Abstract
Corona virus disease-2019 (COVID-19) caused by severe acute respiratory syndrome corona virus-2 (SARS CoV-2), a highly contagious single stranded RNA virus genetically related to SARS CoV. The lungs are the main organs affected leading to pneumonia and respiratory failure in severe cases that may need mechanical ventilation. Occasionally patient may present with gastro-intestinal, cardiac and neurologic symptoms with or without lung involvement. Pathologically, the lungs show either mild congestion and alveolar exudation or acute respiratory distress syndrome (ARDS) with hyaline membrane or histopathology of acute fibrinous organizing pneumonia (AFOP) that parallels disease severity. Other organs like liver and kidneys may be involved secondarily. Currently the treatment is principally symptomatic and prevention by proper use of personal protective equipment and other measures is crucial to limit the spread. In the midst of pandemic there is paucity of literature on pathological features including pathogenesis, hence in this review we provide the current pathology centered understanding of COVID-19. Furthermore, the pathogenetic pathway is pivotal in the development of therapeutic targets.
Keywords: COVID-19, SARS CoV-2, Pathology, ARDS, AFOP, Therapeutic targets
1. Introduction
The current pandemic of corona virus disease-2019 (COVID-19) caused by severe acute respiratory syndrome corona virus-2 (SARS CoV-2) led to complete lockdown in many countries contributing to major socio-economic crisis and irreparable recession, globally. SARS CoV-2, a novel β CoV was first identified in adults presenting with acute lower respiratory tract infection of unexplained etiology in China [1]. Though no age group is spared, severe forms occur in patients older than 60 years specifically with co-morbidities. The majority of the infected individuals are asymptomatic or with mild form of disease and are potential transmitters. This disease is highly contagious and mainly spread through respiratory droplets, close contact with infected cases or materials (fomites) and nosocomially to other patients and health care workers in the hospitals [2,3]. COVID-19 has a much lower case fatality ratio and significantly greater transmission rate than 2003 SARS pandemic [4,5]. Currently RT-PCR of upper and lower respiratory swabs or samples is the gold standard diagnostic test. Serological tests based on antibody detection, though not helpful during the early phases of disease, can be used to confirm infection in later phase. A thorough literature search (PubMed, preprint servers and google scholar) using terms COVID-19 and pathology/pathogenesis, SARS CoV-2 and pathology/pathogenesis and 2019-nCoV and pathology/pathogenesis was done to maximize the yield of literature, which ended on 24 May 2020. In this review, we have comprehensively discussed all aspects of COVID-19 with special emphasis on the pathology including pathogenesis and therapeutic targets. It forms a ready resource for clinicians, pathologists, and researchers including epidemiologists aiding them in the diagnosis and treatment of these patients, and may also pave way to further research.
2. Epidemiology
The earliest case of SARS CoV-2 infection currently known was reported on 31 st December 2019 in Wuhan, Hubei province of China [1]. After this it spread rapidly to other parts of China as well as internationally affecting over 185 countries as of 23 April 2020, leading to the current global pandemic [2]. The World Health Organization declared COVID-19 to be a Public Health Emergency of International Concern on 30 January 2020, and recognized it as a pandemic on 11 March 2020 [6,7]. As of 24 May 2020, globally 5.48 million cases of COVID-19 have been reported, resulting in 346,071 deaths and 2,290,776 people have recovered [8].
The basic reproduction number (R0) of the SARS CoV-2 is estimated to be between 1.4 and 3.9, indicating its highly contagious nature [9,10]. The R0 may be even higher in places of public gatherings like in cruise ships, religious/political/academic/business congregations as well as in hospitals non-compliant with personal protective measures [[9], [10], [11]]. The incubation period and serial interval is estimated at 5–6 days and 8 days, respectively, which is similar to that for SARS CoV and MERS CoV [9,[12], [13], [14]]. Early in the pandemic, the case-fatality rate (CFR) was estimated to be between 0.9 % and 3% [15,16], lower than other HCoVs (SARS CoV (6%–17 %) and MERS CoV (20 %–40 %)) [[17], [18], [19]]. However, by the 24th of May 2020 many countries exhibited exponential rise in CFR [8] (Table 1).
Table 1.
Statistics of top 10 Countries affected by COVID-19 as of 24th May 2020.
| SNo. | COUNTRY | TOTAL CASES | TOTAL DEATHS | TOTAL RECOVERED | Case Fatality Rate (CFR) % |
|---|---|---|---|---|---|
| 1 | USA | 1,682,599 | 99,226 | 451,392 | 5.89 |
| 2 | BRAZIL | 357,839 | 22,500 | 142,587 | 6.28 |
| 3 | RUSSIA | 344,481 | 3541 | 113,299 | 1.02 |
| 4 | SPAIN | 282,852 | 28,752 | 196,958 | 10.16 |
| 5 | UK | 259,559 | 36,793 | N/A | 14.17 |
| 6 | ITALY | 229,858 | 32,785 | 140,479 | 14.26 |
| 7 | FRANCE | 182,584 | 28,367 | 64,617 | 15.53 |
| 8 | GERMANY | 180,328 | 8371 | 160,300 | 4.64 |
| 9 | TURKEY | 156,827 | 4340 | 118,694 | 2.76 |
| 10 | INDIA | 138,536 | 4024 | 57,692 | 2.90 |
N/A Information is missing on recovery data for UK.
2.1. Routes of transmission
Unlike SARS CoV, the high percentage of SARS CoV-2 infected individuals manifest as asymptomatic or pauci-symptomatic infection who escape detection and become potential transmitters [20,21]. It is important to note that, not all close contacts are infected suggesting a role for individual genetic susceptibility [[22], [23], [24]]. In humans, the virus usually gains entry through upper aero-digestive tract. More recently SARS CoV-2 was isolated from the feces of patients, indicating the possibility of fecal-oral spread [24,25]. Furthermore, SARS CoV-2 infection in pregnant women raised a possibility of vertical transmission [26]. However, the vertical transmission was ruled out based on negative testing for the virus on the swabs collected from the amniotic fluid, cord blood, neonatal throat and breast milk of the six infected pregnant women [26]. The long range airborne transmission is also speculated which depends on flow dynamics of the virus from the infected person and also on ventilation status of the area [27]. Moreover, the expansion and spread of COVID-19 can be visualized by mapping techniques like cartograms [28]. The understanding of modes of transmission of SARS CoV-2 will enable application of appropriate containment measures.
2.2. Susceptibility
Though there is generalized susceptibility to SARS CoV-2 infection for all age groups, the disease severity and mortality is less in children [29]. In adults, due to increased plasma ACE2 levels in women they are less commonly affected than men [30]. The elderly population specifically, with co-morbidities such as diabetes, hypertension, stroke, chronic pulmonary/cardiac/renal disease are highly susceptible to severe form of infection owing to low body defense against infection as well as their underlying age related organ system compromise [22,[31], [32], [33]]. Similar to SARS CoV, a recent study reported non- O blood group specifically group A had higher infection and death rates due to COVID-19 owing to absence of protective anti-A IgM antibodies [34,35]. Many uncertainities still persist in the SARS CoV-2 epidemiology especially virus-host interaction including host susceptibility and the evolution of epidemic.
3. Virology
The corona viruses (CoVs) are classified into α and β (seen in mammals including humans); γ and δ (seen in avian species) [36]. They derive the name due to their resemblance to crown (coronam in Latin) at electron microscopic level due to presence of spike protein on their envelop. They are capable of causing respiratory, gastrointestinal, hepatic, and central nervous system infection in avian and mammalian species including humans [37,38]. In general, estimates suggest that 2% of the population are healthy carriers of the CoVs and that these viruses are responsible for about 5%–10% of acute respiratory infections [39]. It is postulated that, when these viruses pass from natural hosts to humans through an intermediate amplifying host, they undergo rapid mutation and recombination leading to generation of novel CoVs that are virulent and cause human disease [39]. Such novel CoVs have caused severe acute respiratory syndrome (SARS - SARS CoV) in China (2002–2003) and Middle East respiratory syndrome (MERS - MERS CoV) in Saudi Arabia (2012) resulting in pandemics with pulmonary and extra-pulmonary manifestations in the last few decades [36,40]. In children, the CoVs usually present as co-infection with other respiratory viral infections but can be the sole pathogen causing infection in children suffering from underlying chronic diseases [12]. Thus, CoVs have become major pathogens of emerging respiratory disease outbreaks.
The SARS CoV-2 belongs to family: coronaviridae, order: nidovirales genus: beta corona virus and lineage B of subgenus: sarbecovirus [36]. WHO’s “novel coronavirus 2019″ (2019-nCoV) was officially renamed to SARS CoV-2 by the International Committee on Taxonomy of Viruses (ICTV) and the resultant disease was called COVID-19 by WHO on February 11, 2020 [41]. Phylogenetically, SARS CoV-2 exhibited 80 %–99.8 % genetic similarity with CoVs of Rhinolopus bats, pangolin and civets [[42], [43], [44], [45]]. Additionally, the receptor-binding domain (RBD) of the Spike/S protein through which SARS CoV-2 infects the host is virtually identical to pangolin CoV with a difference of only one amino acid [46]. Recently another study suggested pangolin involvement in SARS CoV-2 origin due to evidence of re-assortment in CoVs [47]. SARS CoV-2 differs from other β CoVs by the presence of unique polybasic cleavage site that contributes to increased pathogenicity and transmissibility [48].
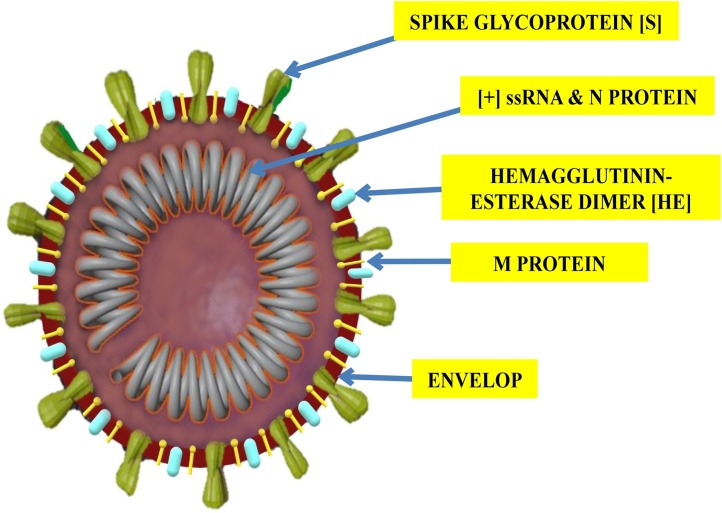
Each virion is a enveloped, non-segmented, positive sense single stranded RNA virus (+ssRNA) with nucleocapsid. Its virion measures 50−200 nm in diameter with a round or elliptic form with 5′‐cap structure and 3′‐poly‐A tail (Fig. 1 ). The single‐stranded positive‐sense RNA contains 29,891 nucleotides, encoding 9860 amino acids. Like any typical CoV the genome of SARS CoV-2 contains six open reading frames (ORFs). The first ORF (2/3 of whole genome;ORF1a/b), encodes 16 nonstructural proteins (nsps) (nsp1‐16). Intervening ORF1a and 1b exhibits −1 frameshift which leads to formation of two polypeptides: pp1a and pp1ab which are further processed into 16 nsps by virally encoded proteases (chymotrypsin‐like protease (3CLpro) and one or two papain‐like proteases) that helps in the viral genome replication, formation of double membrane vesicles (DMVs) and proofreading [[49], [50], [51]]. Rest 1/3 of the genome near the 3′‐ terminus mainly has genes encoding four structural proteins (spike (S), membrane (M), envelope (E), and nucleocapsid (N)) and for accessory proteins (HE, 3a/b and 4a/b proteins) that inhibit host defenses [52]. The virus is sensitive to ultraviolet rays, heat and can be effectively inactivated by lipid solvents like ether (75 %), ethanol, per-oxyacetic acid, chloroform, and chlorine-containing disinfectants except chlorhexidine [2].
Fig. 1.
Structure of SARS CoV-2 Virion.
Spike (S) protein - helps in attachment to host cell. Membrane (M) protein - nutrient transport. Envelope (E) - virion outermost layer. " helps in viral assembly and budding " for the E protein function in the legend. Hemagglutinin esterase (HE) - inhibits host defenses. Nucleo-capsid (N) - encloses and protects viral genome. Viral genome- positive sense single stranded RNA.
4. Pathogenesis
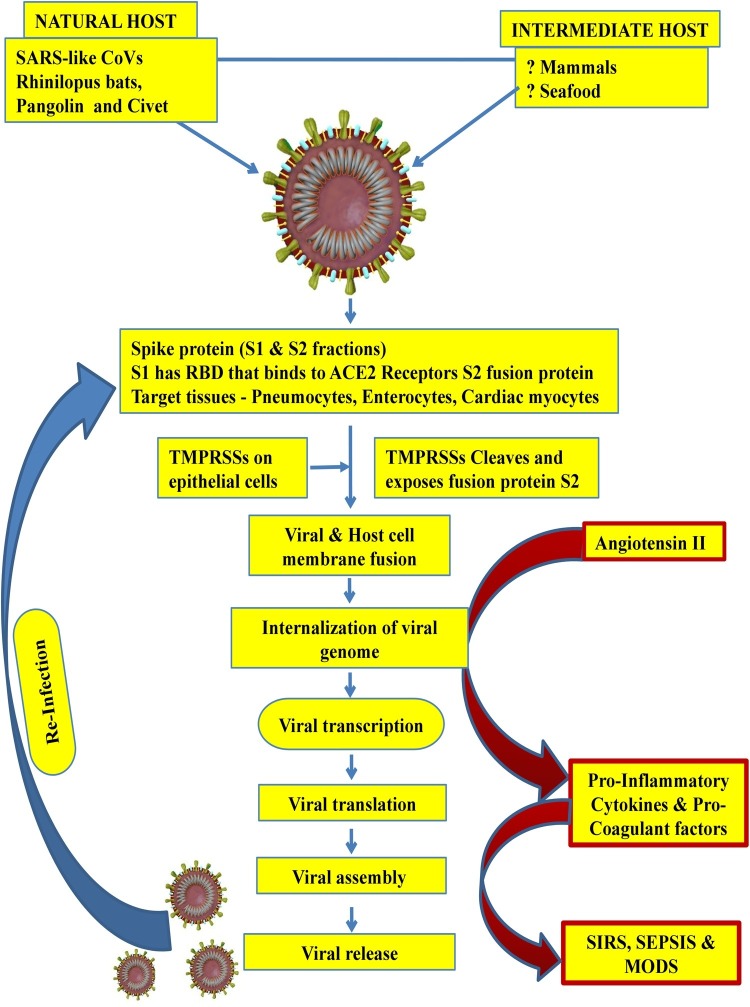
The SARS CoV-2 because of its similarity with SARS CoV is presumed to infect human cells through its densely glycosylated spike (S) proteins S1 fraction with receptor binding domain (RBD) which binds to the angiotensin-converting enzyme 2 receptor (ACE-2 R) with 10–20 fold higher affinity than SARS CoV [53]. ACE-2 R are principally expressed in human alveolar epithelial cells (type II > type I) [42,54,55]. The ACE-2 R is also expressed on endothelial cells, gastrointestinal (esophageal & intestinal) epithelium and cardiac myocytes [56,57]. After the virus gets attached to this receptor, the SARS CoV-2 with its unique polybasic S1/S2 protease cleavage site with SPRR insertion on the spike protein which is recognized and cleaved by transmembrane protease serine (TMPRSSs) expressed on host cells to expose the fusion protein (S2 fraction) that enables the fusion of both viral and the host cell membrane [56]. It has been demonstrated that ACE-2 R and TMPRSSs are highly co-expressed in alveolar type 2 pneumocytes, epithelium of upper esophagus and absorptive enterocytes, forming the basis of speculation that the SARS CoV-2 can gain access into host through esophageal and intestinal epithelium apart from alveolar epithelium. Hence, the potential target tissues for SARS CoV-2 should co-express ACE-2 R and TMPRSSs [56]. This membrane fusion enables internalization of viral RNA into the host cell cytoplasm which then forms new viral proteins, by replication and translation. The final step before virions are released from the infected cells is the viral assembly in the form of nucleocapsid (N) proteins binding to RNA molecules which gets further covered by the envelope and membrane proteins leading to formation of complete virions capable of infecting other cells.
With the establishment of SARS CoV-2 infection type II pneumocytes (responsible for tissue repair and surfactant bio-synthesis) are damaged causing increased surface tension resulting in dyspnoea. In addition, these damaged type II pneumocytes compromise the alveolar immunologic balance function by inappropriately triggering a cascade of local and systemic inflammatory response due to excessive cytokine synthesis and release (cytokine storm) by the activated inflammatory cells owing to accumulation of uncleaved angiotensin II [[58], [59], [60]]. This cytokine excess when severe, causes wide spread tissue damage due to systemic inflammatory response syndrome (SIRS). In addition, there is widespread activation of pro-coagulant factors leading to microthrombi in various tissues/organs resulting in ARDS, multiple organ dysfunction syndrome (MODS), ischemia and high mortality. This phenomenon is evidenced by the presence of significantly increased pro-inflammatory cytokines (Interleukins 1-β, 1RA, 7, 8, 9, 10, b FGF2, GCSF, GMCSF, IFNγ, IP10, MCP1, MIP1α, MIP1β, PDGFβ, TNFα, and VEGFA.) in severe COVID-19 patients [61] (Fig. 2 ).
Fig. 2.
Pathogenesis of COVID-19.
SARS CoV-2 infection in humans is possibly acquired from Rhinolopus bats or Pangolins or Civets and through an currently unknown intermediate host. The viral entry into host cell is mediated initially by S1 fraction of Spike protein binding to ACE2-R on target cells and subsequently by S2 fraction cleavage by TMPRSS2 (host cell origin) enabling viral internalization. Inside host cells viral genome undergoes replication with the help of RNA dependent RNA polymerase to form new virions, which infects other cells. In response to this there is excessive synthesis and release of pro-inflammatory factors mediated by angiotensin-II which is responsible for clinical presentation.
5. Pathology
The current understanding of pathology stems from few case reports and autopsy case studies. The gross features include heavy and boggy lungs, patchy consolidation along with pleural fibrinous exudate and/or fibrosis, sometimes with purulent inflammation due to secondary bacterial infection with/without evidence of pericarditis [62].
The microscopic features depend on stage and severity of the disease. Early stages (asymptomatic/mildly symptomatic patients) show non-specific changes including pulmonary edema, focal pneumocyte hyperplasia, focal chronic inflammatory infiltrate and multinucleated giant cells with absence of prominent hyaline membrane formation [63]. As, the disease progress there is diffuse alveolar damage with transparent hyaline membrane formation and severe pulmonary edema. However, in SARS CoV-2, there is firbomyxoid exudates with visible fibrinous cords along with mucous plugging of bronchioles which has a bearing with respect to oxygen therapy. There is also widespread interstitial inflammatory infiltrates with severe epithelial damage,diffuse type II pneumocyte hyperplasia consistent with ARDS [[64], [65], [66], [67], [68]]. One study reported massive pulmonary interstitial fibrosis with variable degree of hemorrhagic necrosis, chronic inflammation with multinucleate giant cells and intracytoplasmic viral inclusion bodies in severe cases [69]. Interestingly, another study showed features of lymphocytic viral pneumonia in a patient who died early in the disease (5th day after development of symptoms), whereas five other patients who succumbed later (20th day after development of symptoms) exhibited acute fibrinous and organizing pneumonia (AFOP) showing extensive fibrinous deposits forming balls/mounds but not hyaline membrane in their alveoli. These patients also showed prominent vascular injury evidenced by endothelial cell detachment and prominent intracytoplasmic vacuolization in small and medium-sized pulmonary blood vessels [70]. Also, severe COVID-19 infection has been associated with a novel pulmonary-specific vasculopathy known as pulmonary intravascular coagulopathy (PIC), that parallels disease severity [71]. These findings may be considered as important indicators of disease severity and prognosis.
The liver shows mild lobular lymphocytic infiltration and moderate micro-vesicular steatosis along with mild lobular activity, possibly related to the viral infection itself and ischemia. There were no obvious histological changes in heart tissue except for mild interstitial chronic mononuclear infiltrate [57,64,65]. Hence the changes in the liver and heart are more likely secondary or related to the underlying diseases [64]. The pathology in other organs have not been elucidated.
It is too early to determine the specificity and consistency of these histopathological findings with respect to the stage and severity of the COVID-19 owing to the paucity of information obtained from few biopsy/autopsy case reports. In addition, the histopathological features may be modified or altered by patients’ immunity, presence of co-morbidities, secondary infections and therapy given to these patients especially steroids.
6. Clinical presentation
The COVID-19 encompasses varied clinical spectrum, and during the incubation period (average∼2weeks) the patients are either asymptomatic or present with mild upper respiratory symptoms in 2–4 days after being infected [16]. The symptoms include sneezing, coughing, runny nose, fatigue, sore throat and fever. One important feature which raises the suspicion of COVID-19, is dyspnoea, which is seen in over 50 % of patients [55 %] and may take 2–14 days to develop. The most common clinical symptoms include fever (86.0 %), cough (63.9 %), malaise or fatigue (34.7 %), productive cough (28.9 %), shortness of breath (19.7 %) and myalgia (18.8 %). Only few patients present with gastrointestinal symptoms like diarrhea (5.7 %) and nausea/vomiting (6.1 %) [72]. In 26.7 % of COVID-19 patients there was at least one underlying co-morbidity(hypertension, diabetes, chronic cardiovascular/pulmonary/renal disease and cancer) [72]. The severe form is characterized by ARDS that necessitates mechanical ventilatory support in an intensive care unit (ICU), and also leads to multiorgan involvement resulting in shock, septicemia, and MODS with high mortality [73,74]. A substantial proportion of patients developed diarrhea during hospitalization, potentially aggravated by various drugs including antibiotics [75]. These patients may also present with cardiac sounding chest pain due to myocarditis and myocardial infarction [76]. Neurological manifestations due to acute ischemic stroke or cerebral venous sinus thrombosis or cerebral hemorrhage may sometimes precede pulmonary manifestations and include symptoms like headache, dizziness, ataxia, seizures, confusion, hyposmia and hypogeusia as a result of primary infection [77]. Secondarily the convalescing patients may manifest with post-infective demyelinating neurological complications like acute myelitis and Gullian Barre syndrome [78,79]. Very rarely patients may present with ocular complications like viral conjunctivitis [80]. COVID-19 patients also have increased risk of thrombo-embolism including disseminated intravascular coagulation (DIC). Cutaneous manifestations like erythematous exanthem, livedo reticularis, Cutaneous vasculitis, Urticaria, Chickenpox -like blisters and Covid toes (digital infarcts) were also documented [81].
6.1. COVID-19 in children
Children are either asymptomatic or pauci-symptomatic (fever (50 %), cough (38 %), fatigue, rhinorrhoea or nasal congestion) and are less likely to have severe infections [82,83]. Gastrointestinal symptoms like diarrhoea, abdominal cramps and vomiting, common in children, particularly infants and newborns [84]. A rare inflammatory syndrome affecting children displaying overlapping signs of severe COVID-19, toxic shock syndrome and Kawasaki disease has been reported in many Western countries. These children present with abdominal pain, other gastrointestinal symptoms, and signs of myocardial damage (elevated troponin and pro-B-type natriuretic peptide levels) as well as coronary artery inflammation similar to Kawasaki disease. [85].
6.2. Laboratory parameters
COVID-19 positive patients frequently exhibit hematologic abnormalities in the form of lymphopenia, leukopenia, and thrombocytopenia, along with elevated levels of liver enzymes, lactate dehydrogenase, prothrombin time and D-dimers [72]. Lymphopenia is associated with disease severity and mortality [1]. Acute phase reactants such as CRP, ferritin and procalcitonin and pro-inflammatory cytokine levels were higher in COVID-19 than healthy adults [86]. COVID-19 patients needing ICU management when compared to non-ICU patients had higher plasma levels of pro-inflammatory cytokines (IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα), increased total WBC and neutrophil counts, higher levels of D-dimer, creatine kinase, and creatinine [31,74]. Similar laboratory findings were seen in Children with COVID-19 [29].
6.3. Radiological features
Findings on chest imaging in SARS CoV-2 pneumonia seems to be similar to ordinary viral pneumonia, with some peculiarities. Chest X-ray and CT changes may be seen even before the detection of the virus from swab. In contrast, the chest X-ray may be normal in 31 % of laboratory confirmed COVID-19 cases [87]. The commonest feature on chest X-ray is presence of bilaterally symmetrical ground glass opacities with or without associated consolidation in the posterior and peripheral lung fields [32]. However, the CT findings vary with the duration of symptoms [88]. In the initial Phase (Days 2–4) basal multifocal peripheral ground-glass opacities are noted. With disease progression (mid Phase (Days 4–7) there is linear opacities developing on a background of ground-glass opacities (crazy pavement pattern). In the late Phase (Days 8–14) the central ground-glass opacities become surrounded by denser crescentic shaped consolidation (forming more than three-fourths of a circle) or form complete ring of at least 2 mm in thickness- called as ‘reversed halo sign’ or ‘atoll sign’ [88] Children also exhibit similar radiologic features [29]. Chest CT suggesting COVID-19 had 97 % sensitivity in concordance with positive RT-PCR. However, in 75 % of negative RT-PCR patients, Chest CT alone suggested COVID-19 diagnosis indicating higher sensitivity for COVID-19 diagnosis by CT. But as RT-PCR is the gold standard for confirming diagnosis it should be repeated in such circumstances [89].
7. Diagnosis
Though testing of all suspected COVID-19 cases is ideal to contain the virus spread early but the limited diagnostic capacity falls short needing a priority testing criteria. The various health authorities including WHO, Centers for Disease Control and Prevention (CDC) of USA have formulated criteria. The criteria for testing by CDC [90] is given in Supplement: I. Numerous diagnostic tests are available including molecular diagnostic tests (conventional RT-PCR, point of care testing and high throughput screening including next generation sequencing (NGS)), detection of viral antigens and anti-viral antibodies by serology and viral isolation with culture. The molecular diagnostic tests are based on detection of three genes- Envelop, RNA dependent RNA polymerase and Nucleocapsid by conventional RT-PCR [91]. This test has high sensitivity and specificity and hence considered as gold standard and is the recommended test for confirmation of SARS CoV-2 infection [91,92]. The preferred samples for this test are nasopharyngeal and throat swabs as well as lower respiratory samples. The lower respiratory samples yield higher positive rates when compared to upper respiratory samples [93,94]. These tests may give false negative results especially when the viral load is low and hence it should be repeated again with a gap of at least 24 h in patients with high index of clinical suspicion. These tests are complex, time consuming, expensive and need expertise to perform as well as to interpret [91,95,96]. The molecular test based on point of care testing using cartridges are rapid and needs less expertise. High throughput technologies including NGS can be used for simultaneous screening of large number of samples but its application is limited to research only due to high expenditure [97]. The serological tests detects either the viral antigens (spike protein and nucleo-capsid being target antigens), or the antibody response to the virus by immuno-chromatography and ELISA methods. Specifically, the antibody testing is not helpful in the early phases of infection. Though simple, cost effective, easy to perform and interpret, there are chances of false positives especially due to cross reactive antibodies against other HCoVs. Additionally, a negative antibody test does not exclude SARS CoV-2 infection [95,98]. The role of virus isolation and culture as well as detection of the virus by its cytopathic effects on cell lines is highly limited due to requirements of bio-safety level-3 facility [99,100]. Hence it is not recommended by WHO for diagnostic purpose [101] Currently the diagnostic tests for detecting SARS CoV-2 infection is variable and non-uniform owing to the use of different probes, kits and reagents.
8. Prevention and therapeutic options
Though there are numerous reports claiming efficacy of various drugs and vaccine against COVID-19, none are effective and safe to receive approval by regulatory authorities. The management of COVID-19 mainly relies on effective implementation of infection preventive and control measures and delivery of timely supportive care including oxygen therapy and mechanical ventilation as and when indicated.
8.1. Prevention and control
As the R0 value is >1 (range 1.4–3.9) [9,10], the main aim of prevention is to bring down the R0 to <1. The strategies include identification of the infected individuals and their contacts (contact tracing) and quarantine them under surveillance, proper infection control at the hospital and community levels. At the hospital level, the control measures include creating isolation wards and intensive care units for admission of symptomatic infected cases, following strict hygiene, proper use of personal protective equipment including wearing masks, proper sample collection, testing and disposal of potentially infectious bio-hazards. At the community level, school closures, avoidance of public gatherings, maintaining prescribed norms of social distancing, wearing protective masks, emphasis on frequent hand washing with soap water/sanitizers and health screening which could be achieved by proper health education [2]. Efficiency of intervention strategies such as screening of incoming people, wearing masks, quarantine for travellers has already been proved [102]. Specifically, reducing travel volume to and from China has had a positive impact on transmission dynamics of COVID-19 [103].
Though preventive vaccines against SARS CoV-2 can be developed targeting the spike (S) glycoprotein or its receptor-binding domain (RBD), these are made ineffective due to generation of altered immunogens in the target proteins owing to rapid mutations and recombinations [104,105]. In SARS CoV, live attenuated vaccine with the deleted structural E gene mutant was effective in producing neutralizing antibodies which lowered viral loads and reduced disease severity [106]. The development of inactivated vaccines against SARS CoV was hindered due to occurrence of harmful immune and/or inflammatory responses post challenge [[107], [108], [109]]. Sub-unit vaccines (purified proteins combined with adjuvants) and viral vector (adeno virus) vaccines against S glycoprotein or its RBD and N protein of SARS CoV and MERS CoV elicited higher humoral response as well as enhanced mucosal immunity with intranasal administration [[110], [111], [112]]. Furthermore, DNA based vaccine against S glycoprotein of MERS CoV also showed robust neutralizing antibody response and is currently under clinical trial [113]. Based on these reports vaccines for SARS CoV-2 is likely possible. However, its efficacy and safety has to be proved before approval.
8.2. Therapeutic options
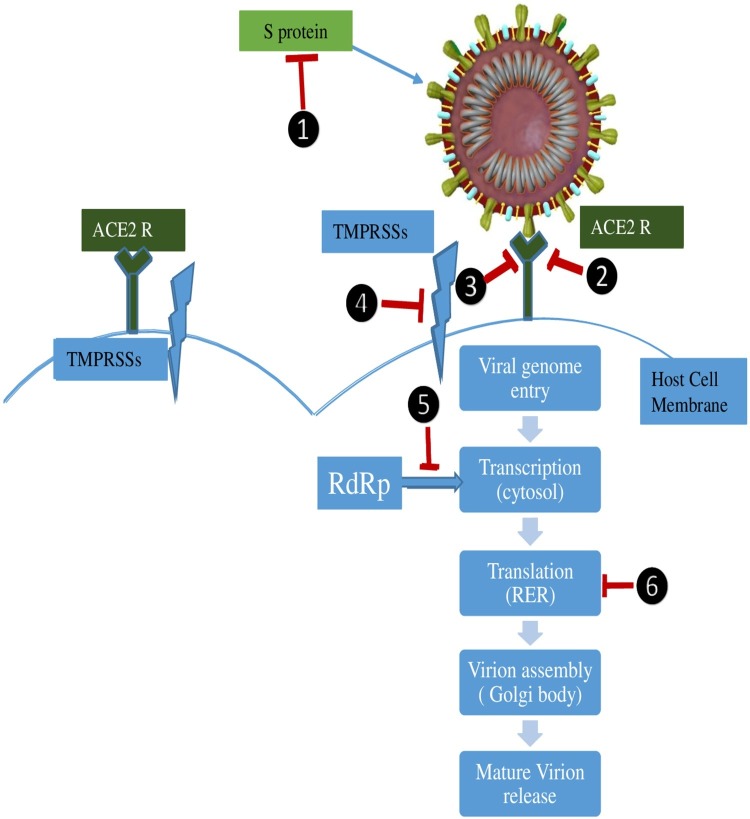
In the absence of specific anti-viral therapy, treatment is mainly symptomatic and supportive that includes oxygen therapy, conservative fluid management, hemodynamic support and/or mechanical ventilation. Mechanical ventilatory support with low tidal volume and low inspiratory pressure is indicated when the respiratory distress is refractory to conventional oxygen therapy or NIV [2]. Extracorporeal membrane oxygenation (ECMO) is indicated in patients with refractory hypoxemia despite prone position mechanical ventilation [2]. A recent retrospective study identified older age, high sequential organ failure assessment score (SOFA) score, and D-dimer greater than 1 μg/mL as poor prognostic factors which aid the clinician early in instituting aggressive treatment and monitoring for such patients [114]. Steroids, and injudicious antibiotic use should be discouraged. Some studies report effective use of RNA polymerase inhibitors Remdesivir and Immucillin-A as prophylactic and therapeutic agents against HCoVs including SARS CoV-2 [115,116]. Anti-malarial drug chloroquine and its analogue may show protective effect against virus by decreasing intracellular pH but may cause cardiac arrythmias owing to prolonged QTc interval in some patients [117]. Monoclonal antibodies against interleukin-6 (IL-6) like Sarilumab, Siltuximab, Tocilizumab and interleukin-1 (IL-1) inhibitor like Anakinra may be useful in severe cases and may control the effects of SIRS which is the main culprit in the pathogenesis of severe cases [118]. Currently, there are 1673 studies registered in clinical trials involving various investigational drugs and vaccine apart from those mentioned above and are still at phase-I level [119]. Theoretically, molecules involved at each step of SARS CoV-2 pathogenesis may become potential therapeutic targets (Fig. 3 ).
Fig. 3.
Preventive and Therapeutic Targets for COVID-19.
There are various structural and functional preventive and therapeutic targets (1) Spike protein by sub-unit vaccine. (2) ACE2-R saturation by exogenous soluble ACE which makes the receptor unavailable for viral binding.(3)ACE R binding by monoclonal antibodies and drugs blocking viral attachment. (4) TMPRSS2 inhibitors prevent viral entry into host cells by blocking cleavage of S2 fraction of S protein important for membrane fusion. (5) RNA dependent RNA polymerase inhibitors block viral transcription. (6) Viral protein synthesis inhibitors block viral translation.
9. Conclusion
To conclude, SARS CoV-2 is highly infective and its control depends on strict implementation of preventive measures. Though RT-PCR is the gold standard for SARS CoV-2 diagnosis, the results are variable and there is scope of false negatives owing to either sampling errors or due to usage of different primers and reagents by different vendors. Serologic estimations of antibody titres though not helpful for diagnosis, may be useful for prognostication and follow-up. Though currently available pathologic data is limited, it is of prime importance to unveil the pathogenesis which will enable the development of therapeutic options. However, studies on larger cohorts are needed to validate the findings obtained for generalized application. Importantly, due to limited availability of time and resources for research during the current emergency situation there is a huge lag and gap in understanding COVID-19; hence the current review removes the lag and bridges the gap and provides pathology centered understanding of the disease. Furthermore, the currently approved clinical trials that are in various stages of development on all aspects of COVID-19 including vaccines and potential therapeutic targets will shed light in future.
Author contributions
Chandrakumar Shanmugam (CS), Abdul Rafi Mohammed (ARM), Swarupa Ravuri (SR), Vishwas Luthra (VL), Narasimhamurthy Rajagopal (NR) and Saritha Karre (SK) designed and structured the overall review; CS, SR, VL and SK contributed to epidemiology, virology, pathologenesis and pathology sections; CS, ARM, and NR contributed to clinical, radiological presentations and preventive and therapeutic sections of the manuscript; CS, ARM and VL were involved in the preparation of the illustrations.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We thank Dr. Vaseemuddin Mohammad (Consultant nephrologist, Chicago, Ilinois, USA), Dr. Hari Gopinath (Consultant pediatrician, Hyderabad, India), Dr. Yuvaraj Doraiswamy(Consultant Anesthetic, Carmarthen, NHS, UK) and Dr. N Harikrishna Reddy (Consultant Dermatologist, Inverness, Scotland, NHS, UK) for their critical review of this manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.prp.2020.153222.
Appendix A. Supplementary data
The following are Supplementary data to this article:
XXXXX
XXXXX
References
- 1.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;(395):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cascella M., Rajnik M., Cuomo A., et al. 2020. Features, Evaluation and Treatment of Corona Virus (COVID-19) Statpearls NCBI- Bookshelf 6 April.https://www.ncbi.nlm.nih.gov/books/NBK554776/#_NBK554776_pubdet_ [Google Scholar]
- 3.https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
- 4.Ruan S. Likelihood of survival of Corona virus disease 2019. Lancet. 2020;30(March) doi: 10.1016/S1473-3099(20)30257-7. [DOI] [Google Scholar]
- 5.Mahase Elizabeth. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 6.https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
- 7.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-Accessed 11 March 2020.
- 8.]https://www.worldometers.info/coronavirus/Accessed 24 May 2020.
- 9.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(January 13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25(January 4) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocklöv J., Sjödin H., Wilder-Smith A. COVID-19 outbreak on the Diamond Princess cruise ship: estimating the epidemic potential and effectiveness of public health countermeasures. J. Travel Med. 2020;(February) doi: 10.1093/jtm/taaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X., Rayner S., Luo M.H. Does SARS-CoV-2 has a longer incubation period than SARS and MERS? J. Med. Virol. 2020:1–3. doi: 10.1002/jmv.25708. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauch C.T., Lloyd-Smith J.O., Coffee M.P., Galvani A.P. Dynamically modeling SARS and other newly emerging respiratory illnesses: past, present and future. Epidemiology. 2005;16(November 6):791–801. doi: 10.1097/01.ede.0000181633.80269.4c. [DOI] [PubMed] [Google Scholar]
- 14.Lipsitch M., Cohen T., Cooper B., et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 48. Available at: https://www.who.int/docs/default- source/coronaviruse/situation-reports/20200308-sitrep-48-covid-19. [Google Scholar]
- 16.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and pre- vention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Leung G.M., Hedley A.J., Ho L.M., et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann. Intern. Med. 2004;(141):662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lau E.H., Hsiung C.A., Cowling B.J., et al. A comparative epidemiologicanalysis of SARS in Hong Kong, Beijing and Taiwan. BMC Infect. Dis. 2010;10(50) doi: 10.1186/1471-2334-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., et al. Epidemiological, demo- graphic, and clinical characteristics of 47 cases of Middle East respira- tory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothe C., Schunk M., Sothmann P., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10) doi: 10.1056/NEJMc2001468. 2020 0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahase E. China coronavirus: mild but infectious cases may make it hard to control outbreak, report warns. BMJ. 2020;368:m325. doi: 10.1136/bmj.m325. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.Y., Ko J.H., Kim J.Y., et al. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J. Korean Med. Sci. 2020;35(7):e86. doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang K.W., Ho P.L., Ooi G.C., et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 24.Poutanen S.M., Low D.E., Henry B., et al. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348(20):1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 25.Holshue M.L., Debolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10) doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(March 10226) doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morawska L., Tang J.W., Bahnfleth W., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao P., Zhang H., Wu Z., Wang J. Visualising the expansion and spread of coronavirus disease 2019 by cartograms. Environ. Plann. A: Econ. Space. 2020;52(4):698–701. doi: 10.1177/0308518X20910162. [DOI] [Google Scholar]
- 29.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19. An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020 doi: 10.1097/INF.0000000000002660. XX:00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciaglia E., Vecchione C., Puca A.A. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front. Pediatr. 2020;8(April):1–3. doi: 10.3389/fped.2020.00206. Article 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [Published online February 7, 2020] JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aprahamian I., Cesari M. Geriatric Syndromes and SARS-COV-2: More than Just Being Old. J. Frailty Aging. 2020;14(April):1–3. doi: 10.14283/jfa.2020.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W.J., Ni Z.Y., Hu Y., et al. China medical treatment expert group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China [Published online February 28, 2020] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y., Cheng G., Chui C.H., et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293(March 12):1450–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J., Yang Y., Huang H., et al. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv preprint. 2020 doi: 10.1101/2020.03.11.20031096. [DOI] [Google Scholar]
- 36.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L.F., Shi Z., Zhang S., Field H., Daszak P., Eaton B. Review of bats and SARS. Emerg Infect Dis. 2006;12(12):1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge X.Y., Li J.L., Yang X.L., et al. Isolation and characterization of a bat SARS‐like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(April 4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cauchemez S., Van Kerkhove M.D., Riley S., Donnelly C.A., Fraser C., Ferguson N.M. Transmission scenarios for Middle East respiratory syndrome coronavirus (MERS‐CoV) and how to tell them apart. Euro Surveill. 2013;18(24) pii: 20503. [PMC free article] [PubMed] [Google Scholar]
- 41.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 42.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(February 7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. "Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(February 10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30(19 March 7):1346–1351. doi: 10.1016/j.cub.2020.03.022. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cyranoski D. Mystery deepens over animal source of coronavirus". Nature. 2020;579(26 February 7797):18–19. doi: 10.1038/d41586-020-00548-w. Bibcode: 2020 Natur.579…18C. [DOI] [PubMed] [Google Scholar]
- 46.Xiao K., Zhai J., Feng Y. 2020. Isolation and Characterization of 2019-nCoV-like Coronavirus from Malayan Pangolins. February bioRxiv (preprint) [DOI] [Google Scholar]
- 47.Wong M.C., Cregeen S.J., Ajami N.J., Petrosino J.F. 2020. Evidence of Recombination in Coronaviruses Implicating Pangolin Origins of nCoV-2019. February bioRxiv (preprint) [DOI] [Google Scholar]
- 48.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176(February) doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snijder E.J., van der Meer Y., Zevenhoven‐Dobbe J., et al. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80(12):5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double‐membrane vesicles. mBio. 2013;4(4) doi: 10.1128/mBio.00524-13. pii: e00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus‐encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81(Pt 4):853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 53.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian X.L., Huang A., Xia S., et al. 2020. Potent Binding of Novel Coronavirus 2019 Spike Protein by a SARS Coronavirus-specific Human Monoclonal Antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gui M., et al. Cryo-electron microscopy structures of the SARS-CoVspike glycoprotein reveal a prerequisite conformational state for receptorbinding. Cell Res. 2017;27:119–129. doi: 10.1038/cr.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng T., Cao H., Zhang H., et al. 2020. The Insert Sequence in SARS-CoV-2 Enhances Spike Protein Cleavage by TMPRSS. bioRxiv (preprint server) [DOI] [Google Scholar]
- 57.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;(March):18. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barkauskas C.E., et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/jci68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroetz D.N., et al. Type I interferon induced epigenetic regulation of macrophages suppresses innate and adaptive immunity in acute respiratory viral infection. PLoS Pathog. 2015;11:e1005338. doi: 10.1371/journal.ppat.1005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nabhan A.N., Brownfield D.G., Harbury P.B., Krasnow M.A., Desai T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science (New York, N.Y.) 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanley B., Lucas S.B., Youd E., et al. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206522. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Tian S., Hu W., Niu L., et al. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020 doi: 10.1016/j.jtho.2020.02.010. S1556-0864(20)30132-30135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian S., Xiong Y., Liu H., et al. Pathological study of the 2019 novel corona virus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020 doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome -case report. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Qian W.R., Guoqiang Qu, Yunyun Wang, et al. A report on the general observation of the necropsy of a newly developed corona virus pneumonia. Fa YiXue Za Zhi. 2020 doi: 10.12116/j.issn.1004-5619.2020.01.00. 1004-5619 (2020) 01-0. [DOI] [Google Scholar]
- 67.Ding Y., Wang H., Shen H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng D.L., Al Hosani F., Keating M.K., et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates. Am. J. Pathol. 2014;2016(April 186):652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo W., Yu H., Gou J., et al. 2020. Clinical Pathology of Critical Patient with Novel Coronavirus Pneumonia (COVID-19)www.preprints.org [Google Scholar]
- 70.Copin M.C., Parmentier E., Duburcq T., et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06057-8. 23 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGonagle D., O’Donnell J.S., Sharif K., et al. Why the immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia are distinct from macrophage activation syndrome with disseminated intravascular coagulation. Lancet Rheum. 2020 doi: 10.1016/S2665-9913(20)30121-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mao Y., Lin W., Wen J., Chen G. Clinical and pathological characteristics of 2019 novel coronavirus disease(COVID-19): a systematic review. medRxiv preprint. 2020 doi: 10.1101/2020.02.20.20025601. [DOI] [Google Scholar]
- 73.Wang Y., Chen Y., Q Qin. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures [Epub ahead of print] J. Med. Virol. 2020 doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang C., Wang Y., Li X., Ren L., Zhao J., y Hu, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;(395):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin L., Jiang X., Zhang Z., et al. 2020. Gastrointestinal Symptoms of 95 Cases With SARS-CoV-2 Infection Gut Published Online First: 02 April. [DOI] [PubMed] [Google Scholar]
- 76.Basu-Ray I., Soos M.P. Cardiac Manifestations Of Coronavirus (COVID-19) Statpearls (internet) https://www.ncbi.nlm.nih.gov/books/NBK556152/#_article-95199_s4_ [PubMed]
- 77.Lahiri D., Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020;12(4):e7889. doi: 10.7759/cureus.7889. April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao K., Huang J., Dai D., et al. Acute myelitis after SARS-CoV-2 infection: a case report. MedRxiv. 2020 doi: 10.1101/2020.03.16.20035105. [DOI] [Google Scholar]
- 79.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L., et al. Br. J. Ophthalmol. 2020;0:1–4. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [Google Scholar]
- 81.Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16387. Accepted Author Manuscript. March. [DOI] [PubMed] [Google Scholar]
- 82.Bi Q., Wu Y., Mei S., et al. Epidemiology and transmission of COVID-19 in Shenzhen China: analysis of 391 cases and 1,286 of their close contacts. medRxiv. 2020 doi: 10.1101/2020.03.03.20028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X.F., Yuan J., Zheng Y.J., et al. [Clinical and epidemiological character- istics of 34 children with 2019 novel coronavirus infection in Shenzhen] Zhonghua Er Ke Za Zhi. 2020;58:E008. doi: 10.3760/cma.j.issn.0578-1310.2020.0008. [DOI] [PubMed] [Google Scholar]
- 84.Dong Y., Mo X., Hu Y., et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;(March 16) [Epub ahead of print] [Google Scholar]
- 85.Jones V.G., Mills M., Suarez D., et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020 doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 86.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;(395):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong H.Y.F., Lam H.Y.S., et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019;27(March) doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang Y., Zhang H., Xu Y., Xie J., Pang P., Ji W. CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html.8may2020.
- 91.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [PMID: 31992387] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng M.P., Papenburg J., Desjardins M., et al. Diagnostic testing for severe acute respiratory syndrome–Related Coronavirus-2. Ann. Intern. Med. 2020;13(april) doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y., Yang M., Shen C., et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. 2020 February 11. [Google Scholar]
- 95.To K.K., Tsang O.T., Leung W.S., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. March 23 pii:S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerg. Microbes Infect. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pfefferle1 S., Reucher S., Nörz D., et al. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000152. pii=2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo L., Ren L., Yang S., et al. Profiling early humoral response to diagnose Novel coronavirusdisease(COVID-19) Clin. Infect. Dis. 2020;(March 21):ciaa310. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.World Health Organization . 3rd ed. WHO; Geneva: 2004. Laboratory Biosafety Manual; p. 186. [Google Scholar]
- 100.Mäkelä M.J., Puhakka T., Ruuskanen O., et al. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36(February 2):539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.World Health Organization . WHO; Geneva: 2020. Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases.https://apps.who.int/iris/bitstream/handle/10665/331329/WHO-COVID-19-laboratory-2020.4-eng.pdf [Google Scholar]
- 102.Zhang N., Cheng P., Jia W., et al. Impact of intervention methods on COVID-19 transmission in Shenzhen. Build. Environ. 2020;180 doi: 10.1016/j.buildenv.2020.107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anzai A., Kobayashi T., Linton N.M., et al. Assessing the impact of reduced travel on exportation dynamics of novel coronavirus infection (COVID-19) J. Clin. Med. 2020;9(2):601. doi: 10.3390/jcm9020601. Published 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He Y., Li J., Du L., et al. Identification and characterization of novel neutral- izing epitopes in the receptor-binding domain of SARS-CoV spike protein: revealing the critical antigenic determinants in inactivated SARS-CoV vac- cine. Vaccine. 2006;24:5498–5508. doi: 10.1016/j.vaccine.2006.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeDiego M.L., Alvarez E., Almazán F., et al. A severe acute respiratory syn- drome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J. Virol. 2007;81:1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin J.T., Zhang J.S., Su N., et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. (Lond.) 2007;12:1107–1113. [PubMed] [Google Scholar]
- 108.Agarwal A.S., Tao X., Algaissi A., et al. Immunization with inactivated Middle East respiratory syndrome coronavirus vaccineleads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.e Y., Zhou Y., Siddiqui P., et al. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem. Biophys. Res. Commun. 2004;325:445–452. doi: 10.1016/j.bbrc.2004.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jaume M., Yip M.S., Kam Y.W., et al. SARS CoV subunit vaccine: body-mediated neutralisation and enhancement. HongKong Med J.anti. 2012;18(suppl. 2):31–36. [PubMed] [Google Scholar]
- 111.Ma C., Li Y., Wang L., et al. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immuniza- tion: implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–2108. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.See R.H., Zakhartchouk A.N., Petric M., et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J. Gen. Virol. 2006;87(pt 3):641–650. doi: 10.1099/vir.0.81579-0. [DOI] [PubMed] [Google Scholar]
- 113.Addo M. 2018. Safety, Tolerability and Immunogenicity of Vaccine Candidate MVA-MERS-S. Available at: https://clinicaltrials.gov/ct2/show/ NCT03615911#outcomemeasures. (Accessed 22 February 2020) [Google Scholar]
- 114.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviralcompound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295(April 15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Warren T.K., Wells J., Panchal R.G., et al. Protection against filovirus dis- eases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rebeaud M.E., Zores F. SARS-CoV-2 and the use of chloroquine as an antiviral treatment. Front. Med. (Lausanne) 2020;7:184. doi: 10.3389/fmed.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ingraham N.E., Emran S.L., Bk Thielen, et al. Immunomodulation in COVID-19. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30226-5. Published Online May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.https://clinicaltrials.gov/ct2/results?cond=COVID-19 (Accessed 24 May 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
XXXXX
XXXXX