Abstract
Background
Acute pulmonary embolism (PE) has been described as a frequent and prognostically relevant complication of COVID-19 infection.
Aim
We performed a systematic review and meta-analysis of the in-hospital incidence of acute PE among COVID-19 patients based on studies published within four months of COVID-19 outbreak.
Material and Methods
Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in abstracting data and assessing validity. We searched Medline, Scopus and Web of Science to locate all articles published up to August 1, 2020 reporting the incidence of acute PE (or lung thrombosis) in COVID-19 patients. The pooled in-hospital incidence of acute PE among COVID-19 patients was calculated using a random effects model and presenting the related 95% confidence interval (CI). Statistical heterogeneity was measured using the Higgins I2 statistic.
Results
We analysed data from 7178 COVID-19 patients [mean age 60.4 years] included in twenty-three studies. Among patients hospitalized in general wards and intensive care unit (ICU), the pooled in-hospital incidence of PE (or lung thrombosis) was 14.7% of cases (95% CI: 9.9–21.3%, I2=95.0%, p<0.0001) and 23.4% (95% CI:16.7–31.8%, I2=88.7%, p<0.0001), respectively. Segmental/sub-segmental pulmonary arteries were more frequently involved compared to main/lobar arteries (6.8% vs18.8%, p<0.001). Computer tomography pulmonary angiogram (CTPA) was used only in 35.3% of patients with COVID-19 infection across six studies.
Conclusions
The in-hospital incidence of acute PE among COVID-19 patients is higher in ICU patients compared to those hospitalized in general wards. CTPA was rarely used suggesting a potential underestimation of PE cases.
Keywords: Pulmonary embolism, Covid-19, Epidemiology, Meta-analysis
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) remains a severe public health emergency of international concern. Over the past months, several investigations have suggested an association between the COVID-19 pathogenesis and a pro-coagulant pattern that seems to be implicated in a higher risk of both arterial and venous thrombotic events [1], [2], [3], [4], [5], [6], [7]. In this regard, acute pulmonary embolism (PE) has emerged as a potential severe complication of the infection and both American and European consensus statement have suggested general recommendations to deal with these clinical events [8], [9], [10], [11]. However, the actual in-hospital incidence of acute PE in these patients has not yet been determined, but autopsy studies suggested that PE or lung thrombosis may represent a frequent cause of death in COVID-19 patients (Ref Ann Int Med). Indeed, radiological assessment with CT pulmonary angiography (CTPA) was not always feasible, especially in patients hospitalized in intensive care units (ICUs) during the first months of the pandemics, also due to critical illness and the frequent need of pronation during mechanical ventilation [12]. A more reliable estimation of the extent of this complication appears essential to guide the management of these patients. The aim of the present study is to perform a systematic review and meta-analysis on the in-hospital incidence of acute PE in COVID-19 patients hospitalized in general wards and ICUs based on studies published so far.
2. Material and methods
2.1. Study design and eligibility criteria
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplementary file 1) [13]. Data were obtained searching MEDLINE, Scopus and Web of Science for all investigations published any time to August 1, 2020 reporting the occurrence of acute PE in COVID-19 patients during the hospitalization.
2.2. Outcomes
The in-hospital incidence of acute PE in COVID-19 patients hospitalized into intensive care unit (ICU) and general wards was chosen as the primary outcome. Conversely, the anatomic location of thromboembolism within the pulmonary arterial vasculature and the use of CTPA for the diagnosis of acute PE were selected as the secondary outcomes.
2.3. Data extraction and quality assessment
The selection of studies to be included in our analysis was independently conducted by 2 authors (L.R., M.Z.) in a blinded fashion. Any discrepancies in study selection was resolved by consulting a third author (P.Z.). The following MeSH terms were used for the search: “COVID-19″ AND (“Pulmonary embolism” OR “Thrombosis” OR “Venous thromboembolism”). Moreover, we searched the bibliographies of target studies for additional references. Case reports, review articles, abstracts, editorials/letters, and case series with less than 10 participants were excluded. Data extraction was independently conducted by 2 authors (M.Z., P.Z). Studies were excluded from the meta-analysis if they did not provide data regarding the incidence of acute PE among COVID-19 patients. For all studies reviewed we extracted the number of patients enrolled, the mean age, male gender, prevalence of common cardiovascular comorbidities (if reported), the number of acute PE observed in patients hospitalized in ICU or general wards, the use of CTPA and the anatomic location of pulmonary emboli. The quality of included studies was graded using the Newcastle-Ottawa quality assessment scale [14].
2.4. Data synthesis and analysis
Continuous variables were expressed as mean ± standard deviation (SD) or as median with corresponding interquartile range, categorical variables as counts and percentages. The cumulative in-hospital incidence of acute PE (n/N), defined as the ratio between patients experiencing acute PE (n) and the number of patients enrolled in each study (N), hospitalized in general wards and ICUs were pooled using a random effects model and presented with the corresponding 95% confidence interval (CI). Statistical heterogeneity was measured using the Higgins I2 statistic. A I2=0 was considered to indicate no heterogeneity, values of I2 as <25%, 25–75% and above 75% to indicate low, moderate, and high degrees of heterogeneity, respectively [15]. To evaluate publication bias both Egger's test and funnel plots were computed. Data regarding the anatomical distribution of intraluminal pulmonary artery filling defects and the use of CTPA were calculated by extracting numerators and denominators separately and independently from the individual studies. The difference between the main/lobar versus segmental/subsegmental pulmonary arteries was compared using the Pearson's χ2 test. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat, USA).
3. Results
3.1. Search results and included studies
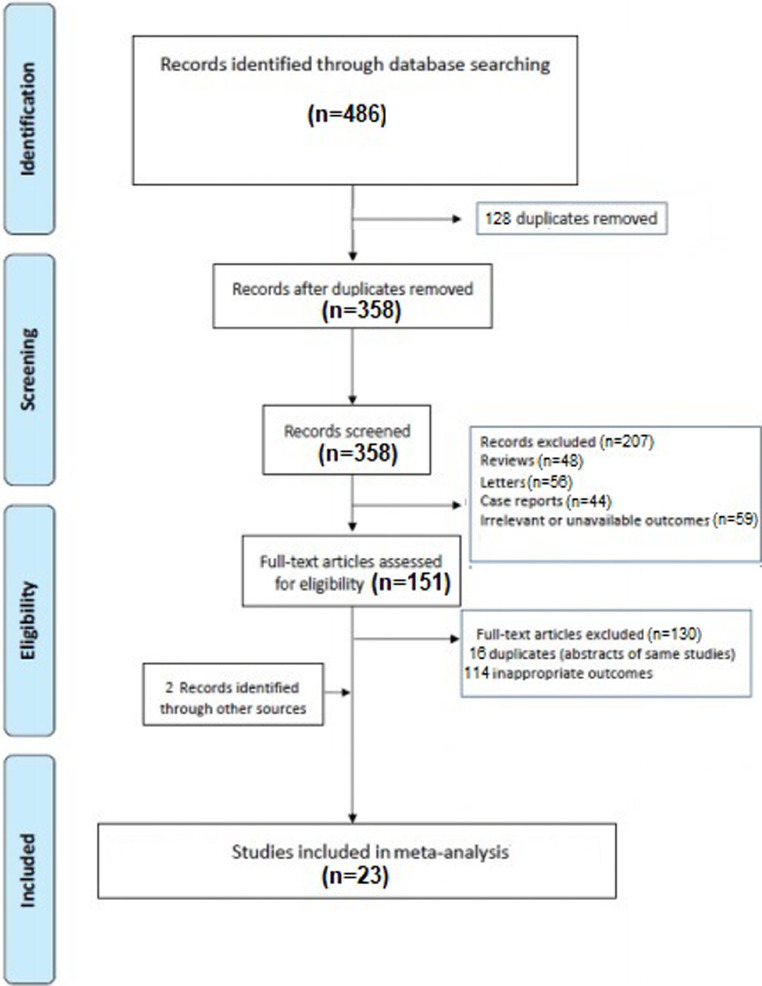
A total of 486 articles were obtained with our search strategy. After excluding duplicates and preliminary screening, 151 full-text articles were assessed for eligibility and 130 studies were excluded for not meeting the inclusion criteria, leaving 23 investigation fulfilling the inclusion criteria (Fig. 1 ) [1,3,4,8,12,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33].
Fig. 1.
Flow diagram of selected studies for the meta-analysis according to the Preferred reporting items for systematic reviews and meta-analyses (PRISMA).
3.2. Characteristics of the population and quality assessment
Overall, 7178 COVID-19 patients [mean age 60.4 years] were included in the analysis. The general characteristics of the studies included are showed in Table 1 . Although the concomitant comorbidities were not systematically recorded by all investigations, active cancer and previous venous thromboembolic events were reported in a small percentage of cases. Fourteen studies considered ICU patients [1,3,4,8,[16], [17], [18], [19], [20],22,25,26,28] while fifteen provided data of subjects hospitalized in general wards [1,8,17,20,21,23,24,[26], [27], [28], [29], [30], [31], [32], [33]. Seven studies reported the data of both ICU and general wards patients [1,8,17,19,20,26,28]. Quality assessment showed that all studies were of moderate-high quality according to the NOS scale (Supplementary file 2) [14].
Table 1.
General characteristics of the population enrolled. The summary datarefer to the entire population of each study. Frequencies are reported as count (%). []: Interquartile range; ICU: Intensive care unit; NOS: Newcastle-Ottawa quality assessment scale; NR: Not reported; SD: Standard deviation: VTE: Venous Thromboembolism.
| Author | Study design | Mean age (years) | Number of patients | Males N, (%) | Arterial hypertension N, (%) | Diabetes N, (%) | Active cancer N, (%) | Cerebrovascular disease | Previous VTE | Setting | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICU | General wards | |||||||||||
| Lodigiani et al. [1] | Retrospective Single center |
66 [55–75] | 388 | 264 (58) |
183 (47) |
88 (23) |
25 (6) |
20 (5) |
12 (3) |
X | X | 8 |
| Poissy et al. [16] | Retrospective Single center |
57 | 107 | 13/22⁎⁎ (59.1) |
NR | NR | NR | NR | NR | X | 7 | |
| Grillet et al. [17] | Retrospective Single center |
66 (SD:13) | 100 | 70 (70) |
NR | 20 (20) |
20 (20) |
NR | NR | X | X | 7 |
| Leonard-Lorant [8] | Retrospective Double-center |
63.5 | 106 | 70 (66) |
NR | NR | NR | NR | NR | X | X | 7 |
| Llitjos et al. [3] | Retrospective Double-center |
68 [51.7–74.5] | 26 | 20 (77) |
22 (85) |
NR | 0 | NR | 1 (4) |
X | 7 | |
| Klok et al. [18] | Retrospective multicenter | 64 (SD:12) | 184 | 139 (76) |
NR | NR | 5 (3) |
NR | NR | X | 8 | |
| Thomas et al. [19] | Retrospective Single center | 59 (SD:13) | 63 | 44 (69) |
NR | NR | NR | NR | NR | X | X | 7 |
| Middeldorp et al. [20] | Retrospective single center | 61 (SD:14) | 198 | 130 (66) |
NR | NR | 7 (3) |
NR | 11 (5) |
X | X | 8 |
| Helms et al. [4] | Retropsective Multicenter | 63 [53–71] | 150 | 122 (81) |
NR | 30 (20) |
9 (6) |
72 (48) |
8 (5) |
X | 8 | |
| Galeano-Valle et al. [21] | Prospective Single center |
64.3 (SD:14.4) | 24 | 14 (58.) |
NR | NR | 1 (4) |
NR | NR | X | 8 | |
| Bompard et al. [12] | Retrospective Double center |
64 [64–76] | 135 | 94 (70) |
NR | NR | NR | NR | NR | X * | 7 | |
| Soumagne et al. [22] | Retrospective Multicenter |
63.5 (SD:10.1) |
375 | 288 (77) |
216 (58) |
99 (26) |
44 (12) |
NR | NR | X | 7 | |
| Freund et al. [23] | Retrospective Multicenter |
61.0 (SD: 19) |
3253 | 1558 (47.8) |
1294 (40) |
NR | 442 (13.5) |
NR |
385 (11.8) |
X° | 7 | |
| Chen et al. [24] | Retrospective Single center |
65 [56.5–70] | 25 | 15 (60) |
10 (40) |
5 (20.0) |
0 | NR | NR | X | 7 | |
| Longhcamp et al. [25] | Retrospective Single center |
68 (SD: 11) |
25 | 16 (64) |
10 (40) |
1 (4) |
2 (8) |
NR | 0 | X | 6 | |
| Whyte et al. [26] | Retrospective Single center |
61.5 | 214 | 129 (60.2) |
NR | NR | 16 (7) |
NR | 21 (10) |
X | X | 7 |
| Marone et al. [27] | Retrospective Single center |
NR | 101 | NR | NR | NR | NR | NR | NR | X | 5 | |
| Fauvel et al. [28] | Retrospective Multicenter |
64 (SD:17) |
1240 | 721 (58) |
559 (45) |
268 (22) |
167 (13.5) |
94 (8) |
98 (8) |
X | X | 8 |
| Van den Heuvel [29] | Retrospective Single center |
63 [51–68] |
51 | 41 (80) |
21 (41) |
9 (18) |
NR | 2 (4) |
NR | X | 6 | |
| Mestre-Gomez et al. [30] | Retrospective Single center |
65 [56–73] |
29 | 21 (72) |
12 (41) |
3 (10.0) |
5 (17) |
1 (3) |
1 (3.4) |
X | 7 | |
| van Dam et al. [31] | Retrospective Single center |
63 (SD:6.4) |
23 | 16 (70) |
NR | NR | 1 (4) |
NR | 1 (4) |
X | 7 | |
| Gervaise et al. [32] | Retrospective Single center |
62.3 (SD:17.8) |
72 | 54 (75) |
NR | NR | NR | NR | NR | X | 6 | |
| Trimaille et al. [33] | Retrospective Single center |
62.2 (SD:17.0) |
289 | 171 (59) |
132 (46) |
59 (20) |
8 (3) |
NR | 28 (10) |
X | 8 | |
Only ICU patients were considered in the analysis since some cases of acute pulmonary embolism in non-ICU setting were also observed in outpatients.
Referred to patients with acute Pulmonary embolism.° Emergency department (ED).
3.3. Pooled in-hospital incidence of acute pulmonary embolism in icu and general wards
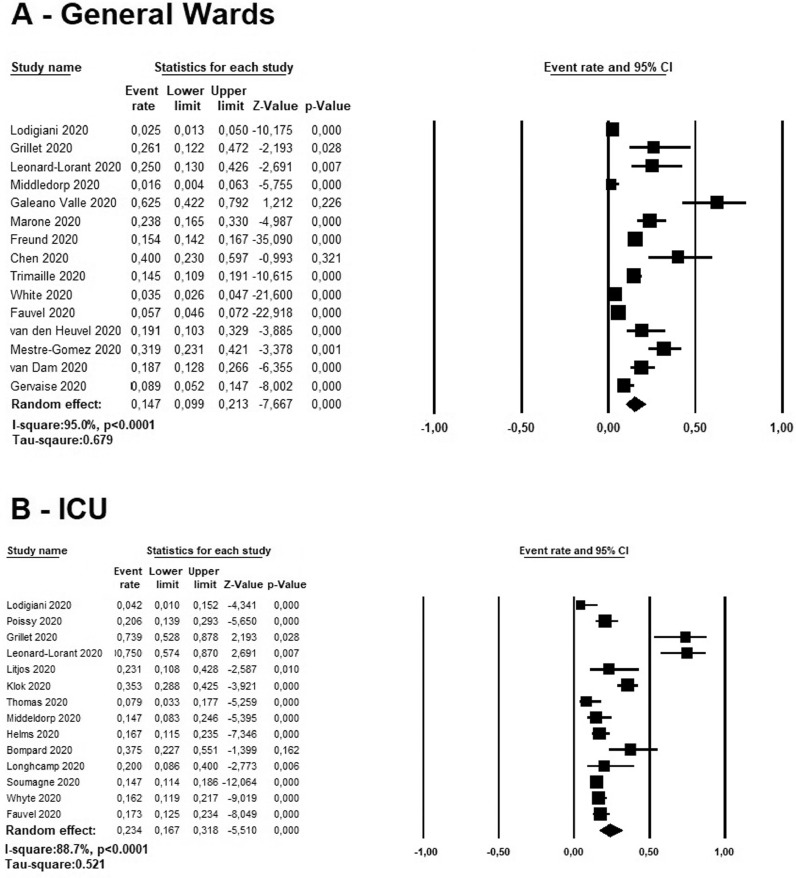
The cumulative in-hospital rate of acute PE in COVID-19 patients hospitalized in general wards ranged between 1.6 to 62.5% among six studies [1,8,17,20,21,23,24,[26], [27], [28], [29], [30], [31], [32], [33]. A random effect model revealed a pooled incidence of acute PE in 14.7% of cases (95% CI: 9.9–21.3%, I2=95.0%) (Fig. 2 , panel A). Higher rates were reported in ICU patients, ranging between 4.2 to 75.0% in the ten studies reviewed [1, 3,4,8,[16], [17], [18], [19], [20],22,25,26,28]. In these patients, a pooled cumulative incidence rate of acute PE was 23.4% (95% CI:16.7–31.8%, I2=88.7%) (Fig. 2, panel B).
Fig. 2.
Forest plots investigating the pooled incidence of acute pulmonary embolism in COVID-19 patients hospitalized in ICU (A) and in general wards (B).
3.4. Assessment of publication bias
The Egger's tests revealed no evidences of publication bias in estimating the pooled incidence of acute PE among patients admitted in general wards or ICU (t = 0.065, p = 0.978 and t = 0.591, p = 0.565, respectively). A visual assessment of the funnel plot cannot reassure about the presence of an asymmetry with studies characterized by higher PE rate being missing at the basis of the triangle (Supplementary file 3).
3.5. Imaging techniques adopted and deep vein thrombosis
Most of the studies reviewed used CTPA for the diagnosis of PE. Only one study reported the use of transthoracic echocardiography in two patients for the diagnosis [3]. Prophylactic and therapeutic anticoagulation resulted largely used in the studies reviewed using different drugs such as enoxaparin, dalteparin and unfractionated heparin (UFH) as well as different regimens. However, very few investigations reported the number of PE patients treated before the diagnosis of acute PE, as shown in Table 2 . The analysis of the prevalence of concomitant DVT [1,[21], [22], [23], [24], [25], [26], [27], [28],30,33] ranged between 1.5% [8] to 33.3% [27].
Table 2.
Anatomical sites of acute pulmonary embolism and percentages of imaging assessment performed to assess pulmonary thromboembolic events.NR not reported; NA: not applicable (retrospective studies); CTPA: Computed tomography pulmonary angiography; CUS: Compression ultrasonography. Follow-up was available only in prospective studies but one of this did not reported the length [21].
| Author | Imaging techniques | Thromboprophylaxis | DVT | Follow-up | Sites of intraluminal pulmonary arterial filling defects | Imaging test performed (CTPA) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Main (%) | Lobar (%) | Segmental (%) | Subsegmental (%) | (%) | ||||||
| Lodigiani et al. [1] | CTPA Two point CUS in ICU Whole leg ultrasound in general wards |
Enoxaparin or Nadroparin NR for PE patients (only reported 100% of ICU patients) |
PE±DVT and isolated DVT reported separately | NA | NR | 30.0 | 10.0 | 33 | ||
| Poissy et al. [16] | CTPA | LWMH or UFH In 20/22 patients NR for PE patients |
3/22 (13.6) | NA | 10.0 * 40.0⁎⁎ |
55.0 | NR | 31.8 | ||
| Grillet et al. [17] | CTPA | NR | NR for PE patients | NA | 0 | 43.4 | 100 | 0 | 35.7 | |
| Leonard-Lorant [8] | CTPA | LMWH 25/32 (78%) |
NR for PE patients | NA | 21.8 | 34.3 | 28.1 | 15.6 | 63.0 | |
| Llijtos et al. [3] | CTPA (in 4 patients) TEE (in 2 patients) Limb ultrasound |
8 (31%) prophylactic anticoagulation 18 (69%) therapeutic anticoagulation NR for PE patients |
NR for PE patients | NA | NR | NR | NR | NR | NR | |
| Klok et al. [18] | CTPA Limb Ultrasound |
Nadroparin in all patients with different regimens | NR for PE patients | NA | 70.7 | 29.2 | NR | |||
| Thomas et al. [19] | CTPA Limb Ultrasound |
Prophylactic dalteparin in all patients NR for PE patients |
NR for PE patients | NA | 20 | 0 | 60.0 | 20 | 17.4 | |
| Middeldorp et al. [20] | CTPA Limb ultrasound |
Thromboprophylaxis with nadroparin in 167 patients (84%) 19 patients (9.6%) continued therapeutic anticoagulation |
Defined as PE±DVT | 17 | 7.6 | 76.9 | 15.3 | NR | ||
| Helms et al. [4] | CTPA | LMWH or UFH Prophylactic dose 105 (70) Therapeutic dose 45 (60) |
NR for PE patients | 7 | 37.5 | 33.3 | 20.8 | 12.5 | NR | |
| Galeano-Valle et al. [21] | CTPA CUS |
Enoxaparin or Bemiparin In 19/24 patients |
4/11 (36.3) | NR | 13.3 | 46.6 | 86.6 | 46.6 | NR | |
| Bompard et al. [12] | CTPA | Enoxaparin in all patients at prophylactic dose | NR for PE patients | 26 | 31.2 | 65.2 | 12.5 | 53 ° | ||
| Soumagne et al. [22] | CTPA | NR | 35 (9.3) |
NR | NR | NR | NR | 14.6 | ||
| Freund et al. [23] | CTPA | NR | 101 (11) |
NR | NR | NR | NR | 15 | ||
| Chen et al. [24] | CTPA | NR | 1 (4) |
NR | 0 | 25 (100) | 25 (100) | 0 | 100 | |
| Longhcamp et al. [25] | CTPA | Intravenous heparin infusion or enoxaparin |
6 (24) |
0 | 3 (60) |
2 (40) |
0 | 28 | ||
| Whyte et al. [26] | CTPA | Enoxaparing or UFH |
7 (8.7) |
NR | 3 (3.7) |
NR | 28 (35) |
13 (16.2) |
14.4 | |
| Marone et al. [27] | CTPA CUS |
LMWH | 8 (33.3) |
10 | NR | NR | NR | NR | NR | |
| Fauvel et al. [28] | CTPA | LMWH 738 (63.0) |
18 (1.5) |
NR | NR | NR | NR | NR | 43.0 | |
| Van den Heuvel [29] | CTPA | NR | NR | NR | NR | NR | NR | NR | 92 | |
| Mestre-Gomez et al. [30] | CTPA | LMWH 23 (79.3) |
2 (6.9) |
NR | 9 (31) |
20 (69) |
NR | |||
| van Dam et al. [31] | CTPA | (100) Not specified the drug |
0 | NR | 4 (17) |
16 (70) |
3 (13) |
NR | ||
| Gervaise et al. [32] | CTPA | NR | NR | NR | 2 (15) |
4 (30) |
7 (55) |
0 | 49.3 | |
| Trimaille et al. [33] | CTPA | Enoxaparin | 12 (24.5) |
NR | NR | NR | NR | NR | 34.6 | |
Defined as proximal;.
Defined as bilateral. °Performed due to clinical deterioration.
3.6. Anatomical location of acute pulmonary embolism and use of ctpa
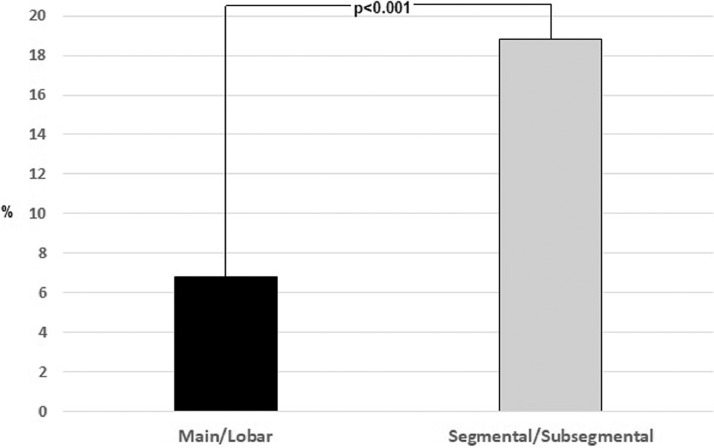
The studies reviewed did not systematically report the anatomical location of the pulmonary emboli in the arterial tree or classified the anatomical location heterogeneously. In fifteen studies that reported the former information, arterial filling defects at CTPA, calculated by extracting numerators and denominators separately and independently from the individual studies, involved the main, lobar, segmental and subsegmental pulmonary arteries in 8.3% (n = 85/1023), 7.8%, (n = 102/1299), 12.2% (n = 189/1544) and 11.4 (n = 107/1025) of cases, respectively [1,4,8,[16], [17], [18], [19], [20], [21],24,25,27,30,31]. Segmental/sub-segmental were more frequently involved compared to main/lobar arteries (6.8% vs18.8%, p<0.001) (Fig. 3 ). Moreover, in the thirteen studies that reported how many patients underwent CTPA, it was used only in 35.3% (n = 1957/5532) of patients with COVID-19 infection (Table 2) [1,8,12,16,17,19,[24], [25], [26],28,[31], [32], [33].
Fig. 3.
Comparison of the proximal and distal distribution of intraluminal pulmonary arterial filling defects.
4. Discussion
We performed a pooled analysis of the rate of acute PE in COVID-19 patients including data collected during the first months after the COVID-19 outbreak. The in-hospital rate of acute PE was higher in ICU patients than in those hospitalized in general wards. The most common sites in which pulmonary filling defect were observed using CTPA appeared to be the lobar and segmental pulmonary arteries. However, CTPA was only used in a selected group of patients, approximately one-third of total, indicating that underdiagnosis was likely and, consequently, missed PE events may have contributed to the high mortality recorded among COVID-19 hospitalized patients. This uncertainty is reflected by the extreme clinical and statistical heterogeneity of these results.
Previous analyses have estimated a significant lower incidence of acute PE in the ICU population, which generally increase in mechanically ventilated patients [34,35]. The high incidence of acute PE in critically ill patients may reflect a more severe pro-coagulant state [36], [37], [38], [39]. Indeed, as shown by the general characteristics of the patients reviewed, both active cancer and previous venous thromboembolic events were uncommon and unlikely to explain the burden of thromboembolic complications beyond a contributing role.
Our results have several implications for clinical practice. First, the high rate of acute PE in COVID-19 patients makes it urgent to establish the optimal antithrombotic regimen that may minimize the risk of thromboembolic events in these patients. In this regard, recent analyses and perspectives have proposed different therapeutic and prophylactic regimens but the debate is still ongoing [40,41]. Second, it appears clear that the diagnosis of acute PE is largely underestimated in COVID-19 patients. Indeed, only one third of patients underwent CTPA for diagnostic purposes. Yet, recent autoptic studies performed in COVID-19 patients have demonstrated the presence of arterial emboli involving both major pulmonary arteries and microthrombi involving the more distal arterial vessels [42], [43], [44]. Indeed, these two scenarios may coexist: local “immunothrombosis” triggered by the viral infection and “classic” venous thromboembolism caused by major transient provoking risk factors, including bed rest, the presence of catheters, and hypoxemia, as well as age and the presence of concomitant conditions. Moreover, local endothelial cell dysfunction in the pulmonary microvasculature also seems to play a substantial role in the thromboinflammatory processes. In this regard, both cytokine storm and/or macrophage activation syndrome (MAS) could trigger the expression of active tissue factor (TF) within the lungs, further activating the coagulation cascade [45]. It remains to be elucidated whether “immunotrombosis” can be prevented by standard thromboprophylaxis and can be cured by available anticoagulant regiments.
During the current COVID-19 pandemic, the traditional diagnostic algorithms have been frequently overturned to limit the risk of infection for both operators and outpatients, limiting the execution of radiological examinations to minimize intrahospital transfers [46]. Indeed, there are obvious difficulties in perform CTPA in mechanical ventilated patients, especially when it requires pronation. Some of the reviewed studies evidenced that CTPA was performed in the event of further clinical and/or respiratory deterioration in ICU patients [3,12]. To reduce the burden of acute PE in these patients it seems essential to promote serial assessment using bedside transthoracic echocardiographic (TTE), electrocardiograms, assessment of myocardial injuries biomarkers [47,48] and compression ultrasonography (CUS), which may detect early, indirect signs that raise the suspicion of acute PE. A low threshold to suspect PE appears reasonable in this setting. At the same time, separate intra-hospital paths for the transfer of patients to radiological wards would permit the diagnosis of acute PE while minimizing the risk of infection.
A multinational registry, the COVID-19 Registry on Thrombosis and Thromboembolic complications (CORE-THROMBOSIS), is recruiting to provide representative data on the magnitude of the problem and enable us to formulate robust hypotheses to be tested in future trials [49].
4.1. Limitations
Our study has several limitations related to the observational nature of the studies reviewed and their own limitations with all inherited biases. In particular, potential underestimation could derive from detection bias if PE was not searched systematically or suspected based on systematic criteria, and CTPA may only have been carried out in patients with a clinical condition severe enough to raise the suspicion that other factors than the infection were at play. Sampling bias by the competing risk of death may also have led to underestimation of the real cumulative incidence of PE. At the same manner, we cannot assess if an adequate prophylactic anticoagulation was consistently administered in each study because these data were not systematically provided in the review investigations. Moreover, the hospitalization length can represent another potential source of bias since is strictly related immobilization [50]. This late aspect could explain the higher pooled cumulative in-hospital PE incidence in ICUs compared to general wards since ICU hospitalization, and immobilization, is generally longer. Few investigations on the COVID-19 infection have analysed the incidence of acute PE as a complication of COVID-19 infection, limiting the number of the studies included into the meta-analysis and the corresponding number of patients.
5. Conclusions
The pooled incidence of acute PE among COVID-19 patients was higher in ICU patients compared with patients hospitalized in general wards. Available data may underestimate the real incidence of acute PE as a complication of COVID-19 infection. A clinical and radiological distinction between acute PE and local “immunothrombosis” is impossible based on the available data and its therapeutic consequences remain to be investigated. Appropriate diagnostic strategies must be promoted to enhance the diagnosis of acute PE in these patients to reduce the mortality rate [51].
Declaration of Competing Interest
S.B. reports personal fees from Biocompatibles Group UK and Bayer HealthCare, non-financial support from Bayer HealthCare and Daiichi Sankyo, outside the submitted work.
S.V.K. reports grants and non-financial support from Bayer AG; grants and personal fees from Boehringer Ingelheim, personal fees from Bayer AG, grants and personal fees from Actelion, grants and personal fees from Daiichi-Sankyo, grants and personal fees from Biocompatibles Group UK, personal fees from Pfizer—Bristol-Myers Squibb, grants and personal fees from MSD, outside the submitted work
The other authors have no conflicts of interest to report.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2020.09.006.
References
- 1.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Alexia B., Sandri M.T., Barco S., Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020 doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.M., Meziani F., CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia [published online ahead of print. J Thromb Haemost. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranucci M., Ballotta A., Di Dedda U., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Yan X., Fan Q., et al. d-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O., et al. Acute Pulmonary Embolism in COVID-19 Patients on CT Angiography and Relationship to d-Dimer Levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;S0049-3848(20):30120–30121. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and Thrombotic or Thromboembolic Disease: implications for Prevention, Antithrombotic Therapy, and Follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. S0735-1097 (20) 35008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2020. https://www.escardio.org/static_file/Escardio/Education-General/Topic%20pages/Covid-19/ESC%20Guidance%20Document/ESC-Guidance-COVID-19-Pandemic.pdf.
- 12.Bompard F., Monnier H., Saab I., Tordjman M., Abdoul H., Fournier L., Sanchez O., Lorut C., Chassagnon G., Revel M.P. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56 doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 14.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses, 2012. http://www.ohrica/programs/clinical_epidemiology/oxfordasp, Accessed May 5, 2020.
- 15.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poissy J., Goutay J., Caplan M., et al. Pulmonary Embolism in COVID-19 Patients: awareness of an Increased Prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 17.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected by Pulmonary CT Angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K., Lavinio A., Bessera M. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., Bouman C.C.S., Beenen L.F.M., Kootte R.S., Heijmans J., Smits L.P., Bonta P.I., van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galeano-Valle F., Oblitas C.M., Ferreiro-Mazón M.M., Alonso-Muñoz J., del-Toro-Cervera J., Demelo-Rodríguez P. Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soumagne T., Lascarrou J.B., Hraiech S., Horlait G., Higny J., d’Hondt A., et al. Factors Associated With Pulmonary Embolism Among Coronavirus Disease 2019 Acute Respiratory Distress Syndrome: a Multicenter Study Among 375 Patients. Crit Care Explor. 2020;2:e0166. doi: 10.1097/CCE.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freund Y., Drogrey M., Ò Miró, Marra A., Féral-Pierssens A.L., Penaloza A., Lara Hernandez B.A., Beaune S., Gorlicki J., Vattinada Ayar P., Truchot J., Pena B., Aguirre A., Fémy F., Javaud N., Chauvin A., Chouihed T., Montassier E., Claret P.G., Occelli C., Roussel M., Brigant F., Ellouze S., Le Borgne P., Laribi S., Simon T., Lucidarme O., Cachanado M., Bloom B., IMPROVING EMERGENCY CARE FHU Collaborators Association between Pulmonary Embolism and COVID-19 in ED patients Undergoing CTPA: the PEPCOV international retrospective study. Acad Emerg Med. 2020 doi: 10.1111/acem.14096. [DOI] [PubMed] [Google Scholar]
- 24.Chen J., Wang X., Zhang S., Lin B., Wu X., Wang Y., Wang X., Yang M., Sun J., Xie Y. Characteristics of Acute Pulmonary Embolism in Patients With COVID-19 Associated Pneumonia From the City of Wuhan. Clin Appl Thromb Hemost. 2020 doi: 10.1177/1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longchamp A., Longchamp J., Manzocchi-Besson S., Whiting L., Haller C., Jeanneret S., et al. Venous thromboembolism in critically Ill patients with COVID-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4:842–847. doi: 10.1002/rth2.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whyte M.B., Kelly P.A., Gonzalez E., Arya R., Roberts L.N. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res. 2020;195:95. doi: 10.1016/j.thromres.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marone E.M., Bonalumi G., Curci R., Arzini A., Chierico S., Marazzi G., et al. Characteristics of Venous Thromboembolism in COVID-19 Patients: a Multicenter Experience from Northern Italy. Ann Vasc Surg. 2020;S0890-5096(20) doi: 10.1016/j.avsg.2020.07.007. 30598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauvel C., Weizman O., Trimaille A., Mika D., Pommier T., Pace N., Douair A., Barbin E., Fraix A., Bouchot O., Benmansour O., Godeau G., Mecheri Y., Lebourdon R., Yvorel C., Massin M., Leblon T., Chabbi C., Cugney E., Benabou L., Aubry M., Chan C., Boufoula I., Barnaud C., Bothorel L., Duceau B., Sutter W., Waldmann V., Bonnet G., Cohen A., Pezel T., Critical Covid-19 France Investigators Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020:ehaa500. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Heuvel F.M.A., Vos J.L., Koop Y., van Dijk A.P.J., Duijnhouwer A.L., de Mast Q., van de Veerdonk F.L., Bosch F., Kok B., Netea M.G., Hoogerwerf J., Hoefsloot W., Tjwa E.T.T.L., de Korte C.L., van Kimmenade R.R.J., Nijveldt R. Cardiac function in relation to myocardial injury in hospitalised patients with COVID-19. Neth Heart J. 2020;28:410–417. doi: 10.1007/s12471-020-01458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mestre-Gómez B., Lorente-Ramos R.M., Rogado J., Franco-Moreno A., Obispo B., Salazar-Chiriboga D., Saez-Vaquero T., Torres-Macho J., Abad-Motos A., Cortina-Camarero C., Such-Diaz A., Ruiz-Velasco E., Churruca-Sarasqueta J., Muñoz-Rivas N., Infanta Leonor Thrombosis Research Group Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dam L.F., Kroft L.J.M., van der Wal L.I., Cannegieter S.C., Eikenboom J., de Jonge E., et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gervaise A., Bouzad C., Peroux E., Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020 doi: 10.1007/s00330-020-06977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trimaille A., Curtiaud A., Marchandot B., Matsushita K., Sato C., Leonard-Lorant I., Sattler L., Grunebaum L., Ohana M., Von Hunolstein J.J., Andres E., Goichot B., Danion F., Kaeuffer C., Poindron V., Ohlmann P., Jesel L., Morel O. Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thromb Res. 2020;193:166–169. doi: 10.1016/j.thromres.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahloul M., Chaari A., Kallel H., Abid L., Hamida C.B., Dammak H., et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med. 2010;5:97–103. doi: 10.4103/1817-1737.62473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C., Zhang Z., Mi J., Wang X., Zou Y., Chen X., Nie Z., Luo X., Gan R. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore) 2019;98:e15833. doi: 10.1097/MD.0000000000015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zochios V., Keeshan A. Pulmonary embolism in the mechanically-ventilated critically ill patient: is it different? JICS. 2013;14:36–44. [Google Scholar]
- 37.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., Navalesi P., Simioni P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. 2020 doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis. J Thromb Haemost. 2020 doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Criel M., Falter M., Jaeken J., Van Kerrebroeck M., Lefere I., Meylaerts L., Mesotten L., vander Laenen M., Fivez T., Thomeer M., David R. Venous thromboembolism in SARS-CoV-2 patients: only a problem in ventilated ICU patients, or is there more to it? Eur Resp J. 2020 doi: 10.1183/13993003.01201-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020 doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: a Prospective Cohort Study. Ann Intern Med. 2020 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolhnikoff M., Duarte-Neto A.N., de Almeida Monteiro R.A., Ferraz da Silva L.F., Pierre de Oliveira E., Nascimento Saldiva P.H., Mauad T., Marcia Negri E. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Why the immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia are distinct from macrophage activation syndrome with disseminated intravascular coagulation. Lancet Rheum. 2020 doi: 10.13140/RG.2.2.19782.83521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rigatelli G., Zuin M., Rigatelli A., Zuliani G., Roncon L. Intubation and Ventilation amid COVID-19: comment Anesthesiology. 2020; doi:10.1097/ALN.0000000000003374. [DOI] [PMC free article] [PubMed]
- 47.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., Guo G.Y., Du J., Zheng C.L., Zhu Q., Hu M., Li X.Y., Peng P., Shi H.Z. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 50.Roncon L., Zuin M., Zonzin P. Age-adjusted d-dimer cut-off levels to rule out venous thromboembolism in COVID-19 patients. Thromb Res. 2020;190:102. doi: 10.1016/j.thromres.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barco S., Konstantinides S.V. Thrombosis and Thromboembolism Related to COVID‐19 A clarion call for obtaining solid estimates from large‐scale multicenter data. Res Pract Thromb Haemost. 2020 doi: 10.1002/rth2.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]