Highlights
-
•
Mitochondrion plays a critical role in controlling exercise-induced adaptive responses through coordination of redox signaling.
-
•
Mitostasis is regulated by mitochondrial biogenesis, fusion and fission dynamics, and mitophagy, and their functional integrity is crucial for muscle contraction.
-
•
Muscle immobilization could promote ubiquitin proteolysis, mitophagy, and inflammation via activating nuclear factor-κB and forkhead box O signaling.
-
•
Stimulation of PGC-1α signaling through exercise or experimental approach enhances muscle protein preservation, mitochondrial stability, and resistance to oxidative stress and inflammation.
-
•
Future research will continue to explore the mechanism and application of improved mitochondrial homeostasis in health issues that induce muscle atrophy.
Keywords: Antioxidant, Exercise, Peroxisome-proliferator-activated receptor γ coactivator 1-α, Reactive oxygen species, Redox signaling, Skeletal muscle
Abstract
In the past, contraction-induced production of reactive oxygen species (ROS) has been implicated in oxidative stress to skeletal muscle. As research advances, clear evidence has revealed a more complete role of ROS under both physiologic and pathologic conditions. Central to the role of ROS is the redox signaling pathways that control exercise-induced major physiologic and cellular responses and adaptations, such as mitochondrial biogenesis, mitophagy, mitochondrial morphologic dynamics, antioxidant defense, and inflammation. The current review focuses on how muscle contraction and immobilization may activate or inhibit redox signalings and their impact on muscle mitochondrial homeostasis and physiologic implications.
1. Introduction
Exercise is a powerful stimulus to physiologic adaptations of the body, including cardiovascular, pulmonary, neuromuscular, endocrinal, and musculoskeletal systems.1 During exercise performed at proper intensity and duration, the reactive oxygen species (ROS) are generated within the muscle cells and play an important role in regulating a wide range of biologic functions that directly or indirectly affect these adaptations.2 The balance of ROS generation and cellular antioxidant defense may determine cellular redox status, which can influence either the de novo gene expression of new proteins or the post-translational modification of existing proteins, or both. In most cases, the influence of ROS is exerted via signal transduction pathways wherein transcription factors (TF) are synthesized and/or covalently modified via an oxidoreductive (redox) process. Redox signaling is an important branch of exercise physiology that describes the processes and mechanisms by which ROS participate in and control exercise-induced adaptive responses.3
As a complicated biologic integration, redox signaling requires almost all cellular components, such as the cell membrane, nucleus, ribosome, mitochondrion, lysosome, and proteasome, to participate in. Research during the past several decades reveals that mitochondrion plays a critical role in the coordination of redox signaling.4 This is because 1) mitochondrion is the organelle where all energy substrates are finally metabolized to generate ATP, the only energy form that various cellular functions depend on; 2) the majority of ROS (mainly H2O2 and NO) is generated within the mitochondria, which to a large extent determines the cellular redox status5; and 3) mitochondrion itself undergoes constant turnover controlled primarily by 3 processes: biogenesis, fission and fusion dynamics, and autophagy (mitophagy).6 In addition, a healthy and stable mitochondrial population (mitostasis) is important to other cell functions in the skeletal muscle, such as force production, glucose deposition, lipid metabolism, inflammation, apoptosis, and aging.7 The current review, obviously, is not able to cover all aspects of mitochondrial properties. The authors focus on several areas in which mitostasis and redox signaling depend on each other, with a special reference to exercise adaptation of skeletal muscle health, which has been known as hormesis.8 For detailed reviews, readers are referred to several recent and more comprehensive reviews.9, 10, 11
2. Mitochondrial biogenesis: the role of peroxisome-proliferator-activated receptor γ coactivator 1-α (PGC-1α)
When animals are engaged in long-term physical work with a high oxygen consumption, mitochondrial volume, density, and oxidative enzyme activity in the skeletal muscle increase.1 The observed increase in mitochondrial population is accomplished primarily by enhanced mitochondrial biogenesis, regulated by complex signaling pathways that require the synthesis, import, and incorporation of proteins and other molecules into the existing mitochondrial reticulum, as well as replication of mitochondrial DNA.12 PGC-1α serves as a master transcriptional coactivator, which not only controls the biogenesis of mitochondria but also participates in the adaptation of antioxidant defense, control of fusion and fission dynamics, and mitophagy.13
A wealth of literature has shown that endurance increases PGC-1α levels in the skeletal muscle.14, 15, 16 This training adaptation is usually, but not always, accompanied by elevated nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM) levels, 2 required elements for increases in nuclear-encoded mitochondrial proteins and mitochondrial proliferation. Thus, trained muscle demonstrates predictable physiologic and biochemical outcomes, such as elevated mitochondrial oxygen consumption and ATP production, mitochondrial morphologic changes, increased oxidative enzymes, and enhanced fatty acid oxidation.17 A cross-sectional study showed that endurance-trained human subjects had 7-fold higher PGC-1α, 5-fold higher TFAM, and more than 2-fold higher NRF1 protein contents in their leg muscles than their sedentary counterparts.18 It is noteworthy that although mitochondrial biogenesis is at least partially dependent on intact PGC-1α signaling,19 PGC-1α is not absolutely required for training adaptation; some studies using PGC-1α knockout (KO) mice still derived significant improvement in mitochondrial oxidative markers.10 Interestingly, repeated intermittent sprinting bouts were also shown to increase PGC-1α content by 6-fold in rat muscle, along with a 2-fold increase in NRF1 and TFAM.20 This phenomenon may explain why high-intensity interval training is an effective way to achieve results that are comparable to those achieved with long-term chronic training.9
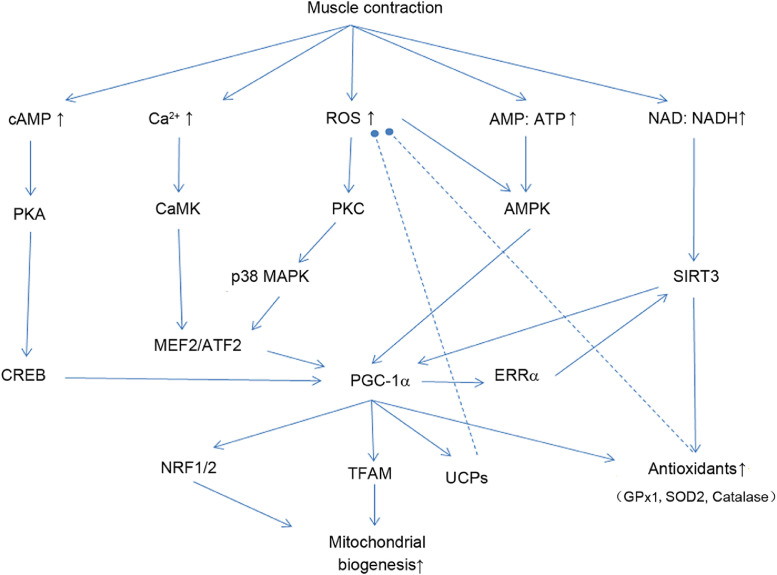
To date, research indicates that contraction-induced PGC-1α expression is partly dependent on the activation of Ca2+/calmodulin-dependent protein kinase IV,15 which phosphorylates cyclic adenosine monophosphate-response element binding protein (CREB), and calcineurin, which activates myocyte enhancer factor 2 (MEF2).13 Muscle contraction also increases ROS production in the mitochondria that activates p38 mitogen-activated protein kinase (MAPK) via protein kinase c (PKC). p38 phosphorylates MEF2 and activates transcription factor 2 (ATF2). ATF2-CREB dimerization appears to bind to PGC-1α promoter as a required step.14 Alternatively, during strenuous exercise, adenosine 5’ monophosphate-activated protein kinase (AMPK) is activated due to the elevated AMP/ATP ratio that enhances coactivation of PGC-1α.16 Cellular redox status can profoundly influence the gene expression and coactivating ability of PGC-1α in the muscle. Sprinting exercise-induced upregulation of PGC-1α content was severely hampered when rats were injected with ammonium pyrrolidinedithiocarbamate (PDTC), an antioxidant and nuclear factor-κB (NF-κB) inhibitor.18 Furthermore, when rats were treated with PDTC daily during a chronic training program, the elevation of muscle PGC-1α was hampered.18
Besides mitochondrial biogenesis, PGC-1α also stimulates a range of other cell functions connected to estrogen-related receptor-α (ERα) binding, fatty acid β-oxidation, and antioxidant enzyme regulation. Reduced mRNA levels of superoxide dismutase 1 (SOD1, or CuZnSOD), SOD2 (or MnSOD), and glutathione peroxidase 1 (GPx1), as well as SOD2 protein content, were observed in skeletal muscle from PGC-1α KO mice, whereas mice with PGC-1α overexpression showed higher SOD2.21 Recent research reveals that PGC-1α plays a direct role in the ROS-activated mitochondrial oxidative and antioxidant enzyme (SOD2) gene expression.22 Nuclear receptor corepressor (NcOR1), as a conserved protein, normally binds to peroxisome proliferator-activated receptor β (PPARβ) of the target gene promoter to suppress transcription. Upon exposure to H2O2, the inhibition is relieved, allowing transcription to take place. Furthermore, PGC-1α promotes sirtuin (SIRT) 3 gene expression, which deacetylates and activates mitochondrial enzymes, including SOD2, through a post-translational mechanism.23 Taken together, PGC-1α is probably the most important pathway that confers a wide range of cellular functions that adapt to acute and chronic exercise (Fig. 1). Some additional results of PGC-1α signaling are mentioned in subsequent sections.
Fig. 1.
Contraction-activated cell signaling pathways that lead to increased PGC-1α and mitochondrial biogenesis in the skeletal muscle. Arrow-headed lines represent activation; dot-ended lines represent inhibition. AMP:ATP = adenosine monophosphate/adenosine triphosphate ratio; AMPK = AMP-activated protein kinase; ATF = activating transcription factor; CaMK = Ca2+/Calmodulin-activated protein kinases; cAMP = cyclic adenosine monophosphate; CREB = cAMP response element-binding protein; ERRα = estrogen-related receptor α; GPx1 = glutathione peroxidase 1; MAPK = mitogen-activated protein kinases; MEF2 = myocyte enhancer factor-2; NAD:NADH = nicotinamide adenine dinucleotide / nicotinamide adenine dinucleotide hydrogen ratio; NRF = nuclear respiratory factor; PGC-1ɑ = peroxisome proliferator-activated receptor γ coactivator 1-α; PKA = protein kinase A; PKC = protein kinase C; ROS = reactive oxygen species; SIRT3 = sirtuin 3; SOD = superoxide dismutase; TFAM = mitochondrial transcription factor A; UCPs = uncoupling proteins.
3. Mitochondrial fusion and fission dynamics
Mitochondria are organized into dynamic networks extending throughout the cytosol in close contact with the nucleus, endoplasmic reticulum, and Golgi network, thus facilitating the crosstalk among these organelles. It is now known that mitochondria undergo constant fusion and fission dynamics, and the morphologic changes are closely related to their function, stability, and turnover.24 Mitochondrial fusion in mammals requires mitofusins (Mfn1 and Mfn2), with the assistance of optic atrophy protein 1.25 Mitochondrial fission is controlled by the interaction of 2 proteins: dynamin-related protein 1 (DRP1) and human fission protein 1 (Fis1). Balanced mitochondrial fusion and fission events are beneficial for oxidative phosphorylation and optimal metabolic output in the muscle. Fusion provides a chance for mitochondria for rapid transmission of Ca2+ signals, whereas fission of the mitochondrial network into individual units is necessary for efficient mitophagy to eliminate damaged mitochondria.26 This process also stimulates mitochondrial biogenesis to ensure a healthier pool of functional mitochondria.
Recent research shows that mitochondrial dynamics are controlled by redox signaling. ROS, PGC-1α, forkhead box O (FoxO), and NF-κB have been identified as potential regulators during metabolic disturbance such as exercise.27 H2O2-stimulated Mfn1/2 expression is attenuated in PGC-1α KO C2C12 muscle cells, whereas PGC-1α overexpression enhances Mfn2 mRNA and protein production.28 PGC-1β induces fission protein expression in C2C12 myoblasts and myotubes, and increases the length of mitochondrial tubules.12 An acute bout of prolonged exercise has been shown to increase Fis1 expression but decrease Mfn1/2 in a duration-dependent manner.29 These findings suggest that heavy exercise may lead to a fragmented mitochondrial network, which compromises energy-production efficiency. On the other hand, endurance exercise was reported to increase mRNA levels of Mnf1/2 and DRP1 in rats30 and in human muscle.31,32 However, the effect of training on mitochondrial dynamics is not always predictable. Some studies have shown that chronic exercise promotes Fis1 and Mfn expression in the same direction, suggesting that fission may be required to reorganize the mitochondrial network in response to metabolic demand.33 ROS appear to modulate mitochondrial dynamics, as Mfn2 protein content decreased in leg muscle of trained rats, but the reduction was blocked by PDTC.19 These results indicate that mitochondrial fusion and fission may be an important process underlining the functional adaption to endurance training. Depending on the mode, intensity, and duration of exercise, as well as the species under investigation, the outcome on mitochondrial dynamics may be different.
4. Redox control of mitophagy
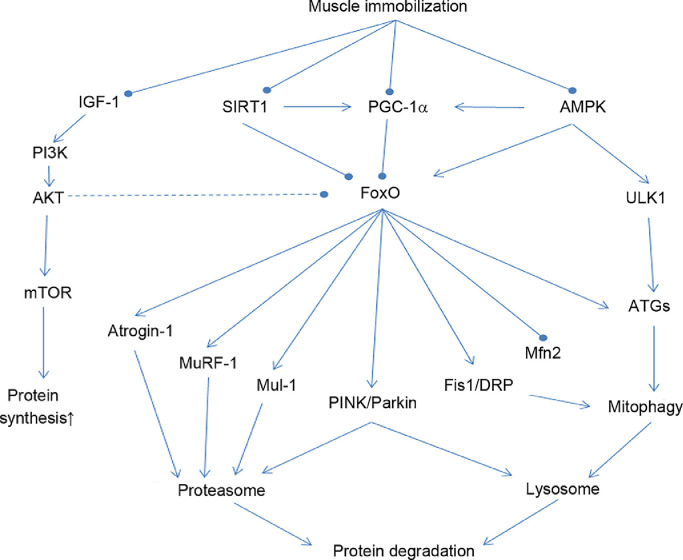
Mitochondrial quality and quantity control (mitostasis) in response to exercise is regulated not only by mitochondrial biogenesis but also by selective degradation of dysfunctional mitochondria that may be caused by ROS and oxidative damage via the autophagy-lysosome pathway (mitophagy).34, 35, 36, 37, 38 Activation of mitophagy may be viewed as a protective mechanism because accumulation of damaged mitochondria may reduce metabolic function of the cell and even stimulate apoptosis by membrane permeabilization and release of cytochrome c, thus activating caspase-3.39 A decline of mitochondrial inner membrane potential (Δψm) may serve as the internal signal during the initial phase of mitophagy, when unc-51 like autophagy activating kinase 1 (ULK1) is activated and recruits Beclin-1 to form a double-membraned phagophore, the preform of autophagosome. Phagophore attracts the binding of microtubule-associated protein 1 light chain 3-II (LC3II), B-cell lymphoma 2/adenovirus E1B 19kDa interacting-protein 3, and the autophagy adaptor protein, p62/sequestosome 1 (hereafter p62) to form autophagosomes, which engulf damaged mitochondria and fuse with lysosome, which contains rich proteases that degrade mitochondrial proteins.40 FoxO family proteins play a central role in activating both the ubiquitin-proteasome pathway and the mitophagy pathway.41 Activation of FoxO can take place when insulin-like growth factor-I/Akt/the mammalian target of rapamycin (IGF/Akt/mTOR) signaling is weakened when muscle is immobilized or when AMPK is activated during strenuous muscle contraction.42 PGC-1α attenuates proteolysis due to its ability to upregulate mitochondrial biogenesis and antioxidant enzymes, reduce ROS and oxidative stress, and inhibit FoxO pathway.43,44 PGC-1α is also known to regulate Mfn2 expression, thus partially controlling mitochondrial fission.45 PGC-1α overexpression with transgenic approach or in vivo transfection has been shown to prevent muscle from catabolic wasting during disuse and aging.23,46,47 Fig. 2 illustrates the role of FoxO in promoting proteolysis and mitophagy as well as factors that regulate FoxO in muscle cells.
Fig. 2.
Role of FoxO signaling in activating ubiquitin-proteolysis and autophagy-lysosomal pathway when skeletal muscle is immobilized. Various factors that activate or inhibit FoxO signaling are also illustrated. Arrow-headed lines represent activation; dot-ended lines represent inhibition. AKT = protein kinase B; AMPK = adenosine monophosphate-activated protein kinase; ATGs = autophagy-related genes; DRP1 = dynamin-related protein 1; Fis1 = mitochondrial fission 1 protein; FoxO = forkhead box transcription factor; IGF-1 = insulin-like growth factor 1; Mfn2 = mitofusin 2; Mul-1 = mitochondrial E3 Ubiquitin protein ligase 1; MuRF-1 = muscle RING-finger protein-1; mTOR = mammalian target of rapamycin; PI3K = phosphoinositide 3-kinase; PGC-1ɑ = peroxisome proliferator-activated receptor γ coactivator 1-α; PINK = phosphatase and tensin homolog-induced kinase; SIRT1 = sirtuin 1; ULK1 = unc-51-like autophagy activating kinase.
An acute bout of exercise has been shown to activate mitophagy in skeletal muscle. The evidence was derived primarily by the observation that LC3I was converted to LC3II by lipidation and that p62 content was reduced.48 This early phase of autophagosome formation requires the activation of AMPK, which phosphorylates ULK1, a major initiator of mitophagy.49 During exercise at high intensity, Parkin accumulation on mitochondrial membrane has been reported, which is presumably recruited by phosphatase and tensin homolog-induced kinase 1 (PINK1), a kinase responsive to membrane damage and reduction of ∆ψm.50 Prolonged exhaustive exercise has also been shown to increase activation of FoxO1 and FoxO3, strong stimulators of mitophagy.41 A number of studies have reported an exercise-induced increase in autophagy and mitophagy;51 however, these studies shared a common limitation: they report mainly changes in protein content of various autophagy pathway components shortly after exercise, whereas the flux of autophagy during exercise remains largely unknown.51 It is noteworthy that the effect of chronic exercise training on mitophagy may appear different from that of acute exercise; several studies have shown a decrease of mitophagy flux after endurance training.48,52,53 Training is known to improve mitochondrial morphologic and functional integrity with less damage, so the signals to activate mitophagy may be lessened in the trained muscle. Additionally, training induces mitochondrial and cellular antioxidant defense systems, resulting in a more stable redox status, which also softens the redox-sensitive signals for the mitophagy pathway.
5. Redox adaptation of uncoupling proteins (UCPs)
Mitochondrial production of ROS is partly dependent on the cross-membrane proton motive force (Δψm). UCPs are a heterogeneous family of proteins that partially dissipate the proton electrochemical gradient, thereby reducing ROS production.54 Several members of the UCP family are found in the brown adipose tissue of rodents for adaptive thermogenesis (UCP1), in the skeletal muscle (UCP3), and elsewhere in the body (UCP2). UCP3 appears to be a potential regulator of transmembrane proton potential to influence oxidative phosphorylation during muscle contraction.55 The exact implication of and mechanism for exercise-induced UCP3 are still elusive. UCP3 gene expression was increased by an acute bout of exercise in mouse muscle, and the upregulation depended on mitochondrial H2O2 production.30,56 Although H2O2 stimulated UCP3 in wild type muscle cells, PGC-1α in KO abolished these effects.28 Thus, UCP gene expression seems to be redox sensitive, and PGC-1α could be an important mediator in the upregulation of UCP3. To explore the physiological significance of UCP3 upregulation, Chen et al.50 examined the time course of UCP3 level along with mitochondrial respiration and ROS production during a prolonged bout of exercise in rat skeletal muscle. The increase of UCP3 mRNA and protein peaked at 150 min, when a reduction of ROS production and state 4 respirations in the mitochondria were observed. In the UCP3-/- mice, exercise failed to increase mitochondrial uncoupling respiration.56 The authors suggest that control of UCP3 expression may shunt protons outside the inner membranes, thereby decreasing Δψm and ROS production to protect mitochondria from oxidative stress.
6. Redox signaling of antioxidant enzymes
An abundance of literature has reported muscle antioxidant adaptation to chronic exercise training.2,3 Mitochondrial SOD (SOD2) demonstrates the most predictable and robust upregulation, showing increases in both protein content and activity. Furthermore, an acute bout of exercise can stimulate the mRNA levels of SOD2, suggesting a transcriptional activation of its gene expression.57 Gluthathione peroxidase activity also responds to endurance training, but it is not clear whether the increased activity is entirely due to enzyme protein increase or post-translational modification. ROS seem to be a required molecular signal for exercise-induced SOD2 upregulation in muscle, whereas the initial studies revealed that NF-κB and MAPK signaling pathways are heavily involved.57,58 Inhibition of NF-κB with PDTC severely diminished the inhibitor of nuclear factor κB (IκB)-induced kinase (IKK) activation, IκB phosphorylation, and p50 nuclear accumulation in response to exercise, while allopurinol, the inhibitor of xanthine oxidase, abolished exercise-activated NF-κB, extracellular signal-regulated kinases 1/2 (ERK1/2), and p38 signaling during sprinting in rats.58 Importantly, allopurinol partially blocked the exercise-induced mRNA of SOD2 and induced nitric oxide synthase, both enzymes requiring NF-κB signaling. These studies emphasized the critical role of mitochondria in modulating antioxidant defence that directly affect ROS production and intracellular redox status, which can impact on all redox signaling pathways in the cell.3
Recent research strongly suggests that, as the master regulator of mitochondrial function, PGC-1α also controls antioxidant enzyme expression in the mitochondria. PGC-1α KO fibroblasts exhibit a decrease in SOD2, catalase, and GPx1 mRNA contents compared to wild type fibroblasts,28 whereas reduced mRNA levels of SOD1, SOD2, and GPx1, as well as SOD2 content, were observed in skeletal muscle from PGC-1α KO mice.21 However, mice with PGC-1α overexpression showed higher SOD2 levels in both young and aged muscles. Furthermore, PGC-1α promotes SIRT gene expression, which deacetylates and activates mitochondrial enzymes, including SOD2.59 In immobilized (IM) skeletal muscle, local transfection of PGC-1α DNA was shown to boost SOD2 expression and recover SOD2 activity due to SIRT3 upregulation and deacetylation.23 Significantly, local overexpression of PGC-1α was capable of restoring age-associated loss of SOD2 protein content and activity, as well as stimulating catalase and GPx1 expression in old mice.60 The molecular mechanism by which PGC-1α regulates antioxidant gene expression is still under heavy investigation, but recent research in this area demonstrates that in C2C12 muscle cells, SOD2 gene promotor contains several PPAR receptor elements (PPREs) that are normally occupied by NCoR1, a conserved repressor of SOD2 transactivation.22,61 An increase in intracellular PGC-1α can overcome PPREs binding by NcOR1, allowing SOD2 mRNA to be transcribed. Although it is still unclear how cellular redox status may affect the exchange of PGC-1α and NcOR1, this finding has provided us with significant insight into the ways in which muscle responds to oxidative stress during exercise.
7. The role of mitochondria in muscle immobilization atrophy
Muscle atrophy caused by prolonged IM is characterized by decreased muscle fiber cross-sectional area, reduced force production, increased fatigability, and insulin resistance.62 Atrophy may result from a decreased protein synthesis or an increased protein degradation, or both.63 Recent research indicates that IM-induced protein degradation can be activated by 2 separate but related redox signaling pathways: the ubiquitin-proteolysis and the autophagy-lysosomal pathway.63 During muscle IM, the signaling of IGF1/Akt/mTOR axis is withdrawn, causing FoxO dephosphorylation and nuclear sequestration. FoxO is the most important activator of both of the above-mentioned catabolic pathways (Fig. 2).41 Two weeks of hindlimb IM in mice has been shown to enhance the mRNA and protein levels of Atrogin-1 and muscle RING-finger protein-1, which target at both regulatory (e.g., calcineurin and myogenic differentiation 1) and structural (e.g., myosin and troponin I) proteins, along with elevated dephosphorylated/phosphorylated FoxO1/3 ratio.23,64,65 FoxO also appears to promote mitophagy-related protein expression, with increased PINK1, Parkin, and LC3II levels. Importantly, mitochondrial ubiquitination was enhanced, and total mitochondrial density decreased by 50% in the IM muscle. In addition, mitochondrial quality was severely compromised, indicated by decreases of citric synthase and cytochrome c oxidase activities, along with a dramatic decline of ATP production rate (per unit of mitochondrial protein).23
A downregulation of PGC-1α was observed in muscle atrophy of different models and was thought to be a major molecular mechanism for enhanced FoxO phosphorylation, NF-κB activation, and protein loss.63 PGC-1α plays a protective role against protein catabolism and muscle wasting, with a variety of ramifications. For instance, denervation-induced muscle atrophy and the effects of Duchenne muscular dystrophy are ameliorated when the PGC-1α level is preserved or elevated.66 Inactivity-induced deficit of PGC-1α in skeletal muscle results in a chronic systemic inflammatory state, evidenced by increased tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) expression and by NF-κB activation.21 However, in the absence of chronic disease, despite NF-κB activation, overproduction of proinflammatory cytokines, and oxidative stress, inflammation does not consistently lead to muscle atrophy.67 On the other hand, PGC-1α overexpression via local transfection was demonstrated to preserve mitochondrial volume and function and to reverse atrophy-associated inflammation.23 These findings highlight the critical role of mitochondria in the preservation of protein balance and prevention of muscle atrophy.
Recent literature has revealed that mitophagy may be activated during muscle IM and contribute to the loss of muscle mass.67 Activation of the FoxO pathway stimulates the PINK1-Mfn2-Parkin axis that facilitates the removal of damaged mitochondria to maintain a healthier mitochondrial pool. However, increased mitophagy during IM may be a double-edged sword; removal of damaged mitochondria maintains a smaller but more intact mitochondrial population but in the short term sacrifices mitochondrial volume. In a recent study, we have shown that 2 weeks of IM increased both PINK1 and Parkin expression several-fold, whereas the mitochondrial ubiquitin ligase mitochondrial E3 ubiquitin protein ligase 1 level was dramatically elevated.23,64 The Mfn2 level plunged, but optic atrophy protein 1 expression increased in the IM muscle. Importantly, ubiquitinated Mfn2 and mitochondrial contents more than doubled with IM. After in vivo transfection of PGC-1α, tibialis anterior muscle overexpressing PGC-1α showed decreases in PINK1, Parkin, and LC3II protein levels.64 In the above study, the role of AMPK, a strong activator of FoxO, was not evaluated; but SIRT levels were upregulated by PGC-1α transfection. SIRT1 is known to regulate the activity of NFκB and FoxO, the key controllers for proinflammatory cytokine expression and mitophagy.68 SIRT1 deacetylates and activates PGC-1α, perceivably forming a feed-forward cycle to inhibit FoxO. Furthermore, SIRT3 deacetylates SOD2 in the mitochondria, thus controlling ROS emmision.69 Therefore, the role of SIRTs in PGC-1α-induced mitochondrial biogenesis and inhibition of mitophagy should not be underestimated in muscle IM atrophy.
8. The role of redox signaling in muscle inflammation
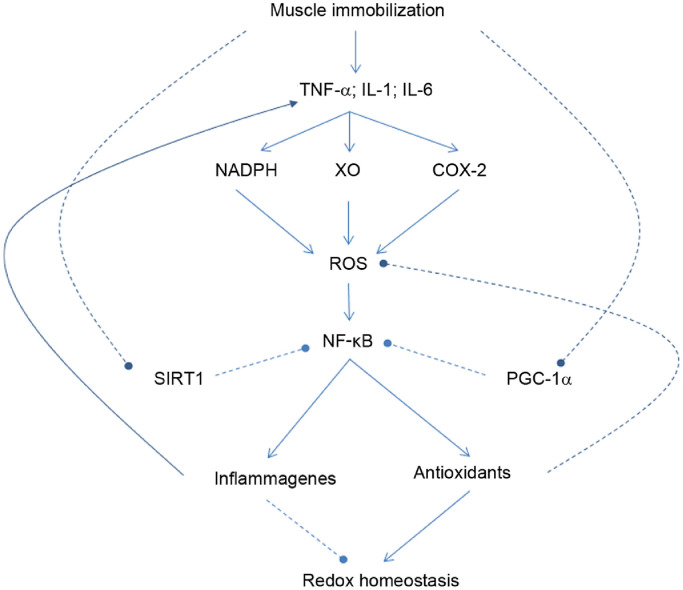
Inflammation represents a pathophysiologic state that substantially alters cellular oxidative-antioxidant homeostasis. During inflammation, ROS production is boosted by the action of certain enzymes, such as nicotinamide adenine dinucleotide phosphate hydrogen oxidase (NADPH), cyclooxygenase-2, and xanthine oxidase, which activate NF-κB, the primary redox-sensitive signaling pathway for inflammation (Fig. 3).70,71 Exercise at moderate intensity does not usually stimulate NF-κB activation because ROS are mostly removed by cellular antioxidant systems. Furthermore, elevated PGC-1α is known to inhibit p65 DNA binding.59 Moreover, SIRT1 activation during muscle contraction deacetylates p65, preventing its nuclear entry and binding.72 However, if exercise intensity is high or if exercise has a significant component of muscle-lengthening contraction, such as in downhill running and heavy weightlifting, local myofibril injury may activate NAPDH oxidase to produce superoxide radicals (O2.−) and, consequently, other ROS, including NO, H2O2, and hypochlorous acid. Elevated plasma levels of TNF-α may also stimulate mitochondria to produce O2.− and H2O2.73 Activation of PKC and NF-κB-induced kinase, a member of the MAPK family, is known to activate IKK, which phosphorylates the IκB submit of NF-κB complex and unleashes p50/p65 for nuclear binding.70,74 A direct and significant outcome is the enhanced gene expression of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 from immune cells and/or damaged muscle tissues. Proinflammatory cytokines also promote the expression of adhesion molecules, such as vascular cell adhesion molecule 1 (VCAM-1), cytokine-induced neutrophil chemoattractant-1 (CINC-1), and monocyte chemoattractant protein-1 (MCP-1), as well as NOS expression.75 The increased blood flow due to NO production and the chemotactic effects of adhesion molecules facilitate polymorphonutrophil and circulating cytokines to infiltrate into the affected area. Elevated proinflammatory cytokines inevitably affect mitochondrial ROS generation and metabolic function. In a study conducted by Liao et al.,73 rats subjected to downhill running (−10%) with increasing duration demonstrated progressive increases in muscle TNF-α concentration and myeloperoxidase activity, a clear indication of inflammation. Meanwhile, there was a steady decrease of mitochondrial aconitase activity, whereas SOD2 activity was not altered.
Fig. 3.
Role of NFκB signaling in causing inflammation and loss of redox homeostasis in skeletal muscle cells. Arrow-headed lines represent activation; dot-ended lines represent inhibition. COX-2 = cyclooxygenase-2; IL = interleukin; NADPH = nicotinamide adenine dinucleotide phosphate hydrogen; NF-κB = nuclear factor-κB; PGC-1ɑ = peroxisome proliferator-activated receptor γ coactivator 1-α; ROS = reactive oxygen species; SIRT1 = sirtuin 1; TNF-α = tumor necrosis factor-α; XO = xanthine oxidase.
Recent research indicates that mitochondria are involved in the inflammatory process and that PGC-1α plays a role as an anti-inflammatory agent. Studies in PGC-1α KO animals demonstrate that PGC-1α modulates local or systemic inflammation by regulating the expression of proinflammatory cytokines, such as TNF-α and IL-6.76 PGC-1α KO mice showed higher basal mRNA expression of TNF-α and IL-6 in skeletal muscle, as well as higher serum IL-6 levels.13 In addition, PGC-1α overexpressed mice showed lower expression of TNF-α and IL-6.21 These data suggest that PGC-1α plays a protective role in inflammatory response by reducing proinflammatory cytokine production. Moreover, a single exercise bout increased skeletal muscle TNF-α mRNA and serum TNF-α content in PGC-1α KO mice but not in wild type mice.13 When mouse hindlimb muscle was immobilized followed by remobilization, clear signs of inflammation were observed in the muscle, such as NF-κB activation, increased mitochondrial H2O2 production, and elevated TNF-α and IL-6 contents. These responses were largely abolished when the muscle was transfected with PGC-1α.23 These findings echoed an earlier study showing that PGC-1α transgenic mice had lower muscle proinflammatory cytokine production and oxidative damage.21
9. Conclusion
Evolution has turned ROS into an essential component of cellular life through redox signaling. In skeletal muscle, contraction-induced ROS production underscores numerous adaptations in response to exercise, as documented during the past half century. Muscle contraction can dramatically change the balance between ROS production and antioxidant defense, often resulting in a small surplus of ROS, which activates several signal transduction pathways including, but not limited to, NF-κB, MAPK, PGC-1α, FoxO, and SIRT. Exercise at appropriate intensity and duration stimulates PGC-1α signaling that promotes mitochondrial biogenesis and antioxidant adaptation, whereas rigorous exercise, especially eccentric contraction, can lead to hyperactivation of NF-κB and FoxO, promoting ubiquitin proteolysis and mitophagy, as well as stimulating inflammation. These catabolic signaling pathways are also activated in IM muscle. Mitochondrial homeostasis (mitostasis) is regulated by mitochondrial biogenesis, fusion and fission dynamics, and mitophagy; and their morphological and functional integrity is essential for muscle performance during exercise. PGC-1α is a master mitochondrial regulator that exerts profound influences on many other redox signaling pathways. Experimental overexpression of PGC-1α has already demonstrated positive effects on muscle contractile function, protein preservation, mitochondrial stability, and resistance to oxidative stress and inflammation. We hope that future research will continue to explore the mechanism and application of improved mitostasis on a number of health issues, such as cancer cachexia, muscle disuse atrophy, and sarcopenia.
Acknowledgments
Authors' contributions
LLJ elaborated the plan of the article, reviewed the literature, and drafted the manuscript; DY conducted the literature review and drafted the manuscript; CK and TZ helped draft the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Holloszy J.O., Coyle E.F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 2.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji L.L. Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol. 2007;42:582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Yun J., Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers S.K., Nelson W.B., Hudson M.B. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picca A., Lezza A.M.S., Leeuwenburgh C., Pesce V., Calvani R., Bossola M. Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenat Res. 2018;21:350–359. doi: 10.1089/rej.2017.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji L.L., Kang C., Zhang Y. Exercise-induced hormesis and skeletal muscle health. Free Radic Biol Med. 2016;98:113–122. doi: 10.1016/j.freeradbiomed.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Ji L.L., Kang C. Role of PGC-1α in sarcopenia: etiology and potential intervention: a mini-review. Gerontology. 2015;61:139–148. doi: 10.1159/000365947. [DOI] [PubMed] [Google Scholar]
- 10.Hood D.A., Tryon L.D., Carter H.N., Kim Y., Chen C.C. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J. 2016;473:2295–2314. doi: 10.1042/BCJ20160009. [DOI] [PubMed] [Google Scholar]
- 11.Palikaras K., Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol. 2014;56:182–188. doi: 10.1016/j.exger.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Handschin C., Spiegelman B.M. The role of exercise and PGC1α in inflammation and chronic disease. Nature. 2008;454:463−9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handschin C. Peroxisome proliferator-activated receptor-γ coactivator-1α in muscle links metabolism to inflammation. Clin Exp Pharmacol Physiol. 2009;36:1139–1143. doi: 10.1111/j.1440-1681.2009.05275.x. [DOI] [PubMed] [Google Scholar]
- 14.Baar K., Wende A.R., Jones T.E., Marison M., Nolte L.A., Chen M. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 15.Liesa M., Palacín M., Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 16.Durham W.J., Arbogast S., Gerken E., Li Y.P., Reid M.B. Progressive nuclear factor-κB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve. 2006;34:298–303. doi: 10.1002/mus.20579. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 18.Feng H., Kang C., Dickman J.R., Koenig R., Awoyinka I., Zhang Y. Training-induced mitochondrial adaptation: role of peroxisome proliferator‐activated receptor γ coactivator-1α, nuclear factor-κB and β‐blockade. Exp Physiol. 2012;98:784–795. doi: 10.1113/expphysiol.2012.069286. [DOI] [PubMed] [Google Scholar]
- 19.Derbré F., Gomez-Cabrera M.C., Nascimento A.L., Sanchis-Gomar F., Martinez-Bello V.E., Tresguerres J.A. Age-associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age. 2012;34:669–679. doi: 10.1007/s11357-011-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanza I.R., Short D.K., Short K.R., Raghavakaimal S., Basu R., Joyner M.J. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanai J., Cao P., Tanksale P., Imamura S., Koshimizu E., Zhao J. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto H., Williams E.G., Mouchiroud L., Cantó C., Fan W., Downes M. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147:827–839. doi: 10.1016/j.cell.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang C., Goodman C.A., Hornberger T.A., Ji L.L. PGC-1α overexpression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. FASEB J. 2015;29:4092–4106. doi: 10.1096/fj.14-266619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang C., O'Moore K.M., Dickman J.R., Ji L.L. Exercise activation of muscle peroxisome proliferator-activated receptor-γ coactivator-1α signaling is redox sensitive. Free Radic Biol Med. 2009;47:1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Hom J., Sheu S.S. Morphological dynamics of mitochondria: a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009;46:811–820. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandemange S., Herzig S., Martinou J.C. Mitochondrial dynamics and cancer. Semin Cancer Biol. 2009;19:50–56. doi: 10.1016/j.semcancer.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Koopman W.J., Verkaart S., van Emst-de Vries S.E., Grefte S., Smeitink J.A., Nijtmans L.G. Mitigation of NADH: ubiquinone oxidoreductase deficiency by chronic Trolox treatment. Biochim Biophys Acta. 2008;1777:853–859. doi: 10.1016/j.bbabio.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Yu T., Robotham J.L., Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jäger S. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Ding H., Jiang N., Liu H., Liu X., Liu D., Zhao F. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800:250–256. doi: 10.1016/j.bbagen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Garnier A., Fortin D., Zoll J., N'Guessan B., Mettauer B., Lampert E. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005;19:43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 32.Bo H., Zhang Y., Ji L.L. Redefining the role of mitochondria in exercise: a dynamic remodeling. Ann N Y Acad Sci. 2010;1201:121–128. doi: 10.1111/j.1749-6632.2010.05618.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y., Triolo M., Hood D.A. Impact of aging and exercise on mitochondrial quality control in skeletal muscle. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/3165396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonaldo P., Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25−39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 36.Lokireddy S., Wijesoma I.W., Teng S., Bonala S., Gluckman P.D., McFarlane C. The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab. 2012;16:613–624. doi: 10.1016/j.cmet.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Bodine S.C., Baehr L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Green D.R., Van Houten B. SnapShot: mitochondrial quality control. Cell. 2011;147:950. doi: 10.1016/j.cell.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez A.M., Candau R.B., Bernardi H. FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci. 2014;71:1657–1671. doi: 10.1007/s00018-013-1513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greer E.L., Oskoui P.R., Banko M.R., Maniar J.M., Gygi M.P., Gygi S.P. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 43.Sandri M., Lin J., Handschin C., Yang W., Arany Z.P., Lecker S.H. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackman R.W., Kandarian S.C. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 45.James D.I., Parone P.A., Mattenberger Y., Martinou J.C. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 46.Kang C., Ji L.L. PGC-1α overexpression via local transfection attenuates mitophagy pathway in muscle disuse atrophy. Free Radic Biol Med. 2016;93:32–40. doi: 10.1016/j.freeradbiomed.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 47.Kang C., Ji L.L. Muscle immobilization and remobilization downregulates PGC-1α signaling and the mitochondrial biogenesis pathway. J Appl Physiol (1985) 2013;115:1618–1625. doi: 10.1152/japplphysiol.01354.2012. [DOI] [PubMed] [Google Scholar]
- 48.Guan Y., Drake J.C., Yan Z. Exercise-induced mitophagy in skeletal muscle and heart. Exerc Sport Sci Rev. 2019;47:151–156. doi: 10.1249/JES.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laker R.C., Drake J.C., Wilson R.J., Lira V.A., Lewellen B.M., Ryall K.A. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8:548. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C.C.W., Erlich A.T., Crilly M.J., Hood D.A. Parkin is required for exercise-induced mitophagy in muscle: impact of aging. Am J Physiol Endocrinol Metab. 2018;315:E404–E415. doi: 10.1152/ajpendo.00391.2017. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez A.M., Candau R., Bernardi H. Recent data on cellular component turnover: focus on adaptations to physical exercise. Cells. 2019;8:E542. doi: 10.3390/cells8060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carter H.N., Kim Y., Erlich A.T., Zarrin-Khat D., Hood D.A. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J Physiol. 2018;596:3567–3584. doi: 10.1113/JP275998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C.C.W., Erlich A.T., Hood D.A. Role of Parkin and endurance training on mitochondrial turnover in skeletal muscle. Skelet Muscle. 2018;8:10. doi: 10.1186/s13395-018-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams V., Nehrhoff B., Späte U., Linke A., Schulze P.C., Baur A. Induction of iNOS expression in skeletal muscle by IL-1β and NFκB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 55.Krauss S., Zhang C.Y., Lowell B.B. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 56.Jiang N., Zhang G., Bo H., Qu J., Ma G., Cao D. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: a potential antioxidant function. Free Radic Biol Med. 2009;46:138–145. doi: 10.1016/j.freeradbiomed.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Cabrera M.C., Borrás C., Pallardo F.V., Sastre J., Ji L.L., Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartoni R., Léger B., Hock M.B., Praz M., Crettenand A., Pich S. Mitofusins 1/2 and ERRα expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 60.Yeo D., Kang C., Gomez-Cabrera M.C., Vina J., Ji L.L. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1alpha overexpression in vivo. Free Radic Biol Med. 2019;130:361–368. doi: 10.1016/j.freeradbiomed.2018.10.456. [DOI] [PubMed] [Google Scholar]
- 61.Pérez -Schindler J., Summermatter S., Salatino S., Zorzato F., Beer M., Balwierz P.J. The corepressor NCoR1 antagonizes PGC-1α and estrogen-related receptorα in the regulation of skeletal muscle function and oxidative metabolism. Mol Cell Biol. 2012;32:4913–4924. doi: 10.1128/MCB.00877-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irrcher I., Ljubicic V., Kirwan A.F., Hood D.A. AMP-activated protein kinase-regulated activation of the PGC-1α promoter in skeletal muscle cells. PLoS One. 2008;3:e3614. doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kandarian S.C., Jackman R.W. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 64.Kang C., Yeo D., Ji L.L. Muscle immobilization activates mitophagy and disrupts mitochondrial dynamics in mice. Acta Physiol (Oxf) 2016;218:188–197. doi: 10.1111/apha.12690. [DOI] [PubMed] [Google Scholar]
- 65.Marzetti E., Calvani R., Cesari M., Buford T.W., Lorenzi M., Behnke B.J. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whidden M.A., McClung J.M., Falk D.J., Hudson M.B., Smuder A.J., Nelson W.B. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol (1985) 2009;106:385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hunter R.B., Stevenson E., Koncarevic A., Mitchell-Felton H., Essig D.A., Kandarian S.C. Activation of an alternative NF-κB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 68.Olmos Y., Sánchez-Gómez F.J., Wild B., García-Quintans N., Cabezudo S., Lamas S. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tao R., Coleman M.C., Pennington J.D., Ozden O., Park S.H., Jiang H. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh S., Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109(Suppl. 1):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 71.Jiang B., Xu S., Hou X., Pimentel D.R., Brecher P., Cohen R.A. Temporal control of NF-κB activation by ERK differentially regulates interleukin-1β-induced gene expression. J Biol Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 72.Dali‐Youcef N., Lagouge M., Froelich S., Koehl C., Schoonjans K., Auwerx J. Sirtuins: the magnificent seven, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 73.Liao P., Zhou J., Ji L.L., Zhang Y. Eccentric contraction induces inflammatory responses in rat skeletal muscle: role of tumor necrosis factor-α. Am J Physiol Regul Integr Comp Physiol. 2010;298:R599–R607. doi: 10.1152/ajpregu.00480.2009. [DOI] [PubMed] [Google Scholar]
- 74.Li Q., Engelhardt J.F. Interleukin-1β induction of NFκB is partially regulated by H2O2-mediated activation of NFκB-inducing kinase. J Biol Chem. 2006;281:1495–1505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- 75.Luo G., Hershko D.D., Robb B.W., Wray C.J., Hasselgren P.O. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-κB. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1249–R1254. doi: 10.1152/ajpregu.00490.2002. [DOI] [PubMed] [Google Scholar]
- 76.Aoi W., Naito Y., Takanami Y., Kawai Y., Sakuma K., Ichikawa H. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic Biol Med. 2004;37:480–487. doi: 10.1016/j.freeradbiomed.2004.05.008. [DOI] [PubMed] [Google Scholar]