Abstract
The p53 transcription factor confers its potent tumor suppressor functions primarily through the regulation of a large network of target genes. The recent explosion of next generation sequencing protocols has enabled the study of the p53 gene regulatory network (GRN) and underlying mechanisms at an unprecedented depth and scale, helping us to understand precisely how p53 controls gene regulation. Here, we discuss our current understanding of where and how p53 binds to DNA and chromatin, its pioneer-like role, and how this affects gene regulation. We provide an overview of the p53 GRN and the direct and indirect mechanisms through which p53 affects gene regulation. In particular, we focus on delineating the ubiquitous and cell type-specific network of regulatory elements that p53 engages; reviewing our understanding of how, where, and when p53 binds to DNA and the mechanisms through which these events regulate transcription. Finally, we discuss the evolution of the p53 GRN and how recent work has revealed remarkable differences between vertebrates, which are of particular importance to cancer researchers using mouse models.
INTRODUCTION
The TP53 gene encoding the tumor suppressor p53 is the most frequently mutated gene in human cancers (1,2). p53 is also deactivated or repressed in a large proportion of tumors containing wild-type p53 through diverse mechanisms including aberrant degradation, deregulation of activators, effectors, or repressors such that most, if not all, cancers circumvent the p53 signaling pathway. p53 is the eponymous member of the p53 transcription factor (TF) family that evolved from an ancestral p63/p73 gene that can be found in most invertebrates (3,4). Functionally the ancestral p63/p73 gene protects organismal integrity and the germ line by inducing cell death in cells with genome damage. In higher vertebrates all three p53 family members can be found and their function has diversified. While p53 largely functions as a tumor suppressor, p63 and p73 play important developmental roles in addition to context-dependent tumor suppressive and tumor promoting activities.
p53 functions as a sequence-specific TF, which is activated in normal cells in response to a diverse range of stress-induced stimuli, in particular DNA damage, to regulate a large network of target genes through which it exerts the majority of its tumour suppressive functions (5,6). The transcriptional output of the complex p53 gene regulatory network determines whether cells pause until stress/damage is resolved or repaired, terminally arrest, or die. Consistent with its tumor suppressive TF function, cancers most frequently harbor mutation in the region of TP53 that encodes the DNA binding domain (DBD) (7).
In the following paragraphs we discuss what we know about p53 binding to DNA and chromatin and how this relates to gene regulation. Particularly, we focus on recent advances in our understanding of how and where p53 binds to DNA and the mechanisms through which these events regulate transcription.
THE p53 GENE REGULATORY NETWORK—A UNIVERSE OF TARGETS
A TF gene regulatory network (GRN) comprises all genes regulated by a given TF: through binding directly to its cognate gene regulatory DNA elements or indirectly through the effects of its direct targets on downstream signaling pathways and transitional TFs. Importantly, myriad studies over the last decade have demonstrated that the majority of TFs, including p53 and its family members p63/p73, can affect their GRNs through binding to both proximal promoters or distal enhancers, the latter of which are connected to the respective gene by long-range interactions.
As described further below, recent meta-analyses of high-throughput datasets generated over the last decade suggest that p53 predominantly activates expression of direct targets through binding to proximal promoter regions, and have begun to define the broader p53 binding landscape and disentangle the direct and indirect regulatory events within the p53 GRN. Surprisingly, integrating multiple gene expression and chromatin-immunoprecipitation (ChIP) datasets revealed that only ∼11% of the >3000 genes whose mRNAs were consistently altered in response to p53 activation exhibit reproducible p53 binding proximal to their transcriptional start sites (TSS) (thus predicting them as direct p53 targets) (6,8). Two websites enable researchers to quickly query their gene of interest for information on its p53-dependent regulation and p53 binding near the gene locus (www.targetgenereg.org; (8)) and https://www.niehs.nih.gov/research/resources/databases/p53/index.cfm; (6)). Notably, while p53 regulates a substantial set of genes through gene proximal regions, it binds to many more distal locations, the function of which is harder to discern, and may regulate even more genes.
Canonically, p53 is recruited through a DNA motif called the p53 response element (p53RE) comprising two decameric half-sites of the consensus sequence RRRCWWGYYY (R = A/G, W = A/T, Y = C/T). Productive p53 binding leading to transcriptional activation predominantly occurs at p53REs within 2.5 kb of the TSS of the regulated gene, which also can be situated in the first intron (8,9). Recent integrative ChIP-seq studies suggested that proximal p53 binding events are predominantly associated with target gene up-regulation in response to p53 activation (10–13), which is strongly supported by recent meta-analyses (6,8,14,15). Conversely, these studies also indicate that the vast majority of genes down-regulated in response to p53 activation are not associated with proximal p53 binding events indicating they are either regulated through indirect mechanisms or distal binding events. While this does not preclude p53 exerting context-specific direct repressive effects on some genes, it strongly supports a model wherein p53 functions mostly as an activator of transcription reconciling many years of research and debate.
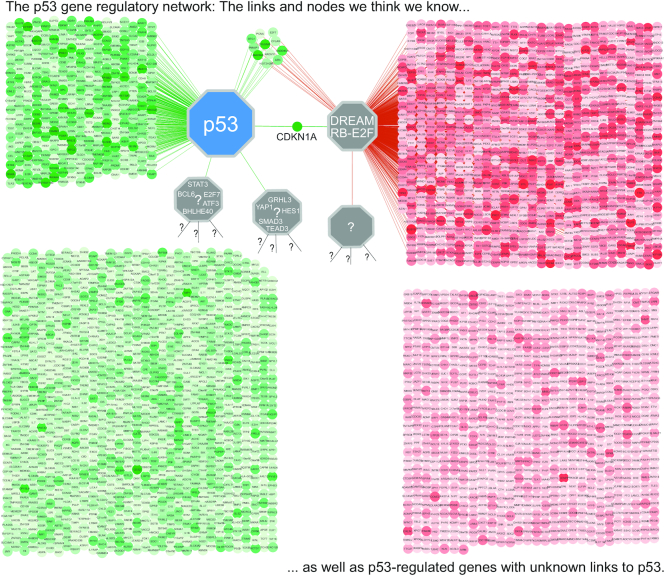
The largest fraction of genes indirectly regulated by p53 is represented by cell cycle genes, which are repressed in response to p53 activation. These genes are largely regulated through the p53–p21–DREAM/RB pathway (16–19), which is highly conserved between mouse and human (20) and may control up to one thousand cell cycle genes (8). This has been complemented through studies that challenged multiple trans-repression mechanisms proposed for p53 and rectified how p53 regulates several genes (13,14,16,21,22). Additional mechanisms of indirect gene regulation through p53 include the direct activation of non-coding RNAs such as micro RNAs. This includes mir-34a that is a tumor suppressor in its own right (23), as well as several long non-coding RNAs (24). Moreover, p53 activation directly affects the mRNA levels of many other TFs and their regulators, including ATF3, BCL6, BHLHE40, E2F7, HES1, GRHL3, SMAD3, STAT3, TEAD3, and YAP1, which could cumulatively contribute to the p53-dependent regulation of a large number of genes (8). Such indirect effects are particularly difficult to decipher, and the mechanisms that mediate p53-dependent expression of many genes remain to be uncovered (Figure 1).
Figure 1.
The p53 gene regulatory network. Many genes are frequently and reproducibly up-regulated (n = 1392, green nodes) or down-regulated (n = 1707, red nodes) by p53. The common p53-regulated genes include a subset of genes directly regulated by p53 (n = 311, upper left cluster). In these cases, p53 binds to the gene's proximal promoter within 2.5 kb from the TSS. The best-known indirect regulatory mechanism by p53 involves its direct target CDKN1A that encodes for p21 and leads to reactivation of the cell cycle trans-repressor complexes DREAM and RB-E2F. Following their activation by p21, DREAM and RB-E2F are particularly important to down-regulate cell cycle genes (n = 888, upper right cluster). For many other genes differentially regulated by p53 the underlying regulatory mechanism remains to be uncovered (bottom clusters). Green and red nodes are respectively up- and down-regulated by p53. Saturation indicates the p53 Expression Score (threshold ±5), which is calculated as the number of datasets reporting significant gene up-regulation minus the number of datasets reporting significant gene down-regulation upon p53 activation. Thus, high saturation indicates p53-dependent regulation across cell types and treatments. Green and red edges represent respective direct target gene up- and down-regulation through proximal promoter binding by p53 or DREAM/RB. Distance contains no information. Data from (8).
Our current understanding of gene regulation by p53 binding events has been biased by a logical focus on proximal promoter binding events, since assigning longer-range regulatory events presents significant technical challenges. Hence, the information content required to recruit p53 to DNA and to mediate p53-dependent gene regulation remains elusive. For example, in silico motif searches in the mouse and the human genome, using position weight matrices without spacers, identified 124 313 and 98 553 potential canonical p53REs respectively (20). However, less than 8% of these sites are found to recruit p53 across disparate datasets, with an even smaller subset of those p53-bound sites to reproducibly confer direct regulation of the nearest gene. While a large proportion of direct p53-induced target genes are regulated through p53 binding to proximal p53REs, we are beginning to understand that expression of a number of p53-regulated genes may be directly influenced by distal p53RE binding events (12,25–29). A recent genome-wide screen for distal p53 binding events that influence cell proliferation and survival after doxorubicin treatment identified productive p53 binding events up to 250 kb from the nearest gene (30). Moreover, we are beginning to understand that p53 can bind to distinct genomic loci in a cell type-specific manner as exemplified by a subset of squamous-specific p53REs bound by its sibling p63 (31–33), to which p53 can bind to only in squamous cells (12). Notably, our current understanding likely underestimates p53 interactions with difficult to map repetitive regions of the genome, an important consideration since at least a subset of p53REs are thought to have evolved from retroviral elements (34–38).
THE UBIQUITOUS DNA BINDING LANDSCAPE OF p53
The last decade of genome-scale studies has provided deep insights into the genomic locations and contexts in which p53 binds to DNA. To date, at least 132 (Table 1) human p53 wild-type ChIP-seq datasets have been produced by independent labs examining chromatin binding of p53. While efforts to robustly integrate and reconcile the large amounts of ChIP-seq and gene expression data generated have been limited by the diversity of cell types, duration, and nature of stimuli analyzed to date, important patterns have begun to emerge from these meta-analyses (6,8,9,14,15). The largest meta-analysis to-date compared similarly processed genomic data and identified a reproducible subset of >1000 genomic locations bound by p53, present in at least 20 of 41 datasets (6), which are highly concordant with those found in earlier meta-analyses (8,15). Similar efforts to catalogue p53-binding sites in mouse cells, also identified a ‘default set’ of p53 binding sites regardless of activating stimulus or cellular context (20,39), providing strong evidence for the existence of a conserved ubiquitous group of p53:DNA interactions that are commonly induced when p53 is stabilized. Despite this, significant differences were observed between the DNA binding landscapes of human and mouse p53 (20).
Table 1.
132 ChIP-seq datasets of human wild-type p53 published using multiple cell lines cultured with various conditions
| Cells | Treatment | public accession | Reference |
|---|---|---|---|
| A498 | IR (4 Gy, 2 h) | GSM2677375 | (52) |
| A549 | IR (4 Gy, 2 h) | GSM2677380 | (52) |
| A549 | Nutlin (5 μM, 2 h) | GSM3771330 | (52) |
| A549 | Nutlin (5 μM, 2 h) | GSM3771331 | (52) |
| A549 | TGFβ (2.5 ng/ml, 5 days) + Nutlin (5 μM, 2 h) | GSM3771332 | (52) |
| A549 | TGFβ (2.5 ng/ml, 5 days) + Nutlin (5 μM, 2 h) | GSM3771333 | (52) |
| A2780 | Cisp (2 μM, 3 days) | GSM3720408 | (53) |
| A2780 | vehicle | GSM3720407 | (53) |
| BJ | IR (10 Gy, 6 h) | GSM1348340 | (54) |
| BJ | RasV12+control vector | GSM508793 | (55) |
| BJ | untreated | GSM1348339 | (54) |
| CAL51 | IR (5 Gy, 1 h) | ERR375899 | (56) |
| CAL51 | IR (5 Gy, 2 h) | ERR375900 | (56) |
| CAL51 | untreated | ERR375898 | (56) |
| Calu-1 | DMSO (0.01%, 48 h) | GSM3682106 | (57) |
| Calu-1 | Belinostat (0.1 μM, 48 h) + Cisp (10 μM, 48 h) | GSM3682107 | (57) |
| FSF | DXR (0.2 μg/ml, 12 h) | GSM1342488 | (47) |
| FSF | DXR (0.2 μg/ml, 12 h) | GSM1342494 | (47) |
| GM06993 + GM11992 | DXR (0.5 μM, 18 h) | GSM1142696 | (58) |
| GM06993 + GM11992 | untreated | GSM1142697 | (58) |
| GM12878 | IR (10 Gy, 4 h) | GSM1142702 | (58) |
| GM12878 | Nutlin (10 μM, 18 h) | GSM1142700 | (58) |
| H460 | IR (4 Gy, 2 h) | GSM2677379 | (52) |
| HCT116 | 5-FU (350 μM, 12 h) | GSM1412744 | (59) |
| HCT116 | 5-FU (350 μM, 6 h) | SRR1343581 | (60) |
| HCT116 | 5-FU (375 μM, 6 h) | GSM1417250 | (61) |
| HCT116 | 5-FU (50 μg/ml, 24 h) | - | (62) |
| HCT116 | Camptothecin (CPT) (1.5 μM, 4h) | SRR2817469 | (63) |
| HCT116 | Camptothecin (CPT) (5 μM, 8 h) | SRR2967009,SRR2967010 | (64) |
| HCT116 | DMSO (0.05%) control | SRR1343582 | (60) |
| HCT116 | DMSO (0.2%, 12 h) | SRR4090090 | (9) |
| HCT116 | DMSO (4 h) | SRR2817470 | (63) |
| HCT116 | DXR (1.6 μM, 6 h) | SRR1343583 | (60) |
| HCT116 | IR (4Gy, 2 h) | GSM2677381 | (52) |
| HCT116 | IR (8 h) | SRR1539836 | (65) |
| HCT116 | IR (8 h) | SRR1539837 | (65) |
| HCT116 | Negative control siRNA (3 days) | GSM3103907 | (66) |
| HCT116 | Nutlin (10 μM, 6 h) | SRR1343584 | (60) |
| HCT116 | Nutlin (10 μM, 12 h) | SRR4090091 | (9) |
| HCT116 | Nutlin (12 h) | GSM3103906 | (66) |
| HCT116 | siRNA against iASSP (3 days) | GSM3103908 | (66) |
| HCT116 | Untreated | GSM1412743 | (59) |
| HCT116 | Untreated | SRR1539838 | (65) |
| HCT116 | Untreated | SRR2967011,SRR2967012 | (64) |
| HCT116 | Untreated | GSM3103905 | (66) |
| HDF | DMSO (48 h) | SRR3125899 | (67) |
| HDF | Nutlin (10 μM, 48 h) | SRR3125901 | (67) |
| HepG2 | control AdV (48 h) + UVC (24 h) | GSM1581946 | (68) |
| HepG2 | Hepatitis B (HBx)-expressing AdV (48 h) + UVC (24 h) | GSM1581947 | (68) |
| hESC | DXR (6 h) | GSM981236 | (51) |
| hESC | RA (1 μM, 48 h) | GSM981237 | (51) |
| hESC | Untreated | GSM981235 | (51) |
| HFK | Cisp (25 μM, 24 h) | GSM1366691,GSM1366697 | (12) |
| HFK | DXR (350 nM, 24 h) | GSM1366690,GSM1366696 | (12) |
| HFK | Untreated | GSM1366689,GSM1366695 | (12) |
| HuSkFib | Nutlin (5 μM, 6 h) | GSM3020116, GSM3378524 | (48) |
| HuSkFib | DMSO (6 h) | GSM3020115, GSM3378522 | (48) |
| IMR90 | 5-FU (375 μM, 6 h) | GSM783262 | (69) |
| IMR90 | DMSO control | GSM1418969 | (25) |
| IMR90 | Etop (100 μM, 6 h) | SRR7357223 | (70) |
| IMR90 | Etop (100 μM, 24 h) | GSM1294880,GSM1294881,GSM1294893,GSM1294882,GSM1294883 | (71) |
| IMR90 | Nutlin (5 μM, 6 h) | GSM1418970 | (25) |
| IMR90 | O/E E1A/RasG12V | GSM1294879,GSM1294885,GSM1294891 | (71) |
| IMR90 | O/E RasG12V | GSM1294877,GSM1294878,GSM1294890 | (71) |
| IMR90 | Senescent (HRasV12) | GSM1048851 | (72) |
| IMR90 | Untreated | GSM1294876,GSM1294884 | (71) |
| IMR90 | Untreated | GSM1048850 | (72) |
| LOXIMVI | IR (4 Gy, 2 h) | GSM2677373 | (52) |
| Lymphocyte_BS104 | DMSO (0.1%, 24 h) | GSM2988942 | (6) |
| Lymphocyte_BS104 | DXR (0.3 μg/ml, 24 h) | GSM2988944 | (6) |
| Lymphocyte_BS104 | Nutlin (10 μM, 24 h) | GSM2988946 | (6) |
| Lymphocyte_BS116 | DMSO (0.1%, 24 h) | GSM2988948 | (6) |
| Lymphocyte_BS116 | DXR (0.3 μg/ml, 24 h) | GSM2988950 | (6) |
| Lymphocyte_BS116 | Nutlin (10 μM, 24 h) | GSM2988952 | (6) |
| Lymphocyte_BS45 | DMSO (0.1%, 24 h) | GSM2988930 | (6) |
| Lymphocyte_BS45 | DXR (0.3 μg/ml, 24 h) | GSM2988932 | (6) |
| Lymphocyte_BS45 | Nutlin (10 μM, 24 h) | GSM2988934 | (6) |
| Lymphocyte_BS90 | DMSO (0.1%, 24 h) | GSM2988936 | (6) |
| Lymphocyte_BS90 | DXR (0.3 μg/ml, 24 h) | GSM2988938 | (6) |
| Lymphocyte_BS90 | Nutlin (10 μM, 24 h) | GSM2988940 | (6) |
| MALME3 | IR (4 Gy, 2 h) | GSM2677378 | (52) |
| MCF7 | 5-FU (100 μM, 8 h) | SRR287799 | (73) |
| MCF7 | Decitabine (2 μM, 5 days) | GSM2740046 | (52) |
| MCF7 | DMSO (0.2%, 12 h) | SRR4090093 | (9) |
| MCF7 | IR (4 Gy, 2 h) | GSM2677372 | (52) |
| MCF7 | IR (10 Gy, 1 h) | SRR5690016 | (74) |
| MCF7 | IR (10 Gy, 2.5 h) | SRR5690017 | (74) |
| MCF7 | IR (10 Gy, 4 h) | SRR5690018 | (74) |
| MCF7 | IR (10 Gy, 5 h) | SRR5690019 | (74) |
| MCF7 | IR (10 Gy, 7.5 h) | SRR5690020 | (74) |
| MCF7 | IR (10 Gy, 7.5 h), then sequential Nutlin | SRR5690021 | (74) |
| MCF7 | neocarzinostatin (NCZ) (400 ng/ml, 3 h) | SRR5857009,SRR5857012 | (75) |
| MCF7 | Nutlin (5 μM, 2 h) | GSM2677384 | (52) |
| MCF7 | Nutlin (5 μM, 24 h) | GSM1146168 | (76) |
| MCF7 | Nutlin (10 μM, 8 h) | SRR287800 | (73) |
| MCF7 | Nutlin (10 μM, 12 h) | SRR4090094 | (9) |
| MCF7 | RITA (0.1 μM, 8 h) | SRR287797 | (73) |
| MCF7 | RITA (1 μM, 8 h) | SRR287798 | (73) |
| MCF7 | Untreated | SRR287796 | (73) |
| MCF7 | Untreated | SRR5690015 | (74) |
| MCF7 | Untreated | SRR5857010,SRR5857013 | (75) |
| MCF7 | Untreated | GSM2740045 | (52) |
| MCF7 | Untreated | GSM1429753 | (77) |
| MCF10A | Nutlin (5 μM, 6 h) | GSM3020136, GSM3378513 | (48) |
| MCF10A | DMSO (6 h) | GSM3020135, GSM3378510 | (48) |
| MDA-MB-175VII | Untreated | GSM1429754 | (77) |
| PBMC | 5-FU (50 μg/ml, 24 h) | - | (62) |
| SaOS-2 | O/E GFP | ERR206782,ERR206794,ERR206795 | (11) |
| SaOS-2 | O/E p53-wt (18 h) | ERR206781,ERR206779,ERR206784 | (11) |
| SaOS-2 | O/E p53-wt (24 h) | GSM501691,GSM501692 | (78) |
| SaOS-2 | O/E p53-wt (24 h) | GSM1241481 | (79) |
| SJSA | DMSO (0.2%, 12 h) | SRR4090096 | (9) |
| SJSA | Nutlin (10 μM, 12 h) | SRR4090097 | (9) |
| SKMEL5 | IR (4 Gy, 2 h) | GSM2677377 | (52) |
| SW480 | Empty vector | SRR1920910 | (80) |
| SW480 | p53 wt O/E | SRR1920909 | (80) |
| UACC62 | IR (4 Gy, 2 h) | GSM2677382 | (52) |
| UACC257 | IR (4 Gy, 2 h) | GSM2677383 | (52) |
| UACC257 | Nutlin (5 μM, 2 h) | GSM2677385 | (52) |
| U2OS | ActD (5 nM, 24 h) | GSM545807 | (81) |
| U2OS | DMSO (0.1%, 24 h) control | GSM1133482 | (82) |
| U2OS | DXR (0.6 μg/ml, 24 h) | GSM1133484 | (82) |
| U2OS | Etop (10 μM, 24 h) | GSM545808 | (81) |
| U2OS | IR (4 Gy, 2 h) | GSM2677376 | (52) |
| U2OS | Nutlin (10 μM, 24 h) | GSM1133486 | (82) |
| U2OS | Untreated | SRR1344509,SRR1343579 | (60) |
| U2OS | Untreated | GSM1133488 | (82) |
| U2OS | Untreated | ERR359700,ERR359705 | (83) |
| U2OS | UV (50 J, 6 h) | SRR1344510,SRR1343580 | (60) |
| U2OS | UVC (20 J/m2, 16 h) | ERR359704,ERR359706 | (83) |
| U2OS | UVC (20 J/m2, 8 h) | ERR359699,ERR359702 | (83) |
| UO31 | IR (4 Gy, 2 h) | GSM2677374 | (52) |
Abbreviations: 5-FU, 5-fluorouracil; ActD, actomycin D; Cisp, cisplatin; DXR, doxorubicin; Etop, etoposide; IR, ionizing radiation; nutlin, Nutlin-3; RITA, reactivation of p53 and induction of tumor cell apoptosis; UV, ultraviolet radiation; p53 O/E, p53 overexpression; RA, retinoic acid; Ras O/E, Ras overexpression; DMSO, dimethyl sulfoxide; TGFβ, Transforming growth factor beta; AdV, adenovirus.
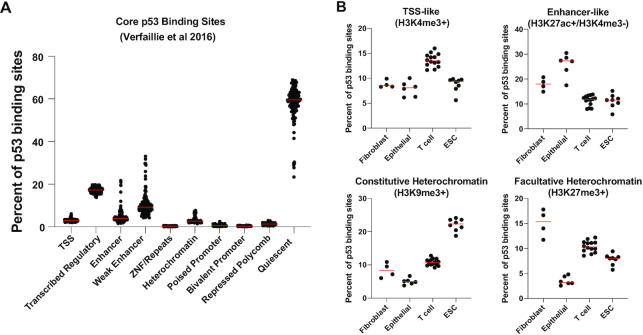
Despite the identification of a ubiquitous group of p53 binding sites, the molecular mechanisms that facilitate these common p53 binding events across experimental conditions and cell lines remain unclear. Analysis of genomic context of p53 binding sites based on enrichment of particular histone modifications or accessibility based on DNAse-I hypersensitivity or Assay for Transposase-Accessible Chromatin followed by sequencing (ATAC-seq) as a proxy to identify active regulatory regions (40–46) indicates that p53 binds within at least three observable genomic contexts: (i) accessible DNA, (ii) constitutive nucleosome-rich regions, and (iii) nucleosome-rich regions that display some level of nucleosome eviction in a p53-dependent manner (25,27,47). p53 binding events within accessible DNA are also enriched for histone modification patterns that indicate an active transcriptional regulatory region, such as an enhancer or promoter (25,26,47,48). In agreement with this prediction, examination of the chromatin context of ubiquitous p53 binding events derived from chromHMM (chromatin Hidden Markov Model) across 127 unique cell types (49) shows that a median of approximately one-third of ‘ubiquitous’ p53 binding sites reside within transcriptionally-associated genomic regions across all cell types (Figure 2A, TSS, transcribed regulatory, enhancer, or weak enhancer). The chromatin context of p53 binding sites varies significantly between cell types, even at binding locations that are ubiquitous across all cell types tested to date (15). These cell type differences are not limited to transcriptionally-associated chromatin features, as common p53 binding sites across embryonic stem cells (hESC) and primary fibroblasts are enriched for constitutive and facultative (polycomb-mediated) heterochromatin features (Figure 2B). CDKN1A/p21 expression is repressed in hESC through polycomb-mediated H3K27me3 at the promoter which can be alleviated by H3K27me3 methyltransferase inhibition (50). Other p53-bound gene promoters were also decorated with this repressive modification in hESC, suggesting that the chromatin state of a p53 binding site can alter p53-dependent transcriptional activation (51). Consistent with previous work (25,27), the majority of common p53 binding events (median of 59% across all cell types) actually fall within quiescent chromatin (Figure 2A). These chromatin regions are devoid of active and repressive histone modifications and lack features of open or accessible chromatin. The potential function of these regions is a rich area for future investigation given the total number and broad conservation of these binding events across cell types and treatment conditions.
Figure 2.
The local chromatin modification state of core p53 binding sites. (A) Integration of ubiquitous (core) p53 binding sites identified by (15) with ChromHMM-assigned chromatin features across 127 unique cell types (ChromHMM = chromatin Hidden Markov Model). These results are qualitatively similar when the ubiquitous p53 binding sites from the Nguyen et al. meta-analysis are used (6). Each dot represents the percent of p53 binding sites in each cell type that have the listed local chromatin environment. The 25-state ChromHMM model was collapsed into 10 distinct groups similar to (252). Red lines represent the median percentage of p53 binding sites with the given chromatin state across the 127 cell types assayed. (B) The percent of core p53 binding sites with the given chromatin state for primary fibroblasts, epithelial cell types, T cell types and embryonic stem cells (ESC).
DNA BINDING IN THE CONTEXT OF CHROMATIN: PIONEER-LIKE p53
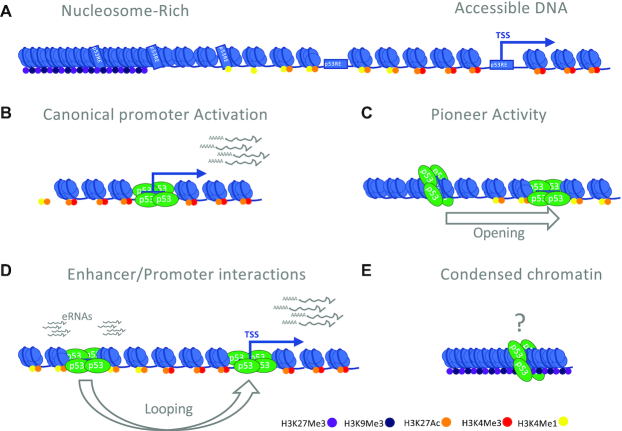
DNA wrapped around a histone octamer is called a nucleosome. DNA in a nucleosomal context is generally unable to be recognized and bound by sequence-specific TFs. Therefore, nucleosomes often act as direct barriers to TF binding and biochemical reactions on DNA, such as transcription and DNA replication/repair (84). Extensive evidence now indicates that p53, in addition to canonical binding to accessible promoter and enhancer regions (Figure 3A, B), also can bind to nucleosomal DNA with high affinity. As such, p53 falls into a functionally defined group of proteins called pioneer factors, which can interact with and recognize specific DNA information even when the DNA resides in the context of nucleosomes (85). Pioneer factors can use this nucleosome binding activity to initiate a local chromatin remodeling cascade, ultimately establishing DNA accessibility at cognate transcriptional regulatory regions (Figure 3C) (86). This ability is uncommon amongst sequence-specific TFs, with analyses from ENCODE showing that DNA binding for most TFs is strongly inhibited by the presence of nucleosomes (40,41).
Figure 3.
Chromatin binding states of p53. (A) A general schematic of the range of chromatin states with observed p53 binding. (B) Binding of p53 to gene proximal promoters represents the canonical model for p53 engagement with gene regulatory elements. p53-bound promoters are generally characterized by accessible (nucleosome-free) chromatin and histone modification-based hallmarks such as H3K4 trimethylation (H3K4me3). (C) p53 can bind to DNA in the context of a nucleosome using pioneer factor-like activity. Pioneer factor activity reflects two related functions: the recognition of a TF motif in nucleosomal DNA and the facilitation of nucleosome remodeling to create an accessible DNA element. p53 recognizes its RE across multiple nucleosomal contexts, although binding is strongly disfavored when the p53RE is at the nucleosome dyad. The majority of nucleosome binding sites remain nucleosome-enriched (closed), although this may be context or treatment-specific. p53 binding to closed chromatin in fibroblasts, for example, leads to a subset of regions that become accessible and correlate with transcriptional enhancer activity. (D) p53 also binds to genomic regions with hallmarks of transcriptional enhancers, which includes accessible DNA, the presence of H3K4me1 and H3K27ac, and the absence of H3K4me3. p53 binding to these regions elicits transcription of bidirectional enhancer RNA (eRNA). Although very limited data currently exists regarding p53, one mechanism for distal gene regulatory element activity is through chromatin looping, which brings these elements in physical proximity to target gene promoters. (E) The final class of binding events is to condensed/closed chromatin, which can either be characterized as actively repressed heterochromatin (containing the histone modifications H3K9me3 or H3K27me3) or quiescent. Quiescent chromatin is condensed but does not have the stereotypical histone modifications associated with heterochromatin. Quiescent chromatin represents the largest individual chromatin state bound by p53, although their function is unknown (Figure 2A).
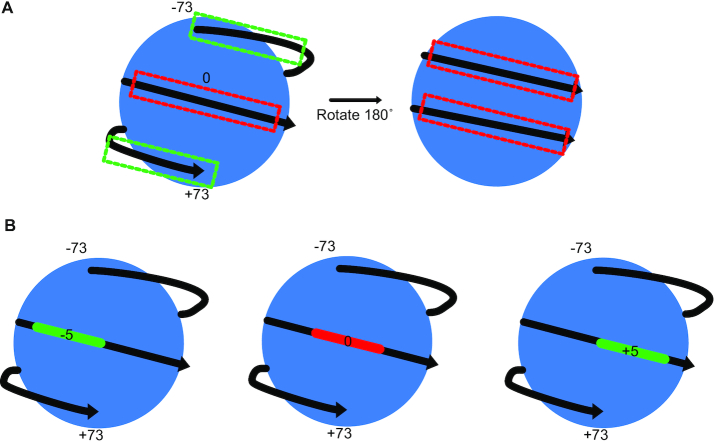
A key question regarding p53’s pioneer factor function relates to how nucleosomal positioning and occupancy affect p53 binding and transcriptional activity. Initial evidence for p53 pioneer factor activity comes from in vitro studies demonstrating direct binding to the p53RE in the CDKN1A promoter even when the DNA template is within a nucleosome (87). Biochemical reconstitution of p53RE DNA into mono-nucleosomes confirmed the direct p53 binding to DNA within chromatin (88). Both an updated, highly-parallel sequencing approach (89) and DNase I-mediated footprinting of the native CDKN1A promoter sequence came to similar conclusions about preferred p53:nucleosome orientations (90). p53 prefers binding to nucleosome orientations where the p53RE is near the nucleosome edges and disfavors binding near the nucleosome dyad or core (Figure 4A) (89,90). Whether this preference is due to dynamic unwrapping/‘breathing’ of DNA near nucleosome edges or a specific recognition mechanism of p53 for DNA at nucleosome edges remains to be directly experimentally validated (91).
Figure 4.
The location and orientation of p53 response elements within nucleosomal DNA influences p53 binding affinity (A) 146 nucleotides of DNA wrap around a histone octamer (blue) with the nucleosome dyad normally denoted as position 0. In vitro nucleosome binding experiments and in vivo ChIP-seq analyses suggest p53 affinity for nucleosomal DNA is higher when the p53 response element is within linker DNA (non-nucleosome associated) or near the nucleosome edges (illustrated with a green box). p53 binding near the dyad or to the nucleosome gyre (opposite side when rotated 180°) is disfavored relative to other positions (marked with red boxes). Of note, p53 binding to the disfavored positions still occurs at nanomolar Kdvalues and can also occur in the absent of a p53RE, suggesting intrinsic affinity of p53 for nucleosomal DNA. (B) p53 affinity for nucleosomal DNA depends partially on the rotational position of the p53RE relative to the nucleosome dyad. When the center of the 20bp p53RE is at rotational position 0 (middle), binding is disfavored relative to when the rotational position is shifted in increments of five nucleotides (left or right). This binding preference periodicity has been observed for other pioneer transcription factors, like the homeodomain and forkhead factors (46).
Binding of p53 to nucleosomal DNA is also influenced by the rotational position of the p53RE relative to the nucleosome dyad (Figure 4B). Strongest p53 binding in vitro was observed when the DNA minor groove of the p53RE half site was exposed to solvent (88). Within a nucleosome, this would happen when the p53RE is centered 5bp from the dyad, repeating every 10bp after that. Rotational position does not supersede location around the nucleosome, as nucleosome edge binding, even in disfavored rotational positions, was stronger than any rotational position within the nucleosome core (89). p53REs at some pro-apoptotic p53 targets appear to exist in a non-preferred nucleosome rotational position relative to some cell cycle arrest targets (92) and this has been proposed to explain differential transcription activation kinetics between these two gene classes (92,93). This possibility has not been directly tested on a broad scale, although the advent of both high-throughput in vitro nucleosome binding assays and more sensitive genome-scale chromatin structure measurements should allow such analyses in the future.
The binding of p53 to native nucleosomal DNA sequences within cells is supported by examination of p53 binding using ChIP coupled to highly parallel analysis approaches (Figure 3A and E). Using ChIP-coupled microarray (ChIP-chip), p53 was shown to bind to high-affinity p53REs within approximately 2,000 nucleosome-rich regions in cells (94). Subsequent ChIP-seq studies confirmed that p53 can bind to genomic locations with high nucleosome occupancy (25,27), and that many p53:nucleosome interactions take place at nucleosome edges, similar to in vitro observations (89). Taken together, these biochemical and genomic observations demonstrate the capacity of p53 to bind to nucleosomal DNA, and that this is influenced by the context of the p53RE. Context-dependent p53 binding based on nucleosome positioning provides intriguing avenues for studying the biology of p53, including how p53RE positioning within a nucleosome influences differential transcriptional activation and p53-dependent phenotypes.
Another key question regarding p53’s pioneer activity relates to the molecular mechanisms allowing p53 to recognize and bind to nucleosomal DNA. Sequence-specific nucleosomal DNA binding activity of p53, perhaps unsurprisingly, is dependent on the p53 DBD. The p53 DBD alone can bind to p53RE in many of the same contexts as full-length p53 (88). Notably, full-length p53 is capable of non-specific binding to nucleosomal DNA lacking a recognizable p53RE (88), whereas deletion of the disordered and posttranslational modification-rich C-terminal domain (CTD) diminishes this non-specific binding (88,89,95). The CTD appears critical for binding to p53RE that deviate from the consensus sequence (96), a finding that has been further confirmed by SELEX (systematic evolution of ligands by exponential enrichment)-based high-throughput analysis (97). Non-specific DNA binding has also been proposed to allow p53 to scan DNA for high affinity p53REs (98); however, the ultimate functional relevance of the CTD mediating non-specific binding to both naked and nucleosomal DNA is unclear and requires additional study.
The molecular mechanisms of other pioneer TFs may provide some clues into p53 family pioneer activity. For example the pioneer factors SOX2 and KLF4 display extensive non-specific nucleosome binding that may aid sliding-based DNA motif searching and binding to exposed partial DNA motifs within nucleosomal DNA (99). Pioneer factor binding to nucleosomal DNA is aided by the ability to differentially recognize full and optimal or degenerate sequence motifs (100). p53 binding preferences display defined periodicity from the nucleosome dyad, as has been demonstrated for both forkhead and homeobox pioneer factors (46). Thus, the mechanisms used by other well-studied pioneer factors may provide context into specific molecular functions needed for p53-dependent nucleosome binding. Understanding the mechanisms used by p53 to recognize nucleosomal DNA and how differential binding of p53 to various nucleosomal DNA contexts affects transcriptional activation and other biological processes are key questions for future research.
CHROMATIN-ENGAGED COFACTORS OF p53
To enact its chromatin modifying roles, p53 interacts with an extensive list of transcriptional cofactors and can mediate their recruitment directly to p53REs. Amongst these cofactors are a number of chromatin modifying and remodeling enzymes with known co-activator and co-repressor roles, involved in nucleosome eviction, regulation of chromatin structure, and the activity of transcriptional regulatory regions. This includes key modulators of histone modification state, such as CBP and p300 (mediating H3K27ac) (101,102), TIP60 and hMOF (H4K16ac) (103,104), GCN5/PCAF (H3K9ac) (105), and the H3K27me3 demethylase KDM6B (54). p53 can also directly interact with components of chromatin remodeling complexes, such as RSF1 (106), BRG1 (107), ARID1A (108), BRD7 (55), and TRRAP (109). These interactions are not limited to those supporting p53-dependent trans-activation, as p53 also associates with known transcriptional repressors, such as histone deacetylases (HDACs) (110) as well as modulators of repressive chromatin and heterochromatin including PCL1 (111), KMT5A/SETD8 (112), LSD1 (113), SMYD2 (114), SUV39H1 (115), USP7 (116), as well as EHMT1 and EHMT2 (117). Such interactions are mediated through both transactivation domains (TADs) of p53 as well as the post-translational modification-rich CTD, whose modification state (similar to a histone tail) can be read by chromatin regulators such as SET (118), 53BP1 (119), and PHF20 (120,121). While the list of known chromatin regulators interacting with p53 is extensive, key questions remain regarding their roles in modulating p53 activity. Many of the above factors post-translationally modify p53 itself (122) to affect changes in p53 DNA binding, stability, and interactions with cofactors.
Acetylation of multiple lysine residues in p53’s DNA-binding and oligomerisation domains by histone acetyltransferase (HAT) p300, potentiates DNA binding in vitro (102), which in turn elicits localized effect on histones, both of which are competitively removed by the activities of HDACs. HDACs have been shown to both interact with MDM2 to enhance p53 degradation and directly deacetylate the p53 C-terminus to influence its transcriptional activity (123–127). These C-terminal lysine residues are targeted by additional HATs, such as CBP and PCAF, to facilitate p53 activity, whereas they are also validated substrates for lysine methyltransferases and ubiquitin ligases, whose activity can variably repress or activate p53 function in vivo (113,117,128,129). Delineating the importance of specific post-translational modifications (PTM) and the residues on which they are found has been extremely complex. However, recent data suggest that the overall net charge of the p53 C-terminus may play an important role in repressing its activity, with an un-acetylated ‘acidic’ C-terminus being read by SET, much like a histone, inhibiting its acetylation (118). Modulating the activities of regulators of p53 PTMs, such as inhibitors of HDACs and other co-factors, have shown potential to promote p53 induced cell death alone or in combination with DNA-damaging agents (130–133), but the underlying mechanisms remain poorly understood.
In fact, direct evidence for roles of the modifying enzymes in facilitating or impeding p53 interactions with the p53RE through remodeling and regulation of local chromatin structure are currently limited. One such example are the two H4K16 targeting acetyltransferases hMOF and TIP60, which when bound to p53 acetylate adjacent nucleosomes (25), and H4K16ac has been previously described as one of the only histone modification that directly alters local chromatin compaction (134–136). The breadth and depth of p53 interactions with chromatin regulatory proteins will certainly require an extensive, but necessary, unraveling to better understand how these factors are related to potential pioneer factor activity versus canonical transcriptional co-activator and co-repressor activities.
FUNCTIONAL CONSEQUENCES OF p53 BINDING IN VARIED GENOMIC CONTEXTS
Generally, transcriptional regulatory regions such as enhancers and promoters are believed to function through the combined activity of multiple TFs binding to accessible DNA elements (137,138) and can be characterized by their histone modification patterns and the presence of transcribed enhancer RNA (139). p53 binds to regulatory regions with these characteristics, but also pervasively binds to DNA that appears inaccessible and devoid of evidence suggestive of transcriptional regulatory potential (Figure 3B–E). Thus, a key outstanding question centers on understanding how local transcription factors and varying chromatin contexts of p53 binding relate to subsequent gene activation.
Recent work utilizing massively parallel reporter screens (MPRA) to assay the transcriptional activation potential of hundreds of p53 binding sites indicated that p53 alone can activate transcription of plasmid-based reporters (15). These data suggest that any p53 genomic binding event can lead to transcriptional activation as p53 would not need any other local transcription factors. This interpretation is supported by observation that the presence of other TF motifs surrounding a p53RE are not predictive of transcriptional activation upon p53 binding (27). Further support is found in the extensive evidence that p53 is a strong transcriptional activator in yeast, where other cooperating TFs are presumably absent (140,141). This model is intriguing, as it would suggest that p53 binding to all genomic contexts, including to nucleosomal or closed chromatin, can support transcriptional activation. Additional experimental approaches are necessary to determine whether these p53 binding events are productive in their native genomic and chromatin context.
Considerable evidence, however, suggests that p53 is unlikely to act in the ‘single factor model’ at all genomic binding sites. For example p53 frequently binds to regulatory regions such as enhancers and promoters, the canonical chromatin features of which are established independent of p53 activity (25–27,48,142). Furthermore, combinatorial activity of TFs with p53 has been extensively reported in the literature (143–148). Another MPRA approach provided additional evidence for the role of other TFs in regulating p53 transcriptional activity. (149). Screening the transcriptional activity of thousands of variants of p53-bound regulatory elements revealed that other motifs flanking a p53RE contribute to p53-dependent activation. One such example was binding of ATF3 and p53 to an enhancer element regulating expression of GDF15 (149). ATF3 and p53 have been previously shown to co-occupy many genomic locations, with ATF3 activity contributing to p53-dependent activation of numerous downstream targets (63,150). A novel CRISPR-based screen identified CEBPB as a key regulator of the p53-responsive enhancer controlling CDKN1A expression and the establishment of a senescent cell state (151). Analysis of this CRISPR screen using multiple machine learning approaches identified additional TF motifs predicted to contribute to the activity of enhancers bound by p53 (152). p53 binding to nucleosome rich loci correlates with a stronger adherence to a consensus p53RE, whereas p53 binding to regions with high accessibility are linked to lower scoring or non-canonical p53RE motifs (25,26). These observations might be related to the need for additional TFs to assist in establishing DNA accessibility and to facilitate binding of p53 to these elements to drive transcriptional activation. Additional evidence for the role of local chromatin accessibility and co-occupancy of other transcription factors on p53 activity is still needed, but the integration of high-throughput biochemical and genetic methods with computational approaches should help delineate the regulatory logic of p53-bound genomic loci.
Does p53, then, bind to regions with high nucleosome occupancy as a precursor to the eventual displacement of nucleosomes and establishment of DNA accessibility (Figure 3C)? Activation and binding of p53 has indeed been associated with reduced nucleosome occupancy at the CDKN1A promoter (90,153), and ChIP-chip analysis in the MCF7 cell line and recent ATAC-seq in primary fibroblasts have demonstrated that p53-dependent nucleosome displacement occurs in additional locations (27,94). Together, these results suggest that p53 binding to nucleosomal DNA can in certain contexts mediate chromatin accessibility and can alter transcriptional activation. However, analysis of the sequences encoding p53 regulated regions identified by ATAC-seq did not reveal any DNA sequence-based information that might suggest the context required for p53-mediated nucleosome eviction (27) consistent with other computational approaches (15). Yet, while p53 nucleosome binding can lead to eviction and establishment of accessible DNA elements, extensive data suggest that this activity is limited and context dependent. For example, in colorectal cells p53 sites associated with bona fide p53REs (CDKN1A, SFN) were accessible, both before and after p53 activation and with no change observed in p53-null cell lines (142). p53 binding sites often have pre-established histone modification patterns before p53 activation and in cells with genetic or biochemical depletion of p53 (25,26,48). Moreover, the large majority of p53 binding sites in dermal or lung fibroblasts were either accessible before p53 binding or were constitutively inaccessible even after binding (25,27). These data indicate that increased chromatin accessibility is not always a direct consequence of p53 binding and suggest a limited and context-dependent role for p53 in mediating chromatin accessibility (25–27).
Activation of transcription subsequent to p53 binding to TSS proximal sites has been suggested to differ for specific promoter elements (87,154) at least in part due to variation between p53REs (155–157). Recent single-molecule and cryo-EM experiments indicate that p53 mediates recruitment of the transcription pre-initiation complex member TFIID to central promoter elements that can be further affected by promoter sequence and thus alters stabilization of TFIID necessary for increasing transcription initiation (158). Interestingly, these studies also suggest that upon successful interaction of TFIID with DNA elements in the promoter, p53 dissociates from the p53RE. Thus, the rate of interaction and cycling of p53 interactions with different p53REs and their capacity to engage TFIID recruitment to cognate promoter sequences, likely plays an important role in differential binding and activation of p53 targets. In support of this model, promoters of p53 target genes show differential transcriptional activation kinetics in response to natural p53 protein pulses which may alter residence time and frequency of interaction with promoters (159). More recent work demonstrates that stochastic transcription of p53 target genes is controlled by transcription burst frequency, a parameter controlled by transcription initiation rates (160). Live cell imaging suggests that elevated p53 protein expression increases the likelihood a gene target will be transcribed (burst frequency), but does not influence the magnitude of the transcriptional response (burst size) (161). These data also suggest that affinity-based models of promoter activity are unlikely to fully describe p53 regulated transcription, with p53 CTD modifications controlling some of the observed kinetic differences between p53 target genes. It is thus unclear to what degree transcriptional activation kinetics are determined by p53 protein stability and modification state, intrinsic sequences at p53 binding sites, or other promoter-associated factors, as all have the strong potential to alter p53 interaction frequency and activation of promoters. Advances in single molecule imaging approaches and single-cell RNA velocity measurements have the potential to advance our understanding of how multiple local factors and sequence context alter p53’s ability to activate promoters and drive the observed kinetics in mRNA synthesis.
In addition, there is also evidence to suggest a significant number of p53 binding sites may not be directly associated with regulatory elements of genes, long non-coding RNAs, or micro-RNAs. The lack of traditional signifiers of transcriptional regulatory potential (such as particular histone modification patterns) might suggest these binding events are evolutionary remnants, could have context-dependent transcriptional roles, or could function in non-traditional roles. One possibility is that p53 might act locally to influence DNA repair and protect genome integrity, such as in the case where p53 binds to both human telomeres and other fragile genetic sites to influence chromatin structure that protects DNA from damage (64,162). Protection of these regions from DNA damage requires local p53-dependent transcription to establish the appropriate repressive heterochromatin features, but does not appear to require expression of any common p53 target gene. Further, p53 pervasively binds to p53REs derived from retrotransposons or other mobile genetic elements (34–38,163) and can suppress transcription of retroelements (164–167). The viral origin of these elements may also lead to an undercount of p53 binding sites in the human genome as these regions tend to be highly repetitive and thus difficult to identify using traditional ChIP-seq approaches. The p53-mediated mechanisms conferring protection from retroelements is also conserved through evolution, having recently been identified within Drosophila (167). Certainly, p53 has been shown to have other roles in DNA context, such as playing an important role in replication restart (168) and replication fork progression (169,170). The absence of these p53-dependent processes can lead to further genomic instability. While these p53 binding events do not appear to influence p53-dependent gene regulation, they have key roles in the maintenance of genome fidelity in the germline and tumor suppression.
PIONEER ACTIVITY OF p63 AND p73 AND THE IMPLICATIONS FOR p53
Full-length p63 (TAp63) functions as a haplo-insufficient tumor suppressor (171) that also ensures quality control in germ line cells (172). The shorter p63 isoform ΔNp63 is overexpressed or amplified in almost all squamous cancers and serves as master regulator of stratifying epithelia that is crucial for limb, mammary gland, prostate, and epidermal development (173). Like TAp63, full-length p73 (TAp73) is a haplo-insufficient tumor suppressor and it functions as a key regulator of neuronal development, multi-ciliated cell differentiation, and metabolism (171,174). The p53 family shares highly-related DNA binding domains with similar, but not identical, RE sequence preferences (88,175). Consequently, the p53 TF family shares many binding sites; however, all three family members also display substantial subsets of unique target genes (12,175,176). Understanding the interplay between p53, p63, and p73 is complicated by the differential function of several N- and C-terminal isoforms of all three family members that exhibit overlapping targets with often antagonistic function within and between the family members (177,178). We are only beginning to understand how distinct binding and gene regulation by the p53 family members and their isoforms is coordinated, a better understanding of which may shed light on outstanding questions highlighted above in relation to full-length canonical TAp53α.
Pioneer factors play crucial roles in organismal development and differentiation events, with many pioneer factors directing critical lineage determination events during development or cellular reprogramming (86,179). Mice lacking p53, while cancer prone like the human p53 mutation carrying Li-Fraumeni syndrome patients (180), generally develop normally, although variably penetrant developmental defects have been observed (181–183). This suggests that p53 and its putative pioneer factor activity are not strictly required for the establishment of chromatin states and transcriptional networks needed for proper development. In contrast, genetic evidence from mouse models and human genetically linked conditions/syndromes studies strongly indicate that the p53 family members p63 and p73 are key lineage determination factors for basal (p63) and ciliated (p73) epithelial cells (184–186). Recent studies suggest that the ΔNp63 isoform certainly shares the ability to bind to inaccessible chromatin and exhibits extensive capacity for opening chromatin at these sites (187–190), but unlike p53, this pioneer activity is critical for development (191). Specifically, p63 is required for the establishment of enhancer accessibility and activation of an extensive squamous cell-specific gene expression network (31,48,190,192). Moreover, p63-dependent chromatin remodeling is required for expression of key epithelial lineage-specific genes in two cellular reprogramming paradigms (33,188), and conversely depletion of p63 in terminally differentiated epithelial cell types led to decreases in histone modification abundance at regulatory regions (48). In epithelial keratinocytes, loss of p63 activity led to a nucleosome sliding into normally accessible regulatory regions, similar to the chromatin bookmarking activity previously proposed (187,192). Appropriate nucleosome structure at p63-bound regulatory regions was related to p63 interactions with BAF chromatin remodeling complex (187), providing a direct biochemical mechanism for these observed activities. Although not fully investigated, these data strongly suggest that the pioneer factor activity of p63 is essential for epithelial lineage determination and that developmental phenotypes associated with loss of p63 function may be due to decreased chromatin accessibility at regulatory regions.
Similarly, p73 is required for the establishment of ciliated epithelial cell identity through control of cell type-specific regulatory elements and gene expression (184,185), although direct binding to nucleosomal DNA has not yet been demonstrated. Why then does p63 (and potentially p73) have the ability to modulate chromatin accessibility in specific cell types while the broadly expressed p53 has a more limited role? The ability of p63 to initiate and maintain chromatin accessibility requires an intact C-terminal sterile alpha-motif (SAM) domain as disease-associated mutants in the p63 SAM domain fail to establish enhancer accessibility and activate downstream gene expression (33,191). Unlike p63 and p73, p53 lacks a SAM domain, suggesting this domain may be critical for the broad chromatin remodeling and pioneer activities of p63 and p73. What is clear is that the p53 family of TFs share a common ability to bind to nucleosomal DNA and mediate local chromatin remodeling. Continued investigation into the unique and shared mechanisms used by members of the p53 family of TFs to facilitate chromatin remodeling is likely to reveal significant insight into numerous biological processes and human disease states.
DISSECTING UBIQUITOUS AND CELL TYPE-SPECIFIC DIRECT p53 TARGET GENES
To date, identification of direct p53 target genes largely relies on correlating proximal p53 binding events, such as within 2.5 or 5 kb from the TSS, with genes significantly differentially expressed upon p53 activation. The identification is complicated by defining relevant thresholds for robust peak calling, distances to genes, and differential gene expression. Nonetheless, extensive genomic and transcriptomic analyses have enabled the identification of common human p53 target genes that exist across multiple cell contexts, such as cell type, chromatin/epigenetic state, activating signal, and time (6,8,9). These ubiquitous p53 target genes are all up-regulated in response to p53 activation supporting the view that p53 primarily functions in gene activation (14). These ubiquitous targets could be examined in the context of the pioneering function of p53, especially since in the absence of stress a number of these p53 target genes are also pre-bound, and whether chromatin accessibility affects gene transcription. Thus, ubiquitous p53 targets could provide a more effective assessment of p53 function in human cancers not based only on TP53 gene mutations, particularly since p53 can be inactivated in cancers even though it is not mutated (193). In support of this idea, it has been shown that sensitivity of cell lines to an MDM2 inhibitor could be predicted by a set of 13 direct p53 target genes (194), most of which represent ubiquitous p53 targets. Although the study received critique for misclassifying the p53 status of several cell lines (195), the authors amended their analyses and provided evidence that after revising the annotation of cell lines’ p53 status, the gene set still was at least as predictive as the p53 status was (196). A more recent set of 175 genes predicting sensitivity to MDM2 inhibition (197) also contains several ubiquitous p53 targets. In contrast to ubiquitous direct p53 target genes that are normally up-regulated, cell cycle genes are commonly down-regulated by p53 and a recent analysis of data from The Cancer Genome Atlas showed that, similar to most cancers (198), p53 mutant cancers display up-regulation of cell cycle genes with a 4-gene subset promising prognostic value (193). The ubiquitous p53 target genes could also act as biomarkers to assess the effectiveness of standard chemotherapeutics that lead to p53 activation. The use of ubiquitous p53 target genes as biomarkers will also be beneficial in establishing the potential efficacy of cancer therapies focused on reestablishing wild-type p53 activity in cancers with mutant or aggregation-prone p53 (199,200). It is currently unknown the extent to which these drugs restore full, wild-type function and activate the same wild-type target genes.
In addition to TF binding events proximal to TSS, it is well-established that TF binding to enhancers affects the expression of many genes (201). Within the ubiquitous set of p53 binding events, only ∼30% map to the proximal 5 kb surrounding annotated TSS (6). While a strong correlation between the presence of a gene proximal p53 binding event and increased transcriptional activity of that gene has been reported (8,9,202), binding near a TSS appears to be neither necessary nor sufficient for gene activation. Certainly, close proximity to promoters (either near the TSS or within the first intron) is a well-described and accepted feature of p53 binding events associated with canonical p53-induced genes. However, while many such events are detected in meta-analyses of binding sites alone (15) and their integration with gene expression studies (6,8,9,14), the majority (∼70%) of p53 binding sites are detected in gene distal regions (Figure 3) (11,12,25,26,61,69,73,81). Recent analysis of the relationship between p53 binding sites and their distance to the nearest p53-regulated gene also suggested that p53 binding events within 2.5 kb of a TSS are much more likely to predict transcriptional activation and the number of p53 binding events beyond this distance with experimentally verified trans-activation potential is low (9). Directly connecting distal elements to transcription is technically challenging and we have likely severely underestimated the contribution of distal p53 binding events in gene regulation. Advances in computational and experimental approaches have improved our ability to make these connections. Genome-scale and targeted chromatin conformation capture approaches can identify potential distal p53 binding site interactions with target gene promoters. The chromatin conformation capture (3C) approach identified a p53 binding element, which regulates the expression of three pro-apoptotic genes in Drosophila over a distance of nearly 330 kb (203). Use of the 4C (3C on chip) approach identified dynamic reorganization of chromatin contacts across a 100kB region centered on CDKN1A after p53 stabilization (204), suggesting that looping and structure of chromatin domains are involved in p53-dependent gene regulation. As previously discussed, the use of locus-specific transcriptional repressors based on CRISPR/Cas9 technology revealed multiple distal p53 binding sites over 200kb from the nearest gene that were required for p53-dependent cell cycle arrest (30), suggesting p53 can operate over long genomic distances. These experimental approaches, along with advancements in data curation such as the GeneHancer, FOCS, InTAD, and HACER projects (205–208), provide a rational basis for the future inquiry into the role of distal p53 binding sites in the regulation of gene expression. Recent efforts integrating TF binding, histone modification, and gene expression profiling data indicate that p53 may indeed function over long genomic distances (209). Given the frequency of distal p53 binding events and the general lack of information regarding their spatial configuration within the nucleus, the universe of p53 target genes will inevitably become larger and its regulation more complex. Much work remains to understand potential longer-range regulatory events and regulation of non-coding RNA-species, including how topologically associating domains influence p53-dependent activities and phenotypes across cell types, tissues, and organisms. (210,211).
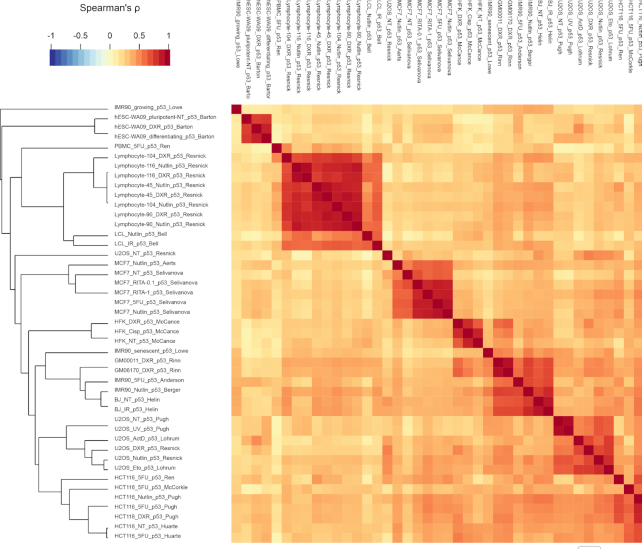
Beyond the ubiquitous p53 binding sites and target genes, unsupervised clustering demonstrates greater similarity within one cell type than with a common treatment for both DNA binding by p53 (Figure 5) and direct p53 target genes (6). Thus, while there are many shared binding sites and target genes between cells and conditions, there are also strong cell-type/fate differences. For example, in human embryonic stem cells (hESC) p53 binding and target gene activation differed between retinoic acid-induced differentiation and DNA damage conditions, suggesting the treatment affected cell fate to endow the p53-binding profile of a ‘different cell type’ (51). In addition, p53-dependent gene expression also differed between hESC and human mesenchymal stem cells (hMSC), with the primary difference attributed to chromatin modification patterns at p53-bound promoters (50).
Figure 5.
Hierarchical clustering of read coverage signal across p53 peaks that appear in ≥20 datasets was performed considering the Spearman correlation. Clustering results are displayed in a heatmap; the color of individual cells describes the correlation value between two datasets and the cluster distances between each sample shown using a dendrogram. Data from (6).
These differential effects of p53 activation are likely a combination of tissue specific accessibility of p53 target and differential ‘priming’ of cell types for cell death (212), as has recently been shown with HDAC inhibitors that synergistically induce p53-dependent cell death in combination with Nutlin-3a or chemotherapy. This is mediated through HDAC inhibitors blocking activation of a subset of p53 targets including the pro-survival caspase-8 inhibitor FLIP, which releases the brake on ‘priming’ of pro-apoptotic targets TRAILR2, BAX, and PUMA (130). Similarly, treatment of MCF7 cells with the DNA methylation inhibitor decitabine led to de novo chromatin accessibility and p53 binding, suggesting that changes in cell state and pharmaceutical treatments can alter the binding and activity of p53 (52). This study also showed that accessible chromatin differences between cell types influenced p53 occupancy and correlates with unique cell type-specific binding events (52). Cell type-specific chromatin accessibility is likely related to the observed differential chromatin modification state at p53 binding sites. How chromatin accessibility and modification state differ across cell types or after drug treatments and their influence on p53 occupancy requires more investigation. Future experiments utilizing ATAC-seq or NoMe-seq (nucleosome occupancy and methylome sequencing) in these cells may provide more information to identify cell type- and fate-specific features (213,214). Together, these data suggest that p53 recruitment may be affected by ‘availability’ of p53REs defined by tissue-specific regulatory elements expanding the repertoire of genes that can be affected by p53 binding events, as has been shown for p63 (12). Notably, differential gene regulation irrespective of p53 binding shows treatment-specific instead of cell type-specific clusters (8). This is likely influenced by biological phenomena related to additional signals as well as temporal and treatment-specific effects.
In vivo studies in Drosophila and mice demonstrate tissue-specific transcriptional responses after exposure to similar p53-activating stimuli (215,216). The overlap in p53-dependent gene expression is minimal between Drosophila tissues (215), suggesting a permissive role for cell type in the p53 response. Similarly, p53 activation in disparate tissue types in the developing mouse embryo yielded different cell fates, such as the classical choice between apoptosis and cell cycle arrest, but regulates the same set of genes in different mouse tissues (216). Intriguingly, the magnitude of p53-dependent gene induction or repression appears to be a major difference between the tissues and may play a role in the disparity in cell phenotypes. Developmental timing can also alter p53-dependent target gene expression and cell fates. Pre-migratory and migrating neural crest are highly sensitive to p53-dependent apoptosis, whereas those cells that reached their final destination in the pharyngeal arch were highly resistant to apoptosis triggered by p53 (216). These data suggest that some of the differences between p53-dependent outcomes may be due to developmental timing and differences in p53 target gene activation between cell types, both of which are likely influenced by gene regulatory elements and local chromatin structure.
In a similar fashion to chromatin structure, DNA methylation likely plays a regulatory role in the control of p53-dependent transcription. Among the occupied sites that are common across cell types and near TSS, 40% are not associated with differential expression of the nearest gene, suggesting that p53 binding alone in many cases is not sufficient to drive expression of the nearest gene but that additional information and regulatory mechanisms can be required (6). These might include mechanisms regulating mRNA half-life, protein degradation, and protein folding/activation. For example, the Toll like receptor-8 (TLR8) gene, which is a direct p53 target, is undetectable in cancer cells possibly because of promoter methylation (personal observation). Other direct p53 targets such as IGFBP7 (217), 14–3-3 σ (also known as SFN) (218,219), and the long non-coding RNA TP53TG1 (220) have also been shown to be hypermethylated in cancer. Additionally, viruses may also use this mechanism to silence downstream p53 targets (221), suggesting that methylation or other epigenetic modifications might affect differential cell type responses to p53 activation.
EVOLUTION OF THE p53 GENE REGULATORY NETWORK IN VERTEBRATES
All p53 protein family members contain a highly conserved DBD (222), and p53 homologs from multiple species have been shown to specifically trans-activate common p53REs (223). Similar to p53 family proteins, most TFs display evolutionary conservation of their sequence-specific DNA binding (224–226). Conservation of protein function is key when animal models are employed to understand mechanisms that underlie human diseases. In case of p53, mouse models are frequently used to unravel the p53 signaling pathway and to inform anti-cancer strategies (183,227,228). Gene regulatory networks of many TFs, however, have diverged substantially between mouse and human (229). For TFs investigated in the ENCODE project only an average of 44% of regulatory TF to gene associations have been identified as conserved between mouse and human (224). Consistently, early studies comparing p53REs of several direct p53 target genes identified only limited conservation across species (230,231). The small number of genes reported to differ in their p53-dependent regulation between mice and humans included the well-established p53 targets GADD45A, RRM2B, DDB2, APAF1, XPC, and PCNA, which were identified as direct p53 targets in human (231–234) but not in mouse (231,234). Another example includes the LIF gene that functions in reproduction and that is a direct p53 target gene in human (235), but not in elephants (236). Instead, elephant p53 directly regulates the LIF pseudogene LIF6, which functions in apoptosis rather than in reproduction (236). Elephants also possess up to 18 copies of p53 pseudogenes which are under positive selection, suggesting functionality in the evolution of cancer resistance in this long-lived mammal (237,238). In blind mole rats, p53 carries an R174K substitution identical to known tumor-associated mutations and is unable to induce multiple pro-apoptotic genes, such as APAF1, but hyperactivates cell cycle arrest genes. Presumably, this enables blind mole rat cells to favor a reversible cell cycle arrest over hypoxia-induced apoptosis (239). A recent study provided a meta-analysis of genome-wide p53 binding data and p53-dependent transcriptome regulation data from mouse and compared the mouse data to similar data of human. Strikingly, >1000 genes have been identified to differ substantially in their response to p53 activation and these genes largely function in DNA damage response, metabolism, and cell cycle regulation (20). Thus, the p53-dependent regulation of these processes may at least partially differ between mouse and human.
Mechanistically, the comprehensive comparison of the p53 GRN between mouse and human revealed that evolutionary turnover of p53REs underlies broad variation in p53 binding profiles that are causative for different p53-dependent gene up-regulation (20). Concordantly, evolutionary turnover of p63REs and p63 binding was found to substantially alter the p63 GRN (240). Taking only genomic regions into account that are present in both human and mouse, the meta-analysis found less than 13% of p53 binding sites to be conserved between the two species (20). This number is even smaller when also mouse- and human-specific genome regions are considered. For example, it was reported that p53REs can be shaped by long terminal repeats from endogenous retroviruses (34,35), long interspersed nuclear repeats (LINEs; (37)), and ALU repeats (36,38) in humans and fuzzy tandem repeats in mice (163,241). It was shown that p53 oscillates faster in mouse and rat cells than in cells from human, monkey, or dog. It is hypothesized that faster p53 oscillations in mouse cells are triggered by a stronger negative feedback loop between p53 and MDM2, which in turn may be caused by a subtle change in the p53RE controlling the expression of MDM2 (242). Different p53 oscillation patterns influence p53-dependent gene expression (74), but to what extent the different p53 oscillation patterns between mouse and human contributes to differences in the p53 GRN remains unclear. Interestingly, p53 and p63 binding sites have undergone more evolutionary turnover as compared to binding sites of the DREAM complex (20) or most TFs that have been investigated by ENCODE (224). A possible explanation for the high turnover could be the exceptionally large size of the p53 and p63 REs, which contain two decameric half sites totaling 20 bp. TF recognition motifs that exceed 10 base pairs show substantially decreased evolutionary stability (243,244), and thus the long p53 and p63 REs may be altered more rapidly during evolution.
The lack of evolutionary conservation of a number of important direct p53 target genes suggests that regulating these individual genes may not be critical or can be compensated by alternative p53-regulated genes in the same pathway to conserve p53’s tumor suppressor function. For example, trans-activation-mutants of p53 that could up-regulate only subsets of direct p53 target genes were sufficient to suppress spontaneous tumor development in mice (245). Similarly, mice expressing a triple acetylation mutant form of p53 (p533KR), which is unable to up-regulate many direct p53 target genes, are also protected from spontaneous tumor development (246). Interestingly, a triple-knockout of the conserved key p53 target genes p21 (also known as Cdkn1A), Puma (Bbc3), and Noxa (Pmaip1) was not sufficient to impair p53’s tumor suppressor function (247). In addition to the set of 86 evolutionary conserved direct p53 target genes that has been identified (20), species-specific direct p53 targets may contribute substantially to the tumor suppressor function of p53. Particularly noteworthy are mouse- and human-specific direct p53 target genes that can serve a similar function in the p53 response. The Y-family translesion DNA synthesis polymerases POLH and POLK can both allow for DNA replication despite the presence of DNA damage (248). In human solely POLH and in mouse solely Polk was identified as direct p53 target gene (20). Together the findings suggest that many species-specific direct p53 target genes may be dispensable for the tumor suppressor function of p53, and in some cases p53 employs different species-specific targets to serve a similar function. However, it is important to note that only limited analyses of epithelial tissue and in tumor initiating contexts have been conducted to date in mouse and our assumptions of target conservation may as a result be slightly underestimated.
Mouse models serve as the gold standard in basic cancer research, but it is known that their translation potential to human is limited (249–251). For example, mice expressing p533KR displayed impaired apoptosis, cell cycle arrest, and senescence but retained the ability to regulate metabolic genes, which may have protected these mice from spontaneous tumor development (246). However, p53-regulated genes involved in metabolic control have been particularly subjected to alterations in their p53 response between mouse and human (20). Thus, p53’s ability to control metabolic processes is different in human compared to mouse and may not be sufficient to suppress tumor formation in humans.
The assumption with mouse models has been that p53 functions largely similar to human. While p53 is undoubtedly a tumor suppressor across mammals, the specific genes and regulatory networks required for this broad activity are quite different. Consequently, single nucleotide variations in p53REs and p53 mutants could have very different effects in humans than in mice. In turn, a drug acting on the p53 signaling pathway could affect a mouse very differently than a human. If we want to translate findings from animal models to humans more efficiently, we have to understand their differences more precisely. The marked differences that have been revealed encourage the use of advanced animal and cell culture models. While in clinical research patient derived xenografts and humanized mouse models help to narrow the gap between animal and human, in basic research organoids are promising tools to generate more meaningful data.
CONCLUSION
The last decade of research has greatly expanded our understanding of how p53 functions as a transcription factor and shed light on the composition of its GRN, but as highlighted above, it is clear that much remains to be uncovered to fully understand the molecular mechanisms that shape the p53 GRN in a given cell or tissue. Further investigation of the role of distal p53 binding sites in the regulation of gene expression, especially the role of local and long-range enhancer:gene interactions, will be necessary to fully assess the direct transcriptional effects elicited upon p53 binding to DNA (Figure 3D). Future work on identifying TFs cooperating with p53 to establish transcriptional networks, such as those working downstream of p53 activation, will expand our understanding of the expansive gene regulatory repertoire (Figure 1).
Despite the vast body of work, it is still unclear how p53 activation induces differential cell fates at a single cell level and how this is affected by cellular identity, stage of cell cycle, and concurrent activation of other stress induced survival/death programs. This will inevitably be enlightened by studies using cutting edge technologies to evaluate single-cell transcriptional dynamics. Such studies coupled with other high throughput modalities like modern 3D organoid culture will be crucial to dissect cell/tissue and condition/treatment-specific p53 activity. This will in turn help us to understand how p53, its isoforms, and family members function as pioneer factors and in other non-canonical roles associated with their interaction with the genome. Cumulatively, these insights will allow us to better understand what these diverse activities of p53 might mean for its tumor suppressor and non-tumor suppressor role, enable us to exploit p53 de-regulation in cancer; as well as providing a more complete picture on emergent roles for the p53 family in cell lineage commitment, cell lineage transitions in cancer, stem cell reprogramming, and developmental disorders.
ACKNOWLEDGEMENTS
We thank Carl W. Anderson and Daniel Menendez for critical feedback on the manuscript. We thank Sara A. Grimm and C. Andy Lavender of the Integrative Bioinformatics core at NIEHS for assistance with Figure 5 and Table 1.
Contributor Information
Morgan A Sammons, Department of Biological Sciences and The RNA Institute, University at Albany, State University of New York, 1400 Washington Avenue, Albany, NY 12222, USA.
Thuy-Ai T Nguyen, Genome Integrity & Structural Biology Laboratory and Immunity, Inflammation and Disease Laboratory, National Institute of Environmental Health Sciences/National Institutes of Health, 111 TW Alexander Drive, Research Triangle Park, NC 27709, USA.
Simon S McDade, Patrick G Johnston Centre for Cancer Research, Queen's University Belfast, 97 Lisburn Road, Belfast BT9 7AE, UK.
Martin Fischer, Computational Biology Group, Leibniz Institute on Aging – Fritz Lipmann Institute (FLI), Beutenbergstraße 11, 07745 Jena, Germany.
FUNDING
M.A.S. is supported by the National Institutes of Health (NIH) [R15 GM128049]; T.T.N. and her research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (NIEHS) [Z01-ES065079, Z01-ES100557, in part]; S.S.M. is supported by Cancer Research UK (CRUK) [C11884/A24367]; Biotechnology and Biological Sciences Research Council (BBSRC) [BB/T002824/1]; Prostate Cancer UK (PCUK)-Movember Centre of Excellence; M.F. is supported by the German Research Foundation (DFG) [FI 1993/2–1]. Funding for open access charge: Leibniz Institute on Aging - Fritz Lipmann Institute (FLI); The FLI is a member of the Leibniz Association and is financially supported by the Federal Government of Germany and the State of Thuringia.
Conflict of interest statement. None declared.
REFERENCES
- 1. Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R., Meyerson M., Gabriel S.B., Lander E.S., Getz G.. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014; 505:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-cancer analysis of whole genomes. Nature. 2020; 578:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belyi V.A., Ak P., Markert E., Wang H., Hu W., Puzio-Kuter A., Levine A.J.. The origins and evolution of the p53 family of genes. Cold Spring Harb. Perspect. Biol. 2010; 2:a001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rutkowski R., Hofmann K., Gartner A.. Phylogeny and function of the invertebrate p53 superfamily. Cold Spring Harb. Perspect. Biol. 2010; 2:a001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer M. Census and evaluation of p53 target genes. Oncogene. 2017; 36:3943–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen T.-A.T., Grimm S.A., Bushel P.R., Li J., Li Y., Bennett B.D., Lavender C.A., Ward J.M., Fargo D.C., Anderson C.W. et al.. Revealing a human p53 universe. Nucleic Acids Res. 2018; 46:8153–8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouaoun L., Sonkin D., Ardin M., Hollstein M., Byrnes G., Zavadil J., Olivier M.. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum. Mutat. 2016; 37:865–876. [DOI] [PubMed] [Google Scholar]
- 8. Fischer M., Grossmann P., Padi M., DeCaprio J.A.. Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F target gene analyses identifies cell cycle gene regulatory networks. Nucleic Acids Res. 2016; 44:6070–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrysik Z., Galbraith M.D., Guarnieri A.L., Zaccara S., Sullivan K.D., Pandey A., MacBeth M., Inga A., Espinosa J.M.. Identification of a core TP53 transcriptional program with highly distributed tumor suppressive activity. Genome Res. 2017; 27:1645–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kenzelmann Broz D., Mello S.S., Bieging K.T., Jiang D., Dusek R.L., Brady C.A., Sidow A., Attardi L.D.. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013; 27:1016–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schlereth K., Heyl C., Krampitz A.M., Mernberger M., Finkernagel F., Scharfe M., Jarek M., Leich E., Rosenwald A., Stiewe T.. Characterization of the p53 Cistrome - DNA Binding Cooperativity Dissects p53’s Tumor Suppressor Functions. PLoS Genet. 2013; 9:e1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDade S.S., Patel D., Moran M., Campbell J., Fenwick K., Kozarewa I., Orr N.J., Lord C.J., Ashworth A.A., McCance D.J.. Genome-wide characterization reveals complex interplay between TP53 and TP63 in response to genotoxic stress. Nucleic Acids Res. 2014; 42:6270–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen M.A., Andrysik Z., Dengler V.L., Mellert H.S., Guarnieri A., Freeman J.A., Sullivan K.D., Galbraith M.D., Luo X., Kraus W.L. et al.. Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. Elife. 2014; 3:e02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer M., Steiner L., Engeland K.. The transcription factor p53: Not a repressor, solely an activator. Cell Cycle. 2014; 13:3037–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verfaillie A., Svetlichnyy D., Imrichova H., Davie K., Fiers M., Atak Z.K., Hulselmans G., Christiaens V., Aerts S.. Multiplex enhancer-reporter assays uncover unsophisticated TP53 enhancer logic. Genome Res. 2016; 26:882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer M., Quaas M., Steiner L., Engeland K.. The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res. 2016; 44:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischer M., Müller G.A.. Cell cycle transcription control: DREAM/MuvB and RB-E2F complexes. Crit. Rev. Biochem. Mol. Biol. 2017; 52:638–662. [DOI] [PubMed] [Google Scholar]
- 18. Uxa S., Bernhart S.H., Mages C.F.S., Fischer M., Kohler R., Hoffmann S., Stadler P.F., Engeland K., Müller G.A.. DREAM and RB cooperate to induce gene repression and cell-cycle arrest in response to p53 activation. Nucleic Acids Res. 2019; 47:9087–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schade A.E., Fischer M., DeCaprio J.A.. RB, p130 and p107 differentially repress G1/S and G2/M genes after p53 activation. Nucleic Acids Res. 2019; 47:11197–11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer M. Conservation and divergence of the p53 gene regulatory network between mice and humans. Oncogene. 2019; 38:4095–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benson E.K., Mungamuri S.K., Attie O., Kracikova M., Sachidanandam R., Manfredi J.J., Aaronson S.A.. p53-dependent gene repression through p21 is mediated by recruitment of E2F4 repression complexes. Oncogene. 2014; 33:3959–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fischer M., Quaas M., Nickel A., Engeland K.. Indirect p53-dependent transcriptional repression of Survivin, CDC25C, and PLK1 genes requires the cyclin-dependent kinase inhibitor p21/CDKN1A and CDE/CHR promoter sites binding the DREAM complex. Oncotarget. 2015; 6:41402–41417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat. Rev. Cancer. 2012; 12:613–626. [DOI] [PubMed] [Google Scholar]
- 24. Grossi E., Sánchez Y., Huarte M.. Expanding the p53 regulatory network: LncRNAs take up the challenge. Biochim. Biophys. Acta - Gene Regul. Mech. 2016; 1859:200–208. [DOI] [PubMed] [Google Scholar]
- 25. Sammons M.A., Zhu J., Drake A.M., Berger S.L.. TP53 engagement with the genome occurs in distinct local chromatin environments via pioneer factor activity. Genome Res. 2015; 25:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su D., Wang X., Campbell M.R., Song L., Safi A., Crawford G.E., Bell D.A.. Interactions of chromatin context, binding site sequence content, and sequence evolution in stress-induced p53 occupancy and transactivation. PLoS Genet. 2015; 11:e1004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Younger S.T., Rinn J.L.. p53 regulates enhancer accessibility and activity in response to DNA damage. Nucleic Acids Res. 2017; 45:9889–9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melo C.A., Drost J., Wijchers P.J., van de Werken H., de Wit E., Vrielink J.A.F.O., Elkon R., Melo S.A., Léveillé N., Kalluri R. et al.. ERNAs are required for p53-dependent enhancer activity and gene transcription. Mol. Cell. 2013; 49:524–535. [DOI] [PubMed] [Google Scholar]
- 29. Léveillé N., Melo C.A., Rooijers K., Díaz-Lagares A., Melo S.A., Korkmaz G., Lopes R., Akbari Moqadam F., Maia A.R., Wijchers P.J. et al.. Genome-wide profiling of p53-regulated enhancer RNAs uncovers a subset of enhancers controlled by a lncRNA. Nat. Commun. 2015; 6:6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borys S.M., Younger S.T.. Identification of functional regulatory elements in the human genome using pooled CRISPR screens. BMC Genomics. 2020; 21:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDade S.S., Henry A.E., Pivato G.P., Kozarewa I., Mitsopoulos C., Fenwick K., Assiotis I., Hakas J., Zvelebil M., Orr N. et al.. Genome-wide analysis of p63 binding sites identifies AP-2 factors as co-regulators of epidermal differentiation. Nucleic Acids Res. 2012; 40:7190–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kouwenhoven E.N., van Heeringen S.J., Tena J.J., Oti M., Dutilh B.E., Alonso M.E., de la Calle-Mustienes E., Smeenk L., Rinne T., Parsaulian L. et al.. Genome-wide profiling of p63 DNA-binding sites identifies an element that regulates gene expression during limb development in the 7q21 SHFM1 locus. PLoS Genet. 2010; 6:e1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin-Shiao E., Lan Y., Welzenbach J., Alexander K.A., Zhang Z., Knapp M., Mangold E., Sammons M., Ludwig K.U., Berger S.L.. p63 establishes epithelial enhancers at critical craniofacial development genes. Sci. Adv. 2019; 5:eaaw0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang T., Zeng J., Lowe C.B., Sellers R.G., Salama S.R., Yang M., Burgess S.M., Brachmann R.K., Haussler D.. Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc. Natl. Acad. Sci. U.S.A. 2007; 104:18613–18618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bourque G., Leong B., Vega V.B., Chen X., Lee Y.L., Srinivasan K.G., Chew J.-L., Ruan Y., Wei C.-L., Ng H.H. et al.. Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res. 2008; 18:1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zemojtel T., Kielbasa S.M., Arndt P.F., Chung H.R., Vingron M.. Methylation and deamination of CpGs generate p53-binding sites on a genomic scale. Trends Genet. 2009; 25:63–66. [DOI] [PubMed] [Google Scholar]
- 37. Harris C.R., DeWan A., Zupnick A., Normart R., Gabriel A., Prives C., Levine A.J., Hoh J.. P53 responsive elements in human retrotransposons. Oncogene. 2009; 28:3857–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cui F., Sirotin M.V., Zhurkin V.B.. Impact of Alu repeats on the evolution of human p53 binding sites. Biol. Direct. 2011; 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tonelli C., Morelli M.J., Sabò A., Verrecchia A., Rotta L., Capra T., Bianchi S., Campaner S., Amati B.. Genome-wide analysis of p53-regulated transcription in Myc-driven lymphomas. Oncogene. 2017; 36:2921–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thurman R.E., Rynes E., Humbert R., Vierstra J., Maurano M.T., Haugen E., Sheffield N.C., Stergachis A.B., Wang H., Vernot B. et al.. The accessible chromatin landscape of the human genome. Nature. 2012; 489:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J., Zhuang J., Iyer S., Lin X., Whitfield T.W., Greven M.C., Pierce B.G., Dong X., Kundaje A., Cheng Y. et al.. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res. 2012; 22:1798–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neph S., Vierstra J., Stergachis A.B., Reynolds A.P., Haugen E., Vernot B., Thurman R.E., John S., Sandstrom R., Johnson A.K. et al.. An expansive human regulatory lexicon encoded in transcription factor footprints. Nature. 2012; 489:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Project Consortium E. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012; 489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roadmap Epigenomics Consortium Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A., Heravi-Moussavi A., Kheradpour P., Zhang Z., Wang J. et al.. Integrative analysis of 111 reference human epigenomes. Nature. 2015; 518:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nie Y., Cheng X., Chen J., Sun X.. Nucleosome organization in the vicinity of transcription factor binding sites in the human genome. BMC Genomics. 2014; 15:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu F., Farnung L., Kaasinen E., Sahu B., Yin Y., Wei B., Dodonova S.O., Nitta K.R., Morgunova E., Taipale M. et al.. The interaction landscape between transcription factors and the nucleosome. Nature. 2018; 562:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Younger S.T., Kenzelmann-Broz D., Jung H., Attardi L.D., Rinn J.L.. Integrative genomic analysis reveals widespread enhancer regulation by p53 in response to DNA damage. Nucleic Acids Res. 2015; 43:4447–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karsli Uzunbas G., Ahmed F., Sammons M.A.. Control of p53-dependent transcription and enhancer activity by the p53 family member p63. J. Biol. Chem. 2019; 294:10720–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ernst J., Kellis M.. Large-scale imputation of epigenomic datasets for systematic annotation of diverse human tissues. Nat. Biotechnol. 2015; 33:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Itahana Y., Zhang J., Göke J., Vardy L.A., Han R., Iwamoto K., Cukuroglu E., Robson P., Pouladi M.A., Colman A. et al.. Histone modifications and p53 binding poise the p21 promoter for activation in human embryonic stem cells. Sci. Rep. 2016; 6:28112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akdemir K.C., Jain A.K., Allton K., Aronow B., Xu X., Cooney A.J., Li W., Barton M.C.. Genome-wide profiling reveals stimulus-specific functions of p53 during differentiation and DNA damage of human embryonic stem cells. Nucleic Acids Res. 2014; 42:205–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hafner A., Kublo L., Tsabar M., Lahav G., Stewart-Ornstein J.. Identification of universal and cell-type specific p53 DNA binding. BMC Mol. Cell Biol. 2020; 21:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma Q., Yang F., Mackintosh C., Jayani R.S., Oh S., Jin C., Nair S.J., Merkurjev D., Ma W., Allen S. et al.. Super-enhancer redistribution as a mechanism of broad gene dysregulation in repeatedly drug-treated cancer cells. Cell Rep. 2020; 31:107532. [DOI] [PubMed] [Google Scholar]
- 54. Williams K., Christensen J., Rappsilber J., Nielsen A.L., Johansen J.V., Helin K.. The histone lysine demethylase JMJD3/KDM6B is recruited to p53 bound promoters and enhancer elements in a p53 dependent manner. PLoS One. 2014; 9:e96545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Drost J., Mantovani F., Tocco F., Elkon R., Comel A., Holstege H., Kerkhoven R., Jonkers J., Voorhoeve P.M., Agami R. et al.. BRD7 is a candidate tumour suppressor gene required for p53 function. Nat. Cell Biol. 2010; 12:380–389. [DOI] [PubMed] [Google Scholar]
- 56. Rashi-Elkeles S., Warnatz H.-J., Elkon R., Kupershtein A., Chobod Y., Paz A., Amstislavskiy V., Sultan M., Safer H., Nietfeld W. et al.. Parallel profiling of the transcriptome, cistrome, and epigenome in the cellular response to ionizing radiation. Sci. Signal. 2014; 7:doi:10.1126/scisignal.2005032. [DOI] [PubMed] [Google Scholar]
- 57. Kong L.R., Ong R.W., Tan T.Z., Nur A., Salleh M., Thangavelu M., Chan J.V., Yong L., Koh J., Periyasamy G. et al.. Targeting codon 158 p53-mutant cancers via the induction of p53 acetylation. Nat. Commun. 2020; 11:2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zeron-Medina J., Wang X., Repapi E., Campbell M.R., Su D., Castro-Giner F., Davies B., Peterse E.F.P., Sacilotto N., Walker G.J. et al.. A polymorphic p53 response element in KIT ligand influences cancer risk and has undergone natural selection. Cell. 2013; 155:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sánchez Y., Segura V., Marín-Béjar O., Athie A., Marchese F.P., González J., Bujanda L., Guo S., Matheu A., Huarte M.. Genome-wide analysis of the human p53 transcriptional network unveils a lncRNA tumour suppressor signature. Nat. Commun. 2014; 5:5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang G.S., Chen X.A., Park B., Rhee H.S., Li P., Han K.H., Mishra T., Chan-Salis K.Y., Li Y., Hardison R.C. et al.. A comprehensive and high-resolution genome-wide response of p53 to stress. Cell Rep. 2014; 8:514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Botcheva K., McCorkle S.R.. Cell context dependent p53 genome-wide binding patterns and enrichment at repeats. PLoS One. 2014; 9:e113492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang B., Niu D., Lam T.H., Xiao Z., Ren E.C.. Mapping the p53 transcriptome universe using p53 natural polymorphs. Cell Death Differ. 2014; 21:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao J., Li X., Guo M., Yu J., Yan C.. The common stress responsive transcription factor ATF3 binds genomic sites enriched with p300 and H3K27ac for transcriptional regulation. BMC Genomics. 2016; 17:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tutton S., Azzam G.A., Stong N., Vladimirova O., Wiedmer A., Monteith J.A., Beishline K., Wang Z., Deng Z., Riethman H. et al.. Subtelomeric p53 binding prevents accumulation of DNA damage at human telomeres. EMBO J. 2016; 35:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Desantis A., Bruno T., Catena V., De Nicola F., Goeman F., Iezzi S., Sorino C., Gentileschi M.P., Germoni S., Monteleone V. et al.. Che-1 modulates the decision between cell cycle arrest and apoptosis by its binding to p53. Cell Death Dis. 2015; 6:e1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen S., Wu J., Zhong S., Li Y., Zhang P., Ma J., Ren J., Tan Y., Wang Y., Au K.F. et al.. IASPP mediates p53 selectivity through a modular mechanism fine-tuning DNA recognition. Proc. Natl. Acad. Sci. U.S.A. 2019; 116:17470–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Menietti E., Xu X., Ostano P., Joseph J.M., Lefort K., Dotto G.P.. Negative control of CSL gene transcription by stress/DNA damage response and p53. Cell Cycle. 2016; 15:1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chan C., Thurnherr T., Wang J., Gallart-Palau X., Sze S.K., Rozen S., Lee C.G.. Global re-wiring of p53 transcription regulation by the hepatitis B virus X protein. Mol. Oncol. 2016; 10:1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Botcheva K., McCorkle S.R., McCombie W.R., Dunn J.J., Anderson C.W.. Distinct p53 genomic binding patterns in normal and cancer-derived human cells. Cell Cycle. 2011; 10:4237–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Catizone A.N., Good C.R., Alexander K.A., Berger S.L., Sammons M.A.. Comparison of genotoxic versus nongenotoxic stabilization of p53 provides insight into parallel stress-responsive transcriptional networks. Cell Cycle. 2019; 18:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kirschner K., Samarajiwa S.A., Cairns J.M., Menon S., Pérez-Mancera P.A., Tomimatsu K., Bermejo-Rodriguez C., Ito Y., Chandra T., Narita M. et al.. Phenotype specific analyses reveal distinct regulatory mechanism for chronically activated p53. PLoS Genet. 2015; 11:e1005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aksoy O., Chicas A., Zeng T., Zhao Z., McCurrach M., Wang X., Lowe S.W.. The atypical E2F family member E2F7 couples the p53 and RB pathways during cellular senescence. Genes Dev. 2012; 26:1546–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nikulenkov F., Spinnler C., Li H., Tonelli C., Shi Y., Turunen M., Kivioja T., Ignatiev I., Kel A., Taipale J. et al.. Insights into p53 transcriptional function via genome- wide chromatin occupancy and gene expression analysis. Cell Death Differ. 2012; 19:1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hafner A., Stewart-Ornstein J., Purvis J.E., Forrester W.C., Bulyk M.L., Lahav G.. p53 pulses lead to distinct patterns of gene expression albeit similar DNA-binding dynamics. Nat. Struct. Mol. Biol. 2017; 24:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Porter J.R., Fisher B.E., Baranello L., Liu J.C., Kambach D.M., Nie Z., Koh W.S., Luo J., Stommel J.M., Levens D. et al.. Global inhibition with specific activation: how p53 and MYC redistribute the transcriptome in the DNA double-strand break response. Mol. Cell. 2017; 67:1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Janky R., Verfaillie A., Imrichová H., van de Sande B., Standaert L., Christiaens V., Hulselmans G., Herten K., Naval Sanchez M., Potier D. et al.. iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLoS Comput. Biol. 2014; 10:e1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu J., Sammons M.A., Donahue G., Dou Z., Vedadi M., Getlik M., Barsyte-Lovejoy D., Al-Awar R., Katona B.W., Shilatifard A. et al.. Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature. 2015; 525:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koeppel M., van Heeringen S.J., Kramer D., Smeenk L., Janssen-Megens E., Hartmann M., Stunnenberg H.G., Lohrum M.. Crosstalk between c-Jun and TAp73 / contributes to the apoptosis-survival balance. Nucleic Acids Res. 2011; 39:6069–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dashzeveg N., Taira N., Lu Z.G., Kimura J., Yoshida K.. Palmdelphin, a novel target of p53 with Ser46 phosphorylation, controls cell death in response to DNA damage. Cell Death Dis. 2014; 5:e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hünten S., Kaller M., Drepper F., Oeljeklaus S., Bonfert T., Erhard F., Dueck A., Eichner N., Friedel C.C., Meister G. et al.. p53-regulated networks of protein, mRNA, miRNA, and lncRNA expression revealed by integrated pulsed stable isotope labeling with amino acids in cell culture (pSILAC) and next generation sequencing (NGS) analyses. Mol. Cell. Proteomics. 2015; 14:2609–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Smeenk L., van Heeringen S.J., Koeppel M., Gilbert B., Janssen-Megens E., Stunnenberg H.G., Lohrum M.. Role of p53 Serine 46 in p53 target gene regulation. PLoS One. 2011; 6:e17574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Menendez D., Nguyen T.A., Freudenberg J.M., Mathew V.J., Anderson C.W., Jothi R., Resnick M.A.. Diverse stresses dramatically alter genome-wide p53 binding and transactivation landscape in human cancer cells. Nucleic Acids Res. 2013; 41:7286–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu Y., Lin J.C., Piluso L.G., Dhahbi J.M., Bobadilla S., Spindler S.R., Liu X.. Phosphorylation of p53 by TAF1 Inactivates p53-Dependent Transcription in the DNA Damage Response. Mol. Cell. 2014; 53:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Perlmann T., Wrange O.. Specific glucocorticoid receptor binding to DNA reconstituted in a nucleosome. EMBO J. 1988; 7:3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zaret K.S., Mango S.E.. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr. Opin. Genet. Dev. 2016; 37:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zaret K.S., Carroll J.S.. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011; 25:2227–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Espinosa J.M., Verdun R.E., Emerson B.M.. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol. Cell. 2003; 12:1015–1027. [DOI] [PubMed] [Google Scholar]
- 88. Sahu G., Wang D., Chen C.B., Zhurkin V.B., Harrington R.E., Appella E., Hager G.L., Nagaich A.K.. p53 binding to nucleosomal DNA depends on the rotational positioning of DNA response element. J. Biol. Chem. 2010; 285:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yu X., Buck M.J.. Defining TP53 pioneering capabilities with competitive nucleosome binding assays. Genome Res. 2019; 29:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Laptenko O., Beckerman R., Freulich E., Prives C.. p53 binding to nucleosomes within the p21 promoter in vivo leads to nucleosome loss and transcriptional activation. Proc. Natl. Acad. Sci. U.S.A. 2011; 108:10385–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Polach K.J., Widom J.. Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J. Mol. Biol. 1995; 254:130–149. [DOI] [PubMed] [Google Scholar]
- 92. Cui F., Zhurkin V.B.. Rotational positioning of nucleosomes facilitates selective binding of p53 to response elements associated with cell cycle arrest. Nucleic Acids Res. 2014; 42:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gomes N.P., Espinosa J.M.. Disparate chromatin landscapes and kinetics of inactivation impact differential regulation of p53 target genes. Cell Cycle. 2010; 9:3428–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lidor Nili E., Field Y., Lubling Y., Widom J., Oren M., Segal E.. p53 binds preferentially to genomic regions with high DNA-encoded nucleosome occupancy. Genome Res. 2010; 20:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weinberg R.L., Freund S.M.V., Veprintsev D.B., Bycroft M., Fersht A.R.. Regulation of DNA binding of p53 by its C-terminal domain. J. Mol. Biol. 2004; 342:801–811. [DOI] [PubMed] [Google Scholar]
- 96. Laptenko O., Shiff I., Freed-Pastor W., Zupnick A., Mattia M., Freulich E., Shamir I., Kadouri N., Kahan T., Manfredi J. et al.. The p53 C terminus controls site-specific DNA binding and promotes structural changes within the central DNA binding domain. Mol. Cell. 2015; 57:1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rastogi C., Rube H.T., Kribelbauer J.F., Crocker J., Loker R.E., Martini G.D., Laptenko O., Freed-Pastor W.A., Prives C., Stern D.L. et al.. Accurate and sensitive quantification of protein-DNA binding affinity. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tafvizi A., Huang F., Fersht A.R., Mirny L.A., van Oijen A.M.. A single-molecule characterization of p53 search on DNA. Proc. Natl. Acad. Sci. U.S.A. 2011; 108:563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Soufi A., Garcia M.F., Jaroszewicz A., Osman N., Pellegrini M., Zaret K.S.. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015; 161:555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Meers M.P., Janssens D.H., Henikoff S.. Pioneer factor-nucleosome binding events during differentiation are motif encoded. Mol. Cell. 2019; 75:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lill N.L., Grossman S.R., Ginsberg D., DeCaprio J., Livingston D.M.. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997; 387:823–827. [DOI] [PubMed] [Google Scholar]
- 102. Gu W., Roeder R.G.. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997; 90:595–606. [DOI] [PubMed] [Google Scholar]
- 103. Sykes S.M., Mellert H.S., Holbert M.A., Li K., Marmorstein R., Lane W.S., McMahon S.B.. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol. Cell. 2006; 24:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tang Y., Luo J., Zhang W., Gu W.. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell. 2006; 24:827–839. [DOI] [PubMed] [Google Scholar]
- 105. Liu L., Scolnick D.M., Trievel R.C., Zhang H.B., Marmorstein R., Halazonetis T.D., Berger S.L.. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 1999; 19:1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Min S., Kim K., Kim S.-G., Cho H., Lee Y.. Chromatin-remodeling factor, RSF1, controls p53-mediated transcription in apoptosis upon DNA strand breaks. Cell Death Dis. 2018; 9:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Naidu S.R., Love I.M., Imbalzano A.N., Grossman S.R., Androphy E.J.. The SWI/SNF chromatin remodeling subunit BRG1 is a critical regulator of p53 necessary for proliferation of malignant cells. Oncogene. 2009; 28:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Guan B., Wang T.-L., Shih I.-M.. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011; 71:6718–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barlev N.A., Liu L., Chehab N.H., Mansfield K., Harris K.G., Halazonetis T.D., Berger S.L.. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell. 2001; 8:1243–1254. [DOI] [PubMed] [Google Scholar]
- 110. Murphy M., Ahn J., Walker K.K., Hoffman W.H., Evans R.M., Levine A.J., George D.L.. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 1999; 13:2490–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Brien G.L., Healy E., Jerman E., Conway E., Fadda E., O’Donovan D., Krivtsov A.V., Rice A.M., Kearney C.J., Flaus A. et al.. A chromatin-independent role of polycomb-like 1 to stabilize p53 and promote cellular quiescence. Genes Dev. 2015; 29:2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shi X., Kachirskaia I., Yamaguchi H., West L.E., Wen H., Wang E.W., Dutta S., Appella E., Gozani O.. Modulation of p53 function by SET8-mediated methylation at lysine 382. Mol. Cell. 2007; 27:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Huang J., Sengupta R., Espejo A.B., Lee M.G., Dorsey J.A., Richter M., Opravil S., Shiekhattar R., Bedford M.T., Jenuwein T. et al.. p53 is regulated by the lysine demethylase LSD1. Nature. 2007; 449:105–108. [DOI] [PubMed] [Google Scholar]
- 114. Huang J., Perez-Burgos L., Placek B.J., Sengupta R., Richter M., Dorsey J.A., Kubicek S., Opravil S., Jenuwein T., Berger S.L.. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006; 444:629–632. [DOI] [PubMed] [Google Scholar]
- 115. Chen L., Li Z., Zwolinska A.K., Smith M.A., Cross B., Koomen J., Yuan Z.-M., Jenuwein T., Marine J.-C., Wright K.L. et al.. MDM2 recruitment of lysine methyltransferases regu… [EMBO J. 2010] - PubMed result. EMBO J. 2010; 29:2538–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mungamuri S.K., Qiao R.F., Yao S., Manfredi J.J., Gu W., Aaronson S.A.. USP7 enforces heterochromatinization of p53 target promoters by protecting SUV39H1 from MDM2-mediated degradation. Cell Rep. 2016; 14:2528–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huang J., Dorsey J., Chuikov S., Pérez-Burgos L., Zhang X., Jenuwein T., Reinberg D., Berger S.L.. G9a and Glp methylate lysine 373 in the tumor suppressor p53. J. Biol. Chem. 2010; 285:9636–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang D., Kon N., Lasso G., Jiang L., Leng W., Zhu W.-G., Qin J., Honig B., Gu W.. Acetylation-regulated interaction between p53 and SET reveals a widespread regulatory mode. Nature. 2016; 538:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Roy S., Musselman C.A., Kachirskaia I., Hayashi R., Glass K.C., Nix J.C., Gozani O., Appella E., Kutateladze T.G.. Structural insight into p53 recognition by the 53BP1 tandem Tudor domain. J. Mol. Biol. 2010; 398:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Klein B.J., Wang X., Cui G., Yuan C., Botuyan M.V., Lin K., Lu Y., Wang X., Zhao Y., Bruns C.J. et al.. PHF20 readers link methylation of histone H3K4 and p53 with H4K16 acetylation. Cell Rep. 2016; 17:1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cui G., Park S., Badeaux A.I., Kim D., Lee J., Thompson J.R., Yan F., Kaneko S., Yuan Z., Botuyan M.V. et al.. PHF20 is an effector protein of p53 double lysine methylation that stabilizes and activates p53. Nat. Struct. Mol. Biol. 2012; 19:916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nguyen T.-A., Menendez D., Resnick M.A., Anderson C.W.. Mutant TP53 posttranslational modifications: challenges and opportunities. Hum. Mutat. 2014; 35:738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Harms K.L., Chen X.. Histone deacetylase 2 modulates p53 transcriptional activities through regulation of p53-DNA binding activity. Cancer Res. 2007; 67:3145–3152. [DOI] [PubMed] [Google Scholar]
- 124. Ito A., Kawaguchi Y., Lai C.-H., Kovacs J.J., Higashimoto Y., Appella E., Yao T.-P.. MDM2–HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002; 21:6236–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Juan L.J., Shia W.J., Chen M.H., Yang W.M., Seto E., Lin Y.S., Wu C.W.. Histone deacetylases specifically down-regulate p53-dependent gene activation. J. Biol. Chem. 2000; 275:20436–20443. [DOI] [PubMed] [Google Scholar]
- 126. LeBoeuf M., Terrell A., Trivedi S., Sinha S., Epstein J.A., Olson E.N., Morrisey E.E., Millar S.E.. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev. Cell. 2010; 19:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang H., Zhou W., Zheng Z., Zhang P., Tu B., He Q., Zhu W.G.. The HDAC inhibitor depsipeptide transactivates the p53/p21 pathway by inducing DNA damage. DNA Repair (Amst.). 2012; 11:146–156. [DOI] [PubMed] [Google Scholar]
- 128. Loewer A., Batchelor E., Gaglia G., Lahav G.. Basal dynamics of p53 reveal transcriptionally attenuated pulses in cycling cells. Cell. 2010; 142:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Honda R., Tanaka H., Yasuda H.. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997; 420:25–27. [DOI] [PubMed] [Google Scholar]
- 130. Lees A., McIntyre A.J., Crawford N.T., Falcone F., McCann C., Holohan C., Quinn G.P., Roberts J.Z., Sessler T., Gallagher P.F. et al.. The pseudo-caspase FLIP(L) regulates cell fate following p53 activation. Proc. Natl. Acad. Sci. U.S.A. 2020; 117:17808–17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Adams P.D., Latif A.L., Newcombe A., Robertson N., Terradas M., Stewart H., Reid C., Rishi Delori L., Chevassut T., Huang X. et al.. BET inhibitors potentiate activation of p53 and killing of AML by MDM2 inhibitors — a candidate combination therapy. Blood. 2018; 132:3912–3912. [Google Scholar]
- 132. Sanz G., Singh M., Peuget S., Selivanova G.. Inhibition of p53 inhibitors: progress, challenges and perspectives. J. Mol. Cell Biol. 2019; 11:586–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang D., Kon N., Lasso G., Jiang L., Leng W., Zhu W.G., Qin J., Honig B., Gu W.. Acetylation-regulated interaction between p53 and SET reveals a widespread regulatory mode. Nature. 2016; 538:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dorigo B., Schalch T., Kulangara A., Duda S., Schroeder R.R., Richmond T.J.. Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science. 2004; 306:1571–1573. [DOI] [PubMed] [Google Scholar]
- 135. Shogren-Knaak M., Ishii H., Sun J.M., Pazin M.J., Davie J.R., Peterson C.L.. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006; 311:844–847. [DOI] [PubMed] [Google Scholar]
- 136. Robinson P.J.J., An W., Routh A., Martino F., Chapman L., Roeder R.G., Rhodes D.. 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. J. Mol. Biol. 2008; 381:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Reiter F., Wienerroither S., Stark A.. Combinatorial function of transcription factors and cofactors. Curr. Opin. Genet. Dev. 2017; 43:73–81. [DOI] [PubMed] [Google Scholar]
- 138. Spitz F., Furlong E.E.M.. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 2012; 13:613–626. [DOI] [PubMed] [Google Scholar]
- 139. Azofeifa J.G., Allen M.A., Hendrix J.R., Read T., Rubin J.D., Dowell R.D.. Enhancer RNA profiling predicts transcription factor activity. Genome Res. 2018; 28:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schärer E., Lggo R.. Mammalian p53 can function as a transcription factor in yeast. Nucleic Acids Res. 1992; 20:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Inga A., Monti P., Fronza G., Darden T., Resnick M.A.. p53 mutants exhibiting enhanced transcriptional activation and altered promoter selectivity are revealed using a sensitive, yeast-based functional assay. Oncogene. 2001; 20:501–513. [DOI] [PubMed] [Google Scholar]
- 142. Braastad C.D., Han Z., Hendrickson E.A.. Constitutive DNase I hypersensitivity of p53-regulated promoters. J. Biol. Chem. 2003; 278:8261–8268. [DOI] [PubMed] [Google Scholar]
- 143. Daino K., Ichimura S., Nenoi M.. Both the basal transcriptional activity of the GADD45A gene and its enhancement after ionizing irradiation are mediated by AP-1 element. Biochim. Biophys. Acta. 2006; 1759:458–469. [DOI] [PubMed] [Google Scholar]
- 144. Koutsodontis G., Tentes I., Papakosta P., Moustakas A., Kardassis D.. Sp1 plays a critical role in the transcriptional activation of the human cyclin-dependent kinase inhibitor p21(WAF1/Cip1) gene by the p53 tumor suppressor protein. J. Biol. Chem. 2001; 276:29116–29125. [DOI] [PubMed] [Google Scholar]
- 145. Li H., Zhang Y., Ströse A., Tedesco D., Gurova K., Selivanova G.. Integrated high-throughput analysis identifies Sp1 as a crucial determinant of p53-mediated apoptosis. Cell Death Differ. 2014; 21:1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Thornborrow E.C., Manfredi J.J.. The tumor suppressor protein p53 requires a cofactor to activate transcriptionally the human BAX promoter. J. Biol. Chem. 2001; 276:15598–15608. [DOI] [PubMed] [Google Scholar]
- 147. Menendez D., Inga A., Snipe J., Krysiak O., Schönfelder G., Resnick M.a. A single-nucleotide polymorphism in a half-binding site creates p53 and estrogen receptor control of vascular endothelial growth factor receptor 1. Mol. Cell. Biol. 2007; 27:2590–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Menendez D., Inga A., Resnick M.A.. Estrogen receptor acting in cis enhances WT and mutant p53 transactivation at canonical and noncanonical p53 target sequences. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Catizone A.N., Uzunbas G.K., Celadova P., Kuang S., Bose D., Sammons M.A.. Locally acting transcription factors regulate p53-dependent cis-regulatory element activity. Nucleic Acids Res. 2020; 48:4195–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tanaka Y., Nakamura A., Morioka M.S., Inoue S., Tamamori-Adachi M., Yamada K., Taketani K., Kawauchi J., Tanaka-Okamoto M., Miyoshi J. et al.. Systems analysis of ATF3 in stress response and cancer reveals opposing effects on pro-apoptotic genes in p53 pathway. PLoS One. 2011; 6:e26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Korkmaz G., Lopes R., Ugalde A.P., Nevedomskaya E., Han R., Myacheva K., Zwart W., Elkon R., Agami R.. Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9. Nat. Biotechnol. 2016; 34:192–198. [DOI] [PubMed] [Google Scholar]
- 152. Niu X., Deng K., Liu L., Yang K., Hu X.. A statistical framework for predicting critical regions of p53-dependent enhancers. Brief. Bioinform. 2020; doi:10.1093/bib/bbaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Espinosa J.M., Emerson B.M.. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol. Cell. 2001; 8:57–69. [DOI] [PubMed] [Google Scholar]
- 154. Morachis J.M., Murawsky C.M., Emerson B.M.. Regulation of the p53 transcriptional response by structurally diverse core promoters. Genes Dev. 2010; 24:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tomso D.J., Inga A., Menendez D., Pittman G.S., Campbell M.R., Storici F., Bell D.A., Resnick M.A.. Functionally distinct polymorphic sequences in the human genome that are targets for p53 transactivation. Proc. Natl. Acad. Sci. U.S.A. 2005; 102:6431–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Inga A., Storici F., Darden T.A., Resnick M.A.. Differential transactivation by the p53 transcription factor is highly dependent on p53 level and promoter target sequence. Mol. Cell. Biol. 2002; 22:8612–8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Menendez D., Inga A., Jordan J.J., Resnick M.A.. Changing the p53 master regulatory network: ELEMENTary, my dear Mr Watson. Oncogene. 2007; 26:2191–2201. [DOI] [PubMed] [Google Scholar]
- 158. Coleman R.A., Qiao Z., Singh S.K., Peng C.S., Cianfrocco M., Zhang Z., Piasecka A., Aldeborgh H., Basishvili G., Liu W.L.. p53 dynamically directs TFIID assembly on target gene promoters. Mol. Cell. Biol. 2017; 37:e00085-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Harton M.D., Koh W.S., Bunker A.D., Singh A., Batchelor E.. p53 pulse modulation differentially regulates target gene promoters to regulate cell fate decisions. Mol. Syst. Biol. 2019; 15:e8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Friedrich D., Friedel L., Finzel A., Herrmann A., Preibisch S., Loewer A.. Stochastic transcription in the p53-mediated response to DNA damage is modulated by burst frequency. Mol. Syst. Biol. 2019; 15:e9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hafner A., Reyes J., Stewart-Ornstein J., Tsabar M., Jambhekar A., Lahav G.. Quantifying the central dogma in the p53 pathway in live single cells. Cell Syst. 2020; 10:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tutton S., Deng Z., Gulve N., Vladimirova O., Beishline K., Wiedmer A., Murphy M., Lieberman P.M.. Elevated telomere dysfunction in cells containing the African-centric Pro47Ser cancer-risk variant of TP53. Oncotarget. 2019; 10:3581–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Simeonova I., Lejour V., Bardot B., Bouarich-Bourimi R., Morin A., Fang M., Charbonnier L., Toledo F.. Fuzzy tandem repeats containing p53 response elements may define species-specific p53 target genes. PLoS Genet. 2012; 8:e1002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hagan C.R., Rudin C.M.. DNA cleavage and Trp53 differentially affect SINE transcription. Genes Chromosomes Cancer. 2007; 46:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chang N.-T., Yang W.K., Huang H.-C., Yeh K.-W., Wu C.-W.. The transcriptional activity of HERV-I LTR is negatively regulated by its cis-elements and wild type p53 tumor suppressor protein. J. Biomed. Sci. 2007; 14:211–222. [DOI] [PubMed] [Google Scholar]
- 166. Leonova K.I., Brodsky L., Lipchick B., Pal M., Novototskaya L., Chenchik A.A., Sen G.C., Komarova E.A., Gudkov A.V. p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:E89–E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wylie A., Jones A.E., D’Brot A., Lu W.J., Kurtz P., Moran J.V., Rakheja D., Chen K.S., Hammer R.E., Comerford S.A. et al.. p53 genes function to restrain mobile elements. Genes Dev. 2016; 30:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Roy S., Tomaszowski K.-H., Luzwick J.W., Park S., Li J., Murphy M., Schlacher K.. p53 orchestrates DNA replication restart homeostasis by suppressing mutagenic RAD52 and POLθ pathways. Elife. 2018; 7:e31723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hampp S., Kiessling T., Buechle K., Mansilla S.F., Thomale J., Rall M., Ahn J., Pospiech H., Gottifredi V., Wiesmüller L.. DNA damage tolerance pathway involving DNA polymerase ι and the tumor suppressor p53 regulates DNA replication fork progression. Proc. Natl. Acad. Sci. U.S.A. 2016; 113:E4311–E4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Klusmann I., Rodewald S., Müller L., Friedrich M., Wienken M., Li Y., Schulz-Heddergott R., Dobbelstein M.. p53 Activity Results in DNA Replication Fork Processivity. Cell Rep. 2016; 17:1845–1857. [DOI] [PubMed] [Google Scholar]
- 171. Flores E.R., Sengupta S., Miller J.B., Newman J.J., Bronson R., Crowley D., Yang A., McKeon F., Jacks T.. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell. 2005; 7:363–373. [DOI] [PubMed] [Google Scholar]
- 172. Gebel J., Tuppi M., Krauskopf K., Coutandin D., Pitzius S., Kehrloesser S., Osterburg C., Dötsch V.. Control mechanisms in germ cells mediated by p53 family proteins. J. Cell Sci. 2017; 130:2663–2671. [DOI] [PubMed] [Google Scholar]
- 173. Soares E., Zhou H.. Master regulatory role of p63 in epidermal development and disease. Cell. Mol. Life Sci. 2018; 75:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nemajerova A., Amelio I., Gebel J., Dötsch V., Melino G., Moll U.M.. Non-oncogenic roles of TAp73: from multiciliogenesis to metabolism. Cell Death Differ. 2018; 25:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Riege K., Kretzmer H., McDade S.S., Hoffmann S., Fischer M.. Dissecting the DNA binding landscape and gene regulatory network of p63 and p53. 2020; bioRxiv doi:12 June 2020, preprint: not peer reviewed 10.1101/2020.06.11.145540. [DOI] [PMC free article] [PubMed]
- 176. Lin Y.-L., Sengupta S., Gurdziel K., Bell G.W., Jacks T., Flores E.R.. p63 and p73 transcriptionally regulate genes involved in DNA repair. PLoS Genet. 2009; 5:e1000680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 177. Murray-Zmijewski F., Lane D.P., Bourdon J.-C.. P53/P63/P73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006; 13:962–972. [DOI] [PubMed] [Google Scholar]
- 178. Joruiz S.M., Bourdon J.C.. p53 isoforms: key regulators of the cell fate decision. Cold Spring Harb. Perspect. Med. 2016; 6:a026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Iwafuchi-Doi M., Zaret K.S.. Cell fate control by pioneer transcription factors. Development. 2016; 143:1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Guha T., Malkin D.. Inherited TP53 mutations and the Li-fraumeni syndrome. Cold Spring Harb. Perspect. Med. 2017; 7:a026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Armstrong J.F., Kaufman M.H., Harrison D.J., Clarke A.R.. High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 1995; 5:931–936. [DOI] [PubMed] [Google Scholar]
- 182. Sah V.P., Attardi L.D., Mulligan G.J., Williams B.O., Bronson R.T., Jacks T.. A subset of p53-deficient embryos exhibit exencephaly. Nat. Genet. 1995; 10:175–180. [DOI] [PubMed] [Google Scholar]
- 183. Jackson J.G., Lozano G.. The mutant p53 mouse as a pre-clinical model. Oncogene. 2013; 32:4325–4330. [DOI] [PubMed] [Google Scholar]
- 184. Marshall C.B., Mays D.J., Beeler J.S., Rosenbluth J.M., Boyd K.L., Santos Guasch G.L., Shaver T.M., Tang L.J., Liu Q., Shyr Y. et al.. p73 is required for multiciliogenesis and regulates the Foxj1-associated gene network. Cell Rep. 2016; 14:2289–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nemajerova A., Kramer D., Siller S.S., Herr C., Shomroni O., Pena T., Gallinas Suazo C., Glaser K., Wildung M., Steffen H. et al.. TAp73 is a central transcriptional regulator of airway multiciliogenesis. Genes Dev. 2016; 30:1300–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yang A., Schweitzer R., Sun D., Kaghad M., Walker N., Bronson R.T., Tabin C., Sharpe A., Caput D., Crum C. et al.. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999; 398:714–718. [DOI] [PubMed] [Google Scholar]
- 187. Bao X., Rubin A.J., Qu K., Zhang J., Giresi P.G., Chang H.Y., Khavari P.A.. A novel ATAC-seq approach reveals lineage-specific reinforcement of the open chromatin landscape via cooperation between BAF and p63. Genome Biol. 2015; 16:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Li L., Wang Y., Torkelson J.L., Shankar G., Pattison J.M., Zhen H.H., Fang F., Duren Z., Xin J., Gaddam S. et al.. TFAP2C- and p63-dependent networks sequentially rearrange chromatin landscapes to drive human epidermal lineage commitment. Cell Stem Cell. 2019; 24:271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lin-Shiao E., Lan Y., Coradin M., Anderson A., Donahue G., Simpson C.L., Sen P., Saffie R., Busino L., Garcia B.A. et al.. KMT2D regulates p63 target enhancers to coordinate epithelial homeostasis. Genes Dev. 2018; 32:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Pattison J.M., Melo S.P., Piekos S.N., Torkelson J.L., Bashkirova E., Mumbach M.R., Rajasingh C., Zhen H.H., Li L., Liaw E. et al.. Retinoic acid and BMP4 cooperate with p63 to alter chromatin dynamics during surface epithelial commitment. Nat. Genet. 2018; 50:1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Qu J., Tanis S.E.J., Smits J.P.H., Kouwenhoven E.N., Oti M., van den Bogaard E.H., Logie C., Stunnenberg H.G., van Bokhoven H., Mulder K.W. et al.. Mutant p63 affects epidermal cell identity through rewiring the enhancer landscape. Cell Rep. 2018; 25:3490–3503. [DOI] [PubMed] [Google Scholar]
- 192. Kouwenhoven E.N., Oti M., Niehues H., van Heeringen S.J., Schalkwijk J., Stunnenberg H.G., Bokhoven H., Zhou H.. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 2015; 16:863–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Donehower L.A., Soussi T., Korkut A., Liu Y., Schultz A., Cardenas M., Li X., Babur O., Hsu T.-K.K., Lichtarge O. et al.. Integrated analysis of TP53 gene and pathway alterations in the cancer genome atlas. Cell Rep. 2019; 28:1370–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Jeay S., Gaulis S., Ferretti S., Bitter H., Ito M., Valat T., Murakami M., Ruetz S., Guthy D.A., Rynn C. et al.. A distinct p53 target gene set predicts for response to the selective p53- HDM2 inhibitor NVP-CGM097. Elife. 2015; 4:e06498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Sonkin D. Expression signature based on TP53 target genes doesn’t predict response to TP53-MDM2 inhibitor in wild type TP53 tumors. Elife. 2015; 4:e10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Jeay S., Gaulis S., Ferretti S., Bitter H., Ito M., Valat T., Murakami M., Ruetz S., Guthy D.A., Rynn C. et al.. Correction: a distinct p53 target gene set predicts for response to the selective p53-HDM2 inhibitor NVP-CGM097. Elife. 2016; 5:e19317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ishizawa J., Nakamaru K., Seki T., Tazaki K., Kojima K., Chachad D., Zhao R., Heese L., Ma W., Ma M.C.J. et al.. Predictive gene signatures determine tumor sensitivity to MDM2 inhibition. Cancer Res. 2018; 78:2721–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Whitfield M.L., George L.K., Grant G.D., Perou C.M.. Common markers of proliferation. Nat. Rev. Cancer. 2006; 6:99–106. [DOI] [PubMed] [Google Scholar]
- 199. Bykov V.J.N., Issaeva N., Shilov A., Hultcrantz M., Pugacheva E., Chumakov P., Bergman J., Wiman K.G., Selivanova G.. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat. Med. 2002; 8:282–288. [DOI] [PubMed] [Google Scholar]
- 200. Soragni A., Janzen D.M., Johnson L.M., Lindgren A.G., Thai-Quynh Nguyen A., Tiourin E., Soriaga A.B., Lu J., Jiang L., Faull K.F. et al.. A designed inhibitor of p53 aggregation rescues p53 tumor suppression in ovarian carcinomas. Cancer Cell. 2016; 29:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Schoenfelder S., Fraser P.. Long-range enhancer–promoter contacts in gene expression control. Nat. Rev. Genet. 2019; 20:437–455. [DOI] [PubMed] [Google Scholar]
- 202. Riley T., Sontag E., Chen P., Levine A.. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 2008; 9:402–412. [DOI] [PubMed] [Google Scholar]
- 203. Link N., Kurtz P., O’Neal M., Garcia-Hughes G., Abrams J.M.. A p53 enhancer region regulates target genes through chromatin conformations in cis and in trans. Genes Dev. 2013; 27:2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Millau J.-F., Wijchers P., Gaudreau L.. High-resolution 4C reveals rapid p53-dependent chromatin reorganization of the CDKN1A locus in response to stress. PLoS One. 2016; 11:e0163885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Fishilevich S., Nudel R., Rappaport N., Hadar R., Plaschkes I., Iny Stein T., Rosen N., Kohn A., Twik M., Safran M. et al.. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database (Oxford). 2017; 2017:bax028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hait T.A., Amar D., Shamir R., Elkon R.. FOCS: a novel method for analyzing enhancer and gene activity patterns infers an extensive enhancer–promoter map. Genome Biol. 2018; 19:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Wang J., Dai X., Berry L.D., Cogan J.D., Liu Q., Shyr Y.. HACER: an atlas of human active enhancers to interpret regulatory variants. Nucleic Acids Res. 2019; 47:D106–D112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Okonechnikov K., Erkek S., Korbel J.O., Pfister S.M., Chavez L.. InTAD: chromosome conformation guided analysis of enhancer target genes. BMC Bioinformatics. 2019; 20:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chen C.-H., Zheng R., Tokheim C., Dong X., Fan J., Wan C., Tang Q., Brown M., Liu J.S., Meyer C.A. et al.. Determinants of transcription factor regulatory range. Nat. Commun. 2020; 11:2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Barrington C., Georgopoulou D., Pezic D., Varsally W., Herrero J., Hadjur S.. Enhancer accessibility and CTCF occupancy underlie asymmetric TAD architecture and cell type specific genome topology. Nat. Commun. 2019; 10:2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dixon J.R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J.S., Ren B.. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012; 485:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Sarosiek K.A., Fraser C., Muthalagu N., Bhola P.D., Chang W., McBrayer S.K., Cantlon A., Fisch S., Golomb-Mello G., Ryan J.A. et al.. Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell. 2017; 31:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ho Y.-T., Shimbo T., Wijaya E., Ouchi Y., Takaki E., Yamamoto n, Kikuchi Y., Kaneda Y., Tamai K.. Chromatin accessibility identifies diversity in mesenchymal stem cells from different tissue origins. Sci. Rep. 2018; 8:17765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Nordström K.J., Schmidt F., Gasparoni N., Salhab A., Gasparoni G., Kattler K., Müller F., Ebert P., Costa I.G. DEEP consortium et al.. Unique and assay specific features of NOMe-, ATAC- and DNase I-seq data. Nucleic Acids Res. 2019; 47:10580–10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kurtz P., Jones A.E., Tiwari B., Link N., Wylie A., Tracy C., Krämer H., Abrams J.M.. Drosophila p53 directs nonapoptotic programs in postmitotic tissue. Mol. Biol. Cell. 2019; 30:1339–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Bowen M.E., McClendon J., Long H.K., Sorayya A., Van Nostrand J.L., Wysocka J., Attardi L.D.. The spatiotemporal pattern and intensity of p53 activation dictates phenotypic diversity in p53-driven developmental syndromes. Dev. Cell. 2019; 50:212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Suzuki H., Igarashi S., Nojima M., Maruyama R., Yamamoto E., Kai M., Akashi H., Watanabe Y., Yamamoto H., Sasaki Y. et al.. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010; 31:342–349. [DOI] [PubMed] [Google Scholar]
- 218. París R., Henry R.E., Stephens S.J., McBryde M., Espinosa J.M.. Multiple p53-independent gene silencing mechanisms define the cellular response to p53 activation. Cell Cycle. 2008; 7:2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ferguson A.T., Evron E., Umbricht C.B., Pandita T.K., Chan T.A., Hermeking H., Marks J.R., Lambers A.R., Futreal P.A., Stampfer M.R. et al.. High frequency of hypermethylation at the 14-3-3 sigma locus leads to gene silencing in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 2000; 97:6049–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Diaz-Lagares A., Crujeiras A.B., Lopez-Serra P., Soler M., Setien F., Goyal A., Sandoval J., Hashimoto Y., Martinez-Cardús A., Gomez A. et al.. Epigenetic inactivation of the p53-induced long noncoding RNA TP53 target 1 in human cancer. Proc. Natl. Acad. Sci. U.S.A. 2016; 113:E7535–E7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Soria C., Estermann F.E., Espantman K.C., Oshea C.C.. Heterochromatin silencing of p53 target genes by a small viral protein. Nature. 2010; 466:1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Chillemi G., Kehrloesser S., Bernassola F., Desideri A., Dötsch V., Levine A.J., Melino G.. Structural evolution and dynamics of the p53 proteins. Cold Spring Harb. Perspect. Med. 2017; 7:a028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Lion M., Raimondi I., Donati S., Jousson O., Ciribilli Y., Inga A., Maeso I., Irimia M., Tena J., Casares F. et al.. Evolution of p53 transactivation specificity through the lens of a yeast-based functional assay. PLoS One. 2015; 10:e0116177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Stergachis A.B., Neph S., Sandstrom R., Haugen E., Reynolds A.P., Zhang M., Byron R., Canfield T., Stelhing-Sun S., Lee K. et al.. Conservation of trans-acting circuitry during mammalian regulatory evolution. Nature. 2014; 515:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Cheng Y., Ma Z., Kim B.-H., Wu W., Cayting P., Boyle A.P., Sundaram V., Xing X., Dogan N., Li J. et al.. Principles of regulatory information conservation between mouse and human. Nature. 2014; 515:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Nitta K.R., Jolma A., Yin Y., Morgunova E., Kivioja T., Akhtar J., Hens K., Toivonen J., Deplancke B., Furlong E.E.M. et al.. Conservation of transcription factor binding specificities across 600 million years of bilateria evolution. Elife. 2015; 4:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Toledo F., Wahl G.M.. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat. Rev. Cancer. 2006; 6:909–923. [DOI] [PubMed] [Google Scholar]
- 228. Kaiser A.M., Attardi L.D.. Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ. 2018; 25:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D. et al.. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014; 515:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Horvath M.M., Wang X., Resnick M.A., Bell D.A.. Divergent evolution of human p53 binding sites: Cell cycle versus apoptosis. PLoS Genet. 2007; 3:1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Jegga A.G., Inga A., Menendez D., Aronow B.J., Resnick M.A.. Functional evolution of the p53 regulatory network through its target response elements. Proc. Natl. Acad. Sci. U.S.A. 2008; 105:944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kastan M.B., Zhan Q., El-Deiry W.S., Carrier F., Jacks T., Walsh W.V., Plunkett B.S., Vogelstein B., Fornace A.J.. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992; 71:587–597. [DOI] [PubMed] [Google Scholar]
- 233. Tanaka H., Arakawa H., Yamaguchi T., Shiraishi K., Fukuda S., Matsui K., Takei Y., Nakamura Y.. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000; 404:42–49. [DOI] [PubMed] [Google Scholar]
- 234. Tan T., Chu G.. p53 Binds and activates the xeroderma pigmentosum DDB2 gene in humans but not mice. Mol. Cell. Biol. 2002; 22:3247–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hu W., Feng Z., Teresky A.K., Levine A.J.. p53 regulates maternal reproduction through LIF. Nature. 2007; 450:721–724. [DOI] [PubMed] [Google Scholar]
- 236. Vazquez J.M., Sulak M., Chigurupati S., Lynch V.J.. A zombie LIF gene in elephants is upregulated by TP53 to induce apoptosis in response to DNA damage. Cell Rep. 2018; 24:1765–1776. [DOI] [PubMed] [Google Scholar]
- 237. Abegglen L.M., Caulin A.F., Chan A., Lee K., Robinson R., Campbell M.S., Kiso W.K., Schmitt D.L., Waddell P.J., Bhaskara S. et al.. Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA Damage in Humans. JAMA - J. Am. Med. Assoc. 2015; 314:1850–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Sulak M., Fong L., Mika K., Chigurupati S., Yon L., Mongan N.P., Emes R.D., Lynch V.J.. TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. Elife. 2016; 5:e11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Ashur-Fabian O., Avivi A., Trakhtenbrot L., Adamsky K., Cohen M., Kajakaro G., Joel A., Amariglio N., Nevo E., Rechavi G.. Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation. Proc. Natl. Acad. Sci. U. S. A. 2004; 101:12236–12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Sethi I., Gluck C., Zhou H., Buck M.J., Sinha S.. Evolutionary re-wiring of p63 and the epigenomic regulatory landscape in keratinocytes and its potential implications on species-specific gene expression and phenotypes. Nucleic Acids Res. 2017; 45:8208–8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Morin A., Bardot B., Simeonova I., Lejour V., Bouarich-Bourimi R., Toledo F.. Of mice and men. Transcription. 2013; 4:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Stewart-Ornstein J., Cheng H.W.J., Lahav G.. Conservation and divergence of p53 oscillation dynamics across species. Cell Syst. 2017; 5:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Stewart A.J., Hannenhalli S., Plotkin J.B.. Why transcription factor binding sites are ten nucleotides long. Genetics. 2012; 192:973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Tuğrul M., Paixão T., Barton N.H., Tkačik G., Fay J., Wittkopp P., Zheng W., Gianoulis T., Karczewski K., Zhao H. et al.. Dynamics of transcription factor binding site evolution. PLoS Genet. 2015; 11:e1005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Brady C.A., Jiang D., Mello S.S., Johnson T.M., Jarvis L.A., Kozak M.M., Broz D.K., Basak S., Park E.J., McLaughlin M.E. et al.. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011; 145:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Li T., Kon N., Jiang L., Tan M., Ludwig T., Zhao Y., Baer R., Gu W.. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012; 149:1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Valente L.J., Gray D.H.D., Michalak E.M., Pinon-Hofbauer J., Egle A., Scott C.L., Janic A., Strasser A.. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep. 2013; 3:1339–1345. [DOI] [PubMed] [Google Scholar]
- 248. Lange S.S., Takata K., Wood R.D.. DNA polymerases and cancer. Nat. Rev. Cancer. 2011; 11:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Rangarajan A., Weinberg R.A.. Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat. Rev. Cancer. 2003; 3:952–959. [DOI] [PubMed] [Google Scholar]
- 250. de Jong M., Maina T.. Of Mice and humans: are they the same?–implications in cancer translational research. J. Nucl. Med. 2010; 51:501–504. [DOI] [PubMed] [Google Scholar]
- 251. Ellis L.M., Fidler I.J.. Finding the tumor copycat: therapy fails, patients don’t. Nat. Med. 2010; 16:974–975. [DOI] [PubMed] [Google Scholar]
- 252. Ernst J., Kellis M.. ChromHMM: automating chromatin-state discovery and characterization. Nat. Methods. 2012; 9:215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]