Abstract
Background:
Subthalamic nucleus deep brain stimulation (STN DBS) surgery is clinically effective for treatment of cervical dystonia (CD) however the underlying physiology has not been examined. We used transcranial magnetic stimulation (TMS) to examine the effects of STN DBS on sensorimotor integration, sensorimotor plasticity and motor cortex excitability which are identified as the key pathophysiological features underlying dystonia.
Methods:
TMS paradigms of short latency afferent inhibition (SAI) and long latency afferent inhibition (LAI) were used to examine the sensorimotor integration. Sensorimotor plasticity was measured with paired associative stimulation (PAS) paradigm and motor cortex excitability was examined with short latency intracortical inhibition (SICI) and intracortical facilitation (ICF). DBS was turned OFF and ON to record these measures.
Results:
STN DBS modulated SAI and LAI which correlated well with the acute clinical improvement. While there were no changes seen in the motor cortex excitability, DBS was found to normalize the sensorimotor plasticity however there was no clinical correlation.
Conclusion:
Modulation of sensorimotor integration is a key contributor to clinical improvement with acute stimulation of STN. Since the motor cortex excitability didnot change and the change in sensorimotor plasticity didnot correlate with clinical improvement, STN DBS has restrictions in physiological effects.
Keywords: Deep brain stimulation, cervical dystonia, subthalamic nucleus
INTRODUCTION
Subthalamic nucleus (STN) deep brain stimulation (DBS) is an effective target for control of cervical dystonia (CD).1 Ostrem et al found STN DBS improved CD symptoms by greater than 50% when followed for 36 months after surgery2 however the range of clinical improvement was wide (30–80%) and the underlying physiological changes were not examined.
Transcranial magnetic stimulation (TMS) studies in dystonia have identified an abnormal sensorimotor integration, an excessive sensorimotor plasticity and an increased motor cortex excitability as key pathophysiological features.3, 4 Short and long latency afferent inhibition (SAI and LAI) are effective TMS measures for examination of sensorimotor integration. SAI is defined as inhibition of motor evoked potentials (MEP) recorded from hand muscle when peripheral nerve stimulation at the wrist precedes delivery of TMS pulse over the contralateral motor cortex by ~20ms. If the peripheral nerve stimulation precedes the TMS pulse by ~200ms, it is defined as LAI.5 STN DBS in Parkinson’s disease has been found to restore the SAI and the LAI to approximately normal levels6 but the effects in dystonia are not known.
Many studies in dystonia have found an increased sensorimotor plasticity which is effectively measured with a paired associative stimulation (PAS) paradigm involving repetitive pairing of peripheral sensory stimulation with a TMS pulse to the motor cortex. .7, 8,. Short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) determined with paired pulse paradigms at specific interstimulus interval (ISI) are established TMS measures of the motor cortex excitability. ;). We examined the effects of STN DBS on these key pathophysiological features in a cohort of CD patients and determine if there was a clinical correlation..
MATERIALS AND METHODS
We enrolled 10 medication-refractory predominantly CD patients with bilateral STN DBS in an IRB approved TMS study (details in supplemental methods). Ten healthy controls (mean age ± SD, 46.7 ± 16.2; five males, five females, age range 22–68 years) were enrolled for normative TMS data.
CD subjects were examined on two consecutive days during DBS OFF (turned OFF for 4–8 hours) and DBS ON conditions assigned in random order. TMS recordings followed by clinical measures consisting of Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) and visual analogue scale (VAS) were performed each day. For DBS OFF recordings,
TMS recordings
Standard paired stimulation TMS techniques were used for SAI at interstimulus interval (ISI) of 20 and 30ms, LAI at 150 and 200ms, SICI at 2 and 3ms and ICF at 10 and 15ms. For PAS, 90 pairs of median nerve stimulation and TMS pulse at 25ms ISI were delivered over 30 minutes.11
Statistical analysis
.SAI, LAI, SICI and ICF measures were all expressed as ratio of conditioned (with preceding pulses) to the unconditioned (test pulse alone) motor evoked potential (MEP). Ratios <1 represented inhibition, and ratios >1 indicated facilitation.
Two-way repeated measures ANOVA (SPSS 24) with statistical significance set to a p ≤ 0.05 was used to compare the effects of DBS, and ISI for each TMS measure. Post-hoc tests corrected for multiple comparisons were performed. PAS plasticity was expressed as the percent change in both mean and maximum MEP compared to the pre-PAS--protocol MEP size. , . TMS measures were correlated with clinical scores using the Spearman correlation coefficient.
RESULTS
Seven males and three females with CD, mean age 45.6 years ± 16.2 (SD) age range 20–77 years, mean disease duration 14.8 ± 9.4 years and mean DBS duration 2.9 ± 1.6 years participated in the study. (Table 1).
Table:
Clinical profile of patients
| Age | Sex | Dystonia phenotype | Medications | Duration of dystonia in years | Duration of DBS in years | DBS settings | Battery type | TWSTRS severity OFF, ON | TWSTRS pain OFF, ON | VAS disability OFF, ON | VAS pain OFF, ON | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | ||||||||||||
| 1 | 20 | M | segmental dystonia | none | 8 | 4 | C+1–3-; 3V; 130Hz; 60PW; 862Ω | 0–1-2+; 2.4V; 130Hz; 60PW; 1719Ω | Activa PC | 12, 7 | 2, 2 | 7, 2 | 2, 1 |
| 2 | 55 | M | segmental dystonia | clonazepam 3mg, alprazolam 3mg | 30 | 2 | C+11-; 1.9V; 125Hz; 60PW; 790Ω | 2+ 0–1-; 3.2V; 125Hz; 60PW; 862Ω | Activa PC | 21, 5 | 11, 3 | 7, 1 | 5, 2 |
| 3 | 45 | M | segmental dystonia, dyt1 | 23 | 3 | C+8–9-10-; 1.5V; 160Hz; 150PW; 898Ω | C+1-; 1.6V; 130Hz; 60PW; 980Ω | Activa PC | 21, 4 | 0, 0 | 7, 1 | 0, 0 | |
| 4 | 60 | F | segmental dystonia | buproprion 75mg, fluoxetine10mg | 9 | 5 | 5+6-; 2V; 130Hz; 60PW; 1024Ω | 2+3-; 3.2V; 130Hz; 60PW; 1011Ω | Activa PC | 15, 5 | 10, 3 | 6, 1 | 3, 0 |
| 5 | 29 | M | segmental dystonia | 16 | 6 | C+5-; 2.5V; 140Hz; 60PW; 765Ω | C+1-; 2.5V; 140Hz; 60PW; 863Ω | Activa PC | 12, 9 | 6, 3 | 5, 4 | 2, 0 | |
| 6 | 77 | F | segmental dystonia | diazepam 5mg,baclofen 10mg, benadryl 25mg | 13 | 3 | 2+1-; 3V; 90Hz; 90PW; 1035Ω | 3+2-; 2.4V; 130Hz; 90PW; 1310 Ω | Soletra | 25, 16 | 12, 7 | 8, 6 | 7, 2 |
| 7 | 65 | M | cervical dystonia + blepharospasm | none | 3 | 2 | C+2–3-; 2V; 135Hz; 90PW; 662Ω | C+1–2-; 2.2V; 135Hz; 90PW; 750Ω | Activa SC | 25, 6 | 13.5, 3 | 8, 3 | 4, 0 |
| 8 | 69 | M | cervical dystonia + blepharospasm | trihexyphenidyl 2mg | 17 | 2 | C+2-; 2.2V; 135Hz; 90PW; 795Ω | 3+2-; 2.1V; 135Hz; 90PW; 1229Ω | Activa SC | 22, 11 | 12, 4.5 | 2, 1 | 2, 1 |
| 9 | 44 | F | cervical dystonia + blepharospasm | trazodone 100mg, trihexyphenidyl 6mg, clonazepam 1.5mg | 3 | 1 | C+1-; 1.5V; 100Hz; 150PW; 1038Ω | 2+1-; 2.1V; 135Hz; 60Hz; 1093Ω | Activa SC 37603 | 27, 14 | 21, 8 | 8, 5 | 8, 4 |
| 10 | 66 | M | cervical dystonia | diazepam 5mg | 26 | 2 | C+10-; 2V; 135Hz; 90PW; 1088Ω | C+0-; 2V; 135Hz; 120PW; 1304Ω | Activa PC | 22, 5 | 12, 4 | 8, 3 | 8, 4 |
The main effects for DBS (F 1,9 = 7.32, p = 0.024), and ISI (F 1,9 = 11.9, p = 0.007) were significant for SAI however the interaction effects were not significant; DBS x ISI (p = 0.57), Whereas for LAI, the main effects for DBS (F 1, 9 = 0.08, p = 0.78) were not significant, however they were significant for ISI (F 1,9 = 5.7, p = 0.04) and interaction effects for DBS x ISI (F 1,9 = 6.6, p = 0.03) were significant Post hoc testing showed that LAI 200 OFF was significantly reduced compared to LAI 200 ON (p = 0.01). All the remaining comparisons were not significant (LAI 150 OFF and LAI 150 ON, p = 0.61; LAI 150 OFF and LAI 150 ON, p = 0.84; LAI 200 OFF and LAI 200 ON, p = 0.41) (Figure).
Figure 1:
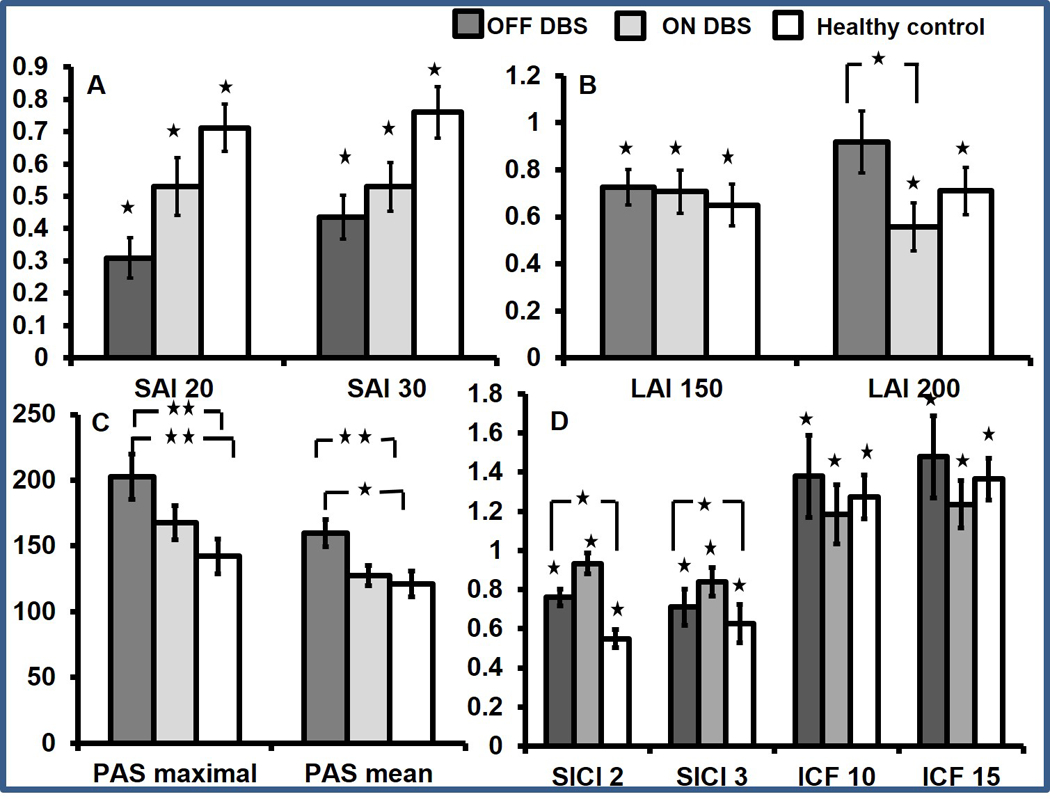
Figure 1 (A) Group average data on short latency afferent inhibition (SAI) in patients with cervical dystonia during DBS OFF and ON and in healthy controls. Compared with test pulse, SAI is significant only when single stimulation paradigm is applied. In comparison with healthy controls, SAI is significantly increased. SAI is found to be increased at interstimulus interval (ISI) of 20 and 30 ms in the presence of single median nerve stimulation when DBS is turned off. The x-axis indicates the groups and the ISIs studied. The y-axis shows afferent inhibition as ratios of the conditioned (test stimulus with preceding peripheral nerve stimulation) to the unconditioned (test stimulus alone) MEP amplitude. Values <1 indicate afferent inhibition. Dark grey bar represents DBS OFF, light grey represents DBS ON, and white bar represents healthy control subjects. Error bars represent SEs. Asterisks above the bars indicate significant inhibition compared with test pulse alone. Asterisks between the bars indicate significant difference between the groups (factorial ANOVA and post hoc testing). *P<0.05. (B) Group average data on long latency afferent inhibition (LAI) in patients with cervical dystonia during DBS OFF and ON and in healthy controls. Compared with test pulse LAI is significant only when single stimulation paradigm is applied. LAI is found to be reduced at an ISI of 200 ms in the presence of single median nerve stimulation when DBS is turned off. LAI does not change with DBS when dual nerve stimulation is used. The x-axis indicates the groups and the ISIs studied. The y-axis shows afferent inhibition as ratios of the conditioned (test stimulus with preceding peripheral nerve stimulation) to the unconditioned (test stimulus alone) MEP amplitude. Values <1 indicate afferent inhibition. Dark grey bar represents DBS OFF, light grey represents DBS ON, and white bar represents healthy control subjects. Error bars represent SEs. Asterisks above the bars indicate significant inhibition compared with test pulse alone. Asterisks between the bars indicate significant difference between the groups (factorial ANOVA and post hoc testing). *P<0.05. (C) Paired associative stimulation (PAS) MEP amplitudes are shown. PASmaximal and PASmean (MEP average over three time points) recorded from the Abductor pollicis brevis (APB) muscle after the PAS protocol is employed. The data are plotted as a ratio to the baseline MEP amplitude. Ratios higher than 1 indicate facilitation and ratios below 1 indicate inhibition. PASmaximal and PASmean are seen to increase when DBS is turned off. Error bars represent SEs. Asterisks between the bars indicate significant difference between the groups (factorial ANOVA and post hoc testing). *P<0.05, **P<0.01. (D) Group average data on short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) in patients with cervical dystonia during DBS OFF and ON and in healthy controls. Compared with test pulse SICI and ICF are significant for all three groups. SICI significantly increased compared with healthy controls during DBS OFF. However, there are no effects of DBS on SICI and ICF. The x-axis indicates the groups and the ISIs studied. The y-axis shows afferent inhibition as ratios of the conditioned (test stimulus with preceding peripheral nerve stimulation) to the unconditioned (test stimulus alone) MEP amplitude. Values <1 indicate inhibition and values >1 indicate facilitation. Dark grey bar represents DBS OFF, light grey represents DBS ON, and white bar represents healthy control subjects. Error bars represent SEs. Asterisks above the bars indicate significant inhibition compared with test pulse alone. *P<0.05. ANOVA, analysis of variance; DBS, deep brain stimulation; MEP, motor evoked potential.
The change in SAI with STN DBS had a significant linear correlation with change in TWSTRS severity scores (r = 0.56, p = 0.01), TWSTRS pain scores, (r = 0.62, p = 0.007), VAS disability scores (r = 0.76, p = 0.01) and VAS pain scores (r = 0.78, p = 0.008). Similarly, the change in LAI correlated with change in TWSTRS measures (severity r = 0.75, p = 0.025; pain r = 0.51, p = 0.03), however correlations with VAS measures only approached significance (disability r = 0.45, p = 0.05; pain r = 0.38, p = 0.054).
There was a significant increase in PASmean (p = 0.001) and PASmaximal (p = 0.002) when DBS was turned OFF (PASmean 139.1 ± 32.2; PASmaximal182.3 ± 54.2) compared to DBS ON (PASmean 127.6 ± 24.1; PASmaximal 167.7 ± 41.8) in CD subjects. PASmean and PASmaximal were significantly different between DBS OFF and healthy controls (PASmean 121.2 ± 31.5, p = 0.03; PASmaximal 142.1 ± 41.6, p = 0.01). There was no correlation between DBS induced change in PAS and clinical scores (TWSTRS severity r = 0.26, p = 0.45; TWSTRS pain r = − 0.42, p = 0.22; VAS disability r = −0.5, p = 0.13; VAS pain r = − 0.32, p = 0.37)
There were no significant main effects of DBS and ISI on SICI (DBS p = 0.67; ISI p = 0.23) and ICF (DBS p = 0.12; ISI p = 0.22) There were no significant interaction effects. Compared to healthy controls, SICI during DBS OFF in CD subjects was significantly reduced (SICI 2, p = 0.04; SICI 3, p = 0.04) however there was no significant difference in ICF.
DISCUSSION
The main findings of the study were as follows. STN DBS modulated SAI and LAI which correlated well with clinical improvement. STN DBS normalized the PAS plasticity however there was no correlation with clinical improvement and STN DBS had no effects on SICI and ICF. These findings suggest modulation of sensorimotor integration plays an important role whereas change in plasticity has a restricted role and modulation of motor excitability doesnot contribute to acute changes in dystonia symptoms.
The SAI is an inhibitory cortical phenomenon elicited when a peripheral sensory input interacts with the motor cortex either through direct thalamocortical projections or a short travel involving few processing steps whereas LAI involves activation of many sensory areas12, 13 probably In a writer’s cramp study, SAI was observed to increase in the abductor digiti minimi muscle during phasic contraction of FDI muscle, which was speculated to reflect a compensatory mechanism to prevent overflow of dystonia from the index finger to the little finger.14 Similarly an increased SAI in our study during DBS OFF which corrected with stimulation probably represented a compensatory mechanism in response to worsening of dystonia symptoms. However Zittel et al found SAI to be reduced in cervical dystonia which is in contrast to our DBS OFF findings.15 We believe a lack of complete washout from the effects of DBS may likely explain the discrepant finding. STN DBS also normalized a significantly reduced LAI (compared to healthy controls) which correlated well with clinical improvement. STN likely modulates sensorimotor integration (SAI and LAI) through alteration of corticomotor output in response to peripheral sensory input. We speculate these effects could be either mediated through orthodromic thalamocortical pathway or through antidromic activation of the hyperdirect pathway projecting from the motor cortex to the STN. A future study involving DBS tractography is necessary to shed further insights.
Although Ruge et al found the PAS plasticity with GPi stimulation did not change despite DBS turned OFF for nearly two days, we found acute STN stimulation led to a reduced PAS plasticity. These differences could be attributed to different stimulation target (STN versus GPi), the underlying phenotype (focal versus generalized DYT1 positive dystonia) and the duration of DBS OFF (4 hours versus 2 days) STN DBS modulate the pathway mediating this plasticity however17 a lack of clinical correlation suggests that unlike SAI and LAI, sensorimotor plasticity measured with PAS does not appear to drive the acute clinical improvements with STN DBS. We also did not find any significant effects of STN DBS on the motor cortex excitability although a previous study found SICI to normalize after several months of GPi DBS 18
We speculate that a limitation in the capacity of DBS to modulate all pertinent pathophysiological features may possibly contribute to the wide range of clinical improvements We acknowledge the study was limited by a moderate sample size, DBS OFF time was relatively short and maynot have achieved a complete washout and the study focusses on acute effects of STN DBS. Nevertheless, our study is unique and enhances the understanding of the physiology underpinning the clinical effects of STN DBS. Future studies should longitudinally track the physiology to identify the potential markers of DBS outcomes.
Supplementary Material
Acknowledgment:
NIH K23 NS092957-01A1 (AWS)
Financial Disclosure:
Dr. Aparna Wagle Shukla – Reports grants from the NIH and has received grant support from Benign Essential Blepharospasm Research foundation, Dystonia coalition, Dystonia Medical Research foundation, National Organization for Rare Disorders and grant support from NIH (NIH KL2 TR000065)
Dr Jill Ostrem - Received research support from St. Jude Medical, Boston Scientific, Cala Health, and Google and received educational grant support from Medtronic, Boston Scientific, Merz, Allergan, and AbbVie. She has also received grant support from the Michael J Fox Parkinson’s Disease Foundation
Dr. David E. Vaillancourt – Reports grant support from NIH, Bachmann-Strauss Foundation, and Tyler’s Hope Foundation. He is co-founder and manager of Neuroimaging Solutions, LLC.
Dr Robert Chen - Received research support from the Canadian Institutes of Health Research, Catherine Manson Chair in Movement Disorders, the Weston Foundation, Medtronic Inc and Merz. He was a consultant for Merz, Allergan and UCB.
Dr Kelly Foote- Received research support and fellowship support from Medtronic, and has received an honorarium from Medtronic for chairing an expert DBS practitioners’ symposium. He has received research support from, and serves on the neurosurgery advisory board for Neuropace. He has also received research support from St. Jude, Boston Scientific, and Functional Neuromodulation. All listed research support has been granted to the University of Florida and Dr. Foote has derived no personal financial gain from these studies
Dr. Michael S. Okun – Serves as consultant for the National Parkinson’s Foundation, and has received research grants from the National Institutes of Health, National Parkinson’s Foundation, Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, and UF Foundation. Dr. Okun has previously received honoraria, but in the past > 60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36 months) sponsored by PeerView, Prime, Quantia, Henry Stewart, and the Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
References
- 1.Kleiner-Fisman G, Liang GS, Moberg PJ, et al. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: Impact on severity, neuropsychological status, and quality of life. J Neurosurg 2007;107:29–36. [DOI] [PubMed] [Google Scholar]
- 2.Ostrem JL, San Luciano M, Dodenhoff KA, et al. Subthalamic nucleus deep brain stimulation in isolated dystonia: A 3-year follow-up study. Neurology 2017;88:25–35. [DOI] [PubMed] [Google Scholar]
- 3.Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord 2013;28:958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagle Shukla A, Vaillancourt DE. Treatment and physiology in parkinson’s disease and dystonia: Using transcranial magnetic stimulation to uncover the mechanisms of action. Curr Neurol Neurosci Rep 2014;14:449–014-0449–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in parkinson’s disease. Brain 2003;126:1883–1894. [DOI] [PubMed] [Google Scholar]
- 6.Sailer A, Cunic DI, Paradiso GO, et al. Subthalamic nucleus stimulation modulates afferent inhibition in parkinson disease. Neurology 2007;68:356–363. [DOI] [PubMed] [Google Scholar]
- 7.Weise D, Schramm A, Stefan K, et al. The two sides of associative plasticity in writer’s cramp. Brain 2006;129:2709–2721. [DOI] [PubMed] [Google Scholar]
- 8.Quartarone A, Bagnato S, Rizzo V, et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain 2003;126:2586–2596. [DOI] [PubMed] [Google Scholar]
- 9.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: A consensus update. Mov Disord 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Chen R, Ashby P. Safety of transcranial magnetic stimulation in patients with implanted deep brain stimulators. Mov Disord 1999;14:157–158. [DOI] [PubMed] [Google Scholar]
- 11.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000;123 Pt 3:572–584. [DOI] [PubMed] [Google Scholar]
- 12.Di Lazzaro V, Oliviero A, Profice P, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 2000;135:455–461. [DOI] [PubMed] [Google Scholar]
- 13.Tokimura H, Di Lazzaro V, Tokimura Y, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 2000;523 Pt 2:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson SP, Bliem B, Lomarev M, Shamim E, Dang N, Hallett M. Changes in short afferent inhibition during phasic movement in focal dystonia. Muscle Nerve 2008;37:358–363. [DOI] [PubMed] [Google Scholar]
- 15.Zittel S, Helmich RC, Demiralay C, Munchau A, Baumer T. Normalization of sensorimotor integration by repetitive transcranial magnetic stimulation in cervical dystonia. J Neurol 2015;262:1883–1889. [DOI] [PubMed] [Google Scholar]
- 16.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain 2004;127:4–20. [DOI] [PubMed] [Google Scholar]
- 17.Wagle Shukla A, Moro E, Gunraj C, et al. Long-term subthalamic nucleus stimulation improves sensorimotor integration and proprioception. J Neurol Neurosurg Psychiatry 2013;84:1020–1028. [DOI] [PubMed] [Google Scholar]
- 18.Ruge D, Tisch S, Hariz MI, et al. Deep brain stimulation effects in dystonia: Time course of electrophysiological changes in early treatment. Mov Disord 2011;26:1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


