative of enhanced antigen presentation and activated T-cell phenotypes.
Conclusions:
Abemaciclib plus anastrozole demonstrated biological and clinical activity with generally manageable toxicities in patients with HR+/HER2− early breast cancer. Abemaciclib led to potent cell-cycle arrest, and in combination with anastrozole, enhanced immune activation.
Introduction
Hormone receptor–positive (HR+) breast cancer is the most common subtype of breast cancer (1). Endocrine therapy (ET) including the nonsteroidal aromatase inhibitor anastrozole is a highly effective therapy for early- and late-stage disease (2). The major limitation of ET is the innate or acquired resistance associated with molecular pathways including the constitutive activation of cyclin-dependent kinases 4 and 6 (CDK4/6; ref. 3).
The discovery that inhibition of CDK4/6 significantly decreases in vitro and in vivo growth of hormone receptor (HR)–positive, human epidermal growth factor receptor 2–negative (HR+/HER2) breast cancer cells has changed the treatment landscape of this disease subtype (4–6). CDK4 and CDK6 promote progression from G1 phase to S phase of the cell cycle and are essential for regulation of cell proliferation (7). Inhibition of CDK4/6 with a small molecule prevents cell-cycle progression through G1 and arrests tumor growth. Abemaciclib is one of three currently approved selective small-molecule inhibitor of CDK4/6 for treatment of HR+/HER2− metastatic breast cancer, the other two being palbociclib and ribociclib (8–14). In preclinical studies, short-term inhibition caused temporary G1 arrest in vitro and in vivo (9, 13). Continuous inhibition with the drug results in sustained growth arrest by preventing phosphorylation of retinoblastoma protein (Rb; ref. 11) and the release of the transcription elongation factor 2 (E2F) required for cell-cycle progression from G1 to S resulting in apoptosis or senescence. In addition, preclinical studies suggest the potential for CDK4/6 inhibitors to promote antitumor immunity (15, 16).
Expression of the proliferation marker Ki67 is commonly used in early stage breast cancer as a prognostic marker (17). Studies of neoadjuvant ET demonstrated that a change in Ki67 after 2 weeks of therapy can be used as a pharmacodynamic marker of efficacy and correlated with recurrence-free survival (18–20). Aside from estrogen receptor status (ER+), predictive biomarkers of potential clinical benefit from CDK4/6 inhibitors remain elusive (21–23).
We evaluated the biological and clinical effects of treatment with abemaciclib alone and in combination with anastrozole compared with anastrozole monotherapy in postmenopausal women with early-stage HR+/HER2− breast cancer. We hypothesized that the addition of a CDK4/6 inhibitor to ET would significantly improve Ki67 responses. Given the known role of CDK4/6 inhibitors in the cell cycle and their suggested role in antitumor immunity, we also hypothesized changes in cell cycle–associated genes (CCAG) and immune response–related biomarkers would be observed, and changes in these biomarkers may be predictive of response. Therefore, we assessed the change in Ki67 expression after 2 weeks of treatment in addition to the radiologic, pathologic, and clinical response at the end of treatment (EOT) and evaluated the modulation of cell cycle and immune-associated genes.
Patients and Methods
Clinical trial design and tumor biopsies
neoMONARCH, a multicenter, randomized, open-label, phase II study compared the biological effects of 2 weeks of treatment with abemaciclib (150 mg orally every 12 hours) in combination with anastrozole (1 mg orally once daily) to those of abemaciclib monotherapy and anastrozole monotherapy for postmenopausal women with early-stage [stage I (tumor ≥1 cm), II, IIIA, or IIIB] HR+/HER2− breast cancer. Eligible patients underwent core biopsies prior to randomization in a 1:1:1 ratio to 1 of 3 treatment arms for 2 weeks (Supplementary Fig. S1A). Randomization was stratified by progesterone receptor (PR) status and tumor size. Patients received treatment for 2 weeks (cycle 1), followed by a second core biopsy. All patients then received abemaciclib and anastrozole combination therapy for 14 weeks followed by a third biopsy at completion of treatment (16 weeks). Clinical, radiologic, and pathologic responses, as well as safety profiles were assessed following 16 weeks of therapy. Patients who experienced a benefit from study therapy could remain on treatment for up to 2 additional cycles (8 weeks; extension period) at the discretion of the treating physician. Surgery at EOT was not a requirement of this study. During the first 28 days, prophylactic loperamide (2 mg orally) was given with each dose of abemaciclib, then at the discretion of the treating physician.
Study participants were required to have adenocarcinoma of the breast deemed suitable for treatment with neoadjuvant endocrine monotherapy. Exclusion criteria included bilateral invasive, metastatic, or inflammatory breast cancer; prior systemic therapy or radio-therapy of the current cancer; prior antiestrogen therapy with raloxifene, tamoxifen, aromatase inhibitor, or other selective ER modulator; history of any other malignancy within the past 5 years, with the exception of nonmelanoma skin cancer or carcinoma in situ of the cervix; significant cardiac history; and active infection. Study was conducted at 53 sites in 10 countries in compliance with the principles of good clinical practice, applicable laws and regulations, and the Declaration of Helsinki. Patients provided written consent before enrollment. Study protocol was approved by each institution’s review board and was registered with ClinicalTrials.gov (NCT02441946).
Ki67 IHC and quantification
The primary objective was to determine the change in Ki67 expression from baseline to 2 weeks of therapy. A Ki67 response at 2 weeks was predefined in the protocol as Ki67 ≤2.7%. Formalin-fixed, paraffin-embedded (FFPE) breast tumors (from core biopsies or surgical tissue) were stained for Ki67 at each time point by IHC at the University of Southern California CAP/CLIA–certified laboratory using the anti-Ki67 antibody (DAKO clone MIB-1). Samples were blindly assessed by apathologist usingstandard scoring guidelines (24). Baseline Ki67 measurement of at least 5% and a valid 2-week measurement of any magnitude were used for the analysis of all Ki67-derived endpoints.
For exploratory biomarker analyses, complete cell-cycle arrest (CCCA) was defined as Ki67 ≤2.7%, as described previously (18, 25–27). Tumors were categorized by posttreatment Ki67 expression as either sensitive (Ki67 ≤2.7%) or resistant (Ki67 ≥7.4%), as described previously (25). Tumors intrinsically resistant to therapy were identified as Ki67 ≥7.4% after 2 and 16 weeks of therapy.
Safety analyses and tumor response
Secondary objectives assessed radiologic, pathologic, and clinical response, as well as safety and tolerability from baseline to 16 weeks of therapy. The safety analysis was based on summaries of adverse events (AE) reported in Common Terminology Criteria for Adverse Events (CTCAE version 4.0) and possible relationship to study drug as assessed by the investigators. The secondary endpoint of pathologic complete response (pCR) was defined as no invasive cancer in the breast and sampled axillary lymph nodes following completion of neoadjuvant systemic therapy. The secondary endpoints of clinical objective response (OR) and radiologic response were evaluated according to RECIST v1.1. Clinical tumor assessments were performed at baseline and the start of each cycle using skin calipers. Tumor assessments by imaging were performed at baseline and the end of study treatment or during study treatment at the discretion of the treating physician. Acceptable imaging modalities were ultrasound and magnetic resonance imaging.
Gene expression analysis (MODAplex and RNA-seq)
Exploratory biomarker analyses evaluated the early and late cell cycle and immune-modulating effects of abemaciclib. Gene expression analyses were performed to identify markers of drug sensitivity and resistance. Extracted RNA from FFPE tumor biopsies at each time-point was subjected to a CCAG expression panel using the MODAplex platform (Qiagen) in the Clinical Diagnostic Laboratory at Eli Lilly and Company (Indianapolis, IN) or sequenced using the Illumina Truseq RNA exome RNA sequencing (RNA-seq) at Insight Genetics (Nashville, TN). See Supplementary Materials and Methods for assay details.
PIK3CA sequencing
DNA was isolated from baseline FFPE tumor tissue with the BioSprint 96 DNA Kit (Qiagen). Droplet digital PCR (ddPCR) was performed by Asuragen, Inc. on the BioRad QX200 platform to evaluate 4 PIK3CA mutations: E542K, E545K, H1047L, and H1047R. The cut-off points for mutations defined as “POSITIVE” were ≥2% mutant allele frequency for the PIK3CA E542K, H1047L, and H1047R assays and ≥5% mutant allele frequency for the PIK3CA E545K assay, based on empirically determined lower limit of detection for each probe.
Data sharing statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Statistical analysis
With a minimum of 50 patients in each arm, this design provided 82% power, assuming a change in geometric mean Ki67 expression from −82% in the control arm to −91% in either experimental arm at a 1-sided alpha level of 0.1. To enroll 50 evaluable patients per arm, or 150 patients in total, approximately 220 patients were randomized. The primary endpoint of the percent change in Ki67 expression from baseline to 2 weeks of treatment was evaluated on the log Ki67 ratio scale. The analysis was done using a linear model with treatment, PR status, and tumor size as fixed effects. The least square mean estimates obtained from the model were back-transformed to the percentage change scale.
The pCR rate was estimated from patients (regardless of initial 2-week therapy) who underwent surgery (n = 190) and was compared with a historical control rate of 5%. Comparison was performed using a 1-sided exact binomial test at a 1-sided alpha of 0.1.
The RNA-seq data from available samples were subjected to differential gene expression analyses per treatment arm per time point versus a pooled baseline of patients using a one-way ANOVA model (LIMMA ver3.34.9), followed by a Gene Set Enrichment Analysis (GSEA Preranked ver6.0.10 and MSigDb datasets [Reactome.v6.1, h.all.v6.1]). For MODAplex analyses, the longitudinal expression data were analyzed using a mixed-effect model for repeated measurements under the missing at random assumption. In particular, the least squares mean estimates of expression change from baseline to postbaseline timepoints were estimated using a model with treatment arm, time point, interaction of treatment arm with time point, and baseline expression level as covariates. In exploratory biomarker analyses limited by small sample size, the baseline samples were pooled.
Results
Patients and treatment
A total of 224 postmenopausal women with early-stage breast cancer were enrolled and randomly assigned to receive a 2-week lead-in of abemaciclib plus anastrozole (n = 74), abemaciclib monotherapy (n = 76), or anastrozole monotherapy (n = 74; Supplementary Fig. S1A). One patient who was randomized did not receive treatment. In total, 97% of patients completed cycle 1 (2 weeks of abemaciclib, anastrozole, or combination) and entered cycle 2, when all patients received abemaciclib plus anastrozole treatment for remainder of study treatment (14 weeks). Among patients who started cycle 2, 85% of patients completed study treatment (16 weeks) while 15% of patients discontinued study treatment for the following reasons: AEs (n = 15), patient decision (n = 9), disease progression (n = 5), and other (n = 3). In addition, 23% of patients entered the extension period and received 8 additional weeks of study treatment. After treatment discontinuation, 85% of the intent-to-treat (ITT) population underwent definitive surgery.
Demographic and baseline characteristics were balanced across treatment arms (Table 1). The overall median age was 64 years (range, 42–92 years). The majority of patients had invasive ductal carcinoma (65%) and 17% had PR-negative tumors. The median tumor size was 3 cm with 18% of patients with tumor size <2 cm, 57% with ≥2 cm and <5 cm, 25% with ≥5 cm.
Table 1.
Patient and disease baseline characteristics.
| Variable, n (%) | Abemaciclib + ANZ (n = 74) | Abemaciclib (n = 76) | ANZ (n = 74) | Total (n = 224) |
|---|---|---|---|---|
| Age, years, median (range) | 63 (52–92) | 62 (51–86) | 65 (42–83) | 64 (42–92) |
| Race, na | 73 | 76 | 74 | 223 |
| Asian, n (%) | 12 (16) | 16 (21) | 16 (22) | 44 (20) |
| Caucasian, n (%) | 55 (75) | 57 (75) | 55 (74) | 167 (75) |
| Other, n (%) | 6 (8) | 3 (4) | 3 (4) | 12 (5) |
| ECOG PS, na | 74 | 75 | 74 | 223 |
| 0, n (%) | 69 (93) | 70 (93) | 64 (87) | 203 (91) |
| 1, n (%) | 5 (7) | 5 (7) | 10 (14) | 20 (9) |
| Disease stage, na | 73 | 75 | 72 | 220 |
| I, n (%) | 10 (14) | 11 (15) | 12 (17) | 33 (15) |
| II, n (%) | 53 (73) | 55 (73) | 48 (67) | 156 (71) |
| III, n (%) | 10 (14) | 9 (12) | 12 (17) | 31 (14) |
| Tumor grade, na | 74 | 75 | 73 | 222 |
| 1, n (%) | 9 (12) | 8 (11) | 10 (14) | 27 (12) |
| 2, n (%) | 33 (45) | 43 (57) | 42 (58) | 118 (53) |
| 3, n (%) | 20 (27) | 11 (15) | 13 (18) | 44 (20) |
| Undetermined, n (%) | 12 (16) | 13 (17) | 8 (11) | 33 (15) |
| Tumor size, na | 74 | 75 | 72 | 221 |
| Median in cm (range) | 3 (0.5–10) | 3 (0.5–10) | 3 (1–10) | 3 (0.5–10) |
| < 2 cm, n (%) | 16 (22) | 10 (13) | 13 (18) | 39 (18) |
| ≥ 2 cm and <5 cm, n (%) | 44 (60) | 45 (60) | 37 (51) | 126 (57) |
| ≥ 5 cm, n (%) | 14 (19) | 20 (27) | 22 (31) | 56 (25) |
| Histology, n (%) | ||||
| Invasive ductal | 49 (66) | 54 (72) | 42 (58) | 145 (65) |
| Invasive lobular | 7 (10) | 6 (8) | 14 (19) | 27 (12) |
| Other | 18 (24) | 15 (20) | 17 (23) | 50 (23) |
| Lymph node involvement, na | 74 | 75 | 73 | 222 |
| NX, n (%) | 1 (1) | 0 | 1 (1) | 2 (1) |
| N0, n (%) | 38 (51) | 44 (59) | 40 (55) | 122 (55) |
| N1, n (%) | 33 (45) | 27 (36) | 27 (37) | 87 (39) |
| N2, n (%) | 2 (3) | 3 (4) | 5 (7) | 10 (5) |
| N3a, n (%) | 0 | 1 (1) | 0 | 1 (0.5) |
| Baseline Ki67, n | 65 | 66 | 64 | 195 |
| Median in % (range)b | 24 (5–58) | 26 (6–60) | 26 (5–59) | 25 (5–60) |
| Hormone receptor status na | 74 | 75 | 74 | 223 |
| ER+; PR+, n (%) | 62 (84) | 59 (79) | 64 (87) | 185 (83) |
| ER+; PR−, n (%) | 11 (15) | 16 (21) | 10 (14) | 37 (17) |
| ER−; PR+, n (%) | 1 (1) | 0 | 0 | 1 (0.4) |
Abbreviations: ANZ, anastrozole; ECOG PS, Eastern Cooperative Oncology Group performance status.
Missing data for some patients, used as denominator.
Patients with Ki67 ≥5%.
Percentage change in Ki67 expression
The primary endpoint of the study was to compare the percent change in Ki67 expression from baseline to 2 weeks between the combination of abemaciclib plus anastrozole or abemaciclib alone to anastrozole alone. Of the 224 ITT patients, baseline tumor Ki67 expression was available for 208 (93%) with Ki67 ≥5% in 195 (87%) of the tumors.
Ki67 expression was assessed at baseline and after 2 weeks of treatment in tumors from 75% of patients including 80% in the combination arm, 69% in the abemaciclib arm, and 76% in the anastrozole arm (Fig. 1A; Supplementary Table S1). Ki67 expression decreased by a geometric mean change of −93% [90% confidence interval (CI), −95 to −90] in the combination arm, −91% (90% CI, −93 to −87) in the abemaciclib arm, and −63% (90% CI, −73 to −49) in the anastrozole alone arm (Fig. 1B). The geometric mean ratios for abemaciclib plus anastrozole versus anastrozole were 0.2 (90% CI, 0.1–0.3; P < 0.001) and 0.3 (90% CI, 0.2–0.4; P < 0.001) for abemaciclib versus anastrozole. In total, 47% of tumors demonstrated CCCA, achieved by more patients in the abemaciclib plus anastrozole arm (68%) and abemaciclib arm (58%) compared with the anastrozole arm (14%; Fig. 1C). The differences between the abemaciclib-containing arms and the anastrozole monotherapy arm were statistically significant with an odds ratio for the combination therapy versus anastrozole of 13 (90% CI, 5–31; P < 0.001) and for abemaciclib versus anastrozole at 8 (90% CI, 3–20; P < 0.001). These data support the mechanism of action for abemaciclib as a potent cell-cycle inhibitor administered in HR+/HER2− early-stage breast cancer.
Figure 1.
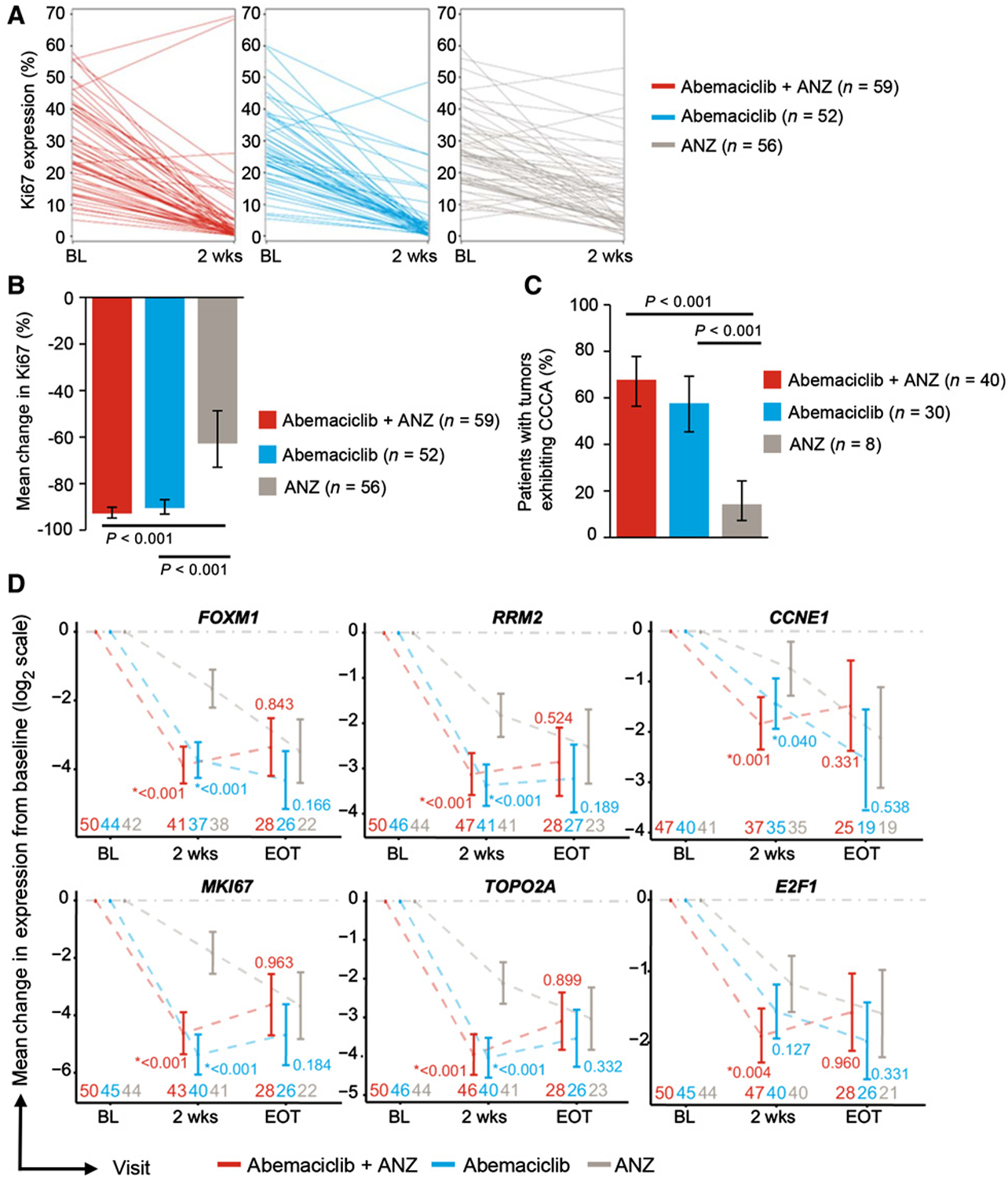
Antiproliferative effects of abemaciclib, anastrozole (ANZ), and combination therapy on HR+/HER2− breast cancer. A, Individual paired Ki67 expression at baseline and 2 weeks after treatment with abemaciclib + ANZ, abemaciclib, and anastrozole. B, Geometric mean percentage suppression of Ki67 from baseline to 2 weeks by treatment arms. P values are based on a one-sided hypothesis test from a linear model with treatment, progesterone receptor status (positive vs. negative/unknown), and tumor size (<2 cm vs. ≥2 cm and <5 cm vs. ≥5 cm) as fixed effects. C, Mean percentage rate of response as determined by complete cell-cycle arrest (CCCA, Ki76 ≤2.7) at week 2 by treatment arms. P value is calculated by Fisher exact test of a 1-sided hypothesis. D, Gene expression by MODAplex mRNA assay of cell cycle–associated genes at 2 weeks and EOT. P value compared abemaciclib-containing treatment arms versus anastrozole. E, CCAGs between resistant versus sensitive subgroups. All patients received anastrozole therapy for the 2 weeks of initial treatment, and then abemaciclib + anastrozole to the EOT. Plots show the expression of selected genes. Pairwise P values compare, respectively, the mean gene expression between (1/2/3): 1, abemaciclib + anastrozole-sensitive versus anastrozole-sensitive; 2, abemaciclib + anastrozole-sensitive versus abemaciclib + anastrozole-resistant; 3, anastrozole-sensitive versus abemaciclib + anastrozole-resistant. Baseline samples were pooled. F, CCAGs between intrinsically resistant versus sensitive tumor samples. Tumors were classified by posttreatment Ki67 expression as intrinsically resistant (Ki67 ≥7.4 at 2 weeks and EOT) or sensitive (Ki67 ≤2.7% at 2 weeks and EOT) to therapy regardless of initial 2 weeks of treatment. Baseline samples were pooled. Abbreviations: BL, baseline; n, tumor specimens; ns, nonsignificant (P > 0.05); *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; wks, weeks.
Clinical response, radiologic response, and pCR
Secondary objectives of the study assessed clinical, radiologic, and pathologic response from baseline to 16 weeks of therapy. Caliper response at EOT was available for 71% of patients. At the completion of study treatment, a total of 8% of patients achieved CR and 46% a partial response (PR) for an overall response rate (ORR) of 54% in the ITT population (Supplementary Fig. S1B).
Radiologic assessments at EOT, using the same method used at baseline, were available for 79% of patients. The radiologic ORR was 46% in the ITT population with 5% of patients having a CR and 42% a PR (Supplementary Fig. S1C).
Of the 190 patients who underwent surgery at EOT, 4% of patients had no evidence of invasive disease in the breast and axillary lymph nodes (Supplementary Fig. S1D).
Safety
Safety and tolerability were evaluated as a secondary objective. Overall, the majority of patients (96%) experienced at least 1 treatment-emergent adverse event (TEAE) during the study (Table 2). The most frequent TEAEs (≥20% of patients) included diarrhea, constipation, nausea, fatigue, abdominal pain, decreased appetite, and neutropenia. Grade 3 TEAEs were reported in 36% of patients. Grade 3 TEAEs reported in ≥5% of patients included neutropenia (9%) and increased alanine aminotransferase (ALT; 5%). Grade 4 TEAEs were reported for 2% of patients and included ALT elevation (1%), leukopenia (0.4%), and aspartate aminotransferase (AST) elevation (0.4%).
Table 2.
Treatment-emergent adverse events at the end of treatment.
| Investigator-assessed TEAEs ≥ 10% (N = 223) | CTCAE grade, n (%) | ||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | All grades | |
| Patients ≥ 1 TEAE | 44 (20) | 87 (39) | 80 (36) | 4 (2) | 215 (96) |
| Diarrhea | 92 (41) | 34 (15) | 11 (5) | 0 | 137 (61) |
| Constipationa | 72 (32) | 21 (9) | 4 (2) | 0 | 97 (44) |
| Nausea | 66 (30) | 22 (10) | 5 (2) | – | 93 (42) |
| Fatigue | 57 (26) | 25 (11) | 6 (3) | – | 88 (40) |
| Abdominal pain | 35 (16) | 7 (3) | 8 (4) | – | 50 (22) |
| Decreased appetite | 32 (14) | 12 (5) | 4 (2) | 0 | 48 (22) |
| Neutropenia | 6 (3) | 19 (9) | 21 (9) | 0 | 46 (21) |
| ALT increased | 13 (6) | 3 (1) | 12 (5) | 3 (1) | 31 (14) |
| Vomiting | 21 (9) | 8 (4) | 2 (0.9) | 0 | 31 (14) |
| Anemia | 13 (6) | 14 (6) | 2 (0.9) | 0 | 29 (13) |
| Hot flush | 24 (11) | 5 (2) | 0 | – | 29 (13) |
| Dysgeusia | 18 (8) | 7 (3) | – | – | 25 (11) |
| Rash | 14 (6) | 7 (3) | 3 (1) | 0 | 24 (11) |
| AST increased | 8 (4) | 9 (4) | 5 (2) | 1 (0.4) | 23 (10) |
| Headache | 20 (9) | 2 (0.9) | 1 (0.4) | – | 23 (10) |
| Leukopenia | 14 (6) | 7 (3) | 1 (0.4) | 1 (0.4) | 23 (10) |
Note: No Grade 5 TEAE was observed.
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events v4.0.
Two micrograms of loperamide was administered prophylactically with each abemaciclib dose for the first 28 days and then at the investigator’s discretion.
This study also evaluated incorporation of prophylactic antidiarrheal therapy into the abemaciclib regimen. Patients received loperamide prophylactically with each abemaciclib dose for the first 28 days and then at the investigator’s discretion. Any grade diarrhea occurred in 61% of patients including 5% of patients with grade 3 diarrhea. All grades of constipation were observed in 44% of patients, with grade 3 events in 2% of patients. No grade ≥4 events were observed.
Pharmacodynamic effects of treatment and cell-cycle gene expression
To evaluate the modulation of CCAGs as a result of treatment with abemaciclib, anastrozole, and combination therapy, exploratory analyses with MODAplex technology for quantitative mRNA analysis were performed on available RNA extracted from FFPE tumor biopsies. Treatment with abemaciclib alone or in combination with anastrozole led to a statistically significant decrease in expression of FOXM1 (P < 0.001; P < 0.001), RRM2 (P < 0.001; P < 0.001), CCNE1 (P = 0.04; P = 0.001), MKI67 (P < 0.001; P < 0.001), and TOPO2A (P < 0.001; P < 0.001) from baseline to 2 weeks when compared with treatment with anastrozole alone (Fig. 1D). Treatment with anastrozole alone resulted in decreased gene expression but not to the extent of abemaciclib alone or in combination at 2 weeks. By EOT, the difference in expression of these genes across treatment groups converged after all patients received combination treatment for 14 weeks. These exploratory data demonstrate that abemaciclib’s cell-cycle inhibition correlated with an antiproliferative effect on cancer cells.
Next, we evaluated the effect of adding abemaciclib to anastrozole (only)-treated tumors (n = 14) that did not achieve CCCA at 2 weeks (anastrozole-resistant: Ki67 ≥7.4% at 2 weeks; Supplementary Fig. S2A and S2B). MODAplex mRNA analysis available for a small subset of these tumor samples (n = 8) showed that anastrozole-resistant tumors displayed numerically higher expression of FOXM1, RRM2, CCNE1, MKI67, TOPO2A, and E2F1 at 2 weeks compared with anastrozole-sensitive tumors, although this was not statistically significant (P > 0.05; Fig. 1E). The addition of abemaciclib to anastrozole-resistant tumors at 2 weeks led to CCCA in a majority (64%) of tumors, with a subsequent decrease in expression of the CCAGs at EOT in tumors with available MODAplex data (Fig. 1E; Supplementary Fig. S2A and S2B). The anastrozole-resistant tumors not achieving CCCA despite the addition of abemaciclib to anastrozole displayed higher levels of CCAGs at 2 weeks and EOT compared with sensitive tumors (Fig. 1E), suggesting a lack of cell cycle inhibition and intrinsic resistance to abemaciclib and anastrozole.
Finally, we explored expression of the CCAGs by MODAplex in the intrinsically resistant tumors (Ki67 ≥7.4% at 2 weeks and EOT regardless of 2-week lead in; Supplementary Fig. S2C and S2D). Intrinsically resistant subgroups displayed persistently high levels of Ki67 at 2 weeks and EOT regardless of treatment compared to sensitive subgroups (Ki67 ≤2.7% at 2 weeks and EOT; Supplementary Fig. S2C and S2D). Moreover, intrinsically resistant subgroups displayed higher levels of CCAG at 2 weeks and EOT than sensitive subgroups, consistent with lack of cell-cycle inhibition (Fig. 1F). These findings suggest early changes in gene expression may predict response to therapy between intrinsically resistant and sensitive tumor samples.
Response by baseline clinical, pathologic, and molecular characteristics
To explore the potential association between treatment-induced pharmacodynamic effects and baseline characteristics, clinical, pathologic, and molecular characteristics were assessed. Subgroup analyses did not demonstrate significant association between the abemaciclib-driven change in Ki67 expression at 2 weeks in any of the clinical, pathologic, and molecular subgroups (Table 3; Supplementary Fig. S3A). For the small subset of samples for which molecular subtyping was performed, we observed CCCA in both luminal B and luminal A tumors (Table 3).
Table 3.
Rate of CCCA at 2 weeks by treatment arms and key subgroups.
| Abemaciclib + ANZ (n = 59) | Abemaciclib (n = 52) | ANZ (n = 56) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N1 | n1 | CCCA rate (A) | N1 | n1 | CCCA rate (B) | N1 | n1 | CCCA rate (C) | P value (A vs. C; B vs. C) |
| All KE patients | 40 | 68 | 30 | 58 | 8 | 14 | P < 0.001; P < 0.001 | |||
| Stage | ||||||||||
| I | 7 | 4 | 57 | 7 | 4 | 57 | 5 | 0 | 0 | P = 0.071; P = 0.071 |
| II | 44 | 29 | 66 | 38 | 24 | 63 | 41 | 7 | 17 | P < 0.001; P < 0.001 |
| III | 7 | 6 | 86 | 7 | 2 | 29 | 8 | 1 | 13 | P = 0.009; P = 0.446 |
| Grade | ||||||||||
| 1 | 9 | 8 | 89 | 5 | 4 | 80 | 6 | 3 | 50 | P = 0.143; P = 0.348 |
| 2 | 24 | 16 | 67 | 32 | 20 | 63 | 31 | 5 | 16 | P < 0.001; P < 0.001 |
| 3 | 16 | 8 | 50 | 5 | 3 | 60 | 10 | 0 | 0 | P = 0.008; P = 0.022 |
| Tumor size | ||||||||||
| < 2 cm | 9 | 7 | 78 | 6 | 3 | 50 | 8 | 1 | 13 | P = 0.012; P = 0.175 |
| ≥ 2 cm and <5 cm | 39 | 23 | 59 | 31 | 17 | 55 | 29 | 6 | 21 | P = 0.002; P = 0.007 |
| ≥ 5 cm | 11 | 10 | 91 | 15 | 10 | 67 | 19 | 1 | 5 | P < 0.001; P < 0.001 |
| Hormone receptor status | ||||||||||
| ER+; PR+ | 49 | 36 | 74 | 40 | 22 | 55 | 48 | 6 | 13 | P < 0.001; P < 0.001 |
| ER+; PR− | 9 | 4 | 44 | 12 | 8 | 67 | 8 | 2 | 25 | P = 0.373; P = 0.085 |
| Histology | ||||||||||
| Invasive ductal | 44 | 29 | 66 | 42 | 24 | 57 | 32 | 7 | 22 | P < 0.001; P = 0.002 |
| Invasive lobular | 5 | 4 | 80 | 2 | 2 | 100 | 10 | 1 | 10 | P = 0.017; P = 0.045 |
| Other | 10 | 7 | 70 | 8 | 4 | 50 | 13 | 0 | 0 | P < 0.001; P = 0.012 |
| BL LN involvement | ||||||||||
| No | 29 | 18 | 62 | 29 | 21 | 72 | 26 | 3 | 12 | P < 0.001; P < 0.001 |
| Yes | 29 | 21 | 72 | 23 | 9 | 39 | 28 | 5 | 18 | P < 0.001; P = 0.084 |
| Molecular subtype | ||||||||||
| Luminal A | 5 | 4 | 80 | 5 | 3 | 60 | 9 | 1 | 11 | P = 0.023; P = 0.095 |
| Luminal B | 2 | 1 | 50 | 11 | 7 | 64 | 5 | 0 | NE | P = 0.286; P = 0.029 |
| PIK3CA | ||||||||||
| Mutant | 19 | 11 | 58 | 10 | 7 | 70 | 15 | 2 | 13 | P = 0.009; P = 0.007 |
| Wild type | 21 | 15 | 71 | 30 | 17 | 57 | 27 | 4 | 15 | P < 0.001; P = 0.001 |
| BL Ki67 Index | ||||||||||
| <20 | 21 | 19 | 91 | 14 | 10 | 71 | 19 | 5 | 26 | P < 0.001; P = 0.013 |
| ≥20 | 38 | 21 | 55 | 38 | 20 | 53 | 37 | 3 | 8 | P < 0.001; P < 0.001 |
Note: A responder is a patient with an ln(Ki67) value of less than 1.
Abbreviations: ANZ, anastrozole; BL, baseline; KE, Ki67-evaluable; LN; lymph node; N1, subgroup patients; n1, patients who achieved a response at 2 weeks (Ki67 index < 2.7%).
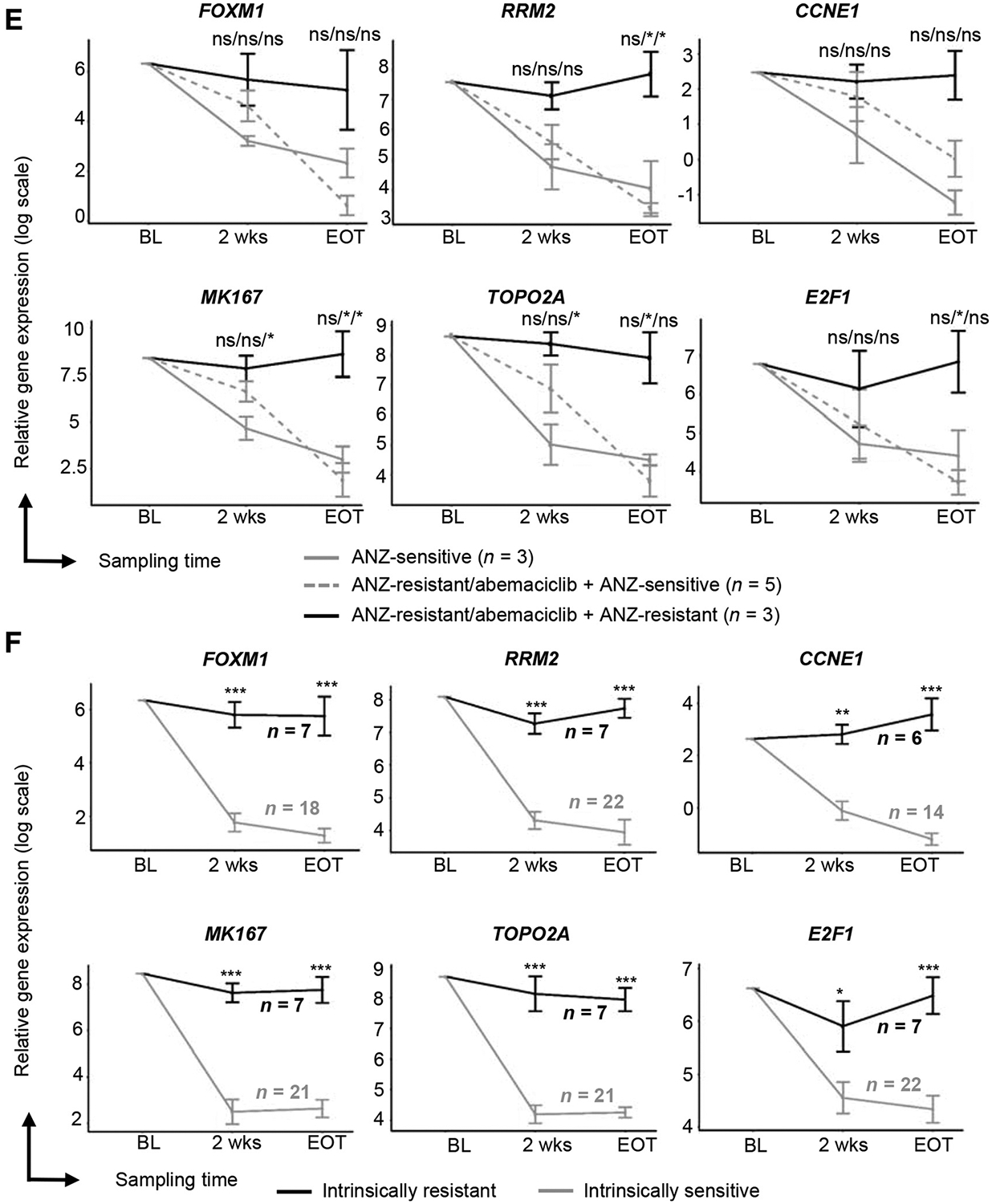
In patients with Ki67 analysis available at EOT (Supplementary Fig. S3B), the decrease in Ki67 expression from baseline to EOT was similar regardless of the initial 2 weeks of therapy (Supplementary Fig. S3C) and a similar rate of CCCA was observed at EOT across all arms (Supplementary Fig. S3D). To determine the extent to which abemaciclib-containing treatment suppresses tumor cell proliferation in high grade tumors, we examined Ki67 expression at baseline, 2 weeks, and EOT as a function of tumor grade (n = 138). Pathologic grade 2 and grade 3 tumors exhibited higher baseline Ki67 and most tumors, regardless of grade or baseline Ki67 expression, showed a reduction in Ki67 with treatment at 2 weeks, with a larger reduction observed in the abemaciclib-containing treatment arms compared to anastrozole alone (Fig. 2A; Supplementary Table S2).
Figure 2.
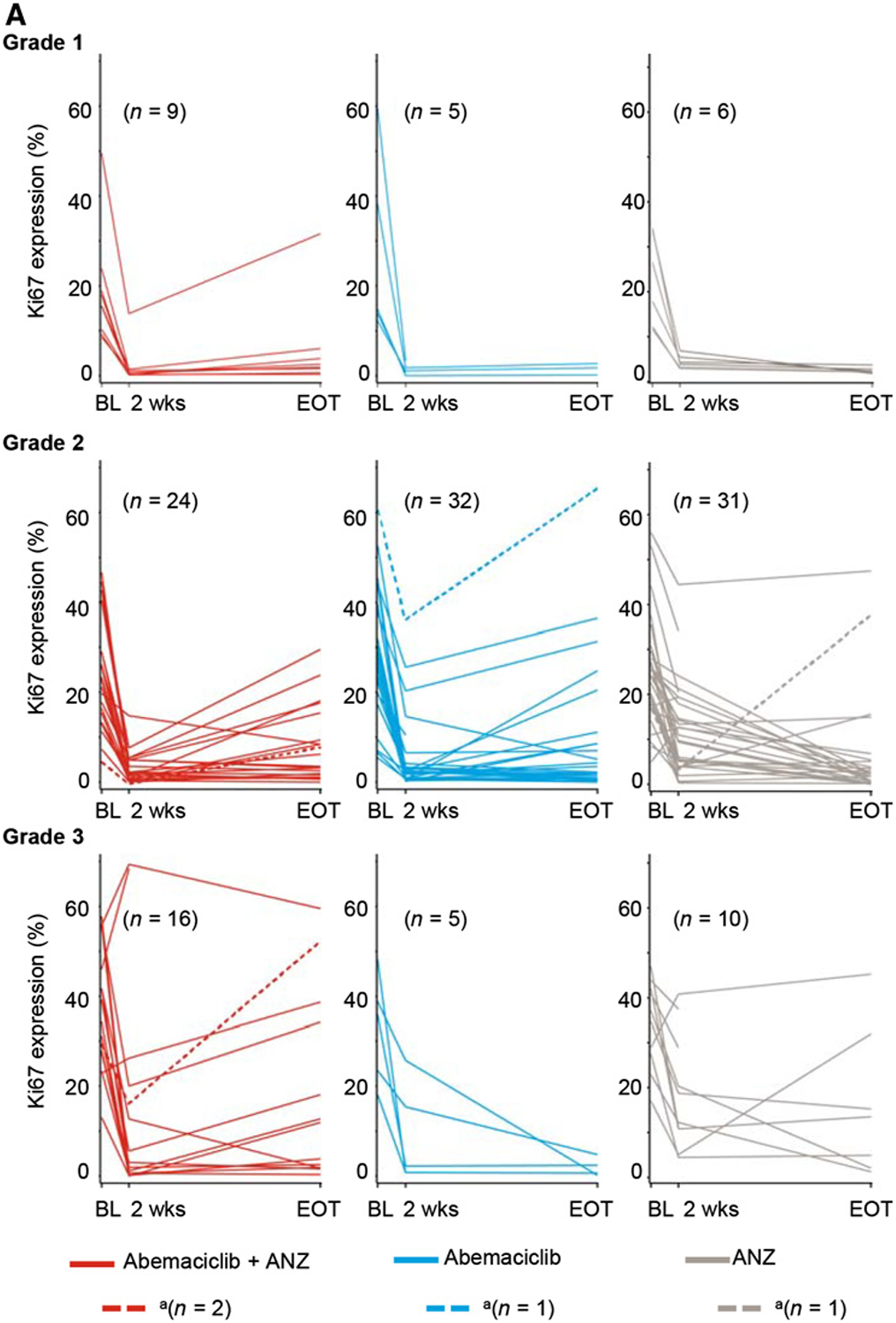
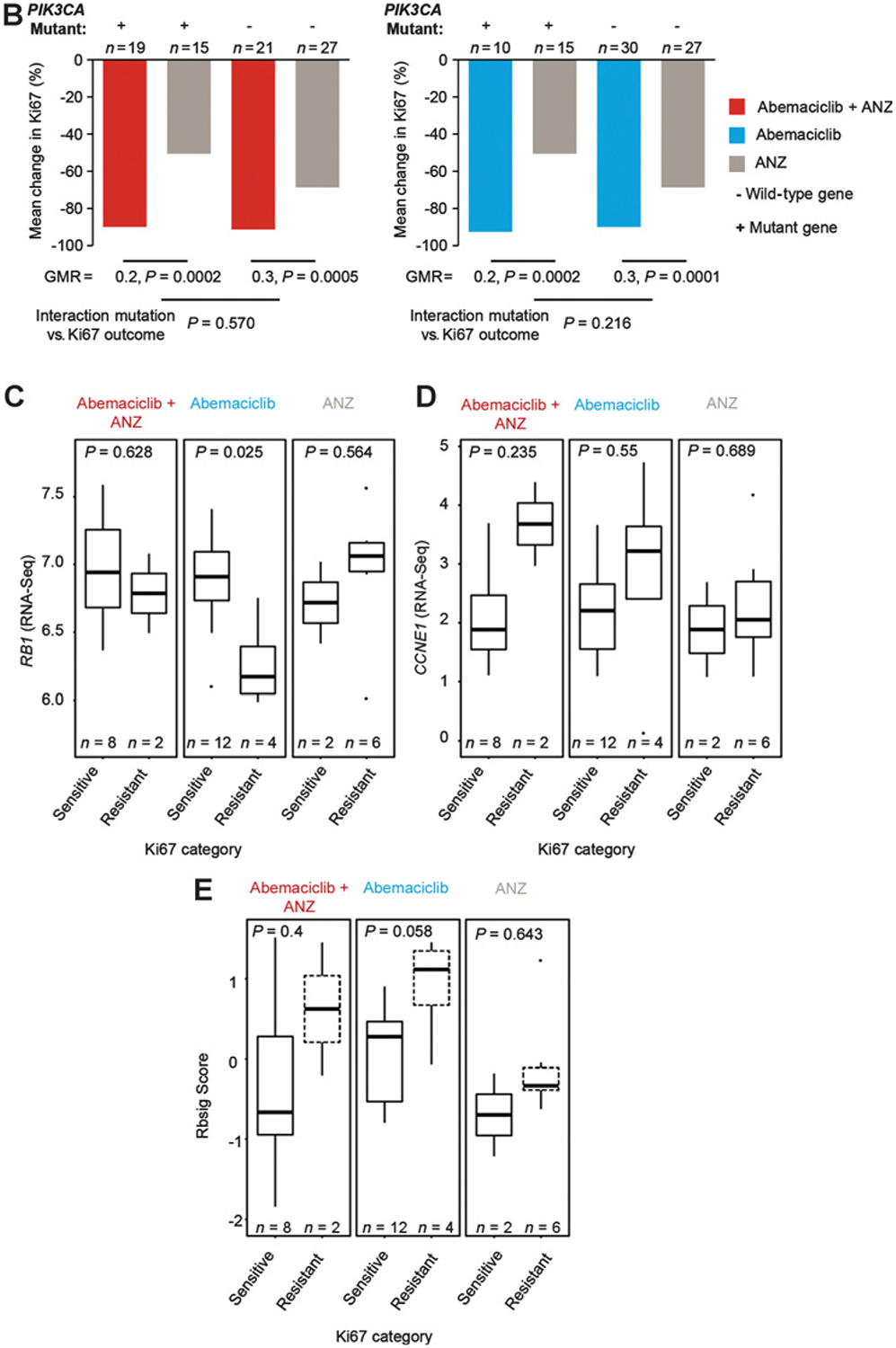
Ki67 expression and response by baseline tumor grade, PIK3CA mutation status, and gene expression analysis. A, Changes in Ki67 expression classified by baseline tumor grade. aPatient off treatment at EOT assessment. B, Changes in Ki67 expression classified by PIK3CA mutation status. C–E, RNA sequencing of baseline tumors: relative gene expression of RB1 (C) and CCNE1 (D) are reported in log2 (mean exon reads) scale; gene expression signature of Rb loss-of-function (E) is reported as RBsig score. Tumors were classified by treatment response at 2 weeks, defined by posttreatment Ki67 expression. Sensitive samples were defined as Ki67 ≤2.7% and resistant samples as Ki67 ≥7.4%. Abbreviations: ANZ, anastrozole; BL, baseline; CCNE1, Cyclin E1; GMR, geometric mean ratios; RB, retinoblastoma; RBsig, Rb-loss-of-function gene expression signature; wks, weeks.
PIK3CA mutation status was evaluable for 122 tumors; 36% of tumors harbored a PIK3CA-activating mutation (Supplementary Table S1). There were more PIK3CA mutant tumors in the combination arm (32%) than in the abemaciclib (19%) and anastrozole (27%) arms. The presence of a PIK3CA mutation had no significant effect on Ki67 expression change from baseline to 2 weeks, including the rate of CCCA in response to abemaciclib alone (P = 0.570) or in combination compared to anastrozole (P = 0.216; Table 3; Fig. 2B).
Deregulation of the Rb pathway and the expression of cyclin E1 (CCNE1) have been described as mediators of response to CDK4/6 inhibitors (21, 28, 29). Therefore, we analyzed the mRNA expression of RB1 and CCNE1, as well as a gene expression signature of Rb loss-of-function (30) from available RNA sequencing of baseline tumors (Fig. 2C–E). Tumors were categorized by the 2-week Ki67 as sensitive (Ki67 ≤2.7%) or resistant (Ki67 ≥7.4%), whereas tumors in the intermediate category (Ki67 >2.7% and <7.4%) were excluded from this analysis (18, 25, 27). Low expression of RB1 mRNA was associated with tumors resistant to abemaciclib monotherapy (Fig. 2C). In this limited sample size, this association was not observed with response to anastrozole or the combination therapy, consistent with Rb being the primary target of the CDK4/6 mechanism of action. Resistant tumors displayed numerically higher expression of CCNE1 and Rb loss-of-function score than sensitive tumors, although this was not statistically significant (Fig. 2D and E). In addition, anastrozole-treated tumors that did not respond to the addition of abemaciclib and intrinsically resistant tumors displayed numerically higher levels of CCNE1 at 2 weeks and EOT compared with tumors that responded to combination therapy (Fig. 1E and F). Given the role of CCNE1 in inducing E2F transcription factor and cell proliferation, these data support a mechanism of resistance to ET and CDK4/6 inhibitors, as described previously (31–33).
Impact of treatment discontinuation on Ki67 expression
To evaluate the potential relevance of treatment discontinuation on cell proliferation, we conducted an exploratory analysis assessing patients (n = 32) with tumors that achieved CCCA at 2 weeks irrespective of the assigned treatment arm who subsequently were documented as having stopped study treatment (both abemaciclib and anastrozole) prior to the EOT biopsy. Among these 32 patients, 59% had discontinued for up to 4 days and 41% had discontinued treatment for more than 4 days (range: 5 to 25 days) before the EOT biopsy. We observed when patients stopped study treatment for >4 days, the Ki67 rebounded (Ki67 >2.7%) in 69% of the tumors compared with patients who remained on study treatment (11%) or had stopped for 1 to 4 days (32%; Supplementary Fig. S3E and S3F) prior to biopsy. Generally, the rebound in Ki67 expression also resulted in increased expression of CCAGs (data not shown). Taken together, these data suggest that continued treatment with a CDK4/6 inhibitor in combination with ET may be necessary to maintain cell-cycle inhibition and antiproliferation of tumor cells.
Effect of abemaciclib on cell cycle and immune-related gene signatures
Recent studies have demonstrated the potential for CDK4/6 inhibitors to promote antitumor immunity and intratumor immune inflammation (15, 16, 34). To further explore this observation, we first examined whether study treatment had any effect on the presence of tumor-infiltrating lymphocytes (TIL) in the tumor stroma (Supplementary Fig. S4). Consistent with previous reports evaluating TILs in HR+ breast cancer (35), at baseline most of the tumors (88%, in combination arm; 92%, in abemaciclib arm; 94%, in anastrozole arm) had low TILs (0%–10% lymphoid cells) while a small subset (≤10%) of tumors had TILs in the 10% to 40%, and >40% ranges. Treatment with abemaciclib, anastrozole, or abemaciclib plus anastrozole had no effect on the percentage of stroma TILs either at 2 weeks (Supplementary Fig. S4A) or 16 weeks (EOT; Supplementary Fig. S4B).
We next evaluated the early and late effects of abemaciclib, anastrozole, and the combination in a subset of tumors (N = 114) for which whole transcriptome RNA-seq was available (Supplementary Fig. S5A). Consistent with the known activity of CDK4/6 inhibitors and antiestrogens to inhibit the cell cycle, E2F targets, G2–M checkpoint, and cell cycle–related pathways were the most downregulated gene sets across all treatment arms at 2 weeks and at EOT (Fig. 3A, highlighted example of E2F shown in Fig. 3B; Supplementary Fig. S5B). Genes involved in the early and late estrogen response pathways were also significantly downregulated in all treatment arms at 2 weeks and EOT (Fig. 3A and B). As previously reported (16), treatment with the combination of abemaciclib plus anastrozole resulted in upregulation of the allograft rejection, inflammatory response, and IFNg response Hallmark gene sets at 2 weeks and EOT (Fig. 3A and C). Many other immune pathway gene sets were also enriched, including the PD-1 pathway gene set, highlighting a potential increase in the presence of activated T cells (Fig. 3C; Supplementary Fig. S5C). Although these changes were consistent across early and late time points for the abemaciclib plus anastrozole combination arm, inflammatory immune gene changes were not significantly changed for those evaluable tumors treated with abemaciclib or anastrozole monotherapy during the 2-week lead-in period (Fig. 3A).
Figure 3.
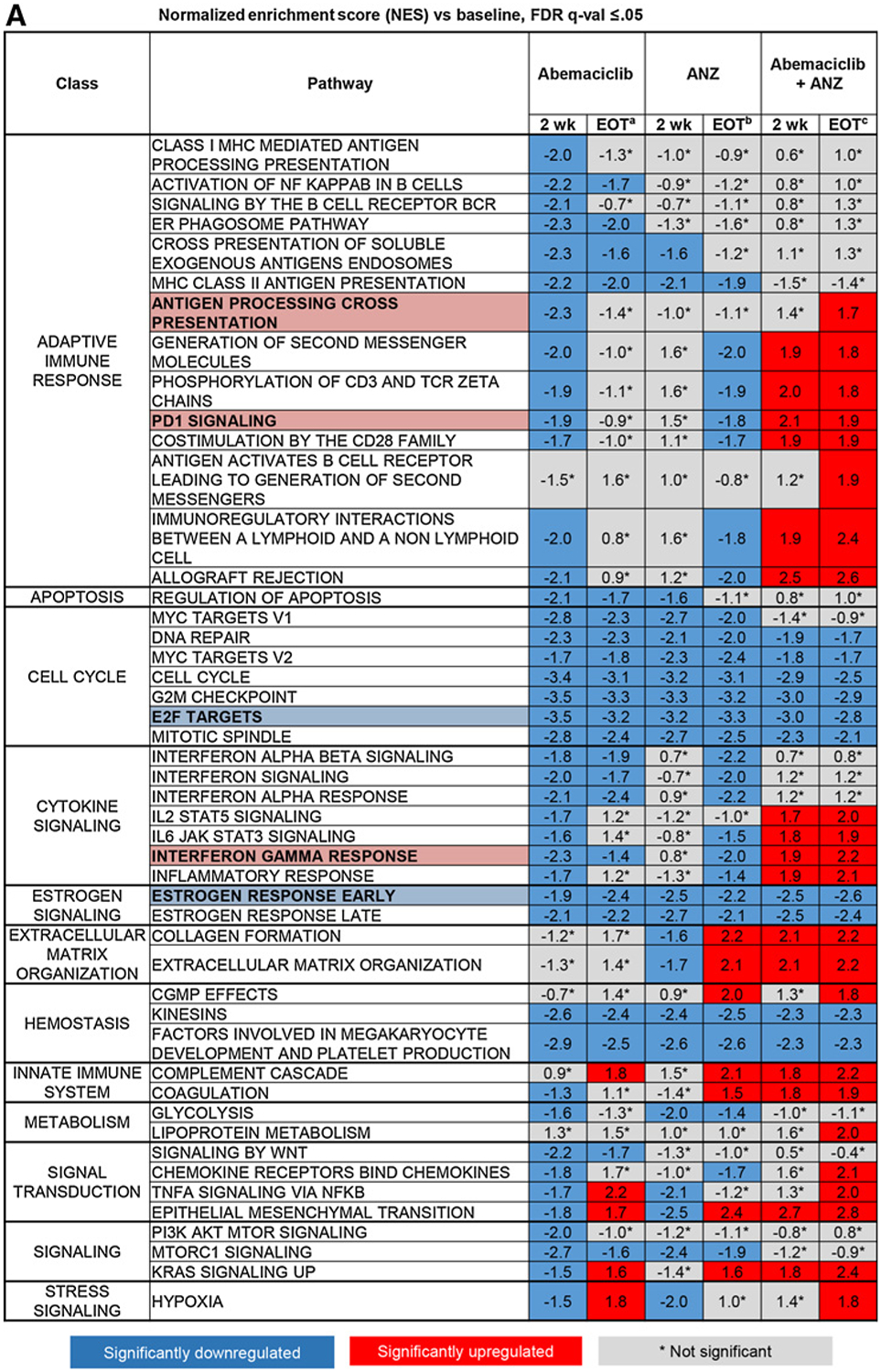
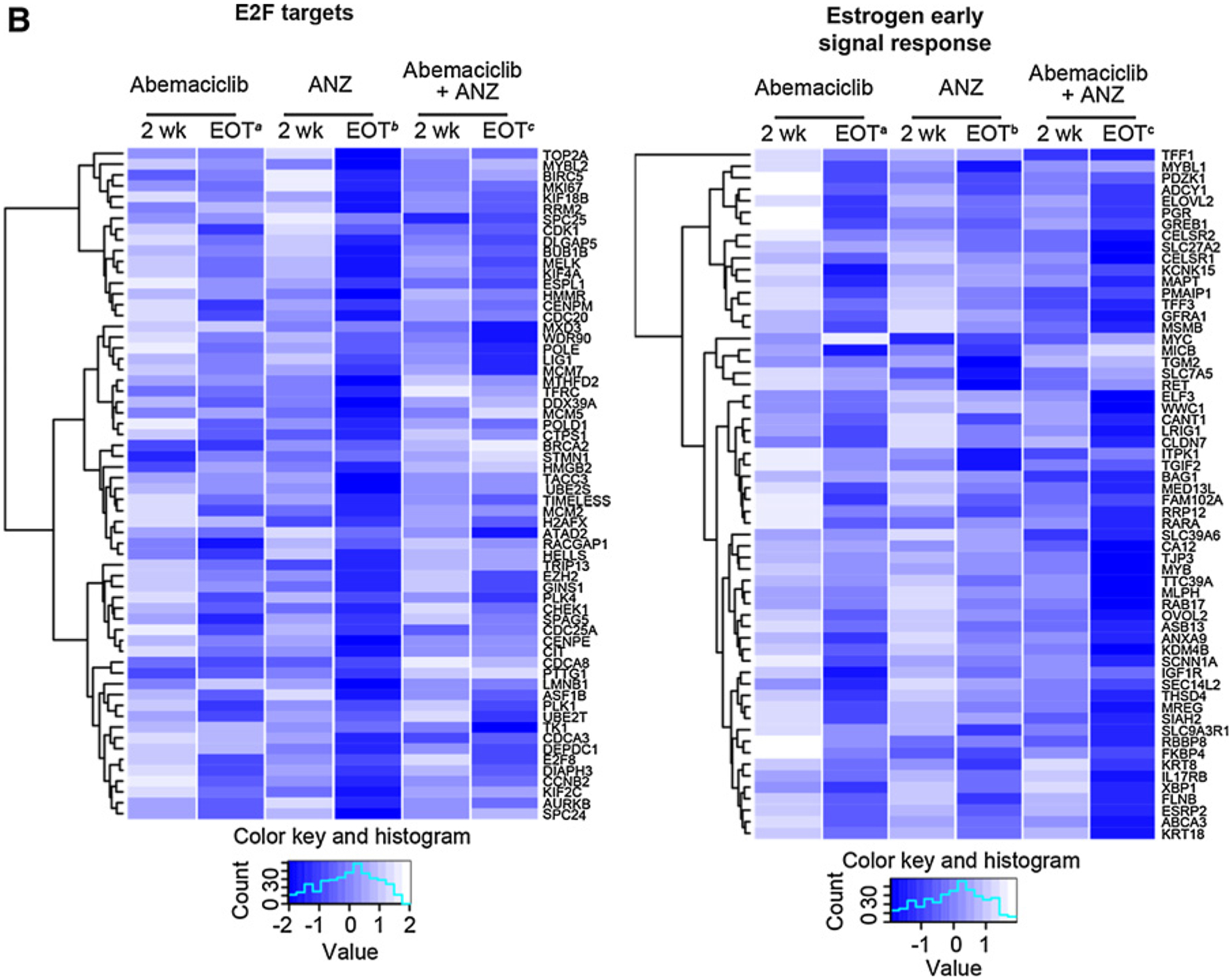
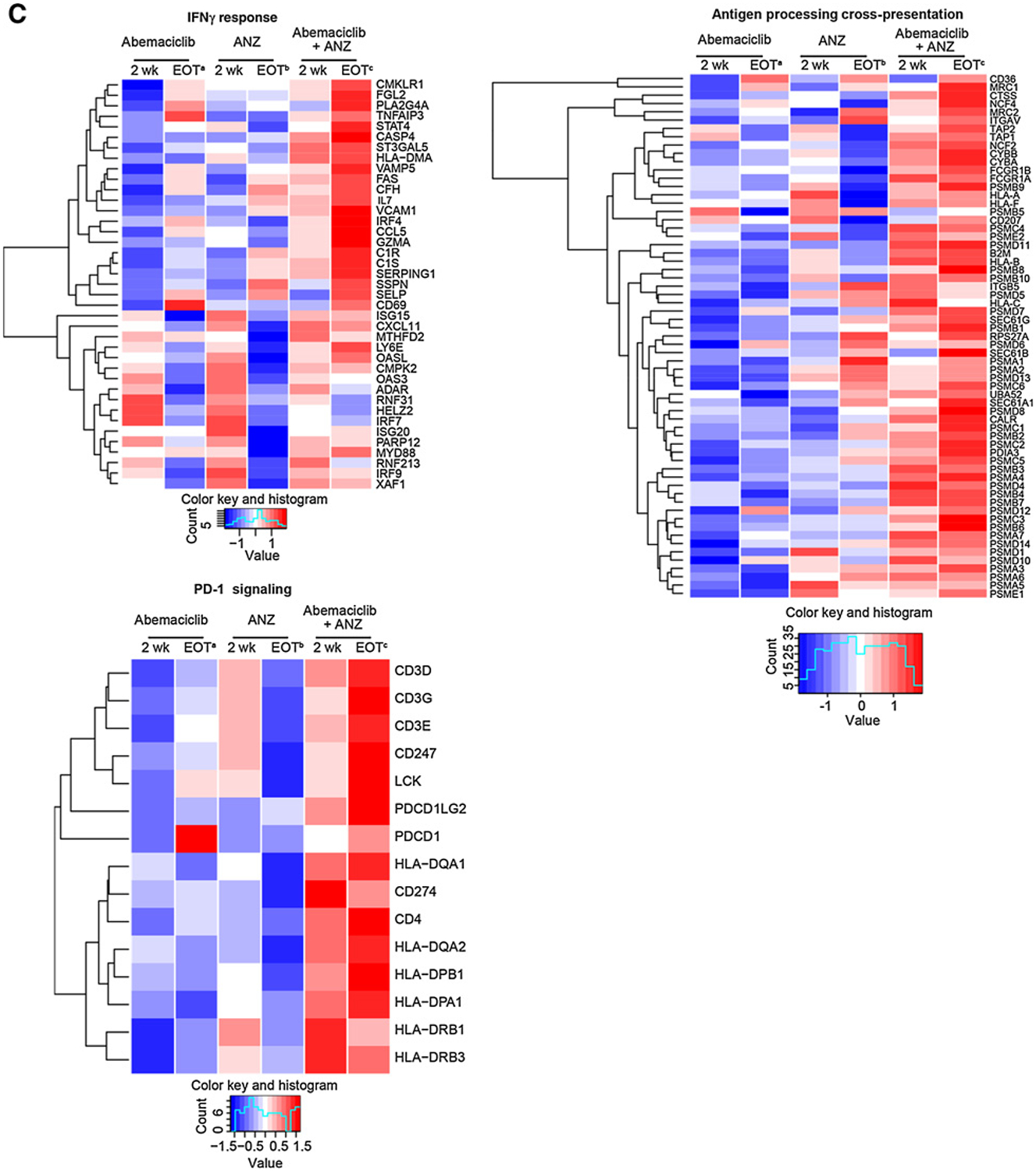
Combined treatment with abemaciclib and anastrozole (ANZ) downregulates cell-cycle processes and estrogen signaling and upregulates immune-response gene expression in HR+/HER2− early-stage breast cancer. A, Topmost enriched GSEA pathways across treatment arms and timepoints. *Nonsignificant effect. B, Heatmap of core-enriched genes in cell cycle–related and estrogen signaling gene sets. C, Heatmap of core-enriched genes in immune-related gene sets. Genes that were significantly differentially expressed across treatment arms and time points are shown in B and C. Abbreviations: wk, week. a2 weeks of initial therapy with abemaciclib followed by 14 weeks with combination therapy; b2 weeks of initial therapy with anastrozole followed by 14 weeks with combination therapy; c2 weeks of initial therapy with abemaciclib + anastrozole followed by 14 weeks with combination therapy.
Discussion
neoMONARCH met its primary endpoint of change in Ki67 expression by demonstrating a greater decrease in expression of the tumor Ki67 levels after 2 weeks of abemaciclib alone (−91%) or in combination with anastrozole (93%) compared with anastrozole alone (−63%). These data corroborated the previously described in vivo antiproliferative effect of abemaciclib (9) and are consistent with previous studies (26, 27). In contrast to studies in metastatic breast cancer, we did not observe a synergistic effect on CCCA response with combination treatment. It is possible that 2 weeks is not sufficient to see synergy with combination treatment versus abemaciclib alone. Alternatively, abemaciclib alone can potently inhibit cell-cycle progression resulting in CCCA, and because Ki67 is already suppressed we are not able to observe this synergistic effect by measuring Ki67 expression. Subgroup analysis of the percent change in Ki67 from baseline to 2 weeks of treatment showed that abemaciclib alone or in combination with anastrozole benefitted all groups of HR+/HER2− breast tumors including those exhibiting persistent tumor proliferation with ET alone, including high-grade and luminal B tumors.
Treatment with abemaciclib led to potent cell-cycle arrest in patients with HR+/HER2− early breast cancer. Complete cell-cycle arrest was achieved in most patients after 2 weeks of treatment with abemaciclib either alone or in combination with anastrozole as compared with anastrozole alone, supporting the notion that a cell-cycle inhibitor such as a CDK4/6 inhibitor is important in potent cell-cycle arrest and antitumor activity in HR+/HER2− breast cancer. Ki67 and gene expression analysis identified tumors that may benefit most from abemaciclib plus anastrozole. In this study, baseline expression of CCAGs including CCNE1 did not predict response to treatment; however, early changes in CCAG expression from baseline to 2 weeks correlated with Ki67 response. Therefore, gene expression assessment before and on-treatment could potentially be utilized to identify and select patients who may derive the most benefit from the addition of a CDK4/6 inhibitor to ET.
This study adds further credence for continued treatment with a CDK4/6 inhibitor to maintain cell-cycle inhibition. Among patients with CCCA response at 2 weeks, Ki67 expression rebounded at EOT mainly in patients who discontinued abemaciclib and anastrozole for more than 4 days prior to the EOT biopsy. In contrast, the majority of patients who remained on abemaciclib and anastrozole on the day of biopsy did not exhibit a rebound in Ki67. Three patients’ tumors, however, demonstrated a numerical increase in EOT Ki67 (Ki67: 34%→0.1%→12%; Ki67: 29%→2%→3%; Ki67: 13%→3%→4%) despite remaining on study treatment on the day of biopsy. The mechanism and clinical significance of this increase is unclear but the possibility of acquired resistance between 2 and 16 weeks of therapy cannot be excluded. The observation of Ki67 rebound upon discontinuation of a CDK4/6 inhibitor is in line with previously reported data from the neoadjuvant study of palbociclib (NeoPalAna), showing the antiproliferative effect measured by Ki67 decrease was reversible after patients stopped palbociclib treatment but continued ET until surgery (27). In aggregate, these data suggest that continuous CDK4/6 inhibition is important in maintaining antiproliferative effects in cancer cells.
Analyses of gene expression changes after therapy confirmed the ability of abemaciclib, anastrozole, and the combination to down-regulate genes associated with cell cycle and estrogen signaling. We did not detect any histologic changes in the evaluation of TILs in tumors across treatment arms after 2 or 16 weeks of treatment. However, interrogation of gene expression using RNA-seq in a limited dataset revealed an upregulation of inflammatory and T cell–related pathways following abemaciclib plus anastrozole treatment. The effects on these pathways (PD-1 signaling, IFNγ, and allograft rejection) are similar to those previously reported (15, 16, 27). It is unclear why 2 weeks of abemaciclib or anastrozole monotherapy followed by 14 weeks of combination therapy did not demonstrate the same effects at EOT as the combination arm since all patients received 14 weeks of abemaciclib plus anastrozole. Further study is needed to explore this observation, as well as the effect of sequencing of therapy with CDK4/6 inhibitors and ET. The observation that treatment did not increase stroma TILs despite enhancement of the immune response genes suggests abemaciclib plus anastrozole treatment may lead to increased immune activation in the tumor immune environment through activation of available TILs rather than through TIL proliferation. These clinical data are consistent with the immune-modulating potential of abemaciclib plus anastrozole previously described preclinically in in vitro and in vivo (15, 16). Given the limited sample size available from the current exploratory biomarker analyses, these data should be interpreted with caution, and further studies are warranted to confirm the translational biomarker observations.
This study demonstrated a pCR of 4% (7/190) in the breast and lymph nodes with abemaciclib plus anastrozole in the neoadjuvant setting (26, 27, 36). PALLET, a large randomized trial (N = 307) of palbociclib in the neoadjuvant setting, reported a pCR rate of 3% in the breast with any nodal status and 1% in the breast and nodes (26) while a pCR rate of 0% was reported in NeoPalAna (27). One limitation of studies like these is the short treatment duration. Although 4 to 6 months is the typical duration for neoadjuvant therapy, there is a general consensus that neoadjuvant treatment less than 6 months is insufficient to reach maximal tumor response with ET-based regimens. In addition, there is a lack of long-term follow-up for survival outcome data to correlate with the 2-week Ki67 response. Nevertheless, the encouraging role of CDK4/6 inhibitors in combination with ET provided support for a number of global phase III studies evaluating the combination of a CDK4/6 inhibitor with standard of care ET in patients with early-stage HR+/HER2− breast cancer (NCT02513394, NCT03155997, NCT03701334).
The overall safety profile observed in neoMONARCH was consistent with abemaciclib clinical trials conducted in the metastatic setting. No new safety signals were observed, with the exception of a higher incidence of constipation, consistent with the use of scheduled prophylactic loperamide for at least 28 days. Although liver enzyme AEs (increased ALT or AST) were reported, most were of low grade and none of these patients showed concurrent increase of total bilirubin. The incidence of neutropenia AEs in neoMonarch was lower than the incidence reported in patients with metastatic BC in MONARCH 3 (14). This may simply be due to differences in early-stage disease versus metastatic disease, or potentially due to different regenerating capacities of the bone marrow in patients with metastatic disease who have received prior adjuvant chemotherapy, compared with patients with early stage disease who are treatment naïve.
In summary, neoMONARCH demonstrated a positive benefit-risk profile for abemaciclib in combination with anastrozole in patients with HR+/HER2− early-stage breast cancer, demonstrating biological and clinical activity in a variety of breast cancer subtypes including high-grade, high proliferating tumors. Treatment with the combination of abemaciclib plus anastrozole resulted in upregulation of several immune-related gene sets. Further studies are warranted to evaluate the potential of abemaciclib and ET in early-stage breast cancer and in combination with immune therapies.
Supplementary Material
Translational Relevance.
Endocrine therapy (ET) in combination with CDK4 and CDK6 inhibitors has improved outcomes in patients with hormone receptor–positive (HR+), HER2− advanced breast cancer. Abemaciclib is a selective CDK4 and CDK6 inhibitor approved for treatment of patients with HR+/HER2− advanced or metastatic breast cancer. neoMONARCH evaluated the biological effect of neoadjuvant administration of abemaciclib in combination with ET in patients with early-stage breast cancer. Abemaciclib, alone and in combination with anastrozole, led to potent inhibition of cell-cycle progression measured by Ki67 expression. Gene expression analyses at early and late time points demonstrated a down-regulation of cell cycle–associated genes and an upregulation of genes related to immune response. Combination therapy maintained inhibition of cell proliferation and led to activation of T-cell immune response. Gene expression analyses of treatment-resistant or -sensitive tumors defined by Ki67 expression may help identify gene signatures for CDK4 and 6 treatment sensitivity.
Acknowledgments
The authors thank the patients and their families and caregivers for participating in the neoMONARCH trial. They also acknowledge the neoMONARCH study steering committee, in addition to the investigators and their support staff, who generously participated in the neoMONARCH trial. This trial was funded by Eli Lilly and Company. The authors thank Angela Santiago (USC) for technical assistance with histology, performing Ki67 IHC, and assisting with recording of the results, as well as Ivonne Villalobos (USC) for data management. They also thank Lacey Litchfield for data interpretation/analysis and for reviewing the manuscript, Gerard Joseph Oakley III for his assistance with the image acquisition and histologic review as a Lilly pathologist, David A. Schaer for his scientific input in the analysis of the immune response data, and Shawn Estrem for his expertise on biomarkers. Annie-Carole Trampont (Eli Lilly and Company) provided medical writing assistance, and Purnima Chandra (Eli Lilly Services India Private Limited) provided editorial assistance. Medical writing support was provided by Prudence Stanford, and editorial support was provided by Barbara Jackson of Syneos Health, funded by Eli Lilly and Company. This study was funded by Eli Lilly and Company.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Trial registration (clinicaltrials.gov): NCT02441946
Disclosure of Potential Conflicts of Interest
S.A. Hurvitz reports receiving commercial research grants from Ambrx, Amgen, Bayer, Boehringer-Ingelheim, Daiichi-Sankyo, Genentech/Roche, GlaxoSmithKline, Immunomedics, Lilly, Novartis, Pfizer, Macrogenics, OBI Pharma, Pieris, PUMA, Sanofi, Seattle Genetics, Medivation, and Merrimack, and is an advisory board member/unpaid consultant for GlaxoSmithKline, Novartis, AstraZeneca, Lilly, Amgen, and Daiichi Sankyo. M. Martin is a paid consultant for Roche, Novartis, PUMA, AstraZeneca, Amgen, Taiho Oncology, Pharmamar, Eli Lilly, and Daiichi Sankyo, and reports receiving commercial research grants from Roche, Novartis, and PUMA. M.F. Press is a paid consultant for Biocartis, Puma Biotechnology, Cepheid, Karyopharm Therapeutics, Science Branding Communications, Novartis, and Zymeworks, Inc., and reports receiving commercial research grants from Cepheid, Eli Lilly, F. Hoffmann-La Roche AG, and Novartis, and other remuneration from Amgen, Inc. M. Fernandez-Abad is an advisory board member/unpaid consultant for Eli Lilly. E. Petru is an advisory board member/unpaid consultant for Abemaciclib. V. Guarneri reports receiving speakers bureau honoraria from and is an advisory board member/unpaid consultant for Eli Lilly. C. Huang reports receiving commercial research grants from Eli Lilly; other commercial research support from Roche, Novartis, MSD, EirGenix, OBI Pharma, Daiichi Sankyo, AstraZeneca, and Pfizer; and speakers bureau honoraria from Amgen, Pfizer, Roche, and Novartis, and is an advisory board member/unpaid consultant for Eli Lilly, Pfizer, Roche, and Amgen. S. Barriga, P.J. Ebert, A. Aggarwal, and V.M. Jansen are employees/paid consultants for and hold ownership interest (including patents) in Eli Lilly. S.R. Wijayawardana, A. Hossain, and J. Liu are employees/paid consultants for Eli Lilly. D.J. Slamon is a paid consultant for Eli Lilly, Novartis, and Pfizer; reports receiving commercial research grants from Novartis; and holds ownership interest (including patents) in Pfizer, Amgen, Seattle Genetics, and BioMarin. No potential conflicts of interest were disclosed by the other authors.
References
- 1.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365:60–2. [DOI] [PubMed] [Google Scholar]
- 3.Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmgenomics Pers Med 2014;7:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncolo 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 6.Iwata H. Clinical development of CDK4/6 inhibitor for breast cancer. Breast Cancer 2018;25:402–6. [DOI] [PubMed] [Google Scholar]
- 7.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 2003;36: 131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin Cancer Res 2017;23:5218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–46. [DOI] [PubMed] [Google Scholar]
- 11.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–53. [DOI] [PubMed] [Google Scholar]
- 12.Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–84. [DOI] [PubMed] [Google Scholar]
- 13.Torres-Guzman R, Calsina B, Hermoso A, Baquero C, Alvarez B, Amat J, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 2017;8:69493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston S, Martin M, Di Leo A, Im S-A, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep 2018;22:2978–94. [DOI] [PubMed] [Google Scholar]
- 16.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson H, Hills M, Zabaglo L, A’Hern R, Leary AF, Haynes BP, et al. Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Ann Oncol 2011;22:1770–6. [DOI] [PubMed] [Google Scholar]
- 18.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167–70. [DOI] [PubMed] [Google Scholar]
- 19.Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 2017;35:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100: 1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrido-Castro AC, Goel S. CDK4/6 inhibition in breast cancer: mechanisms of response and treatment failure. Curr Breast Cancer Rep 2017;9:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 2016;13:417–30. [DOI] [PubMed] [Google Scholar]
- 23.Lynce F, Shajahan-Haq AN, Swain SM. CDK4/6 inhibitors in breast cancer therapy: current practice and future opportunities. Pharmacol Ther 2018;191: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011;103:1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giltnane JM, Hutchinson KE, Stricker TP, Formisano L, Young CD, Estrada MV, et al. Genomic profiling of ER(+) breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci Transl Med 2017;9:pii:eaai7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor–positive early breast cancer: PALLET Trial. J Clin Oncol 2019;37:178–89. [DOI] [PubMed] [Google Scholar]
- 27.Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res 2017;23:4055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guarducci C, Bonechi M, Boccalini G, Benelli M, Risi E, Di Leo A, et al. Mechanisms of resistance to CDK4/6 inhibitors in breast cancer and potential biomarkers of response. Breast Care 2017;12:304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol 2019;37:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malorni L, Piazza S, Ciani Y, Guarducci C, Bonechi M, Biagioni C, et al. A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget 2016;7:68012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang H, Huang D, Yang F, Guan X. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat 2018;168:287–97. [DOI] [PubMed] [Google Scholar]
- 32.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res 2016;76:2301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansen VM, Bhola NE, Bauer JA, Formisano L, Lee KM, Hutchinson KE, et al. Kinome-wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer. Cancer Res 2017; 77:2488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teo ZL, Versaci S, Dushyanthen S, Caramia F, Savas P, Mintoff CP, et al. Combined CDK4/6 and PI3Kalpha inhibition is synergistic and immunogenic in triple-negative breast cancer. Cancer Res 2017;77:6340–52. [DOI] [PubMed] [Google Scholar]
- 35.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2016;2:1354–60. [DOI] [PubMed] [Google Scholar]
- 36.Cottu P, D’Hondt V, Dureau S, Lerebours F, Desmoulins I, Heudel PE, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol 2018;29:2334–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


