Abstract
Wildlife vaccination is of urgent interest to reduce disease-induced extinction and zoonotic spillover events. However, several challenges complicate its application to wildlife. For example, vaccines rarely provide perfect immunity. While some protection may seem better than none, imperfect vaccination can present epidemiological, ecological, and evolutionary challenges. While anti-infection and antitransmission vaccines reduce parasite transmission, antidisease vaccines may undermine herd immunity, select for increased virulence, or promote spillover. These imperfections interact with ecological and logistical constraints that are magnified in wildlife, such as poor control and substantial trait variation within and among species. Ultimately, we recommend approaches such as trait-based vaccination, modeling tools, and methods to assess community- and ecosystem-level vaccine safety to address these concerns and bolster wildlife vaccination campaigns.
Keywords: vaccination, wildlife, spillover, conservation, imperfect immunity
Highlights
Vaccines vary in their efficacy and can be categorized as conferring waning, binary, or partial immunity. Some imperfect vaccines may indirectly increase parasite transmission or virulence.
Target vaccine coverage depends on wildlife disease control objectives, for example, spillover prevention or conservation.
Understanding the ecological drivers of variation in exposure (e.g., trait-based behaviors) and physiological response to vaccination (e.g., species identity, age class, genotype) is critical to developing efficient vaccine deployment strategies.
Features of vaccine or host–parasite biology should drive the choice of modeling framework between classic compartment models versus individual-based models (IBMs). Susceptible-infected-resistant (SIR) models are useful for modeling binary imperfection, but IBMs are better for vaccines with partial imperfection.
The Potential of Wildlife Vaccines
Vaccination, the process of exposing the immune system to an antigen to induce pathogen resistance, is a powerful tool for controlling disease. The benefits of vaccination are twofold: recipients are directly protected against infection and unvaccinated hosts are indirectly protected through herd immunity (see Glossary), which reduces transmission and parasite-mediated harm to host populations [1]. Vaccination has been vastly successful for humans and livestock [2,3]. Successful vaccination campaigns against rabies in raccoons (Procyon lotor), red foxes (Vulpes vulpes), gray foxes (Urocyon cinereoargenteus), and coyotes (Canis latrans) suggest that vaccination efforts could be directed towards emerging infectious diseases (EIDs) that cause devastating host declines, for example, amphibian chytridiomycosis, white nose syndrome, Tasmanian devil facial-tumor disease, and Ebola [4., 5., 6., 7., 8., 9., 10.]. The success of vaccination in human and livestock populations, the pressing need for disease-control tools in wildlife conservation, and the ever-increasing threat of zoonotic spillover events support a clear need to develop vaccination as an intervention tool for wildlife disease control. However, several outstanding challenges and questions remain before vaccination can emerge as a reliable tool for wildlife disease control. We argue that accounting for the limitations of imperfect vaccines, host and non-host ecology, and individual physiology in the development of vaccination campaigns is vital for harnessing the potential of wildlife vaccines successfully.
Objectives of Wildlife Vaccination
Biodiversity conservation and the prevention of pathogen spillover are two urgent concerns of wildlife disease control. Emerging diseases of wildlife threaten population and species persistence and contribute significantly to the ongoing loss of biodiversity [11]. Additionally, wildlife populations are reservoir hosts for many zoonotic pathogens such as rabies, Nipah virus, and coronaviruses that threaten the health of humans [12].
Controlling disease in wildlife reservoir populations can reduce spillover transmission, but complete prevention of spillover risk from a known pathogen requires elimination or eradication of a parasite within a reservoir host to prevent zoonotic transmission. Vaccines may be able to achieve this objective, but given the inherent antigenic specificity of all known vaccines they will not prevent novel pathogen emergence. Theory underlying eradication often identifies a critical level of vaccine coverage, which drives the effective reproductive ratio (R eff) of a pathogen below the threshold value of one [1]. Combating rinderpest virus reintroduction during the eradication campaign exemplifies the intense effort needed for eradication [3].
By contrast, vaccination for conservation aims to maximize the persistence of host populations and communities by decreasing the risk of disease-induced extinction, rather than through achieving parasite elimination. Wildlife populations can generally withstand small-scale disease outbreaks, and so conservation-motivated vaccination does not always require pathogen eradication [13]. Thus, vaccination coverage required for conservation-motivated disease control tends to be lower than that required for spillover prevention. For example, modeling estimates suggest that maintaining low vaccination coverage, between 20% and 40%, will stave off rabies-induced extinction of Ethiopian wolves (Canis simensis) [13].
Vaccine Efficacy and Modes of Imperfection
Despite their potential for controlling wildlife disease, vaccines rarely provide perfect immunity, which can compromise herd immunity or contribute to the evolution of increased parasite virulence [14]. For example, a prototype vaccine partially protects amphibians from Batrachochytrium dendrobatidis; vaccination decreases, but does not eliminate, parasite proliferation [15]. By contrast, a theoretically perfect vaccine would provide permanent and complete resistance to infection for all recipients, but vaccines considered for wildlife often fall short of this definition [14]. Three broad aspects of vaccine imperfection are often discussed in the literature: waning, leaky, and partial immunity. However, 'leaky' immunity is used inconsistently and imprecisely, generating confusion. One reason for this is that modeling frameworks, such as susceptible-infected-resistant (SIR) compartment models can make it difficult to incorporate some types of vaccine imperfections. Therefore, we suggest a clarified categorization based on waning, binary, and partial immunity. Importantly, these categories are not mutually exclusive, and we discuss the impacts of these varying levels of immunity on wildlife populations, vaccine efficacy, and modeling frameworks.
Waning Immunity
Waning describes the loss of resistance to infection over time. Individuals can vary in their waning rate, and immunity can be restored by subsequent exposures, that is, 'boosters'. Vaccine-induced immunity often wanes faster than immunity generated from natural infection, which can leave vaccinated individuals at higher risk during recurrent or cyclical epidemics [16]. For example, eastern equine encephalitis virus vaccination in sandhill cranes (Grus americana) and whooping cranes (Grus canadensis) waned rapidly, requiring booster vaccination within 30 days [17]. Life history traits, immune boosting sources, and waning rate interact to determine vaccine utility [18]. Waning immunity is routinely and relatively easily incorporated into SIR compartment models by allowing resistant individuals to re-enter the susceptible class.
Binary Immunity
Binary immunity occurs when vaccination does not induce immunity in all recipients [19]. This generates a binary outcome, wherein hosts are either resistant or susceptible, with no intermediate outcome. Binary outcomes of immunization have also been described as an 'all-or-nothing qualitative response' [20]. For example, high rates of binary vaccine outcomes for the varicella vaccine in humans prompted the recommendation for a second dose within months of the first [21]. Differences in vaccine immunogenicity, adjuvants, vaccine storage, dosage, administration, host infection status, competence of the host’s immune system, and host genetics can all shape binary immunity [19,22]. Random binary immunization outcomes are often incorporated into SIR models by effectively lowering vaccination coverage by the proportion of binary failures [23]. However, if certain host types are more prone to vaccine failure, then it might be critical to address how these different failure rates among different host classes affect disease dynamics [24].
Partial Immunity
In contrast to binary efficacy, which assumes that a vaccine either succeeds in inducing an acquired immune response or fails, vaccines that provide partial immunity may not completely prevent infection, disease symptoms, or transmission in an immunized host. Partial immunity allows for vaccine efficacy to be measured on a proportional gradient from 0 to 1, rather than as a qualitative all-or-nothing response [25,26]. One critical complication is that partial immunity may impact a number of infection outcomes, such as resistance to infection, disease attributed to infection, and infectiousness [27]. The functional consequences of these changes are detailed below. Partial immunity is less easily incorporated into SIR-type models and has therefore been relatively neglected compared with other modes of imperfection. Individual-based models (IBMs), which explicitly track individual traits and histories, may be much better suited to investigate this vaccine imperfection.
Functional Mechanisms and Consequences of Imperfect Vaccines
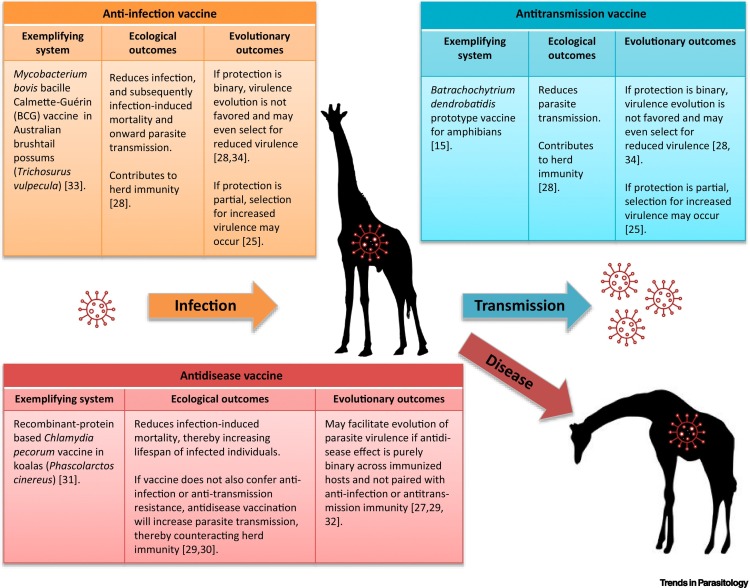
Different resistance responses to imperfect vaccines have unique ecological and evolutionary consequences. Imperfect immunization can confer the following three phenotypic types of resistance response: (i) antidisease, (ii) anti-infection, and (iii) antitransmission (Figure 1 ). These are also not mutually exclusive, and they can be assessed using either binary (qualitative) or partial (quantitative) metrics [26,28,29]. Because the majority of vaccines are imperfect, anticipating and addressing their potential deleterious consequences is a priority in determining vaccination feasibility in a wildlife context. For example, the imperfect-vaccine hypothesis postulates that partial immunity upon vaccination could drive the evolution of increased pathogen virulence, and the risk of vaccine-driven virulence evolution is dependent on the vaccination phenotype and efficacy [29].
Figure 1.
Ecological and Evolutionary Outcomes of the Resistance Phenotypes.
Imperfect vaccines can be categorized by the phenotypic resistance effects on vaccinated hosts, such as anti-infection, antidisease, and antitransmission. Each of these nonexclusive categories can influence epidemiology and pathogen evolution. The figure cites [15,25,27., 28., 29., 30., 31., 32., 33., 34.].
Antidisease Vaccines
Antidisease vaccines reduce virulence (i.e., increase host tolerance) without necessarily reducing the risk of infection or subsequent transmission. Therefore, these vaccines directly benefit recipients, but can counteract herd immunity if the infectious period is lengthened. Studies on Marek’s disease in poultry, and helminth and tuberculosis coinfections in African buffalo, show that interventions which reduce the mortality of infected hosts, without decreasing infection or transmission rates, increase parasite transmission in populations by extending the infectious period [29,30]. Despite this potential for increased transmission, antidisease vaccines may still be effective for conservation if their net effect reduces total parasite-induced mortality or reproductive costs. A prototype anti-Chlamydia pecorum vaccine for koala (Phascolarctos cinereus) conservation offers potential as a therapeutic vaccine as it reduces disease in unexposed and infected koalas, with some reduction in infection incidence and loads [31]. However, antidisease vaccines are unlikely to reduce spillover risk, precisely because they can promote transmission.
Evolutionarily, lengthening the infectious period through antidisease vaccination is theorized to relax selection against high virulence [27,29]. This prediction, derived from the transmission-virulence trade-off hypothesis, arises because limiting host death allows for otherwise highly virulent genotypes to persist and even be favored by selection [29]. While experimental evidence explicitly demonstrating increased virulence driven by vaccination is lacking, a recent study on house finches (Haemorhous mexicanus) parasitized by the bacterium Mycoplasma gallisepticum demonstrated that an antidisease phenotype conferred by a natural primary infection facilitated a twofold increase in the fitness advantage of a high-virulence strain during secondary infections [32]. However, antidisease vaccines that vary in degree of protection among immunized individuals may be less risky for vaccine-driven virulence evolution, as variance in host protection will not uniformly favor the evolution of increased parasite virulence [27].
Anti-infection and Antitransmission Vaccines
Vaccines that prevent or reduce parasite establishment in an immunized host are considered anti-infection vaccines. Antitransmission vaccines, on the other hand, may permit infection but prevent or reduce onward transmission from the recipient. Both phenotypes contribute to herd immunity, and epidemiological models predict that parasite elimination can be achieved with high rates of coverage and efficacy [28]. Thus, both anti-infection and antitransmission vaccines can be effective for spillover prevention and conservation. The Mycobacterium bovis bacille Calmette–Guérin (BCG) vaccine, used to prevent spillover of M. bovis into livestock, confers anti-infection resistance in Australian brushtail possums (Trichosurus vulpecula), and the transmission-reducing prototype B. dendrobatidis vaccine offers promise for use in amphibian conservation [15,33].
The evolutionary consequences of these vaccines depend crucially on the mode of imperfection. Binary anti-infection or antitransmission vaccines do not favor virulence evolution and can, at times, even reduce selection for parasite virulence, by preventing coinfections for example [28,34]. Conversely, partial anti-infection or antitransmission vaccines can select for increased virulence [25]. Partial anti-infection and antitransmission phenotypes effectively increase the exposure dose required for establishment (i.e., the infectious dose), which can select for increases in parasite reproduction rate [25,28]. Theory suggests that this type of anti-infection resistance favors virulence evolution by encouraging the increase in intrinsic parasite reproduction for successful infection establishment [25].
Ecological and Logistical Challenges of Vaccination Exacerbated in Wildlife
Vaccines have strong potential to achieve disease control in wildlife. However, imperfect vaccines must also overcome physiological, behavioral, and ecological factors to succeed. Thus, complications arise from two primary factors: vaccine imperfections and vaccine administration. Lack of control and intraspecific, interspecific, and environmental heterogeneity are central sources of uncertainty in vaccine delivery, uptake, and response (Box 1 ). Vaccination success hinges on high coverage of doses that induce a durable immune response without harming recipients [1]. In complex ecological communities, indirect deployment (i.e., oral baiting) campaigns risk simultaneously over- and under-dosing many organisms because wildlife can vary in (i) the amount of inoculum consumed or encountered, and (ii) their physiological response to a given dose.
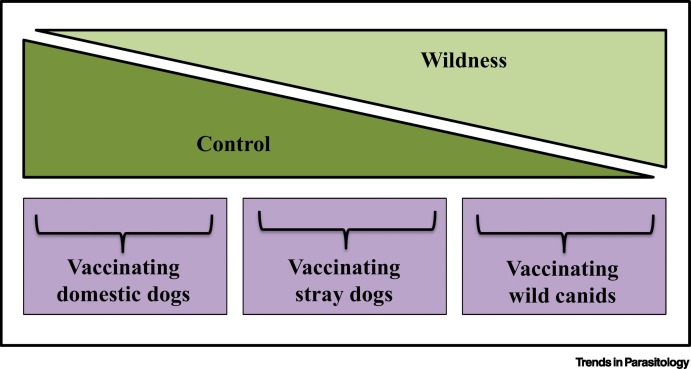
Box 1. Canid Rabies Vaccination Campaigns: Limitations to Control.
Rabies vaccination of canids has been used to both prevent spillover transmission into human populations and protect endangered wildlife [51]. Rabies vaccination of domestic dogs, stray dogs, and wild canids demonstrates vaccination across a gradient of control and wildness (Figure I). Globally, domestic dogs are the main source of rabies transmission to humans [52]. Consequently, owned-dog vaccination is used to interrupt dog-to-human transmission and, largely due to the control afforded by ownership, has been successful in eliminating enzootic canine rabies in the USA [53]. However, the unconstrained movement of stray dogs allows contact with wildlife, owned dogs, and humans, amplifying their importance in rabies transmission [54]. Difficulty catching stray dogs contributed to poor coverage, and hence failure, in a mass rabies vaccination campaign in Bangkok, Thailand [55]. Furthermore, high population growth, turnover, and translocation rates of stray dogs intensifies the challenge of achieving and maintaining vaccination coverage sufficient for herd immunity [54., 55., 56.]. Combining vaccination with neutering can combat these challenges [57].
Vaccination of wildlife against rabies, to prevent spillover into humans and domestic animals, has also involved hugely successful campaigns – locally eliminating rabies in red foxes and coyotes, while decreasing its prevalence in gray foxes [4., 5., 6.]. This success is undoubtedly driven by the advent of oral bait vaccines, which can be distributed across large geographic scale [6]. Yet, although oral vaccination reduces the need for wildlife control via capture and handling, and increases the geographic scale of administration, successful oral vaccination requires ecological knowledge of target and non-target foraging behaviors and home ranges for baiting, population turnover rates for estimating length of vaccination protection, and species-specific immunological responses [6,58,59]. Rabies vaccination has also been implemented as a conservation measure for endangered wild canids, such as the Ethiopian wolf (Canis simensis) and African wild dogs (Lycaon pictus) [56,60].
In these canid vaccination campaigns, control at the individual level, such as compliance, handling, and capture, proves most challenging. Thus, strategies that prioritize population-level measures, that is, economic incentives through government support for owned-dog vaccination, managing stray dog populations through neutering, and oral baiting of free-roaming and wild canids, significantly enhance vaccination success.
Figure I.
Rabies Vaccination on a Gradient of Wildness.
Alt-text: Box 1
Heterogeneity in host behavior, morphology, and habitat use all influence infection risk, and probability of vaccine exposure [35., 36., 37.]. Assessing vaccine exposure in target and non-target wildlife can be done using biomarkers, such as fluorescent Rhodamine B [38]. Moreover, the immunological traits of most wildlife hosts remain poorly known, and even closely related species can exhibit marked variation in response to vaccination [39]. In vaccination campaigns using indirect deployment, assessing vaccine safety and impact on non-target hosts and non-hosts is a critical step to anticipating and preventing harmful unintended consequences on ecological communities and ecosystem functioning. Dose–response profiles are a useful and routine tool for assessing consequences of over- and under-dosing wildlife. Specifically, dose–response profiles can be useful for quantifying differences in dose-specific immune responses for distinct classes of hosts (e.g., species identity, developmental stage, age class, genotype). Additionally, the effect of vaccination on non-target wildlife can be evaluated by tracking community diversity metrics (e.g., abundance, richness, and evenness) and ecosystem function pre- and post-administration in both placebo and vaccinated environments [38]. Furthermore, trait-based vaccination may help to overcome issues related to patchy coverage and dosing.
Trait-Based Vaccination
Which hosts should be prioritized for vaccination? Host factors such as age, immunity, behavior, and genetics all influence host competence [40]. These heterogeneous factors contribute significantly to disparities in parasite susceptibility and transmission between hosts, leading to relatively few individuals being responsible for most parasite transmission in a population [41]. This observation can be harnessed to tailor control methods using trait-based vaccination.
Random mixing is a fundamental assumption of classic vaccination and transmission models, but network analyses of wildlife show that traits such as territoriality or sociality often reveal nonrandom contacts, elevating the importance of accounting for contact and home range heterogeneity in vaccination [42,43]. Targeted vaccination of superspreaders has been continually proposed as a method to reduce required immunization coverage [44,45]. For example, targeted vaccination of socially central chimpanzees, determined by detailed behavioral data, or approximated using trait-based estimates, can significantly reduce the vaccination coverage threshold [44]. Incorporating contact networks into transmissible vaccine models, using an individual-based approach, could assess if behaviors associated with superspreading, such as gregariousness or boldness, increase vaccine transmission [46,47]. Alternatively, vaccination for conservation could target individuals that are disproportionately important to population growth or persistence [48].
Modeling Wildlife Vaccination
SIR models are the most common models used for predicting vaccination outcomes [27]. While valuable for modeling waning and binary modes of imperfection, SIR models cannot capture the complexities of partial immunity, especially when spatial dynamics, social interactions, or individual history are important [23,27,49]. Limitations of modeling partial immunity using ordinary differential equations (ODEs) can be overcome by using IBMs, which are able to incorporate different host immune responses and space-based behaviors such as territoriality and migration [49]. For example, in the case of fox rabies control in Europe, IBM predictions recommended the use of a lower coverage vaccination strategy relative to an SIR model [50]. This lower coverage strategy was carried out successfully and saved considerable resources [49].While the simplicity and analytical tractability of ODE models can offer considerable advantages, we advocate for the increased consideration of IBMs in the study of wildlife disease because they can represent individual-level physiology, connect seamlessly with transmission networks or spatially explicit movement models, and accommodate individual history and heterogeneity [49].
Concluding Remarks
Vaccines can advance biodiversity conservation and spillover control. However, vaccine imperfections can substantially compromise the achievement of herd immunity or promote the evolution of increased virulence, yet these factors are not always accounted for in theory, planning, or analysis of vaccine use in wildlife. Wildlife vaccination offers a frontier to explore advancing questions in ecoimmunology, imperfect immunity, and disease-control innovation. The biological factors shaping vaccination success, feasibility, and efficacy should be as central to decisions regarding wildlife vaccination as logistical limitations and financial resources (see Outstanding Questions). Thorough empirical assessment of vaccine–host–parasite biology can both (i) prevent impractical vaccination campaigns and (ii) ameliorate challenges regarding vaccine dose and coverage, saving time and limiting adverse outcomes.
Disentangling potential modes of imperfection is critical for predicting outcomes of vaccination. Incorporating these effects into models and experiments can predict otherwise counterintuitive deleterious outcomes, such as increased transmission caused by antidisease resistance. We suggest that IBMs should be selected for vaccines conferring partial immunity or systems in which space-based behaviors drive disease dynamics. Additionally, vaccination outcomes should be simultaneously studied across ecological scales and evolutionary time. Imperfect vaccines impose subtle tension between individual- and population-level benefits, and deeper theoretical examination can help to prevent the implementation of unfeasible or potentially harmful vaccines.
Furthermore, wild hosts and parasites are inherently heterogeneous and poorly controlled. Dose–response profiles and community diversity metrics should be used to account for heterogeneity when calculating safe and effective vaccine doses for wildlife individuals, populations, communities, and ecosystems. Trait-based vaccination approaches could prioritize hosts that disproportionately contribute to population persistence or parasite transmission, thus minimizing coverage required for parasite eradication or host population viability. Ecological complexities and evolutionary consequences of imperfect immunity provide an abundance of challenges when vaccinating wildlife; but pursuing wildlife vaccination for use in conservation or spillover prevention is by no means foolish if informed by the system’s underlying physiology and ecology.
Outstanding Questions.
What imperfections do candidate vaccines exhibit? How do these imperfections complicate or undermine disease management objectives?
Are vaccine imperfections consistent across host ages, life stages, genotypes, or species?
Are vaccine imperfections consistent across environmental contexts, such as parasite exposure histories, variation in diet quality and quantity, or environmental stressors?
How can vaccine deployment schemes minimize underdosing, overdosing, and non-target effects? What are the effects of combining trait-based and transmissible vaccines on required coverage?
What are the consequences of under- and over-dosing? How can non-target, community- or ecosystem-level adverse consequences of vaccines be quantified?
What are the downstream impacts of wildlife vaccination on population dynamics and community diversity? How do costs of defense factor into broad outcomes of wildlife vaccination (e.g., immunity–reproduction tradeoffs, helminth–microorganism immunity tradeoffs, parasite coinfection dynamics)?
What traits drive variation in uptake or exposure to vaccines and subsequent immune responses? Do these traits covary? Will these trait combinations enhance or reduce variation in immune phenotypes in wild populations?
Which hosts are most important to vaccinate? How does this depend on the management objective?
How do transmission dynamics (epizootic vs enzootic), timing of vaccination (proactive vs reactive), and baseline host immunity factor into vaccination campaign objectives and delivery?
How will wildlife vaccination complement other disease management techniques?
Which modeling frameworks will best contribute to vaccination campaign success and the anticipation of potential evolutionary responses?
Will predicted evolutionary outcomes of imperfect vaccines be observed in wildlife vaccination scenarios?
Alt-text: Outstanding Questions
Acknowledgments
We thank Drs Levi Morran, Karen Levy, and Sarah Bowden for their valuable feedback on the manuscript. We also thank members of the Civitello laboratory for their comments. K.M.B. and D.J.C. were supported by National Science Foundation (NSF) IOS-1755002. K.M.B. was also supported by NSF GRFP DGE-1937971. D.J.C. was also supported by National Institutes of Health (NIH) 1R01 AI150774-01. Any opinions, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF.
Glossary
- Adjuvants
vaccine additives to increase its immunogenicity.
- Coinfections
two or more parasite species simultaneously infecting the same host.
- Dose–response profiles
quantifying an organism’s physiological response to varying doses of vaccine.
- Effective reproductive ratio (Reff)
the number of secondary infections a primary infection contributes in a population with resistant individuals.
- Enzootic
refers to a pathogen endemic in non-human animals.
- Herd immunity
indirect protection of susceptible hosts by resistant hosts.
- Host competence
the relative ability of a host to become infected by and transmit a parasite.
- Host tolerance
decreased mortality or pathology in response to infection.
- Immunogenicity
a vaccine’s ability to induce an acquired immune response.
- Imperfect-vaccine hypothesis
theory suggesting that, depending on the phenotype of resistance, partial vaccination may select for increased parasite virulence.
- Parasite virulence
host death or pathology induced by infection.
- Reservoir host
a population of organisms that serve as an infection source for another host population.
- Resistance phenotype
categories of incomplete immunity, including antidisease immunity, anti-infection immunity, and antitransmission immunity.
- Spillover
transmission of parasites from a non-human host species to humans.
- Superspreader
an individual that disproportionately contributes to parasite transmission within a given population.
- Trait-based vaccination
vaccine distribution prioritizing individuals with specific characteristics.
- Transmissible vaccines
vaccines that autonomously spread from treated to untreated individuals.
- Transmission-virulence trade-off hypothesis
a hypothesis derived from the assumption that transmission rate and virulence are correlated, predicting that an intermediate level of virulence is favored by selection.
- Zoonotic pathogen
a parasite able to be transmitted from non-human animals to humans.
References
- 1.Anderson R.M., May R.M. Vaccination and herd immunity to infectious diseases. Nature. 1985;318:323–329. doi: 10.1038/318323a0. [DOI] [PubMed] [Google Scholar]
- 2.Rappuoli R. Vaccines, new opportunities for a new society. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12288–12293. doi: 10.1073/pnas.1402981111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Swart R.L. Rinderpest eradication: lessons for measles eradication? Curr. Opin. Virol. 2012;2:330–334. doi: 10.1016/j.coviro.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert A.T., Chipman R.B. Rabies control in wild carnivores. In: Fooks A.R., Jackson A.C., editors. Rabies: Scientific Basis of the Disease and Its Management. 4th edn. Elsevier; 2020. pp. 605–654. [Google Scholar]
- 5.MacInnes C.D. Elimination of rabies from red foxes in Eastern Ontario. J. Wildl. Dis. 2001;37:119–132. doi: 10.7589/0090-3558-37.1.119. [DOI] [PubMed] [Google Scholar]
- 6.Slate D. Oral rabies vaccination in North America: opportunities, complexities, and challenges. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheele B.C. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science. 2019;363:1459–1463. doi: 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- 8.Hoyt J.R. Long-term persistence of Pseudogymnoascus destructans, the causative agent of white-nose syndrome, in the absence of bats. EcoHealth. 2015;12:330–333. doi: 10.1007/s10393-014-0981-4. [DOI] [PubMed] [Google Scholar]
- 9.Flies A.S. An oral bait vaccination approach for the Tasmanian devil facial tumor diseases. Expert Rev. Vaccines. 2020;19:1–10. doi: 10.1080/14760584.2020.1711058. [DOI] [PubMed] [Google Scholar]
- 10.Leendertz S.A.J. Ebola in great apes – current knowledge, possibilities for vaccination, and implications for conservation and human health. Mammal Rev. 2017;47:98–111. [Google Scholar]
- 11.Smith K.F. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 2006;20:1349–1357. doi: 10.1111/j.1523-1739.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 12.Letko M. Bat-borne virus diversity, spillover and emergence. Nat. Rev. Microbiol. 2020;18:461–471. doi: 10.1038/s41579-020-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydon D.T. Low-coverage vaccination strategies for the conservation of endangered species. Nature. 2006;443:692–695. doi: 10.1038/nature05177. [DOI] [PubMed] [Google Scholar]
- 14.Plumb G. Vaccination in conservation medicine. Rev. Sci. Tech. Int. Off. Epizoot. 2007;26:229–241. [PubMed] [Google Scholar]
- 15.McMahon T.A. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature. 2014;511:224–227. doi: 10.1038/nature13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffernan J.M., Keeling M.J. Implications of vaccination and waning immunity. Proc. R. Soc. B Biol. Sci. 2009;276:2071–2080. doi: 10.1098/rspb.2009.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark G.G. Antibody response of Sandhill and Whooping cranes to an Eastern Equine Encephalities virus vaccine. J. Wildl. Dis. 1987;23:539–544. doi: 10.7589/0090-3558-23.4.539. [DOI] [PubMed] [Google Scholar]
- 18.Morris S.E. Demographic buffering: titrating the effects of birth rate and imperfect immunity on epidemic dynamics. J. R. Soc. Interface. 2015;12 doi: 10.1098/rsif.2014.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heininger U. The concept of vaccination failure. Vaccine. 2012;30:1265–1268. doi: 10.1016/j.vaccine.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 20.Gandon S., Michalakis Y. Evolution of parasite virulence against qualitative or quantitative host resistance. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:985–990. doi: 10.1098/rspb.2000.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michalik D.E. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J. Infect. Dis. 2008;197:944–949. doi: 10.1086/529043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mentzer A.J. Searching for the human genetic factors standing in the way of universally effective vaccines. Philos. Trans. R. Soc. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine P. 'Herd immunity': a rough guide. Clin. Infect. Dis. 2011;52:911–916. doi: 10.1093/cid/cir007. [DOI] [PubMed] [Google Scholar]
- 24.te Kamp V. Responsiveness of various reservoir species to oral rabies vaccination correlates with differences in vaccine uptake of mucosa associated lymphoid tissues. Sci. Rep. 2020;10:2919. doi: 10.1038/s41598-020-59719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Roode J.C. Virulence evolution in response to anti-infection resistance: toxic food plants can select for virulent parasites of monarch butterflies: Resistance and virulence evolution. J. Evol. Biol. 2011;24:712–722. doi: 10.1111/j.1420-9101.2010.02213.x. [DOI] [PubMed] [Google Scholar]
- 26.Miller I.F., Metcalf C.J.E. Evolving resistance to pathogens. Science. 2019;363:1277–1278. doi: 10.1126/science.aaw8710. [DOI] [PubMed] [Google Scholar]
- 27.Miller I.F., Metcalf C.J. Vaccine-driven virulence evolution: consequences of unbalanced reductions in mortality and transmission and implications for pertussis vaccines. J. R. Soc. Interface. 2019;16 doi: 10.1098/rsif.2019.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandon S. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:6. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- 29.Read A.F. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezenwa V.O., Jolles A.E. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science. 2015;347:175–177. doi: 10.1126/science.1261714. [DOI] [PubMed] [Google Scholar]
- 31.Waugh C. A prototype recombinant-protein based Chlamydia pecorum vaccine results in reduced chlamydial burden and less clinical disease in free-ranging koalas (Phascolarctos cinereus) PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0146934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming-Davies A.E. Incomplete host immunity favors the evolution of virulence in an emergent pathogen. Science. 2018;359:1030–1033. doi: 10.1126/science.aao2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buddle B.M. Efficacy and safety of BCG vaccine for control of tuberculosis in domestic livestock and wildlife. Front. Vet. Sci. 2018;5:259. doi: 10.3389/fvets.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choisy M., de Roode J.C. Mixed infections and the evolution of virulence: effects of resource competition, parasite plasticity, and impaired host immunity. Am. Nat. 2010;175:E105–E118. doi: 10.1086/651587. [DOI] [PubMed] [Google Scholar]
- 35.Rocke T.E. Age at vaccination may influence response to sylvatic plague vaccine (SPV) in Gunnison’s prairie dogs (Cynomys gunnisoni) EcoHealth. 2015;12:278–287. doi: 10.1007/s10393-014-1002-3. [DOI] [PubMed] [Google Scholar]
- 36.Tripp D.W. Season and application rates affect vaccine bait consumption by prairie dogs in Colorado and Utah, USA. J. Wildl. Dis. 2014;50:224–234. doi: 10.7589/2013-04-100. [DOI] [PubMed] [Google Scholar]
- 37.Herrera J., Nunn C.L. Behavioural ecology and infectious disease: implications for conservation of biodiversity. Philos. Trans. R. Soc. B Biol. Sci. 2019;374 doi: 10.1098/rstb.2018.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bron G.M. Impact of sylvatic plague vaccine on non-target small rodents in grassland ecosystems. EcoHealth. 2018;15:555–565. doi: 10.1007/s10393-018-1334-5. [DOI] [PubMed] [Google Scholar]
- 39.Curlee J.F. Cross-species vaccination in wild and exotic animals. Adv. Vet. Med. 1999;49:551–556. doi: 10.1016/s0065-3519(99)80041-x. [DOI] [PubMed] [Google Scholar]
- 40.Gervasi S.S. The context of host competence: a role for plasticity in host–parasite dynamics. Trends Parasitol. 2015;31:419–425. doi: 10.1016/j.pt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanderWaal K.L., Ezenwa V.O. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct. Ecol. 2016;30:1606–1622. [Google Scholar]
- 42.White L.A. Using contact networks to explore mechanisms of parasite transmission in wildlife: Contact networks: wildlife parasite transmission. Biol. Rev. 2017;92:389–409. doi: 10.1111/brv.12236. [DOI] [PubMed] [Google Scholar]
- 43.McClure K.M. Variation in host home range size decreases rabies vaccination effectiveness by increasing the spatial spread of rabies virus. J. Anim. Ecol. 2020;89:1375–1386. doi: 10.1111/1365-2656.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rushmore J. Network-based vaccination improves prospects for disease control in wild chimpanzees. J. R. Soc. Interface. 2014;11 doi: 10.1098/rsif.2014.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paull S.H. From superspreaders to disease hotspots: linking transmission across hosts and space. Front. Ecol. Environ. 2012;10:75–82. doi: 10.1890/110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basinski A.J. A little goes a long way: Weak vaccine transmission facilitates oral vaccination campaigns against zoonotic pathogens. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smithson M.W. Transmissible vaccines whose dissemination rates vary through time, with applications to wildlife. Vaccine. 2019;37:1153–1159. doi: 10.1016/j.vaccine.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crouse D.T. A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology. 1987;68:1412–1423. [Google Scholar]
- 49.Railsback S., Grimm V. Princeton University Press; 2012. Agent-Based and Individual-Based Modeling: A Practical Introduction. [Google Scholar]
- 50.Thulke H.-H., Eisinger D. The strength of 70%: revision of a standard threshold of rabies control. Dev. Biol. 2008;131:291–298. [PubMed] [Google Scholar]
- 51.WHO Expert Consultation on Rabies: Third Report. World Health Organization; 2018. [Google Scholar]
- 52.Hampson K. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velasco-Villa A. Successful strategies implemented towards the elimination of canine rabies in the Western Hemisphere. Antivir. Res. 2017;143:1–12. doi: 10.1016/j.antiviral.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hampson K. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasempimolporn S. Prevalence of rabies virus infection and rabies antibody in stray dogs: A survey in Bangkok, Thailand. Prev. Vet. Med. 2007;78:325–332. doi: 10.1016/j.prevetmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Randall D.A. An integrated disease management strategy for the control of rabies in Ethiopian wolves. Biol. Conserv. 2006;131:151–162. [Google Scholar]
- 57.Taylor L.H. The role of dog population management in rabies elimination – a review of current approaches and future opportunities. Front. Vet. Sci. 2017;4:109. doi: 10.3389/fvets.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wandeler A.I. Oral immunization of wildlife against rabies: concept and first field experiments. Clin. Infect. Dis. 1988;10:S649–S653. doi: 10.1093/clinids/10.supplement_4.s649. [DOI] [PubMed] [Google Scholar]
- 59.Sidwa T.J. Evaluation of oral rabies vaccination programs for control of rabies epizootics in coyotes and gray foxes: 1995–2003. J. Am. Vet. Med. Assoc. 2005;227:785–792. doi: 10.2460/javma.2005.227.785. [DOI] [PubMed] [Google Scholar]
- 60.Canning G. Rabies outbreak in African wild dogs (Lycaon pictus) in the Tuli region, Botswana: interventions and management mitigation recommendations. J. Nat. Conserv. 2019;48:71–76. doi: 10.1016/j.jnc.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]