Abstract
Background
Chronic obstructive respiratory disorders (ORD) are linked to increased rates of cancer related deaths. Little is known about the effects of hypercapnia (elevated CO2) on pancreatic ductal adenocarcinoma (PDAC) development and drug-resistance.
Study Design
Two PDAC cell-lines were exposed to normocapnic (5% CO2) and hypercapnic (continuous/intermittent 10% CO2) conditions, physiologically similar to patients with active ORD. Cells were assessed for proliferation rate, colony formation, and chemo/radiotherapeutic efficacy. In a retrospective clinical study design, patients with PDAC who have undergone pancreatic resection between the years of 2002–2014 were reviewed. Active smokers were excluded in order to remove possible smoking-related pro-tumorigenic influences. Clinical data, pathological findings, and survival endpoints were recorded. Kaplan-Meier and Cox regression analyses were performed.
Results
Exposure to hypercapnia resulted in an increased colony formation and proliferation rate, in-vitro in both cell lines (MIA-PaCa-2:111% increase and Panc-1:114% increase, P<0.05). Hypercapnia exposure induced a 2.5-fold increase in oxaliplatin resistance (P<0.05) in both cell lines and increased resistance to ionizing radiation in MIA-PaCa-2 cells (P<0.05). Five hundred and seventy-eight patients were included [52% males, median age was 68.7 years (IQR 60.6–76.8 years)]. Cox regression analysis, assessing TNM-staging, age, gender and ORD status, identified ORD as an independent risk factor for both overall survival (HR 1.64, 95%CI 1.2–2.3, P<0.05) and disease-free survival (HR 1.68, 95%CI 1.06–2.67).
Conclusions
PDAC cells exposed to hypercapnic environments, common to patients with ORD, showed tumor proliferation, radioresistance and chemoresistance. Patients with a history of ORD had a worse overall prognosis, suggesting that hypercapnic conditions play a role in the development and progression of PDAC and stressing the need for patient-tailored care.
Precis
Exposure of pancreatic cancer cells to 10% CO2 hypercapnic environment resulted in increased proliferation, radioresistance, and oxaliplatin chemoresistance. Clinically, pancreatic cancer patients with history of obstructive respiratory diseases had increased decreased survival and increased rates of recurrence.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) has become the 3rd leading cause of cancer mortality in the U.S., estimated to account for over 56,000 new cases in 2019 [1]. Smoking, a major risk factor for development of PDAC [2], also plays a key role in the development of pathologic respiratory conditions (e.g., emphysema and chronic bronchitis [3]) resulting in hypoxia and hypercapnia. Both hypoxia and hypercapnia, combined with acidosis, have been described as common features of the tumor microenvironment [4–6]. Hypoxia, specifically, has been reported in numerous studies as a strong facilitator of PDAC resistance to therapy [7, 8]. However, studies of the biologic effects of hypercapnia are limited. In some reports, pathophysiologic high levels of CO2 were associated with increased tumor proliferation and resistance to chemotherapy in other cancer types [5, 9]. Furthermore, some recent studies suggest hypoxia, hypercapnia, and respiratory acidosis as being important modulators and suppressors of anti-cancer immune responses [8, 10, 11].
Clinically, large studies have alluded to a possible link between obstructive respiratory diseases (ORD, such as bronchitis, asthma and emphysema) and increased rates of non-tobacco related cancers [12, 13]. Additionally, a few studies investigating obstructive sleep apnea, which is also characterized by transient hypercapnia and hypoxia have also suggested a connection to increased cancer risks [14, 15]. In regards to chronic exposure of lung parenchyma to smoke, significant resultant changes in respiratory physiology include prolonged hypercapnia, transient hypoxia, and chronic inflammation which can lead to permanent destruction of lung tissues [16, 17], and the development of a chronic obstructive pulmonary disease (COPD) [3]. These pathologic changes can systemically affect distal tissue oxygenation and carbon dioxide accumulation [18]. This notion is supported by several large population studies which support the association of hypoxic/hypercapnic respiratory conditions with cancer susceptibility [19, 20]. In fact, Kornum et al [12] have shown an association of COPD with increased cancer rates, including non-tobacco related cancers, in a large Danish nationwide cohort (N=236,494). Similar findings were also supported by a long-term prospective study, showing increased risks for breast cancer, prostate cancer, non-melanoma skin cancers and hematological malignancies [13]. Herein, we tested whether the effects of chronic and repeated exposure to high levels of CO2, as seen in patients with ORD, were associated with worse clinical outcomes in patients with PDAC. We also hypothesized that such exposures would result in a more aggressive and therapeutic resistant cancer phenotype.
MATERIALS AND METHODS
Cell culture & CO2 Exposure
PDAC cell lines (MIA-PaCa-2 and PANC-1) were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and were grown in standard DMEM based media, supplemented with 10 % FBS, 1 % Penicillin-Streptomycin, 1 % L-Glutamine (Thermo Fisher Scientific, Waltham, MA, USA). All cells were regularly tested for mycoplasma using LookOut® Mycoplasma PCR Detection Kit (Millipore-Sigma, St. Louis, MO, USA). The cells were cultured in normal incubator conditions (i.e., 37 °C in 5 % humidified CO2 incubators), unless otherwise indicated. To mimic hypercapnia, cultures were subjected to either constitutive 10 % CO2 environment or “intermittent” exposure (three consecutive hours daily), as indicated. A control arm of regular 5 % CO2 with no exposure to hypercapnic conditions was also assessed.
Cell proliferation and survival assays
Cells were seeded in 96-well plates in triplicate and were separated into three groups after 24 hours (5 % CO2 Control, Continuous 10 % CO2, Intermittent 10 % CO2 exposure). Cells were treated with either ionizing radiation or chemical cellular stressors such as DNA damaging chemotherapeutics (gemcitabine and oxaliplatin, Millipore-Sigma, St. Louis, MO, USA). Cell count was assessed using double stranded DNA quantification with PicoGreen® staining (Thermo Fisher Scientific, Waltham, MA, USA). Assays were analyzed with a GloMax® microplate reader (Promega, Madison, WI, USA). Fold change and growth rate were calculated and GR50 [21] was determined through a non-linear regression analysis. Statistical analysis was performed using GraphPad® software (2017, GraphPad Inc., California, USA)
Flow Cytometry assessment of proliferation rate
MIA PaCa-2 cells were pre-cultured at 5 % CO2, Continuous 10 % CO2 or intermittent exposure to 10 % CO2 (3 hrs/day). Cells were labelled with CellTrace™ Violet fluorescent dye (Thermo Fisher Scientific, Waltham, MA, USA) as per manufacturer recommendations and left to grow in their respective conditions for 5 days. Cells were then collected and assessed using a BD FACS Celesta™ Flow cytometer (Becton, Dickinson and Company [BD], Warwick, Rhode Island, USA) using an excitation/emission of 405nm/450nm. Data were analysed using the FlowJO software (FlowJo LLC, Ashland, OR, USA).
Colony formation assays
Cells were seeded in 6-well plates and exposed to increased doses of ionizing radiation. Cells were washed with PBS and fixed with 80 % methanol solution. The cells were then stained with 0.5 % Crystal Violet solution (in 20 % methanol), washed with deionized water and left to air dry. Individual wells were assessed for colony count, surface area coverage and overall staining intensity using ImageJ (Version 1.52P, NIH, USA). Daily assessment experiments were performed, in which at the 4th day post seeding, the plates were exposed to increasing doses of radiation and singles wells were analysed in a daily regimen to interrogate the time-dependent dynamics of the radiation effects. Parameters were normalized to the first day of collection (5th day).
Clinical data analyses
The clinical study was approved by the Thomas Jefferson University Hospital Institutional Review Board. Clinical data from an institutional database were used to retrospectively identify PDAC patients who had undergone pancreatic resection in the years 2002–2014. Self-reported current smokers were excluded to remove possible pro-tumorigenic influences from active cigarette smoking. Demographic data, comorbidities, pathologic findings and follow-up end points were recorded.
Statistical Analyses
Categorical data were compared by χ2 test or Fisher’s exact test. Continuous variables were assessed for normality of distribution with the Kolmogorov-Smirnov test. Normally distributed continuous variables were compared using the Student’s T-test. In cases of non-normal distribution patterns, comparisons between groups were performed using the Mann-Whitney test. Kaplan-Meier analysis (with log-rank comparisons) was used to compare between different survival risk factors for overall survival (OS) and disease-free survival (DFS). Factors with P value (<0.1) were subsequently included in a Cox multivariate hazard model. P values (≤0.05) were considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences (IBM SPSS, Ver.20, SPSS Inc., Chicago, IL, USA).
RESULTS
Cohort Description
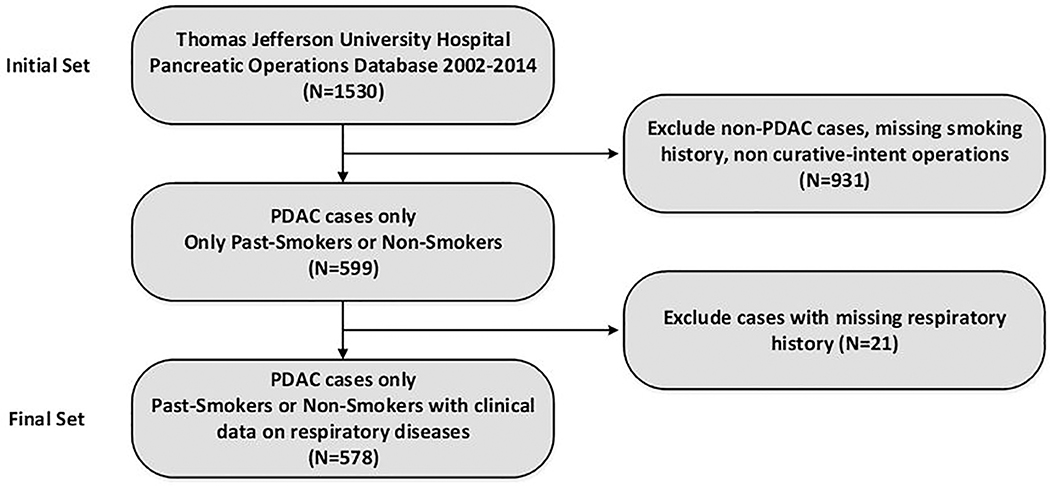
An initial patient set composed of 1530 patients which underwent pancreatic operations at the Thomas Jefferson University Hospital was created. The cases were then selected for PDAC, and curative-intent surgery resulting in a set of 599 patients (52 % males). The median age was 68.9 years (IQR 60.7–76.8 years). Smoking history was positive in 254 patients (42.4 %) of the study cohort and 48 patients (8 %) in the cohort had a history of obstructive respiratory disease (asthma, chronic bronchitis or emphysema). Twenty-one patients (3.5 %) had missing data or unclear status regarding ORD and were excluded from further analysis (final cohort = 578 patients, as seen in Figure 1). The ORD group had significantly more female patients (62.5 % vs. 46.2 %, P<0.05) and higher rates of history of tobacco use (66.7 % vs. 39.6 %, P<0.01). A detailed description of the final cohort and comparison between study arms is shown in Table 1.
Figure 1.
Cohort selection flow diagram. PDAC, pancreatic ductal adenocarcinoma
Table 1.
Cohort Characteristics (n = 578)
| Characteristic | Overall (n = 578) | No obstructive respiratory disease (n = 530) | Obstructive respiratory disease (n = 48) | p Value* |
|---|---|---|---|---|
| Age, y, median (IQR) | 68.7 (60.6–76.8) | 68.5 (60.1–76.9) | 70.6 (64.3–76.8) | NS (0.1) |
| Sex, n (%) | 0.033 | |||
| Male | 303 (52.4) | 285 (53.8) | 18 (37.5) | |
| Female | 275 (47.6) | 245 (46.2) | 30 (62.5) | |
| Race, n (%) | NS | |||
| African American | 44 (7.6) | 43 (8.1) | 1 (2.1) | |
| Asian | 17 (2.9) | 17 (3.2) | 0 (0) | |
| White | 503 (87.0) | 456 (86) | 47 (97.9) | |
| Other | 2 (0.3) | 2 (0.4) | 0 (0) | |
| Unknown | 12 (2.1) | 12 (2.3) | 0 (0) | |
| Smoking history, n (%) | 0.001 | |||
| Nonsmoker | 336 (58.1) | 320 (60.4) | 16 (33.3) | |
| Past smoker | 242 (41.9) | 210 (39.6) | 32 (66.7) | |
| Obstructive respiratory disease, n (%) | — | |||
| No lung disease | 530 (88.5) | — | — | |
| COPD | 29 (4.8) | — | — | |
| Asthma | 19 (3.2) | — | — | |
| Unknown | 21 (3.5) | — | — | |
| Tumor staging | ||||
| T | NS | |||
| T1 | 107 (18.5) | 101 (19.1) | 6 (12.5) | |
| T2 | 317 (54.8) | 288 (54.3) | 29 (60.4) | |
| T3 | 138 (23.9) | 125 (23.6) | 13 (27.1) | |
| TX | 16 (2.8) | 16 (3) | 0 (0) | |
| N | NS | |||
| N0 | 191 (33) | 171 (32.3) | 20 (41.7) | |
| N1 | 212 (36.7) | 202 (38.1) | 10 (20.8) | |
| N2 | 175 (30.0) | 157 (29.6) | 18 (37.5) | |
IQR, interquartile range.
A p value < 0.05 is considered statistically significant.
Patient data: Obstructive respiratory diseases are associated with decreased survival
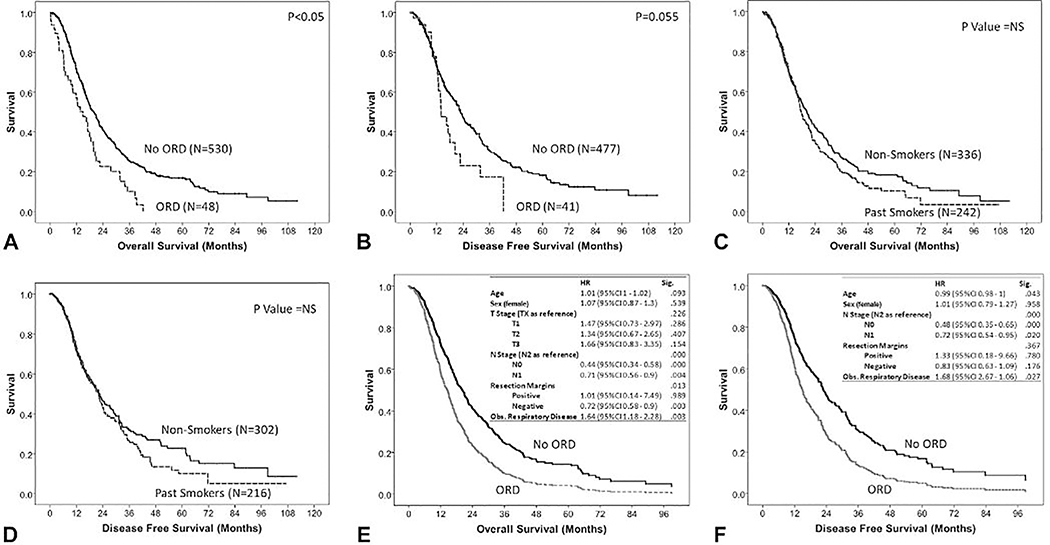
The median overall survival duration of the entire cohort (N=578) was 18.9±0.9 months. T stage, N stage and tumor margin positivity were found to be significant risk factors (P<0.01) for mortality. Patients with obstructive respiratory disease had a significantly reduced OS (14.9±2.3 months vs. 19.8±1.1 months, P<0.05, Figure 2A). Disease recurrence data were missing in 60 patients (10.3%). The median disease-free survival for the evaulable cohort (N=518) was 22.2±1.2 months. T staging was not found to correlate with DFS. N staging and tumor margin positivity were found to be significant risk factors for DFS (P<0.01). Patients in the ORD group had a shorter duration of DFS (13.9±2.2 months vs. 22.7±1.1 months, P=0.055, Figure 2B), however this finding was only borderline significant. Multivariate Cox survival regression analysis revealed ORD to be an independent risk factor for OS (HR 1.64, 95 % CI 1.18–2.3, P<0.01) and DSF (HR 1.68, 95 % CI 1.06–2.67, P<0.05) as shown in figures 2C and 2D, respectively.
Figure 2.
Thomas Jefferson University cohort (N=578) survival analysis showing Kaplan-Meier plots comparing obstructive respiratory disorders (ORD) and non-ORD groups for (A) overall survival and (B) disease-free survival; Kaplan-Meier plots comparing smoking history groups for (C) overall survival and (D) disease-free survival; and Cox regression models for (E) overall survival and (F) disease-free survival. HR, hazard ratio; NS, not significant
Hypercapnic microenvironment promotes cell proliferation
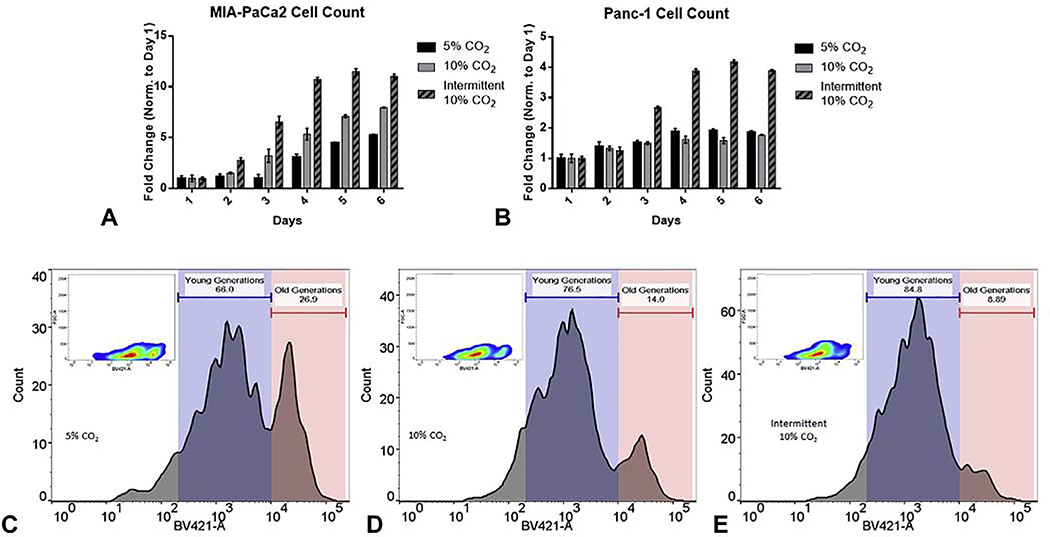
Exposure of MIA-PaCa2 cells to 10 % CO2 resulted in a significant increase in proliferation rate (replications/day) for both the continuous and intermittent exposure arms as compared to the 5 % CO2 control arm (49.3 % ±8.2 % and 111.3 % ±4.7 %, respectively P<0.05). Exposure of PANC-1 cells to 10 % CO2 resulted in a significant increase in proliferation rate only for the intermittent exposure arm as compared to the 5% CO2 controls (114.3 % ±8.2 %, respectively, P<0.05) as seen in Figures 3A–B. Flow cytometry analysis showed a significant decrease in established (older) parental cells and a marked increase in newer generations (Figure 3C–E).
Figure 3.
Proliferation assays. daily quantification based on dsDNA measurement in (A) MIA-Paca2 and (B) Panc-1 pancreatic cancer cell lines exposed to intermittent or continuous 10% CO2 compared to control conditions of 5% CO2 (Mean ±SEM). (C-E) Flow cytometry of labeled MIA-Paca2 cells after long-term exposure to intermittent or continuous 10% CO2 compared to 5% CO2 controls (p<0.05)
Hypercapnic microenvironment promotes radioresistance
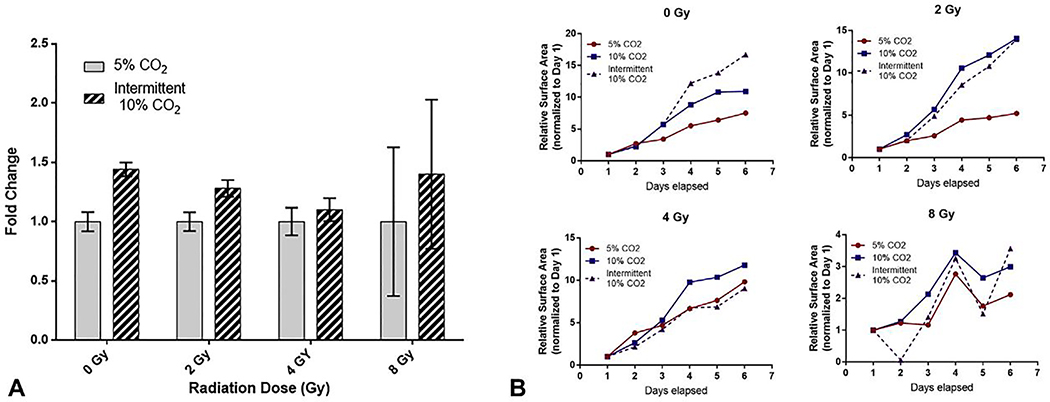
MIA-PaCa2 cells were exposed to increasing doses of ionizing radiation as assessed in clonogenic assays. Intermittent exposure to 10 % CO2 resulted in significantly greater colony surface area coverage as compared with 5 % CO2 in non-irradiated cells and in the 2 Gy groups (fold change 1.44±0.06 and 1.28±0.07, respectively. P<0.05, Figure 4A). Of note, in cells treated with 4 Gy of radiation, 10 % CO2 exposure resulted in a non-significant increase in colony surface area (fold change 1.1±0.1, P=NS). Daily assessment of colony formation post-radiation treatment revealed increased growth rates for cells exposed to 10 % CO2 as compared with 5 % CO2 controls after treatment with radiation (Figure 4B).
Figure 4.
Radiotoxicity clonogenic assays in MIA-Paca2 cancer cells seeded in 6-well plates. (A) Surface area measurement comparison between intermittent exposure to 10% CO2 and 5% CO2 controls (Mean ±SEM). Statistical significance (P<0.05) noted at 0 Gy and 2 Gy of radiation dose. (B) Normalized daily growth trends in response to ionizing radiation comparing 10% CO2 intermittent, 10% CO2 continuous and 5% CO2 continuous exposures.
Hypercapnic microenvironment promotes chemoresistance
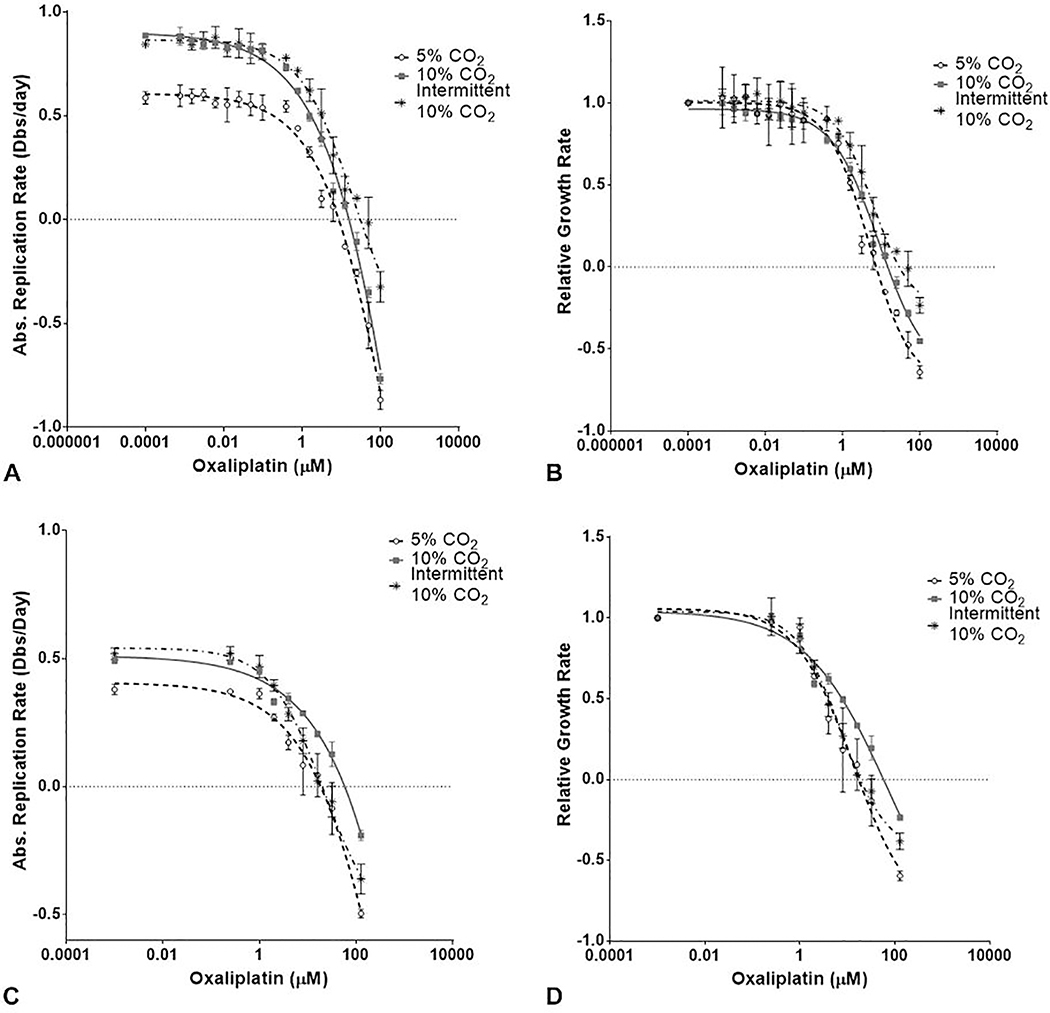
MIA-PaCa2 and PANC-1 cells were assessed for chemoresistance after exposure to increasing amounts of clinically relevant DNA damaging therapeutics (i.e., gemcitabine and oxaliplatin) cultured under normal or elevated CO2 (continuous and intermittent 10 % CO2), as shown in Figure 5. In the MIA-PaCa2 cells, GR50 values for oxaliplatin indicated increased resistance upon exposure to hypercapnic conditions (continuous: 2.47 μM and intermittent: 3.8μM vs. controls:1.53 μM, P<0.05). GR50 values for gemcitabine did not show significant change upon exposure to hypercapnic conditions as compared with 5 % CO2 exposure. However, cells exposed to intermittent 10 % CO2 displayed greater susceptibility to a high dose of gemcitabine (P<0.05). In PANC-1 cells, GR50 values for oxaliplatin indicated increased resistance upon exposure to continuous 10 % hypercapnic conditions compared with 5 % CO2 controls (10.42 μM vs. 3.03 μM, P<0.05). Intermittent exposure to 10 % CO2 resulted in a non-significant increase in GR50 for oxaliplatin as compared with 5 % CO2 controls (3.74 μM vs. 3.03 μM, P=NS).
Figure 5.
Oxaliplatin response in cell viability assays absolute replication rate and GR50 in MIA-PaCa 2 cells (A,B) and Panc-1 cells (C,D).
DISCUSSION
Smoking is an established major risk factor for the development of PDAC, which can account for nearly a 2.4-fold increase in PDAC risk in a dose-dependent manner [22, 23]. Other respiratory diseases associated with hypoxia and hypercapnia, such as COPD and obstructive sleep apnea are associated with an increased risk of cancer [12–15, 19]. While tumor hypoxia has been repeatedly shown to be a critical factor in PDAC therapy resistance [24–27], only a few studies have attempted to look at hypercapnia as a cancer resistance promoting factor. For example, Obata et al [9] have shown that transient hypercarbia increases cell invasion in colon cancer cells, possibly through an upregulation of matrix metallopeptidase 2 and 9 (MMP2/MMP9) protein expression. Moreover, abdominal insufflation of 100 % CO2 was shown to promote cell proliferation [28] and cell invasion [29] in mouse colon cancer models, in vivo.
In this study we have chosen to investigate whether COPD and asthma, commonly associated with hypercapnia, have a prognostic role in PDAC survival. We included only non-smokers or past smokers in our analysis, thereby excluding possible confounding effects from active smoking or inhaled chemicals (e.g, nicotine[30–33]). Our results indicate that ORD are associated with increased mortality risk and shorter disease-free survival. We have then further assessed, in vitro, the different phenotypic changes that occur in PDAC cells in the presence of hypercapnia. We have found that intermittent and continuous exposure to 10 % CO2 induced increased proliferation and resistance to radiotherapy. Furthermore, such exposures resulted in relative resistance to oxaliplatin therapy, as seen in Figure 5.
Concordant with our findings, Kikuchi et al [5] have described a non-pH dependent resistance to reactive oxygen species stress and to platinum-based chemotherapies in lung cancer cells exposed to hypercapnic conditions (e.g., 15 % CO2). These data support our clinical findings that show increased disease progression (i.e., a worse prognosis) in patients that have increased CO2 retention, as is common in COPD and severe asthma patients. Overall, our study reveals hypercapnia to be another disease modifier in PDAC patients with ORD, distinct from hypoxia, but sharing some common phenotypes. These findings may be clinically relevant as they offer a potential personalized approach for ORD patients. In fact, we propose a future prospective clinical trial to optimize ventilatory function as part of patient pre-habilitation prior to oncologic treatment. The interventions would be aimed at reducing the CO2 load through improving ventilation with pharmacologic agents, intensive respiratory therapy, and/or dietary modifications aimed to adjust the respiratory quotient (RQ)[34–36].
This study has several limitations which merit discussion. Clinically, the study is based on a single center, retrospective design, limiting its generalizability and interpretation. Smoking history data were based on self-reports and thus are prone to bias. Additionally, ORD status was determined based upon data in the electronic medical records, rendering the data open for potential misclassification. As noted, hypoxia is a known promoter of cancer resistance and its occurrence in ORD may serve as a confounding factor in the interpretation of the survival data. Our study did not control for additional non-cigarette smoke harmful particulates (e.g., occupational exposures). Other possible confounders such as gender and age were also identified. However, these were included in our regression models to account for their effects. It is important to note that while our in vitro data corroborates our clinical findings, we have yet to validate our results in an in vivo model.
CONCLUSIONS
Hypercapnic conditions promote in vitro pancreatic cancer cell proliferation and resistance. Patients with PDAC who have obstructive respiratory disorders associated with CO2 retention have increased mortality and shorter disease-free survival. These findings support the need for a clinical trial that may set the stage for a personalized therapeutic approach for patients with ORD and PDAC.
Acknowledgments
Support: This work was supported by a Mary Halinski Fellowship (to Dr Nevler) and the W Kim Foster Pancreatic Cancer Research Endowment, and, in part, with grant support from the Newell Devalpine Foundation. Dr Brody was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number P30CA056036 SKCC Core Grant (Thomas Jefferson University).
Abbreviations and Acronyms
- DFS
disease-free survival
- GR50
growth rate inhibition
- ORD
obstructive respiratory disease
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
Disclosure Information: Nothing to disclose
REFERENCES
- 1.SEER Database, National Cancer Institute (NCI) - Cancer Stat Facts: Pancreatic Cancer. 2019.
- 2.Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014. August 28;20(32):11182–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lokke A, Lange P, Scharling H, et al. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006. November;61(11):935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ. Targeting the metabolic microenvironment of tumors. Adv Pharmacol. 2012;65:63–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuchi R, Iwai Y, Tsuji T, et al. Hypercapnic tumor microenvironment confers chemoresistance to lung cancer cells by reprogramming mitochondrial metabolism in vitro. Free Radic Biol Med. 2019. April;134:200–14. [DOI] [PubMed] [Google Scholar]
- 6.Vaupel P The pathophysiology of the tumor microenvironment: Coping with hostile conditions and spatio-temporal heterogeneities. Frontiers in Pharmacology. 2014. February;5. [Google Scholar]
- 7.Erkan M, Kurtoglu M, Kleeff J. The role of hypoxia in pancreatic cancer: a potential therapeutic target? Expert Review of Gastroenterology & Hepatology. 2016. 2016/03/03;10(3):301–16. [DOI] [PubMed] [Google Scholar]
- 8.Daniel SK, Sullivan KM, Labadie KP, Pillarisetty VG. Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clin Transl Med. 2019;8(1):10–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obata S, Goi T, Nakazawa T, et al. Changes in CO2 concentration increase the invasive ability of colon cancer cells. Anticancer Res. 2013. May;33(5):1881–5. [PubMed] [Google Scholar]
- 10.Keogh CE, Scholz CC, Rodriguez J, et al. Carbon dioxide-dependent regulation of NF-kappaB family members RelB and p100 gives molecular insight into CO2-dependent immune regulation. J Biol Chem. 2017. July 7;292(27):11561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzeng YS, Wu SY, Peng YJ, et al. Hypercapnic acidosis prolongs survival of skin allografts. J Surg Res. 2015. May 1;195(1):351–9. [DOI] [PubMed] [Google Scholar]
- 12.Kornum JB, Svaerke C, Thomsen RW, et al. Chronic obstructive pulmonary disease and cancer risk: a Danish nationwide cohort study. Respir Med. 2012. June;106(6):845–52. [DOI] [PubMed] [Google Scholar]
- 13.Chiang CL, Hu YW, Wu CH, et al. Spectrum of cancer risk among Taiwanese with chronic obstructive pulmonary disease. Int J Clin Oncol. 2016. October;21(5):1014–20. [DOI] [PubMed] [Google Scholar]
- 14.Brenner R, Kivity S, Peker M, et al. Increased Risk for Cancer in Young Patients with Severe Obstructive Sleep Apnea. Respiration. 2019;97(1):15–23. [DOI] [PubMed] [Google Scholar]
- 15.Palamaner Subash Shantha G, Kumar AA, Cheskin LJ, Pancholy SB. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med. 2015. October;16(10):1289–94. [DOI] [PubMed] [Google Scholar]
- 16.Xu F, Zhuang J, Wang R, et al. Blunted ventilatory response to hypoxia/hypercapnia in mice with cigarette smoke-induced emphysema. Respir Physiol Neurobiol. 2007. August 15;158(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildebrandt W, Sauer R, Koehler U, et al. Lower hypoxic ventilatory response in smokers compared to non-smokers during abstinence from cigarettes. BMC Pulm Med. 2016. November 24;16(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fricker M, Goggins BJ, Mateer S, et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight. 2018. February 8;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aamli Gagnat A, Gjerdevik M, Gallefoss F, et al. Incidence of non-pulmonary cancer and lung cancer by amount of emphysema and airway wall thickness: a community-based cohort. Eur Respir J. 2017. May;49(5). [DOI] [PubMed] [Google Scholar]
- 20.Hsu WL, Chen HY, Chang FW, Hsu RJ. Does chronic obstructive pulmonary disease increase the risk of prostate cancer? A nationwide population-based study. Int J Chron Obstruct Pulmon Dis. 2019;14:1913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafner M, Niepel M, Chung M, Sorger PK. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat Methods. 2016. June;13(6):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008. July;393(4):535–45. [DOI] [PubMed] [Google Scholar]
- 23.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012. July;23(7):1880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daijo H, Hoshino Y, Kai S, et al. Cigarette smoke reversibly activates hypoxia-inducible factor 1 in a reactive oxygen species-dependent manner. Sci Rep. 2016. September 29;6:34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco FF, Jimbo M, Wulfkuhle J, et al. The mRNA-binding protein HuR promotes hypoxia-induced chemoresistance through posttranscriptional regulation of the proto-oncogene PIM1 in pancreatic cancer cells. Oncogene. 2016. May;35(19):2529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Wang J, Wei W, et al. Hypoxia regulates ABCG2 activity through the activivation of ERK1/2/HIF-1alpha and contributes to chemoresistance in pancreatic cancer cells. Cancer Biol Ther. 2016;17(2):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Han H, Rong Y, et al. Hypoxia potentiates gemcitabine-induced stemness in pancreatic cancer cells through AKT/Notch1 signaling. J Exp Clin Cancer Res. 2018. November 28;37(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen MY, Huang IP, Chen WS, et al. Influence of pneumoperitoneum on tumor growth and pattern of intra-abdominal tumor spreading: in vivo study of a murine model. Hepatogastroenterology. 2008. May-Jun;55(84):947–51. [PubMed] [Google Scholar]
- 29.Zhao J, Lv Y, Cai Y, et al. Hyperthermic carbon dioxide pneumoperitoneum reinforces the inhibition of 5-FU on the proliferation and invasion of colon cancer. Oncol Rep. 2017. January;37(1):492–500. [DOI] [PubMed] [Google Scholar]
- 30.Bersch VP, Osvaldt AB, Edelweiss MI, et al. Effect of nicotine and cigarette smoke on an experimental model of intraepithelial lesions and pancreatic adenocarcinoma induced by 7,12-dimethylbenzanthracene in mice. Pancreas. 2009. January;38(1):65–70. [DOI] [PubMed] [Google Scholar]
- 31.Chipitsyna G, Gong Q, Anandanadesan R, et al. Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells. International journal of cancer Journal international du cancer. 2009. July 15;125(2):276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermann PC, Sancho P, Canamero M, et al. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology. 2014. November;147(5):1119–33 e4. [DOI] [PubMed] [Google Scholar]
- 33.Momi N, Ponnusamy MP, Kaur S, et al. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through alpha7nAChR-mediated MUC4 upregulation. Oncogene. 2013. March 14;32(11):1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akrabawi SS, Mobarhan S, Stoltz RR, Ferguson PW. Gastric emptying, pulmonary function, gas exchange, and respiratory quotient after feeding a moderate versus high fat enteral formula meal in chronic obstructive pulmonary disease patients. Nutrition. 1996. April;12(4):260–5. [DOI] [PubMed] [Google Scholar]
- 35.Kuo CD, Shiao GM, Lee JD. The effects of high-fat and high-carbohydrate diet loads on gas exchange and ventilation in COPD patients and normal subjects. Chest. 1993. July;104(1):189–96. [DOI] [PubMed] [Google Scholar]
- 36.Ramires BR, de Oliveira EP, Pimentel GD, et al. Resting energy expenditure and carbohydrate oxidation are higher in elderly patients with COPD: a case control study. Nutr J. 2012. June 6;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]