Abstract
Nowadays, COVID-19 also known as novel coronavirus has become a global pandemic by causing severe respiratory tract infections in humans without any definite treatment or vaccine. Therefore, disease control measures include slowing down or averting the transfer of this viral infection from person to person. Continuous efforts are carried out to avoid the transmission of this disease to frontline healthcare personnel using single-use personal protective equipment (PPE). However, a critical shortage in this equipment around the world is becoming an alarming concern. Therefore, it is vital to present a possible alternative to overcome the acute shortage of protective gear such as face masks against this infectious disease which can have universal accessibility and is easily available. Additive manufacturing (AM), also known as 3D printing, is a possible solution to overcome the shortage of protective gear and can play a vital role in supporting their conventional production supplies during this global pandemic situation. In this context, this paper provides a brief background study of COVID-19, its conventional preventive measure, and a detailed overview regarding the latest AM efforts including designers’ providers and makers in the 3D printing community. Moreover, numerous inquiries and questions such as technical factors, testing recommendations and characterization methods and biological concerns such as biocompatibility and sterilization for the AM manufactured medical devices are addressed in this paper. In the end, two examples of AM medical devices, i.e., face mask and Ambu bag ventilator, are presented and studied through numerical simulations.
Keywords: COVID-19, Coronavirus, PPE, Frontline healthcare workers, Additive manufacturing, Face mask, Ventilators
Introduction
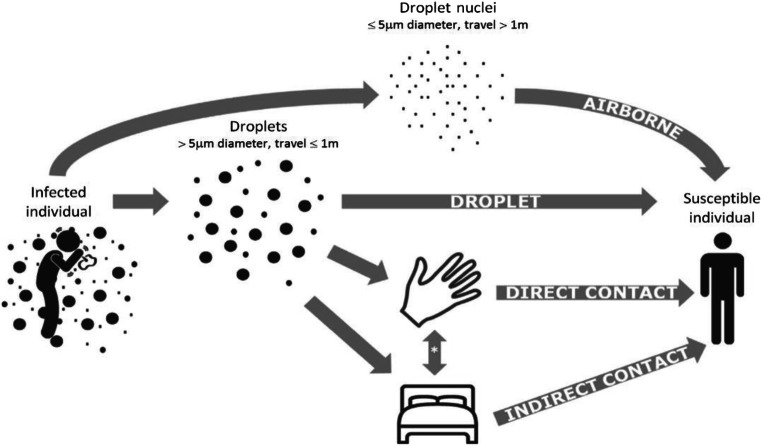
Currently, the world is experiencing a pandemic situation caused by the widespread of the viral disease known as “severe acute respiratory syndrome (SARS-CoV)” or “Coronavirus Disease 2019” (COVID-19) [1–3]. This virus initiates from the family of coronaviridae and caused almost 741,000 death with overall recorded cases of 20.3 million; that is why, it was declared as a global pandemic by WHO on 31 January 2020 [4–7]. Moreover, clinical symptoms and complications of COVID-19 are similar to the viral infections of the same origin and circulate among different animals with the ability of genetic mutation [8, 9]. This virus can be transmitted among human through human touch, air, or droplet inhalation and thus has high transmissibility. Moreover, it is found that in addition to droplet transmission, this virus can be transmitted through airborne transmission (Fig. 1). Airborne transmission is different from droplet transmission and is a much more effective mode of transmission because microbes in the air have a longer life span and can be transmitted over a large distance without coming in contact with the infected person [11, 12]. In addition, the presence of the virus in the blood and stools of a person presented a possibility of an additional mode of transmission. International reports suggested that COVID-19 was more effective and severe in elderly people or people with secondary health issues because of their weak immune system. Approximately, 50% of the patients had a critical or severe infection which requires monitoring in the intensive care unit, and 10% of these patients could require artificial breathing or mechanical ventilation [9]. Primary symptoms include muscle pain, dry cough, fatigue, and headache, while secondary signs include respiratory distress, fever, abdominal pain, sore throat, and diarrhea [13]. The person with signs of this viral infection or was near confirmed cases of COVID-19 or has any history of travel/stay in an exposed area is recommended for the diagnostic test of COVID-19 [13]. This test requires taking a specimen from nasopharyngeal and lowering respiratory tract like bronchoalveolar lavage for reverse transcriptase-polymerase chain reaction (RT-PCR). Presently, a computed topography (CT) scans of the lungs, high CRP, and lymphopenia are also helpful in the diagnosis of COVID-19; in fact, CT scan is quite useful and sensitive in diagnosing the disease while waiting for the RT-PCR test [14, 15].
Fig. 1.
Modes of transmission of COVID-19 [10]
Currently, there is no vaccine or treatment available for COVID-19; however, various clinical trials are in process to manufacture its cure [13, 16]. Therefore, COVID-19 patients require precautionary measures consisting of two steps precautions, i.e., standard precautions and transmission-based precautions [16, 17]. The former method includes respiratory hygiene and etiquette, hand hygiene, use of protective gear for face and eyes, use of sterilizing medical devices, and clean surroundings. However, latter methods include contact precautions, social distancing, and droplet precautions, i.e., the use of face mask and protective eyewear and airborne precaution (respirator, N95) ([17–37], Fig. 2, 3, and 4). Normally, patient with mild symptoms is asked to stay in self-isolation and avoid social contacts; therefore, it is recommended by healthcare professionals that the infected person should do quarantine for 14 days with a median of 5–6 days [15, 39, 40]. Moreover, self-hygiene, the use of antiseptic gel/alcohol for cleaning, and the use of protective gear such as face mask and gloves are recommended especially for healthcare providers and are advised to use PPE according to the risk of the situation [13, 17, 41].
Fig. 2.
Fig. 3.
Examples of eye and face protection gear [17]. (a) Eye Visor, (b) Goggles, and (c) Face Shield
Fig. 4.
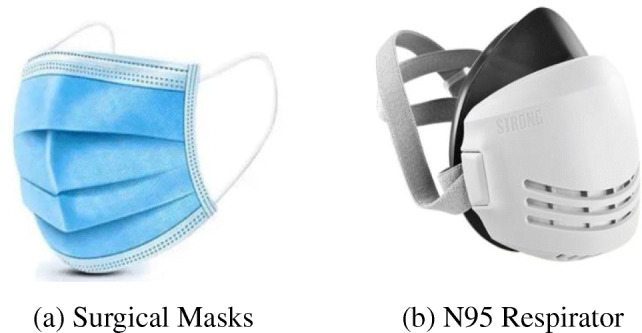
Examples of face masks [17]. (a) Surgical Masks, (b) N95 Respirator
The massive demand of PPE and protective gear during this pandemic situation has caused an acute reduction in their global supply, and significant interruptions in their supply chain left medical personnel and regular people without protection. This poses a challenge to healthcare resources around the world in contrast; according to the World Health Organization (WHO) guidelines, the personal protective material is indispensable for all healthcare workers such as doctors and nurses in hospitals and other healthcare settings. Moreover, hospitals around the world are in urgent need of the necessary medical equipment such as mechanical ventilators to treat patients. So in this context, this global pandemic situation demonstrates emerging requirements for alternatives with flexibility in approaches, such as in situ 3D printing or AM technique of medical devices including PPE in healthcare with an advantage of easy and robust fabrication of any complex geometry or shape. Thus, the present contribution will address and discuss several aspects related to AM that could play an important role in the domain of medical products and devices with an up-to-date overview and categorization of 3D-printed medical products and devices. In addition, in the framework of tackling the shortage in medical supplies against COVID-19, proof of concepts and prototype models will be explored with detailed steps of AM modeling and printing. More specifically, a reusable 3D-printed face mask and respirator accessory based on materials and AM technique processes as examples will be demonstrated.
Medical device shortages during the COVID-19 public health emergency
Since the start of the coronavirus disease 2019 (COVID-19) pandemic, 3D printing has stepped up to become a vital technology to support improved healthcare and our general response to the emergency. The crisis has highlighted how 3D printing can be at the base of a greener and more environmentally friendly future. 3D printing, thanks to the possibility to produce parts on demand, can reduce waste and inventory. Its inherent flexibility and the possibility to modify designs available online are unleashing creative and sustainable solutions that can carry the technology forward in the “new normal.” In this critical situation, the World Health Organization has warned that severe and mounting disruption to the global supply of personal protective equipment (PPE)―caused by rising demand, panic buying, hoarding, and misuse―is putting lives at risk from the new coronavirus. Healthcare systems place orders to selected distributors, and supplies are delivered weekly. The distributors, in turn, buy from manufacturers or, in many instances, contract third parties to manufacture products that the distributors then sell to the healthcare system. At present, US-based PPE production is limited, and more than 70% of respiratory protection supplies used in the USA are manufactured in China. With large spikes in global demand and drastically reduced production in China during early 2020, major distributors have been unable to fill orders. Some healthcare systems currently face estimated delays of 3 to 6 months for requested supplies [42].
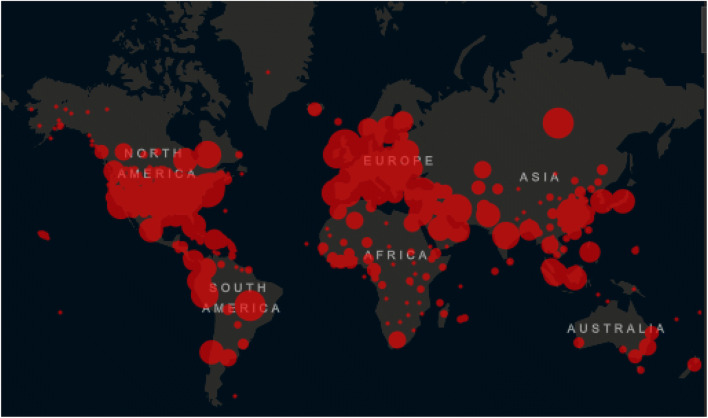
The WHO has reported substantial shortages, compromising their ability to keep healthcare professionals safe while treating increasing numbers of patients. Gloves, face masks, N95 respirators, powered air-purifying respirators, eye protection, and gowns are central to transmission-based precautions. Initial delays in COVID-19 testing increased PPE use, further intensifying demand [43–45]. According to the Centre for Systems Science and Engineering (CSSE) at Johns Hopkins University, as of midnight on 24 April 2020, 2,783,512 cases have been confirmed, including 195,775 deaths. The majority of the deaths are in the USA with 52,654 deaths followed. In Italy, on the same date, 192,994 cases were confirmed, of which 25,969 people died [46]. Figure 5 shows the distribution map of the virus worldwide as of 24 April.
Fig. 5.
COVID-19 situation update worldwide, as of 24 April 2020 [46]
In this circumstance, 3D printing can make a difference and respond to the emergency of this epidemic. Many professions are involved in addressing the global health crisis associated with COVID-19. The 3D printing community has responded to the COVID-19 crisis, pledging to support the production of vital medical equipment for hospitals grappling with this pandemic.
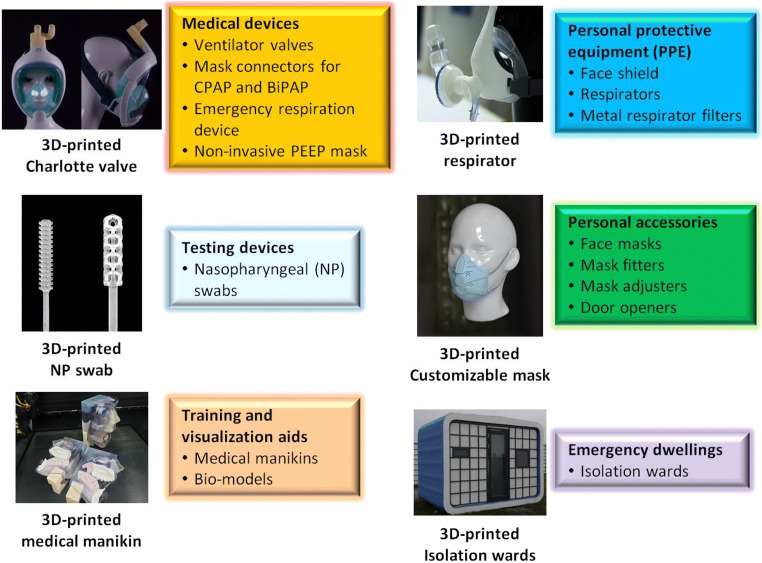
Moreover, the addictive nature of 3D printing enables product customization and complex designs. The broad spectrum of 3D printing applications in the fight against COVID-19 includes PPE, medical and testing devices, personal accessories, visualization aids, and emergency dwellings (Fig. 6). The categories of devices in the device shortage list are [48]:
Personal protective equipment
Testing supplies and equipment
Ventilation-related products
Fig. 6.
Applications of additive manufacturing in the fight against COVID-19 [47]
The latest 3D printing efforts against COVID-19
Many print farms have also been an initiative in China, Europe, and the USA, with 3D printing companies offering their in-house facilities to support with the medical device shortage around the world, using principally three 3D printing types: FDM (fused deposition modeling), SLS (selective laser sintering), and stereolithography (SLA).Table 1 shows a brief review on the latest 3D printing efforts against COVID-19.
Table 1.
AM companies responding to the COVID-19 outbreak [49, 50] (https://www.tctmagazine.com/additive-manufacturing-3d-printing-news/live-blog-how-the-3d-printing-industry-fighting-covid-19/)
| Company | 3D-printed devices | Country |
|---|---|---|
| Renishaw | Respiratory equipment | UK |
| Issinova | Face shields | Italy |
| NGen | Face shields | Canada |
| Fast Radius | Face shield kits | USA |
| Materialize | Oxygen mask | Belgium |
| Prusa | Face shields | Czech |
| BCN3D | Face shields | Spain |
| Titan Robotics | Face shield halos | USA |
| AddiFabb | Mimo face mask adapter | Denmark |
| Trinkle | N95 surgical masks | Germany |
| Prodways | Face shields | France |
| Markforged | Nasopharyngeal testing swabs | USA |
| Forecast 3D |
Face shields, stopgap masks Nasopharyngeal swabs |
USA |
| 3D Systems |
Venturi ventilator valves, stopgap face mask (SFM)-frame with visor Surgical mask, surgical N95 respirator |
UK |
| Carbon | Face shields, nasopharyngeal swabs | USA |
| Blue Origin | Face shield | USA |
| Protolabs | Ventilators | France |
| Essentium | Protective mask kit, filtration media | USA |
| Nexteer | Face mask, face shield | USA |
| Paragon |
Face shields, visors for NHS staff Test kit swabs |
UK |
| MatterHackers | PPE masks | USA |
| Flowbuilt Manufacturing | PPE masks | USA |
| Evonik | Masks, ventilator components | Germany |
| Azul 3D | Face shields | USA |
| Ferrovial | Face mask | Spain |
| EnvisionTEC | Ventilation splitters, PPE testing swabs | USA |
| FATHOM | Ventilator, KN95 masks, face shields | USA |
| CRP Technology | Emergency valves, respiratory masks | Italy |
| Fortify | Masks | USA |
| Roboze | Valves | Italy |
| Freeman Technology | PPE visors | UK |
| Voodoo | Protective face masks | USA |
| Nagami Design | Protective face masks | Spain |
| Sintratec | Hands-free door opener | Switzerland |
| Formlabs | Test swabs | USA |
| SmileDirectClub | Face shields | Canada |
| Volkswagen | Ventilators | Germany |
| Photocentric | Venturi valves for respirators | UK |
| iMakr | Face shields | USA |
| SPEE3D 3D | Anti-microbial copper | Australia |
| Omni3D | Face shields | Poland |
Milan’s Issinova technology is one of the first companies that presented a 3D printable mask connector design, named Charlotte valve, using a selective laser sintering (SLS) printer. This 3D-printed mask connected design was used to attach Decathlon’s Easybreath snorkeling masks with CPAP (Continuous Positive Air Pressure) machines and showed successful testing on patients which led to their mass production [51]. Afterward, the most popular item manufactured and was available online was face shields. In order for the face shields to be suitable for use in COVID-19 centers, they must follow specific standards such as be close on the forehead, allow safe air ventilation, and at the same time be comfortable for long-wearing hours [52]. Moreover, Canadian companies established 3D printing technology to produce critically needed technologies, equipment, and medical devices to fight COVID-19 such as face shields using national supercluster Next Generation Manufacturing Canada (NGen). NGen has invested more than $21 million in manufacturers and companies such as Toronto-based Mosaic to produce an array of products, including 45,000 face shield over 3 months, and Burloak Technologies to produce a full-face shield at volumes of 5000 per week (https://www.tctmagazine.com/additive-manufacturing-3d-printing-news/live-blog-how-the-3d-printing-industry-fighting-covid-19/).
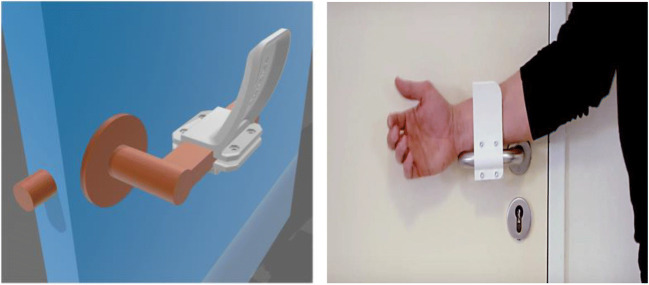
It is well-known by now that serious precautions are required to limit the direct spread of COVID-19 by person-to-person contact or person to objects or surfaces contact such as door handles. Therefore, simple interventions limiting such transmission can have extensive significances such as transmission from door handles in public and in medical centers which usually are designed for patient privacy, ward control, and periods of isolation during pandemics [53]. In addition to surface cleaning with meticulous cleansers, modifications of a range of handles, an alternative for opening doors without direct skin-to-surface contact, have been recently developed and can be manufactured on most 3D printing platforms rapidly (Fig. 7).
Fig. 7.
Materialize designed a hands-free 3D-printed door opener for protection against COVID-19 [52]
In addition, we aim here to provide an overview of the materials as being through 3D printing for use in a medical application in response to COVID-19 and briefly describe the most important ones. Copper3D team has designed a mask, viz., NanoHack 2.0 [54] (see Fig. 8). This mask has been made up of a strong and hermetic monoblock structure, which is 3D printed with PLActive to provide maximum protection against the external environment. The PLActive is a recyclable and biocompatible polymer that contains a copper nanocomposite that has shown antimicrobial properties. The frame is sealed with a 3D-printed edge with MDFlex, an antimicrobial TPU. MDFlex is an innovative nanocomposite developed with a high-quality TPU98A and a patented nano-copper additive, scientifically validated and highly effective.
Fig. 8.
NanoHack mask printed with a recyclable and biocompatible polymer PLActive [54]
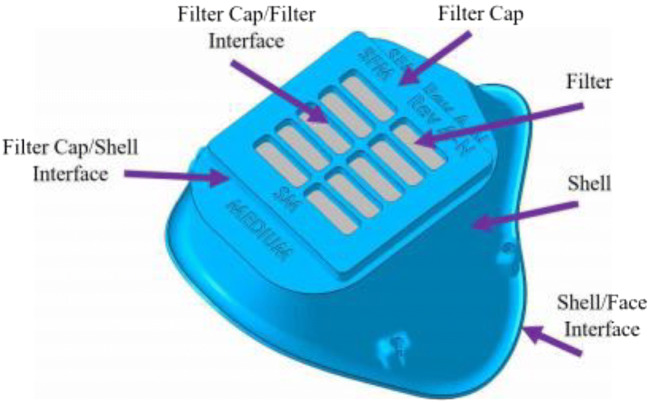
Recently, the concept and prototype of a reusable printed face mask that can be adopted and used all worldwide when needed are revealed by (https://www.governmentciomedia.com/fda-approves-first-3d-printed-mask-COVID-19-support). This mask consists of two reusable 3D-printed components made of polyamide composite (face mask and filter membrane support) and other two disposable parts (head fixation band and filter membrane). The Veterans Health Administration (VHA) team has created 3D-printed surgical Stopgap face mask that can be used for protection against liquid during COVID-19 [55]. This mask is composed of the 3D-printed mask and filter cover, two elastic strips, and a rectangular patch of filter material (Fig. 9). It is manufactured from medical-grade nylon which can be sterilized with pressurized steam and is compatible with disinfectant cleaners, and the 3D printing method is powder bed fusion via selective laser sintering.
Fig. 9.
Stopgap face mask with terminology
Lowell Makes develops 3D-printed masks, viz., COVID-19 mask [56] shown in Fig. 10. This mask is manufactured using filament (PLA, PETG) with low porosity which provides the possibility of sterilization without damage.
Fig. 10.

COVID-19 mask by Lafactoria3d made of PLA [43]
With respect to the manufacturing of screens or valves which, because of their use, do not need to be composed of antibacterial or biocompatible materials, basic materials can be used in 3D printing, such as PLA or ABS (see Fig. 11).
Fig. 11.
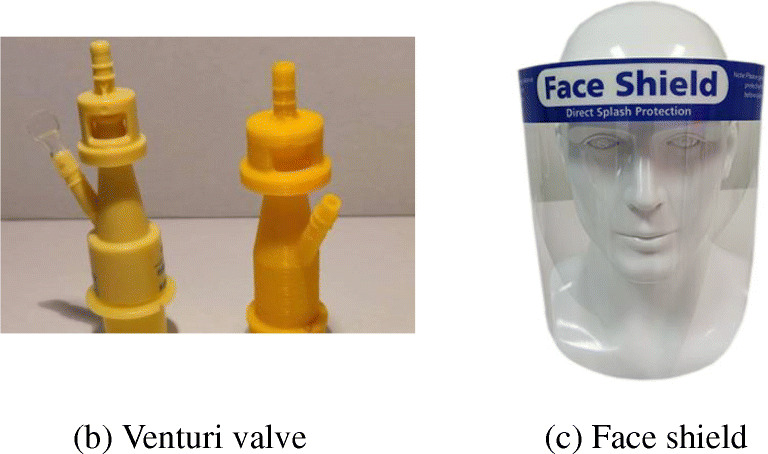
Valves and screens that can be printed using PLA, ABS, or PETG [57]. (a) Venturi valve, (b) Face shield
Regulatory considerations for emergency use authorizations during the COVID-19
An emergency use authorization (EUA) is vital for medical device manufacturers to consider both the short- and long-term rules and regulations related to healthcare efforts because of the current impacts of COVID-19. The manufacturing companies can help healthcare workers in tackling the newfound challenges of this novel disease by working along the Food and Drug Administration (FDA) to accelerate the accessibility of premarket products and technologies [58–66]. The regulatory considerations for emergency use authorizations (EUA) for medical device manufacturers in response to this pandemic consists of four phases: phase 1, in vitro diagnostic products; phase 2, personal protective equipment; phase 3, ventilators and components; and phase 4, other relevant medical devices.
It is rational to accept that the chemical, biological, radiological, and nuclear (CBRN) subject may be effective in response to COVID-19 based on the scientific evidence available to FDA. Moreover, the potential benefits of the CBRN outweigh the risks of such products when used under the conditions defined in the approval [67].
Phase 1: In vitro diagnostic (IVD) products. The Centers for Disease Control and Prevention (CDC) and New York State Department of Public Health (Wadsworth Center NYSDOH) are the first two organizations that were issued EUAs for RT-PCR-based COVID-19 IVD tests in February by FDA. Afterward, more than 60 private and public labs were issued EUAs for the supply of COVID-19 diagnostic tests, and 25 clinical laboratories are included in the EUA list of laboratory-developed, molecular-based tests.
Phase 2: Personal protective equipment (PPE). FDA approved numerous EUAs for air-filtering respirators, approved by the National Institute for Occupational Safety and Health (NIOSH) and non-NIOSH bodies to be used by healthcare professionals to overcome the shortage of hospital PPE supplies. Moreover, devices that can ensure the reuse of PPEs by sterilization and decontamination are also been considered in the PPE category for approvals in emergency.
Phase 3: Ventilators and their components. Various hospitalized COVID-19 patients showed shortness of breath which confirmed that the severe infection of this virus may develop into acute respiratory distress syndrome. Therefore, these patients can require breathing support, including extracorporeal blood purification (EBP) technology, extracorporeal membrane oxygenation, and mechanical ventilation to reduce pro-inflammatory cytokine levels. In this context, FDA has issued EUAs for different respiratory devices including ventilators and accessories such as renal replacement therapy, EBP, and diaphragmatic pacing stimulator systems.
Phase 4: Other relevant medical devices. The Health and Human Services (HHS) approved certain medical devices for the response to the pandemic situation in the early days including IVD COVID-19 tests, PPEs, and ventilators. However, the FDA has now issued EUAs for devices that may assist in reducing PPE usage and provide better care, e.g., on 5 May, FDA issued a EUA for a remote ECG-monitoring device to counter the complications of the COVID-19 treatment with drugs that may cause life-threatening arrhythmia.
Application of 3D printing: medical equipment against COVID-19
As the number of people affected by the virus continues to increases, many countries are in shortage of resources to respond to the COVID-19 epidemic. As mentioned earlier, the lack of protective masks creates a very dangerous situation for frontline workers for the fight against this virus. Despite that many people, associations, and groups are working on the design and manufacture of face masks and other accessories; for the COVID-19 epidemic, many of these designs are not optimized when they are printed using AM technology. For this reason, we exemplify two case studies for which Digimat-AM simulation is employed as a prospect for the optimization of AM design. Through Digimat based AM, it will be possible to simulate the printing process and thus help professional AM providers and designers to identify manufacturing issues [68–70]. This will also allow optimizing the printing parameters for productivity and final part performance before the first medical part is printed, for example, by minimizing the part warpage and residual stresses as a function of the material and process parameters.
Steps to create a 3D medical model using AM
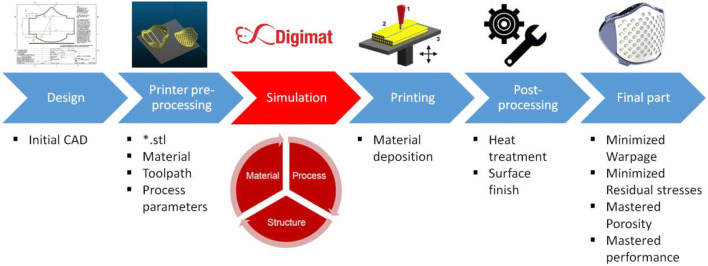
To avoid excessive cost, numerical modeling takes all its importance. Indeed, Digimat is used in our different simulations to analyze the performance of parts produced by additive manufacturing; Fig. 12 gives a summary of the different steps to produce a part for medical applications using numerical simulation [71]. It can be noted that the fabrication of the devices depends on the process, the material, and the heat treatment. Since one cannot control all the parameters, the 3D printing of a part may be slightly different compared with the CAD part, and this is due to the presence of warpage, residual stress, and porosity. Also, if the certification tests are not completed, the whole procedure of designing a part must be repeated, and this can generate large expenses.
Fig. 12.
Additive manufacturing: Digimat workflow [71]
AM process simulation of a face mask
The goals of AM process simulations of a given part is to predict the deformation, the residual stresses, and the microstructure (e.g., porosity) of the final part. Overall, to model the printing process of the underlying face mask intended for a medical purpose (see Fig. 13), it is required to take into account the evolution of the state of the material and also to model the accumulation and the relaxation of stresses which can happen over time. The numerical predictions of warpage require to take into consideration:
Adding thermo-viscoelasticity to take into account stress relaxation after and during printing
Improve accuracy of warpage predictions for heated chamber/bed
Accurately model and optimize cooldown strategy.
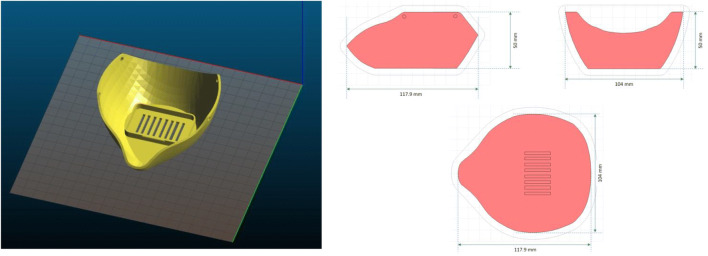
Fig. 13.
Geometry of the original mask structure to be produced by 3D printing
According to the workflow for the printing process simulation given in Fig. 13, defining the part to be printed is the first step; in this case, the CAD geometry is imported as STL file, and the material specifications shall also be characterized. The printing material model chosen is Nylon PA12 because of its biocompatibility that allows printing 3D objects for medical applications. The 3D printing process is well-defined by the toolpath definition and the process parameters and some other process-specific inputs to describe the manufacturing setup. The model put in place is then translated by a numerical simulation of the 3D printing which takes into account the different phenomena of radiation, conduction, and convection (heat transfer mechanisms inside the printer build). For the simulation, it is required a mesh definition, and therefore, the face mask geometry is meshed based on a voxel approach to ease the layer-by-layer modeling of the AM process. Once the finite element analysis is achieved, the residual stresses and the final deformed shape of the face mask can be obtained.
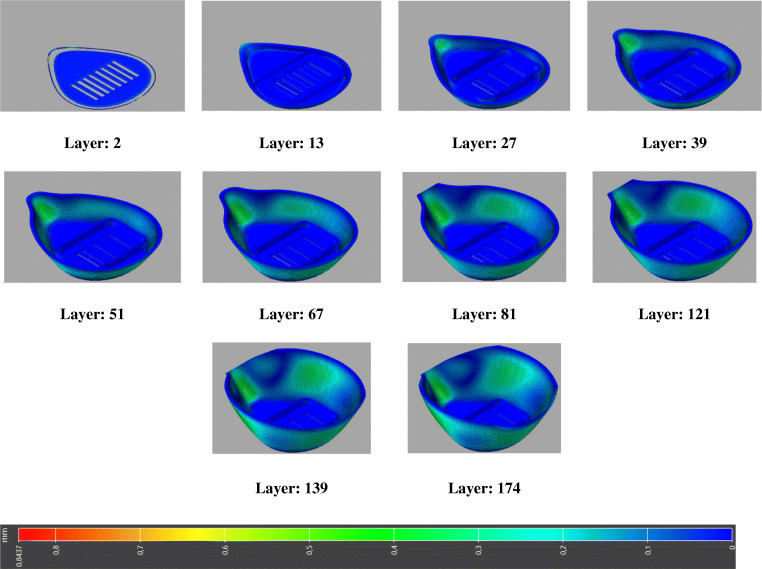
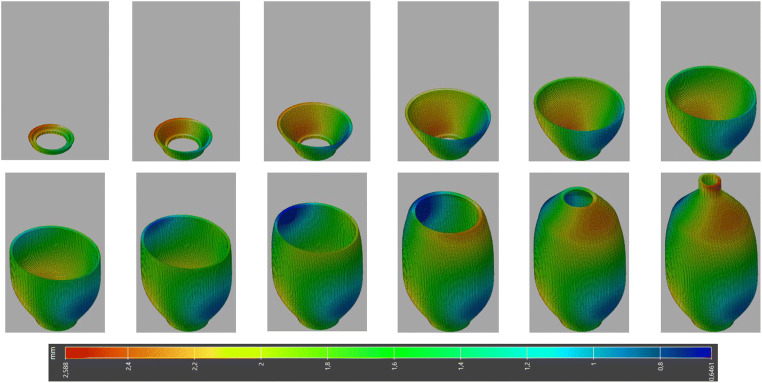
For the printing technology employed, the selective laser sintering (SLS) is chosen. The SLS process includes the sintering of powdered plastic material into a solid structure using a laser heat source. The laser selectively fuses the powder by scanning cross sections generated from a 3D CAD model description of the component. Once sintering a layer, the deposition of a new layer of powder from the powder bed takes place, and a new step of sintering begins, and the process is repeated until the component is completed. The AM process simulations of SLS include a number of steps, which all require to be modeled through the simulation. These steps are heating, layer deposition, laser scanning over the powder bed which heats the powder locally, sintering of powder, heat diffusion, and deposit new layer which could be repeated several times as needed. The printing process and mesh parameters used for face mask printing simulation are summarized in Table 2. These parameters are obtained after conducting an optimization problem, choosing the most appropriate parameters to minimize the warpage and residual stresses. The progressive layer-by-layer manufacturing of the medical face mask is eventually modeled, and global distortion is depicted in Fig. 14.
Table 2.
Process and mesh parameters used for face mask application
| Voxel size (mm) | 0.3 |
|---|---|
| Powder properties | |
| Powder diameter (mm) | 0.05 |
| Power conductivity (mW/mm.°C) | 0.15 |
| Tapped powder density (t/mm3) | 5.1E-10 |
| Manufacturing mesh | |
| Slicing: layer thickness (mm) | 0.12 |
| Chamber temperature (°C) | 160 |
| Laser power (mW) | 48,000 |
| Convection coefficient (mW/mm2.°C) | 0.015 |
Fig. 14.
Layer-by-layer build of the printed face mask
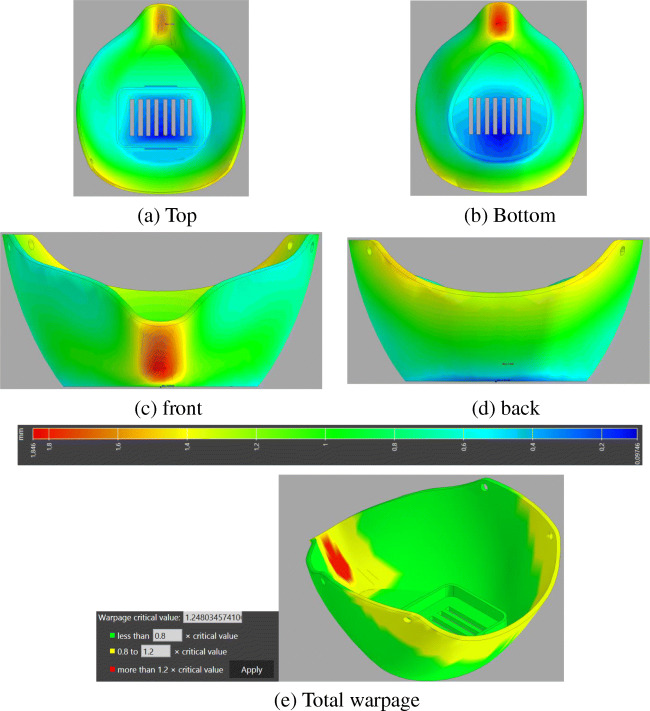
The predicted results of the warpage-inherent strain simulations for the printed mask are presented in Figs. 15 and 16. The accumulated magnitude of deflection of the mask can be displayed in different views in Fig. 15a–d. The maximum value of deformation reached 1.84 mm, with red-colored areas that are undergoing the most important distortion compared with the initial geometry shape. The maximum deflection appears in the area surrounding the nose. The meaning of such warpage deflection is that the actual dimensional geometry of the mask after its printing and temperature cooling process will deviate from the initial configuration. Besides, it is noticeable that the regions printed first accumulate less deflection, while the regions printed last incorporate the more deflection values. This leads to important information about the actual geometry once additively manufactured. For further illustration, the warpage indicator (total out-of-plane deflection) within the printed mask is depicted in Fig. 15e. This figure has been plotted clearly with only three colors being displayed―green, yellow, and red based on an assessment criterion given in Fig. 15e to represent the low, medium, and high warpage, respectively. From this figure, it can also be observed that the warpage is acute at the nose of the mask, wherein this zone suffers an important contraction which makes this region more critical.
Fig. 15.
Warpage deflection results after printing the mask, (a) Top, (b) Bottom, (c) Front, (d) Back, and (e) Total warpage
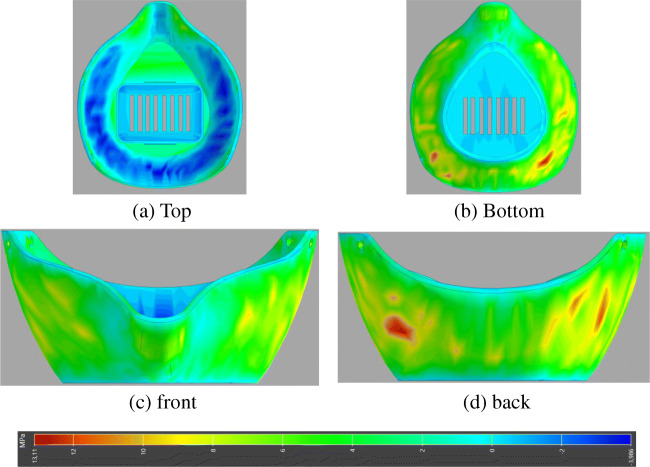
Fig. 16.
Results of stress component (S33) after printing the mask. (a) Top, (b) Bottom, (c) Front, (d) Back
Similarly, the resulting internal stress outcome in principal direction 3 (Z direction) for the printed mask is shown in Fig. 16. The plots show the residual stresses appeared after cooling down the printed mask. This is something that should be taken into account and use it in the final model which can deliver results more precise. For this mask, the most affected areas are the middle portion with the maximum value of internal stress which appears to be 13 MPa, while the lower values appear in the basement portion of the mask, and this probably is due to the lower warpage occurred in that area. This is something to take into account and to use it in the final structural model which can deliver results more accurate.
AM process simulation of Ambu bag ventilator
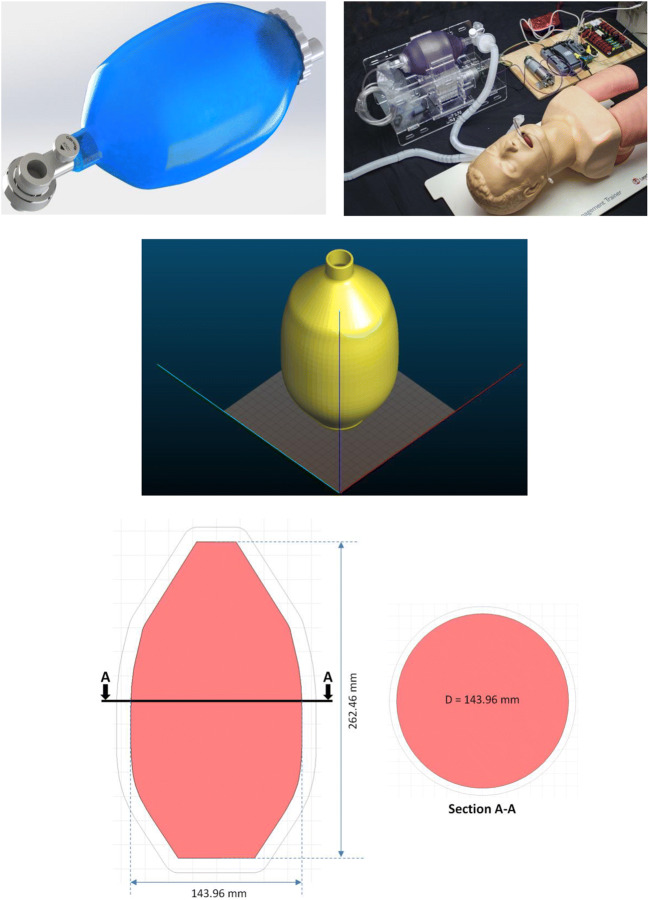
In reaction to the acute shortage of ventilators in the current pandemic situation, 3D printing can help address the shortages in such life-saving ventilation devices. In this subsection, we aim at predicting the warpage and residual stresses while building up the Ambu bag ventilator shown in Fig. 17 during its manufacturing process.
Fig. 17.
CAD geometry of the Ambu bag to simulate its printing process
The manufacturing parameters used for Ambu bag printing simulation are similar to that in Table 2. These parameters are chosen to minimize warpage and residual stresses. To create the mesh defined values, the voxel size is chosen to 1 mm. Once the mesh is created, the number of elements will be 363,767 voxels. For a given combination of material properties and process parameters, the mechanical layer-by-layer structural simulation can then be run to simulate the manufacturing process. The printing material used is Nylon PA12, and it will be set up as a generic printer for the SLS process. The progressive layer-by-layer manufacturing of the Ambu bag is finally modeled, and the stages of its 3D printing with deflection contours are shown in Fig. 18.
Fig. 18.
Stages of 3D printing of Ambu bag using layer-by-layer manufacturing
As a consequence of the printing process simulations, the accumulated warpage deflection and stress in principal direction 3 (Z direction) for the Ambu bag are illustrated in Fig. 19. Both the warpage deflection and internal stresses are present during printing, and certainly, they increase after the cool down. AM involves numerous testing to accomplish the good reliability and predictable performance of the printed component, and this can be easily implemented through the numerical simulations. The printed component could show important warpage deflection due to the thermal gradient which causes the part to be distorted and thereafter the non-guaranteed dimensional tolerance. The results of the warpage simulation show that the maximum value of deformation reached to 2.5 mm; the area most affected is the topmost portion. Regarding the internal stress, the maximum value of stress appears to be close to 9 MPa, and the region in the middle is most affected.
Fig. 19.
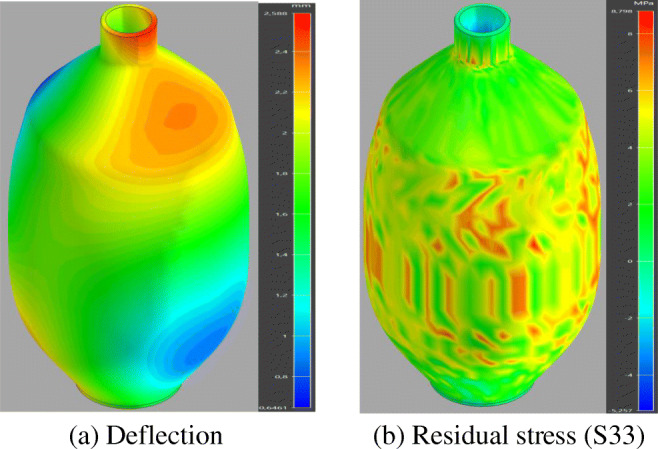
Warpage and stress results after the printing process of the Ambu bag. (a) Deflection, (b) Residual stress (S33)
It can be inferred from the performed simulations that the main interesting issue is to reduce the warpage and the residual stresses as possible. For this reason, efforts shall focus on minimizing/reducing warpage (warpage compensation). And this is all the more important for parts that exhibit significant warping deviations during the printing process. A solution to avoid the warpage consists of the compensation strategy which is based on using previously performed warpage analysis to counter warp the STL.
Conclusion and final remarks
Additive manufacturing, or 3D printing, has supported with the shortage of medical supplies and transforms manufacturing by enabling individuals and companies to produce items in the fight against COVID-19. This research reviewed the AM technology, discussed several advantages of this emerging technology, summarized the significant effects of AM, and underlined significant factors driving high demand for the technology during the COVID-19 pandemic. The 3D printing process is more straightforward and eliminates many steps used in traditional manufacturing. For larger academic medical centers that have partnerships between university-based 3D printing resources and hospitals, this is often already in place; however, appropriate safety protocols should always be reviewed. Currently, there are increasing efforts to prevent or reduce the transmission of coronavirus to the frontline of healthcare providers. AM has emerged as a potential alternative solution that has the advantage of facilitating the fabrication of complex engineering structures such as medical devices including PPE that cannot be easily produced using traditional manufacturing methods. From the perspective that AM could be a potential savior of dwindling medical supplies in the fight against coronavirus, the present contribution has explored several issues around this technology that could play an important role in the medical device field. In this respect, an up-to-date review has been undertaken to determine the ability of AM to provide exclusive benefits to humanity within the medical healthcare supply sector. The impacts of the COVID-19 pandemic on the global AM industry have further been illustrated. In addressing the current shortfall in medical supplies against COVID-19, evidence of conceptual designs and prototype models was provided. The steps toward creating the 3D medical models and the processes of printing 3D solid objects manufacturing using AM based layer-by-layer were introduced. For this purpose, Digimat-AM simulations were carried out for the analysis of the AM process of both the face mask and Ambu bag ventilator for COVID-19 outbreak purpose. The internal procedure of this tool comprises a conjugated thermomechanical analysis performed at the level of microstructure, calculating the stresses and deformations of printed part relying on inherent strain method, substantially minimizing the time of computation for macrostructure analysis. Through these simulations, a set of predictions were performed, including prediction of warpage and residual stress state, improving of printing parameters, optimizing the choice of material and compensate the warpage. Eventually, this emerging technology can effectively contribute in providing ample capacity for medical development in the future through improving the accuracy and reliability of the designs.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
M. Tarfaoui, Email: mostapha.tarfaoui@ensta-bretagne.fr
M. Nachtane, Email: mourad.nachtane@u-bordeaux.fr
I. Goda, Email: ibrahim.goda@u-bordeaux.fr
Y. Qureshi, Email: yumna.qureshi@ensta-bretagne.org
H. Benyahia, Email: h.benyahia.dms@gmail.com
References
- 1.(2020) Disease outbreak news. World Health Organization
- 2.(2020) Situation summary. Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/index.%0Ahtml.%0A. Accessed: 23 Apr 2020
- 3.del Rio C, Malani PN. 2019 novel coronavirus—important information for clinicians. JAMA. 2020;323(11):1039–1040. doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 4.Fauci AS, Lane HC, Redfield RR. Covid-19 — navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(2020) Severe acute respiratory syndrome (SARS). World Health Organization https://www.who.int/csr/don/archive/disease/severe_acute_respiratory_syndrome/en/
- 6.(2020) Middle East respiratory syndrome coronavirus (MERS-CoV). World Health Organization https://www.who.int/%0Aemergencies/mers-cov/en/. Accessed: 30 Apr 2020
- 7.(2020) Rolling updates on coronavirus disease (COVID-19). World Health Organization https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed: 10 May 2020
- 8.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(2020) Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. World Health Organization https://reliefweb.int/sites/reliefweb.int/files/resources/WHO-2019-nCoV-Sci_Brief-Transmission_modes-2020.1-eng.pdf
- 12.Ong SWX et al (2020) Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed]
- 13.(2020) COVID-19 disease (novel coronavirus). INSTITUT PASTEUR https://www.pasteur.fr/en/medical-center/disease-sheets/covid-19-disease-novel-coronavirus. Accessed: 19 May 2020
- 14.Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z, Jiang R, Hu T, Ding Y, Lin L, Gan Q, Luo L, Tang X, Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei-jie G, Zheng-yi N, Yu H, Wen-hua L, Chun-quan O, Jian-xing H, Lei L, Hong S, Chun-liang L, David SCH, Bin D, Lan-juan L, Guang Z, Kwok-Yung Y, Ru-chong C, Chun-li T, Tao W, Ping-yan C, Jie X Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed]
- 16.Roosa K, Lee Y, Luo R, Kirpich A, Rothenberg R, Hyman JM, Yan P, Chowell G. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect Dis Model. 2020;5:256–263. doi: 10.1016/j.idm.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taledo J (2020) “Infection prevention and control and novel coronavirus (COVID-19): standard precautions and use of personal protective equipment. Available: https://www.paho.org/en/documents/presentation-infection-prevention-and-control-and-novel-coronavirus-covid-19-standard. Accessed: 20 May 2020
- 18.Sophie Gallagher (2020) Coronavirus: can latex gloves protect you from catching deadly virus? INDEPENDENT
- 19.Xiao J, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings—personal protective and environmental measures. Emerg Infect Dis J. 2020;26(5):967. doi: 10.3201/eid2605.190994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutter JS, Spronken MI, Fraaij PL, Fouchier RAM, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowling BJ, Leung GM (2020) Epidemiological research priorities for public health control of the ongoing global novel coronavirus (2019-nCoV) outbreak. Eurosurveillance 25(6). 10.2807/1560-7917.ES.2020.25.6.2000110 [DOI] [PMC free article] [PubMed]
- 22.Han Q, Lin Q, Ni Z, You L Uncertainties about the transmission routes of 2019 novel coronavirus. Influenza Other Respi. Viruses, vol. n/a, no. n/a, doi: 10.1111/irv.12735 [DOI] [PMC free article] [PubMed]
- 23.MacIntyre CR, Chughtai AA (2015) Facemasks for the prevention of infection in healthcare and community settings. BMJ 350. 10.1136/bmj.h694 [DOI] [PubMed]
- 24.Ha’Eri GB, Wiley AM (1980) The efficacy of standard surgical face masks: an investigation using “tracer particles”. Clin Orthop Relat Res (148):160–162 [PubMed]
- 25.Patel RB, Skaria SD, Mansour MM, Smaldone GC. Respiratory source control using a surgical mask: an in vitro study. J Occup Environ Hyg. 2016;13(7):569–576. doi: 10.1080/15459624.2015.1043050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis. 2009;49(2):275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- 27.Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ (2013) Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 9(3) [DOI] [PMC free article] [PubMed]
- 28.Leung NHL et al (2020) Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed]
- 29.C. for Disease Control, P. (CDC), and others Fatal Cercopithecine herpesvirus 1 (B virus) infection following a mucocutaneous exposure and interim recommendations for worker protection. MMWR Morb Mortal Wkly Rep. 1998;47(49):1073. [PubMed] [Google Scholar]
- 30.Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. J Am Dent Assoc. 1994;125(5):579–584. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 31.Christensen RP, Robison RA, Robinson DF, Ploeger BJ, Leavitt RW. Efficiency of 42 brands of face masks and 2 face shields in preventing inhalation of airborne debris. Gen Dent. 1991;39(6):414. [PubMed] [Google Scholar]
- 32.Lindsley WG, Noti JD, Blachere FM, Szalajda JV, Beezhold DH. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11(8):509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(2020) The National Institute for Occupational Safety and Health (NIOSH): Infection Control. Centers for Disease Control and Prevention https://www.cdc.gov/niosh/topics/eye/eye-infectious.html. Accessed: 27 May 2020
- 34.Farrier SL, Farrier JN, Gilmour ASM. Eye safety in operative dentistry—a study in general dental practice. Br Dent J. 2006;200(4):218–223. doi: 10.1038/sj.bdj.4813257. [DOI] [PubMed] [Google Scholar]
- 35.Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32(4):372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 36.Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19(1):101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hand hygiene. Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html. Accessed: 27 May 2020
- 38.(2020) Applying the hand sanitizer. Wikihow https://www.wikihow.com/Use-Hand-Sanitizer
- 39.Zhang Y, Chen C, Zhu S, et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Wkly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- 40.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92(6):548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Wang X-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics. 2020;47(2):119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehrotra P, Malani P, Yadav P (2020) Personal protective equipment shortages during COVID-19—supply chain–related causes and mitigation strategies. In JAMA Health Forum (Vol. 1, No. 5, pp. e200553-e200553). American Medical Association [DOI] [PubMed]
- 43.Vordos N et al (2020) How 3D printing and social media tackles the PPE shortage during Covid–19 pandemic. Saf Sci 104870 [DOI] [PMC free article] [PubMed]
- 44.Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19 related supply shortages. Am J Med. 2020;133:771–773. doi: 10.1016/j.amjmed.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gondi S, Beckman AL, Deveau N, Raja AS, Ranney ML, Popkin R, He S. Personal protective equipment needs in the USA during the COVID-19 pandemic. Lancet (London, England) 2020;395(10237):e90. doi: 10.1016/S0140-6736(20)31038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choong YYC, Tan HW, Patel DC, Choong WTN, Chen CH, Low HY et al (2020) The global rise of 3D printing during the COVID-19 pandemic. Nature Reviews Materials:1–3 [DOI] [PMC free article] [PubMed]
- 48.World Health Organization (2020) Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance, 27 February 2020 (No. WHO/2019-nCov/IPCPPE_use/2020.1). World Health Organization
- 49.Mueller T, Elkaseer A, Charles A, Fauth J, Rabsch D, Scholz A, Marquardt C, Nau K, Scholz SG. Eight weeks later—the unprecedented rise of 3D printing during the COVID-19 pandemic—a case study, lessons learned, and implications on the future of global decentralized manufacturing. Appl Sci. 2020;10(12):4135. [Google Scholar]
- 50.Flanagan ST, Ballard DH. 3D printed face shields: a community response to the COVID-19 global pandemic. Acad Radiol. 2020;27(6):905–906. doi: 10.1016/j.acra.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larrañeta E, Dominguez-Robles J, Lamprou DA (2020) Additive manufacturing can assist in the fight against COVID-19 and other pandemics and impact on the global supply chain. 3D printing and additive manufacturing [DOI] [PMC free article] [PubMed]
- 52.Tino R, Moore R, Antoline S, Ravi P, Wake N, Ionita CN, …, Chepelev LL (2020) COVID-19 and the role of 3D printing in medicine [DOI] [PMC free article] [PubMed]
- 53.Armani AM, Hurt DE, Hwang D, McCarthy MC, Scholtz A (2020) Low-tech solutions for the COVID-19 supply chain crisis. Nature Reviews Materials:1–4 [DOI] [PMC free article] [PubMed]
- 54.Swennen GRJ, Pottel L, Haers PE (2019) Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg [DOI] [PMC free article] [PubMed]
- 55.https://lowellmakes.com/3d-printed-masks/. Accessed: 15 Jul 2020
- 56.Di Prima M, Coburn J, Hwang D et al (2016) Additively manufactured medical products - the FDA perspective. 3D Print Med 2. 10.1186/s41205-016-0005-9 [DOI] [PMC free article] [PubMed]
- 57.Belhouideg S (2020) Impact of 3D printed medical equipment on the management of the Covid19 pandemic. The International Journal of Health Planning and Management [DOI] [PMC free article] [PubMed]
- 58.US Food and Drug Administration Public workshop - AMof medical devices: an interactive discussion on the technical considerations of 3D printing, 2014
- 59.FDA (2017) Technical considerations for additive manufactured medical devices -guidance for industry and food and drug administration staff
- 60.(1998) Investigational device exemptions: supplemental applications. Fed Regist 63:64625
- 61.Codifi ed at 21 CFR Sec. 812.35
- 62.U.S. Food and Drug Administration Design control guidance for medical device manufacturers, Guidance for Industry and Food and Drug Administration Staff, issued March 11, 1997
- 63.Clifton W, Damon A, Martin AK (2020) Considerations and cautions for three-dimensional-printed personal protective equipment in the COVID-19 crisis. 3D printing and additive manufacturing [DOI] [PMC free article] [PubMed]
- 64.International Organization for Standardization: ISO/IEC JTC 1 . ISO/IEC 10993:2013 Biological evaluation of medical devices. ISO/IEC, Geneva
- 65.U.S. Department of Health and Human Services. Use of International Standard ISO-10993,
- 66.Gerke S, Shachar C, Chai PR, Cohen IG (2020) Regulatory, safety, and privacy concerns of home monitoring technologies during COVID-19. Nat Med:1–7 [DOI] [PMC free article] [PubMed]
- 67.Hu G, Sharma N (2020) Regulatory considerations for EUA during the COVID-19 public health emergency for medical device manufacturers. Regulatory focus. Regulatory Affairs Professionals Society
- 68.Nachtane M, Tarfaoui M, Ledoux Y, Khammassi S, Leneveu E, Pelleter J (2020) Experimental investigation on the dynamic behavior of 3D printed CF-PEKK composite under cyclic uniaxial compression. Composite structures, 112474
- 69.Jiang J, Xu X, Stringer J. Support structures for additive manufacturing: a review. Journal of Manufacturing and Materials Processing. 2018;2(4):64. [Google Scholar]
- 70.Jiang J, Ma Y. Path planning strategies to optimize accuracy, quality, build time and material use in additive manufacturing: a review. Micromachines. 2020;11(7):633. doi: 10.3390/mi11070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DIGIMAT . User documentation, © e-Xstream engineering. 2009. [Google Scholar]