Abstract
Motor impairments occur frequently in genetic syndromes highly penetrant for Autism Spectrum Disorder (syndromic ASD) and in individuals with ASD without a genetic diagnosis (nonsyndromic ASD). In particular, abnormalities in gait in ASD have been linked to language delay, ASD severity, and likelihood of having a genetic disorder. Quantitative measures of motor function can improve our ability to evaluate motor differences in individuals with syndromic and nonsyndromic ASD with varying levels of intellectual disability and adaptive skills. To evaluate this methodology, we chose to use quantitative gait analysis to study duplication 15q syndrome (dup15q syndrome), a genetic disorder highly penetrant for motor delays, intellectual disability, and ASD. We evaluated quantitative gait variables in individuals with dup15q syndrome (N = 39) and nonsyndromic ASD (N = 21) and compared these data to a reference typically developing cohort. We found a gait pattern of slow pace, poor postural control, and large gait variability in dup15q syndrome. Our findings improve characterization of motor function in dup15q syndrome and nonsyndromic ASD. Quantitative gait analysis can be used as a translational method and can improve our identification of clinical endpoints to be used in treatment trials for these syndromes.
Keywords: Duplication 15q syndrome, Autism Spectrum Disorder, Motor impairments, Gait function, Quantitative gait analysis, Genetic syndrome
Lay summary
Motor impairments, particularly abnormalities in walking, occur frequently in genetic syndromes highly penetrant for Autism Spectrum Disorder (syndromic ASD). Here, using quantitative gait analysis, we find that individuals with duplication 15q syndrome have an atypical gait pattern that differentiates them from typically developing and nonsyndromic ASD individuals. Our findings improve motor characterization in dup15q syndrome and nonsyndromic ASD.
Introduction
Increasing evidence has shown that motor impairments occur frequently in genetic syndromes highly penetrant for Autism Spectrum Disorder (ASD) such as Fragile X, Angelman, and Phelan McDermid syndrome, and these impairments might be some of the earliest signs of atypical development (Bishop et al., 2017; Bishop et al., 2016). These motor impairments, such as abnormal gait, may also serve as an early distinguishing characteristic between individuals with ASD without a known genetic syndrome [nonsyndromic ASD] and individuals with ASD with a known genetic syndrome [syndromic ASD] (Bishop et al., 2016). Within syndromic forms of ASD, impaired motor skills are a more sensitive indicator of genetic mutation severity than intellectual quotient (Buja et al., 2018). Furthermore, the emergence of locomotion and other motor skills are intrinsically linked to the development of language and social communication (Lebarton & Iverson, 2016; Piaget, 1952; Walle & Campos, 2014; West, Leezenbaum, Northrup, & Iverson, 2017). The prevalence of motor impairments among individuals with ASD and the impact of motor development on multiple developmental domains (language, cognition, social communication) supports the need for improved characterization of motor development for both syndromic and nonsyndromic ASD to improve early detection and identify potential treatment targets.
Currently, measurement of motor skills in individuals with syndromic and nonsyndromic ASD has often relied on caregiver reports or on standardized assessments of motor milestones. Although these measurement methods have provided important information on motor development in ASD, they also pose some limitations. Caregiver reports are often focused only on developmental milestones and lack more granularity in measurement. Standardized motor assessments can be time intensive, require subjective interpretation by an assessor, and employ tasks that could be difficult for individuals with intellectual disability to understand (Allen, Bredero, Van Damme, Ulrich, & Simons, 2017; Staples & Reid, 2010). The combination of low intellectual ability and poor motor function frequently leads to “floor effects” on standardized assessments among many individuals with ASD, particularly those with a genetic syndrome (Moss J, Howlin P, 2011; Wilson et al., 2018). Quantitative gait analysis overcomes these limitations by providing objective measurement of motor skills and characterization of subtle motor impairments without the time and cognitive burden posed by standardized motor assessments. This study uses quantitative gait analysis to evaluate and compare the spatiotemporal gait variables of individuals with typical development, nonsyndromic ASD, and dup15q syndrome, a syndromic ASD.
Duplications of 15q11.2-q13.1 (dup15q syndrome) is a genetic syndrome highly penetrant for motor delays, hypotonia, ASD, intellectual disability, and epilepsy (Battaglia, 2008; Finucane BM, et al., 2016; Urraca et al., 2013). It is the most frequent cytogenetic abnormality associated with ASD, occurring in approximately 1% of cases (Depienne et al., 2009). A diagnosis of dup15q syndrome is established by detection of at least one extra maternally derived copy of the Prader-Willi/Angelman critical region, which is approximately 5 Mb long within chromosome 15q11.2-q13.1. There are two primary types of multiplication in dup15q syndrome: a maternal isodicentric supernumerary chromosome that typically consists of two extra copies of 15q11.2-q13.1 and results in tetrasomy for 15q11.2-q13.1, or a maternal interstitial duplication that typically includes one extra copy of 15q11.2-q13.1 and results in trisomy for 15q11.2-q13.1 (Battaglia, 2008; Finucane BM, Lusk L, Arkilo D, 2016). Epilepsy in dup15q syndrome is variable, ranging from controlled seizures to intractable epilepsy, and can have detrimental effects on cognition and adaptive function (Distefano et al., 2016; DiStefano et al., 2020). As many with syndromic forms of ASD, motor impairments such as hypotonia and delayed developmental milestones are extremely prevalent in dup15q syndrome and are often the first presenting symptom of the disorder. In a smaller cohort study of dup15q syndrome, it was found that children with dup15q syndrome scored very low on the fine and gross motor development domains as measured by the Mullen Scales of Early Learning [MSEL] (Distefano et al., 2016). The motor scores were at or near the floor and thus provided little information regarding specific motor profile differences between dup15q syndrome and nonsyndromic ASD. These children also met criteria for ASD based on the Autism Diagnostic Observation Schedule (ADOS-2), but they showed relative strength in social interest and in social behaviors that did not require sustained motor control. Furthermore, motor skills in dup15q syndrome were significantly correlated with language and non-verbal cognition. These findings led the authors to hypothesize that motor impairments are likely impacting social communication skills in individuals with dup15q syndrome, but that more refined measures of domain specific function are needed to understand this relationship (Distefano et al., 2016). Gait is one specific area of gross motor function that has been described as abnormal in dup15q syndrome (Bundey, Hardy, Vickers, Kilpatrick, & Corbett, 2008; Finucane BM, Lusk L, Arkilo D, 2016). Abnormalities in gait have also been quantified in a preclinical study, (Piochon et al., 2014) in which mouse models of the syndrome (mouse homolog chromosome 7q duplication) showed impaired motor coordination when measured on kinematic analysis of gait with the Digigait imaging system. The mice showed greater stance width in the forelimbs, reduced stride frequency, longer stride length, and enhanced (longer) duration of forward propulsion compared to wild type littermates (Piochon et al., 2014). No studies have utilized quantitative analysis to evaluate the gait variables in individuals with dup15q syndrome.
Here, we utilized gait analysis to (1) quantitatively measure gait in individuals with dup15q syndrome with a wide range of intellectual and behavioral function and to (2) evaluate differences in gait between dup15q syndrome, nonsyndromic ASD, and typical development. We focused on domains of pace, postural control, and gait variability. Due to the high penetrance of motor delays and hypotonia, we hypothesized that individuals with dup15q syndrome would show greater abnormalities in gait variables compared to TD and nonsyndromic ASD participants. The ultimate goal of this work is to develop quantitative motor parameters that could serve as translational clinical endpoints for future treatment trials in this syndrome.
Methods
Participants
Participants were recruited from the national Dup15q Alliance, UCLA Dup15q clinic, and the UCLA Early Childhood Partial Hospitalization Program as a part of an ongoing observational study at the University of California, Los Angeles. Gait data for the typically developing (TD) control group was selected from a normative sample collected from research studies at the Gait and Motion Analysis Unit, Hospital Robert Debre, Paris, France (Gouelle, Leroux, & Bredin, 2016). There were a total of 39 individuals with dup15q syndrome ages 36 months to 41 years, 21 children with nonsyndromic ASD (defined as children without a molecular genetic diagnosis) ages 30 months to 11 years, and 131 typically developing individuals ages 36 months-41 years, A subgroup of dup15q syndrome participants and a subgroup of the TD reference group with the same age range as the nonsyndromic ASD group (ages 36 months- 11 years) were identified for group comparisons. Individuals with dup15q syndrome had a confirmed genetic diagnosis of dup15q syndrome (interstitial or isodicentric) based on clinical genetics reports reviewed by the research team. Exclusionary criteria included inability to ambulate without assistance, orthopedic abnormalities of the lower limb, or history of lower limb surgery. For the TD group, a history of neurological conditions was also an exclusionary criterion.
Procedures
All research was approved by the UCLA Institutional Review Board (IRB#15–001565, and IRB# 11–000097). Prior to data collection informed consent was obtained from all families. Cognitive, behavioral, and gait assessments took place at the UCLA Center for Autism Research and Treatment or at the Dup15q Alliance Family Conference in Redondo Beach, CA 2017.
Gait Assessment
The Zeno electronic walkway (Protokinetics, Havertown PA) was used in conjunction with the ProtoKinetics Movement Analysis Software (PKMAS) to obtain and analyze quantitative gait data (Egerton, Thingstad, & Helbostad, 2014; Lynall, Zukowski, Plummer, & Mihalik, 2017). The walkway contains a series of pressure sensors to detect footfalls. We leveraged a large TD cohort collected in previous research studies as our normative sample reference group. We actively collected data on the dup15q syndrome and nonsyndromic ASD groups and ensured that the gait data collection protocol was consistent with the TD cohort. Only the active length of the walkway differed between the current study (16 feet) and studies that produced the TD dataset (20 feet). To minimize any differences, the participants in the current study walked eight full lengths of the walkway compared to the six full lengths for the TD studies. All groups walked an additional four feet on either end of the walkway to account for acceleration and deceleration. The gait paradigm that was analyzed was spontaneous self-paced gait in which the subject can apply and control their natural speed. Participants were given a demonstration of the task and allowed to explore the walkway and environment prior to data collection. Trials were repeated when participants stepped off the recordable area of the walkway or when incomplete footfalls occurred. To account for potential behavioral and mood factors that could influence self-paced gait, the dup15q syndrome and nonsyndromic ASD groups were rated utilizing a mood and attention Likert scale. The scale ranges from 1–5 and evaluates areas such as irritability, hyperactivity, and inattention. A score of 1 indicates that a participant has a content or neutral mood, does not show any verbal or non-verbal protests, is able to be verbally or visually prompted to complete the trial, and is able to be redirected if necessary. The UCLA research team observed all trials and included only those where a self-paced speed was maintained, and the participant displayed a Likert score of 1.
We conceptualized the spatiotemporal gait variables into three domains as follows:
pace: cadence (steps/minutes), step length (centimeters [cm]), normalized step length, velocity (cm/second), normalized velocity (1/second).
postural control: stride width (cm), normalized stride width.
variability: step length coefficient of variation (%), step time coefficient of variation (%), and stride width standard deviation (cm).
The eight self-paced gait trials were averaged together to provide a global mean of all gait trials.
Cognitive Abilities
Cognitive development was assessed with either the Differential Abilities Scale-Second Edition [DAS-II] (Elliot, 2007), the Mullen Scales of Early Learning [MSEL] (Mullen, 1995), or the Wechsler Preschool and Primary Scale of Intelligence-III (Wechsler, 2002) based on age. The MSEL was used to assess participants who were under 68 months of age or, participants who were older but unable to achieve a basal score on the DAS-II. Ratio scores for full-scale developmental quotient (FSDQ), were calculated for each child and based on division of the age-equivalent score by chronological age. Ratio scores were used to account for the scores of children who performed outside of the standardized norms for their chronological age (Table 1).
Table 1.
Participant Characteristics
| TD n = 131 |
TD subgroup n = 89 |
Dup15q all n = 39 |
Dup15q syndrome subgroup n = 17 |
Nonsyndromic ASD n = 21 |
|
|---|---|---|---|---|---|
| Age: M (SD), Range | 137 (93), 36–492 | 89 (35) 36–144 | 162 (122), 36–502 | 74 (27), 36–134 | 67 (28), 36–137 |
| % Male | 40% | 45% | 60% | 65% | 71% |
| % Epilepsy | 51% | 35% | 24% | ||
| FSDQ | 35.2 (6.0) | 65.3 (6.2) | |||
| VABS Communication | 50.5 (6.1) | 63.8 (5.4) | |||
| VABS Daily Living Skills | 57.6 (3.8) | 68.2 (3.8) | |||
| VABS Socialization | 57.7 (5.4) | 62.9 (3.8) | |||
| VABS Motor | 62.3 (4.1) | 73.7 (2.2) | |||
| Height | 140 (2.0) | 127 (18) | 130 (5) | 102 (5) | 117.6 (3.3) |
| Weight | 39 (3) | 52 (5) |
Means and standard deviations. VABS indicates the Vineland Adaptive Behavioral Scale-II.
Adaptive Behavior
Adaptive behavior was assessed via the Vineland Adaptive Behavior Scale-II [VABS-II] (Sparrow, Balla, & Cicchetti, 1984). The VABS-II is a semi-structured interview conducted with the parent and assesses four domains of adaptive behavior: (a) communication, (b) daily living skills, (c) socialization, and (d) motor skills. The VABS-II standard scores are described in this study (Table 1).
ASD Symptoms
Evaluation for autism symptoms was completed using The Autism Diagnostic Observation Schedule- Second Edition (Lord, C., Rutter, M., DiLavore, P.C., Risi, S., Gotham, K., Bishop, 2012) and clinical best estimate. The ADOS-2 was utilized for all individuals with dup15q syndrome and for a majority of the individuals with non-syndromic ASD. In cases where an individual with non-syndromic ASD had recently received an ADOS-2 assessment outside of the study, these scores were obtained and reviewed and a clinical best estimate was completed to confirm an ASD diagnosis.
Statistical Analysis Plan
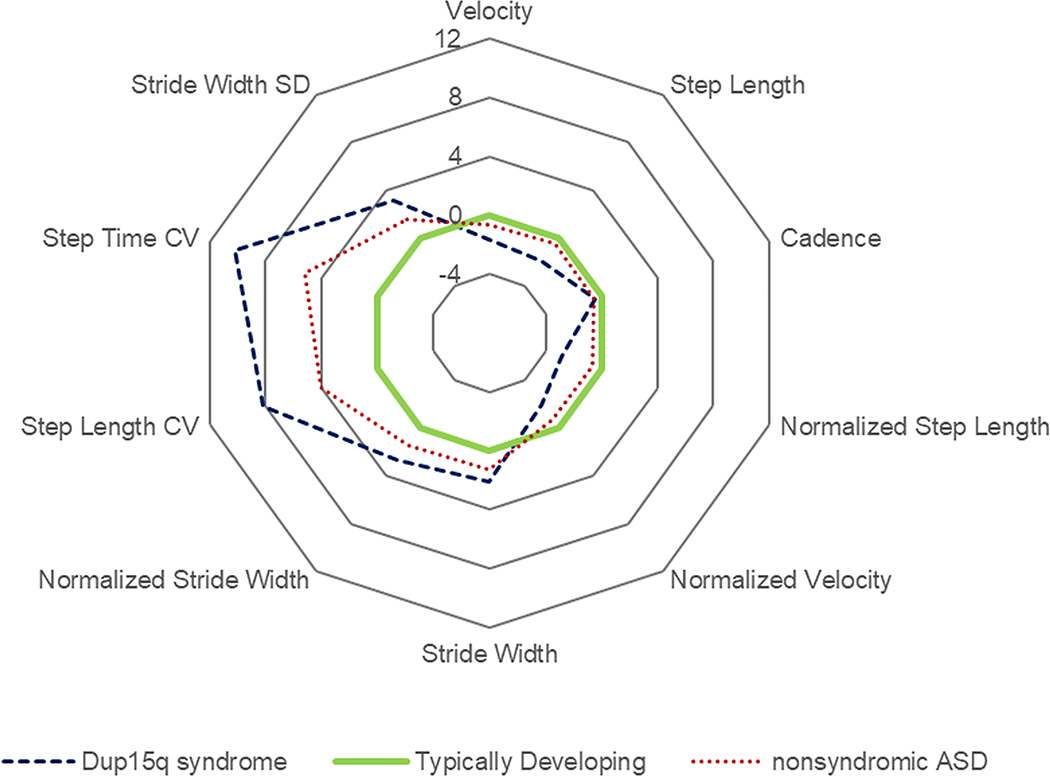
We provide descriptive statistics of age, gender, height, and weight for the three groups. For the dup15q syndrome and nonsyndromic ASD groups, additional descriptive statistics of epilepsy status, cognitive scores, and adaptive behaviors are provided. For linear regression models, we tested assumptions of regression including outliers, normality and homoscedasticity of residuals, and collinearity of predictors. We log transformed the variables that failed these assumptions (normalized stride width, step length coefficient of variation, step time coefficient of variation, and stride width standard deviation) which improved fit. Upon transformation the conclusions did not change so we present the nontransformed coefficients in our results. We constructed 4 sets of linear regression models. Each set of regression models assessed the effects of group and age on the spatiotemporal variables. The first set used the entire dup15 syndrome (n=39) cohort and the TD participants (n=131); the second set used the nonsyndromic ASD group (n = 21) and an age restricted cohort of TD (n= 89); the third set used an age restricted dup15q syndrome subgroup (n=17) and the nonsyndromic ASD participants (n=21); and the fourth set again compares the age restricted dup15q syndrome subgroup and non-syndromic ASD group and adds an additional covariate of FSDQ into the model. In addition to regression outputs, we display the spatiotemporal variables in a radar plot based on z scores with TD as the reference group (Figure 1).
Figure 1:
This radar plot illustrates the difference between dup15q syndrome and nonsyndromic ASD subgroups relative to TD. Comparison is based on z-score, a standardized measure of the distance to the average value of a parameter. Each point represents a separation of 1 Standard Deviation between the parameter mean value in the subgroup and the mean value in typically developing individuals.
Results
Participant Characteristics
Table 1 describes the demographics, epilepsy status, and average cognitive scores and adaptive skills of our sample. There were no differences in age, gender, or height between the dup15q syndrome and the TD cohorts. Compared to the age restricted TD cohort the nonsyndromic ASD cohort was significantly shorter (p = 0.01) and had a higher number of males (p = 0.02). Compared to the nonsyndromic ASD cohort, the age-restricted dup15q cohort had a significantly lower FSDQ (p = 0.001) with scores indicating severely impaired intellectual ability. In addition, the dup15q cohort was significantly lower in weight (p = 0.04), shorter, (p = 0.01) and had lower VABS-II motor scores (p = 0.01). There were no differences in epilepsy status or VABS-II domains of communication, daily living skills, or socialization between the dup15q syndrome and ASD groups.
Spatiotemporal gait variables
Table 2 describes the differences in spatiotemporal gait variables among the three groups within the following domains: pace, postural control, and variability.
Table 2.
Linear Regression models comparing spatiotemporal gait variables
| Spatiotemporal Gait Variables | Dup15q syndrome vs TD | Nonsyndromic ASD vs TD | Dup15q syndrome subgroup vs nonsyndromic ASD | Dup15q syndrome subgroup vs nonsyndromic ASD including FSDQ |
|---|---|---|---|---|
| Pace | ||||
| Velocity (cm/sec) | ||||
| Genetic (Dup15q) | −34 [−41, −27]*** | −11.099 [−20,−2]* | −28 [−44, −12]*** | −16.071 [−33, 0.69] |
| Age | .09 [0.06, 0.12]*** | 0.306 [0.20,0.41] *** | 0.51 [0.23, 0.80]*** | 0.503 [0.21, 0.77]*** |
| FSDQ | 0.393 [0.12, 0.669]** | |||
| Cadence (steps/min) | ||||
| Genetic (Dup15q) | −5 [−1, 0.01]** | −7 [−14,−1]* | −4 [−15, 8] | 0.05 [−14, 14] |
| Age | −0.07 [−0.09, −0.05]*** | −0.3 [−0.4,−0.22]*** | −0.17 [−0.38, 0.05] | −0.17 [−0.39, 0.04] |
| FSDQ | 0.133 [−0.091, 0.356] | |||
| Step Length | ||||
| Genetic (Dup15q) | −15 [−18, −12]*** | −3 [−6,0.5] | −13 [−18, −7]*** | −8 [−14, −3]** |
| Age | 0.07 [0.06–0.08]*** | 0.25 [0.21,0.29]*** | 0.29 [0.19, 0.38]*** | 0.28 [0.20, 0.37]** |
| FSDQ | 0.146 [0.057, 0.236]** | |||
| Normalized Velocity | ||||
| Genetic (Dup15q) | −0.19 [−0.24, −0.14]*** | −0.11 [−0.19,−0.04]** | −0.15 [−0.28, −0.02]** | −0.04 [−0.18, 0.09] |
| Age | −0.00 [−0.00, −0.00]*** | −0.00 [−0.00,−0.00] | 0.00 [−0.00, 0.00] | 0.00 [−0.00, 0.00] |
| FSDQ | 0.003 [0.00, 0.01]** | |||
| Normalized Step Length | ||||
| Genetic (Dup15q) | −0.09 [−0.09, −0.06*** | −0.03 [−0.05,−0.00]* | −0.06 [−0.12, −0.02]** | −0.03 [−0.07, 0.01] |
| Age | 0.00 [−0.00, 0.00] | 0.00 [0.00,0.00]*** | 0.00 [0.00, 0.00]** | 0.00 [0.00, 0.00]*** |
| FSDQ | 0.00 [0.00, 0.00]*** | |||
| Postural Control | ||||
| Stride Width | ||||
| Genetic (Dup15q) | 3.1 [2.2, 4.1]*** | 2 [1,3]*** | 2 [−0.68, 4] | 2 [−1, 4] |
| Age | 0.01 [0.00, 0.01]** | 0.02 [0.01,0.03]** | 0.01 [−0.03, 0.05] | 0.01 [−0.03, 0.05] |
| FSDQ | −0.01 [−0.05, 0.04] | |||
| Normalized Stride Width | ||||
| Genetic (Dup15q) | 0.04 [0.03, 0.05]*** | 0.02 [0.00, 0.02]*** | .04 [0.01, 0.07]** | 0.04 [−0.00,0.07] |
| Age | −0.00 [−0.00,−0.00]** | −0.00 [−0.00, −6.4]* | −0.00 [−0.00, 0,0.00] | −0.00 [−0.00,0.00] |
| FSDQ | −0.00 [−0.00,0.00] | |||
| Variability | ||||
| Step length CV | ||||
| Genetic (Dup15q) | 10 [9,12]*** | 10 [8,13]*** | 7 [3,11]** | 6 [1,10]* |
| Age | −0.02 [−0.02,−0.01]*** | −0.03 [−0.04,−0.02]*** | −0.09 [−0.16,−0.02]* | −0.09[−0.16, −0.01]* |
| FSDQ | −0.03 [−0.11, 0.05] | |||
| Step time CV | ||||
| Genetic (Dup15q) | 9 [8,10]*** | 11 [9,14]*** | 5 [0.60,10]* | 3 [−2, 8] |
| Age | −0.01 [−0.02,−0.01]*** | −0.02 [−0.03,−0.01]*** | −0.04 [−0.12,0.03] | −0.04 [−0.11, 0.04] |
| FSDQ | −0.06 [−0.15, 0.02] | |||
| Stride Width SD | ||||
| Genetic (Dup15q) | 2 [2,2]*** | 1 [0.69,1]*** | 1 [0.13,2]* | 1 [−0.04, 2] |
| Age | −0.00 [−0.00,−0.00]*** | −0.00 [−0.00,0.00] | −0.00 [−0.02,0.01] | −0.00 [−0.02, 0.01] |
| FSDQ | 0.00 [−0.02, 0.02] |
Coefficients and confidence intervals presented for each gait variable.
denotes p<0.05
denotes p<0.01
denotes p<0.001. CV indicates coefficient of variation.
Comparison of dup15q syndrome and nonsyndromic ASD to TD
Compared to the TD cohort, individuals with dup15q syndrome showed significant differences in all gait domains. In particular individuals with dup15q syndrome have (1) a slower pace as characterized by smaller cadence, step length, normalized step length, velocity, and normalized velocity; (2) less postural control as evidenced by larger stride width and normalized stride width; and (3) more variability characterized by larger step length coefficient of variation, step time coefficient of variation, and stride width standard deviation. Similarly, when comparing nonsyndromic ASD to TD there were significant differences in all spatiotemporal variables except for step length in the pace domain.
Comparison of dup15q syndrome and nonsyndromic ASD
Compared to nonsyndromic ASD, individuals with dup15q syndrome demonstrated a slower pace with a smaller velocity, normalized velocity, step length, and normalized step length. There was no significant difference in cadence. In the postural control domain, there was only a difference in normalized stride width. The dup15q syndrome group also had greater stride width standard deviation, step length coefficient of variation, and step time coefficient of variation. When accounting for FSDQ, only step length and step length coefficient of variation remained significantly different. Figure 1 displays the gait profiles of the three groups across the 10 gait variables. As an example, the dup15q syndrome had a step time coefficient of variation that is 10 standard deviations of the mean in typical development.
Discussion
Dup15q syndrome and nonsyndromic ASD compared to TD
Here, we evaluated gait function in individuals with dup15q syndrome and nonsyndromic ASD with a wide range of intellectual ability and adaptive skills. Unlike measures of motor milestones, we quantitatively measured a key motor function that is critical for many aspects of functioning. The quantitative data provided information beyond a binary score of whether an individual can or cannot walk, and instead produced more refined information about specific features of gait. This is also the first large scale study to report spatiotemporal variables of gait function in dup15q syndrome. As hypothesized, the dup15q syndrome gait was characterized by slower pace, poorer postural control, and greater variability in gait compared to that of the TD group. The nonsyndromic ASD gait showed poor postural control and greater gait variability, but a minimal decrease in pace, compared to that of the TD group. In particular, our findings of increased step width and greater variability in gait in nonsyndromic ASD are congruent with what has been reported across many studies (Kindregan, Gallagher, & Gormley, 2015).
Dup15q syndrome compared to nonsyndromic ASD
To our knowledge, this is the first study differentiating gait parameters between a syndromic (dup15q syndrome) and nonsyndromic form of ASD. In terms of postural control, the difference between the two groups was equivocal due to the inconsistency and smaller magnitude of differences across the gait variables of stride width and normalized stride width. While we confirmed that nonsyndromic ASD has variability in gait, the step time coefficient of variability, step length coefficient of variability, and stride width standard deviation in dup15q was even more striking. Slower pace was another defining feature of dup15q syndrome compared to nonsyndromic ASD.
When controlling for cognition (FSDQ) and age, the differences in pace and gait variability between dup15q syndrome and nonsyndromic ASD attenuate, in part due to the strong correlation between the diagnosis of dup15q syndrome and a low IQ and in part because FSDQ is an imperfect measure of IQ. FSDQ not only includes cognitive assessment but also includes measurement of motor skills. Furthermore, accurate measurement of FSDQ in these populations can be impacted due to behavioral difficulties and adjusting to the testing environment (Kasari, Brady, Lord, & Tager-Flusberg, 2013).
Etiology of gait dysfunction
Previous preclinical, clinical, and imaging studies have associated the abnormal gait findings of ASD to deficits in cerebellar pathways (Mahajan & Mostofsky, 2015; Mostofsky et al., 2009). Both poor postural control and variability of gait have been associated with cerebellar abnormalities (Buckley, Mazzà, & McNeill, 2018; Nonnekes et al., 2018). We note that both dup15q syndrome and nonsyndromic ASD had poor postural control and variability in gait, leading us to speculate whether a common deficit in cerebellar function could be responsible. We also postulate that the presence of substantial hypotonia is responsible for the slower pace in dup15q syndrome, since hypotonia reduces the muscular strength and stability needed to maintain gait velocity (Cimolin et al., 2010; Distefano et al., 2016; Finucane BM, Lusk L, Arkilo D, 2016). Hypotonia may also be responsible for the greater variability in gait seen in dup15q syndrome.
Limitations
While our study is an important first step in characterizing motor function in dup15q syndrome and nonsyndromic ASD, it has limitations that should be acknowledged. The data were cross sectional in nature and future studies should include longitudinal measurement of spatiotemporal gait variables to better understand the trajectory of this gross motor function. Our sample had asymmetric FSDQs and ages. While we tried to adjust for this by age restricting our cohorts, a larger sample of age matched and IQ matched cohorts of dup15q syndrome and nonsyndromic ASD would increase our confidence of the interpretation of motor impairments. It is also possible that anti-seizure and psychotropic medications could impact gait function in this population, but due to the wide range of comorbidities and medications used, we could not control for these variables. However, this sample also was a clinically representative sample, the type that would be included in clinical trials or natural history studies.
Future Directions
In the future, we plan to longitudinally follow gait maturation patterns in individuals with dup15q syndrome and other genetic syndromes penetrant for ASD. We hope this approach will improve our understanding of the range of atypical gait patterns and help to differentiate various forms of syndromic ASDs from each other. In addition, we will explore new gait paradigms (beyond self-paced gait) and new gait variables to improve the discriminative function of the quantitative gait analysis. We also plan to evaluate the relationship of gait function to measures of cognition and behavior. These approaches would improve the ability to use these metrics as biomarkers of motor function and as translational clinical endpoints for future treatment trials in this syndrome. The translatable nature of the quantitative gait analysis from the preclinical to the clinical model can also provide a foundation for treatment studies. Although the specific spatiotemporal gait variables cannot be directly translated from the mouse model to the human, the concepts of slowed locomotion and lack of stability can be applied when evaluating changes in gait function. Finally, we will combine quantitative measures of gait with other measures of brain function such as electroencephalogram (EEG) and neuroimaging studies to further our understanding of which brain circuits are implicated in the motor impairments that often define syndromic ASD and that may also lead to variability in motor skills in nonsyndromic ASD (Ewen et al., 2016; Floris, Barber, Nebel, Martinelli, & Lai, 2016; Mahajan & Mostofsky, 2015; Mostofsky et al., 2009). These future approaches can expand our understanding of why motor impairments occur in neurodevelopmental disorders and perhaps the best treatment approaches for various disorders.
Conclusions
Abnormalities in gait function have been described in numerous genetic syndromes highly penetrant for ASD, such as Fragile X Syndrome, Angelman Syndrome, and Phelan-McDermid syndrome. Quantitative motor measures are needed and have been shown to improve precision phenotyping in some of these disorders (Grieco, Gouelle, & Weeber, 2018; O’Keefe et al., 2018; Torres et al., 2016). We utilized an objective and quantitative motor method to phenotype gait function in individuals with dup15q syndrome with a wide range of intellectual ability and adaptive skills. Furthermore, we identified patterns of gait that differentiate and are shared between dup15q syndrome and TD and nonsyndromic ASD comparison groups. This method of quantitative gait anaylsis could be expanded to evaluate numerous other conditions highly penetrant for ID and ASD and serve as valuable measures for treatment trials in these syndromes.
Acknowledgements
The authors wish to thank all the families who generously participated in this study. The present research was supported by grants from the National Institute of Child Health and Human Development (U54HD087101-01), the UCLA Children’s Discovery Institute (CDI-GA-01012018), and the UCLA Clinical and Translational Science Institute (UL1TR001881).
Footnotes
Conflict of Interest: AG is employed by Protokinetics LLC. RBW, DE, BAS, AMW, BS, CH and SSJ do not have any conflict of interest associated with this study.
References
- Allen KA, Bredero B, Van Damme T, Ulrich DA, & Simons J (2017). Test of Gross Motor Development-3 (TGMD-3) with the Use of Visual Supports for Children with Autism Spectrum Disorder: Validity and Reliability. Journal of Autism and Developmental Disorders, 0(0), 1–21. 10.1007/s10803-016-3005-0 [DOI] [PubMed] [Google Scholar]
- Battaglia A (2008). The inv dup (15) or idic (15) syndrome (Tetrasomy 15q). Orphanet Journal of Rare Diseases, 7(15), 1–7. 10.1186/1750-1172-3-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Ph D, Farmer C, Ph D, Bal V, Ph D, … Ph D (2017). Identification of Developmental and Behavioral Markers Associated With Genetic Abnormalities in Autism Spectrum Disorder. Am J Psychiatry, 174(6). 10.1176/appi.ajp.2017.16101115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Thurm A, Farmer C, & Lord C (2016). Autism Spectrum Disorder, Intellectual Disability, and Delayed Walking. PEDIATRICS, 137(3). 10.1542/peds.2015-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley E, Mazzà C, & McNeill A (2018). A systematic review of the gait characteristics associated with Cerebellar Ataxia. Gait and Posture 10.1016/j.gaitpost.2017.11.024 [DOI] [PubMed] [Google Scholar]
- Buja A, Volfovsky N, Krieger AM, Lord C, Lash AE, Wigler M, … Tager-Flusberg HB (2018). Damaging de novo mutations diminish motor skills in children on the autism spectrum. PNAS, 115(8), 1859–1866. 10.1073/pnas.1715427115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundey S, Hardy C, Vickers S, Kilpatrick MW, & Corbett JA (2008). Duplication of the 15q11–13 Region in a Patient with Autism, Epilepsy and Ataxia. Developmental Medicine & Child Neurology, 36(8), 736–742. 10.1111/j.1469-8749.1994.tb11916.x [DOI] [PubMed] [Google Scholar]
- Cimolin V, Galli M, Grugni G, Vismara L, Albertini G, Rigoldi C, & Capodaglio P (2010). JNER JOURNAL OF NEUROENGINEERING AND REHABILITATION Gait patterns in Prader-Willi and Down syndrome patients. Journal of NeuroEngineering and Rehabilitation (Vol. 7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Moreno-De-Luca D, Heron D, Bouteiller D, Gennetier A, Delorme R, … Betancur C (2009). Screening for genomic rearrangements and methylation abnormalities of the 15q11-q13 region in autism spectrum disorders. Biological Psychiatry, 66(4), 349–359. 10.1016/j.biopsych.2009.01.025 [DOI] [PubMed] [Google Scholar]
- Distefano C, Gulsrud A, Huberty S, Kasari C, Cook E, Reiter LT, … Jeste SS (2016). Identification of a distinct developmental and behavioral profile in children with Dup15q syndrome. Journal of Neurodevelopmental Disorders, 1–13. 10.1186/s11689-016-9152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano C, Wilson RB, Hyde C, Cook EH, Thibert RL, Reiter LT, … Jeste S (2020). Behavioral characterization of dup15q syndrome: Toward meaningful endpoints for clinical trials. American Journal of Medical Genetics, Part A, 182(January), 71–84. 10.1002/ajmg.a.61385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton T, Thingstad P, & Helbostad JL (2014). Comparison of programs for determining temporal-spatial gait variables from instrumented walkway data: PKmas versus GAITRite. 10.1186/1756-0500-7-542 [DOI] [PMC free article] [PubMed]
- Elliot CD (2007). Differential Ability Scales: 2nd Edition. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Ewen JB, Lakshmanan BM, Pillai AS, Mcauliffe D, Nettles C, Hallett M, … Ewen JB (2016). Decreased Modulation of EEG Oscillations in High-Functioning Autism during a Motor Control Task, 10(May), 1–11. 10.3389/fnhum.2016.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane BM, Lusk L, Arkilo D, et al. (2016). 15q Duplication Syndrome and Related Disorders. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK367946/
- Floris DL, Barber AD, Nebel MB, Martinelli M, & Lai M (2016). Atypical lateralization of motor circuit functional connectivity in children with autism is associated with motor deficits. Molecular Autism, 1–14. 10.1186/s13229-016-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouelle A, Leroux J, & Bredin J (2016). Changes in Gait Variability From First Steps to Adulthood : Normative Data for the Gait Variability Index Changes in Gait Variability From First Steps to Adulthood : Journal of Motor Behavior, 48(September), 249–255. 10.1080/00222895.2015.1084986 [DOI] [PubMed] [Google Scholar]
- Grieco JC, Gouelle A, & Weeber EJ (2018). Identification of spatiotemporal gait parameters and pressure-related characteristics in children with Angelman syndrome: A pilot study. Journal of Applied Research in Intellectual Disabilities, 31(6), 1219–1224. 10.1111/jar.12462 [DOI] [PubMed] [Google Scholar]
- Kasari C, Brady N, Lord C, & Tager-Flusberg H (2013). Assessing the Minimally Verbal School-Aged Child With Autism Spectrum Disorder. Autism Research, 6(6), 479–493. 10.1002/aur.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindregan D, Gallagher L, & Gormley J (2015). Gait deviations in children with autism spectrum disorders: a review. Autism Research and Treatment, 2015, 741480 10.1155/2015/741480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebarton ES, & Iverson JM (2016). Infant Behavior and Development Full length article Associations between gross motor and communicative development in at-risk infants. Infant Behavior and Development, 44, 59–67. 10.1016/j.infbeh.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual (Part 1): Modules 1–4. Torrance, CA: Western Psychological Services. [Google Scholar]
- Lynall RC, Zukowski LA, Plummer P, & Mihalik JP (2017). Reliability and validity of the protokinetics movement analysis software in measuring center of pressure during walking. Gait & Posture, 52, 308–311. 10.1016/j.gaitpost.2016.12.023 [DOI] [PubMed] [Google Scholar]
- Mahajan R, & Mostofsky SH (2015). Neuroimaging endophenotypes in autism spectrum disorder. CNS Spectrums, (20), 412–426. 10.1017/S1092852915000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Howlin P, O. C. (2011). The Oxford Handbook of Intellectual Disablity and Development. New York: Oxford University Press. [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, & Pekar JJ (2009). Decreased connectivity and cerebellar activity in autism during motor task performance. A JOURNAL OF NEUROLOGY, 132, 2413–2425. 10.1093/brain/awp088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning : AGS edition. American Guidance Service., 18. [Google Scholar]
- Nonnekes J, Goselink RJM, Růzicka E, Fasano A, Nutt JG, & Bloem BR (2018). Neurological disorders of gait, balance and posture: A sign-based approach. Nature Reviews Neurology, 14(3), 183–189. 10.1038/nrneurol.2017.178 [DOI] [PubMed] [Google Scholar]
- O’Keefe JA, Robertson EE, Ouyang B, Carns D, McAsey A, Liu Y, … Hall DA (2018). Cognitive function impacts gait, functional mobility and falls in fragile X-associated tremor/ataxia syndrome. Gait & Posture, 66, 288–293. 10.1016/j.gaitpost.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J (1952). The origins of intelligence in children. New York: International Universities Press. [Google Scholar]
- Piochon C, Kloth AD, Grasselli G, Titley HK, Nakayama H, Hashimoto K, … Hansel C (2014). copy-number variation mouse model of autism. Nature Communications, 5, 1–12. 10.1038/ncomms6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Balla D, & Cicchetti D (1984). The Vineland Adaptive Behavior Scales: Interview edition, survey. In Major psychological assessment instruments (Vol. 2, pp. 199–231). [Google Scholar]
- Staples KL, & Reid G (2010). Fundamental movement skills and autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(2), 209–217. 10.1007/s10803-009-0854-9 [DOI] [PubMed] [Google Scholar]
- Torres EB, Nguyen J, Mistry S, Whyatt C, Kalampratsidou V, & Kolevzon A (2016). Characterization of the Statistical Signatures of Micro-Movements Underlying Natural Gait Patterns in Children with Phelan McDermid Syndrome: Towards Precision-Phenotyping of Behavior in ASD. Frontiers in Integrative Neuroscience, 10, 22 10.3389/fnint.2016.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urraca N, Cleary J, Brewer V, Pivnick EK, Mcvicar K, Thibert RL, … Reiter LT (2013). The Interstitial Duplication 15q11. 2-q13 Syndrome Includes Autism, Mild Facial Anomalies and a Characteristic EEG Signature, (March), 268–279. 10.1002/aur.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle EA, & Campos JJ (2014). Infant Language Development Is Related to the Acquisition of Walking. Developmental Psychology, 50(2), 336–348. 10.1037/a0033238 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2002). The Wechsler Preschool and Primary Scale of Intelligence – WPPSI-111 Technical and Intrepretive Manual 3rd edn. San Antonio, TX: (The Psychological Corporation. [Google Scholar]
- West KL, Leezenbaum NB, Northrup JB, & Iverson JM (2017). The Relation Between Walking and Language in Infant Siblings of Children With Autism Spectrum Disorder. Child Development, 00(0), 1–17. 10.1111/cdev.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RB, Mccracken JT, Rinehart NJ, & Jeste SS (2018). What’s missing in autism spectrum disorder motor assessments? Journal of Neurodevelopmental Disorders, (10). 10.1186/s11689-018-9257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]