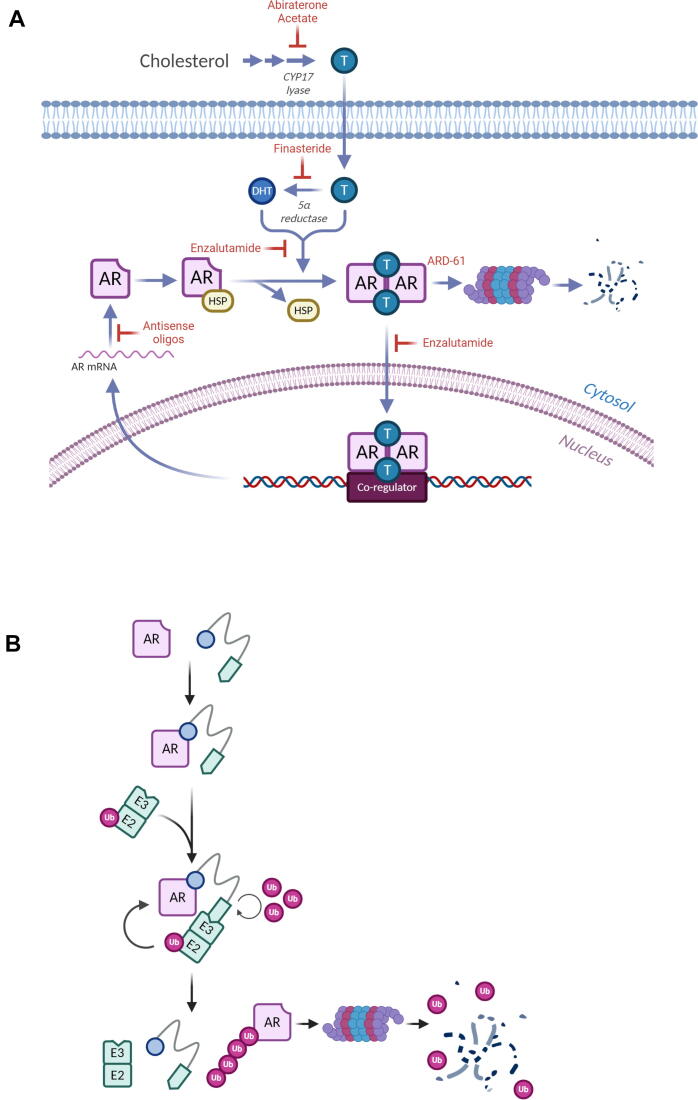
Targeting of the androgen receptor (AR) in prostate cancer has led to a significant decrease in mortality and has been an effective strategy in men with prostate cancer [1]. This success has led to evaluation of AR targeting in other cancers, including brain and breast cancers [2], [3], [4], [10]. More recently, AR has been of increasing interest as a therapeutic target in breast cancer as AR is expressed in 70–90% of estrogen receptor-positive (ER+) breast cancers and approximately 15–30% of triple negative breast cancers (TNBCs) [5]. A variety of inhibitors targeting androgen production and AR signaling have been developed for the treatment of prostate cancer and repurposed for use in breast cancer, including commonly used therapeutics like abiraterone acetate, a CYP17-lyase inhibitor, as well as bicalutamide and enzalutamide, first- and second-generation anti-androgens, respectively (Fig. 1A). More recently, additional drugs, including apalutamide and darolutamide have been approved for use in treatment of non-metastatic castration resistant prostate cancer. These drugs, along with the development of novel compounds and the use of anti-sense oligonucleotides, continue to expand the armamentarium of AR inhibitors available for use.
Fig. 1.
(A) Production of androgens and androgen receptor signaling can be inhibited at various steps using pharmacologic inhibitors, anti-sense oligonucleotides, and PROTACs. (B) PROTACs take advantage of the cell’s ubiquitination machinery to direct target proteins like the androgen receptor (AR) for proteasomal degradation.
Recently, PROteolysis TArgeting Chimera (PROTAC) inhibitors have become a new method of targeting proteins involved in cancer progression by promoting their degradation [6]. PROTAC inhibitors, like ARD-61, use a crosslinker to connect a binding moiety for a protein of interest (POI) to an E3 ligase targeting moiety. Following binding to the POI, the E3 ligase and ubiquitination machinery are recruited, putting the POI in close proximity to the ubiquitination machinery. This results in ubiquitination of the POI and degradation by the proteasome (Fig. 1B). PROTAC inhibitors are highly specific, and unlike traditional inhibitors, PROTACs have the ability to inhibit all protein functions due to the removal of the POI from the cell. The development of PROTAC inhibitors has also been shown to be effective for the degradation of AR in prostate cancer in vitro and in vivo [7], [8], [9]. While patients almost always develop resistance to anti-androgens through various mechanisms that include AR amplification, aberrant post translational modification, bypass of receptor signaling, synthesis of intratumoral androgens, or mutations to AR, degradation of protein by PROTACs has been shown to be effective even in enzalutamide-resistant models of prostate cancer [8]. Therefore, expanded use of PROTAC inhibitors may be effective as a second-line of therapy in AR+ cancers that are resistant to first line AR-targeting therapies.
In this issue, Zhao et al. expands the use of ARD-61 outside of prostate cancer and demonstrates its preclinical utility in a panel of breast cancer cell lines. ARD-61 effectively degrades AR protein at nanomolar concentrations in AR+ breast cancer cell lines, including MDA-MB-453, MCF-7, BT-549, HCC-1428, and MDA-MB-415 cells. The authors demonstrate that ARD-61 is able to degrade both the androgen and progesterone receptors (PR) in T47D and BT-474 cells but does not change levels of the estrogen or glucocorticoid receptors. Further, ARD-61 is more effective than enzalutamide at inhibiting tumor cell growth both in vitro and in vivo in an MDA-MB-453 xenograft model. Treatment with ARD-61 results in an increase in G2/M cell cycle arrest and increased apoptosis while also blocking downstream AR signaling. These findings support the continued development of PROTACs targeting AR for clinical use of AR+ breast cancers.
The development of ARD-61 represents an exciting opportunity to expand the use of an AR PROTAC in breast cancer and adds to a growing list of targeted therapies that may be effective in cancers that rely on androgen receptor signaling. Unlike other strategies, however, the ability of ARD-61 to inhibit both AR and PR is an interesting characteristic of the drug and warrants further investigation into the role of PR in AR+ breast cancers. Further, the role of AR and the effectiveness of AR inhibition in ER+ breast cancers continue to be areas of active investigation. ARD-61 will be a useful tool both for understanding the role of AR in breast cancer and for its potential clinical utility for the subsequent treatment of AR+ breast cancers. This study adds to a growing list of therapeutic options for patients whose cancers are driven by AR and may allow for a broadened indication in men and women with AR+ breast cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Carceles-Cordon M., Kelly W.K., Gomella L., Knudsen K.E., Rodriguez-Bravo V., Domingo-Domenech J. Cellular rewiring in lethal prostate cancer: the architect of drug resistance. Nat Rev Urol. 2020;17:292–307. doi: 10.1038/s41585-020-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speers C., Zhao S.G., Chandler B., Liu M., Wilder-Romans K., Olsen E., Nyati S., Ritter C., Alluri P.G., Kothari V., Hayes D.F., Lawrence T.S., Spratt D.E., Wahl D.R., Pierce L.J., Feng F.Y. Androgen receptor as a mediator and biomarker of radioresistance in triple-negative breast cancer. NPJ Breast Cancer. 2017;3:29. doi: 10.1038/s41523-017-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannelli P., Di Donato M., Galasso G., Di Zazzo E., Bilancio A., Migliaccio A. The androgen receptor in breast cancer. Front Endocrinol (Lausanne) 2018;9 doi: 10.3389/fendo.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni M., Chen Y., Lim E., Wimberly H., Bailey S.T., Imai Y., Rimm D.L., Shirley Liu X., Brown M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119–131. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins L.C., Cole K.S., Marotti J.D., Hu R., Schnitt S.J., Tamimi R.M. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24:924–931. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X., Gao H., Yang Y., He M., Wu Y., Song Y., Tong Y., Rao Y. PROTACs: great opportunities for academia and industry. Signal Transduct Target Ther. 2019;4:1–33. doi: 10.1038/s41392-019-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X., Zhao L., Xiang W., Qin C., Miao B., Xu T., Wang M., Yang C.-Y., Chinnaswamy K., Stuckey J., Wang S. Discovery of highly potent and efficient PROTAC degraders of androgen receptor (AR) by employing weak binding affinity VHL E3 ligase ligands. J Med Chem. 2019;62:11218–11231. doi: 10.1021/acs.jmedchem.9b01393. [DOI] [PubMed] [Google Scholar]
- 8.Kregel S., Wang C., Han X., Xiao L., Fernandez-Salas E., Bawa P., McCollum B.L., Wilder-Romans K., Apel I.J., Cao X., Speers C., Wang S., Chinnaiyan A.M. Androgen receptor degraders overcome common resistance mechanisms developed during prostate cancer treatment. Neoplasia. 2020;22:111–119. doi: 10.1016/j.neo.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han X., Wang C., Qin C., Xiang W., Fernandez-Salas E., Yang C.-Y., Wang M., Zhao L., Xu T., Chinnaswamy K., Delproposto J., Stuckey J., Wang S. Discovery of ARD-69 as a highly potent proteolysis targeting chimera (PROTAC) degrader of androgen receptor (AR) for the treatment of prostate cancer. J Med Chem. 2019;62:941–964. doi: 10.1021/acs.jmedchem.8b01631. [DOI] [PubMed] [Google Scholar]
- 10.Werner C.K., Nna U.J., Sun H., Wilder-Romans K., Dresser J., Kothari A.U., Zhou W., Yao Y., Rao A., Stallard S., Koschmann C., Bor T., Debinski W., Hegedus A.M., Morgan M.A., Venneti S., Baskin-Bey E., Spratt D.E., Colman H., Sarkaria J.N., Chinnaiyan A.M., Eisner J.R., Speers C., Lawrence T.S., Strowd R.E., Wahl D.R. Expression of the androgen receptor governs radiation resistance in a subset of glioblastomas vulnerable to anti-androgen therapy. Mol Cancer Ther. 2020 doi: 10.1158/1535-7163.MCT-20-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]