Abstract
The geographic pattern of cropland is an important risk factor for invasion and saturation by crop-specific pathogens and arthropods. Understanding cropland networks supports smart pest sampling and mitigation strategies. We evaluate global networks of cropland connectivity for key vegetatively propagated crops (banana and plantain, cassava, potato, sweet potato, and yam) important for food security in the tropics. For each crop, potential movement between geographic location pairs was evaluated using a gravity model, with associated uncertainty quantification. The highly linked hub and bridge locations in cropland connectivity risk maps are likely priorities for surveillance and management, and for tracing intraregion movement of pathogens and pests. Important locations are identified beyond those locations that simply have high crop density. Cropland connectivity risk maps provide a new risk component for integration with other factors—such as climatic suitability, genetic resistance, and global trade routes—to inform pest risk assessment and mitigation.
Keywords: cropland connectivity risk, network analysis, invasion and saturation, roots and tubers, banana and plantain
Plant diseases and pests are major threats to food security and wildlands conservation (Anderson et al. 2004, Woolhouse et al. 2005, Fisher et al. 2012, Gonthier and Garbelotto 2013, Aguayo et al. 2014). Understanding which geographic areas have a high risk of pathogen and arthropod pest invasion is an important first step to designing sampling and mitigation strategies (Fears et al. 2014). Climate effects are one component of this risk, and are commonly addressed in species distribution models (Rosenzweig et al. 2001, Anderson et al. 2004, Jeger and Pautasso 2008, Elith and Leathwick 2009, Rodoni 2009, Bebber et al. 2013, Garrett et al. 2014, Hernandez Nopsa et al. 2014, Kroschel et al. 2016). Another important risk component is the structure of trade routes, through which pathogens and pests may move (Anderson et al. 2004, Nakato et al. 2013, Bebber et al. 2014). Habitat connectivity represents a third component that, integrated with these other risk factors and, potentially, additional factors, such as deployment of resistance, can provide a more complete invasion risk assessment. The connectivity of cropland regions helps to determine whether invasive species that are dependent on crops will become established before effective actions can be taken to mitigate them (Margosian et al. 2009, Sutherst 2014). Incorporating cropland connectivity risk with other risk factors for invasion supports a number of integrated pest management and pest risk assessment strategies, from improved methods for detecting and mitigating new invasives to ongoing improvements in policy (With 2004, Margosian et al. 2009, Leung et al. 2015).
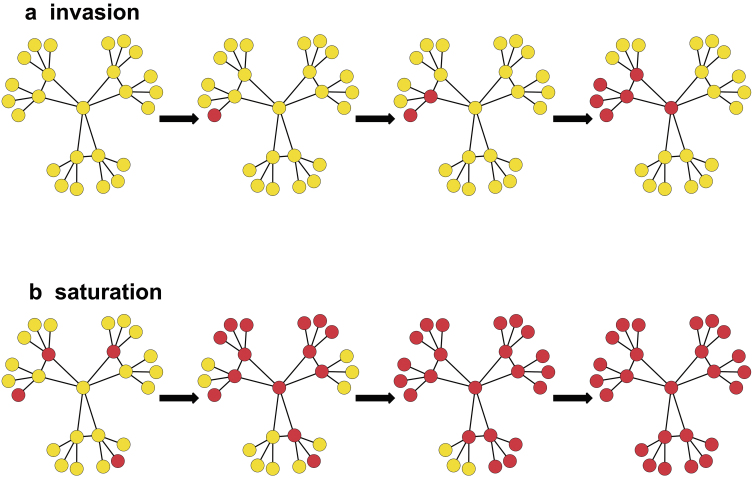
The invasion of species into new countries or continents is a common focus, but local invasion, or saturation, is also important (Cornell and Lawton 1992, Fox et al. 2000, Lion and Gandon 2009). Bebber and colleagues (2014) considered saturation in terms of the fraction of potentially habitable regions that are already occupied by a pest species. Similarly, in the present article, we define saturation as the process by which a species fills in a region, to occupy more and more of the potential habitat within the region. Defining the difference between invasion and saturation is often a question of the spatial resolution and extent being considered (figure 1). From the standpoint of pathogen and arthropod management, a pest may have already invaded a country and be considered endemic, while at the same time there may be some fields it has never reached, and its population may frequently be suppressed by factors such as extreme weather conditions so that it must resaturate. Some pathogens continue to reemerge at different time points (Rugalema et al. 2009, Vurro et al. 2010), such as Phytophthora infestans (Fry et al. 2015). For pathogens such as P. infestans, initial inoculum can be limiting. For example, unusually abundant initial inoculum probably played a key role in the devastating 2009–2010 late blight epidemic in tomato in the northeastern United States (Fry et al. 2013). Similarly, high inoculum associated with synergistic virus species interactions was a key driving factor behind the rapid spread of the cassava mosaic disease pandemic in Africa in the 1990s (Harrison et al. 1997, Legg et al. 2006). High cropland connectivity is a risk factor for saturation and reemergence, as well as novel invasions, when pathogens and arthropods spread from refugia or nearby regions after limiting weather conditions.
Figure 1.
Higher cropland connectivity will tend to increase the risk of both invasion and saturation. In the present figure, invasion and saturation (local invasion) are represented over time, where the pathogen or pest species is present in darker nodes and absent in lighter nodes. (a) In an invasion, a region is initially free of the species. During the process of invasion, the species enters the region and spreads over time. (b) In the process of saturation, a species is already present in a region but not in all potential locations. For example, a restricted subset of nodes in the region may act as refugia for overwintering or oversummering, or for persistence of the species during years with weather less conducive to the species. From these locations, the species can invade linked nodes when conditions are more conducive.
Network analysis offers a number of tools for understanding the strengths and vulnerabilities of network structures. In a geographic network analysis of species invasion or saturation, nodes represent geographic locations and the links between nodes represent functions such as the potential of movement of a pathogen or pest between the nodes (e.g., Buddenhagen et al. 2017, Andersen et al. 2019). Characterizing the network structure of cropland areas acting as sinks or sources can inform the selection of key nodes for surveillance, mitigation, and management improvement (Margosian et al. 2009). Nodes that are linked to many other nodes (nodes that have a high degree) and nodes acting as bridges between cropland regions (nodes with high betweenness centrality) may be particularly important for the spread of pathogens and pests and are important for evaluating invasion risk (Margosian et al. 2009, Hernandez Nopsa et al. 2015). Network traits such as centrality (how important a particular node or link is; Newman 2010), local cohesiveness (how well connected a subset of nodes is compared with their connection to other subsets of nodes; Kolaczyk 2009), and affinity (degree of tendency for nodes to be linked with other nodes of similar centrality; Barrat et al. 2004) can help to identify locations in networks that may be priorities for attention.
Risk assessment for epidemic invasion, coupled with risk-based surveillance and mitigation, are key to efficient control of emerging pests and pathogens (Cameron 2012, Parnell et al. 2014, Hyatt-Twynam et al. 2017). Identification of locations for both surveillance and mitigation is necessary, and they are not always one in the same (Holme 2017, Holme 2018, Andersen et al. 2019). Network analysis has been used to inform surveillance and mitigation in plant (Harwood et al. 2009, Pautasso et al. 2010, Sutrave et al. 2012, Nelson and Bone 2015, Sanatkar et al. 2015, Buddenhagen et al. 2017, Gent et al. 2019, Martinetti and Soubeyrand 2019), animal (Chaters et al. 2019), and human epidemic networks (Keeling and Eames 2005). In most of these plant pathogen and pest network studies, parameters were estimated for models of spread through networks, with links representing pathogen dispersal probability and nodes representing spatial units such as farms, greenhouses, or geographic administrative units. These analyses have typically been conducted for specific crop and pathogen species, with a restricted geographic range. The cropland connectivity analysis described in the present study can be performed before a particular pest or disease becomes important, for general surveillance of a crop or crops. As more information about a particular invasive species problem becomes available, a cropland connectivity analysis can iteratively be made more specific to that problem.
Lack of information about current distribution and dispersal probabilities is a common problem for parameterizing dispersal risk models, especially for new species. More general risk evaluations can draw on models that have proven useful across multiple systems. The inverse power law function is commonly used to model pathogen dispersal. Parameter estimates for six case studies—including plant and human pathogens and distances ranging from experimental field plots (32 meters) to continental scale (9329 km)—ranged from 1.75 to 2.36 (Mundt et al. 2009a). For cucurbit downy mildew, the observed maximum annual disease spread distance ranged from 1914 to 2221 km across 7 years, with inverse power law parameter estimates of approximately 2 or more (Ojiambo et al. 2017). Gravity models are frequently used to describe the risk of movement between two locations, in applications including zoology, ecology, and epidemiology (Jongejans et al. 2014). In dispersal events, the risk of movement between two locations is often a function of the product of the amount of inoculum potentially produced at the source location and the amount of potential host material at the sink location, as in a gravity model (Xia et al. 2004, Sutrave et al. 2012, Jongejans et al. 2014).
Cassava mosaic disease (CMD) is an important example of the likely role of cropland connectivity in the spread of a plant disease epidemic (figure 2). Cassava mosaic begomoviruses (CMBs) cause CMD (Bock and Woods 1983), one of the most damaging constraints to cassava production in Sub-Saharan Africa. Losses have been estimated at more than US$1 billion per year (Legg et al. 2006). CMBs are dispersed via infected planting material and are vectored by the whitefly, Bemisia tabaci (Dubern 1994). Severe disease results when there is coinfection of cassava plants with African cassava mosaic virus and East African cassava mosaic virus (EACMV). A pandemic of severe CMD resulted from rapid spread of these synergistic mixed infections (Harrison et al. 1997, Legg et al. 2011). A heightened risk of disease spread was predicted for areas of widespread cassava cultivation, and slower spread was anticipated where there were major topographical barriers, such as lakes or dense forests (Legg 1999, Legg 2010). More specifically, contrasting rates of CMD spread down the east and west sides of Lake Victoria have been attributed to more contiguous cultivation of cassava on the western side of the Lake, and the physical barrier imposed by the Winam Gulf on the eastern side of Lake Victoria in western Kenya. The limited cassava in central Kenya and central regions of Tanzania likely served as a barrier to the spread of the pandemic associated with the Ugandan strain of EACMV to coastal regions of East Africa (Szyniszewska et al. 2017).
Figure 2.
(a) Cassava mosaic disease in Tay Ninh, Vietnam, and (b) cassava brown streak disease in Mkuranga, Coast Province, Tanzania. Photographs: James Legg.
Evaluating the potential role of locations in spread networks
We evaluate the global cropland connectivity risk associated with cassava and four other crops of particular importance to food security for smallholder farmers in the tropics. These crops are vegetatively propagated, with the associated high risk of transmission of pests and diseases through planting materials. Cropland connectivity captures some elements of the risk of transmission through movement of pathogens and pests independent of crop plants (through flight or passive dispersal in wind, for example), as well as risk due to movement of planting materials and farm equipment. Because information about relevant dispersal kernels is often unavailable, uncertainty quantification (or sensitivity analysis) may be needed to understand how dispersal parameters influence estimates. The objectives of this article are to characterize the network structure of global cropland for banana and plantain, cassava, potato, sweet potato, and yam (Dioscorea spp.); to evaluate the network structure in terms of its potential impact on pest and disease risk due to dispersal, using an index summarizing key metrics for cropland connectivity risk based on a gravity model, with associated uncertainty quantification; and to use the network structure to identify geographic priorities for surveillance and management of emerging pests and diseases, and for saturation of endemic species. We discuss the geographic spread of diseases in the context of cropland connectivity using as examples some key diseases and pests, including banana bunchy top disease, Xanthomonas wilt of bananas, potato yellow vein, and Guatemalan potato tuber moth.
Model of risk of movement between geographic nodes
We described the risk for pathogen and pest movement between each pair of nodes in a gravity model (Jongejans et al. 2014) incorporating the distance between the nodes and the cropping density associated with the nodes. An adjacency matrix summarizes this risk, such that each entry in the matrix corresponds to the risk for a pair of nodes. The distance effect on the risk was calculated as a function of the Vincenty ellipsoid (Hijmans et al. 2017b) distance between the centers of nodes i and j (dij) for two common models of dispersal risk: an inverse power law function, dij–β, and a negative exponential function, exp(–γdij) (Gregory 1968, Campbell and Madden 1990, Mundt et al. 1999, Madden et al. 2007, Mundt et al. 2009b, Severns et al. 2014). For convenience, the distance in meters was further scaled by dividing by 111,319.5 (one degree of Vincenty ellipsoid distance in meters at the equator). Higher values of the parameters β and γ reflect lower likelihood of long-distance dispersal. Three levels (0.5, 1.0, and 1.5) of parameter β were considered for the inverse power law function in uncertainty quantification (table 1), generating a 71%, 50%, and 35% chance of movement, respectively, across a distance of 2 degrees. Five levels (0.05, 0.1, 0.2, 0.3, and 1.0) of parameter γ were evaluated in uncertainty quantification, generating corresponding chances of movement across a distance of 2 degrees of 90%, 82%, 67%, 55%, and 14%, respectively (before adjustment for the proportion harvested area in the two linked pixels). The risk due to greater cropland area for any two nodes i and j was accounted for by multiplying together the mean cropland area (c) associated with each of the nodes (cicj). Therefore, in the first step the weights in the adjacency matrix indicating the overall risk of movement between two geographic nodes were cicjdij–β for the inverse power law function and cicjexp(–γdij) for the negative exponential function. In the uncertainty quantification we also evaluated results across three threshold minimum values (0.001, 0.0001, and 0.00001) for entries in the matrix individually and set the weights below that to be zero. Network models and metrics for the cropland connectivity for each of the five crops were analyzed for the Eastern and Western Hemispheres separately. We used the igraph package (Csárdi and Nepusz 2006) in the R programming environment (R Core Team 2020) to evaluate the networks, as well as the packages raster (Hijmans et al. 2017a), geosphere (Hijmans et al. 2017b) and viridis (Garnier et al. 2018).
Table 1.
Components of a summary measure of cropland connectivity risk index that were evaluated in uncertainty quantification
| Method | Parameter | Levels | Interpretation | |
|---|---|---|---|---|
| Total mean |

C5mingridi is the cropland proportion of the ith 5 minute grid within a 2 degree grid |
C5mingridi | The sum of cropland proportion of all 5 × 5 minute grids within a 2° × 2° grid is divided by the total number of 5 × 5 minute grids aggregated in a 2° × 2° grid | |
| Land mean |
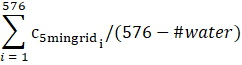
C5mingridi is the cropland proportion of the ith 5 minute grid within a 2 degree grid #water denotes the total number of 5 minute grids with water rather than agricultural land |
C5mingridi | The sum of cropland proportion of all 5 × 5 minute grids within a 2° × 2° grid is divided by the total number of 5 × 5 minute grids containing only land (5 × 5 minute grids with water are excluded) aggregated in a 2° × 2° grid | |
| Dispersal risk model (DRM) DR = cicjdij–β | Inverse power law model dij–β dij is the distance between nodes i and j ci is the fraction harvested area with the crop of interest at the ith node | β | β1 = 0.5 β2 = 1.0 β3 = 1.5 | Potential changes in model to describe different types of pests and dispersal mechanisms |
| DR = cicjexp(–γdij) | Negative exponential model exp(–γdij) dij is the distance between nodes i and j ci is the fraction harvested area with the crop of interest at the ith node | γ | γ1 = 0.05 γ2 = 0.1 γ3 = 0.2 γ4 = 0.3 γ5 = 1.0 | Potential changes in model to describe different types of pests and dispersal mechanisms |
| Cropland proportion | Minimum cropland proportion for inclusion of node in analysis | pc | pc 1 > 0.0015 pc2 > 0.002 pc3 > 0.0025 | Lower thresholds result in more nodes retained in the network |
| Link weight | Minimum link weight for inclusion of link in network | pl | pl 1 > 0.001 pl2 > 0.0001 pl3 > 0.00001 | Lower thresholds result in more links retained in the network |
Note: Each combination of the levels of the values indicated was evaluated. The combinations included varying the form of the mean (total mean or land mean) and varying the dispersal model (inverse power law or negative exponential), as well as the parameters of the model selected.
Network metrics to evaluate invasion and saturation risk. We consider a set of network metrics that have often proven useful for evaluating the role of nodes in network processes. To simplify comparisons, we also summarize across metrics in a cropland connectivity risk index (CCRI). To emphasize the importance of the node as a bridge, the index emphasizes betweenness centrality (supplemental figures S2–S6), based on the number of shortest paths through the network that include the node being evaluated. The other half of the weight is given to other metrics that measure how well connected is a node, its neighbors, and its neighbors’ neighbors: node strength (the sum of a node's link weights), the sum of a node's nearest neighbors’ node degrees (the sum of the number of links associated with each nearest neighbor), and eigenvector centrality (giving each node a score proportional to the sum of the scores of its nearest neighbors and more distant neighbors). The summary index (the CCRI) was calculated as a weighted sum of 1/2 betweenness centrality, 1/6 node strength, 1/6 sum of nearest neighbors’ node degrees, and 1/6 eigenvector centrality, such that each of the four metrics was scaled before summing by dividing by the maximum value observed for that metric. The weighting emphasizes betweenness because betweenness will particularly capture a potential role as a bridge that is not obvious when individual cropland area is considered alone, and also to include connectedness of a node at different scales.
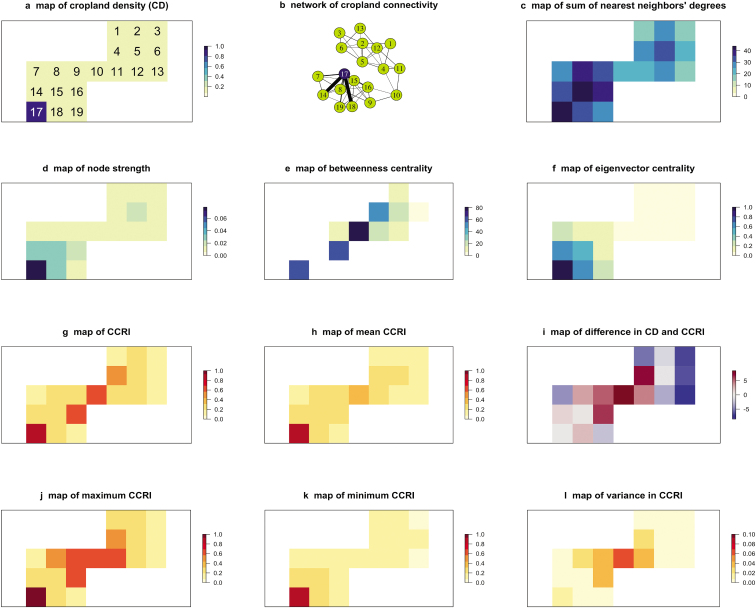
Illustration of features captured by the cropland connectivity risk index. Before we consider cropland connectivity risk for global crop systems, figure 3 is an illustration of how the four metrics described above (betweenness, node strength, the sum of nearest neighbors’ node degree, and eigenvector centrality) capture different elements of cropland connectivity. Suppose this hypothetical map represents cropland density for a target crop species. In this example, most of the cropland units have a low crop proportion (indicated by light green shading), whereas one unit has a high crop proportion (indicated by blue shading). A network (figure 3b) is constructed using the gravity model described above, from the corresponding data for the cropland geographic map (figure 3a), based on one parameter combination for the dispersal model. Note that this is the network for one particular parameter combination, whereas the later components of figure 3 represent summaries across uncertainty quantification, as described more below. The sum of the node degrees for nearest neighbors (figure 3c) captures how well connected the nodes’ neighbors are. Node strength (figure 3d) indicates a node's importance in terms of how connected it is to its neighbors. Betweenness centrality identifies nodes acting as bridges to connect other regions in the network (figure 3e). Eigenvector centrality (figure 3f) shows how well connected a node is through nearest neighbors, their neighbors, and beyond. The CCRI (figure 3g) is the weighted mean of these metrics. In addition to the high-risk locations with high crop density, other locations with high risk because of their role as bridges were identified. The results of an uncertainty quantification (supplemental figure S1) for the CCRI in this hypothetical map are also shown (figure 3h–l), illustrating how a summary across parameter combinations (beyond the parameter combination illustrated in figure 3b) can reveal other features of a cropland landscape. Finally, we identify locations in which CCRI rank among locations is higher than the rank based solely on crop density (figure 3i). These are locations in which the network analysis reveals potentially important roles for a location that would not be as apparent in a simpler point-wise analysis of crop density.
Figure 3.
An illustration of the evaluation of cropland connectivity risk for a simple hypothetical scenario. Note that panels (a–g) illustrate one scenario, whereas panels (h–l) illustrate a summary across multiple scenarios in a sensitivity analysis or uncertainty quantification. (a) The map of cropland density indicates the fraction harvested area for a crop species in a hypothetical small region in which white areas have none of the crop species, light green areas (1–16 and 18–19) have a low fraction of land planted to the crop species, and the blue area (17) has a high fraction planted to the crop species. (b) The network of cropland connectivity that corresponds to the map in a, indicating the links for one set of threshold parameters (negative exponential function with γ = 0.7 was used to calculate the link weight, and a threshold of 0.001 was used to determine whether a link exists). The high density region (blue node 17) and bridging region (light green node 10) are indicated. (c) A map of the sum of nearest neighbors’ degrees for the network in b. Nearest neighbors are those with direct links to a reference node, and node degree is the number of links to a node. (d) A map of node strength for the network in b. Node strength is the sum of the weights of links to a reference node. (e) A map of betweenness centrality for the network in b. Betweenness centrality indicates the number of shortest paths in the network that pass through a reference node. (f) Eigenvector centrality for the network in b. Eigenvector centrality is a measure of how well connected a node is in terms of immediate neighbors, their neighbors, etc. (g) The CCRI for the network in b. CCRI is a weighted mean of the four measures of connectedness in maps c through f. Note that this is the CCRI for one parameter combination, whereas additional combinations are illustrated in the supplemental material (figure S1). (h) A map of the mean CCRI from uncertainty quantification for networks corresponding to map a for a range of parameter combinations (table 1). (i) The difference between ranked values of the mean CCRI from a uncertainty quantification, and the ranked values in map a. This difference indicates locations in which the fraction of land planted to the crop species does not capture all the features of connectivity in the CCRI. Red colors indicate regions in which the CCRI rank is higher than the ranked values of map a, and blue colors indicate regions in which the CCRI is lower. (j) Map of the maximum CCRI from uncertainty quantification. (k) Map of the minimum CCRI from uncertainty quantification. (l) Map of the variance in CCRI across realizations in uncertainty quantification.
Global cropland area
Now we expand this cropland connectivity analysis to the global cropland for five crops. We analyzed two standard data sets representing the global geographic distribution of individual crop species: data representing the conditions circa 2000 from Monfreda and colleagues (2008), referred to in the present article as the Monfreda data set, and IFPRI's Spatial Production Allocation Model (SPAM) data 2005 v3.2 (IFPRI and IIASA 2016), referred to in the present article as the SPAM data set. Each of the two global cropland data sets was analyzed individually using the methods described below (supplemental figures S2b, S3b, S4b, S5b, S6b). We also evaluated a combined data set, “Monfreda and SPAM,” in an ensemble map, based on the mean of the harvested area fraction from the two data sources.
For each of these three data sets, we evaluated the harvested cropland area for banana (supplemental figure S2a), cassava (supplemental figure S3a), potato (supplemental figure S4a), sweet potato (supplemental figure S5a), and yam (supplemental figure S6a). In the present article, we use the term banana to refer to both bananas and plantains (Beed et al. 2012). Data were spatially aggregated by finding the mean harvested area for each crop across 24 × 24 grids of the original 5 × 5 minute grids, for a resolution of 120 × 120 minutes (2 × 2 degrees [°]). We used two methods to calculate the mean of the crop harvested area per grid: the land mean (the sum of the harvested area fractions within an aggregated 2° × 2° unit divided by the number of 5 × 5 minute units within the aggregated unit that contain only land; supplemental figures S2a2, S3a2, S4a2, S5a2, S6a2) and the total mean (the same sum divided by the total number of 5 × 5 minute units within an aggregated 2° × 2° unit; figures supplemental S2a1, S3a1, S4a1, S5a1, S6a1). These two formulations of the mean are different particularly on coastlines and for islands; the uncertainty quantification below addresses both formulations. To focus on more important production areas, we considered three threshold values for inclusion of nodes in the analysis: 0.0015, 0.002, and 0.0025 mean proportion cropland harvested area.
Uncertainty quantification and data quality
We performed an analysis of model sensitivity to parameter shifts to evaluate how consistent results were under changes in model parameters and to determine which nodes had high cropland connectivity risk across all or most model scenarios and which nodes had high risk only for a limited range of model scenarios. On the basis of the combinations of functions, thresholds, and parameters used, 144 cropland connectivity risk index maps were generated for each crop (table 1). For each cell in the maps, we calculated the mean, max, min, and variance across the 144 maps. And, for reference, we summarize the data quality assessment provided by Monfreda and colleagues (2008; supplemental figures S2c, S3c, S4c, S5c, S6c). We also compared how locations rank on the basis of CCRI with how they rank on the basis of harvested crop fraction (crop density). Those locations in which the CCRI rank is substantially higher than the crop density rank are locations that might have particularly important roles in epidemic spread but that would not be identified if analysis looked solely at crop density.
Cropland connectivity and the risk of major pests and diseases of roots, tubers, and bananas
Mapped areas with a higher CCRI are likely to have higher risk for dispersal of pathogens or pests of banana and plantain, cassava, potato, sweet potato, and yam, based on cropland connectivity (figure 4). Cropland connectivity is a risk factor for movement through wind dispersal, active pest movement, vector movement, seed exchange, farm tools, or trade. The locations we identified are candidates for prioritizing surveillance and mitigation programs (Smolinski et al. 2003, Woolhouse et al. 2005), especially if information about weather conduciveness to invasion—for example, suitable temperature (Kroschel et al. 2016)—and other risk factors such as documented trade patterns (Andersen et al. 2019), also support the high-risk designation.
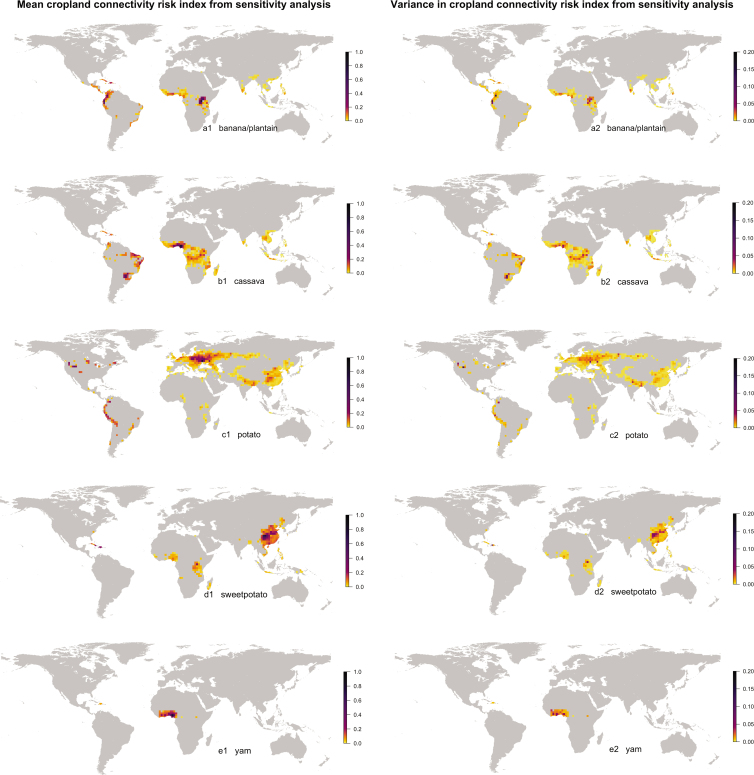
Figure 4.
The mean and variance observed for cropland connectivity risk index components in uncertainty quantification for banana/plantain, cassava, potato, sweet potato, and yam (based on the mean of crop density estimates from Monfreda et al. 2008 and SPAM, IFPRI and IIASA 2016).
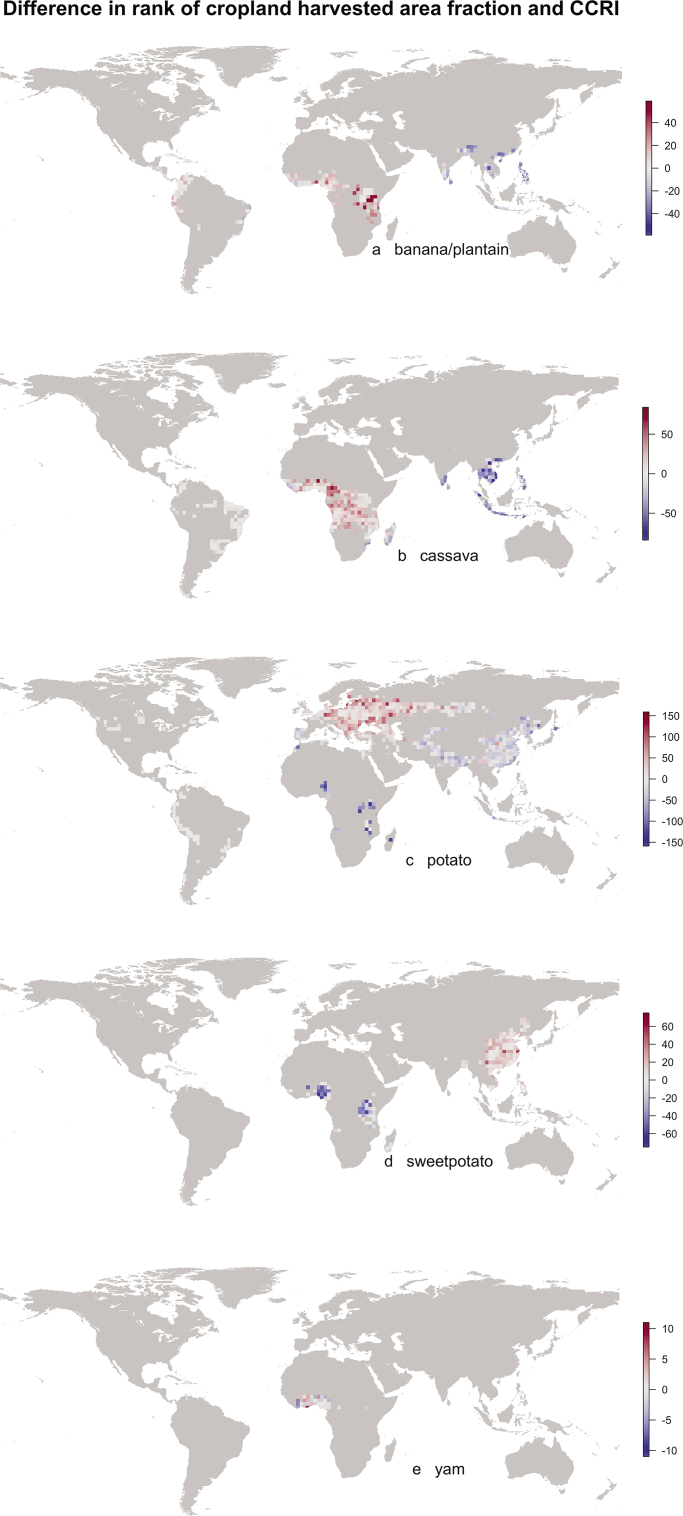
A cropland connectivity risk index will often be an important component of integrated geographic risk assessment, along with weather/climate risk factors, genetic resistance deployment, and trade. We demonstrated how a cropland connectivity risk index can be designed to go beyond simply identifying as high risk those land units that have high crop fraction, especially if the index captures how locations may function as bridges by incorporating a measure such as betweenness centrality. Land units with high crop fraction will tend to be identified as high risk, although less so if they are isolated, because the gravity model weights crop fraction in evaluation of the probability of movement. However, the cropland connectivity risk index also identifies locations that have an important role as bridges between cropping regions, even if the cropping density within the bridge land units is not particularly high (the red regions in figure 3i and figure 5). Therefore, analysis of cropland connectivity can identify additional risk areas on the basis of the larger landscape, beyond those identified through a simple unit-by-unit scan for high cropping density.
Figure 5.
Maps of the difference in cell rank between harvested area fraction and the mean cropland connectivity risk index for banana and plantain, cassava, potato, sweet potato, and yam (based on the mean of crop density estimates from Monfreda et al. 2008 and SPAM, IFPRI and IIASA 2016). The locations at which the CCRI has substantially higher rank than the crop density could have important roles in spread that would not be identified if analyses were limited to crop density.
High variance at a location (figure 4) suggests that more information is needed to be confident about that location's role, such as dispersal model parameter estimates specific to the disease or pest in question, or more information about cropping density to support analysis of the CCRI. Locations with the combination of high mean and low variance may be particularly good candidates for surveillance and mitigation prioritization, because these locations are more likely to have a high risk across all model assumptions evaluated.
Banana and plantain
A combination of high mean CCRI and low variance in CCRI in uncertainty quantification was observed for central, north central and southern Uganda, northwest Tanzania (figure 4a1, 4a2), Rwanda, Burundi, the Inter-Andean valleys in Colombia, central and western Ecuador, and Haiti. The highest global CCRI was found in the border region of Uganda, Rwanda, and Tanzania (figure 4a1). High variance in CCRI was observed in limited regions of Colombia and Ecuador (figure 4a2). The CCRI rank was substantially higher than the rank based on cropland density alone in multiple locations in Africa, particularly in Tanzania (figure 5a).
Two banana diseases illustrate how pathogen invasion and spread can be linked to cropland connectivity. Banana bunchy top disease, caused by Banana bunchy top virus (BBTV, genus Babuvirus), causes devastating losses. Phylogenetic studies of BBTV spread in Africa, along with farmers’ observations, suggest dual introduction events for BBTV—in Egypt and then in the Democratic Republic of Congo—before further virus spread in Sub-Saharan Africa (Kumar et al. 2011, Leung et al. 2015). Movement of planting material, and long-distance spread facilitated by migrant workers, likely have contributed to the gradual expansion of BBTV in Sub-Saharan Africa. Ubiquitous distribution of the vector, the banana aphid (Pentalonia nigronervosa), contributed to local spread of the virus. Xanthomonas wilt of banana (caused by Xanthomonas vasicola pv. musacearum) often causes yield reductions of up to 100% (Tushemereirwe et al. 2004, Ndungo et al. 2006, Tripathi et al. 2009). This disease is mainly spread by infected planting material, insect vectors, farm tools, browsing animals, and occasionally by bats, birds, and weevils (Yirgou and Bradbury 1974, Gold and Bandyopadhyay 2006, Tinzaara et al. 2006, Were et al. 2015). The disease had been limited to the Ethiopian enset growing belt until 2001 when it appeared in banana fields in central Uganda and eastern Democratic Republic of Congo (Tushemereirwe et al. 2004, Ndungo et al. 2006). Highly connected and susceptible Musa ABB type production systems dominate in lower elevation central Uganda, where there is high insect activity, and this, combined with often poor plot management, likely produced the particularly fast spread of the disease. In contrast, and despite a high level of connectivity of banana cultivation zones, disease spread has been slower in the Albertine rift valley mountainous region of east Democratic Republic of Congo. This is probably mainly due to elevation effects on insect vector transmission and the predominance of the Musa AAA-EA subgroup, which are less susceptible to insect vector transmission. In this region, disease spread mainly occurs through garden tool use. The adjacent Congo basin lowlands to the west of the rift valley are dominated by dense tropical forests, with bananas and plantains only in villages along road or river axes, and often low connectivity. As a result, disease spread into the Congo basin has, over a period of nearly 20 years, been very limited.
Cassava
The CCRI for cassava was particularly high throughout southern Nigeria, south central Ghana, western Burundi, northern Brazil (Bahia and Amazonas states), southern Brazil (Parana and Mato Grosso states), southwest Paraguay, and northeast Argentina (figure 4b1). High variance in the CCRI was observed in the border region shared by Paraguay, Brazil, and Argentina (figure 4b2). The CCRI rank was substantially higher than the rank based on cropland density alone in locations in Nigeria, Cameroon, and the Democratic Republic of Congo (figure 5b).
Cassava frogskin disease is an economically important disease of cassava across Latin America and the Caribbean, where up to 90% yield loss was reported in severely infected fields in the 1980s (Pineda et al. 1983). The disease has been associated with several pathogens, and transmission is typically via asymptomatically infected cassava planting material (Alvarez et al. 2009, Carvajal-Yepes et al. 2014). Until 1971, the disease had been found only in Colombia, but since then, it has been reported in Panama, Costa Rica, Venezuela, Peru, and Brazil, (Chaparro-Martinez and Trujillo-Pinto 2001, Calvert and Thresh 2002, Calvert et al. 2012, Di Feo et al. 2015). A more recent threat to cassava production is the reemergent cassava common mosaic disease (CCMD), caused by the mechanically transmitted potexvirus Cassava common mosaic virus. Originally reported to cause significant yield losses in southern Brazil since the 1940s (for a review, see Lozano et al. 2017), recent outbreaks of CCMD have been reported in Peru and Argentina (Di Feo et al. 2015, Fernandez et al. 2017, Zanini et al. 2018). Because of the likely spread of these pathogens through asymptomatic planting material, it is probable that patterns of spread throughout Latin America are related to high intensity areas of production. For example, CCMD is reported in high intensity areas of production in northeast Peru and northeast Argentina, where high CCRI was observed in our analysis.
In contrast to CMD (discussed earlier), cassava brown streak disease (CBSD; figure 2b) is less readily spread by vectors. Its rate of spread into Central Africa was likely also slowed because of a lack of cassava cropland connectivity, associated with the massive barrier of the great forests of the Congo Basin. High levels of cassava cropland connectivity in West Africa, from western Cameroon westward, suggest that if CBSD is introduced to this area, there is likely to be rapid spread further westward. Both cassava pandemics are being driven by superabundant populations of the whitefly vector, Bemisia tabaci. Cropland connectivity is also likely to be important for Bemisia whitefly populations, because the whitefly genotypes occurring on cassava have a strong preference for this crop (Wosula et al. 2017).
Potato
The CCRI for potato was particularly high in northcentral Europe, including northern and central Ukraine, central Poland, central and southern Belarus, and southwestern Russia. CCRI was also high in locations in the United States (Idaho, Washington, Colorado, and the northern Great Lakes region), New Brunswick in Canada, locations in Peru and central Colombia, and central China (figure 4c1). In Africa, the Lake Kivu region has a high CCRI. High variance in the CCRI was observed in central Colombia and central Peru, and around the border between India and Bangladesh (figure 4c2). The CCRI rank was substantially higher than the rank based on cropland density alone in multiple locations in Eastern Europe and Eastern China (figure 5c).
Potato yellow vein is an important potato disease in the northern Andean region caused by Potato yellow vein virus (PYVV). PYVV is transmitted by the greenhouse whitefly (Trialeurodes vaporariorum), through seed potato and underground stem grafts (Salazar et al. 2000). Originally reported in northern Ecuador and west central Colombia, the virus has spread, probably via infected seed tubers, throughout the central Andes, particularly to the most important potato-producing areas of Colombia (Guzmán et al. 2006), Venezuela, and northern Peru. Interestingly, PPVV has not moved further south over the last 20 years despite predicted favorable conditions for whiteflies in these regions (Gamarra et al. 2016). Likely a gap with reduced cropland connectivity and limited potato seed exchange between northern and southern Peru has contributed to this lack of spread, although other factors, such as cultivar resistance, may also be important factors. Central Peru should be a priority area for monitoring to prevent further spread of the disease to southern Peru and Bolivia, whereas spread to other regions is likely only possible through long distance transport of infected potato.
The potato tuber moth, Tecia solanivora, is a challenging potato pest in Central and South America (Kroschel and Schaub 2013). Guatemala is understood to be the country of origin. In 1970, the pest was accidently introduced with infested seed into potato growing regions of Costa Rica—in 1983, into Venezuela and, in 1985, into Colombia. In 2010, T. solanivora was reported for the first time from southern Mexico and, in 1996, from Ecuador. In 1999, T. solanivora appeared on Tenerife, Canary Islands. Since then, the pest has been considered a major threat to potato crops throughout southern Europe and was listed as a quarantine pest by the European and Mediterranean Plant Protection Organization (EPPO 2005). Schaub and colleagues (2016) confirmed the suitable climatic conditions in southern Europe. However, because T. solanivora is strongly monophagous and potato is its only host plant, the movement of infested seed is the main potential pathway of its spread into new potato growing regions, especially if there is not a high level of cropland connectivity among the regions. The pest was therefore contained in Tenerife for many years before it was first detected in mainland Spain in Galicia in 2015 and in Asturias in 2016 (Jeger et al. 2018). In contrast, the South American tomato leafminer, Tuta absoluta, after its transatlantic invasion and first detection in Spain in 2006, rapidly spread across southern Europe, Africa, and Asia (https://gd.eppo.int/taxon/GNORAB/distribution). Compared with T. solanivora, also a Lepidopteran pest of potato, the very rapid spread of T. absoluta is likely due at least in part to its very wide host range in the Solanaceae, and the very high level of connectivity of these combined species. Therefore, considering the low level of connectivity of the potato crop in many regions (e.g, in southern Europe and much of Africa), it will likely be more difficult for a monophagous potato pest such as T. solanivora to invade new potato growing regions.
Sweet potato
The CCRI for sweet potato was high in locations in central China, the Caribbean (Haiti and the Dominican Republic; figure 4d1), and in central Uganda, with central China having the highest ranked global risk. High variance in the CCRI was observed in the area north of Lake Victoria in Uganda (figure 4d2). The CCRI rank was substantially higher than the rank based on cropland density alone in multiple locations in China (figure 5d).
In sweet potato, several weevils important to yield loss exist worldwide. Sweet potato viruses such as sweet potato chlorotic stunt virus (SPCSV), sweet potato feathery mottle virus, and some begomoviruses are already present globally, whereas other viruses such as sweet potato mild mottle virus are found only in certain regions. Some strains of sweet potato viruses, such as the severe EA strain of SPCSV, are geographically localized. Movement of planting material (sweet potato vines) through trade can cover long distances (Rachkara et al. 2017). Whereas some sweet potato pests such as viruses are easily spread through planting material (Gibson and Kreuze 2015) and can form permanent reservoirs in wild host species (Tugume et al. 2008, Tugume et al. 2013, Tugume et al. 2016), others, such as weevils, are not readily spread through planting material, have no known alternative hosts, and are unable to travel long distances by themselves.
Yam
Of the crops evaluated, yam had the lowest overall global harvested area. The highest CCRI observed for yam was in locations in southcentral Nigeria, Benin, Togo, Ghana, and the Ivory Coast, along with locations in the Caribbean including Haiti (figure 4e1). High variance in the CCRI was observed at the border of Mali and Côte d'Ivoire (figure 4e2). The CCRI rank was substantially higher than the rank based on cropland density alone in locations in eastern Nigeria, Togo, western Ivory Coast, and the Dominican Republic (figure 5e).
Yam is a multispecies crop grown for its tubers by millions of smallholder farmers in West Africa. Nearly 94% of global edible yam production is in West Africa (Benin, Cameroon, Côte d'Ivoire, Ghana, Nigeria and Togo), and Nigeria alone produces 66% of global production (FAOstat 2016). Major constraints to yam production in West Africa are mosaic disease caused by Yam mosaic virus and Yam mild mosaic virus (genus Potyvirus), and anthracnose caused by Colletotrichum gloeosporioides. Damage to yam by nematodes—Scutellonema bradys, Pratylenchus spp., and Meloidogyne spp.—is responsible for significant pre- and postharvest deterioration of tubers. All these agents are endemic in all the yam production regions in West Africa, so saturation is the main consideration within that region, and anecdotal evidence clearly links the spread of yam viruses and nematodes to the movement of planting material in West Africa.
Incorporating cropland connectivity in risk assessments
These analyses illustrate general cropland connectivity risk across a large spatial extent and for a fairly coarse spatial resolution. The maps of mean CCRI for each crop are available through links at garrettlab.com/cropland-connectivity, along with information for reading the data into R for potential further analysis or integration with other risk factors. Follow-up analyses for specific locations and particular pathogen or pest species may be useful, especially when more detailed data are available for mapping cropland fraction and for selecting appropriate functions as dispersal kernels for specific time scales, and potentially other system-specific factors. The results presented in this article have a greater confidence for certain crops such as potato and certain regions because of the quality and quantity of the original data available for assembly by Monfreda and colleagues (2008) and in SPAM 2005 v3.2 (IFPRI and IIASA 2016). Examples of the application of network analysis to invasions of particular species include Phytophthora ramorum (Harwood et al. 2009, Shaw and Pautasso 2014) and Phakopsora pachyrhizi (Sutrave et al. 2012, Sanatkar et al. 2015). The role of a land unit will depend on the species of pathogen or pest being considered, and its dispersal kernel. A particular land unit evaluated for species that tend to move only short distances might be isolated, whereas for species that tend to move longer distances it might be an important bridge node (Calabrese and Fagan 2004).
Individual pathogen or pest data and implications for trade can be disseminated via actively updated Regional Pest Risk Assessment working documents, to support standards established by the International Plant Protection Convention to prevent introduction, establishment and spread of pests and diseases, implemented by National and Regional Plant Protection Organizations (Miller et al. 2009, IPPC 2012, Beed et al. 2013). A general measure such as the CCRI can usefully become a standard feature of PRAs, as a starting point in addressing a pest or disease for which there is limited information available. As more attention is focused on the invasive species and data collection increases, scenario analyses of management options, supported by epidemic simulation, could inform increasingly targeted surveillance and mitigation efforts (e.g., Garrett et al. 2018, Andersen et al. 2019).
Other useful points for future research to refine cropland connectivity risk assessments include the following. The current analysis is based on geographic data for 2000 (Monfreda et al. 2008) and 2005 (IFPRI and IIASA 2016), so areas in which crop densities have increased rapidly in recent years are not represented in these global maps yet. An important example is cassava production in Southeast Asia, where production has quickly expanded and is now experiencing an invasion of Sri Lankan cassava mosaic virus (Wang et al. 2015, Delaquis et al. 2018). The cropland density data are summarized across global data sets that vary widely in quality from region to region. The resolution we selected for our analyses was intended to represent a compromise—avoiding too high a spatial resolution because it might have little data to back it up in many regions, and also avoiding too coarse a resolution that might obscure the roles of specific locations. Where more complete data are available or can be collected, more detailed and higher resolution analyses can be performed. Likewise, the current analysis does not take into account geographic features that could have important effects on the likelihood of active or passive movement of pathogens and pests, or weather features such as wind patterns (Sutrave et al. 2012). Roads and rivers may increase pathogen movement, whereas other water bodies, deserts, and mountains may isolate nodes (Meentemeyer et al. 2012). And the distribution of individual crop species captures only some aspects of risk for many pathogens and pests that can use multiple host species. Conversely, if resistance genes are widely deployed, pathogens and pests may only be able to use a subset of the planted fraction (Brown and Hovmoller 2002, Garrett et al. 2017). Extreme weather patterns may be responsible for many important regional or global introductions of pathogens and pests, such as the potential introduction of soybean rust to the United States in hurricane Ivan (Schneider et al. 2005). Flooding may move some soilborne pathogens to new locations. Finally, heterogeneity in time may alter patterns of cropland connectivity. Markets may drive longer term trends in planting patterns, and for shorter season crops such as potato and sweet potato, geographic heterogeneity in planting seasons may disrupt the cropland connectivity suggested when seasons are aggregated.
We present analyses for the crop species individually. However, as an overall measure of cropland connectivity risk for pathogen and pest invasion and saturation for these crops, a cross-crop index constructed by adding the individual species risk indices may be useful for evaluating strategies for general purpose surveillance and management strategies for the set of crops. Combining across host species might also be useful for special cases in which a target pathogen or pest uses multiple host species.
Two regions in Africa, the Great Lakes Region and the region between Ghana and Nigeria, have high cropland connectivity risk for multiple crops. The Ugandan strain of the East African cassava mosaic virus caused famine in East, Central, and West Africa (Anderson et al. 2004), and wheat stem rust race Ug99 also emerged in Uganda (Pretorius et al. 2000). It is an interesting open question if the geographic position of Uganda in cropland networks had some influence on disease emergence, or if it was simply a matter of higher sampling effort that made detection more likely.
Areas identified with low connectivity and low risk can also be prioritized to produce disease-free seed. This has often happened naturally—for example, where particularly dry regions that are not optimal for crop production and, therefore, are often isolated from other crop production regions may produce seed with low disease risk. Seed production areas play an outsize role in the risk of disease spread, even where cropping density is low. The risk of pathogen spread through seed networks is a key component for integration with risk on the basis of cropland connectivity (Garrett et al. 2018, Andersen et al. 2019). The movement of pathogens through the international seed trade is an important risk factor for many crops (Anderson et al. 2004, Wylie et al. 2008, Rodoni 2009). In Sub-Saharan Africa, movement of plant material and farming tools is a key factor for the dispersal of banana diseases such BXW (Tripathi et al. 2009, Beed 2014) and through cuttings for cassava virus diseases, particularly CBSD (Bock 1994, Legg et al. 2015). The United States late blight pandemic in 2009 was caused by the movement of infected tomato plants via trade from a single national supplier (Fry et al. 2013). During 2009–2010, an epidemic of P. infestans in tomato was reported in southwest India with the suggestion that the pathogen was introduced via seed potato imported from the UK and Europe before 2009 (Chowdappa et al. 2013). Cropland connectivity is likely to capture at least a portion of the risk associated with movement of seed, transplant, and agricultural equipment, to the extent that trade and movement of equipment and agricultural workers tends to follow a path through areas that produce a particular crop. Of course, at the same time that cropland connectivity represents a risk for the spread of pathogens and pests, connectivity may also confer advantages for efficiency in deployment of equipment and personnel, as well as marketing of seed and produce.
In summary, cropland connectivity constitutes a risk component important for most pests and diseases. It can complement risk assessments based on the effects of climate, genetic resistance, and formal trade networks. The integration of these risk assessment layers will make the best use of available data to evaluate risk and guide targeted surveillance and mitigation in global strategies such as the proposed Global Surveillance System for plant disease (Carvajal-Yepes et al. 2019). Uncertainty quantification can help in interpreting analyses when information about dispersal kernels is not available, or when a more general analysis is desired, and in targeting data collection to the most important data for improving key parameters. Availability of high quality data related to cropping density, deployment of genetic resistance, and weather patterns will improve risk assessments. Developing optimal methods for integration across cropland connectivity and other risk data layers is an important challenge for risk analysis and offers the promise of more effective risk assessment in the future.
Supplementary Material
Acknowledgments
This research was undertaken as part of and funded by the CGIAR Research Program on Roots, Tubers, and Bananas and was supported by CGIAR Trust Fund contributors www.cgiar.org/funders. We also appreciate support by the Bill and Melinda Gates Foundation (grant no. OPP1080975), the CGIAR Research Program on Climate Change and Food Security, US Department of Agriculture Animal and Plant Health Inspection Service grant no. 11–8453–1483-CA, US National Science Foundation (NSF) grant no. EF-0525712 as part of the joint NSF–National Institutes of Health Ecology of Infectious Disease program, NSF grant no. DEB-0516046, and the University of Florida. We appreciate a helpful discussion with Navin Ramankutty and helpful comments from BioScience reviewers. Yanru Xing and John F. Hernandez Nopsa contributed equally to this work.
Author Biographical
Yanru Xing, John F. Hernandez Nopsa, Kelsey F. Andersen, and Karen A. Garrett (karengarrett@ufl.edu) are affiliated with the Plant Pathology Department, Institute for Sustainable Food Systems, and Emerging Pathogens Institute at University of Florida, Gainesville, USA. John F. Hernandez Nopsa is affiliated with Corporación Colombiana de Investigación Agropecuaria, AGROSAVIA, Mosquera-Bogota, Colombia. Jorge L. Andrade-Piedra, Gregory A. Forbes, Jan F. Kreuze, and Jürgen Kroschel are affiliated with the International Potato Center (CIP), P.O. Box 1558, Lima 12, Peru. Fenton D. Beed is affiliated with the Plant Production and Protection Division, Food and Agriculture Organization of the United Nations (FAO), 00153 Roma, Italy. Guy Blomme is affiliated with Bioversity International, c/o ILRI, Addis Ababa, Ethiopia. Mónica Carvajal-Yepes and Wilmer J. Cuellar are affiliated with the International Center for Tropical Agriculture (CIAT), AA6713, Cali, Colombia. Danny L. Coyne is affiliated with the International Institute of Tropical Agriculture (IITA), Nairobi, Kenya. P. Lava Kumar is affiliated with the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. James P. Legg is affiliated with the International Institute of Tropical Agriculture (IITA), Dar es Salaam, Tanzania. Monica Parker, Elmar Schulte-Geldermann, and Kalpana Sharma are affiliated with the International Potato Center (CIP), Nairobi, Kenya. All authors are affiliated with the CGIAR Research Program on Roots, Tubers, and Bananas. Yanru Xing and John F. Hernandez Nopsa contributed equally to this work.
Contributor Information
Yanru Xing, Plant Pathology Department, Institute for Sustainable Food Systems, and Emerging Pathogens Institute at University of Florida, Gainesville, USA; Yanru Xing and John F. Hernandez Nopsa contributed equally to this work.
John F Hernandez Nopsa, Corporación Colombiana de Investigación Agropecuaria, AGROSAVIA, Mosquera-Bogota, Colombia; Yanru Xing and John F. Hernandez Nopsa contributed equally to this work.
Kelsey F Andersen, Plant Pathology Department, Institute for Sustainable Food Systems, and Emerging Pathogens Institute at University of Florida, Gainesville, USA; CGIAR Research Program on Roots, Tubers, and Bananas.
Jorge L Andrade-Piedra, International Potato Center (CIP), P.O. Box 1558, Lima 12, Peru; CGIAR Research Program on Roots, Tubers, and Bananas.
Fenton D Beed, Plant Production and Protection Division, Food and Agriculture Organization, United Nations (FAO), 00153 Roma, Italy; CGIAR Research Program on Roots, Tubers, and Bananas.
Guy Blomme, Bioversity International, c/o ILRI, Addis Ababa, Ethiopia; CGIAR Research Program on Roots, Tubers, and Bananas.
Mónica Carvajal-Yepes, International Center for Tropical Agriculture (CIAT), AA6713, Cali, Colombia; CGIAR Research Program on Roots, Tubers, and Bananas.
Danny L Coyne, International Institute of Tropical Agriculture (IITA), Nairobi, Kenya; CGIAR Research Program on Roots, Tubers, and Bananas.
Wilmer J Cuellar, International Center for Tropical Agriculture (CIAT), AA6713, Cali, Colombia; CGIAR Research Program on Roots, Tubers, and Bananas.
Gregory A Forbes, International Potato Center (CIP), P.O. Box 1558, Lima 12, Peru; CGIAR Research Program on Roots, Tubers, and Bananas.
Jan F Kreuze, International Potato Center (CIP), P.O. Box 1558, Lima 12, Peru; CGIAR Research Program on Roots, Tubers, and Bananas.
Jürgen Kroschel, International Potato Center (CIP), P.O. Box 1558, Lima 12, Peru; CGIAR Research Program on Roots, Tubers, and Bananas.
P Lava Kumar, International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria; CGIAR Research Program on Roots, Tubers, and Bananas.
James P Legg, International Institute of Tropical Agriculture (IITA), Dar es Salaam, Tanzania; CGIAR Research Program on Roots, Tubers, and Bananas.
Monica Parker, International Potato Center (CIP), Nairobi, Kenya; CGIAR Research Program on Roots, Tubers, and Bananas.
Elmar Schulte-Geldermann, International Potato Center (CIP), Nairobi, Kenya; CGIAR Research Program on Roots, Tubers, and Bananas.
Kalpana Sharma, International Potato Center (CIP), Nairobi, Kenya; CGIAR Research Program on Roots, Tubers, and Bananas.
Karen A Garrett, Plant Pathology Department, Institute for Sustainable Food Systems, and Emerging Pathogens Institute at University of Florida, Gainesville, USA; CGIAR Research Program on Roots, Tubers, and Bananas.
References cited
- Aguayo J, Elegbede F, Husson C et al. 2014. Modeling climate impact on an emerging disease, the Phytophthora alni induced alder decline. Global Change Biology 20: 3209–3221. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Mejía JF, Llano GA et al. 2009. Characterization of a phytoplasma associated with frogskin disease in cassava. Plant Disease 93: 1139–1145. [DOI] [PubMed] [Google Scholar]
- Anderson PK, Cunningham AA, Patel NG et al. 2004. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends in Ecology and Evolution 19: 535–544. [DOI] [PubMed] [Google Scholar]
- Andersen KF, Buddenhagen C, Rachkara P et al. 2019. Modeling epidemics in seed systems and landscapes to guide management strategies: The case of sweetpotato in Northern Uganda. Phytopathology 109: 1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat A, Barthelemy M, Pastor-Satorras R et al. 2004. The architecture of complex weighted networks. Proceedings of the National Academy of Sciences 101: 3747–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebber DP, Ramotowski MAT, Gurr SJ. 2013. Crop pests and pathogens move polewards in a warming world. Nature Climate Change 3: 985–988. [Google Scholar]
- Bebber DP, Holmes T, Gurr SJ. 2014. The global spread of crop pests and pathogens. Global Ecology and Biogeography 23: 1398–1407. [Google Scholar]
- Beed F, Dubois T, Markham R. 2012 A strategy for banana research and development in Africa. Scripta Horticulturae. 107. [Google Scholar]
- Beed F, et al. 2013. Processes and partnerships for effective regional surveillance of banana diseases. Pages 210–215 in Blomme G, Asten Pv, Vanlauwe B, eds. Banana Systems in the Humid Highlands of Sub-Saharan Africa: Enhancing Resilience and Productivity. CABI. [Google Scholar]
- Beed FD. 2014. Managing the biological environment to promote and sustain crop productivity and quality. Food Security 6: 169–186. [Google Scholar]
- Bock K. 1994. Studies on cassava brown streak virus disease in Kenya. Tropical Science 34: 134–145. [Google Scholar]
- Bock KR, Woods RD.. 1983. Etiology of African cassava mosaic disease. Plant Disease 67: 994–995. [Google Scholar]
- Brown JKM, Hovmoller MS.. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297: 537–541. [DOI] [PubMed] [Google Scholar]
- Buddenhagen CE, Hernandez Nopsa JF, Andersen KF et al. 2017. Epidemic network analysis for mitigation of invasive pathogens in seed systems: Potato in Ecuador. Phytopathology 107: 1209–1218. [DOI] [PubMed] [Google Scholar]
- Cameron A.R. 2012. The consequences of risk-based surveillance: Developing output-based standards for surveillance to demonstrate freedom from disease. Preventive Veterinary Medicine 105: 280–286. [DOI] [PubMed] [Google Scholar]
- Calabrese JM, Fagan WF.. 2004. A comparison-shopper's guide to connectivity metrics. Frontiers in Ecology and the Environment 2: 529–536. [Google Scholar]
- Calvert LA, Thresh JM.. 2002. Virus and virus diseases of cassava. Pages 237–206 in Hillocks RJ, Thresh JM, Belloti A, eds. Cassava Biology and Utilization. CABI. [Google Scholar]
- Calvert L, Cuervo M, Lozano I. 2012. Cassava viral diseases of South America. Pages 309–318 in Ospina B, Ceballos H, eds. Cassava in the Third Millennium: Modern Production, Processing, Use, and Marketing Systems. CIAT. [Google Scholar]
- Campbell CL, Madden LV.. 1990. Introduction to Plant Disease Epidemiology. Willey. [Google Scholar]
- Carvajal-Yepes M, Olaya C, Lozano I et al. 2014. Unraveling complex viral infections in cassava (Manihot esculenta Crantz) from Colombia. Virus Research 186: 76–86. [DOI] [PubMed] [Google Scholar]
- Carvajal-Yepes M, et al. 2019. A global surveillance system for crop diseases. Science 364: 1237–1239. [DOI] [PubMed] [Google Scholar]
- Chaparro-Martinez EI, Trujillo-Pinto G.. 2001. First report of frog skin disease in cassava (Manihot esculenta) in Venezuela. Plant Disease 85: 1285. [DOI] [PubMed] [Google Scholar]
- Chaters G.L., Johnson P.C.D., Cleaveland S. et al. 2019. Analysing livestock network data for infectious disease control: An argument for routine data collection in emerging economies. Philosophical Transactions of the Royal Society B 374: 20180264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdappa P, Kumar NBJ, Madhura S et al. 2013. Emergence of 13_A2 blue lineage of Phytophthora infestans was responsible for severe outbreaks of late blight on tomato in South-West India. Journal of Phytopathology 161: 49–58. [Google Scholar]
- Cornell HV, Lawton JH.. 1992. Species interactions, local and regional processes, and limits to the richness of ecological communities: A theoretical perspective. Journal of Animal Ecology 61: 1–12. [Google Scholar]
- Csárdi G, Nepusz T.. 2006. The igraph software package for complex network research. InterJournal, Complex Systems 1695 http://igraph.org. [Google Scholar]
- Delaquis E, et al. 2018. Raising the stakes: Cassava seed networks at multiple scales in Cambodia and Vietnam. Frontiers in Sustainable Food Systems 2: 73. [Google Scholar]
- Di Feo L, Zanini A, Rodríguez Pardina P et al. 2015. First report of Cassava common mosaic virus and cassava frogskin-associated virus infecting cassava in Argentina. Plant Disease 99: 733. [Google Scholar]
- Dubern J. 1994. Transmission of African cassava mosaic geminivirus by the whitefly (Bemisia tabaci). Tropical Science 34: 82–91. [Google Scholar]
- Elith J, Leathwick JR.. 2009. Species distribution models: Ecological explanation and prediction across space and time. Annual Review of Ecology Evolution and Systematics 40: 677–697. [Google Scholar]
- EPPO 2005. Tecia solanivora. EPPO Bulletin; 35: 399–401. [Google Scholar]
- Fears R, Aro E-M, Pais MS et al. 2014. How should we tackle the global risks to plant health? Trends in Plant Science 19: 206–208. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Espinoza I, Lozano I et al. 2017. First report of cassava common mosaic disease and cassava common mosaic virus infecting cassava (Manihot esculenta) in Peru. Plant Disease 101: 1066–1066. [Google Scholar]
- Fisher MC, Henk DA, Briggs CJ et al. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JW, McGrady-Steed J, Petchey OL. 2000. Testing for local species saturation with nonindependent regional species pools. Ecology Letters 3: 198–206. [Google Scholar]
- Fry WE, et al. 2013. The 2009 Late blight pandemic in the eastern United States: Causes and results. Plant Disease 97: 296–306. [DOI] [PubMed] [Google Scholar]
- Fry W, et al. 2015. Five reasons to consider Phytophthora infestans a re-emerging pathogen. Phytopathology 105: 966–981. [DOI] [PubMed] [Google Scholar]
- Gamarra H, Carhuapoma P, Mujica N et al. 2016. Greenhouse whitefly, Trialeurodes vaporariorum (Westwood 1956). Pages 154–168 in Kroschel J, Mujica N, Carhuapoma P, Sporleder M, eds. Pest Distribution and Risk Atlas for Africa: Potential Global and Regional Distribution and Abundance of Agricultural and Horticultural Pests and Associated Biocontrol Agents under Current and Future Climates. International Potato Center. [Google Scholar]
- Garnier S, Ross N, Rudis B et al. 2018. Viridis: Default color maps from “matplotlib.” R package version 0.5.1 https://cran.r-project.org/web/packages/viridis/index.html. [Google Scholar]
- Garrett KA, Thomas-Sharma S, Forbes GA et al. 2014. Climate change and plant pathogen invasions. Pages 22–44 in Ziska LH, Dukes JS, eds. Invasive Species and Global Climate Change. CABI. [Google Scholar]
- Garrett KA, Andersen K, Bowden RL et al. 2017. Resistance genes in global crop breeding networks. Phytopathology 107: 1268–1278. [DOI] [PubMed] [Google Scholar]
- Garrett KA, Alcalá-Briseño RI, Andersen KF et al. 2018. Network analysis: A systems framework to address grand challenges in plant pathology. Annual Review of Phytopathology 56: 559–580. [DOI] [PubMed] [Google Scholar]
- Gent DH, Bhattacharyya S, Ruiz T. 2019. Prediction of spread and regional development of hop powdery mildew: A network analysis. Phytopathology 109: 1392–1403. [DOI] [PubMed] [Google Scholar]
- Gibson RW, Kreuze JF.. 2015. Degeneration in sweetpotato due to viruses, virus-cleaned planting material and reversion: A review. Plant Pathology 64: 1–15. [Google Scholar]
- Gold CS, Bandyopadhyay R.. 2006. Identifying insect vectors and transmission mechanisms for BXW. Final Technical Report R8484. International Institute of Tropical Agriculture, Ibadan, Nigeria. [Google Scholar]
- Gonthier P, Garbelotto M.. 2013. Reducing the threat of emerging infectious diseases of forest trees: Mini review. CAB Reviews 8: 1–2. [Google Scholar]
- Gregory PH. 1968. Interpreting plant disease dispersal gradients Annual Review of Phytopathology 6: 189–212. [Google Scholar]
- Guzmán M, Ruiz E, Arciniegas N et al. 2006. Occurrence and variability of Potato yellow vein virus in three departments of Colombia. Journal of Phytopathology 154: 748–750. [Google Scholar]
- Harrison B, Zhou X, Otim‐;Nape G et al. 1997. Role of a novel type of double infection in the geminivirus‐;induced epidemic of severe cassava mosaic in Uganda. Annals of Applied Biology 131: 437–448. [Google Scholar]
- Harwood TD, Xu X, Pautasso M et al. 2009. Epidemiological risk assessment using linked network and grid based modelling: Phytophthora ramorum and Phytophthora kernoviae in the UK. Ecological Modelling 220: 3353–3361. [Google Scholar]
- Hernandez Nopsa JF, Thomas-Sharma S, Garrett KA. 2014. Climate change and plant disease. Pages 232–243 in Alfen NV, ed. Encyclopedia of Agriculture and Food Systems, vol. 2 Elsevier. [Google Scholar]
- Hernandez Nopsa JF, Daglish GJ, Hagstrum DW et al. 2015. Ecological networks in stored grain: Key postharvest nodes for emerging pests, pathogens, and mycotoxins. BioScience 65: 985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, van Etten J, Mattiuzzi M et al. 2017a. Raster: Geographic data analysis and modeling. R package version 2.6–7 https://cran.r-project.org/web/packages/raster. [Google Scholar]
- Hijmans RJ, Williams E, Vennes C. 2017b. Geosphere: Spherical trigonometry. R package, version 1.5–7 https://cran.r-project.org/web/packages/geosphere. [Google Scholar]
- Holme P. 2017. Three faces of node importance in network epidemiology: Exact results for small graphs. Physical Review E 96: 062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme P. 2018. Objective measures for sentinel surveillance in network epidemiology. Physical Review E 98: 022313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt-Twynam SR, Parnell S, Stutt RO et al. 2017. Risk-based management of invading plant disease. New Phytologist 214: 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [IFPRI and IIASA] International Food Policy Research and International Institute for Applied Systems Analysis 2016. Global Spatially Disaggregated Crop Production Statistics Data for 2005, version 3.2: Harvard Dataverse. [Google Scholar]
- IPPC 2012 IPPC Strategic Framework 2012–2019. Viale delle Terme di Caracalla, 00153 Rome, Italy. www.ippc.int/static/media/files/mediakit/IPPCStrategicFramework-en.pdf. [Google Scholar]
- Jeger MJ, Pautasso M.. 2008. Plant disease and global change: The importance of long–term data sets. New Phytologist 177: 8–11. [DOI] [PubMed] [Google Scholar]
- Jeger M, et al. 2018. Scientific opinion on the pest categorisation of Tecia solanivora. EFSA PLH Panel (EFSA Panel on Plant Health). EFSA Journal 16: 5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejans E, Skarpaas O, Ferrari MJ et al. 2014. A unifying gravity framework for dispersal. Theoretical Ecology 8: 207–223. [Google Scholar]
- Keeling MJ, Eames KTD.. 2005. Networks and epidemic models. Journal of the Royal Society Interface 2: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczyk ED. 2009. Statistical Analysis of Network Data: Methods and Models. Springer. [Google Scholar]
- Kroschel J, Schaub B.. 2013. Biology and ecology of potato tuber moths as major pests of potato. Pages 165–192 in Giordanengo P, Vincent C, Alyokhin A, eds. Insect Pests of Potato: Global Perspectives on Biology and Management. Elsevier. [Google Scholar]
- Kroschel J, Sporleder M, Carhuapoma P. 2016. Pest distribution and risk atlas for Africa. Pages 7–23 in Kroschel J, Mujica N, Carhuapoma P, Sporleder M, eds. Potential Global and Regional Distribution and Abundance of Agricultural and Horticultural Pests and Associated Biocontrol Agents under Current and Future Climates. International Potato Center. [Google Scholar]
- Kumar PL, Hanna R, Alabi OJ et al. 2011. Banana bunchy top virus in sub-Saharan Africa: Investigations on virus distribution and diversity. Virus Research 159: 171–182. [DOI] [PubMed] [Google Scholar]
- Legg J, Owor B, Sseruwagi P et al. 2006. Cassava mosaic virus disease in East and Central Africa: Epidemiology and management of a regional pandemic. Advances in Virus Research 67: 355–418. [DOI] [PubMed] [Google Scholar]
- Legg JP. 2010. Epidemiology of a whitefly transmitted cassava mosaic geminivirus pandemic in Africa. Pages 233–257 in Stansly PA, Naranjo SE, eds. Bemisia: Bionomics and Management of a Global Pest. Springer. [Google Scholar]
- Legg JP. 1999. Emergence, spread and strategies for controlling the pandemic of cassava mosaic virus disease in east and central Africa. Crop Protection 18: 627–637. [Google Scholar]
- Legg JP, et al. 2011. Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Research 159: 161–170. [DOI] [PubMed] [Google Scholar]
- Legg JP, Kumar PL, Makeshkumar T et al. 2015. Cassava virus diseases: Biology, epidemiology, and management. Advances in Virus Research 91: 85–142. [DOI] [PubMed] [Google Scholar]
- Leung W, et al. 2015. Drosophila muller F elements maintain a distinct set of genomic properties over 40 million years of evolution. G3-Genes Genomes Genetics 5: 719–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion S, Gandon S.. 2009. Habitat saturation and the spatial evolutionary ecology of altruism. Journal of Evolutionary Biology 22: 1487–1502. [DOI] [PubMed] [Google Scholar]
- Lozano I, Leiva AM, Jimenez J et al. 2017. Resolution of cassava-infecting alphaflexiviruses: Molecular and biological characterization of a novel group of potexviruses lacking the TGB3 gene. Virus Research 241: 53–61. [DOI] [PubMed] [Google Scholar]
- Madden LV, Hughes G, van den Bosh F. 2007. The Study of Plant Disease Epidemics. American Phytopathological Society. [Google Scholar]
- Margosian ML, Garrett KA, Hutchinson JMS et al. 2009. Connectivity of the American agricultural landscape: Assessing the national risk of crop pest and disease spread. BioScience 59: 141–151. [Google Scholar]
- Martinetti D., Soubeyrand S.. 2019. Identifying lookouts for epidemio-surveillance: Application to the emergence of Xylella fastidiosa in France. Phytopathology 109: 265–276. [DOI] [PubMed] [Google Scholar]
- Meentemeyer RK, Haas SE, Vaclavik T. 2012. Landscape epidemiology of emerging infectious diseases in natural and human-altered ecosystems. Annual Review of Phytopathology 50: 379–402. [DOI] [PubMed] [Google Scholar]
- Miller SA, Beed FD, Harmon CL. 2009. Plant disease diagnostic capabilities and networks. Annual Review of Phytopathology 47: 15–38. [DOI] [PubMed] [Google Scholar]
- Monfreda C, Ramankutty N, Foley JA. 2008. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Global Biogeochemical Cycles 22: GB1022. [Google Scholar]
- Mundt CC, Ahmed HU, Finckh MR et al. 1999. Primary disease gradients of bacterial blight of rice. Phytopathology 89: 64–67. [DOI] [PubMed] [Google Scholar]
- Mundt CC, Sackett KE, Wallace LD et al. 2009a. Aerial dispersal and multiple-scale spread of epidemic disease. Ecohealth 6: 546–552. [DOI] [PubMed] [Google Scholar]
- Mundt CC, Sackett KE, Wallace LD et al. 2009b. Long-distance dispersal and accelerating waves of disease: Empirical relationships. American Naturalist 173: 456–466. [DOI] [PubMed] [Google Scholar]
- Nakato GV, Beed FD, Ramathani I et al. 2013. Risk of banana Xanthomonas wilt spread through trade. Journal of Crop Protection 2: 151–161. [Google Scholar]
- Ndungo V, Eden-Green S, Blomme G et al. 2006. Presence of banana Xanthomonas wilt (Xanthomonas campestris pv. musacearum) in the Democratic Republic of Congo. Plant Pathology 55: 294. [Google Scholar]
- Nelson MF, Bone CE. 2015. Effectiveness of dynamic quarantines against pathogen spread in models of the horticultural trade network. Ecological Complexity 24: 14–28. [Google Scholar]
- Newman MEJ. 2010. Networks: An Introduction. Oxford University Press. [Google Scholar]
- Ojiambo PS, Gent DH, Mehra LK et al. 2017. Focus expansion and stability of the spread parameter estimate of the power law model for dispersal gradients. PeerJ 5: e3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell S, Gottwald TR, Riley T et al. 2014. A generic risk-based surveying method for invading plant pathogens. Ecological Applications 24: 779–790. [DOI] [PubMed] [Google Scholar]
- Pautasso M, Moslonka-Lefebvre M, Jeger MJ. 2010. The number of links to and from the starting node as a predictor of epidemic size in small-size directed networks. Ecological Complexity 7: 424–432. [Google Scholar]
- Pineda B, Jayasinghe U, Lozano JC. 1983. La enfermedad cuero de sapo en yuca (Manihot esculenta Crantz). ASIAVA (Colombia) 4: 10–12. [Google Scholar]
- Pretorius ZA, Singh RP, Wagoire WW et al. 2000. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis. f. sp. tritici in Uganda. Plant Disease 84: 203–203. [DOI] [PubMed] [Google Scholar]
- Core Team R. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rachkara P, Phillips DP, Kalule SW et al. 2017. Innovative and beneficial informal sweetpotato seed private enterprise in northern Uganda. Food Security 9: 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodoni B. 2009. The role of plant biosecurity in preventing and controlling emerging plant virus disease epidemics. Virus Research 141: 150–157. [DOI] [PubMed] [Google Scholar]
- Rosenzweig C, Iglesias A, Yang X et al. 2001. Climate change and extreme weather events: Implications for food production, plant diseases, and pests. Global Change and Human Health 2: 90–104. [Google Scholar]
- Rugalema G, Muir G, Mathieson K et al. 2009. Emerging and re-emerging diseases of agricultural importance: Why local perspectives matter. Food Security 1: 441–455. [Google Scholar]
- Salazar LF, Müller G, Querci M et al. 2000. Potato yellow vein virus: Its host range, distribution in South America and identification as a crinivirus transmitted by Trialeurodes vaporariorum. Annals of Applied Biology 137: 7–19. [Google Scholar]
- Sanatkar MR, Scoglio C, Natarajan B et al. 2015. History, epidemic evolution, and model burn-in for a network of annual invasion: Soybean rust. Phytopathology 105: 947–955. [DOI] [PubMed] [Google Scholar]
- Schaub B, Carhuapoma P, Kroschel J. 2016. Guatemalan potato tuber moth, Tecia solanivora (Povolny 1973). Pages 24–38 in Kroschel J, Mujica N, Carhuapoma P, Sporleder M, eds. Pest Distribution and Risk Atlas for Africa: Potential Global and Regional Distribution and Abundance of Agricultural and Horticultural Pests and Associated Biocontrol Agents under Current and Future Climates. International Potato Center. [Google Scholar]
- Schneider RW, Hollier CA, Whitam HK et al. 2005. First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Disease 89: 774–774. [DOI] [PubMed] [Google Scholar]
- Severns PM, Estep LK, Sackett KE et al. 2014. Degree of host susceptibility in the initial disease outbreak influences subsequent epidemic spread. Journal of Applied Ecology 51: 1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MW, Pautasso M. 2014. Networks and plant disease management: Concepts and applications. Annual Review of Phytopathology 52: 477–493. [DOI] [PubMed] [Google Scholar]
- Smolinski MS, Hamburg MA, Lederberg J. 2003. Microbial Threats to Health: Emergence, Detection, and Response. National Academies Press. [PubMed] [Google Scholar]
- Sutherst RW. 2014. Pest species distribution modelling: Origins and lessons from history. Biological Invasions 16: 239–256. [Google Scholar]
- Sutrave S, Scoglio C, Isard SA et al. 2012. Identifying highly connected counties compensates for resource limitations when evaluating national spread of an invasive pathogen. PLOS ONE 7 (art. e37793). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyniszewska A, Busungu C, Boni S et al. 2017. Spatial analysis of temporal changes in the pandemic of severe cassava mosaic disease in North-Western Tanzania. Phytopathology 107: 1229–1242. [DOI] [PubMed] [Google Scholar]
- Tinzaara W, Gold CS, Ssekiwoko F et al. 2006. Role of insects in the transmission of banana bacterial wilt. African Crop Science Journal 14: 105–110. [Google Scholar]
- Tripathi L, Mwangi M, Abele S et al. 2009. Xanthomonas wilt: A threat to banana production in East and Central Africa. Plant Disease 93: 440–451. [DOI] [PubMed] [Google Scholar]
- Tugume A, Mukasa S, Valkonen J. 2008. Natural wild hosts of Sweet potato feathery mottle virus show spatial differences in virus incidence and virus-like diseases in Uganda. Phytopathology 98: 640–652. [DOI] [PubMed] [Google Scholar]
- Tugume AK, Amayo R, Weinheimer I et al. 2013. Genetic variability and evolutionary implications of RNA silencing suppressor genes in RNA1 of Sweet potato chlorotic stunt virus isolates infecting sweetpotato and related wild species. PLOS ONE 8 (art. e81479). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugume AK, Mukasa SB, Valkonen JPT. 2016. Mixed infections of four viruses, the incidence and phylogenetic relationships of Sweet potato chlorotic fleck virus (Betaflexiviridae) isolates in wild species and sweetpotatoes in Uganda and evidence of distinct isolates in East Africa. PLOS ONE 11 (art. e0167769). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushemereirwe W, Kangire A, Ssekiwoko F et al. 2004. First report of Xanthomonascampestris pv. musacearum on banana in Uganda. Plant Pathology 53: 802. [Google Scholar]
- Vurro M, Bonciani B, Vannacci G. 2010. Emerging infectious diseases of crop plants in developing countries: Impact on agriculture and socio-economic consequences. Food Security 2: 113–132. [Google Scholar]
- Wang HL, Cui XY, Wang XW et al. 2015. First report of Sri Lankan cassava mosaic virus infecting cassava in Cambodia. Plant Disease 100: 1029–1029. [Google Scholar]
- Were E, Nakato GV, Ocimati W et al. 2015. The banana weevil, Cosmopolites sordidus (Germar), is a potential vector of Xanthomonas campestris pv. musacearumin in bananas. Canadian Journal of Plant Pathology 37: 427–434. [Google Scholar]
- With KA. 2004. Assessing the risk of invasive spread in fragmented landscapes. Risk Analysis 24: 803–815. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Haydon DT, Antia R. 2005. Emerging pathogens: The epidemiology and evolution of species jumps. Trends in Ecology and Evolution 20: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosula EN, Chen W, Fei Z et al. 2017. Unravelling the genetic diversity among cassava Bemisia tabaci whiteflies using NextRAD sequencing. Genome Biology and Evolution 9: 2958–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SJ, Coutts BA, Jones MGK et al. 2008. Phylogenetic analysis of Bean yellow mosaic virus isolates from four continents: Relationship between the seven groups found and their hosts and origins. Plant Disease 92: 1596–1603. [DOI] [PubMed] [Google Scholar]
- Xia YC, Bjornstad ON, Grenfell BT. 2004. Measles metapopulation dynamics: A gravity model for epidemiological coupling and dynamics. American Naturalist 164: 267–281. [DOI] [PubMed] [Google Scholar]
- Yirgou D, Bradbury JF.. 1974. A note on wilt of banana caused by the enset wilt organism Xanthomonas musacearum. East African Agricultural and Forestry Journal 40: 111–114. [Google Scholar]
- Zanini AA, Cuellar WJ, Celli MG et al. 2018. Distinct strains of the re-emergent Cassava common mosaic virus (genus: Potexvirus) infecting cassava in Argentina. Plant Pathology 67: 1814–1820. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.