Introduction
The superior vena cava (SVC) is an important target of atrial fibrillation (AF) ablation procedures; its isolation is valuable in patients with triggers arising from it. However, SVC isolation might be difficult to achieve because of the location of the phrenic nerve (PN) and sinus node (SN). A previous study showed that complete SVC isolation was not possible in up to 14% of patients.1 Gianni and colleagues2 reported an alternative ablation strategy that started from the septal aspect of the right atrium (RA)-SVC junction and continued to the posterior and inferior targets sites of early activation until electrical isolation was achieved. PN injury is still observed but can be easily prevented with high-output pacing to exclude a course of the PN. Furthermore, Tanaka and colleagues3 reported a novel strategy of SVC isolation using ultra-high-resolution mapping with SVC foci triggering AF. Patients with the spontaneous conduction block line received point-by-point radiofrequency (RF) applications that were delivered to connect the 2 open ends of the conduction block line, and RF applications were unnecessary along the block line to complete SVC isolation, leading to a reduced number of RF deliveries. This approach is an attractive method to avoid PN and SN injuries; however, the electrical reconduction of this approach is not well described. Herein, we report a case of acute reconnection and reisolation dependent on isoproterenol (ISP) administration following SVC isolation with a novel approach using a spontaneous conduction block.
Case report
A 59-year-old man complained of palpitation and visited our hospital. He had a history of pulmonary vein (PV) isolation for paroxysmal AF. He underwent a second session for recurrent paroxysmal AF 6 months after the first procedure. Written informed consent for the procedure was obtained from the patient.
We created a voltage map of the left atrium during pacing from the RA using a 3-dimensional (3D) mapping system (CARTO 3 system version 6; Biosense Webster, Diamond Bar, CA) and a 20-electrode mapping catheter (PENTARAY; Biosense Webster) via the transseptal approach. No PV reconduction was recorded, and additional SVC isolation was performed.
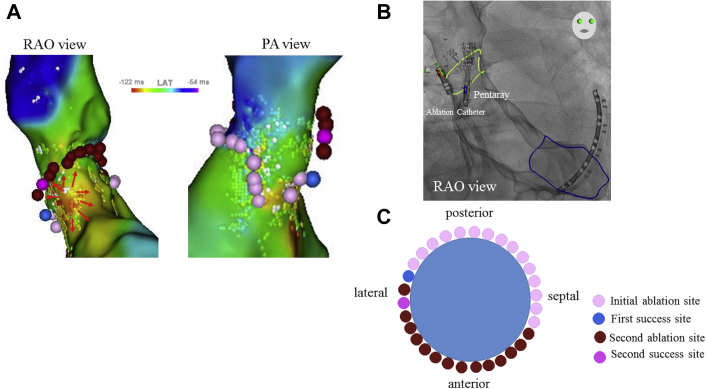
We created an SVC-RA map during sinus rhythm (mapping time, 21 minutes; mapping point, 1312). The SVC-RA activation map confirmed spontaneous conduction block line on the anterior wall of the SVC-RA junction, using a lower threshold function in the CARTO 3 system (lower threshold: 25%) (Figure 1A). Potential records during sinus rhythm mapping hardly showed fractionated electrograms on both the SVC and RA sides of the spontaneous conduction block line (Supplemental Figure 1A). The wavefront propagated to the SVC from the posterior-lateral wall (Supplemental Video 1). The PN site was identified by high-output pacing (20 mA/0.2 mV) using the contact force–sensing irrigated ablation catheter (ThermoCool SmartTouch SF, Biosense Webster), and the PN was not located at the planned ablation site. Point-by-point RF applications were performed at 30 W/30 s from the septal wall and connected to the spontaneous conduction block line at the lateral wall in a counterclockwise manner (Figure 1B). The SVC was isolated following two-thirds of circumferential ablation, excluding the anterior wall direction (RF lesions 16 points, ablation time 552 seconds) (Figure 1A–C).
Figure 1.
Superior vena cava (SVC)–right atrium (RA) activation/voltage map during sinus rhythm before and after initial ablation. A: The SVC-RA activation map showing spontaneous conduction block line at the anterior wall of the SVC (white line) and the wavefront propagation through the SVC from the posterior wall side (black arrow). Creating an ablation line for the septal-posterior-lateral wall resulted in SVC isolation (pink points); the site of the successful isolation is in blue point. B: Voltage map of SVC-RA before the procedure. C: The schema of the initial ablation site from the septal wall connected to the spontaneous conduction block line at the lateral wall. D: The SVC-RA voltage map indicating complete SVC isolation. BI = bipolar; LAT = local activation time; PA = posterior-anterior; RAO = right anterior oblique; SN = sinus node.
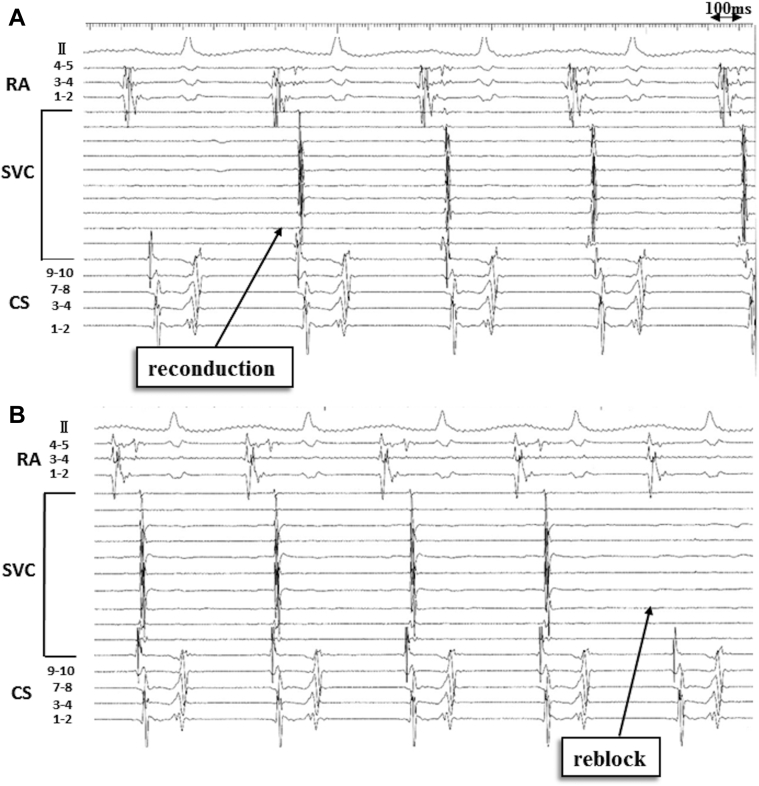
We recreated an SVC-RA voltage map and confirmed SVC isolation during sinus rhythm, as well as the exit block of the automaticity originating in the SVC (Figure 1D). However, acute reconduction of atrial impulses through spontaneous conduction block line to the SVC developed 9 minutes after ISP infusion (heart rate [HR], 140 beats per minute [bpm] on ISP 4 mcg/min; baseline HR = 90 bpm) (Figure 2A). Interestingly, SVC signals disappeared spontaneously 7 minutes after stopping ISP infusion (HR, 120 bpm) (Figure 2B). This ISP-dependent transient reconduction from the RA to the SVC and block was reproducibly observed.
Figure 2.
Isoproterenol (ISP)-dependent superior vena cava (SVC) reconduction and block in intracardiac electrocardiogram. A: Reconduction of the SVC was detected 9 minutes after ISP infusion. B: The SVC was reisolated spontaneously 7 minutes after stopping ISP infusion. CS = coronary sinus; RA = right atrium.
An SVC-RA activation map with ISP infusion showed that the anterior wall side of the spontaneous conduction block line in the baseline disappeared (lower threshold, 25%; Figure 3A). The activation map did not show conduction gap along with the previous ablation lesions. This map demonstrated impulse propagation into the SVC through the anterior wall of the SVC where the spontaneous conduction block line had been present (Supplemental Video 2). No fractionated electrogram was found at the anterior wall of the SVC (Supplemental Figure 1B). Additional ablation at 30 W for the anterior wall of the SVC was performed, and SVC isolation was completed. The successful reisolation site was 22 mm from the first isolation site. Further ablation for 35 seconds was performed to connect with the first ablation line (total ablation time, 488 seconds), and eventually, complete circumferential ablation was necessary (Figure 3B and C).
Figure 3.
Isoproterenol (ISP)-dependent superior vena cava (SVC) reconduction and subsequent SVC reisolation. A: Reconduction of the SVC-right atrium (RA) map with ISP infusion indicating disappearance of the previous spontaneous conduction block line. The wavefront propagated through the SVC from the anterior wall side. SVC reisolation was confirmed by the ablation at the anterior wall side connecting with the first ablation line (red points and purple point). B: An ablation line was created to confirm the potential of the SVC with PENTARAY (Biosense Webster, Diamond Bar, CA; green line). C: Schema showing additional ablation from the anterior wall side at the previous conduction block line, with a full circumferential ablation.
We recreated an SVC-RA voltage map with ISP infusion and confirmed that the SVC isolation was complete (Supplemental Figure 2). Moreover, the exit block was confirmed with the local capture of the SVC potentials. The session ended with confirmation of the bidirectional block. Dormant conduction was not recognized for the infusion of adenosine triphosphate 40 mg. Postoperatively, the patient maintained sinus rhythm without antiarrhythmic drugs during a 7-month follow-up period.
Discussion
To our knowledge, this is the first case of acute SVC reconnection following a novel SVC isolation approach, requiring conventional circumferential lesion set for reisolation after ISP administration. Electrical reconnection of the SVC was frequently observed after standard procedures using angiographic/fluoroscopic and circular mapping catheter guidance and that majority of the conduction gaps were located at the anterolateral part of the SVC-RA junction.4 No study has reported acute or chronic SVC reconnection following the approach using a contemporary 3D mapping system. The ultra-high-resolution mapping system (Rhythmia) allows to identify the detailed pattern of the activation to the SVC, and it is useful for the safe and efficient isolation of the SVC during an AF ablation procedure. Compared with the Rhythmia system, the CARTO 3 system enables automatic visualization of the conduction block as a white block line. This block line is determined based on the difference in conduction times between 2 adjacent mapping points. Using automatic block line visualization for SVC isolation with the CARTO 3 system may reduce the number of RF deliveries with larger isolated areas similar to that with the Rhythmia system; however, whether this spontaneous conduction block is functional or fixed is not known.
ISP is generally used for provocation of ectopic foci in the PV or SVC areas after the isolation procedure and to confirm the exit block of their impulses to the atrial tissues. The reason why we used ISP is explained as follows: The effect of ISP on impulse conduction may be anticipated in the region where incomplete tissue injury caused by the ablation procedure may cause partial depolarization of the resting potentials of the cardiac cells, causing inactivation of the Na+ current to block impulse propagation. Application of ISP on partially depolarized cells can increase the L-type Ca2+ current, which induces the activation of the Ca2+-dependent excitation to resume impulse conduction.5 Therefore, if the application of ISP restores the impulse conduction on the area of the spontaneous conduction block line, the block may be explained as functional rather than fixed. Based on the above assumption, our results of the ISP-dependent reconduction and block agree with the interpretation of the functional nature of the spontaneous conduction block line.
Since March 2019, 19 procedures of SVC isolation were performed in our hospital using the CARTO 3 system (version 6), and in 5 cases acute reconduction of the SVC with ISP infusion was observed. None of the 5 cases had full circumferential ablation owing to the disappearance of the conduction block with ISP infusion. Before the present experience, we ablated the earliest activation site in the SVC guided by the ring catheter and/or the gap sites, which were identified by changing the lower threshold setting in the case of the reconduction of the SVC with ISP infusion. Therefore, we suggest the following to confirm the functional nature of this conduction block line: an activation map of the SVC with ISP infusion should be created at baseline, reisolation should be confirmed with the interruption of ISP infusion, and assessment of reconduction sites should be performed systematically.
In the present case, 1 of the additional causes of RA-SVC reconnection might be the cranial shift of the earliest activation site (ie, pacemaker shift) owing to ISP administration. Modulation of the autonomic tone induces shifts in activation sites during sinus rhythm. Betts and colleagues6 examined shifts in the site of the earliest endocardial depolarization during sinus rhythm and sinus tachycardia using high-density activation mapping. With increasing HR by ISP infusion, the site of earliest endocardial depolarization remained stationary until a sudden shift in a cranial direction, often to sites beyond the histological sinoatrial node.6 In most patients without AF and in approximately half of the patients with AF but without symptomatic bradycardia, the earliest activation site was located in the superior one-third of the crista terminalis at baseline.7 During ISP infusion, the superior SN invariably served as the earliest activation site in all such patients. Because the intracardiac electrograms could not be used to differentiate SN activation and RA activation, the shifts of the SN exit sites and the actual pacemaker sites might look the same on the 3D map. In the present case, the earliest activation sites at baseline and after ISP infusion were not recorded, and the pacemaker shift was not confirmed.
Another possible reason for the SVC reconnection at the block line by ISP infusion is anisotropic conduction. The crista terminalis demonstrates marked anisotropic conduction properties with faster conduction velocity in the longitudinal direction than in the transverse direction. The rate-dependent conduction block of the crista terminalis was found in patients with typical atrial flutter and patients with supraventricular tachycardia.8,9 Further, catheter orientation was another reason for the SVC reconnection. Bipolar electrograms exhibit variable morphology based on the interaction of the wavefront with the orientation of the electrode pair. Catheter orientation to the wavefront may give rise to pseudo block and conduction.10
Another possibility of the transient reconduction of the SVC is dormant conduction. “Dormant conduction” was defined as transient reconduction by adenosine or adenosine triphosphate application at ablation sites. These drugs could increase the outward K+ current to restore the Na+ current–dependent excitation, which was different from the action by ISP. We did not evaluate the effect of adenosine triphosphate infusion during transient reconduction in SVC before the ISP infusion. Therefore, this possibility could not be excluded completely.
Conclusion
To rule out a possible SVC reconnection following the novel SVC isolation approach, assessment of the conduction pattern during ISP infusion is important to identify the conduction gap to the SVC.
Key Teaching Points.
-
•
Although superior vena cava (SVC) isolation is achieved using spontaneous conduction block, acute reconnection and reisolation of the SVC dependent on isoproterenol (ISP) infusion was confirmed in the present case.
-
•
In case of the reconduction of the SVC with ISP infusion, the following is assumed: (1) reconduction owing to lesion gap, and (2) reconduction owing to pacemaker shift or disappearance of functional block line. In the latter case, additional circumferential ablation might be required.
-
•
It is necessary to reevaluate the conduction block pattern by creating an activation map under the ISP infusion as well as under the sinus rhythm.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.06.002.
Appendix. Supplementary data
RA-SVC activation map during sinus rhythm. The spontaneous conduction block line at the anterior wall and the wavefront propagation from the posterior wall of the SVC.
The RA-SVC activation map with ISP infusion. Disappearance of the previous conduction block line and the earliest activation site of the SVC at the anterior wall side.
Supplemental Figure 1.
Potentials at the spontaneous conduction block line under sinus rhythm and ISP infusion (A,B).
Supplemental Figure 2.
The final SVC-RA voltage map after complete SVC isolation.
References
- 1.Corrado A., Bonso A., Madalosso M. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J Cardiovasc Electrophysiol. 2010;21:1–5. doi: 10.1111/j.1540-8167.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 2.Gianni C., Sanchez J.E., Mohanty S. Isolation of the superior vena cava from the right atrial posterior wall: a novel ablation approach. Europace. 2018;20:e124–e132. doi: 10.1093/europace/eux262. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y., Takahashi A., Takagi T. Novel ablation strategy for isolating the superior vena cava using ultra high-resolution mapping. Circ J. 2018;82:2007–2015. doi: 10.1253/circj.CJ-17-1352. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki S., Taniguchi H., Kusa S., Uchiyama T., Hirao K., Iesaka Y. Conduction recovery after electrical isolation of superior vena cava--prevalence and electrophysiological properties. Circ J. 2013;77:352–358. doi: 10.1253/circj.cj-12-0951. [DOI] [PubMed] [Google Scholar]
- 5.Zipes D.P., Jalife H. Saunders; Philadelphia, PA: 2009. Cardiac Electrophysiology: From Cell to Bedside. Fifth Edition; pp. 1–22. [Google Scholar]
- 6.Betts T.R., Roberts P.R., Ho S.Y., Morgan J.M. High density endocardial mapping of shifts in the site of earliest depolarization during sinus rhythm and sinus tachycardia. Pacing Clin Electrophysiol. 2003;26:874–882. doi: 10.1046/j.1460-9592.2003.t01-1-00153.x. [DOI] [PubMed] [Google Scholar]
- 7.Joung B., Hwang H.J., Pak H.-N. Abnormal response of superior sinoatrial node to sympathetic stimulation is a characteristic finding in patients with atrial fibrillation and symptomatic bradycardia. Circ Arrhythm Electrophysiol. 2011;4:799–807. doi: 10.1161/CIRCEP.111.965897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saffitz J.E., Kanter H.L., Green K.G., Tolley T.K., Beyer E.C. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ Res. 1994;74:1065–1070. doi: 10.1161/01.res.74.6.1065. [DOI] [PubMed] [Google Scholar]
- 9.Tai C.T., Chen S.A., Chen Y.C. Conduction properties of the crista terminalis in patients with typical atrial flutter: basis for a line of block in the reentrant circuit. J Cardiovasc Electrophysiol. 1998;9:811–819. doi: 10.1111/j.1540-8167.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 10.Deno D.C., Balachandran R., Morgan D., Ahmad F., Masse S., Nanthakumar K. Orientation-independent catheter-based characterization of myocardial activation. IEEE Trans Biomed Eng. 2017;64:1067–1077. doi: 10.1109/TBME.2016.2589158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RA-SVC activation map during sinus rhythm. The spontaneous conduction block line at the anterior wall and the wavefront propagation from the posterior wall of the SVC.
The RA-SVC activation map with ISP infusion. Disappearance of the previous conduction block line and the earliest activation site of the SVC at the anterior wall side.