Introduction
Catheter ablation is an established first-line therapy for treatment of symptomatic supraventricular tachycardia (SVT), with high success rates and low risk of complications.1 Coronary artery (CA) injury is a rare complication of SVT ablation and is typically seen during radiofrequency ablation of posterior paraseptal accessory pathways inside the coronary sinus ostium (CSO) or its branches.2 The anterior-inferior aspect of the CSO, a site that may be targeted for atrioventricular nodal reentrant tachycardia (AVNRT) ablation, can be near a CA.3 Limited precordial leads displayed at high sweep speeds during standard electrophysiology (EP) procedures coupled with the rarity of the CA injury may result in overlooking this potential complication during AVNRT ablation.
We report a rare case of CA injury during slow pathway ablation for typical AVNRT that manifested as spontaneous ventricular fibrillation (VF) after ablation. We also present a brief review of literature and tips for prevention of this rare complication.
Case report
A 10-year-old male patient (weight 32.5 kg, body surface area 1.15 m2) presented with recurrent episodes of palpitations and adenosine-sensitive narrow complex tachycardia. After carefully evaluating pharmacological and nonpharmacological treatment options with the family, we proceeded with an EP study and catheter ablation. The ablation was performed by the senior author (R.M.), who is US trained and the sole pediatric cardiac electrophysiologist at the Loma Linda University Children’s Hospital. In the year prior to the procedure, 30 ablations in patients <12 years of age were performed by the operator at this center. The procedure was performed under general anesthesia. The following catheters were used for the procedure: coronary sinus: Dynamic XT, decapolar, 6F (Boston Scientific Corp, Maple Grove, MN); right atrium: Supreme Quad, JSN, 4F (Abbott Laboratories, Abbott Park, IL); right ventricle: Supreme Quad, CRD, 4F (Abbott Laboratories); His bundle: HIS CRD2 Supreme (Abbott Laboratories). EP study showed evidence of dual AV nodal physiology and the patient was inducible for typical AVNRT. A “DF” curved nonirrigated 4-mm-tip ablation catheter (Navistar EZ Steer, Biosense Webster, Inc, Diamond Bar, CA) was advanced to the area of the rightward inferior extension of the slow pathway under electroanatomic guidance (CARTO; Biosense Webster, Inc) through a sheath (8F, SEPT; Abbott Laboratories).
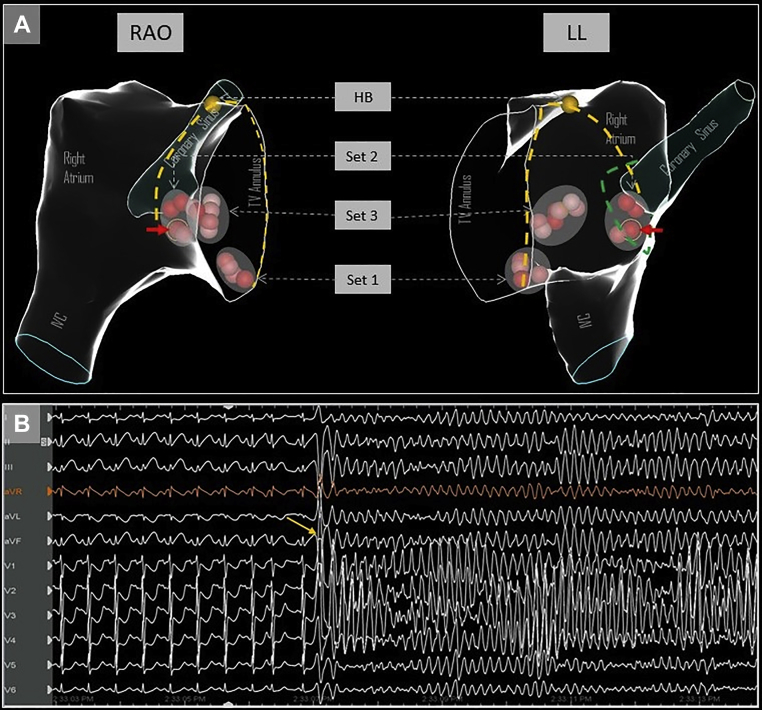
The CSO was defined by a combination of electroanatomic mapping using the ablation catheter, fluoroscopy, near-field atrial electrogram (usually similar or larger amplitude than ventricular signal) and 10 Ω rises in impedance from the baseline. Ablation was performed with a nonirrigated catheter (50 watts, temperature limit 60°C), and this was used for all ablation lesions that are depicted in Figure 1A. Individual lesion characteristics are outlined in Table 1. Breath-hold was utilized for all ablation lesions to avoid respirophasic catheter movement, and there were no appreciable steam pops with any of the lesions. Initial ablation lesions were delivered in the inferior portion of the triangle of Koch (set 1 in Figure 1A) without inducing junctional ectopy. After a few short test lesions in the region indicated by set 3, additional lesions were delivered in an area that was felt to be anterior to the CSO (set 2 in Figure 1A); these lesions were also prematurely terminated owing to impedance rises (>10 Ω) or lack of junctional beats seen. Final ablation lesions were again delivered more anterior to the CSO area, which resulted in junctional beats (set 3 in Figure 1A) and the endpoint of noninducibility.
Figure 1.
A: Electroanatomic views in right anterior oblique (RAO) and left lateral (LL) orientation showing the different sets of ablation lesions delivered, and relationship to His bundle area. The red solid arrow indicates the culprit lesion following which ST changes were seen. The yellow dashed lines mark the anterior and posterior borders of the triangle of Koch. The green dotted line shows the possible outline of a funnel-shaped coronary sinus ostium, indicating that the ablation lesion could have been within the ostium. HB = His bundle; IVC = inferior vena cava; TV = tricuspid valve. B: Ventricular fibrillation induced by R-on-T premature ventricular contraction (yellow arrow).
Table 1.
Reported cases of coronary artery injury following slow pathway ablation of atrioventricular nodal reentrant tachycardia
| Case | Authors/year | Age (years)/ sex | Ablation catheter/ sheath | Recognition | Delay in diagnosis? | Angiographic finding | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | Blaufox et al/ 2004 | 2.5/ male | Unknown | ST elevation during reinduction post ablation | Yes | 80% stenosis of the right posterolateral branch | Intracoronary nitroglycerin Intravenous nitroglycerin, heparin, and solumedrol Stenosis <50% in 48 hours, resolved in 12 months |
| 2 | Garabelli et al/ 2015 | 17/ male | Magnetic navigation catheter / SRO sheath | ST elevation after catheter fell to middle cardiac vein | No | 100% stenosis of right posterolateral branch | Percutaneous coronary intervention with bare metal stent |
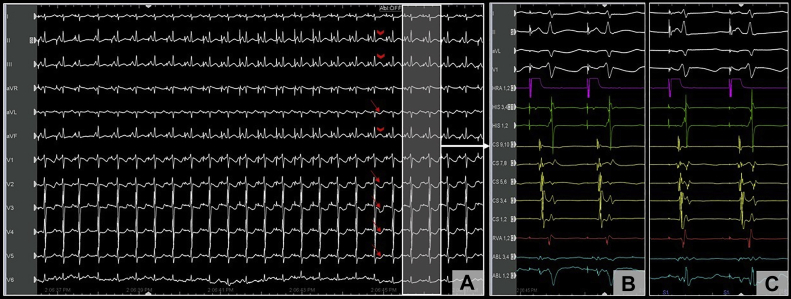
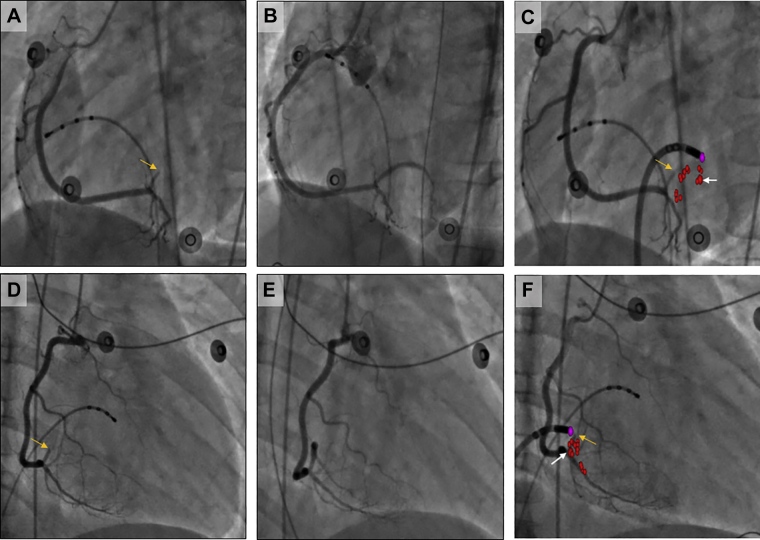
Following this, isoproterenol (10 mcg intravenous bolus) was administered, and testing was repeated with abolition of AH jump. Approximately 4 minutes after isoproterenol administration, the patient developed spontaneous VF from an R-on-T premature ventricular contraction (Figure 1B), requiring defibrillation. This rather unusual finding raised suspicion for CA injury, and review of the surface 12-lead electrocardiogram (EGC) at a 25 mm/s sweep speed showed precordial ST-segment depression (Figure 2A, red arrows) with subtle elevation in the inferior leads (Figure 2A, red arrowheads) after 1 of the ablation lesions (Figure 1A, red arrows). The 12-lead ECG during the entire ablation lesion as well as a plot of biophysical parameters during this ablation lesion are shown in Supplemental Figure S1; there was a gradual increase in impedance of >10 Ω, after which ablation was terminated. These subtle ECG changes post ablation were not evident on 200 mm/s sweep speed that is used during EP study and ablation (Figure 2B and C). Emergent coronary angiography was performed with a JR4 guide catheter, which revealed complete occlusion of the right posterolateral branch of the right CA (Figure 3A and D). Intracoronary nitroglycerin and verapamil were administered through the guide catheter, without improvement in appearance or flow. Percutaneous transluminal coronary angioplasty (PTCA) was performed, which resulted in flow restoration. However, there was residual stenosis followed by complete reocclusion resulting in ST changes and another episode of VF requiring defibrillation. Repeat PTCA was attempted, with administration of additional vasodilators; however, the vessel continued to occlude, suggesting extrinsic compression from myocardial edema or direct thermal injury to the artery. Coronary stenting was performed with a Xience Alpine 2.25/15 drug eluting stent (Abbott Laboratories), with successful resolution of ST changes and TIMI 3 flow with good myocardial blush (Figure 3B and E).
Figure 2.
A: Electrocardiogram at low sweep speed (25 mm/s) showing worsening ST changes during ablation, including ST depression (red arrows) in the precordial leads and subtle ST elevation (red arrowheads) in the inferior leads. These changes (highlighted area with white arrow) were less apparent on the higher sweep speed with limited precordial leads (B) when compared to the ST segment prior to ablation (C).
Figure 3.
Left anterior oblique 60° (A–C) and right anterior oblique 30° (D–F) fluoroscopic images showing coronary occlusion (A and D, yellow arrows), restoration of flow post percutaneous coronary intervention (B and E), and manual overlay of ablation lesions from electroanatomic map on fluoroscopic image using ablation catheter as a reference (C and F, occlusion site: yellow arrow; culprit lesion: white arrow; red dots: ablation lesions; purple dot: reference for catheter tip on electroanatomic map to allow manual overlay).
The patient was placed on weight-based dual antiplatelet therapy with aspirin and clopidogrel. Echocardiogram performed on the following day showed preserved left ventricular ejection fraction without wall motion abnormalities. The patient has remained arrhythmia free 1 year after the procedure and the clopidogrel was discontinued without further events.
Since the center did not have software that automatically combines fluoroscopic images with electroanatomic maps, we planned to perform a manual overlay after the procedure was completed to understand the relationship between the ablation lesion and the site of occlusion. For this purpose, after the initial diagnostic angiogram, while awaiting the interventional cardiologist, the ablation catheter was placed close to the occlusion site using both right and left anterior oblique views on fluoroscopy to use as a reference. Manual overlay was performed using PowerPoint (Microsoft Office; Microsoft Corp, Redmond, WA) at a later stage in an attempt to show the relationship between the site of occlusion and the culprit lesion (Figure 3C and F). The exact distance between these 2 sites, however, could not be determined owing to limitations with performing a manual overlay of electroanatomic maps on fluoroscopic images.
Discussion
We report a rare case of CA injury after slow pathway modification that was initially missed owing to low clinical suspicion for this complication and limited precordial leads displayed at high sweep speeds, as is routinely done during EP procedures. Spontaneous VF seen after ablation prompted us to evaluate for this possibility, and the patient was successfully managed with PTCA and stenting of the right posterolateral branch.
CA injury during SVT ablation is typically seen when targeting posteroparaseptal pathways that are within the coronary sinus, including the middle cardiac vein.2 However, other ablations are also commonly performed in close vicinity to coronary vessels, and although a 5 mm safety distance is advisable during epicardial ablation, multiple endocardial target sites violate this rule. Examples include cavotricuspid isthmus and posterior right ventricular outflow tract ablation, where the right CA and the left main CA can be close to the ablation site, respectively. The anterior-inferior aspect of the CSO is yet another site commonly targeted for AVNRT ablation, and can also be in close proximity (±2 m) to the posterolateral branch of the right CA with right-dominant coronary circulation and left circumflex artery with left-dominant coronary circulation.3
Upon systematic review of literature, we identified 2 reported cases of CA stenosis during ablation of AVNRT (Table 1).4,5 One of these was a 2.5-year-old patient with a body weight of 15 kg, and the other was a patient who underwent inadvertent ablation within the middle cardiac vein. To the best of our knowledge, this is the first case of CA injury in an older, larger child (weight 32 kg) where the ablation lesion appeared to be outside of the CSO, as evidenced by electrogram characteristics (small atrial with large ventricular electrogram), electroanatomic map defining the CSO, and impedance measurements (no significant increase in baseline impedance at the site while creating the electroanatomic map). The possibility of the ablation lesion being within a “funnel-shaped” CSO cannot be ruled out, as high-density electroanatomic mapping as well as venography were not performed.
Acute CA occlusion after ablation may be due to several mechanisms, which include coronary vasospasm, extrinsic compression from surrounding edema, spontaneous plaque rupture or arterial dissection, and direct thermal injury to the artery causing arterial wall edema and inflammation.6,7 A delayed response has also been described, with the development of intimal hyperplasia following medial necrosis and loss of intimal and elastic tissue.8 In this young patient without coronary atherosclerotic disease, neither intracoronary nitroglycerin nor verapamil relieved the occlusion, and the artery reoccluded shortly after PTCA. The mechanism for occlusion was likely direct thermal injury, and flow could only be restored after a stent scaffold. The apparent distance between the ablation lesion and the site of occlusion could be related to the inability to perform a perfect manual overlay of electroanatomic maps on fluoroscopic images (due to x-ray tube rotation, different scales, cardiac cycle gating, or lack of high-density coronary sinus mapping) in addition to proximal extension of radiofrequency energy–related thermal injury and external compression from tissue edema.
The true os of the coronary sinus can be difficult to define with the usual techniques used during ablation procedures (electroanatomic mapping, impedance changes, fluoroscopy, and electrogram characteristics). At the time of ablation, it was felt that electroanatomic mapping performed to define the CSO was sufficient. However, it is possible that more detailed mapping would have revealed that the culprit ablation lesion was within a “funnel-shaped” CSO (Figure 1A; dotted green line indicates possible outline of funnel-shaped CS os), which has been described by CS venography.9 This type of os would potentially not have the same impedance change and signal characteristics as a non-funnel-shaped CSO, and could mislead electrophysiologists into thinking that the catheter tip is outside the coronary sinus. Use of multielectrode catheters for high-density mapping (instead of using an ablation catheter only) and coronary sinus venography can be used to better delineate the true os in select cases; up-front CA angiography prior to ablation should be considered only if ablation is being performed at the CSO. If the distance between the CA and the slow pathway ablation site is <5 mm, an alternative ablation site should be chosen, or cryoenergy should be used. Lower power (for, eg, 30 or 40 watts) can be considered in pediatric patients, especially if the weight is <25 kg.10 Incorporation of additional limb and precordial ECG leads in the standard intracardiac electrogram page during ablation could help identify ST changes earlier at faster sweep speeds. A review of 12-lead ECG at 25 mm/s sweep speed when ablation is performed close to the CSO area is also a reasonable strategy to avoid any delay in diagnosis of this potential complication.
Conclusion
In conclusion, we report a rare case of CA injury that occurred during ablation of typical AVNRT manifesting as VF during isoproterenol testing after ablation. Knowledge of regional anatomy is important to implement during routine ablations, and rapid recognition of this complication can allow for life-saving intervention.
Key Teaching Points.
-
•
The posterolateral branch of the right or left coronary artery can have a close relationship to the coronary sinus ostium, and can be potentially damaged during slow pathway ablation for atrioventricular nodal reentrant tachycardia.
-
•
Electrocardiographic ST-segment changes can be missed with limited precordial leads displayed at a high sweep speed during typical electrophysiology study and ablation procedures.
-
•
It is important to define the extent and morphology of the coronary sinus ostium with high-density electroanatomic mapping, and in select cases venography, prior to performing ablation.
-
•
In cases where potential target sites for ablation appear to be close to the coronary sinus ostium, coronary angiography should be considered, especially in pediatric patients.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors report no conflicts of interest relevant to this publication.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.06.010.
Appendix. Supplementary data
Impedance, power and temperature graph of culprit lesion (#11).
References
- 1.Katritsis D.G., Boriani G., Cosio F.G. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE) Europace. 2017;19:465–511. doi: 10.1093/europace/euw301. [DOI] [PubMed] [Google Scholar]
- 2.Schneider H.E., Kriebel T., Gravenhorst V.D., Paul T. Incidence of coronary artery injury immediately after catheter ablation for supraventricular tachycardias in infants and children. Heart Rhythm. 2009;6:461–467. doi: 10.1016/j.hrthm.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Mao J., Moriarty J.M., Mandapati R., Boyle N.G., Shivkumar K., Vaseghi M. Catheter ablation of accessory pathways near the coronary sinus: value of defining coronary arterial anatomy. Heart Rhythm. 2015;12:508–514. doi: 10.1016/j.hrthm.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaufox A.D., Saul J.P. Acute coronary artery stenosis during slow pathway ablation for atrioventricular nodal reentrant tachycardia in a child. J Cardiovasc Electrophysiol. 2004;15:97–100. doi: 10.1046/j.1540-8167.2004.03378.x. [DOI] [PubMed] [Google Scholar]
- 5.Garabelli P.J., Stavrakis S., Po S.S. A case series and review of the literature regarding coronary artery complications associated with coronary sinus catheter ablation. HeartRhythm Case Rep. 2015;1(5):315. doi: 10.1016/j.hrcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosaka Y., Chinushi M., Takahashi K. Coronary vasospasm triggered ventricular fibrillation delayed after radiofrequency ablation of the right accessory pathway. Europace. 2009;11:1554–1556. doi: 10.1093/europace/eup219. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama H., Nakagawa H., Pitha J.V. Comparison of cryothermia and radiofrequency current in safety and efficacy of catheter ablation within the canine coronary sinus close to the left circumflex coronary artery. J Cardiovasc Electrophysiol. 2005;16:1218–1226. doi: 10.1111/j.1540-8167.2005.50126.x. [DOI] [PubMed] [Google Scholar]
- 8.Paul T., Bökenkamp R., Mahnert B., Trappe H.-J. Coronary artery involvement early and late after radiofrequency current application in young pigs. Am Heart J. 1997;133:436–440. doi: 10.1016/s0002-8703(97)70185-6. [DOI] [PubMed] [Google Scholar]
- 9.Hummel J.D., Strickberger S.A., Man K.C., Daoud E., Niebauer M., Morady F. A quantitative fluoroscopic comparison of the coronary sinus ostium in patients with and without AV nodal reentrant tachycardia. J Cardiovasc Electrophysiol. 1995;6:681–686. doi: 10.1111/j.1540-8167.1995.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 10.Krause U., Backhoff D., Klehs S., Kriebel T., Paul T., Schneider H.E. Catheter ablation of pediatric AV nodal reentrant tachycardia: results in small children. Clin Res Cardiol. 2015;104:990–997. doi: 10.1007/s00392-015-0868-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Impedance, power and temperature graph of culprit lesion (#11).