Abstract
Background/Aims
The importance of hyperplastic polyps during colorectal carcinogenesis is appreciated related to the understanding of serrated pathway. The morphologic subtypes of hyperplastic polyps in carcinogenesis and the nomenculature of lesions with both hyperplastic and adenomatous areas are controversial. We aimed to reveal the molecular properties of hyperplastic polyp subtypes and the molecular changes in polyps containing both hyperplastic and adenomatous areas.
Materials and Methods
49 hyperplastic polyps [19 microvesicular (MVHP), 19 goblet-rich (GRHP), 11 mucin-poor (MPHP)] and 10 mixed hyperplastic and adenomatous polyps were analysed for KRAS, BRAF mutations and MSI with real-time PCR.
Results
68,4% of MVHPs and 81% of MPHPs which were localized in right colon had BRAF mutations. While none of the GRHPs showing a KRAS mutation with a rate of 73% was localized in the ascending colon, 63% of them were localized in the rectosigmoid area. In five (50%) of the mixed polyps, KRAS mutation was detected both in the hyperplastic and adenoma components. There was no BRAF mutation in any of the mixed polyps. However, in two cases, the hyperplastic component was MSI-H and the adenoma area was MSS.
Conclusion
Hyperplastic polyps, even if smaller than 5 mm, are precancerous lesions bearing different mutations. GRHPs with predominant KRAS mutations and MVHPs and MPHSs with predominant BRAF mutations are precancerous. Although the molecular investigations for HPP/SP are not necessary the morphological subtyping should be included if the case is diagnosed with HPP/SP as it will be useful for attracting the gastroenterologist’s attention.
Keywords: Molecular, hyperplastic polyps, colorectal, subgroup
INTRODUCTION
Hyperplastic polyp (HPP) was first described by Morson in 1962 as “metaplastic mucosal polyp,” as Arthur wrote in his article (1). In the following years, various publications emphasizing the difference of these lesions from adenomas (ADs) described these polyps as mucosal lesions with a leafy appearance consisting of dilated tubules showing serration (1–3). Arthur suggested that these lesions may be degenerative and develop due to the aging of the mucosa in adults older than 40 years (2). A few years later, an ultrastructural study also suggested that HPPs comprised normal but overdeveloped absorptive cells (4). In the following years, many studies reported that HPPs up to 5 mm, especially if localized in the left colon, were not precursors of cancer (1, 3, 5). Studies on HPP cases, including adenomatous changes or of the carcinoma area, have been published during the same period of time as reports of the nature of HPPs and nonneoplastic lesions (6–9). Even though most studies classifying colorectal polyps reported that more than 90% of all HPPs were smaller than 5 mm (1–3, 5), Estrada reported that more than half of the polyps up to 5 mm were ADs, and adenomatous changes had been detected in 13% of hyperplastic (HP) changes (7). After Cecilia proposed “polyps with mixed hyperplastic and adenomatous characteristics” terminology, “mixed hyperplastic adenomatous polyp (MHAP)” identification was introduced in the literature (5, 10). In 1990, Longacre emphasized that mixed polyps could be divided into at least two groups. The first group comprised polyps in which traditional HP/AD areas could be sharply circumscribed, and the second group comprised the polyps with AD-like characteristics in glands carrying structural hyperplastic characteristics (MHAP) and the author suggested to term these polyps as “mixed hyperplastic adenomatous polyps/serrated adenoma” (11).
In the next 10 years, different pathways in colon cancer development and molecular changes in HPPs and mixed polyps were identified. Studies showing microsatellite instability (MSI) and KRAS mutations in traditional HPPs increased the evidence of HPPs being neoplastic lesions (12–15). Torlakovic, who grouped all colorectal polyps with serration under the title “serrated polyps,” defined the localization, molecular and morphological characteristics of these polyps. According to him, although serrated polyps had common characteristics, serration, architectural distortion, and nuclear atypia were present in serrated polyps that were localized in the right-sided colon, while thickening in surface basal membrane and muscularis mucosa were more prominent in serrated polyps that were localized in the left-sided colon. Loss of MLH1 and MSH2 was prominent in serrated polyps showing abnormal proliferation without any difference in localization.
As a result of these morphological and molecular findings, Torlakovic et al. emphasized the distinct differences between serrated polyps located in the left and right colons, and according to the mucin amount and distribution type, he proposed the terminologies for the normally proliferated serrated polyps in the left colon as microvesicular serrated polyps, goblet cell serrated polyps, and mucin-poor serrated polyps. For abnormally proliferated polyps, the authors suggested the use of sessile serrated ADs for those located in the right colon and traditional serrated AD for those in the left colon (16).
Studies aiming to elucidate the molecular properties of serrated polyps have revealed that although microvesicular HPs, mucin-poor HPs, and sessile serrated ADs/polyps carry BRAF mutations, they also cause MSI and tumors with CpG island hypermethylation. Furthermore, although goblet-rich polyps carry KRAS mutations more frequently, traditional serrated ADs can have BRAF or KRAS mutations. However, as the name indicates, the molecular properties of mixed adenomatous HPPs are not clear. In this study, we focused on the molecular changes in mixed polyps in which traditional HP/AD components were sharply circumscribed and especially in normally proliferated serrated polyp subtypes.
MATERIALS AND METHODS
Hematoxylin-eosin sections of HPPs and AD lesions of the last 5 years, which were in the archives of the Department of Pathology, were re-evaluated regardless of their localization. Of the samples best representing the morphological definition of Torlakovic, 19 microvesicular-type hyperplastic polyps (MVHPs), 19 goblet cell-rich hyperplastic polyps (GRHPs), and 11 mucin-poor hyperplastic polyps (MPHPs) were included in the study (16, 17). Also, 10 mixed polyps (HP/AD) that were suitable for microdissection and whose HP and AD components could be sharply circumscribed were selected. Five-micrometer-thick sections (n=5) of HPPs were taken in eppendorf tubes. The HP and AD sections of the mixed polyps were separated by “manual microscopic examination” (18) (Figure 1, mixed polyps slide), and 5-μm-thick sections (n=5) of these were taken in eppendorf tubes. DNA was extracted following the manufacturer’s protocols (cobas DNA sample preparation kit, Roche Diagnostics, Risch-Rotkreuz, Switzerland), and KRAS mutations (codons 12, 13, and 61) and BRAF mutation (V600E) were investigated in the DNA samples obtained from the sections by using real-time polymerase chain reaction (RT-PCR). In addition, MSI was investigated in HP and AD parts besides KRAS and BRAF by RT-PCR in mixed polyps (19). Five microsatellite loci (BAT25, BAT26, NR21, NR24, and MONO27) were investigated in mixed polyps, separately in HP and AD areas, as well as in normal tissue.
Figure 1.
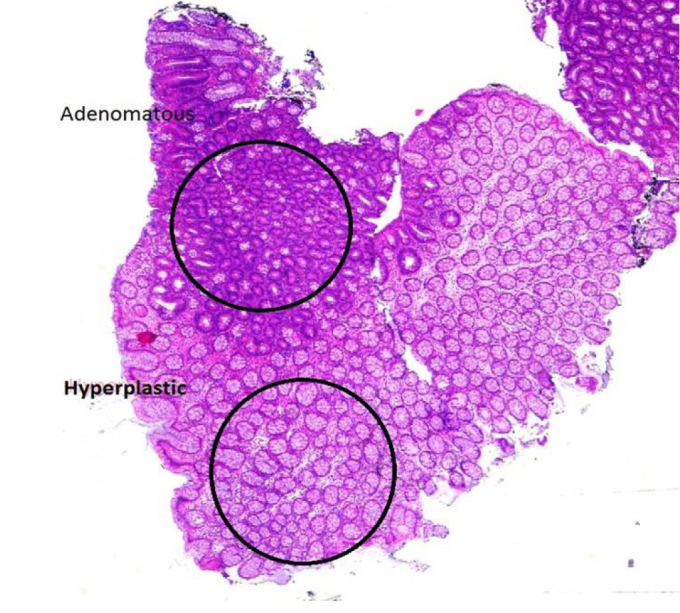
After the microscopic boarding on mixed polyps slide, Slide and paraffin blocks were matched and 2 components were separated from the paraffin block, and then embeded in 2 different blocks (H&Ex2).
MSI in one locus was evaluated as low-frequency MSI (MSI-L), instability in two or more loci as high-frequency MSI (MSI-H), and lack of instability in any of the loci as microsatellite stable (MSS). MSI was not studied in HPPs because of the very low incidence of MSI in HPPs in the literature, and in most of the cases, lesion-free tissue was available. Patients were evaluated according to gender, age (<35, 36–60, and >60 years), and polyp localization. Polyp localizations were divided into two groups as left-sided colon and right-sided colon. The ascending colon, hepatic flexure, transverse colon, and splenic flexure were considered as the right colon, and the descending colon, sigmoid colon, and rectum were considered as the left colon.
The ethics committee of the Dokuz Eylul University Medical School approved the study. Informed consent form was not requested by the ethics committee since this process did not involve interventional procedures.
Statistical Analysis
Demographic, morphological, and mutation results were evaluated by the McNemar chi-square and Kappa analyses, using the Statistical Packages for the Social Sciences (SPSS) (IBM Corp.; Armonk, NY, USA) 15 statistical analysis program, and p values <0.05 were considered significant.
RESULTS
Of the 59 patients included in the study, 31 (52.5%) were male and 28 (47.5%) were female. Two (4.4%) patients were <35 years, 30 (50.8%) were between 36 and 60 years, and 27 (45.8%) were >60 years. Of the serrated polyps, 38 (77.6%) were localized in the left colon (descending colon, sigmoid, and rectum) and 11 (22.4%) were in the right colon (ascending and transverse colons). The left-right localization ratio of the mixed polyps was 50%, each. The diameter of lesions was between 1 and 3 mm in 22 cases, between 4 and 6 mm in 30 cases, and >6 mm in seven cases.
The surface basal membrane and muscularis mucosa were thickened in all the subtypes in varying ranges, except in the mixed group. In this series, there were no GRHPs or MPHPs in the ascending colon. There was no statistically significant relationship between gender, lesion size, and the type of mutations. However, morphological findings and mutation profiling were significantly associated, in concordance with the literature.
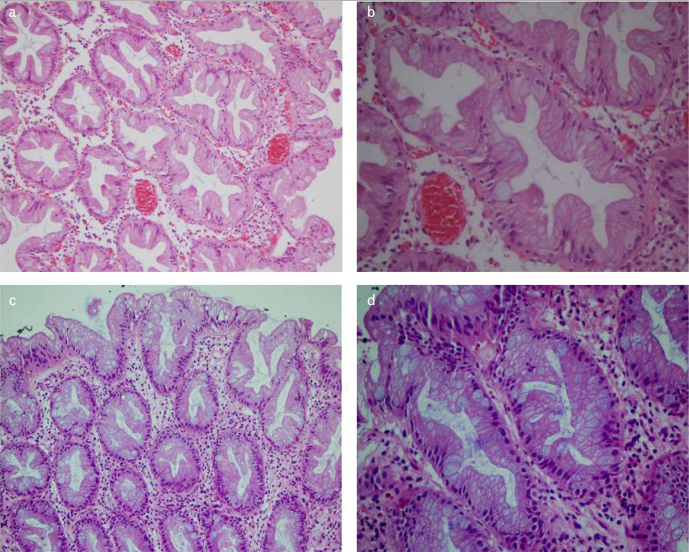
The most characteristic morphological feature in microvesicular HPPs was the presence of apical mucin in the form of small vesicles. There was structurally mild distortion, and approximately one-third of the cases had mild nuclear stratification on the surface (Figure 2, MVHP). A significant proportion of the patients in this group (n = 12, 63.2%) was in the 30–60 age group and in the right colon (n = 6, 31.5%). Furthermore, only one of the MVHPs had a KRAS mutation (6.3%), and 13 (68.4%) had BRAF mutations (p<0,001)
Figure 2. a–d.
Microvesicular hyperplastic polyps(MVHP). The characteristic features are the microvesicular cells and the presence of apical mucin in form of small vesicles (a) and c) HEx20), (b) and d) H&Ex40).
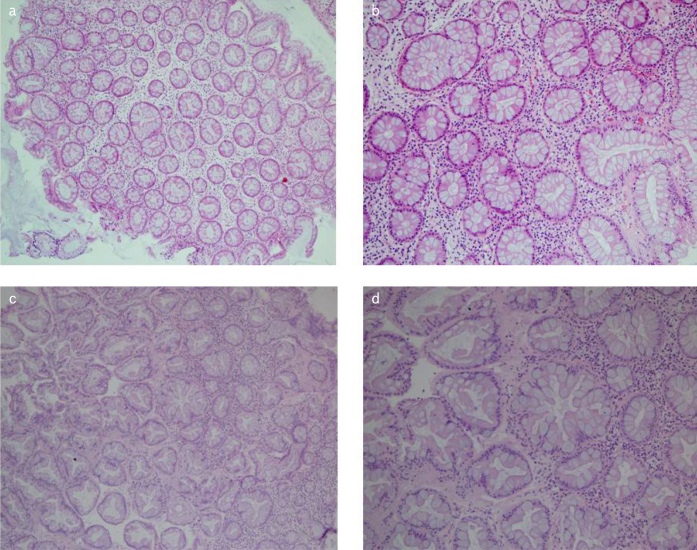
Serration in goblet-rich polyps was not prominent and was generally mild on the surface. The most characteristic morphological feature in goblet cells was apparent hyperplasia. There was no atypia or vesiculation in goblet cells, which were sharply circumscribed and increased in number (Figure 3, GRHP). Among all patients, only two were <35 years of age, both in this group. Ten (52.6%) patients were >60 years of age and 16 (84.2%) of the patients had left colon localization. Furthermore, 14 (73.7%) of the patients with goblet-rich polyps had a KRAS mutation, whereas only one (6.3%) patient had a BRAF mutation (p<00,01)
Figure 3. a–d.
Goblet-rich hyperplastic polyps (GRHP). Numerous goblet cells with no atypia/mitosis or vesiculation (a) and c) HEx10), (b) and d) H&Ex20).
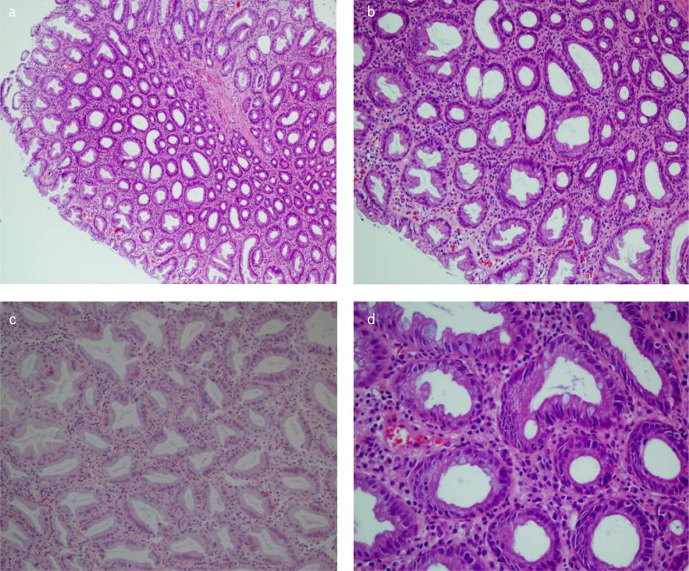
The mucin-poor HPP group was with the least number of patients after re-evaluation. A significant amount of mucin loss, severe loss and irregular distribution in goblet cells, hyperchromatic appearance, stratification, and more inflammation in lamina propria were the most obvious morphological findings, when compared with other HPPs (Figure 4, MPHP). BRAF mutation was detected in 9 (81.8%) of the mucin-poor lesions, and the other two patients had a KRAS mutation (p< 0,001) (p0.00).
Figure 4. a–d.
Mucin-poor hyperplastic polyps (MPHP). Severe loss and irregular distribution in goblet cells, hyperchromatic appearance and stratification (a H&Ex4) (b and c H&Ex10), (d H&Ex20).
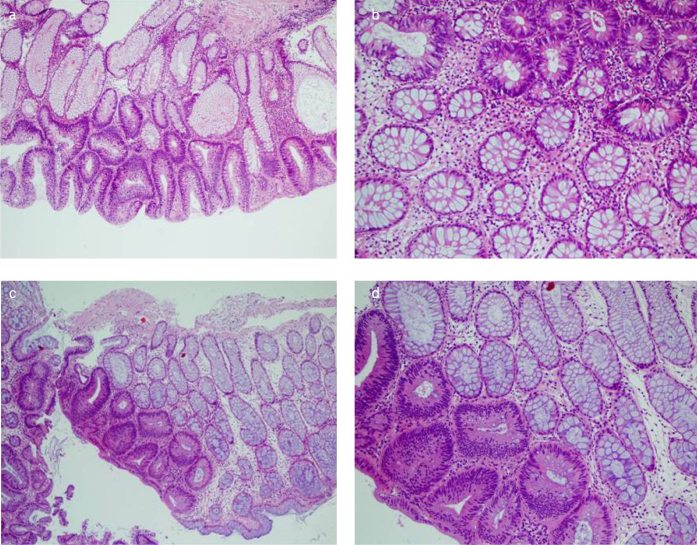
Six (60%) of the mixed polyps (HP/AD) (Figure 5, HP/AD) were rectosigmoid localized, and there was no difference in age or gender. The HP component had GRHP morphology in all patients. KRAS mutation was detected in both HP and AD components in five (50%) of the cases. No BRAF mutation was detected in either component of the mixed polyps. In five cases, both HP and AD components were MSS. In five cases, MSI-L (n=2) and MSI-H (n=3) were detected in the HP component, and the AD components were MSS (Table 1).
Figure 5. a–d.
Mixt polyps (HP/AD); hyperplastic and adenoma areas could be sharply circumscribed. In this study the hyperplastic components was GRHP subtype in the all of the cases (a) H&Ex10) (b) and d) H&Ex20), (c) H&Ex4).
Table 1.
KRAS, BRAF, and MSI status in hyperplastic and adenoma components, respectively, in mixed polyps.
| Mixed polyp (HP/AD) | KRAS | BRAF | MSI |
|---|---|---|---|
| 1 | −/− | −/− | MSI-L/MSS |
| 2 | −/− | −/− | MSS/MSS |
| 3 | +/+ | −/− | MSS/MSS |
| 4 | +/+ | −/− | MSS/MSS |
| 5 | +/+ | −/− | MSS/MSS |
| 6 | −/− | −/− | MSI-L/MSS |
| 7 | −/+ | −/− | MSI-L/MSS |
| 8 | −/− | −/− | MSI-H/MSS |
| 9 | +/+ | −/− | MSI-H/MSS |
| 10 | −/− | −/− | MSS/MSS |
MSI: microsatellite instability; HP/AD: hyperplastic and adenoma; MSS: microsatellite stable; MSI-L: low-frequency MSI; MSI-H: high-frequency MSI.
DISCUSSION
Colorectal cancer (CRC) is the second and third most common cancer in women and men, respectively (20). 84% of sporadic CRCs develop from the chromosomal instability pathway where there is a copy number change in DNA, whereas in the sporadic hypermutated group (13%), MSI forms a molecular pathological basis, and in this group 80%–90% of the cases are accompanied by BRAF mutations (21, 22). This main molecular classification is further elucidated by consensus molecular subtyping, and every day, new publications contribute to the literature (23, 24). Colonoscopy follow-up of the patients with AD occupies the gastroenterologists. Neither gastroenterologists nor pathologists take HPPs into consideration largely, especially in their daily routine. Although it is known that large or multiple HPPs may be precursors of carcinoma especially when they are located in the proximal colon, nonmultiple HPPs smaller than 5 mm, especially if localized in the distal colon, have been considered as harmless lesions without long-term malignancy potential, and this approach is still valid in many centers (7, 25, 26). As new molecular pathways of CRC development are discovered, researchers have also focused on precancerous process and lesions. Although we did not access the original article, we can see that a researcher named David considered HPPs as precancerous in the 1940s because they were frequently seen in the periphery of rectum cancer. Today, by molecular investigations, it would be fair to merit David’s opinion about HPPs (2). MSI and BRAF mutations in right-sided colon cancer and KRAS mutations that were more prominent in the left-sided colon cancer were also observed in HPP/SPs. In this study, 68.4% of MVHPs and 81% of MPHPs localized in the right colon had BRAF mutations. While none of the GRHPs showing a KRAS mutation with a rate of 73% was localized in the ascending colon, 63% of them were localized in the rectosigmoid area. These similarities in terms of the mutation type and location of invasive tumors and HPPs are important clues that HPPs are not completely innocent lesions.
Thus, are all HPPs really HPPs? For example, some of the polypoid colon mucosa taken from the previous polypectomy base/anastomosis line can be easily diagnosed as HPP. However, even though enlargement and proliferation can be observed in these mucosa goblet cells, there is almost no serration. Morphologically, the appearance of reactive mucosal changes/goblet cell hyperplasia, rather than a true HPP, is dominant. For this group of polypoid mucosal changes, the use of HPP terminology, although the molecular profiles have not yet been studied, does not seem very accurate. In de novo HPPs with identified molecular profiles and morphological characteristics, using serrated polyp terminology based on the WHO 2010 classification and reporting the complete morphology as MVHP/MPHP/GRHP will attract the clinician’s attention for the precancerous lesions and HPP terminology will be kept for the diagnosis of lesions in reactive mucosal processes. The definition of mixed polyps is a somewhat controversial for polyps with multiple morphological changes. Grouping these polyps as HP/AD and MHAP and incorporation of MHAPs into the serrated aAD/polyp spectrum can significantly solve the morphological complexity (11).
In this study, we focused on the molecular changes in 10 mixed polyps in which the HP and AD components were sharply circumscribed. Our aim was to find how two components could be found together, as discussed by Longacre and Fenoglio (11, 27). While some of the polyps show adenomatous dysplasia on the surface, HPP appearance at the bottom is a very common finding, which cannot be a coincidence. Does the AD stimulate the HP process in the adjacent mucosa? Or do AD molecular changes accumulate on HP changes? Another question is, are these HP changes only a reactive process or do the molecular properties support the neoplastic process? Although we could not find a clear answer to the first two questions, we can say that we have found a partial answer to the last question. In five (50%) of the mixed polyps, we detected a KRAS mutation both in the HP and AD components. In four cases, there was no KRAS mutation in either component. At least for this type of mutation, we can say that these two different components are actually parallel to each other. The fact is that the HP component was in the GRHP morphology and showed a similar KRAS mutation as GRHP did in all cases, which can be interpreted as the HP component in the mixed polyps reflecting the neoplastic process rather than the reactive process. However, there is no answer as to which morphological component started to develop first, but it is possible to say that, even if the AD component is not present, the GRHPs reflect a neoplastic process, and those who have received this diagnosis deserve follow-ups.
We did not detect any BRAF mutation in any of the mixed polyps. However, in two cases, the HP component was MSI-H and the AD area was MSS. Lino et al. have reported that of the 21 pure HPPs, MSI-L was present in three cases, but there was no MSI-H. Eight of the mixed polyps have shown MSI-L/H in the HP component and only four of the adjacent neoplastic component (serrated AD/traditional AD) have shown MSI. Although MSI-H has not been detected in pure HPPs, either in the literature or in another study by our group, the presence of MSI-H in the HP component observed in the mixed polyps was remarkable. Even though it is suggested that consecutive mutations lead to the formation of components in mixed polyps showing MSI and the other four cases may be due to collision of lesions, it looks quite difficult to distinguish between them just by checking the MSI status (14).
One of the limitations of this study is to include only polyps of a certain size which can be cut manually, since we do not have a laser dissection machine in our laboratory, and also, the number of polyps that are poor in mucin is much less than the other ones.
CONCLUSION
As the information on colorectal carcinogenesis increases, the definitions of precancerous lesions also change. In molecular pathological studies in the CRC area, targeted therapies are perhaps the most popular ones. However, the elaboration of the morphological and molecular features of precancerous lesions is important in terms of prevention and standardization of the follow-up stage prior to the development of invasive carcinoma. The findings of this study suggest that HPPs, even if smaller than 5 mm, are precancerous lesions bearing different mutations. GRHPs with predominant KRAS mutations and MVHPs and MPHSs with predominant BRAF mutations are precancerous.
Although the molecular investigations for HPP/SPs are not necessary to save time and money in daily practice, morphological subtyping should be included if the case is diagnosed with HPP/SPs as it will be useful for attracting the attention of gastroenterologists and establishing a ground for further studies.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Dokuz Eylul University (No: 3804-GOA, 08.02.2018).
Informed Consent: Informed consent is not necessary due to the retrospective nature of this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.Ü., E.U.; Design - M.Ü., Ö.S.; Supervision - S.S.; Resource - M.Ü., Ö.S.; Materials - M.Ü., E.U., G.B.; Data Collection and/or Processing - M.Ü., E.U.; Analysis and/or Interpretation - M.Ü., E.U., Ö.S.; Literature Search - M.Ü.; Writing - M.Ü.; Critical Reviews - S.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Arthur JF. The significance of small mucosal polyps of the rectum. Proc R Soc Med. 1962;55:703–04. doi: 10.1177/003591576205500823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur JF. Structure and significance of metaplastic nodules in the rectal mucosa. J Clin Pathol. 1968;21:735–43. doi: 10.1136/jcp.21.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenoglio CM, Pascal RR. Colorectal adenomas and cancer: pathologic relationships. Cancer. 1982;50:2601–8. [PubMed] [Google Scholar]
- 4.Fenoglio CM, Richart RM, Kaye GI. Comparative electron-microscopic features of normal, hyperplastic, and adenomatous human colonic epithelium. II. Variations in surface architecture found by scanning electron microscopy. Gastroenterology. 1975;69:100–9. doi: 10.1016/S0016-5085(19)32642-3. [DOI] [PubMed] [Google Scholar]
- 5.Urbanski SJ, Kossakowska AE, Marcon N, Bruce WR. Mixed hyperplastic adenomatous polyps--an underdiagnosed entity. Report of a case of adenocarcinoma arising within a mixed hyperplastic adenomatous polyp. Am J Surg Pathol. 1984;8:551–6. doi: 10.1097/00000478-198407000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HS, Patchefsky AS, Marks G. Adenomatous and carcinomatous changes within hyperplastic colonic epithelium. Dis Colon Rectum. 1979;22:152–6. doi: 10.1007/BF02586805. [DOI] [PubMed] [Google Scholar]
- 7.Estrada RG, Spjut HJ. Hyperplastic polyps of the large bowel. Am J Surg Pathol. 1980;4:127–33. doi: 10.1097/00000478-198004000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Franzin G, Novelli P. Adenocarcinoma occurring in a hyperplastic (metaplastic) polyp of the colon. Endoscopy. 1982;14:28–30. doi: 10.1055/s-2007-1021569. [DOI] [PubMed] [Google Scholar]
- 9.Fenoglio CM, Pascal RR. Colorectal adenomas and cancer: pathologic relationships. Cancer. 1982;50:2601–08. [PubMed] [Google Scholar]
- 10.Fenoglio-Preiser CM. Hyperplastic polyps, adenomatous polyps, and mixed hyperplastic adenomatous polyps of the colon: definitions. Prog Clin Biol Res. 1988;279:3–12. [PubMed] [Google Scholar]
- 11.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. J Surg Pathol. 1990;14:524–37. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Jass JR, Cottier DS, Pokos V, Parry S, Winship IM. Mixed epithelial polyps in association with hereditary non-polyposis colorectal cancer providingan alternative pathway of cancer histogenesis. Pathology. 1997;29:28–33. doi: 10.1080/00313029700169494. [DOI] [PubMed] [Google Scholar]
- 13.Otori K, Oda Y, Sugiyama K, et al. High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut. 1997;40:660–3. doi: 10.1136/gut.40.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.lino H, Jass JR, Simms LA, et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52:5–9. doi: 10.1136/jcp.52.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lothe RA, Andersen SN, Hofstad B, et al. Deletion of 1p loci and microsatellite instability in colorectal polyps. Genes Chromosomes Cancer. 1995;14:182–8. doi: 10.1002/gcc.2870140305. [DOI] [PubMed] [Google Scholar]
- 16.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Bosman FT, Carneiro F, Hruban RH, Theise ND. Tumours of colon and rectum, WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC; 2010. pp. 131–81. [Google Scholar]
- 18.Bartley AN, Hamilton SR, Alsabeh R. Members of the Cancer Biomarker Reporting Workgroup, College of American Pathologists. Template for Reporting Results of Biomarker Testing of Specimens from Patients with Carcinoma of the Colon and Rectum. Arch Pathol Lab Med. 2014;138:166–70. doi: 10.5858/arpa.2013-0231-CP. [DOI] [PubMed] [Google Scholar]
- 19.Dietmaier W, Hofstädter F. Detection of microsatellite instability by real time PCR and hybridization probe meltig pointanalysis. Lab Invest. 2001;81:1453–66. doi: 10.1038/labinvest.3780358. [DOI] [PubMed] [Google Scholar]
- 20.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 21.The Cancer Genome Atlas Network (326 collaborators) Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matos P, Jordan P. Targeting Colon Cancers with Mutated BRAF and Microsatellite Instability. Adv Exp Med Biol. 2018;1110:7–21. doi: 10.1007/978-3-030-02771-1_2. [DOI] [PubMed] [Google Scholar]
- 23.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469:125–34. doi: 10.1007/s00428-016-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennazio M, Arrigoni A, Risio M, Spandre M, Rossini FP. Small rectosigmoid polyps as markers of proximal neoplasms. Dis Colon Rectum. 1993;36:1121–5. doi: 10.1007/BF02052260. [DOI] [PubMed] [Google Scholar]
- 26.Ponugoti PL, Cummings OW, Rex DK. Risk of cancer in small and diminutive colorectal polyps. Dig Liver Dis. 2017;49:34–7. doi: 10.1016/j.dld.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Fenoglio-Preiser CM. When is a hyperplastic polyp not a hyperplastic polyp? Am J Surg Pathol. 1999;23:1001–3. doi: 10.1097/00000478-199909000-00001. [DOI] [PubMed] [Google Scholar]