Abstract
Background
The recurrence of the urinary tract infections (UTI), following the antibiotic treatments suggests the pathogen’s resistance to conventional antibiotics. This calls for the exploration of an alternative therapy.
Main body
The anti-uropathogenic and bactericidal activity of many plant extracts was reported by many researchers, which involves only preliminary antibacterial studies using different basic techniques like disk diffusion, agar well diffusion, or minimum inhibitory concentration (MIC) of the crude plant extracts, but reports on the specific action of the phytoconstituents against uropathogens are limited. Vaccinium macrocarpon Aiton (cranberry) is the best-studied home remedy for UTI. Some evidences suggest that proanthocyanins present in cranberry, prevent bacteria from adhering to the walls of the urinary tract, subsequently blocking the further steps of uropathogenesis. Probiotics such as Lactobacillus and Bifidobacterium are beneficial microorganisms that may act by the competitive exclusion principle to defend against infections in the urogenital tracts. Reports on potential vaccine agents and antibodies targeting the different toxins and effecter proteins are still obscure except uropathogenic E. coli.
Conclusion
This review highlights some of the medicinal herbs used by aborigines to prevent or treat acute or chronic urinary tract infections, botanicals with established urobactericidal activity, clinical trials undertaken to compare the efficacy of cranberry products in UTI prevention, and other natural therapeutics reported for UTI.
Keywords: Cranberry, Proanthocyanins, PAC, Urinary tract infections, Uropathogenic Escherichia coli, UTI, UPEC
Background
Urinary tract infection (UTI) is a condition when any part of the urinary tract (urethra, bladder, ureter, and kidney) gets infected with bacteria or occasionally with fungus that evades the host defense barrier and colonize the urinary tract. The effect of UTI ranges from a mild self-limiting sickness to acute sepsis, with a mortality rate of 20-40% [1], which increases inexplicably with age. Both the sexes are prone to develop UTI with a female to male ratio of 2:1 in patients older than 70 years as compared to a 50:1 ratio in younger population [2]. It is the second most common infection after respiratory tract infections. Different methods are practiced to treat and prevent chronic and recurrent UTI, i.e., taking antibiotics, bioactive natural foods, using probiotics, and maintaining good personal hygiene, but still, they are yet to be addressed successfully. As UTI is generally caused by bacteria, they are most frequently treated with antibiotics. But, the type of medication and length of treatment depends on type of bacteria, its level of susceptibility, history, symptoms, and immune status of the patient.
It is not known, what percentage of people are now using alternative therapies, but certainly large numbers of women are drinking cranberry juice or using herbal remedies to enhance their immune status or taking probiotics to restore the normal vaginal flora, which usually gets disturbed after an antibiotic therapy. Vaccine development for organisms other than E. coli still remains obscure [3]. Cranberry, mannose, and probiotics are frequently used for recurrent UTI, and berberine and uva ursi are prescribed for acute UTI. Potassium salt supplements reduce dysuria by alkalinizing the urine. Application of estriol cream and supplement of vitamins A and C were considered to be effective to prevent UTI [4]. Generally, people drink plenty of water to flush out the infectious bacteria. Application of curd water around the urethra can help in getting rid of urinary burning sensation. This present review enlists some ethnobotanicals, which are reported to be beneficial for UTI and other urinary disorders. It covers a list of potential herbs with urobactericidal activity, the in vitro/in vivo and clinical trial studies reported to prove the efficacy of cranberry in treating UTI. It also represents the synopsis of relevant natural therapeutics, those are proven to be useful in both prevention and cure of urological disorders.
Methods
Intense review of literature on the prevalence, mechanism of urinary tract infection, risk factors, preventive measures, and natural therapeutics for UTI were carried out using different databases like Google, Pubmed, and Sciencedirect. The keywords like the preventive and therapeutic role of different plants and their products in uropathogenesis, medicinal plants for acute and recurrent UTI, natural remedies, therapeutics for UTI, and anti-uropathogenic activity of medicinal plants, role of cranberry in acute and recurrent UTI were accessed from Medline, Google, Pubmed, and from different books, electronic, and printed journals, available in the library of Berhampur University, Utkal University, Institute of Life Sciences, and Regional Medical Research Center, Bhubaneswar, Odisha. The different keywords like urinary tract infection, uropathogenic bacteria, uropathogenesis, and UPEC are used in Google, Pubmed, and www.asm.org websites. The language chosen was English and both research and review articles were taken into account.
Botanicals used for UTI
Therapeutic botanicals are defined as plants and their products with medicinal value. Indigenous plants are used for various ailments since time immemorial by mankind and probably we had learned this art from animals, since they have the inherent ability to use natural products for their different health ailments. These natural products are rich in diverse bioactive compounds, which form the basis for the development of new pharmaceuticals. There are immense advantages of using therapeutic botanicals like lesser side effects, more patient approval, less costly, and can be renewed naturally [5]. There are many reports that phytochemicals act as multi-drug resistance inhibitors/modulators that augment the effect of commonly used antibiotics [6, 7]. Diuretics like Solidago spp (goldenrod) herb, Levisticum officinale (lovage) root, Petroselinum crispus (parsley) fruit, and Urtica dioica (stinging nettle) increase urine volume in both healthy and people with urinary disorders that help in flushing out the probable threats. People, who consume antiseptic and anti-adhesive herbs like Arctostaphylos uva-ursi (uva ursi), Juniperus spp (Juniper) leaf, and fruit of Vaccinium macrocarpon (cranberry) excrete antimicrobial compounds, which may directly kill microbes or interfere with their adhesion to epithelial cells, thereby protecting against acute and chronic UTI [8]. The roots of Mahonia aquifolium (Pursh) Nutt. (Oregon grape) (Berberidaceae) and Hydrastis canadensis L. (Goldenseal) (Ranunculaceae) are rich in berberine. Berberine is an important drug against many bacteria and combat infections by preventing the bacteria (E. coli and Proteus species) from adhering to the host cell [9], which suggests their potent role in treating UTI.
Supplement of aqueous extract of corn (Zea mays L.) silk (outer thread-like part) to UTI patients significantly reduced the symptoms by reducing the number of RBCs, pus cells, and crystals in urine without any side effects [10]. It is rich in diverse therapeutic compounds [11]. Plants belonging to family Apiaceae, Fabaceae, Malvaceae followed by Asteraceae and Cucurbitaceae were found to be very effective against UTI [12]. Ethnomedicinal use of some plants against recurrent and chronic UTI is listed in Table 1.
Table 1.
Directory of some important ethnomedicinal plants/plant parts used for UTI
| Botanical name (family) | Parts used | Disorder/disease | Reference |
|---|---|---|---|
| Adiantum lunulatum Burm. f. (Pteridaceae) | Root | Blood discharge in urine | [13] |
| Argemone mexicana L. (Papaveraceae) | Root | Urinary trouble | [14] |
| Clausena excavate Burm. f. (Rutaceae) | Root | Urinary infection | [15] |
| Cucumis melo L. (Cucurbitaceae) | Epicarp | Kidney stone, urinary tract infection | [14] |
| Cucumis sativus L. (Cucurbitaceae) | Seed | Urinary tract infection | [16] |
| Euphorbia thymifolia L. (Euphorbiaceae) | Whole plant | Blood in urine | [17] |
| Mimosa pudica L.(Mimosaceae) | Root, leaf | Urinary infection, burning micturition | [18, 19] |
| Asparagus racemosus Willd. (Asparagaceae) | Roots | Urinary troubles | [20] |
| Azadirachta indica A. Juss. (Meliaceae) | Leaves | Urinary troubles | |
| Cissampelos pareira L. (Menispermaceae) | Roots, leaves | Urinary tract infection, diuretic | |
| Crateva unilocularis Buch.-Ham. (Capparaceae) | Leaves | Urinary diseases, kidney diseases | |
| Malva verticillata L. (Malvaceae) | Root | Urinary tract infection | |
| Mangifera indica L. (Anacardiaceae) | Branch | Urinary diseases, kidney diseases | |
| Phyllanthus urinaria L.(Euphorbiaceae) | Whole plant | Urinary problem | |
| Tinospora sinensis (Lour.) Merr. (Menispermaceae) | Whole plant | Urinary troubles, diuretic | |
| Abutilon indicum (L.) Sweet (Malvaceae) | Leaf | UTI, kidney stone | [21] |
| Crateva nurvel Buch-Ham. (Capparaceae) | Bark | UTI | |
| Cyanodon dactylon (L.) Persoon (Poaceae) | Root | Urolithiasis | |
| Tribulus terrestris L. (Zygophyllaceae) | Root, fruit | Kidney stone | |
| Acacia farnesiana (L.) Willd. (Fabaceae) | Roots | Burning sensation in the urinary tract, UTI oliguria and polyuria | [22] |
| Acanthus ilicifolius L. (Acanthaceae) | Roots | Unclear urine in women | |
| Acrostichum aureum L. (Pteridaceae) | Leaves | Unclear urine in women, UTI | |
| Ageratum conyzoides L. (Asteraceae) | Leaves, roots | UTI | |
| Caesalpinia nuga (L.) Aiton (Caesalpiniaceae) | Plant juice, roots, fruit | Urinary tract disorder, oliguria, and polyuria | |
| Clitoria ternatea L. (Fabaceae) | Leaves | Urinary tract problems | |
| Elephantopus scaber L. (Asteraceae) | Roots | Difficulties in urination | |
| Hemidesmus indicus (L.) R. Br. (Asclepiadaceae) | Leaves | Urinary tract infections | |
| Mimosa pudica L. (Mimosaceae) | Roots, barks | Urinary problems | |
| Moghania macrophylla (Willd.) Kuntze (Fabaceae) | Root | Retrograde ejaculation, painful urination | |
| Melastoma malabathricum L. (Melastomataceae) | Roots, leaves | Burning sensations in the urinary tract, painful urination, oliguria, and polyuria | |
| Nymphaea nouchali Burm. f. (Nymphaeaceae) | Root tops | Urinary ailments | |
| Oroxylum indicum (L.) Kurz (Bignoniaceae) | Bark, fruit | Difficulties in urination, burning sensation, red urination, polyuria, lower abdominal pain | |
| Stephania japonica (Thunb.) Miers (Menispermaceae) | Vines | UTI, diuretic | |
| Urena lobata L. (Malvaceae) | Roots, leaves, bark, flowers | Urinary trouble, burning sensations in the urinary tract | |
| Zizyphus oenoplia (L.) Mill. (Rhamnaceae) | Root | Urinary disorders | |
| Santalum album L. (Santalaceae) | Tender twig | UTI | [23] |
Botanicals with anti-uropathogenic activity
Few Jordanian plants were reported to have antibiotic resistance-modifying activity against MDR E. coli. Especially, methanol extracts of the plant parts improved the effects of cephalexin, doxycycline, neomycin, chloramphenicol, and nalidixic acid against both the standard and resistant strains of E. coli. Extracts of Anagyris foetida L. (Fabaceae) and Lepidium sativum L. (Apiaceae) had differential activity against the standard and resistant strains as it decreased the activity of amoxicillin against the standard strain but increased the activity against resistant strains. Edible plants like Gundelia tournefortii L. (Asteraceae), Eruca sativa Mill. (Brassicaceae), and Origanum syriacum L. (Lamiaceae), augmented clarithromycin activity against the resistant E. coli strain. Perhaps these antibiotics and plant extracts may be prescribed together to treat infections caused by MDR E. coli [24]. There are numerous reports for the anti-uropathogenic and urobactericidal activities of various plants and their products, which are listed in Table 2.
Table 2.
List of medicinal plants with anti-uropathogenic potential
| Plant name (family) | Extract/part used | Name of microorganism | Reference |
|---|---|---|---|
| Ocimum gratissimum L., Salvia officinalis L. (Lamiaceae); Cymbopogon citratus (DC.) Stapf (Poaceae) | Essential oil | Klebsiella pneumoniae; K. oxytoca; E. coli; Enterobacter aerogenes; Morganella morganii; P. mirabilis | [25] |
| Mangifera indica L. (Anacardiaceae) | Water and ethanol extract of seed kernel | Staphylococcus aureus | [26] |
| Zinziber officinale Roscoe (Zinziberaceae); Punica granatum L. (Lythraceae) | Ethanol extract of rhizome and seed, respectively | E. coli | [27] |
| Ocimum gratissimum L. (Lamiaceae) | Ethanol extract of leaf | E. coli; P. mirabilis; S. aureus; Pseudomonas aeruginosa; Candida albicans | [28] |
| Carica papaya L. (Caricaceae) | Water, chloroform, ethanol extract of leaves | K. pneumoniae; E. coli; P. mirabilis | [29] |
| Ibicella lutea (Lindl.) Van Eselt. (Martyniaceae) | Plant extract | P. mirabilis | [30] |
| Allium sativum L. (Liliaceae) | Allicin from clove and leaf | E. coli; S. aureus | [31] |
| Rhizophora apiculata Blume; R. Mucronata Lam.; Bruguiera cylindrical (L.) Blume; Ceriops decandre (Griff.) W.Theob. (Rhizophoraceae); Avicennia marina (Forssk.) Vierh. (Acanthaceae) | Ethanol extract of hypocotyl, bark, collar, and flower | E. coli; K. pneumonia; P. aeruginosa; S. aureus; Enterobacter sp. | [32] |
| Coccinia grandis (L.) Voigt (Cucurbitaceae) | Water, acetone, ethanol extract of leaves | Uropathogenic E. coli (UPEC) | [33] |
| Coleus aromaticus Lour.; Ocimum sanctum L. (Lamiaceae) | Essential oil | E. coli; S. aureus; K. pneumonia; Klebsiella oxytoca; Proteus vulgaris; P. mirabilis; P. aeruginosa | [34] |
| Clitoria ternatea L. (Fabaceae); Achyranthes aspera. L. (Amaranthaceae) | Leaf extract | E. coli; methicillin resistant S. aureus; S. aureus; P. aeruginosa; K. pneumonia; Citrobacter diverses; Serratia liquefaciens; C. albicans | [35] |
| Moringa oleifera Lam. (Moringaceae) | Leaf extract | P. mirabilis | [36] |
| Azadirachta indica L. (Meliaceae); Tinospora cordifolia (Willd.) Miers (Menispermaceae); Euphorbia hirta L. (Euphorbiaceae); Cassia javanica L. (Fabaceae); Phyllanthus niruri L. (Euphorbiaceae); Asparagus racemosus Willd. (Asparagaceae); Eupatorium triplinrrve Blume (Asteraceae) | Chloroform, methanol, acetone, ethanol extract | P. aeruginosa; Staphylococcus epidermis; Serratia marcescens; Enterobacter; Citrobacter | [37] |
| Piptochaetium montevidense (Spreng.) Parodi (Poaceae); Bulbostylis cappilaris (L.) Kunth ex C.B. Clarke (Cyperaceae); Juncus capillaceus Lam. (Juncaceae) | Plant extract | E. coli; K. pneumoniae | [38] |
| Cymbopogon citrates (DC.) Stapf (Poaceae); Syzygium aromaticum (L.) Merr. & L.M. Perry (Myrtaceae) | Essential oil | C. albicans | [39] |
| Seagrass (Halodule pinifolia) (Miki) Hartog; Cymodocea rotundata Asch. & Schweinf. (Cymodoceaceae) | Aqueous methanol (1:4) extract of fresh leaves | E. coli; S. saprophyticus; P. aeruginosa; K. pneumonia; P. mirabilis; Serratia sp | [40] |
| Betula pendula Roth. (Betulaceae); Equisetum arvense L. (Equisetaceae); Herniaria glabra L. (Caryophyllaceae); Galium odoratum (L.) Scop. (Rubiaceae); Urtica dioica L. (Urticaceae); Vaccinium vitis-idae L. (Ericaceae) | Aqueous extract | E. coli | [41] |
| Camellia sinensis (L.) Kuntze (Theaceae) | Leaf extract | E. coli | [42] |
| Aerva lanata (L.) Juss. ex Schult. (Amaranthaceae); Biophytum sensitivum (L.) DC. (Oxalidaceae); Boerhavia diffusa L. (Nyctaginaceae); Myristica fragrans Houtt. (Myristicaceae) | Petroleum ether, chloroform, methanol, water extract of whole plant, and nutmeg nuts | E. coli; S. aureus; S. viridians; P. aeruginosa; K. pneumoniae | [43] |
| Punica granatum L. (Lythraceae); Stevia rebaudiana (Beroni) Bertoni; Allium sativum L. Amaryllidaceae | Alcohol or water extract; basil oil, geranium oil, lemon grass oil, Japanese mint oil | P. mirabilis; P aeruginosa; Acinetobacter; Serratia; Klebsiella | [44] |
| Mangifera indica L. (Anacardiaceae) | Methanol extract of flower | UPEC | [45] |
| Pimenta dioica (L.) Merr. (Myrtaceae); Anacardium occidentale L. (Anacardiaceae) | Leaf and bark extract | E. coli; E. faecalis; P. aeruginosa; S.aureus; K. pneumoniae | [46] |
| Salvia santolinifolia Boiss. (Lamiaceae) | Essential oil | K. pneumoniae; P. mirabilis; P. vulgaris | [47] |
| 20 plants (Betula; Urtica; Orthosiphon; Zea mays; Agropyron repens, etc.) | Leaves | UPEC | [48] |
| Tribulus terrestris L. (Zygoplyllaceae); Cinnamom verum J. Presl. (Lauraceae); Punica granatum L. (Lythraceae) | Aqueous and ethanol extract of dried plant | E. coli; K. pneumoniae; S. aureus from pregnant women | [49] |
| Camellia sinensis (L.) Kuntze (Theaceae) | Leaf extract | E. coli | [50] |
| Callistemon lanceolatus DC. (Myrtaceae) | Petroleum ether, chloroform, ethanol, methanol seed extract | S. aureus; A. baumani; C. feundii; E. faecalis; E. coli; K. pneumoniae | [51] |
| Anacardium occidentale L. (Anacardiaceae) | Fruit juice | P. aeruginosa; E. faecalis; E. coli | [52] |
| Hibiscus sabdariffa L. (Malvaceae) | Calyx extract | C. albicans | [53] |
| Senna sophera (L.) Roxb synonym Cassia sophera L. (Fabaceae) | Alcoholic leaf extract | E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, Citrobacter freundii, Enterococcus faecalis, and S. saprophyticus | [54] |
| Ocimum suave Willd. (Lamiaceae) | Essential oil | S. aureus; K. pneumoniae; E. faecalis; P. aeruginosa; Morganella morgani; Enterobacter; Acinetobacter; Citrobacter | [55] |
| Acanthus montanus (Nees) T. Anderson (Acanthaceae); Aspilia africana C.D. Adams (Asteraceae); Desmodium velutinum (Willd.) DC (Fabaceae) | Ethanol and aqueous extract of leaves | E. coli, P. aeruginosa, S. aureus | [56] |
| Allium sativum L. (Liliaceae); Cinnamomum verum J. Presl (Lauraceae); Syzygium aromaticum (L.) Merrill & Perry (Myrtaceae); Terminalia arjuna (Roxb.) Wight & Arn. (Combretaceae); Zingiber officinale Roscoe (Zingiberaceae) | Ethanol and aqueous extract of rhizome, bark, flower, bark, rhizome, respectively | E. coli, P. aeruginosa, P. vulgaris S. aureus, K. pneumoniae | [57] |
| Syzygium aromaticum (L.) Merr. & L. M. Perry (Myrtaceae), Glycerrhiza glabra L. (Fabaceae), Laurus nobilis L. (Lauraceae), and Brassica rapa L. (Brassicaceae) | Methanol extract of buds, roots, leaves, seeds, respectively | E. coli, Acinetobacter baumannii, and P. aeruginosa | [58] |
| Hemidesmus indicus R. Br. (Asclepiadaceae) | Methanol extract of root | E. coli, K. pneumoniae | [59] |
Cranberry: a potent uroprotective agent
For centuries, cranberries have been used as a treatment for urinary tract diseases and its antibacterial activity was reported long back [60]. It contains > 80% water, 10% carbohydrates (glucose and fructose) [61], and other phytoconstituents like anthocyanins, flavonoids, terpenoids, catechins, organic acids (citric acid, malic acid, and quinic acid, etc.) with small amount of ascorbic acid, benzoic acid, glucuronic acids [62]. Quinic acid was suggested to be responsible for excretion of hippuric acid in urine in large amounts, which is an antibacterial agent and also has the ability to acidify the urine [63, 64]. Moreover, the elucidation of the UTI pathogenesis has opened a new vista to understand the mode of action of cranberry as an anti-adhesive prophylactic and therapeutic agent for UTI [65].
Escherichia coli strains isolated from urine (UPEC) attached three times more efficiently to uroepithelial cells than E. coli isolated from other experimental sources like stool, sputum, or wound. This proves a unique population of E. coli strain responsible for UTI [66]. Antiadherence activity against gram-negative bacteria isolated from urine and other medical sources was observed in volunteers administered with cranberry juice cocktail or urine and uroepithelial cells obtained after drinking the cocktail, which proves its efficacy in treating UTI [66]. Consumption of different cranberry products helped young and elderly women in preventing and protecting them against UTI [67].
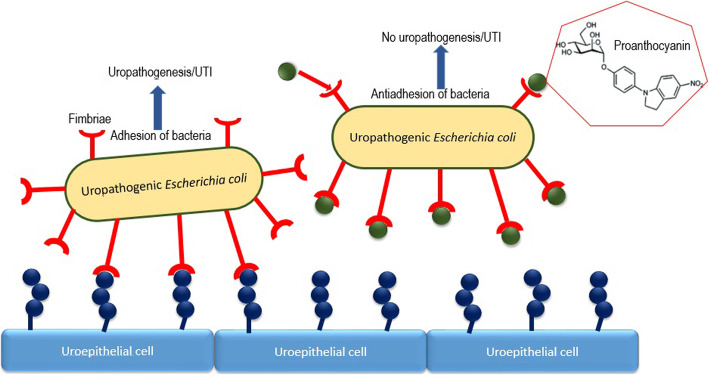
The anthocyanidin/proanthocyanidin biocompounds present in cranberry are reported often to be potent antiadhesive compounds. Since cranberry inhibits the adhesion of type I and P-fimbriated uropathogens (e.g., uropathogenic E. coli) to the uroepithelium, thus, weaken colonization and succeeding infection [68]. Figure 1 depicts the molecular mechanism of antiadhesive property of proanthocyanidins. Due to lack of proper standardization of cranberry products, it becomes extremely complicated to compare products or correlate the results [69]. The in vitro and in vivo studies were summarized in Table 3.
Fig. 1.
Type 1 or P-fimbriae inhibitors (e.g., proanthocyanins) are shown as green balls interfering with binding of bacterial fimbriae to uroepithelial cell
Table 3.
In vitro/in vivo activity of cranberries against UTI causing bacteria
| Study design | Dose | Microorganism | Result | Reference |
|---|---|---|---|---|
| In vitro antiadhesion activity of cranberry (PAC) | 10-50 μg/ml | UPEC | PAC derived from cranberry and blueberry was effective. | [70] |
| In vitro antiadhesion activity of cranberry (PAC) | 60 μg/ml | UPEC | A-linked PAC were more effective than B-linked. | [71] |
| Antiadhesion activity of cranberry vs raisins | 42.5 g | UPEC | 25-50% of reduction in adherence in cranberry gr. None in control or raisin gr. | [72] |
| In vitro antiadhesion activity of cranberry juice | 27% cranberry juice (250 or 750 ml) | E. coli | 45% and 62% decrease in bacterial adhesion to human epithelial cell line in bacteria growing in urine of volunteers administered with 250 and 750 ml of cranberry juice, respectively. | [73] |
| Anti-adhesion activity and prevention of oxidative stress of dried cranberry juice in young women | Dried cranberry juice (400 mg or 1200 mg per day) for 56 days | UPEC | Inhibition of adherence in UPEC with no urine acidity observed in volunteers consuming 1200 mg/day. No effect observed at 400 mg/day. | [74] |
| Anti-adhesion activity of cranberry PAC against bladder and vaginal epithelial cells | 5 to 75 μg/ml of PAC isolated from cranberry powder or extract | E. coli | 50 μg/ml of PAC reduced the mean adherence of E. coli IA2 to vaginal epithelial cells from 18.6 to 1.8 and bladder epithelial cell from 6.9 to 1.6 bacteria per cell | [75] |
| In vitro and in vivo antibacterial and anti-adhesion activity of urine, after cranberry consumption in volunteers | 36 (1 capsule) or 108 mg (3 capsules) of cranberry or placebo per day | E. coli | Better anti-adherence to bladder cell and virulence reduction in E. coli infecting worms when bacteria cultured in urine of volunteer administered with three capsules (108 mg/day) then single capsule (36 mg/day). | [76] |
| Anti-adhesion activity of cranberry juice | Juice or PAC of 0, 64, 128 and 345.8 mg/ml | E. coli | E. coli grown in the presence of PAC repressed adhesion from 50.2 to 7.9 bacteria/cell by altering its surface properties and the effect was reversible. | [77] |
| Antimicrobial activity of urine after cranberry consumption in volunteers | 275 mg of dry, whole cranberries or 25 mg of concentrated, dry cranberries | E. coli, K. pneumonia and C. albicans | ≥ 50% reduction in bacterial number when grown in urine of volunteers after cranberry consumption was found to be 35% (E. coli), 65% (K. pneumoniae), and 45% (C. albicans). | [78] |
| Bacterial anti-adhesion activity of urine collected from cranberry powder administered volunteers | Cranberry capsule of 0, 18, 36, or 72 mg of PAC equivalents per day | E. coli | Dose-dependent decrease in adhesion to bladder cell and reduction in virulence of UPEC in C. elegans model | [79] |
| In vitro anti-adhesion assay in T24 cell line and in vivo virulence assay in C. elegans model | PAC (6-120 mg) plus propolis (170-340 mg) powder | E. coli | Synergistic activity of propolis and proanthocyanidins | [80] |
| In vitro activity of PAC | 4–1024 mg/L | C. albicans | Reduction in biofilm formation due to anti-adherence properties and/or iron chelation at a dose of ≥ 16 mg/L PAC | [81] |
| In vitro activity of A2-linked PAC | 15-100 μg/mL | UPEC, P. mirabilis | Up to 75% reduction of UPEC and P. mirabilis adhesion to HT1376 cell line vs. control. Also drop in motility and urease activity in P. mirabilis. | [82] |
| In vitro and in vivo activity of PAC | 100 μg/mL | P. aeruginosa | Cranberry PACs significantly disrupted the biofilm formation | [83] |
| In vitro activity of oligosaccharides | 0.625-10 mg/mL | E. coli | Reduced biofilm formation by over 50% in pathogenic form and over 60% in nonpathogenic E. coli | [84] |
| Antiadhesive activity of phenolic compounds and their metabolites derived from cranberry | 100–500 μM | UPEC | All the metabolites showed anti-adhesive activity but procyanidin A2, significantly reduced UPEC adherence to uroepithelium at 500 μM (51.3%). | [85] |
| Ex vivo and in vitro antiadhesive activity of PAC and PAC free extract | Standard cranberry extract with 1.24% PAC for ex vivo and 21% PAC for in vitro study | UPEC | 40-50% suppression of UPEC adhesion to human T24 bladder cells. PAC free extract did not influence biofilm and curli formation in UPEC. | [86] |
| In vivo activity of cranberry juice and its organic acids in mice | Cranberry juice/bioactive compounds taken for 7 days | UPEC | Reduction of bacterial number in the bladder of mice drinking fresh cranberry juice, organic acids or both. | [87] |
The recurrence of UTI rates was reduced up to 35% in young to middle-aged women, after the use of cranberry-based compounds. But, in groups with complicated UTI (i.e., young and elderly patients, or patients with neurogenic bladder or with chronic indwelling catheters), the potency of cranberry was unclear. However, these compounds cannot be taken for a longer duration as they have some undesirable effects like weight gain, gastrointestinal problems, and harmful interactions with other drugs [69]. Clinical trials were often complicated and results are not satisfactory in patients with complicated UTI, whereas, cranberry uptake significantly prevented acute cystitis in high-risk females [88]. The clinical trials undertaken with cranberry were summarized in Table 4.
Table 4.
Clinical trials of cranberry products for UTI prevention in different populations
| Experimental design | Dose | N | Result | Reference |
|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled trial | Cranberry juice of 300 ml/day or placebo | 153 elderly women | UTI incidence 15% in cranberry group and 28.1% in placebo group (difference is non-significant) | [89] |
| Randomized, single-blind cross over study | 15 ml juice/kg or water placebo | 21 patients with neuropathic bladder | 9 patients taking cranberry juice and 9 patients taking water showed lowered infection, rest 3 were indifferent. | [90] |
| Randomized, double-blind, crossover trial | Cranberry capsules of 400 mg | 19 female having recurrent UTIs | UTI incidences were 2.4/subject/year in cranberry group and 6.0/subject/year in placebo, 47.4% of withdrawal rate. | [91] |
| Double-blind placebo controlled with crossover | 60 ml/day of cranberry juice or placebo | 15 children under intermittent catheterization | Differences between groups are nonsignificant for bacteriuria or UTI. | [92] |
| Randomized, double-blind, placebo-controlled | 50 ml of cranberry-lingonberry juice (7.5 g), Lactobacillus GG 100 ml/day or placebo | 150 young women with previous UTI | Recurrence rate of UTI reduced in cranberry group, 20% less UTI in cranberry group. | [93] |
| Randomized, double-blind, placebo-controlled | Cranberry juice 250 ml or its tablets | 150 women with recurrent UTIs | Incidence of UTI—30% in juice, 39% in tablets group and 72% in placebo | [94] |
| Randomized, double-blind, placebo-controlled | Cranberry capsules of 8 g or placebo | 135 patients with complicated UTI (multiple sclerosis generated neurogenic bladder) | 34.6% UTI in cranberry group and 32.4% on placebo, no significant difference between the groups and also under intermittent catheterization. | [95] |
| Randomized, double-blind, placebo-controlled | Cranberry capsules of 1 g or placebo | 74 patients with neurogenic bladder induced by spinal cord injury | Insignificant differences in bacteriuria, pyuria, or symptomatic UTIs between the groups, 35% withdrawal rate | [96] |
| Double-blind, placebo controlled with crossover | 400 mg of cranberry tablets for 4 weeks or placebo | 37 patients with neurogenic bladder due to spinal cord injury | 43% of withdrawal rate and no difference were observed between the cranberry and the placebo group. | [97] |
| Randomized, double-blind, placebo-controlled | 25% of cranberry juice (150 ml) and placebo | 376 in door old patients (> 60 years) | 3.7% of UTI incidences in cranberry group of 7.4% with placebo 31% withdrawal rate | [98] |
| Double-blind, randomized, placebo-controlled | 1st group—methenamine hippurate (MH), 2nd—cranberry (800 mg), 3rd—cranberry + MH, and 4th—placebo | 305 patients with spinal cord injury resulted neurogenic bladder | No differences for symptomatic UTI groups to placebo | [99] |
| Randomized, double-blind, placebo-controlled trial | Group A—240 mg of 27% cranberry juice 3 times/day or group B—240 mg daily once or group C—placebo | 188 pregnant women of 16 weeks gestation | No significant differences in UTI occurrence between the groups. Withdrawal rate of 38.8% (A, 50.7%, B, 39.7%, C, 55.5%) | [100] |
| Randomized, double-blind, placebo-controlled trial | Cranberry extract tablet for 6 months | 47 spinal cord injured patients | 0.3 UTI per year in cranberry group vs 1.0 UTI per year in placebo. | [101] |
| Randomized, double-blind, placebo-controlled trial |
cranberry extract (500 mg) or trimethoprim (100 mg) |
137 women with recurrent UTIs—age 45 years | 25 UTIs in cranberry group and14 in trimethoprim group | [102] |
| Randomized controlled trial | Cranberry-lingonberry juice 50 ml/day, Lactobacillus GG 100 ml, 5 days/month or placebo | 84 girls with recurrent UTIs | UTIs incidence 18.5% in 1st group, 42.3% in 2nd, and 48.1% in placebo | [103] |
| Randomized, double-blind, placebo-controlled trial | 27% cranberry juice (8 oz.) | 319 young women with UTI history | UTI recurrence rates—19.3% for cranberry group and 14.6% for placebo | [104] |
| Randomized, double-blind, placebo-controlled trial | Cranberry juice | 263 children cranberry (n = 129), placebo (n = 134) | 0.1% UTI episodes lower in cranberry gr. | [105] |
| Randomized, double-blind, placebo-controlled trial | 200 mg of cranberry | 370 prostate cancer patients | 8.7% UTI in cranberry group, 24.2% in placebo (36% reduction in UTI) | [106] |
| Randomized, double-blind, placebo-controlled trial | Cranberry juice 4, 8 oz/daily, or placebo | 176 patients (120 to cranberry juice and 56 to placebo) | 0.29 UTI in cranberry juice group and 0.37 in the placebo group. P-fimbriated UPEC isolation was 43.5% (10 of 23) in cranberry juice group, 80.0% (8 of 10) in placebo group during the study period | [107] |
| Randomized, double-blind, placebo-controlled trial | 3 capsules of PAC daily for 30 days (108 mg, 72 mg, 36 mg) | 80 women | Dose-dependent reduction in bacteriuria and pyuria | [108] |
| Modified observational study | Sweetened dried cranberry (SDC) of one serving daily for 14 days | 20 women with recurrent UTIs | Mean UTI rate per six months decreased significantly, no UTI observed in > 50% of the patients up to 6 months of SDC consumption | [109] |
| Randomized, double-blind, placebo-controlled multicenter trial | Capsules of cranberry and placebo were taken twice daily for 1 year | 928 women of high and low risk group | Incidence of UTI reduced in cranberry than placebo group (62.8 vs 84.8 per 100 person-years in UTI high risk group). No difference observed in low UTI risk group | [110] |
| Randomized, double-blind, placebo-controlled trial | Two cranberry juice capsules twice daily for 6 weeks or placebo | 160 women undergoing gynecological surgery involving urinary catheterization (80 + 80) | 19% UTI incidence in cranberry group compared to 38% in placebo group | [111, 112] |
| Randomized, double-blind, placebo-controlled trial | 500 mg of whole cranberry fruit powder for 6 months or placebo | Cranberry (n = 89) or a placebo group (n = 93) | UTI occurrence significantly lowered 10.8% vs 25.8% in cranberry and placebo group, respectively | [113] |
| Randomized, double-blind, placebo-controlled trial | 240 ml of cranberry juice per day for 24 weeks or placebo | Cranberry (n = 185) or a placebo (n = 188) | UTI occurrence significantly lowered 21% vs 36% in cranberry and placebo group, respectively | [114] |
Cinnamom verum J. Presl. (cinnamon): a potent botanical for complicated UTI
Chronic recurrent UTI was resulted in patients with urinary catheters due to biofilm formation by MDR UPEC. Trans-cinnamaldehyde (0%, 1%, 1.25%, or 1.5%) was reported to prevent UPEC biofilm formation both on plate culture and indwelling catheters. When trans-cinnamaldehyde was used in catheter lock solution, it inactivated UPEC biofilm formation on catheters. Since the test concentrations had no cytotoxic effects on human bladder epithelial cells, it can be used as a surface coating for catheters or in catheter lock solution to prevent UTI [115]. Trans-cinnamaldehyde significantly reduced uroepithelial cell attachment and invasion by UPEC by inhibiting the expression of major genes associated with its attachment and invasion to host tissue [116]. These findings support the use of cinnamon as a natural remedy for UTI.
Arctostaphylos uva-ursi (L.) Spreng (bearberry)
Arctostaphylos uva-ursi (uva ursi), also known as bearberry or upland cranberry, is a useful herb for bladder infection. Bearberry leaves and preparations made from them have significant antibacterial activity (especially against E. coli) and astringent activity due to its arbutin content and diuretic properties. In a double-blind study of 57 women, five of twenty-seven women had a recurrence in the placebo group while none of thirty women had a recurrence in the uva ursi group after 1 year [117]. Schindler et al. reported that the total amount of urinary excretion of arbutin metabolites (hydroquinone) remained same in all the three groups, after the administration of a single oral dose of bearberry leaves extract or film-coated tablets or an aqueous solution in a randomized crossover study (n = 16) [118].
Probiotics
Probiotics are helpful in establishing and maintaining normal ecology of the vagina, urethra, and bladder and a proper bladder pH and preventing recurrent UTI, which was supported by various in vivo and in vitro studies. Lactobacilli are present predominantly in the urogenital flora of healthy reproductive-aged women. But, the flora is disturbed following long term antibiotic administration and post menstruation temporarily and in post-menopausal women permanently. Supplement of Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum RC-14 appears to be most effective in reducing the risk of intestinal and urogenital infections [119]. The antagonistic activity of five probiotic lactobacilli (L. rhamnosus, L. fermentum, L. acidophilus, L. plantarum, and L. paracasei) and two bifidobacteria (Bifidobacterium lactis, B. longum) against six target pathogens were estimated using different assays. Pyelonephritic E. coli was highly suppressed by L. rhamnosus and both bifidobacterial strains [120]. One hundred thirty-nine women (mean age: 30.5 years) with acute UTI were compared with 185 women of similar age with no episodes of UTIs for 5 years. Frequent consumption of fresh juices, especially berry juices, and fermented milk products containing probiotic bacteria decreased the risk of recurrence of UTI in fertile women. So, dietary supplements can be used to prevent UTI [121].
Preincubation of the uroepithelial cells with Lactobacillus bacterial cell wall fragments inhibited the adherence and colonization of gram-negative uropathogens either completely or partially, which prevented the onset of UTI in female rats. Since the lipoteichoic acid present in the bacterial cell wall is responsible for the adherence of the Lactobacillus cells to uroepithelial cells but its steric hindrance blocked the adherence of uropathogens [122, 123]. Seven strains of lactic acid bacteria (L. paracasei, L. salivarius, two Pediococcus pentosaceus strains, two L. plantarum strains, and L. crispatus) and their fermented probiotic products exhibited clear zones of inhibition against UPEC. This suggests their potential role in adjuvant therapy for prevention and treatment of UTI. The growth of UPEC strains was significantly inhibited after co-culture with lactic acid bacteria and probiotic products in human urine. Oral administration of probiotic products also abrogated the number of viable UPEC in the urine of UPEC-challenged BALB/c mice [124].
Vaccines
Adhesin-based vaccines were very effective in blocking host–pathogen interactions, thereby preventing the establishment of disease [125–127]. In addition to the UPEC adhesins (i.e., pili, fimbriae), adhesins from P. mirabilis, and E. faecalis were also reported as vaccine targets [128]. Vaccination with HlyA (UPEC pore-forming toxin) reduced the rate of renal scaring compared to controls, though it could not prevent UPEC colonization of the kidneys [129]. Several urease inhibitors, i.e., acetohydroxamic acid (AHA), phosphoramidites, benzimidazoles have been used as potent drugs for UTI treatment against urease producing bacterial species like P. mirabilis and S. saprophyticus [130]. Pilicides (type 1 pilus assembly inhibitor) and mannosides (pili function inhibitor) block UPEC colonization, invasion, and biofilm formation and prevent UTI [131, 132].
Discussions
Antibiotics are frequently used to treat and prevent acute and recurrent UTI, but their repeated use can result in dysbiosis of vaginal and intestinal normal flora, as well as antibiotic resistance due to the high mutation ability and horizontal gene transfer capability of different pathogens. Moreover, different mechanisms are used by uropathogens for survival in the bladder under stresses such as starvation and immune responses. Uropathogens undergo morphological changes, invade uroepithelial cells, and form biofilms to persist and cause recurrent infections. Extracellular DNA, exopolysaccharides, pili, flagella, and other adhesive fibers create a niche for a bacterial community that is secluded from antimicrobial agents, immune responses, and other stresses [133]. Thus, it is high time to seek alternative methods for the prevention and treatment of UTIs.
Diuretic botanicals like Asparagus officinalis L. (asparagus), Betula spp. (birch) Elymus repens (L.) Gould (synonym: Agropyron repens) (couch grass), Solidago virgaurea L. (goldenrod), and Equisetum arvense L. (horsetail) work against UTI by increasing urinary volume and supposedly flushing bacteria out of the urinary tract. Ayurvedic herbs like Tribulus terrestris L., Boerhavia diffusa L., Tinospora cordifolia (Willd.) Miers, and Santalum album L. are used since time immemorial for UTI in India. The tribes of Odisha state, India, use the roots of Adiantum lunulatum Burm. f, Argemone mexicana L., Clausena excavata Burm. f, Mimosa pudica L., epicarp of Cucumis melo L., and seeds of Cucumis sativus L. for UTIs. These herbs have proven anti-uropathogenic activities, which were reported enormously by different researchers. However, reports on anti-uropathogenic activity of specific phytoconstituents or their mode of action at the molecular level on uropathogens like enzyme or protein inhibition or degradation, cell membrane, or cell wall disruption or dysfunction of other vital organs of uropathogens are limited. Though the herbal remedies are considered safe to use without any significant side effects yet they are slow in action to be effective in serious acute infections, but they are more effective in preventing recurrence and safeguarding against the post-infectious sequelae.
The safety and efficacy of a product containing two probiotic strains of Lactobacilli plus cranberry extract was reported for impeding recurrent UTIs in pre-menopausal adult women. After 26 weeks, in a randomized, double-blind, placebo-controlled pilot study, a significantly lower number of women experienced recurrent UTIs (9.1 vs 33.3%), those who were administered with the product as compared to placebo [134]. In another study, the efficacy and safety of standardized cranberry capsules as prophylaxis in children with recurrent UTI was reported, where children on cranberry compared to the control group experienced significantly lower percentage of recurrent UTIs, with no side effects. A declined trend of E. coli infections was observed in the cranberry group (83.3% vs. 66.6%), though it was not significant (p = 0.28) [135].
Root extract of Hemidesmus indicus R. Br. (Indian sarsaparilla) (Asclepiadaceae) and seed extract of P. granatum (pomegranate) were reported to have urobactericidal activity against different uropathogens, clinically isolated from patients suffering from urinary tract infections, i.e., Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, and Klebsiella pneumonia [59, 136, 137]. Along with the presence of therapeutic antioxidants, i.e., phenolic compounds, tannins, steroids, terpenes, coumarins, and flavonoids, the extracts were found to be rich in natural glycosides, which are supposed to act as molecular decoys to prevent adhesion of pathogenic bacteria to host cell, thereby inhibiting the future pathogenesis. However, further research is required to confirm it. Till date, there are many reports on scientific evaluations and clinical trials of natural therapeutics for UTI, but they have serious limitations in study design and data interpretation. Most of the products mentioned in this review are based on “in vitro” studies; therefore, more clinical trials should be undertaken in order to assess the efficacy of these alternative preventions and therapeutic methods in humans.
Conclusion
Uroprotective role of cranberry was reported by maximum researchers, yet they suffer from serious drawbacks and fail to prove that cranberry use can prevent or treat acute and recurrent UTI. So, further investigation should focus on the molecular action of various phytochemicals present in cranberry and other potential berries against different uropathogens and uropathogenesis. Supplementation of probiotics was also proven to be effective in both acute and recurrent UTI. However, scientific validation with efficient clinical trial reports will strengthen the practice of using these traditional resources, which will help us in preventing these common yet very discomforting ailments.
Acknowledgements
The author expresses sincere gratitude to the Head, Department of Botany, Berhampur University for providing necessary facilities and thanks to Professor B. B. Panda for his consistent guidance and helpful suggestions during the preparation of this manuscript.
Studies involving plants must include a statement specifying the local, national or international guidelines and legislation, and the required or appropriate permissions and/or licenses for the study
Not applicable
Abbreviations
- MIC
Minimum inhibitory concentration
- MDR
Multidrug resistant
- PAC
Proanthocyanidine
- UTI
Urinary tract infection
- UPEC
Uropathogenic Escherichia coli
Author’s contributions
Author SD had collected all the study material, analyzed, and prepared the complete manuscript. The author(s) read and approved the final manuscript.
Funding
Not applicable
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The author declares that there is no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wagenlehner FM, Lichtenstern C, Rolfes C, Mayer K, Uhle F, Weidner W, Weigand MA (2013) Diagnosis and management for urosepsis. Int J Urol 20:963–970 [DOI] [PubMed] [Google Scholar]
- 2.Cove-Smith A, Almond MK (2007) Management of urinary tract infections in the elderly. Trends in Urology Gynaecol Sex Health 12:31–34 [Google Scholar]
- 3.Reid G (1999) Potential preventive strategies and therapies in urinary tract infection. World J Urol 17(6):359–363 [DOI] [PubMed] [Google Scholar]
- 4.Head KA (2008) Natural approaches to prevention and treatment of infections of the lower urinary tract. Altern Med Rev 13(3):227–244 [PubMed] [Google Scholar]
- 5.Gur S, Turgut-Balik D, Gur N (2006) Antimicrobial activities and some fatty acids of turmeric, ginger root and linseed used in the treatment of infectious diseases. World J Agricultural Sci 2:439–442 [Google Scholar]
- 6.Ahmad I, Aqil F (2007) In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiol Res 162:264–275 [DOI] [PubMed] [Google Scholar]
- 7.Sibanda T, Okoh AI (2008) In vitro evaluation of the interactions between acetone extracts of Garcinia kola seeds and some antibiotics. Afr J Biotechnol 7:1672–1678 [Google Scholar]
- 8.Yarnell E (2002) Botanical medicines for the urinary tract. World J Urol 20(5):285–293 [DOI] [PubMed] [Google Scholar]
- 9.Amin AH, Subbaiah TV, Abbasi KM (1969) Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol 15(9):1067–1076 [DOI] [PubMed] [Google Scholar]
- 10.Sahib AS, Mohammed IH, Hamdan SJ (2012) Use of aqueous extract of corn silk in the treatment of urinary tract infection. J Intercult Ethnopharmacol 1(2):93–96 [Google Scholar]
- 11.Wang GQ, Xu T, Bu XM, Liu BY (2012) Anti-inflammation effects of corn silk in a rat model of carrageenin induced pleurisy. Inflammation 35(3):822–827 [DOI] [PubMed] [Google Scholar]
- 12.Pattanayak S, Das DC, Sinha NK, Parida S (2017) Use of medicinal plants for the treatment of urinary tract infections: a study from Paschim Medinipur district, West Bengal, India. Int J Pharm Bio Sci 8(3):250–259 [Google Scholar]
- 13.Girach RD (1992) Medicinal plants used by Kondha tribe of district Phulbani (Orissa) in eastern India. Ethnobot 4:53–66 [Google Scholar]
- 14.Nayak A, Das NB, Nanda B (1998) Utility of some tribal drugs of Keonjhar and Similipal area. JTR Chem 5(2):53–59 [Google Scholar]
- 15.Brahmam M, Dhal NK, Saxena HO (1996) Ethnobotanical studies among the Tanla of Malyagiri hills in Dhenkanal district Odisha, India. In: Jain SK (ed) Ethnobiology in Human welfare. Deep publication, New Delhi, pp 393–396 [Google Scholar]
- 16.Satapathy KB, Brahmam M (1996) Some medicinal plants used by the tribals of Sundargarh district, Orissa, India. In: Jain SK (ed) Ethnobiology in Human welfare. Deep publications, New Delhi, pp 153–158 [Google Scholar]
- 17.Aminuddin GRD (1993) Observations of the ethnobotany of the Bhunjia – a tribe of Sonabera plateau. Ethnobot 5:83–86 [Google Scholar]
- 18.Girach RD, Aminuddin AM, Mishra MK (1996) Native phytotherapy among rural population of district Bhadrak, Orissa. In: Jain SK (ed) Ethnobiology in human welfare. Deep publications, New Delhi, pp 162–164 [Google Scholar]
- 19.Dash SS, Mishra MK (1999) Plant diversity and sustainable development in a tribal village eco-complex on the eastern ghats of Odisha. J Hum Ecol 10(5-6):415–419 [Google Scholar]
- 20.Bhattarai S, Chaudhary RP, Taylor RSL, Ghimire SK (2009) Biological activities of some Nepalese medicinal plants used in treating bacterial infections in human beings. Nepal J Sci Tech 10:83–90 [Google Scholar]
- 21.Prachi CN, Kumar D, Kasana MS (2009) Medicinal plants of Muzaffarnagar district used in treatment of urinary tract and kidney stones. Ind J Trad Med 8(2):191–195 [Google Scholar]
- 22.Hossan MS, Hanif A, Agarwala B, Sarwar MS, Karim M, Rahman MTU, Jahan R, Rahmatullah M (2010) Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobot Res Appl 8:61–74 [Google Scholar]
- 23.Revathi P, Parimelazhagan T (2010) Traditional knowledge on medicinal plants used by the Irula tribe of Hasanur hills, Erode district, Tamil Nadu, India. Ethnobot Leaflets 2:4 [Google Scholar]
- 24.Darwish RM, Aburjai TA (2010) Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med 10:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereiraa RS, Sumitaa TC, Furlanb MR, Jorgec AOC, Uenod M (2004) Antibacterial activity of essential oils on microorganisms isolated from urinary tract infection. Rev Saude Publica 38(2):1–3 [DOI] [PubMed] [Google Scholar]
- 26.Sowmiya S, Soundarapandian P, Rajan S (2009) Bioactive studies of Mangifera indica against bacteria isolated from urine samples. Curr Res J Biol Sci 1(3):139–143 [Google Scholar]
- 27.Sharma A, Chandraker S, Patel VK, Ramteke P (2009) Antibacterial activity of medicinal plants against pathogens causing complicated urinary tract infections. Indian J Pharm Sci 71(2):136–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nweze EI, Eze EE (2009) Justification for the use of Ocimum gratissimum L. in herbal medicine and its interaction with disc antibiotics. BMC Complement Altern Med 9:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yushau M, Onuorah FC, Murtala Y (2009) In-vitro sensitivity pattern of some urinary tract isolates to Carica papaya extracts. Bayero J Pure Appl Sci 2(2):75–78 [Google Scholar]
- 30.Sosa V, Zunino P (2009) Effect of Ibicella lutea on uropathogenic Proteus mirabilis growth, virulence, and biofilm formation. J Infect Dev Countries 3(10):762–770 [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Sharma VK (2010) Antibacterial activity of allicin from Allium sativum against antibiotic resistant uropathogens. Int J Infect Dis 8(1)
- 32.Ravikumar S, Gnanadesigan M, Suganthi P, Ramalakshmi A (2010) Antibacterial potential of chosen mangrove plants against isolated urinary tract infectious bacterial pathogens. Int J Med Medical Sci 2(3):94–99 [Google Scholar]
- 33.Poovendran P, Vidhya N, Murugan S (2011) Antimicrobial activity of Coccinia grandis against biofilm and ESBL producing uropathogenic E. coli. Global J Pharmacol 5(1):23–26 [Google Scholar]
- 34.Khare RS, Karmakar S, Banerjee S, Nath G, Kundu S, Kundu K (2011) Uropathogen resistant essential oils of Coleus aromaticus and Ocimum sanctum. Int J Pharm Sci Res 2(8):2168–2172 [Google Scholar]
- 35.Balasundaram A, Rathna Kumari P, John G, Selvakumar BN (2011) Antimicrobial activity of the leaf extracts of two medicinal plants against MRSA (Methicilin resistant Staphylococcus aureus) from human urinary tract pathogens. Res J Microbiol 6(7):625–631 [Google Scholar]
- 36.Arun T, Rao CHP (2011) Phytochemical screening and antibacterial activity of Moringa oleifera Lam. against Proteus mirabilis from urinary tract infected patients. Int J Pharm Tech Res 3(4):2118–2123 [Google Scholar]
- 37.Narayanan AS, Raja SS, Ponmurugan K, Kandekar SC, Natarajaseenivasan K, Maripandi A, Mandeel QA (2011) Antibacterial activity of selected medicinal plants against multiple antibiotic resistant uropathogens: a study from Kolli Hills, Tamil Nadu, India. Benefic Microbes 2(3):235–243 [DOI] [PubMed] [Google Scholar]
- 38.Vogel NW, Taschetto AP, Dallagnol R, Weidlich L, Ethur EM (2011) Assessment of the antimicrobial effect of three plants used for therapy of community-acquired urinary tract infection in Rio Grande do Sul (Brazil). J Ethnopharmacol 137(3):1334–1336 [DOI] [PubMed] [Google Scholar]
- 39.Khan MS, Ahmad I (2012) Biofilm inhibition by Cymbopogon citratus and Syzygium aromaticum essential oils in the strains of Candida albicans. J Ethnopharmacol 140(2):416–423 [DOI] [PubMed] [Google Scholar]
- 40.Kannan RR, Arumugam R, Anantharaman P (2012) Chemical composition and antibacterial activity of Indian seagrasses against urinary tract pathogens. Food Chem 135(4):2470–2473 [DOI] [PubMed] [Google Scholar]
- 41.Wojnicz D, Kucharska AZ, Sokol-Lętowska A, Kicia M, Tichaczek-Goska D (2012) Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol Res 40(6):683–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reygaert W, Jusufi I (2013) Green tea as an effective antimicrobial for urinary tract infections caused by Escherichia coli. Front Microbiol 4:162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jagadeesan S, Natarajan V, Rajitha E, Vijayan S, Singaram S (2013) Antibacterial activity of selective plant extracts against urinary tract infection causing organisms. J Microbiol Biotech Res 3(3):1–5 [Google Scholar]
- 44.Rawat S, Ishaq F, Khan A (2013) Antimicrobial effect of drugs, medicinal plant extracts and essential oils against pathogenic bacteria causing urinary tract infection. Global J Biotech Biochem 8(1):15–24 [Google Scholar]
- 45.Poongothai P, Rajan S (2013) Antibacterial properties of Mangifera indica flower extracts on uropathogenic Escherichia coli. Int J Curr Microbiol App Sci 2(12):104–111 [Google Scholar]
- 46.Manasa M, Kambar Y, Swamy HCS, Vivek MN, Kumar TNR, Kekuda TRP (2013) Antibacterial efficacy of Pimenta dioica (Linn.) Merill and Anacardium occidentale L. against drug resistant urinary tract pathogens. J Appl Pharmaceut Sci 3(12):72–74 [Google Scholar]
- 47.Nadir M, Rasheed M, Sherwani SK, Kazmi SU, Ahmad VU (2013) Chemical and antimicrobial studies on the essential oil from Salvia santolinifolia Boiss. Pak J Pharm Sci 26(1):39–52 [PubMed] [Google Scholar]
- 48.Rafsanjany N, Lechtenberg M, Petereit F, Hensel A (2013) Antiadhesion as a functional concept for protection against uropathogenic Escherichia coli: In vitro studies with traditionally used plants with antiadhesive activity against uropathognic Escherichia coli. J Ethnopharmacol 145(2:591–597 [DOI] [PubMed] [Google Scholar]
- 49.Dhanalakshmi J, Selvi S (2013) Antibacterial activity of medicinal plants used against UTI (urinary tract infection) causing pathogens. Int J Res Sci 1(1):01–07 [Google Scholar]
- 50.Noormandi A, Dabaghzadeh F (2014) Effects of green tea on Escherichia coli as a uropathogen. J Tradit Complement Med 5(1):15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kavitha KS, Satish S (2014) Antibacterial activity of seed extracts of Callistemon lanceolatus DC on uropathogenic bacteria. J Acute Med 4(1):6–12 [Google Scholar]
- 52.Vivek MN, Manasa M, Pallavi S, Swamy HCS, Kumar TNR, Kekuda TRP (2014) Antibacterial activity of cashew (Anacardium occidentale L.) apple juice against antibiotic resistant urinary tract pathogens. World J Pharmaceu Sci 2(1):79–82 [Google Scholar]
- 53.Alshami I, Alharbi AE (2014) Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections. Asian Pac J Trop Biomed 4(2):104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jahan N, Khatoon R, Ahmad S (2015) Evaluation of antibacterial potential of medicinal plant Cassia sophera against organisms causing urinary tract infection. Int J Pure App Biosci 3(2):450–455 [Google Scholar]
- 55.Tibyangye J, Okech MA, Nyabayo JM, Nakavuma JL (2015) In vitro antibacterial activity of Ocimum suave essential oils against uropathogens isolated from patients in selected hospitals in Bushenyi District, Uganda. British Microbiology Research Journal 8(3):489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebana RUB, Edet UO, Ekanemesang UM, Etok CA, Ikon GM, Noble MK (2016) Phytochemical screening and antimicrobial activity of three medicinal plants against urinary tract infection pathogens. Asian J Med Health 1(2):1–7 [Google Scholar]
- 57.Samanta P, Sinha NK (2016) Antimicrobial activity of five traditionally used medicinal plants on bacterial infection of urinary tract. Int Res J Basic App Sci 1(2):24–28 [Google Scholar]
- 58.Aziz MA, Adnan M, Rahman H, Allah EFA, Hashem A, Alqarawi AA (2017) Antibacterial activities of medicinal plants against multidrug resistant urinary tract pathogens. Pak J Bot 49(3):1185–1192 [Google Scholar]
- 59.Das S, Naik P, Panda P (2017) Effect of Hemidesmus indicus R.Br. root extract on urinary tract infection causing bacteria. Int J Herbal Med 5(5):160–168 [Google Scholar]
- 60.Bodel PT, Cotran R, Kass EH (1959) Cranberry juice and the antibacterial action of hippuric acid. J Lab Clin Med 54:881–888 [PubMed] [Google Scholar]
- 61.Lenter C (1991) Geigy scientific tables 18th Ed West Caldwell NJCIBA-Geigy.
- 62.Borukh IF, Kirbaba VI, Senchuk GV (1972) Antimicrobial properties of cranberry. Vopr Pitan 31:82 [PubMed] [Google Scholar]
- 63.Kahn HD, Panariello VA, Saeli J, Sampson JR, Schwartz E (1967) Effect of cranberry juice on urine. J Amer Diet Assoc 51:251–254 [PubMed] [Google Scholar]
- 64.Hamilton-Miller JMT (1994) Reduction of bacteriuria and pyuria using cranberry juice. JAMA 272:588 [DOI] [PubMed] [Google Scholar]
- 65.Sobota AE (1984) Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J Urol 131(5):1013–1016 [DOI] [PubMed] [Google Scholar]
- 66.Schmidt DR, Sobota AE (1988) An examination of the anti-adherence activity of cranberry juice on urinary and nonurinary bacterial isolates. Microbios 55(224-225):173–181 [PubMed] [Google Scholar]
- 67.Shmuely H, Ofek I, Weiss EI, Rones Z, Houri-Haddad Y (2012) Cranberry components for the therapy of infectious disease. Curr Opin Biotechnol 23(2):148–152 [DOI] [PubMed] [Google Scholar]
- 68.Zafriri D, Ofek I, Adar R, Pocino M, Sharon N (1989) Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eukaryotic cells. Antimicrob Agents Chemother 33:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guay DR (2009) Cranberry and urinary tract infections. Drugs 69(7):775–807 [DOI] [PubMed] [Google Scholar]
- 70.Howell AB, Vorsa N, Marderosian AD, Foo LY (1998) Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N Engl J Med 339:1085–1086 [DOI] [PubMed] [Google Scholar]
- 71.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M (2005) A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochem 66(18):2281–2291 [DOI] [PubMed] [Google Scholar]
- 72.Greenberg JA, Sara J, Newmann MD, Howell AB (2005) Consumption of sweetened dried cranberries versus unsweetened raisins for inhibition of uropathogenic Escherichia coli adhesion in human urine: a pilot study. J Altern Complement Med 11(5):875–878 [DOI] [PubMed] [Google Scholar]
- 73.Di Martino P, Agniel R, David K, Templer C, Gaillard JL, Denys P et al (2006) Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: a double-blind randomized placebo-controlled cross-over trial. World J Urol 24(1):21–27 [DOI] [PubMed] [Google Scholar]
- 74.Valentova K, Stejskal D, Bednar P, Vostalova J, Cihalik C, Vecerova R et al (2007) Biosafety, antioxidant status, and metabolites in urine after consumption of dried cranberry juice in healthy women: a pilot double-blind placebo-controlled trial. J Agric Food Chem 55(8):3217–3224 [DOI] [PubMed] [Google Scholar]
- 75.Gupta K, Chou MY, Howell A, Wobbe C, Grady R, Stapleton AE (2007) Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol 177(6):2357–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavigne JP, Bourg G, Combescure C, Botto H, Sotto A (2008) In-vitro and in-vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin Microbiol Infect 14(4):350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinzon-Arango PA, Liu Y, Camesano TA (2009) Role of cranberry on bacterial adhesion forces and implications for Escherichia coli-uroepithelial cell attachment. J Med Food 12(2):259–270 [DOI] [PubMed] [Google Scholar]
- 78.Lee YL, Najm WI, Owens J, Thrupp L, Baron S, Shanbrom E et al (2010) Antimicrobial activity of urine after ingestion of cranberry: a pilot study. Evid Based Complement Alternat Med 7(2):227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Howell AB, Botto H, Combescure C, Blanc-Potard AB, Gausa L, Matsumoto T, Tenke P, Sotto A, Lavigne JP (2010) Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis 10:94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lavigne JP, Vitrac X, Bernard L, Bruyere F, Sotto A (2011) Propolis can potentialise the anti-adhesion activity of proanthocyanidins on uropathogenic Escherichia coli in the prevention of recurrent urinary tract infections. BMC Res Notes 4:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rane HS, Bernardo SM, Howell AB, Lee SA (2014) Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm- and adherence-specific mechanisms. J Antimicrob Chemother 69(2):428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicolosi D, Tempera G, Genovese C, Furneri PM (2014) Anti-adhesion activity of A2-type proanthocyanidins (a cranberry major component) on uropathogenic E. coli and P. mirabilis strains. Antibiotics (Basel) 3(2):143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ulrey RK, Barksdale SM, Zhou W, van Hoek ML (2014) Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement Altern Med 14:499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun J, Marais JPJ, Khoo C, LaPlante K, Vejborg RM, Givskov M, Tolker-Nielsen T, Seeram NP, Rowleya DC (2015) Cranberry (Vaccinium macrocarpon) oligosaccharides decrease biofilm formation by uropathogenic Escherichia coli. J Funct Foods 17:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Llano DG, Esteban-Fernandez A, Sanchez-Patan F, Martín-Álvarez PJ, Moreno-Arribas MV, Bartolome B (2015) Anti-adhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic Escherichia coli in bladder epithelial cell cultures. Int J Mol Sci 16(6):12119–12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rafsanjany N, Senker J, Brandt S, Dobrindt U, Hensel A (2015) In vivo consumption of cranberry exerts ex vivo antiadhesive activity against fimH-dominated uropathogenic Escherichia coli: a combined in vivo, ex vivo, and in vitro study of an extract from Vaccinium macrocarpon. J Agric Food Chem 63(40):8804–8818 [DOI] [PubMed] [Google Scholar]
- 87.Jensen HD, Struve C, Christensen SB, Krogfelt KA (2017) Cranberry juice and combinations of its organic acids are effective against experimental urinary tract infection. Front Microbiol 8:542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cimolai N, Cimolai T (2007) The cranberry and the urinary tract. Eur J Clin Microbiol Infect Dis 26(11):767–776 [DOI] [PubMed] [Google Scholar]
- 89.Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA (1994) Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAM 271(10):751–754 [DOI] [PubMed] [Google Scholar]
- 90.Foda MM, Middlebrook PF, Gatfield CT, Potvin G, Wells G, Schillinger JF (1995) Efficacy of cranberry in prevention of urinary tract infection in a susceptible pediatric population. Can J Urol 2(1):98–102 [PubMed] [Google Scholar]
- 91.Walker EB, Barney DP, Mickelsen JN, Walton RJ, Mickelsen RA Jr (1997) Cranberry concentrate: UTI prophylaxis. J Fam Pract 45(2):167–168 [PubMed] [Google Scholar]
- 92.Schlager TA, Anderson S, Trudell J, Hendley JO (1999) Effect of cranberry juice on bacteriuria in children with neurogenic bladder receiving intermittent catheterization. J Pediatr 135(6):698–702 [DOI] [PubMed] [Google Scholar]
- 93.Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M (2001) Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 322(7302):1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stothers L (2002) A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol 9(3):1558–1562 [PubMed] [Google Scholar]
- 95.McGuinness SD, Krone R, Metz LM (2002) A double-blind, randomized, placebo-controlled trial of cranberry supplements in multiple sclerosis. J Neurosci Nursing 34(1):4–7 [Google Scholar]
- 96.Waites KB, Canupp KC, Armstrong S, DeVivo MJ (2004) Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med 27(1):35–40 [DOI] [PubMed] [Google Scholar]
- 97.Linsenmeyer TA, Harrison B, Oakley A, Kirshblum S, Stock JA, Millis SR (2004) Evaluation of cranberry supplement for reduction of urinary tract infections in individuals with neurogenic bladders secondary to spinal cord injury. A prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med 27(1):29–34 [DOI] [PubMed] [Google Scholar]
- 98.McMurdo ME, Bissett LY, Price RJ, Phillips G, Crombie IK (2005) Does ingestion of cranberry juice reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebo-controlled trial. Age Ageing 34(3):256–261 [DOI] [PubMed] [Google Scholar]
- 99.Lee BB, Haran MJ, Hunt LM, Simpson JM, Marial O, Rutkowski SB et al (2007) Spinal-injured neuropathic bladder antisepsis (SINBA) trial. Spinal Cord 45(8):542–550 [DOI] [PubMed] [Google Scholar]
- 100.Wing DA, Rumney PJ, Preslicka CW, Chung JH (2008) Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. J Urol 180(4):1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hess MJ, Hess PE, Sullivan MR, Nee M, Yalla SV (2008) Evaluation of cranberry tablets for the prevention of urinary tract infections in spinal cord injured patients with neurogenic bladder. Spinal Cord 46(9):622–626 [DOI] [PubMed] [Google Scholar]
- 102.McMurdo ME, Argo I, Phillips G, Daly F, Davey P (2009) Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother 63(2):389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferrara P, Romaniello L, Vitelli O, Gatto A, Serva M, Cataldi L (2009) Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scand J Urol Nephrol 43(5):369–372 [DOI] [PubMed] [Google Scholar]
- 104.Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B (2011) Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis 52(1):23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salo J, Uhar M, Helminen M, Korppi M, Nieminen T, Pokka T, Kontiokari T (2012) Cranberry juice for the prevention of recurrences of urinary tract infections in children: a randomized placebo-controlled trial. Clin Infect Dis 54(3):340–346 [DOI] [PubMed] [Google Scholar]
- 106.Bonetta A, Di Pierro F (2012) Enteric-coated, highly standardized cranberry extract reduces risk of UTIs and urinary symptoms during radiotherapy for prostate carcinoma. Cancer Manag Res 4:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stapleton AE, Dziura J, Hooton TM, Cox ME, Yarovaya YY, Chen S, Gupta K (2012) Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc 87(2):143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bianco L, Perrelli E, Towle V, Ness PHV, Mehta MJ (2012) Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc 60(6):1180–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burleigh AE, Benck SM, McAchran SE, Reed JD, Krueger CG, Hopkins WJ (2013) Consumption of sweetened, dried cranberries may reduce urinary tract infection incidence in susceptible women – a modified observational study. Nutr J 12:139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caljouw MAA, van den Hout WB, Putter H, Achterberg WP, Cools HJM, Gussekloo J (2014) Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons: a double-blind randomized placebo-controlled trial in long-term care facilities. J Am Geriatr Soc 62(1):103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foxman B, Cronenwett AE, Spino C, Berger MB, Morgan DM (2015) Cranberry juice capsules and urinary tract infection after surgery: results of a randomized trial. Am J Obstet Gynecol 213(2):194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dieter AA (2015) Cranberry capsules (2 taken twice daily for an average 38 days) reduce the risk of postoperative urinary tract infection in women undergoing benign gynaecological surgery involving intraoperative catheterisation. Evid Based Med 20(4):137 [DOI] [PubMed] [Google Scholar]
- 113.Vostalova J, Vidlar A, Simanek V, Galandakova A, Kosina P, Vacek J, Vrbkova J, Zimmermann BF, Ulrichova J, Student V (2015) Are high proanthocyanidins key to Cranberry efficacy in the prevention of recurrent urinary tract infection? Phytother Res 10:1559–1567 [DOI] [PubMed] [Google Scholar]
- 114.Maki KC, Kaspar KL, Khoo C, Derrig LH, Schild AL, Gupta K (2016) Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection. Am J Clin Nutr 103:1434–1442 [DOI] [PubMed] [Google Scholar]
- 115.Amalaradjou MA, Narayanan A, Baskaran SA, Venkitanarayanan K (2010) Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J Urol 184(1):358–363 [DOI] [PubMed] [Google Scholar]
- 116.Amalaradjou MA, Narayanan A, Venkitanarayanan K (2011) Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J Urol 185(4):1526–1531 [DOI] [PubMed] [Google Scholar]
- 117.Ofek I, Goldhar J, Zafriri D, Lis H, Adar R, Sharon N (1991) Anti-Escherichia coli activity of cranberry and blueberry juices. NEJM 324:1599 [DOI] [PubMed] [Google Scholar]
- 118.Schindler G, Patzak U, Brinkhaus B, von Niecieck A, Wittig J, Krahmer N, Glockl I, Veit M (2002) Urinary excretion and metabolism of arbutin after oral administration of Arctostaphylos uvae ursi extract as film-coated tablets and aqueous solution in healthy humans. J Clin Pharmacol 42(8):920–927 [DOI] [PubMed] [Google Scholar]
- 119.Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B (2001) Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol 30:49–52 [DOI] [PubMed] [Google Scholar]
- 120.Hutt P, Shchepetova J, Loivukene K, Kullisaar T, Mikelsaar M (2006) Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero and uropathogens. J Appl Microbiol 100(6):1324–1332 [DOI] [PubMed] [Google Scholar]
- 121.Kontiokari T, Laitinen J, Jarvi L, Pokka T, Sundqvist K, Uhari M (2003) Dietary factors protecting women from urinary tract infection. Am J Clin Nutr 77:600–604 [DOI] [PubMed] [Google Scholar]
- 122.Chan RCY, Reid G, Irvin RT, Bruce AW, Costerton JW (1985) Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun 47(1):84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reid G, Chan RC, Bruce AW, Costerton JW (1985) Prevention of urinary tract infection in rats with an indigenous Lactobacillus casei strain. Infect Immun 49(2):320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu YH, Ho CY, Huang CC, Tsai CC (2016) Inhibitory effect of lactic acid bacteria on uropathogenic Escherichia coli-induced urinary tract infections. J Prob Health 4(2):144–150 [Google Scholar]
- 125.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner JS, Burlein J, Barren P, Koenig S, Leath S, Jones CH, Hultgren SJ (1997) Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607–611 [DOI] [PubMed] [Google Scholar]
- 126.Langermann S, Möllby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A et al (2000) Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 181:774–778 [DOI] [PubMed] [Google Scholar]
- 127.Asadi Karam MR, Oloomi M, Mahdavi M, Habibi M, Bouzari S (2013) Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine 31:1210–1216 [DOI] [PubMed] [Google Scholar]
- 128.Li X, Erbe JL, Lockatell CV, Johnson DE, Jobling MG, Holmes RK, Mobley HLT (2004) Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect Immun 72:7306–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’Hanley P, Lalonde G, Ji G (1991) Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficacy of an α-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect Immun 59:1153–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kosikowska P, Berlicki L (2011) Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin Ther Pat 21:945–957 [DOI] [PubMed] [Google Scholar]
- 131.Pinkner JS, Remaut H, Buelens F, Miller E, Aberg V, Pemberton N et al (2006) Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci USA 103:17897–17902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cusumano CK, Pinkner JS, Han Z, Greene SE, Ford BA, Crowley JR, Henderson JP, Janetka JW, Hultgren SJ (2011) Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med 3:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3:a010306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koradia P, Kapadia S, Trivedi Y, Chanchu G, Harper A (2019) Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: a controlled pilot study. Exp Rev Anti-Infect Therapy 17(9):733–740 [DOI] [PubMed] [Google Scholar]
- 135.Dotis J, Stabouli S, Pavlaki A, Papachristou F, Printza N (2018) Cranberry standardized capsules may prevent recurrences of urinary tract infections in children. Clin Pediatr 1:1007 [Google Scholar]
- 136.Das S, Panigrahi S, Panda P (2018a) Antiurobacterial activity of Punica granatum L. seed extract. European J Med Plants 22(2):1–12 [Google Scholar]
- 137.Das S, Sahoo KR, Parida B (2018b) Bactericidal activity of Hemidesmus indicus R.Br. root extract against clinically isolated uropathogens. J Med Plant Studies 6(6):180–192 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.