Introduction
Electrical optimization, which is manifested by the abbreviation of the QRS duration, has been associated with improved cardiac resynchronization therapy (CRT) response.1 The introduction of a device-based algorithm (SyncAV) that automatically adjusts the atrioventricular delay (AVD) based on the intrinsic atrioventricular (AV) conduction has been shown to be superior in achieving QRS duration narrowing.2 We report a case in which our patient developed ventricular tachycardia (VT) with an unusual 1:1 atrial tracking of the VT due to the SyncAV algorithm. As a result, antitachycardia therapy was inappropriately withheld.
Case report
A 75-year-old man with an established history of ischemic cardiomyopathy underwent CRT-D (cardiac resynchronization therapy defibrillator) implantation in 2018 (St Jude Quadra Assura CD3367-40QC, Abbott, Singapore).
The device’s pacing mode was DDDR with a base rate of 90 beats per minute (bpm) and cycle length of 667 ms, which in Abbott’s implantable cardioverter-defibrillator (ICD) relies on a modified atrial-based timing. The maximum sensing and tracking rates were programmed at 105 bpm. Tachycardia detection parameters were programmed as follows: VT1 zone 160 bpm, VT2 zone 187 bpm, VF zone 250 bpm. The supraventricular tachycardia (SVT) discrimination timeout feature was programmed off.
He was admitted with VT storm and received multiple shocks. Intravenous amiodarone was initiated and this resulted in the prolongation of his AV nodal conduction to 300 ms at a base rate of 90 bpm. The paced AVD was programmed at 350 ms to allow for appropriate paced AVD search with a 50 ms offset subsequently applied.
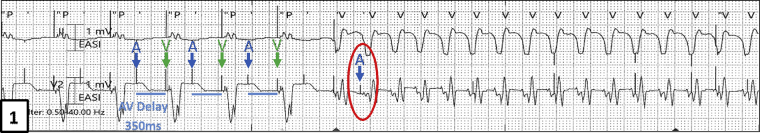
He developed VT at a rate of 163 bpm with a cycle length of 367 ms, as evidenced by the change in morphology seen on the telemetry strip (Figure 1) during his stay and was found unresponsive. Immediate external direct current cardioversion was successful in restoring sinus rhythm. Upon interrogation of his CRT-D, an episode of atrial pacing at a rate of 163 bpm was seen with no antitachycardia therapy delivered from the device (Figure 2A and B). What was the mechanism for the apparent atrial “tracking” of his VT and why was therapy withheld? We further explain in our discussion below.
Figure 1.
Telemetry strip showing APVP with prolonged AV delay (350 ms); subsequent change in rhythm and rate indicating ventricular tachycardia. Atrial paced event circled in red indicates the likely inferred “chamber of onset” of ventricular tachycardia as the atrium.
Figure 2.
A: The earliest recording of tachycardia showed 1:1 atrial-to-ventricular complex association at 163 bpm. Green arrow: Ventricular tachycardia (VT) beat not sensed as it fell into post-atrial ventricular blanking. Blue arrow: Device delivered biventricular pacing, which fused with next VT beat. Purple arrow: Native atrial beat, not tracked. Orange arrow: Functional noncapture of atrial paced event. Ventriculoatrial (VA) interval is demonstrated in second half of strip, with calculated VA interval here: (AP-AP) – (AP-VS) = 367 – 55 = 312 ms. Similar to calculated VA interval 317 ms. B: The recording ended with this strip. When the VT slowed just sufficiently to fall below VT1 zone, following 5 beats of “VS” circled in red, the device declared “return to sinus,” as indicated by the yellow star. The device reverted to DDDR mode as indicated, with a modified atrial-based timing; however, the AP-AP interval highlighted in the blue box was unexpectedly prolonged at 758 ms. The red square boxes of “VS” indicate what the device interpreted as premature ventricular contractions, resulting in a conversion to ventricular-based timing.
Discussion
Figure 2A illustrates the first electrogram recording by the device; unfortunately, the onset of VT was not captured. The electrogram shows VT occurring at a rate of 163 bpm and atrial pacing occurring at the same rate with 1:1 association. Of note, the second VT beat (green arrow) was not sensed by the device as it fell within the post-atrial ventricular blanking (PAVB) period. PAVB in Abbott’s devices are preset at either 44 ms or 52 ms, with 52 ms being the nominal setting, as it was in this case. Following this VT beat that was not sensed, the device delivered biventricular pacing (blue arrow), which fuses with the next VT beat. A native atrial beat then occurs (purple arrow), which was not tracked, followed by a functional noncaptured atrial paced (AP) event (orange arrow).
At baseline, there was normal intrinsic AV nodal conduction. The SyncAV algorithm was switched on with a good abbreviation of the QRS duration. The SyncAV algorithm automatically extends the paced or sense AV delay (350 ms and 325 ms, respectively) for 3 beats after every 256 beats. During this time, the device measures the intrinsic AV interval before applying a programmable AVD offset of 50 ms in our patient’s case. This algorithm cycle repeats every 256 beats, allowing for dynamic adjustment of the paced or sensed AV delay. If the sensed ventricular electrogram timing extends beyond 350 ms during atrial pacing or 325 mg during atrial sensing, which occurred in our patient’s case owing to ongoing amiodarone therapy, SyncAV would maintain the AVD at these values. This is seen in Figure 1 where the AP–biventricular paced interval is 350 ms just before onset of VT.
During VT, Abbott’s devices employ an episodal pacing mode (DDI), which utilizes ventricular-based timing. This is to prevent forced ventricular pacing during the episode, should an atrial sensed event occur. With a base rate of 90 bpm and cycle length of 667 ms, programmed paced AVD at 350 ms (as per the SyncAV algorithm with an AVD prolonged beyond 350 ms), the calculated ventriculoatrial (VA) interval is thus equal to 667 ms minus 350 ms, or 317 ms. This resulted in the apparent 1:1 atrial “tracking” of the VT, where an AP event occurs after every ventricular sense (VS) event with a VS-AP duration of 317 ms. The VT was “tracked” as each VS event occurred 59 ms after an AP event, just after the programmed PAVB of 52 ms.
Dual-chamber discrimination in Abbott’s devices depends on the rate branch algorithm. As there is 1:1 AP-VS correlation during VT, it thus classified this episode under the V=A rate branch arm. Further discrimination then depends on morphology and/or chamber of onset. The chamber of onset was the atrium, which could be inferred as the last AP spike on the telemetry strip in Figure 1 (the arrow circled in red). Even though the morphology of the VT did not match the sinus rhythm template, as both morphology and chamber of onset did not concur, therapy was thus withheld as the “IF ALL” criterion for VT was not met.
It is important to maintain as close to 100% sensitivity for VT detection and therapy as possible, especially in our patient’s case, given his recurrent VT episodes. As such, it would have been prudent to increase diagnostic sensitivity at some expense to specificity. In retrospect, the SVT high rate discrimination timeout feature could have been turned on and the rate branch discriminator set to “IF ANY,” which in this case would have delivered appropriate therapy for the VT episode. The balance between sensitivity and specificity of the device should be applied on a case-by-case basis, considering the patient’s history and likelihood of having episodes of VT vs SVT.
As a result of the above fortuitous events, VT continued, and the device withheld therapy. Subsequently, the VT rate slows for 5 beats to 158 bpm, cycle length of 379 ms which was below the programmed VT1 zone of 160 bpm (Figure 2B). As a result, it triggered a “return to sinus” event, reverting to DDDR mode with modified atrial-based timing. The diagnosis of VT then becomes painfully clear with more V > A. However, therapy was still withheld as the VT rate now has fallen below the programmed VT1 zone.
In modified atrial-based timing during DDDR mode, the cycle length between 2 AP intervals is dictated by the atrial-atrial (A-A) interval, which is 667 ms at a base rate of 90 bpm, and not the VA interval. Interestingly, the AP-AP interval continues at a slower rate of 79 bpm, cycle length of 758 ms and not the expected 90 bpm (Figure 2B). While the first VS episode did not initiate an AP event (DDDR, atrial-based timing), the second VS (Figure 2B, red square), with no preceding AP or atrial sensed event, would be interpreted as a premature ventricular complex by the device, leading to an immediate conversion to a ventricular-based timing. Another AP would then be delivered after a VA interval of 317 ms. The VT continues at this slower rate and the cycle repeats itself. The overall AP-AP cycle length would thus be equal to AP VS 66 ms + VT cycle length 379 ms + VA interval 317 ms, or 762 ms.
After this event, we reprogrammed the device parameters as follows. We reduced the VT1 detection zone from a rate of 160 bpm to 141 bpm as the VT episode occurred at a rate of 158–163 bpm, which was just at the limits of the original VT1 zone. Furthermore, paced AVD was reduced from 350 to 130 ms, sensed AVD was reduced from 325 to 100 ms, and the SyncAV algorithm was turned off. This was owing to the prolonged AV delay in our patient on amiodarone therapy and earlier witnessed deleterious effects of a long AV delay coupled with high programmed base rate and DDI mode during VT. We kept our patient on amiodarone therapy in view of his recurrent VT episodes, as we felt the likelihood of this recurrent event is low given the abovementioned adjustments that were made.
The role of an ICD is to treat malignant ventricular arrhythmias and prevention of sudden cardiac death is its fundamental function. However, with the combination of pacing function in the form of dual-chamber devices or biventricular devices and the advent of increasingly complex device programming algorithms, we need to be careful not to cause intradevice interaction that can potentially undermine the prime purpose of the device.
Strohmer and colleagues3 have reported the association of atrial tachycardia and VT after dual-chamber ICD implantation, as well as how frequent “physiological” AV pacing can lead to proarrhythmic effects in ICD patients. For example, with dual-chamber pacing, an AP ≥48% together with VP >40% was associated with increased likelihood of VT.3 Expanding on this observation, one can imagine that, with the complexities of CRT-D programming, the potential for intradevice interaction is real and hence physicians as well as manufacturers should be cognizant of these risks.
Glikson and colleagues4 reported in a prospective study how a rate-smoothing algorithm resulted in a potential delayed or absent detection of ventricular tachyarrhythmias. When the algorithm is turned on, each R-R interval is used as a reference value and subsequent R-R interval is limited to a programmed percentage variation from the reference. The rate-smoothing algorithm was initially designed to reduce sudden changes in heart rate of patients with pacemakers and subsequently also showed some benefit in reducing the initiation of ventricular arrhythmias through the prevention or shortening of the postextrasystolic pause. Through a complex interplay between rate-smoothing parameters and VT detection, 6 out of 10 cases of monomorphic VT had delayed or absent detection. It was highlighted that VT rates of <220 bpm, long AV delays, and high upper rates have a higher likelihood of affecting VT detection.4
Cooper and colleagues5 also reported a case in which a biventricular ICD system failed to detect VT within its programmed VT detection zone, resulting in inappropriately withheld therapy. The identified cause was once again the rate-smoothing algorithm; however, unlike what Glikson and colleagues4 reported, in this case it was a short AV delay with slow VT (125 bpm) and modest maximum tracking rate of 115 bpm that resulted in impaired VT detection. Owing to the rate-smoothing-down algorithm, which directs the upper rate limit, AP events (using ventricular-based timing) and ventricular blanking periods can be introduced into the VT detection zone and hence affect appropriate VT detection.5
Shalaby6 reported a case of VT with cycle length of 465 ms that was undersensed in a dual-chamber ICD owing to rate-adaptive pacing. This was due to ventricular-based timing, which mandates that atrial pacing occurs at a fixed VA time, which coincidentally occurred close to the VT cycle length. As a result, the subsequent VT beat fell within the PAVB period, resulting in undersensing of the VT and tachycardia therapy being inappropriately withheld.
Conclusion
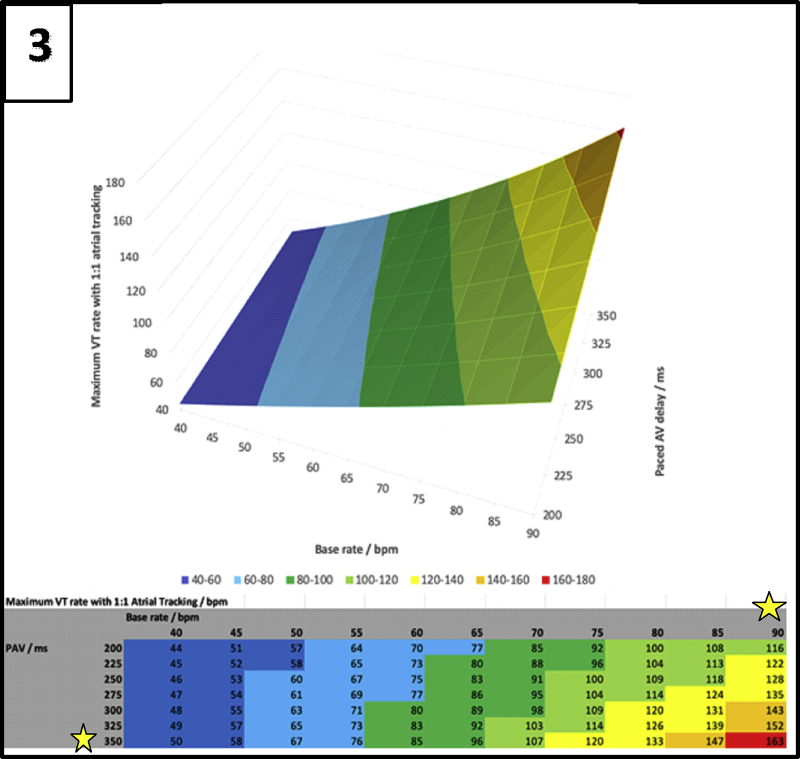
The advent of the SyncAV algorithm allows us to achieve better abbreviation of the QRS complex with CRT. The algorithm necessitates the programming of a long AVD to allow for an appropriate “search” of the intrinsic AVD. A nontracking DDI episodal mode during VT, which utilizes ventricular-based timing, together with a high programmed base rate and long paced AVD, results in a short VA interval and the apparent 1:1 atrial “tracking” of the VT. There is thus a complex interplay between the programmed base rate, AVD, and the VT cycle length (Figure 3). As shown, a VT rate of 163 bpm (in red) would result in 1:1 atrial “tracking” of the VT if a base rate of 90 bpm and a paced AVD of 350 ms is programmed. For any given VT cycle length, we can thus reduce the likelihood of this phenomenon by judiciously avoiding the base rates and AVD intervals, as illustrated in Figure 3. Other possible solutions would be to simply switch off the SyncAV algorithm (at the cost of less QRS abbreviation) and adopt the nominal short paced AVD, or to lengthen the PAVB (if this is a programmable feature).
Figure 3.
Relationship between base rate, paced atrioventricular (AV) delay, and maximum ventricular tachycardia rate with 1:1 atrial tracking. The yellow star indicates the base rate and paced AV delay values that we should avoid programming.
Our patient was fortunate to have had this episode occur during a monitored hospital admission and received prompt treatment. The outcomes could have been significantly different and fatal had it occurred elsewhere. We are reminded through this case that intradevice interactions can occur with detrimental effects.
Key Teaching Points.
-
•
A high programmed base rate together with long paced atrioventricular (AV) delay during ventricular tachycardia (VT) can lead to inadvertent “tracking” of the VT and result in therapy being inappropriately withheld.
-
•
The SyncAV algorithm, though beneficial in achieving QRS duration narrowing in cardiac resynchronization therapy, may not be suitable in situations such as prolonged AV delay and should be avoided.
-
•
In patients at high risk of recurrent ventricular arrhythmia, we should increase sensitivity of defibrillator therapy over specificity. This can include turning on the supraventricular tachycardia discrimination timeout feature and switching rate branch algorithm to “IF ANY.”
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no conflicts of interest to disclose.
References
- 1.Korantzopoulos P., Zhang Z., Li G., Fragakis N., Liu T. Meta-analysis of the usefulness of changes in QRS width to predict response to cardiac resynchronization therapy. Am J Cardiol. 2016;118:1368–1373. doi: 10.1016/j.amjcard.2016.07.070. [DOI] [PubMed] [Google Scholar]
- 2.Varma N., O’Donnell D., Bassiouny M. Programming cardiac resynchronization therapy for electrical synchrony: reaching beyond left bundle branch block and left ventricular activation delay. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strohmer B., Mermi J., Castellanos E. Impact of dual chamber pacing on the incidence of atrial and ventricular tachyarrhythmias in recipients of implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2005;28:S249–S254. doi: 10.1111/j.1540-8159.2005.00002.x. [DOI] [PubMed] [Google Scholar]
- 4.Glikson M., Beeman A.L., Luria D.M., Haydes D.K., Friedman P.A. Impaired detection of ventricular tachyarrhythmias by a rate-smoothing algorithm in dual-chamber implantable defibrillators: intradevice interactions. J Cardiovasc Electrophysiol. 2002;13:312–318. doi: 10.1046/j.1540-8167.2002.00312.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooper J.M., Sauer W.H., Verdino R.J. Absent ventricular tachycardia detection in a biventricular implantable cardioverter-defibrillator due to intradevice interaction with a rate smoothing pacing algorithm. Heart Rhythm. 2004;1:728–731. doi: 10.1016/j.hrthm.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Shalaby A.A. Delayed detection of ventricular tachycardia in a dual chamber rate adaptive pacing implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2004;27:1164–1166. doi: 10.1111/j.1540-8159.2004.00603.x. [DOI] [PubMed] [Google Scholar]