Abstract
Green tea is a popularly consumed beverage worldwide and contains polyphenols, whose antioxidant activities could improve sperm parameters and fertility thereof. We investigated the effect of green tea on the male rat reproductive system as well as its safety. Male Wistar rats were administered 2 and 5% aqueous extract of green tea for 52 days’ ad libitum, while the control group received tap water. Total polyphenol, flavanol, flavonol and soluble solids significantly increased in a concentration-dependent manner in vitro (P < 0.01). Weights of body, testis, epididymis, prostate gland, seminal vesicles, and liver, serum levels of testosterone, ferric reducing antioxidant power, creatinine, and sperm motility, remained unchanged (P > 0.05). Kidney weight, sperm concentration and vitality, spontaneous acrosome reaction increased (P < 0.05), while alanine transaminase and aspartate transaminase levels decreased (P < 0.05). Catalase, superoxide dismutase, glutathione and lipid peroxidation remained unchanged in the testes, liver and kidney (P > 0.05). Histological sections of testis, epididymis, kidney and liver showed no conspicuous alteration. Diameter and epithelial height of seminiferous tubule decreased, while caudal epididymis epithelial height increased (P < 0.01). Consumption of green tea in the conditions used in the present study seems to be safe and improved sperm parameters. However, subtle structural changes observed in the decreased diameter and epithelial height of the seminiferous tubule and increased acrosome reaction needs further investigation.
Subject terms: Cell biology, Physiology, Anatomy
Introduction
Camellia sinensis (L.) Kuntze (Fam. Theaceae) consists of three types based on its level of fermentation (oxidation) as unfermented (white and green teas), partially fermented (oolong tea) and completely fermented (black tea)1. White tea is obtained by an instant steaming of young tea leaves or buds, followed by drying. In contrast, green tea is obtained by immediately steaming freshly harvested mature tea leaves, resulting in the minimal oxidation of the naturally occurring polyphenols in its leaves1,2. Oolong tea is produced by shorter fermentation process while black tea is obtained by rolling of the leaves, followed by a period of 90–120 min’ oxidation3,4.
Green tea is a popularly consumed beverage worldwide, especially in China, Japan and other Asian countries, including India5. It accounts for 20% of tea consumption in the world6, with black tea being the most consumed and white tea, the least7. The leaves of green tea contain 30–40% polyphenols (which contains 80% flavonoids), fibres (26%), proteins (15%), lipids (2–7%), vitamins and minerals (5%), methylxanthines (3–4%) and pigments (1–2%)2,8. Green tea composition may vary and depends on its geographical location, agricultural practices, as well as the properties of the plant itself8. Green tea consumption could be beneficial for risk reduction in cardiovascular diseases, obesity, diabetes, neurodegenerative diseases and cancer as well as weight loss9,10. Although the beneficial effect of consumption of green tea on the overall risk of cancer remains unproven as findings from epidemiological studies revealed inconsistent results11. The health benefits of green tea are attributed mostly to the polyphenolic compounds especially the catechins (e.g. epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG) and epigallocatechin-3-gallate (EGCG)), with EGCG being the most abundant and effective10,12. Unfermented teas have high polyphenolic content, with catechin derivatives, EGCG being the most abundant and powerful antioxidant13. Although, a higher amount of antioxidants, as well as antioxidant potential, are seen in white tea compared to green tea in a dose-dependent manner1,14.
Intake of three to four cups of green tea (equivalent to 2 g) three times a day is to be safe for administration in a long term basis15. Human intervention and bioavailability studies involving low to moderate doses of green tea preparations or EGCG reported no adverse effect16–18. However, other studies revealed that administration of high doses of green tea or EGCG resulted in liver and renal toxicity12,15,19,20, in which its polyphenol catechins could be the causative agent20. High levels of serum aspartate transaminase (AST), alanine transaminase (ALT), and creatinine, respectively indicate liver and renal toxicity or injury as ALT and AST are biomarkers of the liver structure and function, while creatinine is a reliable renal biomarker21,22. The harmful effects of green tea have also been attributed to the pro-oxidative property when consumed at high doses23. Repeated administration of green tea polyphenols (GTPs) (750 mg/kg) caused a hepatic injury24. Oral administration of EGCG (1,500 mg/kg) increased ALT in plasma and reduced survival rate in CF-1 mice9. Mazzanti et al. also showed a link between the intake of green tea supplement and liver damage25. Salminen et al. demonstrated that green tea extract (500 and 1,000 mg/kg) potentiated acetaminophen-induced hepatotoxicity in mice26. Low doses of GTPs (0.01 and 0.1%) but not the high dose of GTPs (1%) ameliorated dextran sulfate sodium (DSS) increased ALT and AST levels in mice22. Administration of high dose GTPs (1%) to male mice caused renal toxicity, as marked by increased serum creatinine level, and increased thiobarbituric acid-reactive substances (TBARS) in both kidney and liver22. Protective effects of green tea extract were also demonstrated against nephrotoxic agents in rats27–29.
The testis performs two crucial functions, that is, spermatogenesis (production of spermatozoa) and steroidogenesis (production of steroid hormones), which are regulated by gonadotropins and locally synthesised factors30. The whole spermatogenic cycle takes 52 days in rats31. Previous studies have indicated inconsistent results on the exposure of green tea to the male reproductive system. Yassa et al. showed that administration of the aqueous green extract (70 mg/kg/day) for 90 days did not affect the body weight, serum testosterone, and the histological appearance of the seminiferous tubules of the testes was normal32. Oral administration of aqueous extract of green tea (70 mg/kg/day) to rats for 63 days did not affect weights of the body and reproductive organs, sperm concentration and viability and diameter of the seminiferous tubule33. Oral administration of 1.25% and 5% green tea extract (polyphenone-60) mixed with powdered CE-2 diet to male rats for 2, 4 and 8 weeks’ ad libitum decreased weight of the body, testes, and prostate gland, but increased levels of luteinising hormone (LH) and testosterone, with no marked difference in the histological sections of the testis at the highest dose34. Consumption of 2.5% and 5% green tea extract (1 ml/100 g body weight) for 26 days resulted in impaired spermatogenesis through the loss of matured and elongated spermatids. Decreased net weight gain and testis weight, sperm count and motility, Δ5 3β- and 17 β-hydroxysteroid dehydrogenase and testosterone levels, with increased follicle-stimulating hormone (FSH) and LH in adult male rats, was also observed35,36.
Furthermore, intraperitoneal injection of EGCG (85 mg/kg body weight) for 2–7 days caused acute body weight loss, and reduction in weight of prostate gland and testis as well as the level of testosterone and LH37. Administration of lower concentrations of EGCG (2 µM and 20 µM) to human sperm enhanced sperm quality through the increased motility, viability and capacitation. In comparison, administration of a high concentration of EGCG (60 µM) caused a deleterious effect38. Supplementation of sperm storage media with tea extract increased sperm viability in a dose-dependent manner1. Other studies have shown the ameliorative or cytoprotective property of green tea following the administration of testicular toxicants such as cadmium and acrylamide or by the induction of oxidative stress in animal models32,33,39–41. In this study, we report on the effect of green tea in the male rat reproductive system after a complete spermatogenic cycle of 52 days as well as its safety by considering the liver and kidney functions.
Results
Chemical analysis of green tea
A significant increase in total polyphenol (P < 0.0001), flavanol content (P < 0.01) and flavonol content (P < 0.0001) were observed in the 5% green tea extract compared to that of 2%. The soluble solid content (P < 0.0001) as well as the ferric reducing power (P < 0.0001) was also higher in the 5% in green tea extract compared to 2% green tea extract) (Table 1).
Table 1.
Chemical analysis of an aqueous extract of green tea.
| Chemical analysis (unit) | 2% Green tea | 5% Green tea | P-value |
|---|---|---|---|
| Total polyphenol (mg Gallic acid equivalent/ml) | 3.49 ± 0.22 | 8.66 ± 0.44 | < 0.0001 |
| Flavanol (mg catechin/ml) | 1.43 ± 0.14 | 4.17 ± 0.51 | 0.0020 |
| Flavonol (mg quercetin equivalent/ml) | 0.06 ± 0.01 | 0.13 ± 0.01 | < 0.0001 |
| FRAP (mM) | 8.25 ± 0.30 | 18.48 ± 1.27 | < 0.0001 |
| Soluble solids (mg/ml) | 4.89 ± 0.35 | 11.81 ± 0.66 | < 0.0001 |
Values are the means ± SEM of 12 replicates.
FRAP ferric reducing antioxidant power.
Fluid intake and weight gain
Overall, fluid intake in male rats exposed to green tea (2% or 5%) did not differ to the control group (Table 2; P > 0.05). Bodyweight gain of animals in all groups increased progressively throughout treatment (Fig. 1). Rats treated with green tea showed no change in the weekly weight gain compared to the control (P > 0.05), although the 2% green tea treated group showed a reduced weight gain at week five compared to the control (Fig. 1; P < 0.0001). The total weight gain of the rats remained unchanged after consumption of green tea for 52 days (Table 2; P > 0.05). Furthermore, the net body weight gain of the control 2% and 5% green tea treated groups were 72.5%, 59.1% and 72.3% respectively (data not shown). Besides, 2% and 5% green tea caused no change in the weight of the testis, epididymis, prostate, seminal vesicles and liver on the male rats (Table 2; P > 0.05), while a substantial rise in the weight of the kidney was observed following exposure to 5% green tea (Table 2; P < 0.05).
Table 2.
Effect of consumption of aqueous extract of green tea for 52 days on fluid intake, body and organ weights of male Wistar rats.
| Control | 2% Green tea | 5% Green tea | |
|---|---|---|---|
| Average daily fluid intake (ml/100 g BW) | 13.43 ± 0.58 | 12.88 ± 0.47 | 12.41 ± 0.60 |
| Total weight gain (g) | 151.80 ± 10.22 | 141.70 ± 9.57 | 158.80 ± 10.06 |
| Testis (g)a | 0.47 ± 0.02 | 0.48 ± 0.04 | 0.46 ± 0.04 |
| Epididymis (g)a | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.14 ± 0.04 |
| Seminal vesicles (g)a | 0.41 ± 0.01 | 0.39 ± 0.05 | 0.38 ± 0.03 |
| Prostate gland (g)a | 0.13 ± 0.01 | 0.13 ± 0.03 | 0.13 ± 0.04 |
| Kidneys (g)a | 0.85 ± 0.02 | 0.87 ± 0.05 | 0.93 ± 0.05* |
| Liver (g)a | 4.57 ± 0.14 | 4.48 ± 0.37 | 4.62 ± 0.25 |
Values represents mean ± SEM of six male rats per group.
BW body weight.
*P < 0.05 compared to the control.
aRelative organ weight = organ weight/final body weight × 100.
Figure 1.
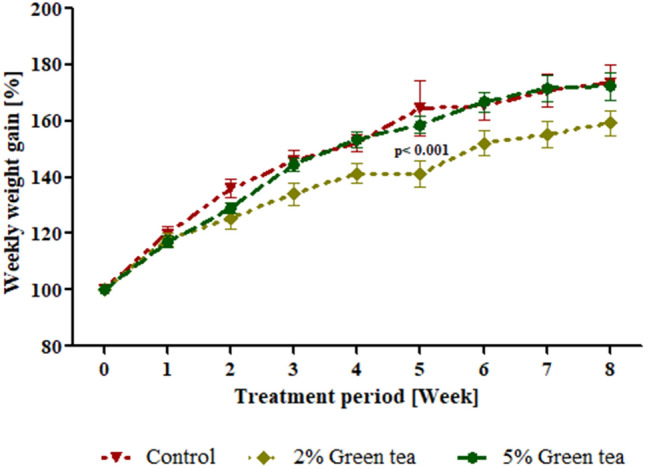
Weekly weight gain for the period of treatment in male rats. Values represented are the mean ± SEM of 6 animals per group. ***p < 0.0001 compared to the control.
Serum analysis
Green tea (2% and 5%) did not affect the level of testosterone produced in the male rats (Table 3; P > 0.05). However, a positive trend in the level of testosterone was observed in a dose-dependent manner (ANOVA-trend analysis, repeated measure: P = 0.029). Ferric reducing antioxidant power in serum remained unchanged following the 52 day’s treatment (Table 3; P > 0.05). Creatinine remained unchanged in its serum level in the treated rats (Table 3; P > 0.05). The AST level in serum, as shown in Table 3 dropped significantly in rat exposed to 2% and 5% green tea (P < 0.05). Repeated measure ANOVA trend analysis showed an increase in the level of AST in serum (P = 0.0050) at the higher tea concentration used. Serum ALT activity decreased significantly in the treated groups (Table 3; P > 0.05). Although not significant, a trend to a decreased level of serum ALT activity was observed with increasing concentrations of green tea (Repeated measure ANOVA-trend analysis: P = 0.1430).
Table 3.
Analysis of serum following treatment of male Wistar rats with green tea.
| Testosterone (ng/ml) | FRAP (µM) | Creatinine (mg/l) | AST (UI/l) | ALT (UI/l) | |
|---|---|---|---|---|---|
| Control | 5.3 ± 0.8 | 328.1 ± 26.0 | 35.4 ± 4.4 | 46.0 ± 3.6 | 47.1 ± 3.2 |
| 2% Green tea | 6.0 ± 1.4 | 329.0 ± 35.0 | 44.6 ± 3.3 | 22.0 ± 2.3*** | 35.5 ± 1.1* |
| 5% Green tea | 8.7 ± 0.7 | 288.0 ± 52.8 | 46.4 ± 6.3 | 26.0 ± 1.1*** | 31.3 ± 1.3** |
Values represented are the mean ± SEM of 6 animals per group.
FRAP ferric reducing antioxidant power, AST aspartate transaminase, ALT alanine transaminase.
*P < 0.05; **P < 0.01, ***P < 0.0001 compared to control.
Sperm parameter analysis
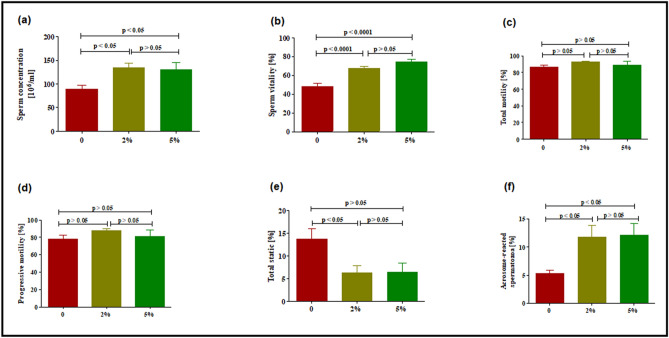
Green tea significantly increased sperm concentration (P < 0.05) (Fig. 2a) and sperm vitality control (P < 0.05). The increase in sperm vitality was up to 40% and 54.8% in the 2% and 5% green tea treated groups, respectively (Fig. 2b). Total motility (Fig. 2c) and total progressive motility (Fig. 2d) remained unchanged (P > 0.05) in both treated groups. Also, green tea caused a significant decline in the percentage of total static spermatozoa (P < 0.05; Fig. 2e). Further to these, sperm velocity parameters, that is, ALH, BCF, LIN, STR, VAP, VCL, VSL, and WOB remained unchanged (P > 0.05; Table 4). following exposure of male rats to 2% and 5% green tea. Lastly, the percentage of acrosome reacted spermatozoa significantly increased (P < 0.05; Fig. 2f).
Figure 2.
Effect of green tea on sperm parameters. (a) Sperm concentration, (b) sperm vitality, (c) total motility, (d) progressive motility, (e) total static sperm, (f) acrosome reacted spermatozoa. Values are represented as mean ± SEM after 52 days’ treatment. The number of rat per group = 6; at least 200 spermatozoa per animal were analysed.
Table 4.
Effect of consumption of aqueous extract of green tea for 52 days on sperm velocity parameters of male Wistar rats.
| Control | 2% Green tea | 5% Green tea | |
|---|---|---|---|
| VSL (µm/s) | 51.7 ± 8.6 | 59.6 ± 3.0 | 61.4 ± 10.7 |
| VCL (µm/s) | 274.0 ± 18.9 | 297.5 ± 20.9 | 274.9 ± 37.7 |
| VAP (µm/s) | 99.2 ± 8.9 | 113.0 ± 7.0 | 105.7 ± 12.7 |
| ALH (µm) | 10.3 ± 0.4 | 10.8 ± 0.3 | 10.1 ± 0.8 |
| LIN (%) | 18.5 ± 1.9 | 20.2 ± 0.6 | 22.1 ± 1.8 |
| STR (%) | 51.9 ± 4.0 | 53.0 ± 1.6 | 57.1 ± 4.9 |
| WOB (%) | 36.1 ± 1.0 | 38.1 ± 0.5 | 38.9 ± 0.8 |
| BCF (Hz) | 6.4 ± 0.6 | 6.1 ± 0.3 | 7.3 ± 1.1 |
Values represented are the means ± SEM of 6 animals per group, and 200 sperm per animal were analysed.
VSL straight-line velocity, VCL curvilinear velocity, VAP average path velocity, ALH the amplitude of lateral head displacement, LIN linearity, STR straightness, WOB wobble, BCF beat cross frequency.
Histology and morphometric measurements
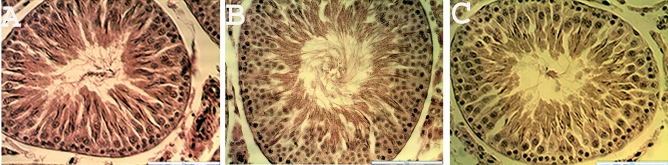
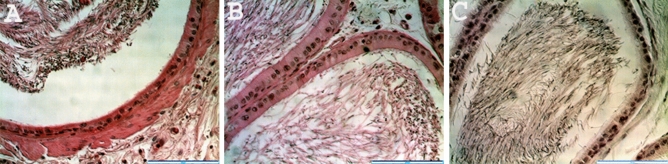
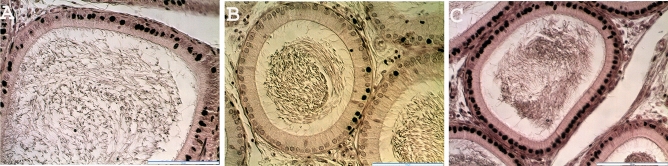
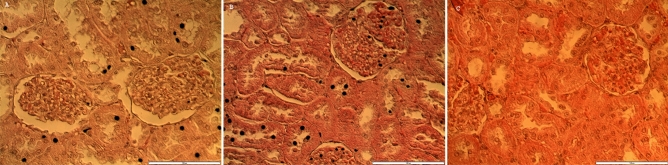
The histological observation of the testes in the treated rats showed no noticeable distortion in the architecture of the cells compared to the control (Fig. 3). All stages of spermatogenesis were present, and copious spermatozoa in the lumen of the seminiferous tubule in the treated groups, like the control. Furthermore, sections of the epididymis (cauda and caput) also showed no difference in structure compared to the control group (Figs. 4 and 5). On the contrary, the diameter (2% and 5%; P < 0.0001) and epithelial heights (2%, P < 0.05) of seminiferous tubules of the treated groups reduced significantly (Table 5). Green tea extract (2%) significantly increased the epithelial height of the cauda epididymis (p < 0.05); however, the epithelial height of the caput epididymis remained unchanged (p > 0.05) (Table 5). Furthermore, the histological sections of the treated kidney and liver depicted standard architecture compared to the control (Figs. 6 and 7).
Figure 3.
Effect of consumption of aqueous extract of green tea for 52 days on the testes of male Wistar rats. (A) Control; (B) 2% Green tea; (C) 5% Green tea. Bar: 100 µm.
Figure 4.
Effect of consumption of aqueous extract of green tea for 52 days on the cauda epididymis of male Wistar rats. (A) Control; (B) 2% Green tea; (C) 5% Green tea. Bar: 100 µm.
Figure 5.
Effect of consumption of aqueous extract of green tea for 52 days on the caput epididymis of male Wistar rats. (A) Control; (B) 2% Green tea; (C) 5% Green tea. Bar: 100 µm.
Table 5.
Effect of consumption of aqueous extract of green tea for 52 days on the measurements of the seminiferous tubules and epididymis.
| Seminiferous tubule | Epididymis epithelial height | |||
|---|---|---|---|---|
| Diameter (µm) | Epithelial height (µm) | Caput (µm) | Cauda (µm) | |
| Control | 281.4 ± 3.0 | 92.8 ± 1.4 | 24.1 ± 0.5 | 16.8 ± 0.5 |
| 2% Green tea | 263. 8 ± 2.4*** | 88.4 ± 1.1* | 26.0 ± 0.8 | 19.4 ± 0.8* |
| 5% Green tea | 262.8 ± 2.7*** | 92.0 ± 1.2 | 25.8 ± 0.6 | 17.9 ± 0.6 |
Values represents mean ± SEM of six male rats per group.
*P < 0.05; ***P < 0.0001.
Figure 6.
Effect of consumption of aqueous extract of green tea for 52 days on the kidney of male Wistar rats. (A) Control; (B) 2% Green tea; (C) 5% Green tea. Bar: 100 µm.
Figure 7.
Effect of consumption of aqueous extract of green tea for 52 days on the liver of male Wistar rats. (A) Control; (B) 2% Green tea; (C) 5% Green tea. Bar: 100 µm.
Antioxidant enzymes and lipid peroxidation in Tissues
Table 6 shows the results on lipid peroxidation and antioxidant enzymes in the testes, liver and kidney following treatment with green tea (2% and 5%). No change in the level of MDA in the testes, liver and kidney (P > 0.05; Table 6). Similarly, GSH, CAT and SOD levels in the kidney, liver and testes remained unchanged (P > 0.05).
Table 6.
Effect of consumption of aqueous extract of green tea for 52 days on the antioxidant activities in the testis, kidney and liver of male Wistar rats.
| Organ | Antioxidant assays (units) | Control | 2% Green tea | 5% Green tea |
|---|---|---|---|---|
| Testis | CAT (µmole/min/mgprot) | 1.5 ± 0.2 | 1.6 ± 0.1 | 1.3 ± 0.1 |
| GSH (mM/mgprot) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
| SOD (U/mgprot) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | |
| MDA (nmole−1/mgprot) | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.1 | |
| Kidney | CAT (µmole/min/mgprot) | 1.8 ± 0.1 | 2.1 ± 0.2 | 1.6 ± 0.1 |
| GSH (mM/mgprot) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
| SOD (U/mgprot) | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.2 | |
| MDA (nmole−1/mgprot) | 2.9 ± 0.3 | 3.1 ± 0.4 | 2.9 ± 0.6 | |
| Liver | CAT (µmole/min/mgprot) | 2.3 ± 0.2 | 1.9 ± 0.2 | 2.4 ± 0.2 |
| GSH (mM/mgprot) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
| SOD (U/mgprot) | 1.0 ± 0.1 | 1.6 ± 0.3 | 1.2 ± 0.2 | |
| MDA (nmole−1/mgprot) | 3.3 ± 0.4 | 4.2 ± 0.8 | 2.9 ± 0.6 |
Values represented are the mean ± SEM of 6 animals per group.
CAT catalase, GSH glutathione, MDA malondialdehyde, SOD superoxide dismutase.
Discussion
The results on the effect of green tea extract or its polyphenols (such as EGCG) on the male reproductive functions and spermatogenesis are conflicting32–35,37. Also, a high dose of green tea extract caused renal toxicity and hepatotoxic effects12,15,19. Hence, we aim to report on the overall effect of consumption of green tea ad libitum on the male reproductive system after the completion of one spermatogenic cycle in rats, as well as on the kidney and liver functions.
Green tea leaves contain 30–40% polyphenols2,8. Similar to previous studies1,14, in vitro analysis of green tea in our study revealed the presence of these polyphenols and further demonstrated a concentration-dependent increase in total polyphenols, flavanol, flavonol, and soluble solids. Further analysis of the antioxidant capacity of green tea in vitro, using FRAP showed a concentration-dependent increase; however, it remained unchanged in the serum of the treated male rats. These were indicating a possible higher antioxidant property with higher concentrations of the extract. Our study however, also showed no change in the serum FRAP after drinking green tea in vivo, which supports the findings of Maxwell & Thorpe42. Contrary to our study, consumption of green tea significantly increased plasma FRAP in humans43. The possible explanation could be due to the differences in bioavailability of green tea, as bioavailability and transformation of EGCG, the predominant catechin in green tea, differs significantly between humans, mice and rats44. Low bioavailability of EGCG was also demonstrated following administration of GTP as the sole source of drinking fluid in rats, with a higher level seen in mice45.
The animals consumed green tea extract (2 and 5%) ad libitum, and their average intake was comparable to the control group and corresponds to approximately 40 cups per day in an 80 kg man. The study demonstrated a progressive rise in the weekly weight of animals, with no obvious signs of clinical toxicity or behavioural changes in the rats, indicating the well-being of the animals during the treatment period. Furthermore, body weight gain of the treated rats also remained unchanged. Similar, to our study, administration of green tea (70 mg/kg/day) to rats for 63 days or 90 days did not affect body weight gain32,33. However, a transient reduction in body weight was observed at week 5 in the 5% treatment group and may be attributed to taste aversion and reduced food intake. Kao et al. however, reported that EGCG (a predominant green tea polyphenol) reduced body weight in rats37. Green tea catechins (especially EGCG) are responsible for the reduced body weight by decreasing the differentiation and proliferation of adipocytes during lipogenesis46. Also, a significant rise in weight loss was observed in humans following consumption of a high dose of green tea for 12 weeks. It may be attributed to inhibition of ghrelin secretion, which leads to increased adiponectin levels47.
Although our study, however, showed a remarkable rise in kidney weight, the creatinine level remained unchanged, suggesting that green tea did not damage renal function. Another study revealed that high concentrations of green tea catechins (5%) increased the kidney weight, with no histopathologcal observation48. Also, l-theanine (a predominant free amino acid found in tea) was shown to increase kidney weight in rats, with no histological correlations49. Higher protein or amino acid intake modulates renal hemodynamic by increasing renal blood flow and elevating intraglomerular pressure that results in an increased glomerular filtration rate (GFR) as as well an increased kidney volume and weight50. The increased weight in our study may be attributed to the catechins or amino acid (l-theanine) content in green tea and was considered not to be toxicologically significant as there was no obvious histopathological changes in the kidney together with a comparable level of creatinine as the control. Besides, the weight of the liver remained unchanged, with significantly reduced levels of AST and ALT. Both of these findings suggest that consumption of green tea at least in rats had no detrimental effects, as also supported by the histological sections of the treated kidneys and liver. Our study is in corroboration with Bun et al.6, as no sign of evidence of distinctive hepatotoxicity was found in green tea treated rats, as this study showed no hepatic functional disorders based on the levels of ALT and AST in serum, as well as histological sections of the liver. The results of the current study are in line with the literature data suggesting hepatoprotective properties of green tea preparations51–53. Takami et al. revealed that prolonged intake (90 days) of high concentrations of green tea catechins (5%) brought about hepatotoxic effects in male rats by the increased levels of ALT and AST48, which could suggest that the use isolated or pure polyphenols could be detrimental to the functioning of these organs. Furthermore, case-reports have associated the use of concentrated green tea-based supplements with liver toxicity in humans54,55.
Our study demonstrated that the level of testosterone (which plays a crucial role in spermatogenesis) remained unchanged, even though, a trend to increased values was observed with increasing concentration of green tea. Other studies showed that green tea extract and EGCG produced a substantial drop in testosterone level in Leydig cells in vitro5,56, while testosterone production in vivo studies showed a reduction35,37, increment34 or remained unchanged32 in male rats. A possible explanation for these variations could be due to the use of different forms of the plant (crude extract, or isolated compounds), modes of administration or length of treatment.
Reproductive organ weights are used as indicators of reproductive toxicity57, and a reduction of testicular weight is a sensitive parameter for interpretation of male gonadal toxicity58. In line with the observed level of testosterone and similar to a previous study33, intake of green tea in this study did not alter the weight of testosterone dependent organs (testis, epididymis, seminal vesicles and prostate gland). Also, histological sections of the testis and epididymis revealed no obvious alteration in their structures. Furthermore, sperm quality is also used as an important indicator of chemical-induced toxicity on testis59. While a study demonstrated that green tea extract did not affect sperm concentration and viability33, our present study showed that green tea significantly increased sperm concentration and sperm vitality, rather, sperm motility and velocity functions were unaffected. However, morphometric measurements in our study showed a significant reduction in the diameter and epithelial height of the seminiferous tubule. In contrast, epithelial heights of cauda epididymis increased, and that of the caput remained unchanged. Contrary to our study, green tea did not affect the diameter of the seminiferous tubule33; however, a reducing trend was observed. Reproductive toxicants are known to reduce the diameter and epithelial height of seminiferous tubule as well as impair spermatogenesis59–61. Our study however, demonstrated an increase in sperm viability, concentration, and epithelial heights of epidiydymis with no dinstinct distortion of the seminiferous tubules or loss of spermatogenic cells. The mechanism of action responsible for these increment is unclear and requires further investigation to understand the impact on the male reproductive functions and fertility outcome.
Bioactive compounds in tea such as catechins, caffeine, and l-theanine62 are shown to possess conflicting effects on the male reproductive system. For instance, low concentrations of caffeine (mainly derived from tea leaves) stimulates lactate (the preferred metabolic substrate for developing germ cells) production (5 and 50 µM), and increases expression of glucose transporters (50 µM; GLUTs) in human Sertoli cells (hSCs)63. In another study, administration of caffeine (200 mg/kg body weight) negatively affected the male reproductive functions as demonstrated by the reduced testicular and epidydimal weight, distortion of the histo-architecture of the seminiferous tubule and loss of spermatogenic cells, sperm count, motility and viability with increase abnormal sperm64,65. In addition, maternal consumption of caffeine during gestation (26 and 45 mg/kg) and lactation (25 and 35 mg/kg) was shown to also negatively affect male reproductive function in the male offspring rats as indicated by a significant reduction in testicular weight, diameter and epithelial heights of seminiferous tubules, testosterone production and sperm quality66. EGCG was shown to have an antiproliferative effect on hSCs (5 and 50 µM), although lactate production was maintained (5 and 50 µM) with an increased consumption of glucose and pyruvate (50 µM). However, it decreased the mitochondrial membrane potential (50 µM) and conversion of pyruvate to alanine (5 and 50 µM), which ensures the regular production of lactate67. l-Theanine, on the other hand, increased hSC proliferation, glucose consumption and mitochondrial membrane potential, although lactate production remained unchanged68. Also, supplementation of sperm storage media for up to 3 days either with caffeine (71 µg/ml), EGCG (82 µg/ml) or l-theanine (19 µg/ml) had no effect on sperm viability, while the supplementation with a combination of these three compounds significantly increased sperm viability, suggesting a synergestic effect of the components of tea rather than an individual bioactive compound69. Hence, a possible explanation for the increased sperm concentration and viability in our study might be attributed to an increased lactate production by the Sertoli cells or increased GLUT protein levels and activities, although further studies are required to validate this.
In order to fertilise an oocyte, capacitated spermatozoa must undergo acrosome reaction, and consists of the release of the hydrolytic enzyme in the secretory vesicle of the sperm acrosome, for the degradation of the zona pellucida of the oocyte. A premature or spontaneous acrosome reaction makes the spermatozoa incompetent to interact with the oocyte and thereby to impair its fertilisation rate70. This study revealed a significant increase in spontaneous acrosome reaction, posing a question on the ability of these spermatozoa to fertilise an oocyte. Production of F-actin during capacitation was shown to be crucial in the prevention of spontaneous acrosome reaction71. An increase in cyclic adenosine monophosphate (cAMP) results in the activation of protein kinase A (PKA) at the onset of capacitation, which indirectly controls the phosphorylation of protein tyrosine, hyperactivated motility and actin polymerisation72. Further to this, calmodulin kinase II (CAMKII) and phospholipase D (PLD) were shown to be major pathways responsible for the polymerisation of actin71. Tsirulnikov et al. further showed that PKA protects the sperm from undergoing spontaneous acrosome reaction by inducing actin polymerisation via PLD and CAMKII73. The possible explanation for the increased spontaneous acrosome reaction as seen in this study could be due to inhibition of PKA, actin polymerisation or CAMKII and PLD pathways, which needs to be further interrogated. Isotani et al. rather reported that acrosin-disrupted spermatozoa equally pierced the zona pellucida as the wild-type spermatozoa, rather, a delayed dispersal rate of cumulus oophorus cells caused a decrease in the number of fertilised oocyte and litter size74. Hence, the effect of green tea or its polyphenolic compounds on fertility rate must be further investigated to understand better the implication of spontaneous acrosome reaction observed in this study.
In comparison to the findings of this study, a previous study showed that black tea increased sperm vitality, motility, the epithelial height of the cauda epididymis, kidney weight, as well as spontaneous acrosome reaction while decreasing the activities of AST and ALT and diameter and epithelial height of the seminiferous tubule75, however, an increased creatinine activity was observed. Another study demonstrated the aphrodisiac property of black tea, accompanied by an increase in testosterone production in rats76. White tea, which is known to have a higher level of antioxidants compared to green, oolong and black teas14, stimulated lactate production in Sertoli cells, which provides nutritional support to the developing germ cells77, as well as increased rat sperm viability1. White tea was also shown to improve prediabetes-induced reproductive dysfunction through increment in glucose transporters 2 and 3 (GLUT 2 and GLUT 3) protein levels and phosphofructokinase 1 (PFK1) activity, sperm motility, with restoration of testicular lactate content and sperm viability78. In another study, white tea improved testiscular antioxidant potential and sperm concentration, and restored sperm quality (motility, morphology and viability) in prediabetic male rats79. These are suggestive of possible effects of the antioxidants in these plants on various aspects of male reproductive functions.
Most of the beneficial effects of green tea are attributed to the antioxidant and free-radical scavenging properties, its polyphenols and flavonols80. Our study showed that the levels of CAT, GSH, MDA, SOD were unaffected by green tea in the liver, kidney and testes of the treated rats, which indicates the safety of the plant and suggests that a balance between the scavenging activity of the antioxidant and generation of ROS is not compromised, thereby preventing oxidative stress under the tested conditions. Another study showed that testicular CAT and GSH remained unchanged while a significant increase in SOD was noted33. Green tea extract was shown to protect testicular tissue against oxidative damage by increasing its antioxidant defence mechanism as demonstrated by the increased SOD and GSH activities with a resultant decrease in lipid peroxidation81. Antioxidant therapy is used to eliminate, take up or reduce the formation of ROS. Antioxidant supplements are categorised based on their activities as a preventive antioxidant (e.g. lactoferrin), which prevents the formation of reactive oxygen species (ROS), and scavenging antioxidants (e.g. vitamins C and E), which removes already present ROS82. The antioxidants are also classified as enzymatic (natural) oxidants (includes glutathione reductase, catalase, superoxide dismutase) and non-enzymatic oxidants, (includes vitamins B, C and E, carotenoid, carnitine, cysteine), which are derived from fruits and vegetables83. For instance, intake of a low amount of vitamin C was associated with increased oxidative stress84, while high levels of antioxidant (supraphysiological level) resulted in reductive stress, which is as destructive to cells as oxidative stress. For instance, administration of high concentrations of ascorbate was shown to have similar effects by causing oxidative stress85. A balance must be attained in the use of medicinal plants with an antioxidant in order to prevent oxidative or reductive stress.
This study demonstrates that continuous and prolonged intake of aqueous extract of green tea improved sperm parameters, specifically sperm concentration and vitality, and appears to be safe in the rats. However, subtle structural changes observed in the reduced diameter and epithelial heights of the seminiferous tubule and increased spontaneous acrosome reaction needs further investigation to understand the implications on fertility outcomes.
Methods
Chemical reagents
All chemicals except mentioned otherwise were obtained from Sigma Aldrich (St. Louis, MO, USA).
Preparation of aqueous extract of green tea
Green tea (Camellia sinensis; Five Roses™) purchased from a retail store in Cape Town, South Africa was infused in freshly boiled tap water for 5 min to final concentrations of 2% and 5% respectively, was filtered through a cheesecloth and Whatman’s filter paper (no 4 and 1, respectively) using a vacuum system (Opuwari & Monsees, 2015).
Chemical analysis of green tea
During the period of treatment, at least three samples of prepared green tea (2% and 5%) were randomly collected at different time points and stored at − 20 °C for further use. In order to determine the quantity of soluble solids in green tea, 1 ml of 2% and 5% green tea (in triplicate) was placed in the dried vials with a known weight. It was then dried overnight at 120 °C, allowed to cool in a desiccator and weighed. The obtained results were expressed as mg/ml of tea.
Total polyphenols in green tea were determined using the Folin–Ciocalteau (F–C) reagent, with Gallic acid as standard86 and the result was expressed as mg Gallic acid equivalent (GAE)/ml of tea. In brief, F–C reagent (10%) and sodium bicarbonate (7.5%) was added to the tea extracts and prepared standards (5:4:1). The thoroughly mixed samples were incubated at 37 °C for 2 h and read at 765 nm.
Flavonol content was determined using quercetin as a standard, according to Mazza et al.87 and expressed as mg Quercetin equivalent/ml of tea. To summarise, 0.1% hydrogen chloride (HCl) in 95% ethanol (50 µl) and 2% HCl (900 µl) was added to blank, standards and diluted samples (50 µl), incubated at 37 °C for 3 min and measured at 360 nm.
Flavanol content was determined using ( +) catechin as standard88 and expressed as mg catechin equivalent/ml of tea. Briefly, 4-(Dimethylamino)-cinnamaldehyde (1,000 µl) was added to blank, standards and tea extract (200 µl) and incubated at 37 °C for 5 min, with absorbance read at 640 nm.
Ferric reducing antioxidant power (FRAP) was determined using Trolox as standard (0, 100, 150, 200, 400 and 600 µM)89, with the result expressed as mM Trolox/ml of tea. In brief, 300 µl FRAP reagent (containing 300 mM sodium acetate buffer, 10 mM 2,4,6-tripyridyl-3-triazine, and 20 mM FeCl3 in a ratio 10:1:1) and dH20 was added to diluted tea extract (10 µl), incubated at 37 °C for 4 min and read at 593 nm using a microplate reader.
Animals
Male Wistar rats sourced from the animal facility of the Medical Bioscience department, University of the Western Cape, had free access to standard rat chow and tap water ad libitum. The animals were kept under standard conditions in a room at a temperature of 21–24 °C with a constant 12 h light/dark cycle. The Biomedical research ethics committee of the University of the Western Cape approved this study (registration No: 11/7/40) and all experiments were performed in accordance with relevant guidelines and regulations.
Treatment protocol
Eighteen male Wistar rats (63-day old; 200–250 g) were randomly divided into three groups (n = 6). Three animals per treatment group were housed in separate cages. Each group received the respective treatment ad libitum using a water bottle for 52 days:
Group one: tap water and served as control.
Group two: 2% green tea.
Group three: 5% green tea.
The amount of daily intake of fluid and the weekly bodyweight of the animals were recorded. At the end of the experiment (day 52), all rats were euthanized. Carbon dioxide was used to anaesthetize and euthanize the rats as it does not interfere with specific sperm parameters 90. To avoid dyspnoea and distress, rats were placed in a glass chamber which was then filled slowly with carbon dioxide from a compressed gas cylinder according to AVMA guidelines91. When rats lost consciousness, gas flow was increased and maintained until no respiratory movements were observed. Death was confirmed by exsanguination of the heart. Final body and organ weights were noted. Blood obtained through a cardiac puncture, allowed to clot for 30 min and centrifuged (3,000×g, 15 min), was stored at − 80 °C for hormonal and biochemical assays. Left cauda epididymis was excised and used for the determination of sperm concentration, vitality, motility and acrosome reaction. Testis, right epididymis, liver and kidney were fixed in Bouin’s solution for histological purposes. Left testis, liver and kidney were frozen at − 80 °C for biochemical assays.
Serum analysis for testosterone and biochemical assays
The level of testosterone in serum obtained from the male rats was measured with an ELISA kit (EIA 1559, DRG Instruments GmBH, Marburg, Germany), as per the manufacturer’s instruction. In brief, enzyme conjugate (200 µl) was added to 25 µl of sample and standard (0, 0.2, 0.5, 1, 2, 6, and 16 ng/ml) well, mixed and incubated at room temperature (RT) for one hour. After that, contents were briskly shaken out and rinsed three times with diluted wash solution (400 µl) and stricken sharply on absorbent paper to remove residual droplet. Substrate solution (200 µl) was added to each well and incubated at RT for 15 min, and the reaction stopped by the addition of stop solution (100 µl). Absorbance was read at 450 nm, and the result expressed as nanogram per millilitre.
Serum creatinine was determined, according to Bartels et al.92 and expressed as mg/ml. In brief, standard solution (0, 15, 30, 60, 90, 120 and 150 mg/l creatinine in dH20) or serum was added to a working solution (0.1 g picric acid in 50 ml dH20 and 50 ml 0.4 NaOH in 4 °C) (1:10), incubated at RT for 15 min and measured at 492 nm.
Levels of ALT and AST in serum was determined93 and expressed as IU/l. ALT or AST substrate (12.5 µl) was added to serum or standard (2.5 µl) and incubated at 37 °C for 1 h. Standard solution for ALT (0, 23, 50, 83, 125 IU/l) and AST ( 0, 20, 55, 95, 145 IU/l). After that, 0.2% 2, 4-dinitrophenylhydrazine in 37% HCl (25 µl) was added to each sample and incubated for 20 min at RT. Following which, 0.4 M NaOH (250 µl) was added and incubated for a further 30 min and read at 492 nm.
Antioxidant activities in the serum were measured using FRAP as described earlier89 and expressed as µM Trolox/ ml of serum.
Analysis of tissue for antioxidant activities
Previously frozen testis (20% w/v), kidney (20% w/v), and liver (20% w/v) tissues were swiftly thawed and homogenised with PRO 200 homogeniser (Proscientific Inc, Oxford, USA), in 1 ml ice-cold Tris-buffered saline (Tris–HCl 20 mM, NaCl 150 mM, pH 7.4) and centrifuged (5,000×g, 30 min and 4 °C). After that, the supernatants stored at − 80 °C and used for antioxidant and biochemical assays.
Lipid peroxidation was assessed according to Yagi94 by measuring malondialdehyde (MDA), a thiobarbituric acid reacted substances (TBARS) and represented as nanomolar of MDA per milligram of protein. In brief, thiobarbituric acid (TBA) reagent (15% v/v trichloroacetic acid and 0.25 N HCl) was added to each sample (2:1), heated at 95 °C for 15 min and allowed to cool. Samples were centrifuged (1,000×g; 10 min) and measured at 532 nm.
Glutathione (GSH) level was analysed as described by Ellman95 and expressed as millimolar thiol per milligram of protein. Briefly, Ellman’s reagent (3 ml) was added to each sample (20 µl), incubated for 15 min and absorbance read at 412 nm.
Superoxide dismutase (SOD) activity was determined by the method of pyrogallol autoxidation96 with a slight modification97. Briefly, samples were mixed with Tris-EDA-HCl buffer and pyrogallol (1:15:1) and incubated at 25 °C for 10 min. After that, 1 N HCl (50 µl) was added, and SOD activity measured at 440 nm. One unit is the amount of enzyme that inhibited the oxidation of pyrogallol by 50%, and the result represented as units per milligram of protein.
Catalase (CAT) activity was evaluated, according to Aebi98. In brief, samples were mixed with PBS and 20 mM H202 (1:4:5) and measured at 25 °C within 2 min. A unit of CAT is the activity of the enzyme that catalysed the reduction of 1 µmol of H2O2 per min per milligram of protein.
Total protein content was performed99 with bovine albumin serum (BSA) as standard (0, 0.2, 0.6, 1,1.4, 2.8, 4,2 and 5.6 mg/ml). To summarise, 25 µl reagent A and 200 µl reagent B (Biorad laboratory, Hercules, USA) was added to each sample and incubated at 37 °C for 30 min. Absorbance was read at 630 nm.
Analysis of rat spermatozoa
Spermatozoa collected from freshly isolated cauda epididymis by a swim out technique was used to analyse the respective parameters100. Sperm diluted with phosphate-buffered saline (PBS) (1:9) was counted with Markler counting chamber (Sefi-Medical Instrument, Haifa, Israel) to determine sperm concentration. Sperm concentration was represented as millions per millilitre (106/ml). Sperm vitality was determined using the fluorescent Duo vital kit (Microptic, Barcelona, Spain) according to the manufacturer’s instruction. In brief, a mixture of fluorochrome solution red (1 µl), fluorochrome solution green (1 µl) and sperm sample (5 µl), was placed on a clean slide. The slide was covered with a coverslip and spermatozoa (100) was analysed under a fluorescence microscope. Spermatozoa fluorescing green or red were respectively scored as alive or dead, and the result expressed as percentage alive. Sperm motility was determined by placing sperm suspension (5 µl) in a Leja slide (Leja Products B.V., Nieuw Vennep, Netherlands) and evaluated with Sperm Class Analyzer software (Microptic, Barcelona, Spain). Sperm parameters assessed included total motility, progressive motility, total static, curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s), average path velocity (VAP, µm/s), the amplitude of lateral head displacement (ALH, µm), and linearity (LIN, %), Wobble (WOB, %), beat cross frequency (BCF, Hz) as defined101. Acrosome reaction was evaluated according to Larson & Miller102. In brief, sperm samples were fixed with 4% p-formaldehyde in PBS (1:5) for 5 min and centrifuged (3,000×g, 3 min). The cells were re-suspended with ammonium acetate (100 µl), smeared on a glass slide, air-dried and stained with Coomassie Brilliant Blue G (0.22%) solution for 2 min at RT. Excess dye was removed and covered with a coverslip and observed under a bright-field microscope (1000X). Spermatozoa head stained blue represented spermatozoa with intact acrosome reaction, while those with partial or no stain, spermatozoa undergoing acrosome reaction.
Histology and morphometric measurement
Following the fixing and tissue processing of the testis, epididymis, kidney and liver, hematoxylin & eosin staining was utilised. The diameter and epithelial heights of the seminiferous tubules of the testes, and epithelial heights of the epididymis were conducted according to Opuwari & Monsees100. In brief, the diameters and epithelial heights of seminiferous tubules in 30 round or nearly round tubular profile per animal as well as epithelial heights of 10 caudal and caput epididymis (100X) were measured with Scope photo 3.0 (MicroImaging Ltd).
Data analysis
Data analysis was completed with GraphPad Prism version 6.0.0 for Windows (GraphPad Software, www.graphpad.com). Kolmogorov–Smirnoff test tested for normal distribution of data. Normally distributed data were analysed with one-way analysis of variance (ANOVA) and Tukey’s multiple comparison test. Not normally distributed data were analysed with the Mann–Whitney test and Dunn’s multiple comparison test. The Independent t-test was used in the analysis of the tea extracts. ANOVA trend analysis was also employed between groups. Data were expressed as mean ± standard error of the mean, and a P-value less than 0.05 was set as statistically significant.
Acknowledgements
We express our appreciation to Gerhard van der Horst and Liana Maree for their assistance with the Computer Assisted Sperm Analysis equipment.
Author contributions
C.S.O. conducted the experiments, performed the statistical analysis and drafted the manuscript. T.K.M. conceptualised the study and revised the manuscript. All authors read and approved the final manuscript.
Funding
The National Research Foundation of South Africa and the Senate Research Council of the University of the Western Cape funded this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chinyerum Opuwari, Email: chinyerum.opuwari@ul.ac.za.
Thomas Monsees, Email: tmonsees@uwc.ac.za.
References
- 1.Dias TR, et al. White tea as a promising antioxidant medium additive for sperm storage at room temperature: a comparative study with green tea. J. Agric. Food Chem. 2014;62:608–617. doi: 10.1021/jf4049462. [DOI] [PubMed] [Google Scholar]
- 2.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 3.Rusak G, Komes D, Likić S, Horžić D, Kovač M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. 2008;110:852–858. doi: 10.1016/j.foodchem.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 4.Del Rio D, et al. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004;52:2807–2815. doi: 10.1021/jf0354848. [DOI] [PubMed] [Google Scholar]
- 5.Figueiroa MS, et al. Green tea polyphenols inhibit testosterone production in rat Leydig cells. Asian J. Androl. 2009;11:362. doi: 10.1038/aja.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bun SS, Bun H, Guédon D, Rosier C, Ollivier E. Effect of green tea extracts on liver functions in Wistar rats. Food Chem. Toxicol. 2006;44:1108–1113. doi: 10.1016/j.fct.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Tea association of the USA, Inc. Tea association of the USA, Inc. Retrieved January24, 2017 (2015).
- 8.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea–a review. J. Am. Coll. Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 9.Lambert JD, et al. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu. Rev. Nutr. 2013;33:161–181. doi: 10.1146/annurev-nutr-071811-150717. [DOI] [PubMed] [Google Scholar]
- 11.Filippini, T. et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Datab. Syst. Rev. (2020). [DOI] [PMC free article] [PubMed]
- 12.Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch. Toxicol. 2015;89:1175–1191. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- 13.Higdon, J. V. & Frei, B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. (2003). [DOI] [PubMed]
- 14.Sharangi, A. B. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.): a review. Food Res. Int.42, 529–535 (2009).
- 15.Schönthal AH. Adverse effects of concentrated green tea extracts. Mol. Nutr. Food Res. 2011;55:874–885. doi: 10.1002/mnfr.201000644. [DOI] [PubMed] [Google Scholar]
- 16.Bettuzzi S, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Can. Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 17.Chow HS, et al. Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol. Prevent. Biomarkers. 2006;15:2473–2476. doi: 10.1158/1055-9965.EPI-06-0365. [DOI] [PubMed] [Google Scholar]
- 18.Lee M-J, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol. Prevent. Biomarkers. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 19.Sarma DN, et al. Safety of green tea extracts. Drug Saf. 2008;31:469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- 20.Wu K-M, Yao J, Boring D. Green tea extract-induced lethal toxicity in fasted but not in nonfasted dogs. International journal of toxicology. 2011;30:19–20. doi: 10.1177/1091581810387445. [DOI] [PubMed] [Google Scholar]
- 21.Hasan KMd, M., Tamanna, N. & Haque, Md. A. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Sci. Hum. Wellness. 2018;7:77–82. [Google Scholar]
- 22.Inoue H, Maeda-Yamamoto M, Nesumi A, Tanaka T, Murakami A. Low and medium but not high doses of green tea polyphenols ameliorated dextran sodium sulfate-induced hepatotoxicity and nephrotoxicity. Biosci. Biotechnol. Biochem. 2013;77:1223–1228. doi: 10.1271/bbb.121003. [DOI] [PubMed] [Google Scholar]
- 23.Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014;557:3–10. doi: 10.1016/j.abb.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Saleh IG, et al. Effect of green tea and its polyphenols on mouse liver. Fitoterapia. 2013;90:151–159. doi: 10.1016/j.fitote.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Mazzanti G, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 26.Salminen WF, et al. Green tea extract can potentiate acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2012;50:1439–1446. doi: 10.1016/j.fct.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Lv J, et al. Protective effect of epigallocatechin gallate, a major constituent of green tea, against renal ischemia–reperfusion injury in rats. Int. Urol. Nephrol. 2015;47:1429–1435. doi: 10.1007/s11255-015-1030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamadin AM, El-Beshbishy HA, El-Mahdy MA. Green tea extract attenuates cyclosporine A-induced oxidative stress in rats. Pharmacol. Res. 2005;51:51–57. doi: 10.1016/j.phrs.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Nasri H, et al. A biochemical study on ameliorative effect of green tea (Camellia sinensis) extract against contrast media induced acute kidney injury. J. Renal Injury Prevent. 2014;3:47. doi: 10.12861/jrip.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.França, L. & Russell, L. The testis of domestic animals. in Male reproduction, a multidisciplinary overview 197–219 (1998).
- 32.Yassa HA, George SM, Refaiy AERM, Moneim EMA. Camellia sinensis (green tea) extract attenuate acrylamide induced testicular damage in albino rats. Environ. Toxicol. 2014;29:1155–1161. doi: 10.1002/tox.21846. [DOI] [PubMed] [Google Scholar]
- 33.Abdelrazek HM, Helmy SA, Elsayed DH, Ebaid HM, Mohamed RM. Ameliorating effects of green tea extract on cadmium induced reproductive injury in male Wistar rats with respect to androgen receptors and caspase-3. Reprod. Biol. 2016;16:300–308. doi: 10.1016/j.repbio.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Satoh K, et al. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem. Toxicol. 2002;40:925–933. doi: 10.1016/s0278-6915(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 35.Chandra, A. K., Choudhury, S. R., De, N. & Sarkar, M. Effect of green tea (Camellia sinensis L.) extract on morphological and functional changes in adult male gonads of albino rats. IJEB 49(09) (2011). [PubMed]
- 36.Das, S. K. & Karmakar, S. N. Effect of green tea (camellia sinensis l.) leaf extract on reproductive system of adult male albino rats. Int. J. Physiol. Pathophysiol. Pharmacol.7, 178–184 (2015). [PMC free article] [PubMed]
- 37.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 38.De Amicis F, Santoro M, Guido C, Russo A, Aquila S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012;56:1655–1664. doi: 10.1002/mnfr.201200190. [DOI] [PubMed] [Google Scholar]
- 39.Sato K, et al. Green tea extracts attenuate doxorubicin-induced spermatogenic disorders in conjunction with higher telomerase activity in mice. J. Assist. Reprod. Genet. 2010;27:501–508. doi: 10.1007/s10815-010-9438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanchi MM, et al. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015;2:252–260. doi: 10.1016/j.toxrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awoniyi DO, Aboua YG, Marnewick J, Brooks N. The effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial rooibos and green tea supplements on epididymal sperm in oxidative stress-induced rats. Phytother. Res. 2012;26:1231–1239. doi: 10.1002/ptr.3717. [DOI] [PubMed] [Google Scholar]
- 42.Maxwell, S. & Thorpe, G. Tea flavonoids have little short term impact on serum antioxidant activity. BMJ313, 229 (1996). [DOI] [PMC free article] [PubMed]
- 43.Benzie IFF, Szeto YT, Strain JJ, Tomlinson B. Consumption of green tea causes rapid increase in plasma antioxidant power in humans. Nutr. Cancer. 1999;34:83–87. doi: 10.1207/S15327914NC340112. [DOI] [PubMed] [Google Scholar]
- 44.Lambert JD, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003;133:4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, et al. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- 46.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol. Nutr. Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 47.Chen I-J, Liu C-Y, Chiu J-P, Hsu C-H. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016;35:592–599. doi: 10.1016/j.clnu.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Takami S, et al. Evaluation of toxicity of green tea catechins with 90-day dietary administration to F344 rats. Food Chem. Toxicol. 2008;46:2224–2229. doi: 10.1016/j.fct.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 49.Borzelleca JF, Peters D, Hall W. A 13-week dietary toxicity and toxicokinetic study with l-theanine in rats. Food Chem. Toxicol. 2006;44:1158–1166. doi: 10.1016/j.fct.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Fouque D, Aparicio M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007;3:383–392. doi: 10.1038/ncpneph0524. [DOI] [PubMed] [Google Scholar]
- 51.Mahmoud, M. F., Fahmy, A. & Auf, M. A. Evaluation of the hepatoprotective effect of green tea extract and selenium on CCL4-induced fibrosis. e-SPEN J.7, e23–e29 (2012).
- 52.Rameshrad M, Razavi BM, Hosseinzadeh H. Protective effects of green tea and its main constituents against natural and chemical toxins: a comprehensive review. Food Chem. Toxicol. 2017;100:115–137. doi: 10.1016/j.fct.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 53.Shivashankara, A. R. et al. Hepatoprotective effects of green tea and its polyphenols: preclinical observations. In: Polyphenols in Human Health and Disease 715–721 (Elsevier, 2014).
- 54.Javaid A, Bonkovsky HL. Hepatotoxicity due to extracts of Chinese green tea (Camellia sinensis): a growing concern. J. Hepatol. 2006;45:334–335. doi: 10.1016/j.jhep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Jimenez-Saenz, M. & del Carmen Martinez-Sanchez, M. Acute hepatitis associated with the use of green tea infusions. J. Hepatol.44, 616–617 (2006). [DOI] [PubMed]
- 56.Opuwari, C. & Monsees, T. Reduced testosterone production in TM3 Leydig cells treated with Aspalathus linearis (Rooibos) or Camellia sinensis (tea). Andrologia47, 52–58 (2015). [DOI] [PubMed]
- 57.Zidan NA. Evaluation of the reproductive toxicity of chlorpyrifos methyl, diazinon and profenofos pesticides in male rats. Int. J. Pharmacol. 2009;5:51–57. [Google Scholar]
- 58.Dutta AL, Sahu CR. Emblica officinalis Garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. SpringerPlus. 2013;2:541. doi: 10.1186/2193-1801-2-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hasanein P, Fazeli F, Parviz M, Roghani M. Ferulic acid prevents lead-induced testicular oxidative stress and suppressed spermatogenesis in rats. Andrologia. 2018;50:e12798. doi: 10.1111/and.12798. [DOI] [PubMed] [Google Scholar]
- 60.Ghanbari, M., Ahmadnia, H., Moradi, M. R. & Khajeh, D. M. Effect of cigarette smoke on spermatogenesis in rats. (2007). [PubMed]
- 61.Kaur R, Kaur K. Effects of dietary selenium (SE) on morphology of testis and cauda epididymis in rats. Indian J. Physiol. Pharmacol. 2000;44:265–272. [PubMed] [Google Scholar]
- 62.de Mejia EG, Ramirez-Mares MV, Puangpraphant S. Bioactive components of tea: cancer, inflammation and behavior. Brain Behav. Immun. 2009;23:721–731. doi: 10.1016/j.bbi.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Dias TR, et al. Dose-dependent effects of caffeine in human Sertoli cells metabolism and oxidative profile: relevance for male fertility. Toxicology. 2015;328:12–20. doi: 10.1016/j.tox.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Bassey RB, Yama OE, Osinubi AA, Noronha CC, Okanlawon A. Effects of Tahitian Noni dietary supplement on caffeine-induced testicular histo-pathological alterations in adult Sprague-Dawley rats. Middle East Ferti. Soc. J. 2011;16:61–66. [Google Scholar]
- 65.Ekaluo, U. B., Ikpeme, E. V., Ibiyang, Y.B. & Omordia, F. O. Effect of Soursop (Annona muricata L.) fruit extract on sperm toxicity induced by caffeine in albino rats. J. Med. Sci.13, 67–71 (2013).
- 66.Dorostghoal M, Majd NE, Nooraei P. Maternal caffeine consumption has irreversible effects on reproductive parameters and fertility in male offspring rats. Clin. Exp. Reprod. Med. 2012;39:144. doi: 10.5653/cerm.2012.39.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dias TR, et al. Implications of epigallocatechin-3-gallate in cultured human Sertoli cells glycolytic and oxidative profile. Toxicol In Vitro. 2017;41:214–222. doi: 10.1016/j.tiv.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Dias TR, et al. L-Theanine promotes cultured human Sertoli cells proliferation and modulates glucose metabolism. Eur. J. Nutr. 2019;58:2961–2970. doi: 10.1007/s00394-019-01999-2. [DOI] [PubMed] [Google Scholar]
- 69.Dias TR, Alves MG, Casal S, Silva BM, Oliveira PF. The single and synergistic effects of the major tea components caffeine, epigallocatechin-3-gallate and l-theanine on rat sperm viability. Food Funct. 2016;7:1301–1305. doi: 10.1039/c5fo01611h. [DOI] [PubMed] [Google Scholar]
- 70.Cummins JM, Yanagimachi R. Development of ability to penetrate the cumulus oophorus by hamster spermatozoa capacitated in vitro, in relation to the timing of the acrosome reaction. Gamete Res. 1986;15:187–212. [Google Scholar]
- 71.Shabtay O, Breitbart H. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol. 2016;415:64–74. doi: 10.1016/j.ydbio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 72.Rotfeld H, Hillman P, Ickowicz D, Breitbart H. PKA and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction. 2014;147:347–356. doi: 10.1530/REP-13-0560. [DOI] [PubMed] [Google Scholar]
- 73.Tsirulnikov E, Huta Y, Breitbart H. PKA and PI3K activities during capacitation protect sperm from undergoing spontaneous acrosome reaction. Theriogenology. 2019;128:54–61. doi: 10.1016/j.theriogenology.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 74.Isotani A, et al. A delayed sperm penetration of cumulus layers by disruption of acrosin gene in rats. Biol. Reprod. 2017;97:61–68. doi: 10.1093/biolre/iox066. [DOI] [PubMed] [Google Scholar]
- 75.Opuwari, C. S. & Monsees, T. K. In vivo effects of black tea on the male rat reproductive system and functions of the kidney and liver. Andrologia e13552 (2020). [DOI] [PubMed]
- 76.Ratnasooriya WD, Fernando TSP. Effect of black tea brew of Camellia sinensis on sexual competence of male rats. J Ethnopharmacol. 2008;118:373–377. doi: 10.1016/j.jep.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 77.Martins, A. D. et al. Effect of white tea (Camellia sinensis (L.)) extract in the glycolytic profile of Sertoli cell. Eur. J. Nutr.53, 1383–1391 (2014). [DOI] [PubMed]
- 78.Dias TR, et al. White tea intake prevents prediabetes-induced metabolic dysfunctions in testis and epididymis preserving sperm quality. J. Nutr. Biochem. 2016;37:83–93. doi: 10.1016/j.jnutbio.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Oliveira PF, et al. White tea consumption restores sperm quality in prediabetic rats preventing testicular oxidative damage. Reprod. Biomed. Online. 2015;31:544–556. doi: 10.1016/j.rbmo.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Mukhtar H, Katiyar SK, Agarwal R. Green tea and skin—anticarcinogenic effects. J. Investig. Dermatol. 1994;102:3–7. doi: 10.1111/1523-1747.ep12371720. [DOI] [PubMed] [Google Scholar]
- 81.Awoniyi, D. O., Aboua, Y. G., Marnewick, J. L., Plesis, S. Du & Brooks, N. L. Protective effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial supplements on testicular tissue of oxidative stressinduced rats. Afr. J. Biotechnol.10, 17317–17322–17322 (2011).
- 82.Lampiao F. Free radicals generation in an in vitro fertilization setting and how to minimize them. World J Obstet Gynecol. 2012;1:29–34. [Google Scholar]
- 83.Bansal, A. K. & Bilaspuri, G. S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. (2011). [DOI] [PMC free article] [PubMed]
- 84.Fraga CG, et al. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. 1991;88:11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J. 1991;273:601–604. doi: 10.1042/bj2730601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 87.Mazza G, Fukumoto L, Delaquis P, Girard B, Ewert B. Anthocyanins, phenolics, and color of cabernet franc, merlot, and pinot noir wines from British Columbia. J. Agric. Food Chem. 1999;47:4009–4017. doi: 10.1021/jf990449f. [DOI] [PubMed] [Google Scholar]
- 88.McMurrough I, McDowell J. Chromatographic separation and automated analysis of flavanols. Anal. Biochem. 1978;91:92–100. doi: 10.1016/0003-2697(78)90819-9. [DOI] [PubMed] [Google Scholar]
- 89.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 90.Stutler SA, et al. Effect of method of euthanasia on sperm motility of mature Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 2007;46:13–20. [PubMed] [Google Scholar]
- 91.Leary, S. et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. 121 (2020).
- 92.Bartels H, Böhmer M, Heierli C. Serum creatinine determination without protein precipitation. Clin. Chim. Acta. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 93.Reitman, S. & Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.28, 56–63 (1957). [DOI] [PubMed]
- 94.Yagi K. Assay for blood plasma or serum. Meth. Enzymol. 1984;105:328–331. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 95.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 96.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 97.Ben Abdallah, F. et al. Effects of date seed oil on testicular antioxidant enzymes and epididymal sperm characteristics in male mice. Andrologia41, 229–234 (2009). [DOI] [PubMed]
- 98.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 99.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 100.Opuwari CS, Monsees TK. In vivo effects of Aspalathus linearis (rooibos) on male rat reproductive functions. Andrologia. 2014;46:867–877. doi: 10.1111/and.12158. [DOI] [PubMed] [Google Scholar]
- 101.van Der Horst G, Seier JV, Spinks AC, Hendricks S. The maturation of sperm motility in the epididymis and vas deferens of the vervet monkey Cercopithecus aethiops. Int. J. Androl. 1999;22:197–207. doi: 10.1046/j.1365-2605.1999.00171.x. [DOI] [PubMed] [Google Scholar]
- 102.Larson JL, Miller DJ. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 1999;52:445–449. doi: 10.1002/(SICI)1098-2795(199904)52:4<445::AID-MRD14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]