Abstract
Background/Aims
Lipodystrophy is a rare metabolic disorder characterized by a near-total or partial lack of subcutaneous adipose tissue and is associated with insulin resistance. We aimed to evaluate the efficacy of magnetic resonance spectroscopy (MRS) imaging to explore the fat content of the liver in patients with lipodystrophy and to determine the relationship between liver fat accumulation and clinical presentations of lipodystrophy.
Materials and Methods
Between July 2014 and February 2016, 34 patients with lipodystrophy were assessed by MRS for the quantification of hepatic steatosis. All patients had metabolic abnormalities associated with insulin resistance. Metabolic parameters and the MRS findings were analyzed to identify potential correlations between liver fat content and disease severity.
Results
The MRS fat ratios (MRS-FRs) were markedly higher, indicating severe hepatic steatosis in lipodystrophy. Patients with generalized and partial lipodystrophy had comparable levels of MRS-FRs, although patients with generalized lipodystrophy were significantly younger. Patients with genetic lipodystrophy had elevated MRS-FRs compared with those with acquired lipodystrophy (p=0.042). The MRS-FR was positively correlated with liver enzyme alanine aminotransferase (p=0.028) and serum adiponectin (p=0.043).
Conclusion
Our data suggest that MRS might be an effective, non-invasive imaging method to quantify hepatic fat content in patients with lipodystrophy. Further studies are needed to validate the technique and threshold values, which would allow accurate comparison of data acquired by different machines and centers.
Keywords: Magnetic resonance imaging, magnetic resonance spectroscopy, lipodystrophy, fatty liver
INTRODUCTION
The first case of lipodystrophy, a rare metabolic disorder characterized by altered adipose tissue distribution, was described in 1885 by Mitchell (1). Later, different subtypes of the disease have been described by several authors (2–4). Lipodystrophy are divided into 2: generalized lipodystrophy (GL) or partial lipodystrophy (PL). Non-human immunodeficiency virus (HIV)-related lipodystrophy is basically classified into 4 subgroups: (i) congenital generalized lipodystrophy (CGL), (ii) acquired generalized lipodystrophy (AGL), (iii) familial partial lipodystrophy (FPLD), and (iv) acquired partial lipodystrophy (APL).
Patients with CGL exhibit near-complete fat loss (5–7). Although recent studies identified several novel genetical etiologies (8–11), CGL has 4 classic subtypes: (i) CGL1 (1-acylglycerol-3-phosphate O-acyltransferase 2 [AGPAT2] gene; chromosome 9q34), (ii) CGL2 (Berardinelli-Seip congenital lipodystrophy 2 [BSCL2] gene; chromosome 11q13), (iii) CGL3 (caveolin 1 [CAV1] gene; chromosome 7q31), and (iv) CGL4 (polymerase I and transcript release factor [PTRF] gene; chromosome 17q21.2) (5).
Patients with FPLD, a subgroup of lipodystrophy inherited in an autosomal dominant manner, usually exhibit normal fat tissue distribution at birth. Commencing after early childhood, progressive and variable fat loss may be noted, especially in the extremities (12). FPLD is mostly caused by heterozygous mutations in several genes. In previous studies, lamin A/C (LMNA) has been reported to be the most common gene responsible for FPLD (13, 14).
APL is characterized by adipose tissue loss from the face and upper extremities that typically starts at childhood (15). AGL is very rare and is associated with near-total adipose tissue loss (16). The literature also contains descriptions of HIV-related lipodystrophy, mandibuloacral dysplasia-associated lipodystrophy, and other rare lipodystrophy conditions (12, 17).
Lipodystrophy is associated with complex metabolic derangements. Patients with lipodystrophy usually present with metabolic abnormalities associated with severe insulin resistance that also include hepatic steatosis that can progress to steatohepatitis and cirrhosis (5, 18). As a result of limited adipocyte expandability and reduced levels of the adipokines such as leptin, the excess energy cannot be efficiently buffered at the adipose depots in patients with lipodystrophy, which leads to severely elevated levels of lipids in circulation and accumulation of lipids in ectopic sites such as the liver (18).
A thorough physical examination is the first step to diagnose liver disease in lipodystrophy. Hepatic involvement can develop at early ages in patients with GL, and hepatomegaly can be easily observed during the physical examination (6). Liver function tests should be ordered in the initial evaluation and during subsequent encounters (19). Hepatic steatosis can be graded and/or quantified by using ultrasound, computed tomography scan, and magnetic resonance imaging (MRI). Ultrasound is often the initial imaging as a non-invasive, non-expensive, and readily available tool. Doppler ultrasound can be added to the examination if the evaluation of the hemodynamics of the portal venous system, the hepatic artery, and the hepatic veins is desired. Ultrasound-based elastography and magnetic resonance (MR) elastography can be used to assess liver fibrosis based on viscoelastic properties of the tissue (20, 21).
Magnetic resonance spectroscopy (MRS) is a non-invasive method that can be used to evaluate hepatosteatosis. MRS reveals hepatic triglyceride accumulation, which has been shown to correlate with liver biopsy data (22, 23). The diagnostic accuracy of MRS for detecting hepatosteatosis was reported to be more than 90% in previous studies (22, 24). Here, we aimed to quantify the liver fat content using the MRS in patients with lipodystrophy in whom hepatic steatosis had been diagnosed during ultrasound. Furthermore, we tried to identify potential correlation between the liver fat content and clinical presentations of lipodystrophy and metabolic disease severity.
MATERIALS AND METHODS
Patients
The study was approved by the Dokuz Eylul University Ethics Committee (protocol no. 2014/28-35). MRI was performed as part of clinical care to diagnose liver disease, and patients gave consent to use anonymous data for research purposes. Data were collected under the oversight of Dokuz Eylul University Ethics Committee. From July 2014 to February 2016, 34 patients (26 women and 8 men) with clinically proven lipodystrophy were included in this study. All patients enrolled in this study had metabolic abnormalities associated with insulin resistance (carbohydrate intolerance, hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol), and hepatosteatosis was detected on ultrasound beforehand.
MRI
MRI was performed using a 1.5-T MRI system (Achieva, Philips Medical Systems, Eindhoven, the Netherlands) fitted with a phased-array coil. To evaluate the liver and other organs of the upper abdomen, we performed T2-weighted fat-saturated spin-echo (SE) (TR/TE/FA, 1,502/70/90°) imaging, T2-weighted SE (1502/70/90°) imaging, T1-weighted in-phase gradient-echo (GRE) (103/4.6/80°) imaging, and T1-weighted opposed-phase GRE (103/2.3/80°) imaging in the transverse plane.
We used a breath-hold, stimulated echo acquisition method-based single-voxel technique (TR, 1,500; TE, 35 ms; bandwidth 1,000 Hz, and 2,048 data points) to obtain spectroscopy data, which minimized the effect of J-coupling. Voxel localization was standardized in all patients to minimize artifacts and to avoid large intra-hepatic vessels. The voxel dimensions were 30×30×30 mm, and the voxel was positioned in the right hepatic lobe at the Couinaud segment V–VI. Automated shimming was used to homogenize the local magnetic field.
MRS analysis
The MRS data were downloaded from the MR scanner and analyzed using Easy Vision MR software (Philips Medical Systems, Eindhoven, The Netherlands). All spectroscopic images were evaluated by 2 radiologists (M.S and C.A) with reference to other sequences, including T1-weighted images (WIs) and T2 WIs.
MRS is a sensitive technique that can identify up to 160 metabolite peaks (25). The most useful liver metabolites are lipids, water, choline, and creatinine. In our protocol, hepatic metabolic content was derived from the signal intensities of the lipid signal at 0.9 ppm (the main CH3[Primary Alkyl] peak) and 1.3 ppm (the main CH2[Secondary Alkyl] peak), the creatinine peak at 3.03 ppm, and the choline peak at 3.23 ppm and the water signal at 4.7 ppm. To describe the MRS fat ratio (MRS-FR), we used the MRS-FR value introduced by Szczepaniak (26, 27). The MRS-FR was defined as follows: MRS-FR = [Signal (lipid)/Signal (lipid + water)] × 100. All measurements were performed twice on all images to take intra-observer variability into account.
Statistical Analysis
All statistical analyses were performed using the Statistical Packages for the Social Sciences (SPSS) for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). The MRS-FR of the lipodystrophy subgroups were compared using the Mann-Whitney U test. The Spearman’s coefficients were used for correlation analyses. The intra-observer reproducibility of the MRS-FR values was calculated. Statistical significance was considered if the p-value was less than 0.05.
RESULTS
The median age was 30 years (range, 11–63 years). Genetic and clinical examinations revealed that 12 patients had CGL (7 caused by AGPAT2, 3 BSCL2, 2 PTRF pathogenic variants), 1 AGL, 14 FPLD (8 caused by LMNA, 1 PPARG pathogenic variants, and 5 patients with FPLD phenotype were negative for pathogenic variants in any of the known lipodystrophy genes), and 7 patients had APL.
The median body mass index (BMI) was 20.81 kg/m2 (range, 16.43–37.05 kg/m2). Twenty-nine patients (85%) had diabetes, and the other patients (5; 15%) had impaired glucose tolerance, which was associated with insulin resistance. All patients had hypertriglyceridemia and low HDL cholesterol. Twelve patients (41%) had elevated levels of liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST), whereas 13 (45%) had elevated gamma-glutamyl transpeptidase.
Table 1 shows the comparison of patients with GL and PL. Patients with GL were significantly younger, had lower BMI, and had more severely suppressed HDL cholesterol, leptin, and adiponectin. The comparison of patients with genetically based lipodystrophy (CGL and FPLD) and acquired lipodystrophy (AGL and APL) is presented in Table 2.
Table 1.
Clinical characteristics and MRS-FR measurements of patients with GL and PL.
| GL (n=13) | PL (n=21) | p | |
|---|---|---|---|
| Age (years) | 21 (14.5–30) | 34.0 (27–41.5) | 0.002 |
| Gender (M/F) | (4/9) | (4/17) | 0.679 |
| BMI (kg/m2) | 19.8 (17.7–21.7) | 21.8 (19.9–26.6) | 0.042 |
| Triglyceride (Normal<150 mg/dL) | 581 (332–1,347) | 377 (213–731) | 0.141 |
| ALT (Normal: 10–37 U/L) | 36 (21–56) | 23.6 (18.5–38) | 0.320 |
| AST (Normal: 10–34 U/L) | 29 (17.5–52.5) | 26 (17.5–39.5) | 0.570 |
| GGT (Normal: 10–44 U/L) | 36 (18–64.5) | 34 (27.5–70) | 0.395 |
| HbA1c (Normal: <5.7%) | 7 (6–10.7) | 8.4 (6.2–9.2) | 0.972 |
| Total cholesterol (Normal: <200 mg/dL) | 169 (147.5–257) | 195 (163–234) | 0.304 |
| HDL (Normal: >40 mg/dL M; >50 F) | 27 (18.5–30.5) | 32 (25–40.5) | 0.030 |
| LDL (Normal: <130 mg/dL) | 97 (70.5–115) | 100 (69.5–122.5) | 0.972 |
| Leptin (ng/mL) | 0.40 (0.1–0.73) | 3.34 (1.39–6.65) | <0.001 |
| Adiponectin (ng/mL) | 0.72 (0.48–3.8) | 3.84(1.65–9.23) | 0.011 |
| MRS-FR (%) | 75% (56–83%) | 72% (66–79%) | 0.901 |
All the values are presented as the median (25th–75th percentiles).
GL: generalized lipodystrophy; PL: partial lipodystrophy; M: male; F: female; BMI: body mass index; ALT: alanine transaminase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transpeptidase; HbA1c: glycosylated hemoglobin; HDL: high-density lipoprotein; LDL: low density lipoprotein; MRS-FR: MRS fat ratio; MRS: magnetic resonance spectroscopy.
Table 2.
Clinical characteristics and MRS-FR measurements of patients with congenital and acquired lipodystrophy.
| Congenital lipodystrophy (n=26) | Acquired lipodystrophy (n=8) | p | |
|---|---|---|---|
| Age (years) | 30.5 (21.7–39.2) | 26.0 (16.7–33.5) | 0.382 |
| Gender (M/F) | (4/22) | (4/4) | 0.066 |
| BMI (kg/m2) | 20.8 (19.3–23.9) | 21.8 (19.1–25.9) | 0.823 |
| Triglyceride (Normal: <150 mg/dL) | 484 (309–866) | 338 (191–1,367) | 0.570 |
| ALT (Normal: 10–37 U/L) | 34 (18.7–50.7) | 23.3 (19.2–43.5) | 0.489 |
| AST (Normal: 10–34 U/L) | 28 (18.7–45.5) | 22 (14–45.2) | 0.310 |
| GGT (Normal: 10–44 U/L) | 29.5 (21–54) | 67.5 (35.7–78.7) | 0.027 |
| HbA1c (Normal: <5.7%) | 8 (6.2–9.2) | 8.7 (6.2–12) | 0.465 |
| Total cholesterol (Normal: <200 mg/dL) | 181 (157–237) | 186 (157–261) | 0.919 |
| HDL (Normal: > 40 mg/dL M; >50 F) | 29 (22–36) | 28.5 (23.7–39.2) | 0.903 |
| LDL (Normal: <130 mg/dL) | 97.5 (70.7–123.7) | 98 (59.5–116.2) | 0.700 |
| Leptin (ng/mL) | 1.05 (0.53–6.22) | 1.59 (1.2–4.37) | 0.626 |
| Adiponectin (ng/mL) | 3.69 (0.86–9.99) | 2.34 (0.95–3.3) | 0.256 |
| MRS-FR (%) | 75% (66–83%) | 57% (17–76%) | 0.042 |
All the values are presented as the median (25th–75th percentiles).
M: male; F: female; BMI: body mass index; ALT: alanine transaminase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transpeptidase; HbA1c: glycosylated hemoglobin; HDL: high-density lipoprotein; LDL: low density lipoprotein; MRS-FR: MRS fat ratio; MRS: magnetic resonance spectroscopy.
The median MRS-FR in the whole study cohort was 73% (25th–75th percentiles: 62%–79%). The intra-observer agreement rate was 0.94. The MRS-FRs of the different lipodystrophy subgroups are presented in Table 3 and Figures 1–4. The MRS-FRs were similar between the groups despite the fact that patients with GL were younger. Patients with genetical lipodystrophy had more severe hepatic steatosis identified by MRS than those with acquired lipodystrophy (p=0.042). The MRS-FR was positively correlated with liver enzyme ALT (r=0.377, p=0.028) and serum adiponectin (r=0.349, p=0.043) in the whole cohort.
Table 3.
Percentage of liver fat in patients with different types of lipodystrophy (MRS, %).
| CGL | AGL | FPLD | APL | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| AGPAT2 | BSCL2 | PTRF | PPARG | LMNA | No pathogenic variant identified | |||
| N | 7 | 3 | 2 | 1 | 1 | 8 | 5 | 7 |
| MRS-FR (%) | 75 (63–83) | 77 (59–84) | 47 (26–68) | 35 | 44 | 75 (67–84) | 78 (70–84) | 66 (12–77) |
|
|
|
|||||||
| 75 (61–83) | 75 (67–81) | |||||||
| 73 (62–79) | ||||||||
Data are presented as median (25th–75th percentiles).
MRS-FR: MRS fat ratio; MRS: magnetic resonance spectroscopy; CGL: congenital generalized lipodystrophy; AGL: acquired generalized lipodystrophy; FPLD: familial partial lipodystrophy; APL: acquired partial lipodystrophy; AGPAT2: 1-acylglycerol-3-phosphate O-acyltransferase 2; BSCL2: Berardinelli-Seip congenital lipodystrophy 2; PTRF: polymerase I and transcript release factor; PPARG: peroxisome proliferator-activated receptor gamma; LMNA, lamin A/C.
Figure 1. a–c.
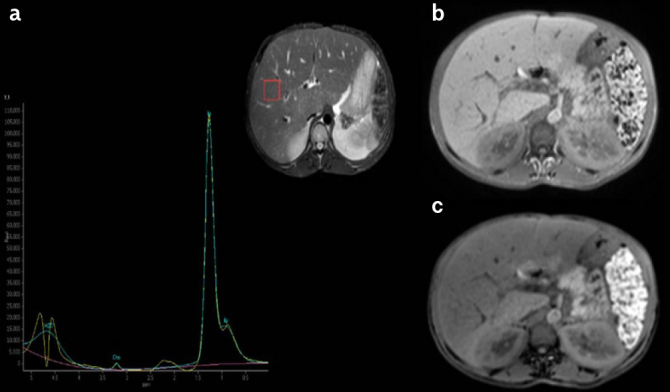
a) MRS spectra, b) IP, and c) OP signal intensity in a patient with CGL type I (30-year-old female). MRS-FR: 83%. MRS-FR: MRS fat ratio; MRS: magnetic resonance spectroscopy; IP: in-phase; OP: out-of-phase; CGL: congenital generalized lipodystrophy.
Figure 2. a–c.
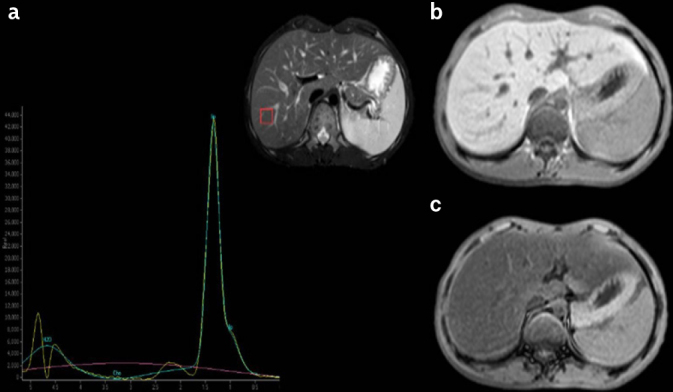
a) MRS spectra, b) IP), and c) OP signal intensity in a patient with CGL type II (16-year-old male). MRS-FR: 84%. MRS-FR: MRS fat ratio; MRS: magnetic resonance spectroscopy; IP: in-phase; OP: out-of-phase; CGL: congenital generalized lipodystrophy.
Figure 3. a–c.
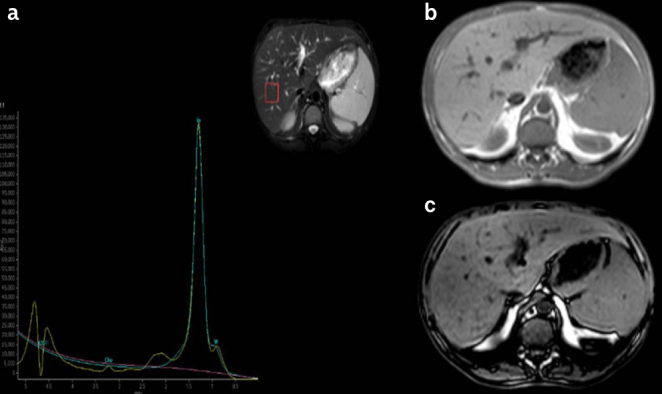
a) MRS spectra, b) IP, and c) OP signal intensity in a patient with FPLD (32-year-old female). MRS-FR: 78%. MRS-FR: MRS fat ratio; MRS: magnetic resonance spectroscopy; IP: in-phase; OP: out-of-phase; FPLD: familial partial lipodystrophy.
Figure 4. a–c.
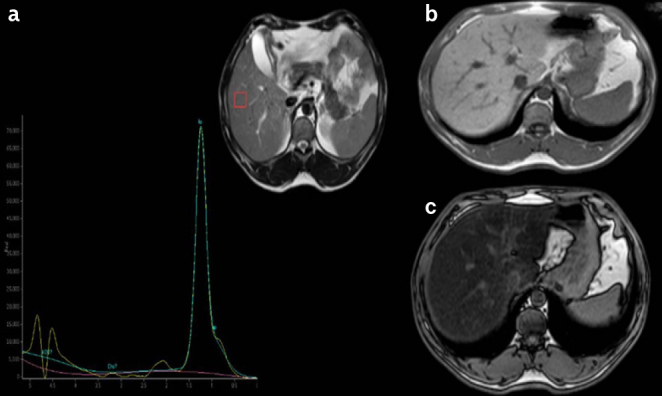
a) MRS spectra, b) IP, and c) OP signal intensity in a patient with APL (45-year-old female). MRS-FR: 80%. MRS-FR: MRS fat ratio; MRS: magnetic resonance spectroscopy; IP: in-phase; OP: out-of-phase; APL: acquired partial lipodystrophy.
DISCUSSION
Lipodystrophy is a complex metabolic disease characterized by the selective loss of adipose tissue, which can be either congenital or acquired (17). Lipodystrophy can be caused by pathogenic variants in several genes; however, the cause of acquired lipodystrophy remains unclear. GL is characterized by a near-total lack of fat, and its prevalence is estimated to be less than 1 case/million (6, 28). Fat loss is selective in patients with PL (6). PL is relatively more common with an estimated prevalence of 2.84 cases/million (29).
Previous studies have suggested that proton MRS is an effective non-invasive imaging method that can be used to analyze hepatic steatosis quantitatively, affording a high diagnostic accuracy (22, 26, 27, 30–32). It has also been shown that MRS, MRS Proton Density Fat Fraction (PDFF), and MRI PDFF measurements show a strong correlation with liver histological findings in terms of quantification of liver fat content (31, 33, 34).
To the best of our knowledge, our study is the first report of the systematic clinical use of MRS in patients with lipodystrophy. The MRS-FRs of this study were markedly higher than those of previous studies that used MRS to quantify liver fat in patients with various diseases (30–37). Szczepaniak et al. (26) considered an MRS-FR value of 5.56% as the upper limit of normal, which corresponded to the 95th percentile of liver lipid content in a subset of 345 individuals without known risk factors for steatosis. This value is comparable with the often-used cut-off level of 5% for the Dixon method, an MRI sequence based on chemical shift and designed to achieve uniform fat suppression, and also corresponds to the histological cut-off level of less than 5% of hepatocytes with steatosis. In contrast, Georgoff et al. (22) reported a value of MRS-FR less than 17% in patients with grade 0 and traced steatosis in a cohort of 52 patients with medically indicated liver biopsy. The amount of hepatic fat was higher than 38.6% in individuals with grade 2 or higher steatosis. The use of MRS to quantify liver fat accumulation has also been elaborated in different clinical settings. Hadigan et al. (35) reported a mean MRS-FR of 14% (range 6.4%–29%) in 33 patients infected with HIV. Ghotb et al. (36) found that HIV/hepatitis C virus co-infected patients had a mean MRS-FR of 11.9% (range 2.8%–20.3%). Several studies have investigated the extent of hepatic steatosis in patients with type 2 diabetes mellitus, where the hepatic fat content ranged from 13%–15% (37).
Previous studies have shown that lipodystrophy is associated with poorly controlled diabetes and severe hypertriglyceridemia, as well as severe hepatic steatosis (17). Although markedly elevated MRS-FR values revealed the presence of severe hepatic steatosis in our cohort, our measurements might have been affected by technical difficulties such as challenging water suppression. Technical differences in the methods used by different centers and the lack of standardization disallow accurate comparison of data acquired by different machines and research groups. In this study, we quantified the lipid and water signal-to-noise ratios (SNRs) of the liver and then calculated single-voxel MRS-FRs using the liver lipid and water SNRs. We utilized the use of breath-hold sequences that potentially helped us improve the quality of spectral data by having clearer images without motion artifacts related to respiratory movements. The field strength of our MR device was 1.5 T, thus reducing the chemical shift effect compared with that of more powerful instruments, although a previous study reported similar metabolite ratios with 1.5-T and 3-T MRS (38). We should also acknowledge the limitation of visual analysis of in-out of phase images for accurate diagnosis of severe hepatic steatosis (39, 40) because some patients in our study population had more than 50% fat in the liver. Our upper abdomen MR imaging protocol basically included conventional T2 WIs, T1-weighted in-phase GRE, and T1-weighted opposed-phase GRE sequences.
The MRS-FRs were similar in patients with generalized and PL, although patients with GL were younger. The MRS-FR was positively correlated with liver enzymes. We also observed that patients with genetically based lipodystrophy (CGL and FPLD) had more severe hepatic steatosis than those with acquired lipodystrophy. In contrast, Misra et al. (16) reported that the hepatic fat content of patients with AGL was remarkably high. We had one patient with AGL in our study who exhibited 35% steatosis upon MRS at the age of 11. We may speculate that the liver was not yet fully affected in her case.
There were certain additional limitations to our study. First, we examined only a limited number of patients with lipodystrophy because the disease is exceedingly rare, and this might have biased our results. We excluded several patients with APL who had apparently normal livers, as seen on performing ultrasound. It is well known that metabolic abnormalities may be less severe in patients with APL (15, 16). Second, there was no healthy control group in our study. Instead, we used literature values for comparison. Third, no histopathological data were available for further correlation analyses.
In conclusion, we found markedly elevated liver MRS-FR measurements in our heterogeneous study cohort that indicates severe hepatic involvement in patients with lipodystrophy. We believe that MRS has a clinical potential for the diagnosis and tracking of hepatic steatosis in patients with various subtypes of lipodystrophy, although further studies with larger samples are needed to validate the technique and threshold values.
MAIN POINTS.
Lipodystrophy, a rare disorder characterized by generalized or partial adipose tissue loss, is associated with severe insulin resistance and nonalcoholic fatty liver disease.
In our study, magnetic resonance spectroscopy (MRS), a non-invasive method to detect hepatic steatosis with high sensitivity and specificity, was shown to be an effective tool to quantify hepatic fat content in patients with lipodystrophy.
MRS can be potentially used to diagnose and monitor hepatic steatosis in patients with various subtypes of lipodystrophy.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Dokuz Eylul University 2014/28-35.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.A., C.A., M.S.; Design - B.A., C.A., M.S.; Supervision - C.A., M.S., B.A.; Resource - E.T.K., E.E., I.Y.S., G.A., T.D., L.D., H.O., H.T., B.S.Y., N.O.K., R.G.; Materials - R.G., N.O.K., B.S.Y., H.T., H.O., L.D., G.A., I.Y.S., E.E., E.T.K, T.D.; Data Collection and/or Processing - R.G., N.O.K., B.S.Y., H.T., H.O., L.D., E.T.K., E.E., I.Y.S., G.A., S.C.A., B.O.S., C.A., T.D.; Analysis and/or Interpretation - S.C.A., B.O.S., C.A.; Literature Search - S.C.A., B.O.S., C.A.; Writing - C.A., B.A., M.S.; Critical Reviews - S.C.A., B.A., C.A., M.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study had received no financial support.
REFERENCES
- 1.Mitchell S. Singular case of absence of adipose matter in the upper half of the body. Am J Med Sci. 1885;90:2. doi: 10.1097/00000441-188507000-00006. [DOI] [Google Scholar]
- 2.Berardinelli W. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab. 1954;14:193–204. doi: 10.1210/jcem-14-2-193. [DOI] [PubMed] [Google Scholar]
- 3.Dunnigan MG, Cochrane MA, Kelly A, Scott JW. Familial lipoatrophic diabetes with dominant transmission. A new syndrome. QJ Med. 1974;43:33–48. [PubMed] [Google Scholar]
- 4.Kobberling J, Dunnigan MG. Familial partial lipodystrophy: two types of an X linked dominant syndrome, lethal in the hemizygous state. J Med Genet. 1986;23:120–7. doi: 10.1136/jmg.23.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akinci B, Meral R, Oral EA. Phenotypic and Genetic Characteristics of Lipodystrophy: Pathophysiology, Metabolic Abnormalities, and Comorbidities. Curr Diab Rep. 2018;18:143. doi: 10.1007/s11892-018-1099-9. [DOI] [PubMed] [Google Scholar]
- 6.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–25. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altay C, Secil M, Demir T, Atik T, Akinci G. Determining residual adipose tissue characteristics with MRI in patients with various subtypes of lipodystrophy. Diagn Interv Radiol. 2017:428–34. doi: 10.5152/dir.2017.17019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain I, Patni N, Ueda M, et al. A Novel Generalized Lipodystrophy-Associated Progeroid Syndrome Due to Recurrent Heterozygous LMNA p.T10I Mutation. J Clin Endocrinol Metab. 2018;103:1005–14. doi: 10.1210/jc.2017-02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mory PB, Crispim F, Kasamatsu T, Gabbay MA, Dib SA, Moises RS. Atypical generalized lipoatrophy and severe insulin resistance due to a heterozygous LMNA p.T10I mutation. Arq Bras Endocrinol Metabol. 2008;52:1252–6. doi: 10.1590/S0004-27302008000800008. [DOI] [PubMed] [Google Scholar]
- 10.Patni N, Xing C, Agarwal AK, Garg A. Juvenile-onset generalized lipodystrophy due to a novel heterozygous missense LMNA mutation affecting lamin C. Am J Med Genet A. 2017;173:2517–21. doi: 10.1002/ajmg.a.38341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montenegro RM, Jr, Costa-Riquetto AD, Fernandes VO, et al. Homozygous and Heterozygous Nuclear Lamin A p.R582C Mutation: Different Lipodystrophic Phenotypes in the Same Kindred. Front Endocrinol (Lausanne) 2018;9:458. doi: 10.3389/fendo.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simha V, Garg A. Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol. 2006;17:162–9. doi: 10.1097/01.mol.0000217898.52197.18. [DOI] [PubMed] [Google Scholar]
- 13.Akinci B, Onay H, Demir T, et al. Clinical presentations, metabolic abnormalities and end-organ complications in patients with familial partial lipodystrophy. Metabolism. 2017;72:109–19. doi: 10.1016/j.metabol.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Ajluni N, Meral R, Neidert AH, et al. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf) 2017;86:698–707. doi: 10.1111/cen.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinci B, Koseoglu FD, Onay H, et al. Acquired partial lipodystrophy is associated with increased risk for developing metabolic abnormalities. Metabolism. 2015;64:1086–95. doi: 10.1016/j.metabol.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003;82:129–46. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Garg A, Misra A. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am. 2004;33:305–31. doi: 10.1016/j.ecl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Akinci B, Sahinoz M, Oral E. Lipodystrophy Syndromes: Presentation and Treatment. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al., editors. Endotext. South Dartmouth (MA): 2000. [PubMed] [Google Scholar]
- 19.Brown RJ, Araujo-Vilar D, Cheung PT, et al. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J Clin Endocrinol Metab. 2016;101:4500–11. doi: 10.1210/jc.2016-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–84. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: clinical applications. J Comput Assist Tomogr. 2013;37:887–96. doi: 10.1097/RCT.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgoff P, Thomasson D, Louie A, et al. Hydrogen-1 MR spectroscopy for measurement and diagnosis of hepatic steatosis. AJR Am J Roentgenol. 2012;199:2–7. doi: 10.2214/AJR.11.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–95. doi: 10.1016/0730-725X(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 24.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma A, Kumar I, Verma N, Aggarwal P, Ojha R. Magnetic resonance spectroscopy - Revisiting the biochemical and molecular milieu of brain tumors. BBA Clin. 2016;5:170–8. doi: 10.1016/j.bbacli.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 27.Weijers G, Wanten G, Thijssen JM, van der Graaf M, de Korte CL. Quantitative Ultrasound for Staging of Hepatic Steatosis in Patients on Home Parenteral Nutrition Validated with Magnetic Resonance Spectroscopy: A Feasibility Study. Ultrasound Med Biol. 2016;42:637–44. doi: 10.1016/j.ultrasmedbio.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Akinci B, Onay H, Demir T, et al. Natural History of Congenital Generalized Lipodystrophy: A Nationwide Study from Turkey. J Clin Endocrinol Metab. 2016;101:2759–67. doi: 10.1210/jc.2016-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiquette E, Oral EA, Garg A, Araújo-Vilar D, Dhankhar P. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375–83. doi: 10.2147/DMSO.S130810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannas P, Kramer H, Hernando D, et al. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers. Hepatology. 2015;62:1444–55. doi: 10.1002/hep.28012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idilman IS, Keskin O, Celik A, et al. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016;57:271–8. doi: 10.1177/0284185115580488. [DOI] [PubMed] [Google Scholar]
- 32.Hwang I, Lee JM, Lee KB, et al. Hepatic steatosis in living liver donor candidates: preoperative assessment by using breath-hold triple-echo MR imaging and 1H MR spectroscopy. Radiology. 2014;271:730–8. doi: 10.1148/radiol.14130863. [DOI] [PubMed] [Google Scholar]
- 33.Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767–75. doi: 10.1148/radiol.13121360. [DOI] [PubMed] [Google Scholar]
- 34.Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–31. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–7. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 36.Ghotb A, Noworolski SM, Madden E, et al. Adipose tissue and metabolic factors associated with steatosis in HIV/HCV coinfection: histology versus magnetic resonance spectroscopy. J Acquir Immune Defic Syndr. 2010;55:228–31. doi: 10.1097/QAI.0b013e3181e1d963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu KN, Gilbert G, Chalut M, Chagnon M, Chartrand G, Tang A. MRI-determined liver proton density fat fraction, with MRS validation: Comparison of regions of interest sampling methods in patients with type 2 diabetes. J Magn Reson Imaging. 2016;43:1090–9. doi: 10.1002/jmri.25083. [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Chang KH, Na DG, et al. Comparison of 1.5T and 3T 1H MR spectroscopy for human brain tumors. Korean J Radiol. 2006;7:156–61. doi: 10.3348/kjr.2006.7.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koplay M, Sivri M, Erdogan H, Nayman A. Importance of imaging and recent developments in diagnosis of nonalcoholic fatty liver disease. World J Hepatol. 2015;7:769–76. doi: 10.4254/wjh.v7.i5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merkle EM, Nelson RC. Dual gradient-echo in-phase and opposed-phase hepatic MR imaging: a useful tool for evaluating more than fatty infiltration or fatty sparing. Radiographics. 2006;26:1409–18. doi: 10.1148/rg.265055711. [DOI] [PubMed] [Google Scholar]


