Abstract
Background
Coronavirus disease 2019 (COVID-19) is a rapidly spreading disease that has caused extensive burden to individuals, families, countries, and the world. Effective treatments of COVID-19 are urgently needed.
Methods and findings
This is the first edition of a living systematic review of randomized clinical trials comparing the effects of all treatment interventions for participants in all age groups with COVID-19. We planned to conduct aggregate data meta-analyses, trial sequential analyses, network meta-analysis, and individual patient data meta-analyses. Our systematic review is based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Cochrane guidelines, and our 8-step procedure for better validation of clinical significance of meta-analysis results. We performed both fixed-effect and random-effects meta-analyses. Primary outcomes were all-cause mortality and serious adverse events. Secondary outcomes were admission to intensive care, mechanical ventilation, renal replacement therapy, quality of life, and nonserious adverse events. We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) to assess the certainty of evidence. We searched relevant databases and websites for published and unpublished trials until August 7, 2020. Two reviewers independently extracted data and assessed trial methodology.
We included 33 randomized clinical trials enrolling a total of 13,312 participants. All trials were at overall high risk of bias. We identified one trial randomizing 6,425 participants to dexamethasone versus standard care. This trial showed evidence of a beneficial effect of dexamethasone on all-cause mortality (rate ratio 0.83; 95% confidence interval [CI] 0.75–0.93; p < 0.001; low certainty) and on mechanical ventilation (risk ratio [RR] 0.77; 95% CI 0.62–0.95; p = 0.021; low certainty). It was possible to perform meta-analysis of 10 comparisons. Meta-analysis showed no evidence of a difference between remdesivir versus placebo on all-cause mortality (RR 0.74; 95% CI 0.40–1.37; p = 0.34, I2 = 58%; 2 trials; very low certainty) or nonserious adverse events (RR 0.94; 95% CI 0.80–1.11; p = 0.48, I2 = 29%; 2 trials; low certainty). Meta-analysis showed evidence of a beneficial effect of remdesivir versus placebo on serious adverse events (RR 0.77; 95% CI 0.63–0.94; p = 0.009, I2 = 0%; 2 trials; very low certainty) mainly driven by respiratory failure in one trial.
Meta-analyses and trial sequential analyses showed that we could exclude the possibility that hydroxychloroquine versus standard care reduced the risk of all-cause mortality (RR 1.07; 95% CI 0.97–1.19; p = 0.17; I2 = 0%; 7 trials; low certainty) and serious adverse events (RR 1.07; 95% CI 0.96–1.18; p = 0.21; I2 = 0%; 7 trials; low certainty) by 20% or more, and meta-analysis showed evidence of a harmful effect on nonserious adverse events (RR 2.40; 95% CI 2.01–2.87; p < 0.00001; I2 = 90%; 6 trials; very low certainty). Meta-analysis showed no evidence of a difference between lopinavir–ritonavir versus standard care on serious adverse events (RR 0.64; 95% CI 0.39–1.04; p = 0.07, I2 = 0%; 2 trials; very low certainty) or nonserious adverse events (RR 1.14; 95% CI 0.85–1.53; p = 0.38, I2 = 75%; 2 trials; very low certainty). Meta-analysis showed no evidence of a difference between convalescent plasma versus standard care on all-cause mortality (RR 0.60; 95% CI 0.33–1.10; p = 0.10, I2 = 0%; 2 trials; very low certainty). Five single trials showed statistically significant results but were underpowered to confirm or reject realistic intervention effects.
None of the remaining trials showed evidence of a difference on our predefined outcomes. Because of the lack of relevant data, it was not possible to perform other meta-analyses, network meta-analysis, or individual patient data meta-analyses. The main limitation of this living review is the paucity of data currently available. Furthermore, the included trials were all at risks of systematic errors and random errors.
Conclusions
Our results show that dexamethasone and remdesivir might be beneficial for COVID-19 patients, but the certainty of the evidence was low to very low, so more trials are needed. We can exclude the possibility of hydroxychloroquine versus standard care reducing the risk of death and serious adverse events by 20% or more. Otherwise, no evidence-based treatment for COVID-19 currently exists. This review will continuously inform best practice in treatment and clinical research of COVID-19.
Sophie Juul and colleagues initiate a living systematic review including meta-analyses on treatments for COVID-19.
Author summary
Why was this study done?
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread rapidly worldwide, causing an international outbreak of the coronavirus disease 2019 (COVID-19).
There is a need for a living systematic review evaluating the beneficial and harmful effects of all possible interventions for treatment of COVID-19.
What did the researchers do and find?
We conducted the first edition of a living systematic review with meta-analyses and trial sequential analyses to compare the effects of all treatment interventions for COVID-19.
One single trial randomizing 6,425 patients showed evidence of a beneficial effect of dexamethasone versus standard care on all-cause mortality and mechanical ventilation.
Meta-analysis of 2 trials showed evidence of a beneficial effect of remdesivir versus placebo on serious adverse events, but we found no effects on all-cause mortality or adverse events considered not serious.
Meta-analysis of 6 trials showed evidence of a harmful effect of hydroxychloroquine on adverse events considered nonserious and did not seem to have any effect on all-cause mortality or serious adverse events.
Five single trials showed statistically significant results but were underpowered to confirm or reject realistic intervention effects.
None of the remaining trials showed evidence of a difference of the experimental interventions on our predefined outcomes.
What do these findings mean?
No certain evidence-based treatment is currently available for COVID-19 patients.
Dexamethasone may reduce all-cause mortality and need for mechanical ventilation in COVID-19 patients.
Remdesivir may reduce serious adverse events in COVID-19 patients, but the clinical effects and the optimal treatment duration need confirmation.
More high-quality, low risk of bias randomized clinical trials are urgently needed.
Introduction
In 2019, a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused an international outbreak of the respiratory illness (coronavirus disease 2019 [COVID-19]) [1]. Since the initial outbreak in China, SARS-CoV-2 has spread globally, and COVID-19 is labeled a public health emergency of global concern by the World Health Organization [2]. The full spectrum of COVID-19 ranges from subclinical infection over mild, self-limiting respiratory tract illness to severe progressive pneumonia, multiorgan failure, and death [3]. Severe disease onset might result in death because of massive alveolar damage and progressive respiratory failure [4–6].
No evidence-based treatment for COVID-19 currently exist to augment widely used supportive care protocols [7]. To control the growing COVID-19 pandemic, we rely on quarantine, isolation, and infection-control measures to prevent disease spread [7] and on supportive care, including oxygen and mechanical ventilation, for infected patients. Many randomized clinical trials assessing the effects of different potential treatments for COVID-19 are currently underway. However, a single trial can rarely validly assess the effects of any intervention, and there is an urgent need to continuously surveil and update the aggregated evidence base so that effective interventions, if such exist, are implemented in clinical practice [8].
The present living systematic review with aggregate meta-analyses and trial sequential analyses aims to continuously inform evidence-based guideline recommendations for the treatment of COVID-19, taking risks of systematic errors (“bias”), risks of random errors (“play of chance”), and certainty of the findings into consideration [9].
Methods
We report this systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (S1 Text) [10,11]. The updated methodology used in this living systematic review is described in detail in The Cochrane Handbook of Systematic Reviews of Interventions [12] and our protocol [9], which was registered in the PROSPERO database (ID: CRD42020178787) prior to the systematic literature search.
Search strategy and selection criteria
Electronic searches
An information specialist searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, Medical Literature Analysis and Retrieval System Online (MEDLINE Ovid), Excerpta Medica database (Embase Ovid), Latin American and Caribbean Health Sciences Literature (LILACS; Bireme), Science Citation Index Expanded (SCI-EXPANDED; Web of Science), Conference Proceedings Citation Index–Science (CPCI-S; Web of Science), BIOSIS (Web of Science), CINAHL (EBSCO host), Chinese Biomedical Literature Database (CBM), China Network Knowledge Information (CNKI), Chinese Science Journal Database (VIP), and Wafang Database to identify relevant trials. We searched all databases from their inception and until August 7, 2020. Trials were included irrespective of language, publication status, publication year, and publication type. For the detailed search strategies for all electronic searches, see S2 Text.
Searching other resources
The reference lists of relevant trial publications were checked for any unidentified randomized clinical trials. To identify unpublished trials, we searched clinical trial registries (e.g., clinicaltrials.gov, clinicaltrialregister.eu, who.int/ictrp, chictr.org.cn) of Europe, USA, and China, and websites of pharmaceutical companies, websites of US Food and Drug Administration (FDA) and European Medicines Agency (EMA). We also searched the COVID-19 Study Registry [13] and the real-time dashboard of randomized trials [14].
We included unpublished and grey literature trials and assessed relevant retraction statements and errata for included trials. We also searched preprint servers (bioRxiv, medRxiv) for unpublished trials. We contacted all trial authors to obtain individual patient data.
Living systematic review
In this living systematic review, 2 independent investigators receive a weekly updated literature search file and continuously include relevant newly published or unpublished trials. The relevant meta-analyses, trial sequential analyses, and network meta-analysis will be continuously updated, and if new evidence is available (judged by the author group), the results will be submitted for publication. Every month, the author group will discuss whether searching once a week is necessary. For a detailed overview of the living systematic review work flow, see our protocol [9]. As this is a living systematic review analyzing results of randomized clinical trials, no ethical approval is required.
Data extraction
Two authors (EEN and JF) independently screened relevant trials. Six authors in pairs (SJ, EEN, JF, FS, CKJ, EB) independently extracted data using a standardized data extraction sheet. Any discrepancies were resolved through discussion, or if required, through discussion with a third author (JCJ). We contacted all trial authors if relevant data were unclear or missing.
Risk of bias assessment
Risk of bias was assessed with the Cochrane Risk of Bias tool–version 2 (RoB 2) [12,15]. Six authors in pairs (SJ, EEN, JF, FS, CKJ, EB) independently assessed risk of bias. Any discrepancies were resolved through discussion or, if required, through discussion with a third author (JCJ). Bias was assessed with the following domains: bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in measurement of outcomes, and bias arising from selective reporting of results [12,15]. We contacted authors of trials with unclear or missing data.
Outcomes and subgroup analyses
Primary and secondary outcomes were predefined in our protocol [9]. Primary outcomes were all-cause mortality and serious adverse events (as defined by the International Conference on Harmonation–Good Clinical Practice [ICH-GCP] guidelines) [9,16]. Secondary outcomes were admission to intensive care (as defined by trialists), receipt of mechanical ventilation (as defined by trialists), receipt of renal replacement therapy (as defined by trialists), quality of life, and nonserious adverse events. We classified nonserious adverse events as any adverse event not assessed as serious according to the ICH-GCP definition.
We chose to add time to clinical improvement as a post hoc outcome. We planned several subgroup analyses, which are described in detail in our protocol [9]. For all outcomes, we used the trial results reported at maximum follow-up.
Assessment of statistical and clinical significance
We performed our aggregate data meta-analyses according to Cochrane [12], Keus and colleagues [17], and the 8-step assessment by Jakobsen and colleagues [18] for better validation of meta-analytic results in systematic reviews. Review Manager version 5.4 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) [19] and Stata 16 (StataCorp LLC, College Station, TX, USA) [20] were used for all statistical analyses. We used risk ratios (RRs) for dichotomous outcomes. We planned to calculate the mean differences (MDs) and standardized mean difference (SMD) with 95% confidence intervals (CI) for continuous outcomes. We assessed a total of 2 primary outcomes, and we therefore adjusted our thresholds for significance [18] and considered a p-value of 0.033 or less as the threshold for statistical significance [9,18]. Because we primarily considered results of secondary outcomes as hypothesis generating, we did not adjust the p-value for secondary outcomes. We conducted both random-effects (Inverse Variance, DerSimonian-Laird) and fixed-effect (Mantel–Haenszel) meta-analyses for all analyses and chose the most conservative result as our primary result [12,18,21,22]. We used trial sequential analysis to control for random errors [23–31]. Trial sequential analysis estimates the diversity-adjusted required information size (DARIS), which is the number of participants needed in a meta-analysis to detect or reject a certain intervention effect. Statistical heterogeneity was quantified by calculating inconsistency (I square) for traditional meta-analyses and diversity (D square) for trial sequential analysis. We used GRADE to assess the certainty of evidence. We downgraded imprecision in GRADE by 2 levels if the accrued number of participants were below 50% of the DARIS and one level if between 50% and 100% of DARIS. We did not downgrade if benefit, harm, futility, or DARIS were reached. Trial sequential analysis will also be used in future updates to adjust the thresholds for significance according to repetitive testing. We used Fisher’s exact test to calculate p-values for all single trial results.
Results
Study characteristics
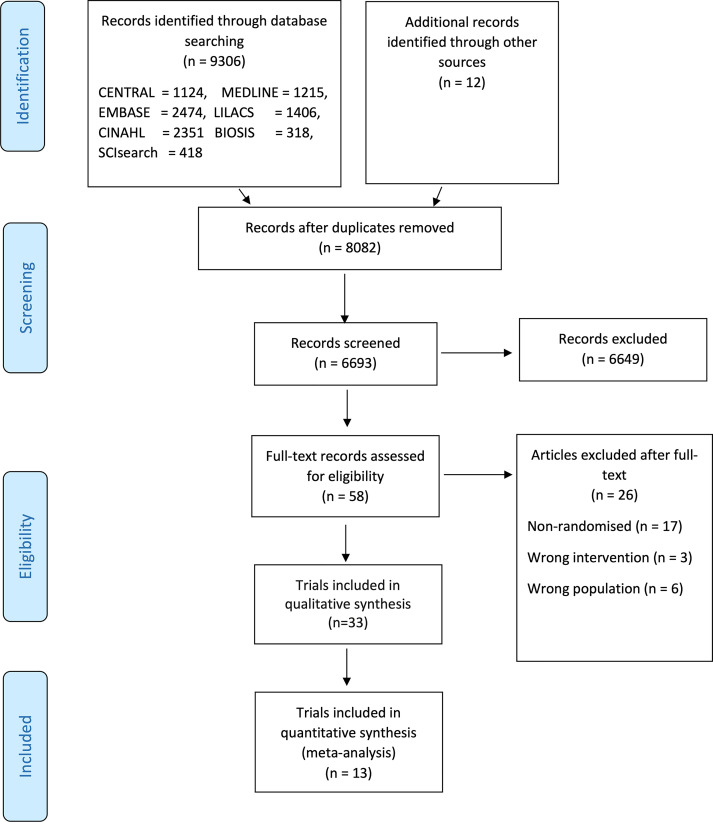
On August 7, 2020, our literature searches identified 8,082 records after duplicates were removed. We included a total of 33 clinical trials randomizing 13,312 participants (Fig 1) [32–65]. We identified several trials, including participants suspected of COVID-19 [66,67]. None of the trials reported separate data on COVID-19 positive participants compared to the remaining participants. We included trials if approximately 50% or more participants had a confirmed COVID-19 diagnosis. We wrote to all authors requesting separate data on COVID-19 confirmed participants, but we have received no responses yet. For at detailed overview of excluded trials, see S1 Table.
Fig 1. PRISMA flow diagram.
BIOSIS, Biosciences Information Services; CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Current Index to Nursing and Allied Health Literature; EMBASE, Excerpta Medica database; LILCAS, Latin American and Caribbean Health Sciences Literature; MEDLINE, Medical Literature Analysis and Retrieval System Online; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; SCIsearch, Science Citation Index Search.
Characteristics of included trials and the trial results can be found in S2 Table. All trials were assessed as at high risk of bias (S3 Table). One trial compared dexamethasone versus standard care [48,53]. Two trials compared remdesivir versus placebo [32,44]. Two trials compared lopinavir–ritonavir added to standard care versus standard care alone [33,41]. Eight trials compared hydroxychloroquine added to standard care versus standard care alone [35,36,43,49,55,56,59,60]. Two trials compared convalescent plasma added to standard care versus standard care alone [40,52]. The remaining trials and comparisons included hydroxychloroquine with and without azithromycin added to standard care versus standard care alone [55], hydroxychloroquine versus placebo [54,68], hydroxychloroquine and chloroquine added to standard care versus standard care alone [49], methylprednisolone added to standard care versus standard care alone [57], lopinavir–ritonavir versus umifenovir and versus standard care [41], favipravir versus umifenovir [34], high-flow nasal oxygenation versus standard mask oxygenation prior to fibreotic tracheal intubation [45], α-lipoic acid versus placebo [47], the combination of novaferon plus lopinavir–ritonavir versus novaferon and versus lopinavir–ritonavir [46], baloxavir marboxil versus favipiravir and versus standard care [42], 5 versus 10 days of remdesivir [38], interferon β-1a added to standard care versus standard care alone [37], colchicine added to standard care versus standard care alone [50], high-dosage with low-dosage chloroquine diphosphate [51], intravenous immunoglobulin added to standard care versus standard care alone [58], ribavirin plus interferon alpha versus lopinavir/ritonavir plus interferon alpha versis ribavirin plus lopinavir/ritonavir plus interferon alpha [62], darunavir/cobicistat plus interferon alpha-2b versus interferon alpha-2b alone [61], lincomycin HCl versus azitromycin [63], 99mTc-methyl diphosphonate (99mTc-MDP) injection added to standard care versus standard care alone [64], and interferon alpha-2b plus gamma versus interferon alpha-2b alone [65].
The maximum follow-up time ranged from 5 [35,36] to 30 [39,58] days after randomization. For several of our outcomes, it was not possible to conduct meta-analysis because of insufficient data.
Glucocorticosteroids versus standard care
We identified one trial, the Randomised Evaluation of COVid-19 thERapY (RECOVERY) trial, randomizing 6,425 participants to dexamethasone versus standard care [48,53]. Maximum follow-up was 28 days after randomization. The trial was assessed at high risk of bias (S3 Table), and the certainty of evidence was assessed at “low” for all-cause mortality, serious adverse events, and mechanical ventilation (S4 Table).
All-cause mortality: 482/2,104 died in the dexamethasone group compared with 1,110/4,321 in the standard care group (age-adjusted rate ratio, 0.83; 95% CI 0.75–0.93; p < 0.001).
Serious adverse events: 482/2,104 experienced one or more serious adverse events in the dexamethasone group compared with 1,110/4,321 in the standard care group (age-adjusted rate ratio, 0.83; 95% CI 0.75–0.93; p < 0.001). This data is based on mortality data only, as suggested by the ICH-GCP definition of a serious adverse event [16].
Intensive care: No data.
Mechanical ventilation: 102/1,780 received invasive mechanical ventilation in the dexamethasone group compared with 285/3,638 in the standard care group (RR 0.77; 95% CI 0.62–0.95).
Renal replacement therapy: No data.
Quality of life: No data.
Nonserious adverse events: No data.
We identified another trial randomizing 63 participants to a different glucocorticoid (methylprednisolone) than the RECOVERY trial [57]. It was not possible to perform meta-analysis, as approximately half of the participants in the experimental group were nonrandomized [57]. We have contacted the trial authors and asked for separate data for all randomized participants, but we have not received a response yet.
Remdesivir versus placebo
We identified 2 trials comparing remdesivir versus placebo [32,44]. Both trials were assessed as at high risk of bias (S3 Table).
Meta-analysis and trial sequential analysis of all-cause mortality
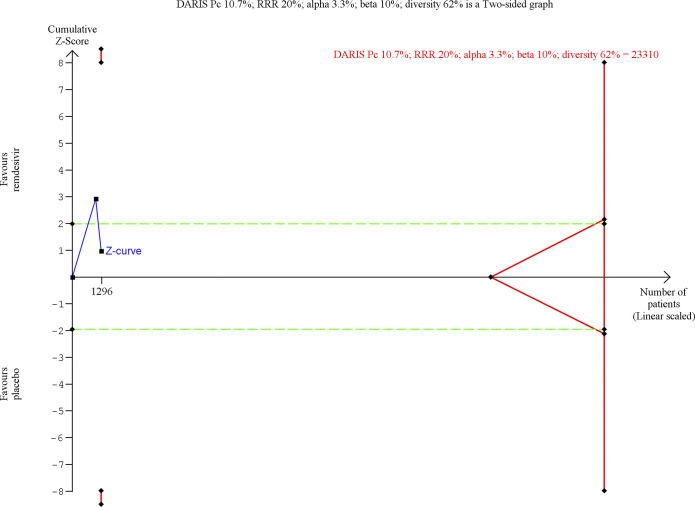
Random-effects meta-analysis showed no evidence of a difference between remdesivir versus placebo on all-cause mortality (RR 0.74; 95% CI 0.40–1.37; p = 0.34, I2 = 58%; 2 trials; very low certainty) (Fig 2; S5 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 58%) indicated heterogeneity. The outcome was assessed 14 days after randomization in the first trial [32] and 28 days after randomization in the second trial [44]. Trial sequential analysis showed that we did not have enough information to confirm or reject that remdesivir versus placebo reduces all-cause mortality with a relative risk reduction of 20% [9] (Fig 3). The fixed-effect meta-analysis showed evidence of a beneficial effect of remdesivir versus placebo on all-cause mortality (RR 0.67; 95% CI 0.47–0.96; p = 0.03; I2 = 58%; 2 trials; very low certainty) (S1 Fig).
Fig 2. Meta-analysis of remdesivir versus placebo on all-cause mortality.
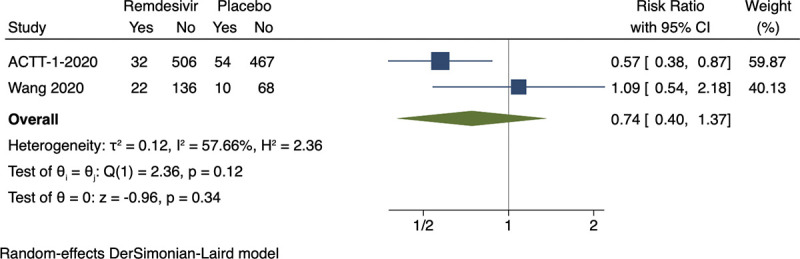
ACTT-1-2020, Adaptive COVID-19 Treatment Trial 1; CI, confidence interval.
Fig 3. Trial sequential analysis of remdesivir versus placebo on all-cause mortality.
Trial sequential analysis on remdesivir versus placebo on all-cause mortality in 2 trials at high risk of bias. The DARIS was calculated based on a mortality proportion in the control group of 10.7%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 62%. The required information size was 23,310 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm. The cumulative Z‐curve did not cross the inner‐wedge futility line (red outward sloping lines nor the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
Meta-analysis and trial sequential analysis of serious adverse events
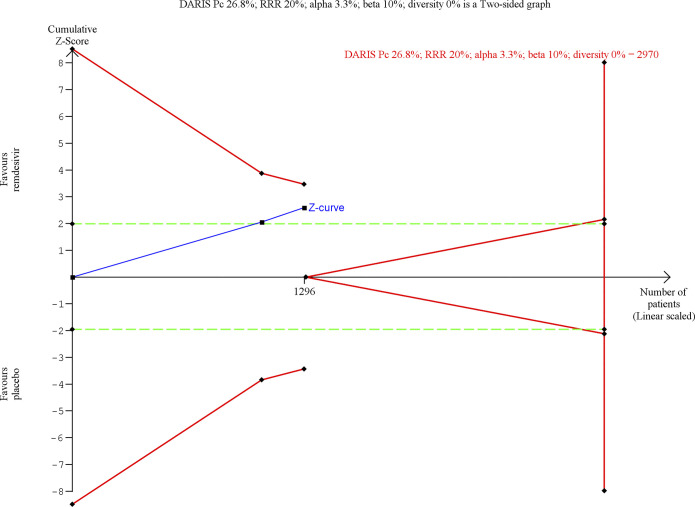
Random-effects meta-analysis showed evidence of a beneficial effect of remdesivir versus placebo on serious adverse events (RR 0.77; 95% CI 0.63–0.94; p = 0.009, I2 = 0%; 2 trials; very low certainty) (Fig 4; S5 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 0%) indicated no heterogeneity. The outcome was assessed 14 days after randomization in the first trial [32] and 28 days after randomization in the second trial [44]. Trial sequential analysis showed that we did not have enough information to confirm or reject that remdesivir versus placebo reduced the risk of serious adverse events with a relative risk reduction of 20% [9] (Fig 5). The difference between groups was mainly driven by a difference in respiratory failure.
Fig 4. Meta-analysis of remdesivir versus placebo on serious adverse events.
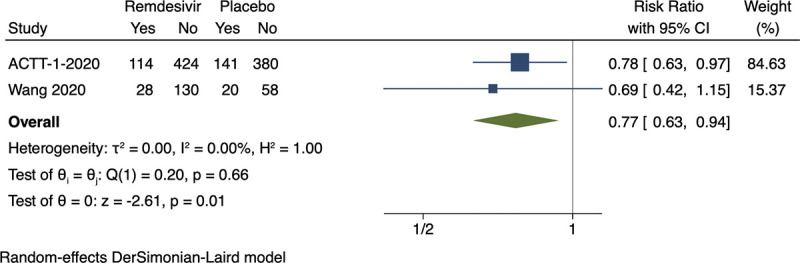
ACTT-1-2020, Adaptive COVID-19 Treatment Trial 1; CI, confidence interval.
Fig 5. Trial sequential analysis of remdesivir versus placebo on serious adverse events.
Trial sequential analysis on remdesivir versus placebo on serious adverse events in 2 trials at high risk of bias. The DARIS was calculated based on a proportion in the control group of 26.8%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 0%. The required information size was 2,970 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines). The cumulative Z‐curve did not cross the inner‐wedge futility line (red outward sloping lines nor the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
Meta-analysis and trial sequential analysis of nonserious adverse events
Random-effects meta-analysis showed no evidence of a difference between remdesivir versus placebo on adverse events not considered serious (RR 0.94; 95% CI 0.80–1.11; p = 0.48, I2 = 29%: 2 trials; low certainty) (S2 Fig; S5 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 29%) indicated no major heterogeneity. The outcome was assessed 14 days after randomization in the first trial [32] and 28 days after randomization in the second trial [44]. Trial sequential analysis showed that we had enough information to reject that remdesivir versus placebo reduced the risk of nonserious adverse events with a relative risk reduction of 20% [9] (S3 Fig).
Meta-analysis of time to clinical improvement/recovery
Random-effects meta-analysis showed evidence of a beneficial effects of remdesivir versus placebo on time to clinical improvement/recovery (log ratio of means −0.28; 95% CI −0.55 to −0.02; p = 0.04; I2 = 0%; 2 trials; very low certainty) (S4 Fig). The 2 trials defined this outcome differently. The first trial analyzed “time to recovery” defined as either discharge from the hospital or hospitalization for infection-control purposes only [32]. The second trial analyzed “time to clinical improvement” defined as the time (in days) from randomization to the point of a decline of 2 levels on a 6-point ordinal scale of clinical status (from 1 = discharged to 6 = death) or discharged alive from hospital, whichever came first [44]. Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 0%) indicated no heterogeneity. The outcome was assessed 14 days after randomization in the first trial [32] and 28 days after randomization in the second trial [44].
One trial assessing the effects of remdesivir reported assessment of viral load. The trial result indicated no evidence of a difference from day 1 to day 28 [44].
Hydroxychloroquine versus standard care
We identified 8 trials comparing hydroxychloroquine added to standard care versus standard care alone [35,36,43,49,55,56,59,60]. We also identified one trial that used placebo as an additional control intervention [54]. All trials were assessed as at high risk of bias (S3 Table). One trial was not eligible for meta-analysis, as the results were not reported in a usable way; i.e., the results were reported as per-protocol, and several participants crossed over [43].
Meta-analysis of all-cause mortality
Fixed-effect meta-analysis showed no evidence of a difference between hydroxychloroquine versus standard care on all-cause mortality (RR 1.07; 95% CI 0.97–1.19; p = 0.17; I2 = 0%; 7 trials; low certainty) (S5 Fig, S6 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 0%) indicated no heterogeneity. The assessment time points varied from 5 [35] to 28 [49,56,59] days after randomization. The trial sequential analysis showed that we had enough information to reject that hydroxychloroquine compared with standard care reduces all-cause mortality with a relative risk reduction of 20% (S6 Fig).
Meta-analysis of serious adverse events
Fixed-effect meta-analysis showed no evidence of a difference between hydroxychloroquine versus standard care on serious adverse events (RR 1.07; 95% CI 0.96–1.18; p = 0.21; I2 = 0%; 7 trials; low certainty) (S7 Fig, S6 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 0%) indicated no heterogeneity. The assessment time points varied from 5 [35] to 28 [49,56,59] days after randomization. The trial sequential analysis showed that we had enough information to reject that hydroxychloroquine compared with standard care reduces all-cause mortality with a relative risk reduction of 20% (S8 Fig).
Meta-analysis of nonserious adverse events
Fixed-effect meta-analysis showed evidence of a beneficial effect of standard care on adverse events not considered serious (RR 2.40; 95% CI 2.01–2.87; p < 0.00001; I2 = 90%; 6 trials; very low certainty) (S9 Fig, S6 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 90%) indicated large heterogeneity. The assessment time points varied from 5 [35,36,54] to 28 [49,56] days after randomization. It was not possible to perform trial sequential analysis due to high diversity [9].
Lopinavir–ritonavir versus standard care
We identified 2 trials comparing lopinavir–ritonavir added to standard care versus standard care alone [33,41]. Both trials were assessed as at high risk of bias (S3 Table).
Meta-analysis and trial sequential analysis of serious adverse events
Random-effects meta-analysis showed no evidence of a difference between lopinavir–ritonavir versus standard care on serious adverse events (RR 0.64; 95% CI 0.39–1.04; p = 0.07, I2 = 0%; 2 trials; very low certainty) (S10 Fig, S7 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 0%) indicated no heterogeneity. The assessment time point was 21 days after randomization in the first trial [41] and 28 days after randomization in the second trial [33]. Trial sequential analysis showed that we did not have enough information to confirm or reject that lopinavir–ritonavir versus standard care reduced the risk of serious adverse events with a relative risk reduction of 20% [9] (S11 Fig).
Meta-analysis of nonserious adverse events
Random-effects meta-analysis showed no evidence of a difference between lopinavir–ritonavir versus standard care on adverse events not considered as serious (RR 1.14; 95% CI 0.85–1.53; p = 0.38, I2 = 75%; 2 trials; very low certainty) (S12 Fig; S7 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 75%) indicated substantial heterogeneity. The assessment time point was 21 days after randomization in the first trial [41] and 28 days after randomization in the second trial [33]. Trial sequential analysis showed that we did not have enough information to confirm or reject that lopinavir–ritonavir compared with standard care reduces nonserious adverse events with a relative risk reduction of 20% [9].
Convalescent plasma versus standard care
We identified 2 trials comparing convalescent plasma added to standard care versus standard care alone [40,52]. Both trials were assessed as at high risk of bias (S3 Table).
Meta-analysis of all-cause mortality
Random-effects meta-analysis showed no evidence of a difference between convalescent plasma versus standard care on all-cause mortality (RR 0.60; 95% CI 0.33–1.10; p = 0.10, I2 = 0%; 2 trials; very low certainty) (S13 Fig; S8 Table). Visual inspection of the forest plot and measures to quantify heterogeneity (I2 = 0%) indicated no heterogeneity. The outcome was assessed 15 days after randomization in the first trial [52] and 28 days after randomization in the second trial [40]. Trial sequential analysis showed that we did not have enough information to confirm or reject that convalescent plasma reduces all-cause mortality with a relative risk reduction of 20% [9].
Remaining trial data
Because of a lack of relevant data, it was not possible to conduct other meta-analyses, individual patient data meta-analyses, or network meta-analysis. Five single trials showed statistically significant results but were underpowered to confirm or reject realistic intervention effects. One trial randomizing 402 participants compared 5 versus 10 days of remdesivir showed evidence of a beneficial effect of 5 days of remdesivir on serious adverse events (p = 0.003 [Fisher’s exact test]) [38]. One trial randomizing 92 participants compared the immunomodulator interferon β-1a added to standard care versus standard care alone showed evidence of a beneficial effect of interferon β-1a on all-cause mortality (p = 0.029) [37]. This trial also showed evidence of a beneficial effect of standard care on nonserious adverse events (p = 0.006) [37]. One single trial randomizing 81 participants compared high-dosage versus low-dosage chloroquine diphosphate showed evidence of a beneficial effect of low-dosage chloroquine on all-cause mortality (p = 0.024) [51]. One single trial randomizing 110 participants compared colchicine added to standard care versus standard care alone showed evidence of a beneficial effect of standard care on adverse events not considered serious (p = 0.003) [50]. One single 3 group trial randomizing 667 participants to hydroxychloroquine with or without azithromycin versus standard care showed evidence of a harmful effect of hydroxychloroquine with azithromycin on adverse events not considered serious (p = 0.015) [55].
None of the remaining single trial results showed evidence of a difference on our predefined review outcomes. Two trials did not report the results in a usable way; one trial reported results of the experimental group with a proportion of participants being nonrandomized [57], and the second trial reported the results as per-protocol, and there was participant crossover [43]. Three trials did not report on our review outcomes [45,63,64]. We have contacted all corresponding authors, but we have not been able to obtain outcomes for our analyses from the trialists yet. All trials were assessed as at high risk of bias (S3 Table). Characteristics of the trials and their results on the review outcomes can be found in S2 Table. Certainty of the evidence was assessed as “low” or “very low” for all outcomes (S9–S26 Tables).
Possible future contributions of ongoing trials
On August 7, 2020, a search on the Cochrane COVID-19 Study Register revealed 1,828 registered randomized clinical trials [13]. From these, 106 different interventions for treatment of COVID-19 patients were identified [13]. The 10 most investigated experimental interventions were hydroxychloroquine (162 trials), convalescent plasma (55 trials), azithromycin (52 trials), lopinavir and ritonavir (40 trials), tocilizumab (33 trials), chloroquine (30 trials), favipiravir (24 trials), remdesivir (15 trials), sarilumab (15 trials), and dexamethasone (13 trials). Eligible trials will continuously be included in the present living systematic review once results become available.
We also identified one press release from the RECOVERY trial that investigates a number of potential treatments for COVID-19 versus standard care, including dexamethasone and hydroxychloroquine [48,53,59,69,70]. The trial also investigates lopinavir–ritonavir (n = 1,596) versus standard care (n = 3,376). According to the press release, there was no significant difference in the primary outcome of 28-day mortality (22.1% lopinavir–ritonavir versus 21.3% usual care; RR 1.04; 95% CI 0.91–1.18; p = 0.58) [70]. There was also no evidence of beneficial effects on the risk of progression to mechanical ventilation or length of hospital stay [70].
Discussion
We performed the first edition of our living systematic review assessing the beneficial and harmful effects of all treatment interventions for COVID-19. We included 33 trials, randomizing a total of 13,312 participants to an experimental versus a control intervention. Our study showed that dexamethasone and remdesivir might be beneficial for COVID-19 patients, but the certainty of the evidence was low to very low, so more trials are needed. We could reject that hydroxychloroquine is beneficial for COVID-19 in reducing death and serious adverse events at the 20% relative risk reduction level.
We identified one trial randomizing 6,425 participants to dexamethasone versus standard care. This trial showed evidence of a beneficial effect of dexamethasone on all-cause mortality and mechanical ventilation [48]. However, the trial was assessed as at high risk of bias and had methodological limitations (S2 Table, S3 Table). First, the trial is described as an adaptive trial, but the reporting of the trial does not comply with the Consolidated Standards of Reporting Trials (CONSORT) extension on adaptive designs [71]. For example, according to the CONSORT extension, trialists should report: “Elements of decision-making rules describing whether, how, and when the proposed trial adaptations will be used during the trial. It involves pre-specifying a set of actions guiding how decisions about implementing the trial adaptations are made given interim observed data (decision rules). It also involves pre-specifying limits or parameters to trigger trial adaptations (decision boundaries)” [71]. However, in the protocol and the statistical analysis plan of the RECOVERY trial, it is stated that the data monitoring committee will request data at a frequency relevant to the emerging data, and no other information is provided. Third, apart from mortality, the trial did not assess other serious or nonserious adverse events as defined by the ICH-GCP guidelines, which limits the validity of the trial. In the trial protocol, it is stated that suspected serious adverse reactions as well as suspected unexpected serious adverse reactions will be recorded, whereas other serious and nonserious adverse events will not be recorded. It is of utmost importance to always assess beneficial and harmful intervention effects on patient-important outcomes. Fourth, the authors emphasize large beneficial effects of dexamethasone on mortality in certain specific subgroups of participants (participants receiving invasive mechanical ventilation or oxygen at baseline). However, these findings should be interpreted with caution because the trialists did not adjust the threshold for significance according to the multiple comparisons including these subgroup analyses, which results in an increased risk of type I errors [72]. Furthermore, these subgroups were not prespecified in any of the trial registries or the trial protocol but appear only in the statistical analysis plan first dated June 9, 2020, a day after the last participant was randomized to the dexamethasone group. Fifth, only 88% in the dexamethasone group and 89% in the standard care group had a confirmed COVID-19 diagnosis at randomization. A total of 12% in the dexamethasone group and 10% in the standard care group had a negative SARS-CoV-2 test result [53]. The relatively large proportion of participants without a confirmed COVID-19 diagnosis included in this trial should be considered when interpreting the trial results. Sixth, the trial was open-label, and hence, the participants, treatment providers, and outcome assessors were not blinded, which might bias the trial results [73]. These limitations need to be considered when interpreting the trial results.
It was possible for us to perform 10 meta-analyses. Meta-analysis showed evidence of a beneficial effect of remdesivir versus placebo on serious adverse events mainly driven by a difference in respiratory failure in the largest trial [32]. Results of the largest trial indicated that remdesivir resulted in a median recovery time of 11 days, compared with a median of 15 days in the placebo group [32]. One single trial compared 5 versus 10 days of remdesivir and showed evidence of a beneficial effect of 5 days of remdesivir on serious adverse events [38]. However, this trial was open-label, did not use blinded outcome assessors, and was assessed at high risk of bias. The effects of remdesivir on other patient-important outcomes are unclear. Furthermore, certainty of the evidence was assessed as “very low” for all-cause mortality and serious adverse events and “low” for nonserious adverse events. Hence, the effects of remdesivir need to be confirmed in future trials at low risk of bias.
Meta-analysis showed no evidence of a difference between hydroxychloroquine versus standard care on all-cause mortality and serious adverse events, and trial sequential analysis showed that we had enough information to reject that hydroxychloroquine reduces the risk of all-cause mortality and serious adverse events with a relative risk reduction of 20%. Meta-analysis showed a harmful effect of hydroxychloroquine on nonserious adverse events. Meta-analysis showed no evidence of a difference between lopinavir–ritonavir versus standard care on serious adverse events and on nonserious adverse events. Meta-analysis showed no evidence of a difference between convalescent plasma versus standard care on all-cause mortality. Because of lack of relevant data, it was not possible to perform other meta-analyses, network meta-analysis, or individual patient data meta-analyses.
A single trial compared the immunomodulator interferon β-1a added to standard care versus standard care alone and showed evidence of a beneficial effect of interferon β-1a on all-cause mortality (p = 0.029) [37]. This trial also showed evidence of a beneficial effect of standard care alone on nonserious adverse events (p = 0.006). One single trial compared high-dosage with low-dosage chloroquine diphosphate [51] showed evidence of a beneficial effect of low-dosage chloroquine diphosphate on all-cause mortality (p = 0.024). One single trial randomizing 110 participants compared colchicine added to standard care versus standard care alone showed evidence of a beneficial effect of standard care on nonserious adverse events (p = 0.003). One single 3 group trial randomizing 667 participants to hydroxychloroquine with or without azithromycin versus standard care showed evidence of a harmful effect of hydroxychloroquine with azithromycin on adverse events not considered serious (p = 0.015) [55]. However, these single trials were underpowered to confirm or reject realistic intervention effects, and they were assessed as at high risk of bias. Therefore, the trial results should be interpreted with great caution [74].
Our living systematic review has a number of strengths. The predefined methodology was based on The Cochrane Handbook for Systematic Reviews of Interventions [12], the 8-step assessment suggested by Jakobsen and colleagues [18], and trial sequential analysis [23]. Hence, this review considers both risks of systematic errors and risks of random errors. Another strength is the living systematic review design, which allows us to continuously surveil and update the evidence base of existing interventions for treatment of COVID-19, resulting in a decreased timespan from evidence to clinical practice. This is particularly important in this international healthcare crisis, in which a large number of new randomized clinical trials are continuously registered and published.
Our living systematic review also has limitations. The primary limitation is the paucity of trials currently available, and the results from the current meta-analyses are of low or very low certainty. This must be considered when interpreting our meta-analysis results. Secondly, the trials that we succeeded in including were all at risks of systematic errors and random errors. Third, it was not possible to perform the planned individual patient data meta-analyses, network-meta-analysis, or the planned subgroup analyses because of the lack of relevant data. We contacted all trial authors requesting individual patient data, but until now, we only received one dataset [39]. Fourth, we included “time to clinical improvement” as an outcome post hoc. Results of this outcome should be interpreted with caution, because it was not predefined and was chosen after the trials were included in the systematic review. We did not include the outcome “time to clinical improvement” in our protocol because this outcome is poorly defined and if outcome assessors are not adequately blinded, assessments of “improvement” may be biased. Furthermore, time to clinical improvement is not one of the most patient-important outcomes, e.g., most patients would rather survive without complications than recover a few days sooner. We chose to meta-analyze this outcome even though the 2 trials defined this outcome differently, i.e., time to clinical improvement [44] and time to clinical recovery [32] (see “Results”). Hence, this outcome result should be interpreted with caution and should only be considered hypothesis generating. Fifth, all trials differed in the included participants’ disease severity at trial intake (mild, moderate, severe, critically ill), which might result in clinical heterogeneity. We will explore disease severity as a subgroup analysis if this is warranted in later stages of this living systematic review. Sixth, the included trials assessed the outcomes at different time points, which might contribute to increased heterogeneity. Seventh, some data are included from preprints, and these might be subject to change following peer review. Therefore, some results, bias risk assessments, and GRADE summaries might change in later editions of this living systematic review following inclusion of the published peer-reviewed manuscripts.
WHO has recently stopped a clinical trial of the antimalaria drug hydroxychloroquine for treating COVID-19 patients [75]. However, this decision applies only to the conduct of the WHO Solidarity trial and does not apply to the clinical use or research evaluation of hydroxychloroquine in pre- or postexposure prophylaxis in patients exposed to COVID-19. The decision was based on the results of a nonrandomized study published by The Lancet on hydroxychloroquine and chloroquine and its effects on hospitalized COVID-19 patients [76]. This study was recently retracted because of several authors questioning the validity of the data used in the study. Based on our data, we can reject that hydroxychloroquine offers benefit to COVID-19 patients.
We have identified 4 important reviews that are comparable to our present project [77–80]. The first is a network meta-analysis just published in BMJ [77]. However, at the time of publication, this project did not include all relevant trials [50,52,54–56,59–61,64], including the pivotal trials assessing the effects of hydroxychloroquine [54,55,59].
The second project is a literature review published in JAMA using PubMed to identify relevant English-language articles published through March 25, 2020, on pharmacological interventions for COVID-19. The search resulted in 1,315 total articles. This is because the authors also included case reports, case series, and review articles if they lacked randomised clinical trials. Moreover, this review was only a narrative review without meta-analytic methods and trial sequential analysis [78].
The third project is a living mapping of ongoing randomized clinical trials with network meta-analysis on all interventions for COVID-19. The authors are producing and disseminating preliminary results through an open platform [79]. This review includes both prevention and treatment and does not use trial sequential analysis or similar methods to handle problems with multiplicity (repeating updating of meta-analysis, multiple comparisons due to inclusion of multiple interventions, assessing multiple outcomes) [9].
The fourth project is a preprint of a rapid review assessing the effectiveness and safety of antiviral antibody treatments for COVID-19 published in medrXiv [80]. Fifty-four studies were included in the review: 3 controlled trials, 10 cohort studies, 7 retrospective medical record/database studies, and 34 case reports or series. These studies included patients with severe acute respiratory syndrome (SARS, n = 33), Middle East respiratory syndrome (MERS, n = 16), COVID-19 (n = 3), and unspecified coronavirus (n = 2). The most common treatment was ribavirin (n = 41), followed by oseltamivir (n = 10), and the combination of lopinavir/ritonavir (n = 7). The authors conclude that current evidence for the effectiveness and safety of antiviral therapies for COVID-19 is inconclusive and suffers from a lack of well-designed prospective trials. Moreover, this review was only a narrative review without meta-analytic methods and trial sequential analysis [9].
Conclusions
Our study showed that dexamethasone and remdesivir might be beneficial for COVID-19 patients, but the certainty of the evidence was low to very low, so more trials are needed. We could reject that hydroxychloroquine versus standard care reduces the risk of death and serious adverse events with 20%. Otherwise, no evidence-based treatment for COVID-19 currently exists. This review will continuously inform best practice in treatment and clinical research of COVID-19. There is an urgent need for additional evidence, especially trials assessing the effects of dexamethasone and remdesivir.
Differences between the protocol and the review
We erroneously reported the adjusted trial sequential analysis alpha as 2% in our published protocol [9]. This has now been corrected to 3.3% according to 2 primary outcomes [18]. Further, we included “time to clinical improvement” as an outcome post hoc.
Supporting information
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
(DOC)
(DOC)
(DOCX)
(XLSX)
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIFF)
(TIFF)
Trial sequential analysis on remdesivir versus placebo on nonserious adverse events in 2 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 37%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 4%. The required information size was 1,970 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines). The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping lines). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(DOCX)
(TIFF)
Trial sequential analysis on hydroxychloroquine versus standard care on serious adverse events in 7 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 20.7%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 0%. The required information size was 4,123 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (inward sloping red lines) nor the conventional naive boundaries. The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping red lines and the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(TIFF)
Trial sequential analysis on hydroxychloroquine versus standard care on serious adverse events in 7 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 21.0%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 0%. The required information size was 4,123 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (inward sloping red lines) nor the conventional naive boundaries. The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping red lines and the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(TIFF)
(TIFF)
Trial sequential analysis on lopinavir–ritonavir versus standard care on serious adverse events in 2 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 27.6%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 0%. The required information size was 2,856 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (inward sloping red lines) nor the conventional naive boundaries. The cumulative Z‐curve did not cross the inner‐wedge futility line (red outward sloping red lines nor the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(TIFF)
(TIFF)
Abbreviations
- CI
confidence interval
- COVID-19
coronavirus diease 2019
- GRADE
Grading of Recommendation, Assessment, Development, and Evaluation
- ICH-GCP
International Conference on Harmonisation–Good Clinical Practice
- MERS
Middle East respiratory syndrome
- RECOVERY
Randomised Evaluation of COVid-19 thERapY
- RR
risk ratio
- SARS
severe acute respiratory syndrome
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Data Availability
The data underlying the results presented in the study are available from the secretary at Copenhagen Trial Unit, Mette Hansen: mette.hansen@ctu.dk. She is not an author of this paper. If we receive individual patient data from a third party, there may apply some restrictions.
Funding Statement
SJ, JCJ, and CG received a grant from The Grundfos Foundation (Grant number: 2020-025, URL: https://www.pdjf.dk/en/). NN received a grant from The Swedish Research Council (Grant number: N/A. URL: https://www.vr.se/english.html). AAV received a European Union's Horizon 2020 grant (Grant number: 754936. URL: https://ec.europa.eu/programmes/horizon2020/en). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Guan W, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Novel Coronavirus (2019-nCOV). Situation Report 51. 2020. [cited 2020 Aug 7]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10
- 3.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JF, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, Rubin EJ. Covid-19—The search for effective therapy. New England Journal of Medicine. 2020. 10.1056/NEJMe2005477 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauci AS, Lane HC, Redfield RR. Covid-19—Navigating the uncharted. N Engl J Med. 2020;382:1268–1269. 10.1056/NEJMe2002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juul S, Nielsen N, Bentzer P, Veroniki AA, Thabane L, Linder A, et al. Interventions for treatment of COVID-19: a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING Project). Syst Rev. 2020;9(1):108 10.1186/s13643-020-01371-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7). 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339(b2700). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. [cited 2020 Aug 7]. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 13.The Cochrane Collaboration. Cochrane COVID-19 Study Register [Internet]. [cited 2020 Aug 7]. Available from: https://covid-19.cochrane.org/
- 14.Thorlund K, Dron L, Park J, Hsu G, Forrest JI, Mills EJ. A real-time dashboard of clinical trials for COVID-19. Lancet Dig Health. 2020;2(6):E286–E287. 10.1016/S2589-7500(20)30086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 16.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Guideline: Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice (ICH-GCP). 2015. [cited 2020 Jul 4]. Available from: https://ichgcp.net/.
- 17.Keus F, Wetterslev J, Gluud C, van Laarhoven CJ. Evidence at a glance: error matrix approach for overviewing available evidence. BMC Med Res Methodol. 2010;10(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol. 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2020.
- 20.StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 21.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2020 Aug 7] Available from: https://handbook-5-1.cochrane.org/. [Google Scholar]
- 22.Higgins JP, Spiegelhalter DJ. Being sceptical about meta-analyses: a Bayesian perspective on magnesium trials in myocardial infarction. Int J Epidemiol. 2002;31(1):96–104. 10.1093/ije/31.1.96 [DOI] [PubMed] [Google Scholar]
- 23.Copenhagen Trial Unit. TSA—Trial Sequential Analysis. [cited 2020 Jul 6]. Available from: http://www.ctu.dk/tsa/.
- 24.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 25.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–9. 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 26.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2008;38(1):287–98. 10.1093/ije/dyn188 [DOI] [PubMed] [Google Scholar]
- 27.Thorlund K, Devereaux P, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2008;38(1):276–86 10.1093/ije/dyn179 [DOI] [PubMed] [Google Scholar]
- 28.Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. 2009;9(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud, C. User manual for trial sequential analysis (TSA). 2011. [cited 2020 Jul 4]. Available from: http://wwwctudk/tsa/files/tsa_manualpdf
- 30.Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. Clin Epidemiol. 2010;2:57 10.2147/clep.s9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imberger G, Thorlund K, Gluud C, Wetterslev J. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review. BMJ Open. 2016;6(8):e011890 10.1136/bmjopen-2016-011890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—Preliminary report. N Engl J Med. 2020. 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 33.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. Epub 2020. 10.1056/NEJMoa200128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus arbidol for COVID-19: A randomized clinical trial. medRxiv. 2020:2020.03.17.20037432. [preprint] [Google Scholar]
- 35.Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang Univ (Med Sci). 2020;49(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020:2020.03.22.20040758. [preprint] [Google Scholar]
- 37.Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, et al. Efficacy and safety of interferon beta-1a in treatment of severe COVID-19: A randomized clinical trial. medRxiv. 2020. 10.1101/2020.05.28.20116467 [Preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. Epub 2020. 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395: 1695–704 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020;324(5):460–470. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Xie Z, Lin W, et al. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Cell Press. 2020. [Pre-proof] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: An exploratory randomized, controlled trial. medRxiv. 2020. 10.1101/2020.04.29.20085761 [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):P1569–1578. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu CN, Xia LZ, Li KH, Ma WH, Yu DN, Qu B, et al. High-flow nasal-oxygenation-assisted fibreoptic tracheal intubation in critically ill patients with COVID-19 pneumonia: a prospective randomised controlled trial. Br J Anaesth. 2020;125(1):E166–E168. 10.1016/j.bja.2020.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng F, Zhou Y, Zhou Z, Ye F, Huang B, Huang Y, et al. A novel protein drug, novaferon, as the potential antiviral drug for COVID-19. medRxiv. 2020. 10.1101/2020.04.24.20077735 [preprint] [DOI] [Google Scholar]
- 47.Zhong M, Sun A, Xiao T, Yao G, Sang L, Zheng X, et al. A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-lipotic acid for critically ill patients with coronavirus disease 2019 (COVID-19). medRxiv. 2020. 2020.04.15.20066266. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19 –Preliminary report. medRxiv. 2020. 10.1101/2020.06.22.20137273 [preprint] [DOI] [Google Scholar]
- 49.Chen L, Zhang Z-Y, Fu J-G, et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study. medRxiv. 2020. 10.1101/2020.06.19.20136093 [preprint] [DOI] [Google Scholar]
- 50.Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136 10.1001/jamanetworkopen.2020.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. JAMA Netw Open. 2020;3(4). [DOI] [PubMed] [Google Scholar]
- 52.Gharbharan A, Jordans CCE, Geurtsvankessel C, et al. Convalescent plasma for COVID-19. A randomized clinical trial. medrRxiv. 2020: 10.1101/2020.07.01.20139857 [Preprint] [DOI] [Google Scholar]
- 53.RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19—Preliminary report. N Engl J Med. Epub 2020. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020. 10.7326/M20-4207. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. Epub 2020. 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild covid-19: a randomized-controlled trial. Clin Infect Dis. Epub 2020. 10.1093/cid/ciaa1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corral-Gudino L, Bahamonde A, Arnaiz delas Revillas F, et al. GLUCOCOVID: A controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. medRxiv. 2020. 10.1101/2020.06.17.20133579 [Preprint] [DOI] [Google Scholar]
- 58.Sakoulas G, Geriak M, Kullar R, et al. Intravenous Immunoglobulin (IVIG) significantly reduces respiratory morbidity in COVID-19 pneumonia: a prospective randomized trial. medRxiv. 2020. 10.1101/2020.07.20.20157891. [Preprint] [DOI] [Google Scholar]
- 59.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020. 10.1101/2020.07.15.20151852. [Preprint] [DOI] [Google Scholar]
- 60.Chen C-P, Lin Y-C, Chen T-C, et al. A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19). medRxiv. 2020. 10.1101/2020.07.08.20148841 [Preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Xia L, Liu L, et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect Dis. 2020;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y-K, Huang Y-Q, Tang S-Q, et al. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus pneumonia: results of a randomized, open-labeled prospective study. SSRN. 2020. 10.2139/ssrn.3576905. [Preprint] [DOI] [Google Scholar]
- 63.Guvenmez O, Keskin H, Ay B, et al. The comparison of the effectiveness of Lincocin® and Azitro® in the treatment of covid-19-associated pneumonia: A prospective study. J Popul Ther Clin Pharmacol. 2020;27(SP1):e5–e10. [DOI] [PubMed] [Google Scholar]
- 64.Yuan X, Yi W, Liu B, et al. Pulmonary radiological change of COVID-19 patients with 99mTc-MDP treatment. medRxiv. 2020. 10.1101/2020.04.07.20054767. [Preprint] [DOI] [Google Scholar]
- 65.Idelsis E-M, Jesus P-E, Yaquelin D-R, et al. Effect and safety of combination of interferon alpha-2b and gamma or interferon alpha-2b for negativization of SARS-CoV-2 viral RNA. Preliminary results of a randomized controlled clinical trial. medRxiv. 2020. 10.1101/2020.07.29.20164251. [Preprint] [DOI] [Google Scholar]
- 66.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davoodi L, Abedi SM, Salehifar E, et al. Febuxostat therapy in outpatients with suspected COVID-19: A clinical trial. Int J Clin Pract. 2020:e13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lofgren SMM, Nicol MR, Bangdiwala AS, et al. Safety of hydroxychloroquine among outpatient clinical trial participants for COVID-19. medRxiv. 2020. 10.1101/2020.07.16.20155531 [Preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on hydroxychloroquine 5 June 2020. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. [cited 2020 Jul 4]. Available from: https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf
- 70.Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) trial on lopinavir-ritonavir 29 June 2020. No clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients studied in RECOVERY. [cited 2020 Jul 4]. Available from: https://www.recoverytrial.net/files/lopinavir-ritonavir-recovery-statement-29062020_final.pdf
- 71.Dimairo M, Pallmann P, Wason J, et al. The Adaptive designs CONSORT Extension (ACE) statement: a checklist with explanation and elaboration guideline for reporting randomised trials that use an adaptive design. BMJ. 2020;369:m115 10.1136/bmj.m115 [Epub ahead of print] 10.1136/bmj.m115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris T, Dahly D, Hood K, et al. Statistical review of effect of dexamethasone in hospitalized patients with COVID-19 –preliminary report. Zenodo. 2020. 10.5281/zenodo.3928540 [DOI] [Google Scholar]
- 73.Savović J, Turner RM, Mawdsley D, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane reviews: the ROBES meta-epidemiologic study. Am J Epidemiol. 2018;187(5):1113–22. 10.1093/aje/kwx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jakobsen JC, Gluud C, Winkel P, et al. The thresholds for statistical and clinical significance–a five-step procedure for evaluation of intervention effects in randomised clinical trials. BMC Med Res Methodol. 2014;14(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.World Health Organization. Coronavirus disease (COVID-19) outbreak 2020. [cited 2020 Jul 5]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 76.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020. 10.1016/S0140-6736(20)31180-6 [Epub ahead of print] [Retracted] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980 10.1136/bmj.m2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;323(18):1824–1836. 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 79.Boutron I, Ravaud P, Chaimani A, Tovey D, Devane D, Meerpohl J, et al. Living mapping and living systematic review of Covid-19 studies. 2020. [cited 2020 Aug 7]. Available from: www.covid-nma.com. [Google Scholar]
- 80.Rios P, Radhakrishnan A, Antony J, Thomas SM, Muller M, Straus SE, et al. Effectiveness and safety of antiviral or antibody treatments for coronavirus: A rapid review. medRxiv. 2020.03.19.20039008 [preprint] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
(DOC)
(DOC)
(DOCX)
(XLSX)
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIFF)
(TIFF)
Trial sequential analysis on remdesivir versus placebo on nonserious adverse events in 2 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 37%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 4%. The required information size was 1,970 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines). The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping lines). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(DOCX)
(TIFF)
Trial sequential analysis on hydroxychloroquine versus standard care on serious adverse events in 7 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 20.7%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 0%. The required information size was 4,123 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (inward sloping red lines) nor the conventional naive boundaries. The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping red lines and the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(TIFF)
Trial sequential analysis on hydroxychloroquine versus standard care on serious adverse events in 7 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 21.0%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 0%. The required information size was 4,123 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (inward sloping red lines) nor the conventional naive boundaries. The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping red lines and the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(TIFF)
(TIFF)
Trial sequential analysis on lopinavir–ritonavir versus standard care on serious adverse events in 2 high risk of bias trials. The DARIS was calculated based on an event rate in the control group of 27.6%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 0%. The required information size was 2,856 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (inward sloping red lines) nor the conventional naive boundaries. The cumulative Z‐curve did not cross the inner‐wedge futility line (red outward sloping red lines nor the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.
(TIFF)
(TIFF)
(TIFF)
Data Availability Statement
The data underlying the results presented in the study are available from the secretary at Copenhagen Trial Unit, Mette Hansen: mette.hansen@ctu.dk. She is not an author of this paper. If we receive individual patient data from a third party, there may apply some restrictions.