Highlights
-
•
Muscular contractions stimulate reactive oxygen species production in active muscle fibers and skeletal muscles are a primary source of reactive oxygen species production during exercise.
-
•
Prolonged or high intensity exercise can result in both: (1) oxidative damage in skeletal muscle fibers and (2) accelerated muscle fatigue.
-
•
Exercise-induced increases in the production of reactive oxygen species in skeletal muscle plays a required role in skeletal muscle adaptation to endurance training.
-
•
Based on the available evidence, it appears unlikely that rigorous and prolonged exercise results in an oxidative stress level that is detrimental to human health.
Keywords: Hormesis, Oxidants, Radicals, Reactive oxygen species, Skeletal muscle
Abstract
The first report demonstrating that prolonged endurance exercise promotes oxidative stress in humans was published more than 4 decades ago. Since this discovery, many ensuing investigations have corroborated the fact that muscular exercise increases the production of reactive oxygen species (ROS) and results in oxidative stress in numerous tissues including blood and skeletal muscles. Although several tissues may contribute to exercise-induced ROS production, it is predicted that muscular contractions stimulate ROS production in active muscle fibers and that skeletal muscle is a primary source of ROS production during exercise. This contraction-induced ROS generation is associated with (1) oxidant damage in several tissues (e.g., increased protein oxidation and lipid peroxidation), (2) accelerated muscle fatigue, and (3) activation of biochemical signaling pathways that contribute to exercise-induced adaptation in the contracting muscle fibers. While our understanding of exercise and oxidative stress has advanced rapidly during the last decades, questions remain about whether exercise-induced increases in ROS production are beneficial or harmful to health. This review addresses this issue by discussing the site(s) of oxidant production during exercise and detailing the health consequences of exercise-induced ROS production.
1. Introduction
The production of high levels of reactive oxygen species (ROS) in cells promotes redox disturbances leading to oxidative damage to cellular components. Indeed, it is clear that chronic oxidative damage is associated with the pathogenesis of cancer, cardiovascular disease, diabetes mellitus, hypertension, and several neurodegenerative diseases.1, 2, 3, 4
Interestingly, while regular physical activity promotes health benefits, rigorous and/or prolonged exercise results in an acute increase in the production of ROS as evidenced by elevated biomarkers of oxidative damage in both blood and skeletal muscles.5 The fact that muscular exercise promotes ROS production appears enigmatic because regular exercise is the only health behavior associated with a decrease in all-cause mortality in humans.6 This review addresses this exercise/oxidative stress paradox by discussing the cellular consequences of exercise-induced ROS production. We begin with a review of the primary oxidants produced in cells followed by a summary of cellular antioxidant systems. We then discuss the sources of ROS production during exercise and debate the question of whether exercise-induced ROS production is beneficial or harmful to health.
2. Redox balance and oxidative stress
The term “oxidative stress” was first defined by Helmut Sies7 as “a disturbance in the pro-oxidant/antioxidant balance in favor of the former”. Although this simple definition has been widely applied in the literature for several decades, this account of oxidative stress has been criticized because of the lack of detail. In particular, given that cellular redox balance is complex, it has been argued that although the term “oxidative stress” does encompass a pro-oxidant vs. antioxidant condition in the cell, this definition does not provide sufficient details about the nature of the cellular redox imbalance. To address this concern, Helmut Sies collaborated with Dean Jones to generate a more detailed definition that defines oxidative stress as “an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control, and/or molecular damage”.8 We have adopted this definition of oxidative stress for this review.
3. Biological radicals—an overview
The observation that living cells produce free radicals (radicals) was first reported in 1954.9 Since this early discovery, many studies have explored the sources and impact of radicals on cells. As a chemical species, radicals are defined as an atom/molecule that contains one or more unpaired electrons.5 The term ROS is a general label that refers to both oxygen-centered radicals and non-radicals that are reactive derivatives of oxygen (e.g., hydrogen peroxide (H2O2)).10 A related term, reactive nitrogen species (RNS), refers to both radical (e.g., nitric oxide (NO)) and non-radical nitrogen species (e.g., peroxynitrite). A brief introduction to the major ROS and RNS follows. For more detailed information on ROS and RNS, the reader is directed to a comprehensive review on this topic.5
Superoxide (O2.−) is the parent molecule of all ROS and is formed by the one-electron reduction of molecular oxygen; this anion is negatively charged and relatively membrane impermeable, and compared to other radical species is relatively unreactive.10
The breakdown (dismutation) of O2.− occurs spontaneously but can also be catalyzed by the enzyme superoxide dismutase (SOD). The dismutation of O2.– provides a major source of the production of H2O2 .10 H2O2 is a non-radical ROS that is permeable to membranes and has a relatively long half-life in the cell. Although H2O2 is considered a weak oxidizing agent, chronically high cellular levels of H2O2 are damaging to cell components and therefore cytotoxic. Importantly, because of the long half-life and high membrane permeability of H2O2, this molecule can diffuse considerable distances in / out of the cell. Together, these properties make H2O2 a primary candidate for ROS-mediated signaling in cells. More details about this topic will be provided elsewhere in this review.
The hydroxyl radical (·OH) has a strong oxidizing potential and is commonly formed by reactions involving both H2O2 and O2.−.11 Because of the high reactivity, ·OH radicals typically oxidize molecules close to their site of production. By comparison to other reactive species, ·OH radicals are considered the most damaging ROS because of their high reactivity.11
NO is the parent molecule of all RNS and is synthesized from the amino acid L-arginine through NO synthase (NOS). Four NOS isoforms exist, and 3 of these isoforms are found in skeletal muscle fibers. Specifically, both neuronal NOS (nNOS/NOS1) and endothelial NOS (eNOS/NOS3) have been identified in skeletal muscle fibers.12 Notably, 2 splice variants of NOS1 (i.e., nNOSβ and nNOSµ) exist in skeletal muscle fibers.13 Moreover, the inducible NOS (iNOS/NOS2) is also found in skeletal muscle during inflammatory conditions such as septic shock.14 These NOSs require several cofactors (e.g., 5,6,7,8-tetrahydrobiopterin and iron) and convert L-arginine into NO and L-citrulline utilizing nicotinamide adenine dinucleotide phosphate (NADPH).15 It is well-established that muscular contractions increase the production of NO within the contracting fibers.16
Once produced, NO is a weak reducing agent that can bind with transition metals located within enzymes to serve as a positive allosteric activator. For example, NO binds to iron located in the enzyme guanyl cyclase; the result of NO binding to iron activates this enzyme, resulting in the formation of cyclic guanosine monophosphate (cGMP). Importantly, NO also reacts rapidly with O2.– to form peroxynitrite (ONOO−). In fact, the reaction between NO and O2.– occurs approximately 3 times faster than the dismutation of O2.−; hence, the formation of ONOO− is the primary reaction of O2.– when NO is present.5
The formation of ONOO− has 2 important biological consequences. First, the formation of ONOO− results in the reduced bioavailability of NO. Second, the formation of ONOO− is also important because this RNS is a strong oxidizing agent that leads to the depletion of thiol groups and nitration of cellular proteins.5
4. Cellular control of ROS
Since regulation of redox balance is critical for cellular health, all mammalian cells are equipped with control systems to regulate oxidation/reduction (redox) balance. A key component of redox control is the cellular antioxidant system. Antioxidants are commonly defined as any substance that significantly delays or prevents oxidation of a substrate. Because a detailed discussion of cellular antioxidants is outside the scope of this review, readers are directed to detailed reviews for more information about antioxidant systems.5 Nonetheless, to provide context for future discussions within this report, a brief overview of cellular antioxidant systems is provided here. Cells contain both enzymatic and non-enzymatic antioxidants that work as a complex regulatory network to control the levels of ROS. Indeed, throughout the cell, antioxidants are compartmentalized in both organelles and the cytoplasm to mitigate ROS and maintain redox balance. Further, antioxidants also exist in the interstitial fluid and blood, and these extracellular antioxidants play a key role in eliminating ROS that exist in extracellular fluids.
Three primary antioxidant strategies are used to protect cells against ROS-mediated damage. First, numerous low-molecular weight molecules capable of scavenging ROS exist in both the extracellular space and within cells. Second, some enzymatic antioxidants act by converting ROS into less reactive molecules; this limits oxidation and prevents the transformation of these ROS to more damaging species. A final antioxidant strategy involves the binding of pro-oxidant transition metals (e.g., iron and copper) via metal binding proteins; these chelating molecules prevent these transition metals from participating in ROS formation.5
4.1. Antioxidant enzymes
SOD, glutathione reductase, and catalase (CAT) are the 3 primary antioxidant enzymes located in cells.17 Nonetheless, it is also clear that the thioredoxin (Trx) and peroxiredoxin (Prx) antioxidant systems also play a supportive role in maintaining redox balance in the cell. SOD is the first line of defense against O2.– and dismutates O2.– to form H2O2 and oxygen (O2). Three isoforms of SOD (SOD1, SOD2, and SOD3) exist in all mammals, and all require a redox active transition metal in the active site for the catalytic breakdown of the superoxide anion.18 Two of the SOD isoforms are located within cells, whereas the third SOD isoform is positioned within the extracellular space.19 SOD1 resides in both the cytosol and the mitochondrial intermembrane space whereas SOD2 is only situated in the mitochondrial matrix. In contrast, SOD3 is located outside the cell in the extracellular space.19,20
Although superoxide radicals are not highly reactive, they remove electrons from cellular components (e.g., biological membranes), resulting in a series of radical-mediated reactions. As mentioned elsewhere in this article, superoxide radicals are also toxic because of their involvement in the generation of hydroxyl radicals. Further, recall that O2.–can also react with NO to form ONOO−. It follows that elimination of superoxide radicals is critical to prevent cellular oxidative injury.
Five glutathione peroxidases (GPXs) exist in mammals (GPX1–GPX5).21 All of these GPX enzymes are responsible for the reduction of H2O2 or organic hydroperoxide to form water (H2O) and alcohol (ROH), respectively; this reaction requires an electron donor, and reduced glutathione (GSH) is the primary electron donor involved in GPX reactions.22
Although all GPX isoforms reduce H2O2, the expression of multiple GPX isoforms is biologically logical because of the different cellular locations of the GPX isoforms. For example, GPX1 is located in both the cytosol and mitochondria. GPX2 is located exclusively in the cytosol, whereas GPX3 is found in both the cytosol and extracellular space.21 Hence, the family of GPX antioxidant enzymes play an important role in redox regulation due to both their substrate specificity and their dispersion across different cellular compartments; the varied cellular locations of GPX is advantageous because ROS are mitigated at their site of production.
The principal function of the antioxidant enzyme CAT is the breakdown of H2O2 into H2O and O2. Similar to GPX and SOD, CAT is also located within multiple compartments of the cell, including the cytosol and mitochondria. CAT differs from the GPXs in 2 major ways. First, CAT does not require an electron donor and, second, CAT only eliminates H2O2 and does not remove organic hydroperoxides.
The Trx antioxidant system is comprised of Trx and Trx reductase. Two isoforms of Trx exist: the cytosolic isoform (Trx1) and the mitochondrial form (Trx2).22 Functionally, Trx is the major ubiquitous disulfide reductase responsible for maintaining proteins in their reduced state.23 Trx maintains the reduced state of proteins via formation of a disulfide bond with the substrate protein and the transfer of 2 of its electrons to the target protein; this results in oxidation of the Trx protein and reduction of the target substrate. Oxidized Trx can then be reduced by electrons from NADPH via Trx reductase, allowing Trx to continue its role as a redox modulator.24 Trx serves several physiological roles: (1) protection against protein oxidation, (2) reduction of transcription factors, and (3) regulation of apoptosis.10 Moreover, Trx reductase also contributes as an antioxidant enzyme by reducing hydroperoxides and functioning as a NADPH-dependent dehydroascorbate reductase to recycle vitamin C.24
Finally, Prxs are a family of peroxidases with mammalian cells expressing 6 different isoforms.25 Using electrons provided by Trx, Prxs catalyze the reduction of H2O2, alkyl hydroperoxides, and peroxynitrite.25 Recent reviews on the function of Prxs in cells has concluded that Prxs appear to be more than simple peroxide-eliminating enzymes and may play an important role in cell signaling in a variety of cell types, including skeletal muscle.25,26 Nonetheless, complete details of how Prxs interact with cellular proteins to regulate redox signaling remain largely unexplained.
4.2. Nonenzymatic antioxidants
Numerous nonenzymatic antioxidants exist in cells (e.g., GSH, uric acid, bilirubin, vitamin E, and vitamin C), and a detailed discussion of this topic is beyond the scope of this review. Nonetheless, because of the importance of GSH in control of redox balance, we provide a brief overview of the vital role that GSH plays in the prevention of oxidative stress. Indeed, as a cellular antioxidant, GSH serves multiple important roles. For example, GSH directly reacts with a variety of radicals by donating a hydrogen atom; this results in a less reactive and less damaging species. Further, as highlighted elsewhere in this review, an important antioxidant action of GSH is to donate electrons for GPX to eliminate H2O2 and organic hydroperoxides. Moreover, GSH is also important because it reduces the antioxidant vitamins E and C. This action of GSH is central because it assists in maintaining the limited cellular resources of vitamins E and C in the reduced state; this reduction of vitamins E and C allows these molecules to continue to act as cellular antioxidants.10
5. Historical overview of research in exercise-induced oxidative stress
The first report that exercise is associated with increased biomarkers of oxidative damage appeared in the literature in 1978;27 this original report revealed that prolonged endurance exercise in humans results in increased biomarkers of oxidative stress. Four years later, this work was followed by the discovery that contracting skeletal muscle produce ROS.28 Using similar techniques (i.e., electron spin resonance), this finding was corroborated by Jackson et al.,29 and numerous studies in the last 4 decades have confirmed that rigorous exercise is associated with oxidative stress in both humans and animals.5,30 More specifically, many types of exercise (prolonged endurance exercise, resistance exercise, high-intensity anaerobic exercise, and eccentric exercise) result in oxidative stress, as evidenced by increased biomarkers of oxidation in both skeletal muscle and blood.31, 32, 33, 34
The first evidence that ROS contribute to skeletal muscle fatigue was reported by 2 independent investigations in 1990.35,36 This important research stimulated many subsequent studies focusing on the mechanisms responsible for ROS-mediated skeletal muscle fatigue.37
Several important studies performed in the 1990s revealed that endurance exercise training and/or high-intensity interval training increased the antioxidant capacity of both cardiac and skeletal muscle myocytes.5 More specifically, these studies revealed that exercise training increases several antioxidant enzymes, including SOD1, SOD2, and GPX1.38, 39, 40, 41, 42, 43, 44, 45, 46 In particular, these early studies show that exercise training increases both SOD1 and SOD2 in the trained skeletal muscles by 20%–110%.5 Similarly, studies also revealed that regular endurance exercise training increases skeletal muscle levels of GPX1 by 20%–180%.5 Although a few studies published in the 1980s and 1990s suggest that exercise training also increases muscle levels of CAT, other reports have failed to demonstrate an exercise-induced increase in CAT. Therefore, whether or not exercise increases CAT levels in cardiac and skeletal muscles remains controversial.5 Finally, to date, limited information exists about the impact of exercise training on the abundance of Prxs isoforms in skeletal muscle, and additional studies will be required to determine if exercise training impacts this antioxidant system in muscle fibers.
Another important discovery that occurred in the 1990s was the observation that skeletal muscle expresses 2 isoforms of NOS and that contracting skeletal muscles produce NO.12,47 This breakthrough paved the way for additional studies on the role that NO plays in skeletal muscle signaling.
In the late 1990s and early 2000s, a paradigm shift occurred in the thinking about the biological impact of exercise-induced ROS production. More specifically, during most of the 1980s, the ROS produced in skeletal muscles during exercise were considered damaging to muscle fibers without positive consequences. However, this view began to change after the recognition that NO was an important biological signaling molecule. Indeed, several important studies published in the early 2000s revealed that ROS are critical signaling molecules that increase gene expression in cultured myotubes, and many studies since have confirmed that ROS alter gene expression in skeletal muscle and other tissues.48, 49, 50 For a detailed overview of the history of research in exercise and oxidative stress.51
6. Sources of oxidants in contracting skeletal muscles
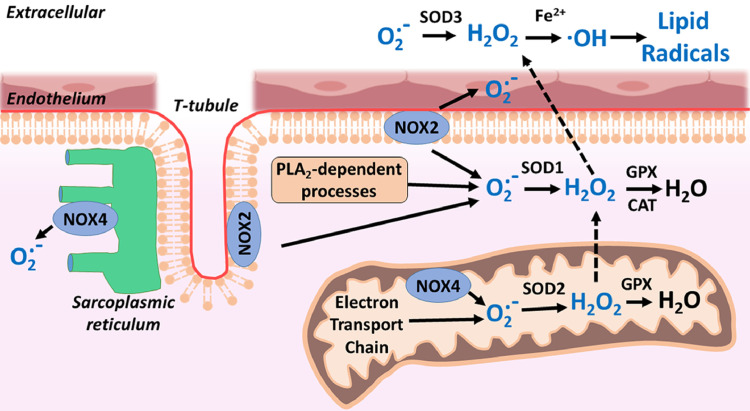
Since the discovery that exercise-induced oxidative stress occurs, many investigators have probed the potential sources of ROS production in a number of tissues. Though oxidants could be produced in a variety of tissues during exercise, it has been established that skeletal muscle is the dominant source of ROS production during exercise.52 Possible sources of exercise-induced ROS production in muscle fibers has been widely investigated and include the following: (1) mitochondria, (2) phospholipase A2 (PLA2), and (3) NADPH oxidases (NOX2 and NOX4) localized in 4 sites within fibers: mitochondria, sarcolemma, sarcoplasmic reticulum, and T-tubules (Fig. 1).53, 54, 55, 56, 57 A brief discussion of each of these potential sites of oxidant production follows.
Fig. 1.
Potential sites of the production or reactive oxygen species in contracting skeletal muscles. CAT = catalase; GPX = glutathione peroxidase; H2O2 = hydrogen peroxide; NOX = NADPH oxidase; O2.− = superoxide; ·OH = hydroxyl radical; PLA2 = phospholipase A2; SOD = superoxide dismutase. Modified from Powers and Jackson.5
Although early studies proposed that mitochondria are the likely source of ROS production in muscle fibers during exercise, this prediction does not appear to be accurate given that ROS production in skeletal muscle mitochondria decreases during exercise. Specifically, based on studies performed in the 1970s, it was estimated that 2%–5% of molecular oxygen consumed in the mitochondria formed O2.−.58 Based on this account, it was then hypothesized that increases in oxidative phosphorylation in the mitochondria within contracting skeletal muscles would result in a proportional increase in O2.− production. Nonetheless, contemporary studies reveal that mitochondria actually produce less O2.− during active State 3 respiration compared to basal State 4 respiration.59, 60, 61 Therefore, the available evidence indicates that mitochondria are not the major site of ROS production in skeletal muscle during exercise.
PLA2 is an enzyme that cleaves membrane phospholipids to release arachidonic acid; free arachidonic acid is a substrate for several ROS-generating enzyme systems, including the lipoxygenases.62 Importantly, activation of PLA2 can activate NADPH oxidases,63 and augmented PLA2 activity in skeletal muscle can also promote ROS production in mitochondria64 and the cytosol.65 Note that both calcium-dependent and calcium-independent forms of PLA2 exist in skeletal muscle and both isoforms are capable of stimulating ROS generation in muscle.64,65 It is hypothesized that the calcium-independent enzymes regulate cytosolic oxidant activity skeletal muscle cells under resting conditions,65 whereas the calcium-dependent isoform of PLA2 stimulates mitochondrial ROS generation during contractile activity.66 Nonetheless, additional research is required to confirm or deny this postulate.
Skeletal muscle expresses 2 isoforms of NADPH oxidase (NOX2 and NOX4).67 NOX2 is located within the sarcolemma and T-tubule, whereas NOX4 is located in both the sarcoplasmic reticulum and the mitochondria.68 NOX4 is constitutively active and does not require association with regulatory subunits.67 In contrast, NOX2 is activated by specific agonists (e.g., angiotensin II, mechanical/contractile stress, and cytokines).67 Therefore, it appears likely that NOX4 contributes to the basal rate of ROS production in muscle fibers, whereas NOX2 is the primary source of NADPH oxidase-mediated ROS production in contracting muscle.67 Indeed, several recent studies point to NOX as a primary contributor to contraction-induced ROS production.56,69,70 For example, investigators using a variety of experimental techniques to examine the subcellular sites responsible for O2.− production in muscle fibers concluded that mitochondria are not responsible for contraction-induced production of O2.− in muscle fibers; instead, they concluded that NOX is a major source of O2.− production both at rest and during contractions.56 Similar conclusions have been reached by others.69,70 Nonetheless, at present, concluding that NOX is the dominant source for ROS in contracting skeletal muscle is complicated by the complexities associated with the study of NOX activity in cells. Clearly, improved methodologies and additional studies are required to clarify this issue. For more information on this topic, we refer the reader to the following references.67,68
7. Cellular consequences of exercise-induced oxidant production in skeletal muscle fibers
It is established that ROS are continually produced in skeletal muscle at both rest and during exercise; importantly, ROS modulate a variety of physiological processes, including regulation of blood flow, muscle force production, and muscle adaptation to exercise training. Investigations into the consequences of ROS production in skeletal muscle fibers have now spanned 4 decades, resulting in a voluminous amount of research. Because of space limitations, we will limit our discussion to 3 issues related to the consequence of endurance exercise-induced ROS production in skeletal muscle: (1) exercise-induced oxidative stress, (2) ROS impact on muscle force production, and (3) ROS influence on muscle adaption to exercise training. We begin with a discussion of the impact of exercise-induced oxidative damage to macromolecules.
7.1. Exercise-induced oxidative stress
Although short-duration (i.e., <1 min) and low-intensity (∼30% maximal oxygen consumption (VO2max) exercise does not appear to promote oxidative stress, it is well-established that acute bouts of prolonged and high-intensity endurance exercise in untrained humans and animals results in increases in biomarkers of oxidative stress (e.g., increased protein oxidation and lipid peroxidation) both in blood and in the active skeletal muscles.71,72 However, both short-term (5 consecutive days) and long-term (12 weeks) endurance exercise training increases antioxidant enzyme activities in the trained muscles and eliminates contraction-induced oxidative stress due to an acute bout of exercise.73,74 Further, a recent meta-analysis concludes that DNA damage occurs in white blood cells immediately following an acute bout of endurance exercise and the damage persists for up to 24 h.75 However, this exercise-induced DNA damage is not detectable several days post-exercise; this is likely attributable to the exercise-induced up-regulation of DNA repair mechanisms.75
7.2. ROS impact on muscle force production
The impact of ROS on muscle force production has been shown to be biphasic and dependent upon the level of ROS within the fiber. Again, the parent molecule in the ROS cascade is the superoxide radical that is dismutated to H2O2, and it appears likely that both O2.− and H2O2 influence muscle contractile function.76 At rest, superoxide radicals are produced at low rates in skeletal muscle fibers. During exercise, the rate of O2.− production in muscle is markedly increased; the amount of total O2.− production in the muscle fiber is dependent upon both the intensity and duration of exercise as well as the temperature of the contracting muscle. In general, relatively high-intensity, prolonged aerobic exercise (i.e., 65%–75% VO2max) results in greater ROS production compared to low-intensity (i.e., <40% VO2max), short duration exercise. Moreover, increased muscle temperature results in higher levels of ROS during contractions.77
As discussed elsewhere in this review, ROS are eliminated by an array of enzymatic and non-enzymatic antioxidants in the muscle fiber. Thus, the impact of ROS production on skeletal muscle function is the balance between the rate of ROS production and the rate of ROS removal by antioxidants.78
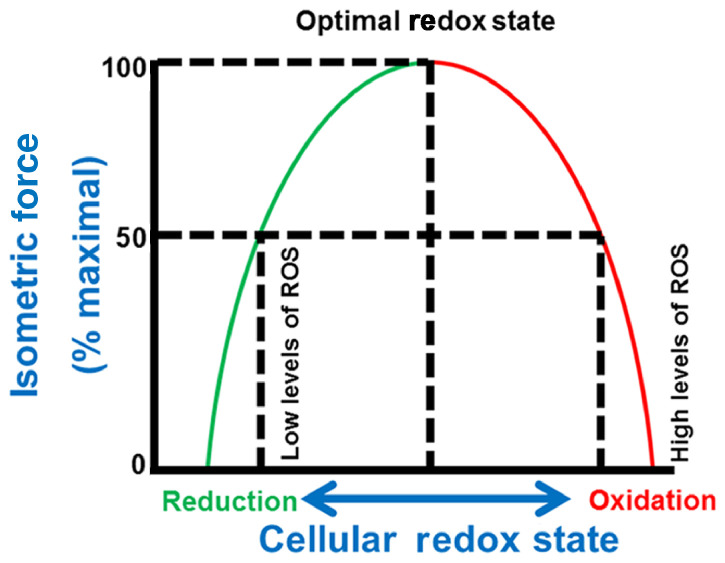
During the 1990s and early 2000s, Reid et al.76,79, 80, 81 published a landmark series of experiments demonstrating that ROS have a biphasic influence on skeletal muscle force production (Fig. 2). Their work reveals that, in unfatigued muscle, an optimum level of ROS is required for muscle fibers to generate 100% of their maximal isometric force production. For example, their work reveals that selective removal of O2.− or H2O2 from the fiber using SOD or CAT, respectively, results in a decrease in muscle maximal force production. Conversely, increasing the ROS levels in the fiber above the optimal point results in a decrease in the muscles’ ability to generate force.78 The fact that administration of the antioxidant N-acetylcysteine delays the rate of muscle fatigue during prolonged exercise provides further support for the concept that high levels of ROS impairs maximal muscle force production.36,82,83 Note that, while these experiments measured maximal isometric force production in muscle, it is likely that an optimum level of ROS is also required for maximal force production during concentric contractions as well.
Fig. 2.
Relationship between cellular redox state and skeletal muscle force production. Note that maximal force production in skeletal muscle requires an optimal redox state. Movement away from the optimal redox state (i.e., an increase in reduction or oxidation) results in a decrease in maximal isometric force production. ROS = reactive oxygen species. Modified from Reid76
What are the mechanisms responsible for the observation that muscle force production can be increased or decreased in response to redox disturbances? This issue remains a debated topic, and a definitive answer to this question does not currently exist. Nonetheless, it is feasible that changes in both free calcium levels in the muscle and myofibrillar sensitivity to calcium contribute to the redox impact on muscle force production. Further, it is possible that a ROS-mediated decrease in Na+/K+ pump activity may also contribute to the decrease in muscle force production that occurs during prolonged endurance exercise. In reference to the role that calcium sensitivity plays in muscle force production, well-controlled studies performed on single skeletal muscle fibers confirm that high levels of oxidants (i.e., H2O2) decrease myofibrillar sensitivity to calcium resulting in decreased muscle force production at any given level of free calcium in the fiber.84,85 This consistent observation explains, at least in part, why high levels of oxidants depress muscle force production. In contrast, the impact of high oxidant levels on cytosolic levels of free calcium during muscle contraction is less clear. Specifically, while it is established that calcium release channels on the sarcoplasmic reticulum (i.e., ryanodine receptors) are redox sensitive,86, 87, 88, 89 the precise impact of redox modulation on these channels remains unclear. For example, evidence exists both for and against the notion that high levels of oxidants disrupt calcium release from the sarcoplasmic reticulum.84,90 The explanation for this experimental discrepancy is unclear but may be due to differences in experimental conditions across numerous studies (e.g., muscle temperature, oxidant levels). Nonetheless, taken together, the experimental evidence indicates that the elevated levels of oxidants in skeletal muscle associated with prolonged exercise is capable of damaging one or more proteins involved in excitation-contraction coupling, resulting in a reduction in muscle force production (i.e., fatigue).
Finally, it is possible that a ROS-mediated decrease in Na+/K+ pump activity also contributes to the reduction in muscle force production that occurs during prolonged endurance exercise.91 Specifically, muscular exercise results in a loss of intracellular K+ and an increase in intracellular Na+ despite a decrease in Na+/K+ pump activity.91 This decrease in intracellular K+ and the reduced trans-sarcolemmal Na+ gradient impairs membrane excitability and, therefore, decreases muscle force production.91 Experimental evidence to support the concept that ROS-mediated depression of Na+/K+ pump activity contributes to muscle fatigue is derived from human experiments confirming that the antioxidant N-acetylcysteine attenuates exercise-induced muscle fatigue, in part by improved regulation of intracellular K+ levels.91
7.3. ROS influence on muscle adaption to exercise training
As discussed elsewhere in this review, it is clear that exercise-induced increases in oxidants contribute to muscle fatigue. However, the production of ROS in skeletal muscle during prolonged endurance exercise also plays an important role in cell signaling pathways involved in muscle adaptation to exercise. Indeed, both human and animal studies demonstrate that prevention of exercise-induced redox signaling blunts the training-induced changes in skeletal muscle fibers.92, 93, 94 A complete discussion of this topic exceeds the scope of this review, and the reader is referred to recent reviews for specific details about redox signaling and skeletal muscle adaptation.93,95,96 Nonetheless, a brief overview of the link between exercise-induced production of ROS and skeletal muscle adaptation to endurance exercise is appropriate.
Skeletal muscle is a highly plastic tissue that undergoes sizeable phenotypic changes in response to endurance exercise training. Remarkably, as few as 5–10 consecutive days of endurance exercise results in substantial increases in both the oxidative and antioxidant capacity of skeletal muscle fibers.73,74,97 During the past 20 years, our understanding of the signaling mechanisms responsible for these changes has increased markedly. Importantly, many of these cell-signaling pathways are initiated, or at least potentiated, by redox signals. Indeed, redox-sensitive pathways result in changes in transcription factor activity, either increasing or decreasing the transcription of target genes. In this regard, it is now clear that exercise-induced production of ROS plays an important role in exercise-induced signaling via nuclear factor-kappa B (NF-κB) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in skeletal muscle fibers.95,96 This is important because both NF-κB and PGC-1α play a required role in exercise-mediated increases in skeletal muscle antioxidants and mitochondrial biogenesis. In addition to NF-κB and PGC-1α redox activation of nuclear factor erythroid 2-related factor 2 plays an important role in promoting exercise-induced expression of many key components involved in the endogenous antioxidant system.98 Indeed, nuclear factor erythroid 2-related factor (Nrf2) controls the expression of key components of the glutathione and Trx antioxidant systems as well as enzymes involved in NADPH generation.99 Together, the available evidence indicates that exercise-induced production of ROS is clearly a requirement for skeletal muscle adaptations induced by endurance exercise training.
Further, evidence exists that ROS signaling is also involved in resistance training-induced hypertrophy.100 For example, in a cell culture model, H2O2-induced oxidant stress can activate protein kinase B, which promotes protein synthesis in cells via down-stream activation of mammalian target of rapamycin (mTOR).101 mTOR activation stimulates protein synthesis via increased translation of contractile protein mRNA. Furthermore, experimental evidence in a plantaris muscle overload model reveals that production of ONOO−, a reaction product of NO and O2.−, triggers a signaling cascade resulting in the direct activation of mTOR.102 Hence, it appears that contraction-induced ROS production is a key signaling molecule in resistance training-induced muscle hypertrophy.
8. Exercise and free radicals: friend or foe?
The question, “Is exercise-induced ROS production beneficial or a detriment to health?” has been debated for more than 3 decades without resolution. The following segment will discuss this perplexing question by examining the (1) concept of exercise-induced hormesis and the (2) association between regular exercise and the risk of chronic diseases.
8.1. Exercise and hormesis
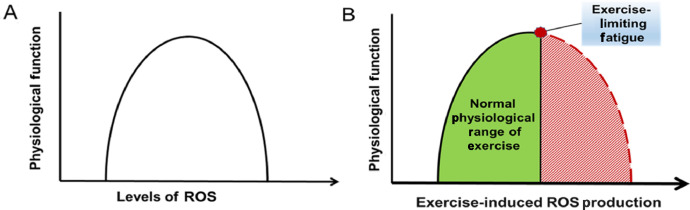
Whether exercise-induced ROS production is damaging or beneficial to health likely depends on the balance between the levels of ROS production during exercise and the competency of the cellular antioxidant systems to protect cells against an oxidant challenge. In this regard, several recent reviews have concluded that regular exercise training does not result in chronic oxidative stress in the active muscles.17,103, 104, 105, 106 Conceptually, this conclusion is supported by the notion of exercise-induced hormesis. The term hormesis is used in biology to describe a biphasic dose–response curve where a transient increase in low levels of a stressor (e.g., radiation, radicals) provides a beneficial adaptive effect on cells whereas a chronic and/or high dose of the stressor results in damage to the cells (Fig. 3A).107,108 Although hormesis research has a long history in biology, the first description of exercise-induced hormesis appeared in 2005.109 This report concluded that low to moderate levels of exercise-induced ROS production plays an essential role in exercise-induced adaptation of skeletal muscle. In contrast, high levels of ROS production results in damage to the muscle and a decline in the physiological benefits associated with low to moderate ROS production. This exercise-induced hormesis concept has received recent support from several investigators.17,106,109,110 As discussed elsewhere in this article, it has been established that exercise-induced increases in the production of ROS in skeletal muscle plays a required role in skeletal muscle adaptation to training. The bell-shaped hormesis curve predicts that increases in exercise-induced production of ROS promotes significant physiological benefits until an optimum level of ROS production is reached. However, if exercise results in a true hormetic effect on the body, after reaching this zenith of physiological benefit, any further increase in exercise-induced ROS production would result in tissue damage and a decline in the exercise-induced adaptations.
Fig. 3.
(A) Relationship between cellular levels of ROS and physiological function. This biphasic bell-shaped curve represents the ROS hormesis curve. (B) Relationship between the exercise-induced muscle fiber levels of reactive oxygen species and physiological function. This figure predicts that training-induced increases in muscle fiber levels of ROS does not reach a detrimental level because of exercise-induced fatigue. ROS = reactive oxygen species.
Recently, Ji et al.17 have questioned whether prolonged, high-intensity exercise reaches the level of ROS production required to result in the downward slope on the hormesis curve illustrated by the right-hand side of Fig. 3A. These researchers support this position by noting that the existing human and animal literature does not provide persuasive evidence that prolonged, high-intensity exercise results in extreme oxidant-mediated damage in cells and a decrease in the antioxidant capacity of the trained muscles.17 Ji et al.17 also support this position by arguing that high-intensity exercise is unlikely to generate extreme levels of oxidant damage in the working muscle fibers for several reasons. First, the exercise intensity that can be sustained for long durations is influenced by both the cardiovascular system's ability to provide blood to the working muscles and the impact of ROS production on muscle fatigue. Thus, together, the cardiovascular limitation, along with exercise-induced muscle fatigue, would ultimately limit the intensity and duration of exercise that can be sustained. It follows that this limitation for exercise would also limit the total production of muscle ROS during an exercise bout. Second, although mitochondria are a known source of muscle ROS production, compared to State 4 (resting) respiration, mitochondrial coupling is higher during State 3 (exercise) respiration, reducing electron spill and ROS production by the mitochondria during exercise. Third, acute exercise has been reported to increase the expression of uncoupling proteins in skeletal muscle mitochondria; this increase in uncoupling proteins moves protons from the intermembrane space into the matrix, diminishing a high mitochondrial proton gradient that favors O2.− formation. Last, regular exercise training results in significant increases in the abundance of key antioxidant enzymes in skeletal muscles and therefore elevates the fibers’ ability to remove ROS during exercise. Together, these observations support the forecast that during exercise, skeletal muscles are not exposed to extreme levels of ROS-mediated damage, and therefore the exercise-induced ROS production hormesis curve is predicted to follow the pattern illustrated in Fig. 3B.17 Although this argument is logical, the thesis that exercise does not result in high levels of oxidative damage in muscles during exercise does not directly answer the question of whether exercise-induced ROS production in muscles increases the risk of chronic diseases. This issue is addressed in Section 8.2.
8.2. Exercise training, oxidative stress, and chronic disease
Scientific interest in the question of whether exercise-induced ROS production is friend or foe has been fueled by the knowledge that oxidative stress is associated with numerous chronic diseases including cancer, cardiovascular disease, hypertension, Alzheimer's disease, and Parkinson's disease.105,111,112 Certainly, if exercise-induced ROS production has negative health consequences, it would be expected that people who engage in regular exercise would experience a higher incidence of chronic diseases that are associated with oxidative stress. However, this is not the case, since many epidemiological studies conclude that lifelong exercise reduces the incidence of several chronic diseases and lowers all-cause mortality. A brief summary of this work follows.
Oxidative stress has long been implicated in various stages of tumorigenesis.113 It follows that reducing oxidative stress has the potential to reduce the risk of cancer.113 In this regard, a large epidemiological study involving 1.44 million patients concluded that regular physical activity reduced the risk of 13 different types of cancer.114 Moreover, exercise has been shown to reduce the risk of recurrence of tumor growth in breast, colon, and prostate cancers.115, 116, 117 The molecular mechanisms responsible for exercise-induced protection against cancer remain unclear, but an upregulation of antioxidant gene expression has been postulated to be a contributory factor.113,118
It is established that oxidative stress plays a role in the development and progression of cardiovascular disease.105 Importantly, regular exercise training reduces the prevalence of heart disease along with several of the cardiovascular risk factors, including hypertension.111,119 Moreover, the exercise-induced reduction in cardiovascular risk factors follows a clear dose–response relationship, with higher volumes and intensities of exercise providing greater health benefits.120 While it is possible that exercise-induced protection against hypertension and cardiovascular disease is directly linked to an exercise-induced reduction in oxidative stress, proving cause and effect is experimentally challenging. Therefore, it remains unclear as to whether exercise-mediated changes in cellular redox balance is the primary factor responsible for exercise-induced protection against heart disease and hypertension.111,119
Oxidative stress has been implicated in several neurodegenerative diseases, including both Alzheimer's disease and Parkinson's disease.112,121, 122, 123 Although ROS may not be the trigger for induction of these neurological disorders, oxidative stress is predicted to exacerbate both disorders.123 The influence of exercise training on the risk of these diseases is clear, since numerous epidemiological studies conclude that an inverse relationship exists between physical activity and the incidence of both Alzheimer's disease and Parkinson's disease.112 Furthermore, exercise training has been reported to slow the progression of both disorders.112 Several mechanisms have been proposed for this exercise-induced benefit, including increased cerebral blood flow and increased antioxidants within the brain.112,124
Given that regular exercise has been shown to reduce the risk of several types of cancers, cardiovascular disease, hypertension, Alzheimer's disease, and Parkinson's disease, it is not surprising that an inverse dose–response relationship exists between the volume of physical activity and all-cause mortality.125, 126, 127 Indeed, the evidence that regular physical activity reduces all-cause mortality has been widely accepted for more than 2 decades. Although the mechanism(s) responsible for exercise-induced protection against chronic disease remains a topic of debate, the undeniable evidence that regular exercise protects against all-cause mortality supports the conclusion that exercise-induced production of ROS is a “friend” and not a “foe” of good health.
9. Conclusions
The fact that high-intensity and/or prolonged exercise promotes oxidative stress in humans was discovered more than 4 decades ago. The tissues most responsible for ROS production during exercise remain debated, but it is clear that contracting skeletal muscles are an important source of ROS production during exercise. The intracellular sites of ROS production in contracting skeletal muscles continue to be an active area of research, but mounting evidence implicates NADPH as an important source of ROS production during exercise.
The consequences of exercise-induced oxidative stress continue to be a controversial topic. In theory, exercise-induced ROS production could be a double-edged sword, whereby a moderate level of ROS production during exercise promotes positive physiological adaptation in the active skeletal muscles (e.g., mitochondrial biogenesis, synthesis of antioxidant enzymes, and stress proteins), whereas high levels of ROS production result in damage to macromolecular structures (e.g., proteins, lipids, and DNA). Although the impact of exercise-induced ROS production in skeletal muscle has been postulated to be a bell-shaped hormesis curve, there is not convincing evidence that prolonged, high-intensity exercise results in tissue damage and impaired physiological function. Indeed, research consistently demonstrates that long duration and high-intensity exercise provides the greatest health benefits. Therefore, based on the available evidence, it appears unlikely that rigorous and prolonged exercise results in an oxidative stress level that is detrimental to human health.
Acknowledgments
Authors’ contributions
SKP developed the outline of the review, reviewed the literature, and wrote the manuscript; TY also reviewed the literature, contributed to figure preparation, and provided editorial suggestions during review; RD, MPB, and HH read the manuscript and provided editorial suggestions during revision, and contributed to figure preparation; MO read the manuscript and provided editorial suggestions during revision. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Kruk J., Aboul-Enein H.Y., Kladna A., Bowser J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radic Res. 2019;53:497–521. doi: 10.1080/10715762.2019.1612059. [DOI] [PubMed] [Google Scholar]
- 2.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Wei W., Liu Q., Tan Y., Liu L., Li X., Cai L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin. 2009;33:370–377. doi: 10.3109/03630260903212175. [DOI] [PubMed] [Google Scholar]
- 5.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus W.E., Powell K.E., Haskell W.L., Janz K.F., Campbell W.W., Jakicic J.M. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med Sci Sports Exerc. 2019;51:1270–1281. doi: 10.1249/MSS.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sies H. Academic Press; London: 1985. Oxidative stress. [Google Scholar]
- 8.Jones D.P. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 9.Commoner B., Townsend J., Pake G.E. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B., Gutteridge J. 4th ed. Oxford Press; Oxford: 2007. Free radicals in biology and medicine. [Google Scholar]
- 11.Hensley K., Floyd R.A. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophy. 2002;397:377–383. doi: 10.1006/abbi.2001.2630. [DOI] [PubMed] [Google Scholar]
- 12.Kobzik L., Reid M.B., Bredt D.S., Stamler J.S. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 13.Balke J.E., Zhang L., Percival J.M. Neuronal nitric oxide synthase (nNOS) splice variant function: insights into nitric oxide signaling from skeletal muscle. Nitric Oxide. 2019;82:35–47. doi: 10.1016/j.niox.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Adams V., Nehrhoff B., Späte U., Linke A., Schulze P.C., Baur A. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappa B activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 15.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33 doi: 10.1093/eurheartj/ehr304. 829–37a-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid M.B. Redox interventions to increase exercise performance. J Physiol. 2016;594:5125–5133. doi: 10.1113/JP270653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji L.L., Kang C., Zhang Y. Exercise-induced hormesis and skeletal muscle health. Free Radic Biol Med. 2016;98:113–122. doi: 10.1016/j.freeradbiomed.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Culotta V.C., Yang M., O'Halloran T.V. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763:747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki K., Ohno H., Oh-ishi S., Kizaki T., Ookawara T., Fukii J. Superoxide dismutases in exercise and disease. In: Sen C.K., Packer L., Hänninen O., editors. Handbook of oxidants and antioxidants and exercise. Elsevier Science; Amsterdam: 2000. pp. 243–295. [Google Scholar]
- 20.Hearn A.S., Tu C., Nick H.S., Silverman D.N. Characterization of the product-inhibited complex in catalysis by human manganese superoxide dismutase. J Biol Chem. 1999;274:24457–24460. doi: 10.1074/jbc.274.35.24457. [DOI] [PubMed] [Google Scholar]
- 21.Brigelius-Flohé R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;38710:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 22.Björnstedt M., Kumar S., Björkhem L., Spyrou G., Holmgren A. Selenium and the thioredoxin and glutaredoxin systems. Biomed Environ Sci. 1997;10:271–279. [PubMed] [Google Scholar]
- 23.Balsera M., Buchanan B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic Biol Med. 2019;140:28–35. doi: 10.1016/j.freeradbiomed.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Arnér E.S., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 25.Rhee S.G., Kil I.S. Multiple functions and regulation of mammalian peroxiredoxins. Annu Rev Biochem. 2017;86:749–775. doi: 10.1146/annurev-biochem-060815-014431. [DOI] [PubMed] [Google Scholar]
- 26.Wadley A.J., Aldred S., Coles S.J. An unexplored role for peroxiredoxin in exercise-induced redox signalling. Redox Biol. 2016;8:51–58. doi: 10.1016/j.redox.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillard C.J., Litov R.E., Savin W.M., Dumelin E.E., Tappel A.L. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 28.Davies K.J., Quintanilha A.T., Brooks G.A., Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 29.Jackson M.J., Edwards R.H., Symons M.C. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta. 1985;847:185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 30.Jackson M.J. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal. 2011;15:2477–2486. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloomer R.J., Goldfarb A.H. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29:245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 32.Groussard C., Rannou-Bekono F., Machefer G., Chevanne M., Vincent S., Sergent O. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur J Appl Physiol. 2003;89:14–20. doi: 10.1007/s00421-002-0767-1. [DOI] [PubMed] [Google Scholar]
- 33.McBride J.M., Kraemer W.J., Triplett-McBride T., Sebastianelli W. Effect of resistance exercise on free radical production. Med Sci Sports Exerc. 1998;30:67–72. doi: 10.1097/00005768-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Scheffer D.L., Silva L.A., Tromm C.B., da Rosa G.L., Silveira P.C., de Souza C.T. Impact of different resistance training protocols on muscular oxidative stress parameters. Appl Physiol Nutr Metab. 2012;37:1239–1246. doi: 10.1139/h2012-115. [DOI] [PubMed] [Google Scholar]
- 35.Novelli G.P., Bracciotti G., Falsini S. Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic Biol Med. 1990;8:9–13. doi: 10.1016/0891-5849(90)90138-9. [DOI] [PubMed] [Google Scholar]
- 36.Shindoh C., DiMarco A., Thomas A., Manubay P., Supinski G. Effect of N-acetylcysteine on diaphragm fatigue. J Appl Physiol (1985) 1990;68:2107–2113. doi: 10.1152/jappl.1990.68.5.2107. [DOI] [PubMed] [Google Scholar]
- 37.Reid M.B. Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic Biol Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Criswell D., Powers S., Dodd S., Lawler J., Edwards W., Renshler K. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc. 1993;25:1135–1140. [PubMed] [Google Scholar]
- 39.Ji L.L., Fu R., Mitchell E.W. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- 40.Ji L.L., Stratman F.W., Lardy H.A. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys. 1988;263:150–160. doi: 10.1016/0003-9861(88)90623-6. [DOI] [PubMed] [Google Scholar]
- 41.Laughlin M.H., Simpson T., Sexton W.L., Brown O.R., Smith J.K., Korthuis R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol. 1990;68:2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- 42.Leeuwenburgh C., Fiebig R., Chandwaney R., Ji L.L. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267:R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- 43.Leeuwenburgh C., Hollander J., Leichtweis S., Griffiths M., Gore M., Ji L.L. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272:R363–R369. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- 44.Powers S.K., Criswell D., Lawler J., Ji L.L., Martin D., Herb R.A. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol. 1994;266:R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- 45.Powers S.K., Criswell D., Lawler J., Martin D., Ji L.L., Herb R.A. Regional training-induced alterations in diaphragmatic oxidative and antioxidant enzymes. Respir Physiol. 1994;95:227–237. doi: 10.1016/0034-5687(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 46.Powers S.K., Criswell D., Lawler J., Martin D., Lieu F.K., Ji L.L. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol. 1993;265:H2094–H2098. doi: 10.1152/ajpheart.1993.265.6.H2094. [DOI] [PubMed] [Google Scholar]
- 47.Kobzik L., Stringer B., Balligand J.L., Reid M.B., Stamler J.S. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun. 1995;211:375–381. doi: 10.1006/bbrc.1995.1824. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L.Z., Johnson A.P., Rando T.A. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]
- 49.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 50.Jackson M.J. Free radicals generated by contracting muscle: by-products of metabolism or key regulators of muscle function? Free Radic Biol Med. 2008;44:132–141. doi: 10.1016/j.freeradbiomed.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Powers S.K., Radak Z., Ji L.L. Exercise-induced oxidative stress: past, present and future. J Physiol. 2016;594:5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson M.J., Vasilaki A., McArdle A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic Biol Med. 2016;98:13–17. doi: 10.1016/j.freeradbiomed.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 53.Espinosa A., Leiva A., Peña M., Müller M., Debandi A., Hidalgo C. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol. 2006;209:379–388. doi: 10.1002/jcp.20745. [DOI] [PubMed] [Google Scholar]
- 54.Javeshghani D., Magder S.A., Barreiro E., Quinn M.T., Hussain S.N. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 55.Sakellariou G.K., Lightfoot A.P., Earl K.E., Stofanko M., McDonagh B. Redox homeostasis and age-related deficits in neuromuscular integrity and function. J Cachexia Sarcopenia Muscle. 2017;8:881–906. doi: 10.1002/jcsm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakellariou G.K., Vasilaki A., Palomero J., Kayani A., Zibrik L., McArdle A. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia R., Webb J.A., Gnall L.L., Cutler K., Abramson J.J. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Physiol Cell Physiol. 2003;285:C215–C221. doi: 10.1152/ajpcell.00034.2002. [DOI] [PubMed] [Google Scholar]
- 58.Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kavazis A.N., Talbert E.E., Smuder A.J., Hudson M.B., Nelson W.B., Powers S.K. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med. 2009;46:842–850. doi: 10.1016/j.freeradbiomed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powers S.K., Hudson M.B., Nelson W.B., Talbert E.E., Min K., Szeto H.H. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson E.J., Neufer P.D. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol. 2006;290:C844–C851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 62.Zuo L., FL Christofi F.L., Wright V.P., Bao S., Clanton T.L. Lipoxygenase-dependent superoxide release in skeletal muscle. J Appl Physiol (1985) 2004;97:661–668. doi: 10.1152/japplphysiol.00096.2004. [DOI] [PubMed] [Google Scholar]
- 63.Zhao X., Bey E.A., Wientjes F.B., Cathcart M.K. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67phox and p47phox. J Biol Chem. 2002;277:25385–25392. doi: 10.1074/jbc.M203630200. [DOI] [PubMed] [Google Scholar]
- 64.Nethery D., Callahan L.A., Stofan D., Mattera R., DiMarco A., Supinski G. PLA2 dependence of diaphragm mitochondrial formation of reactive oxygen species. J Appl Physiol (1985) 2000;89:72–80. doi: 10.1152/jappl.2000.89.1.72. [DOI] [PubMed] [Google Scholar]
- 65.Gong M.C., Arbogast S., Guo Z., Mathenia J., Su W., Reid M.B. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J Appl Physiol. 2006;100:399–405. doi: 10.1152/japplphysiol.00873.2005. [DOI] [PubMed] [Google Scholar]
- 66.Nethery D., Stofan D., Callahan L., DiMarco A., Supinski G. Formation of reactive oxygen species by the contracting diaphragm is PLA2 dependent. J Appl Physiol (1985) 1999;87:792–800. doi: 10.1152/jappl.1999.87.2.792. [DOI] [PubMed] [Google Scholar]
- 67.Ward C.W., Prosser B.L., Lederer W.J. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid Redox Signal. 2014;20:929–936. doi: 10.1089/ars.2013.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferreira L.F., Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic Biol Med. 2016;98:18–28. doi: 10.1016/j.freeradbiomed.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pearson T., Kabayo T., Ng R., Chamberlain J., McArdle A., Jackson M.J. Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PLoS One. 2014;9:e96378. doi: 10.1371/journal.pone.0096378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaelson L.P., Shi G., Ward C.W., Rodney G.G. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve. 2010;42:522–529. doi: 10.1002/mus.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lawler J.M., Powers S.K., Visser T., Van Dijk H., Kordus M.J., Ji L.L. Acute exercise and skeletal muscle antioxidant and metabolic enzymes: effects of fiber type and age. Am J Physiol. 1993;265:R1344–R1350. doi: 10.1152/ajpregu.1993.265.6.R1344. [DOI] [PubMed] [Google Scholar]
- 72.Quindry J.C., Stone W.L., King J., Broeder C.E. The effects of acute exercise on neutrophils and plasma oxidative stress. Med Sci Sports Exerc. 2003;35:1139–1145. doi: 10.1249/01.MSS.0000074568.82597.0B. [DOI] [PubMed] [Google Scholar]
- 73.Vincent H.K., Powers S.K., Demirel H.A., Coombes J.S., Naito H. Exercise training protects against contraction-induced lipid peroxidation in the diaphragm. Eur J Appl Physiol Occup Physiol. 1999;79:268–273. doi: 10.1007/s004210050505. [DOI] [PubMed] [Google Scholar]
- 74.Vincent H.K., Powers S.K., Stewart D.J., Demirel H.A., Shanely R.A., Naito H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur J Appl Physiol. 2000;81:67–74. doi: 10.1007/PL00013799. [DOI] [PubMed] [Google Scholar]
- 75.Tryfidou D.V., McClean C., Nikolaidis M.G., Davison G.W. DNA damage following acute aerobic exercise: a systematic review and meta-analysis. Sports Med. 2019;50:103–127. doi: 10.1007/s40279-019-01181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reid M.B. Invited review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 77.Clanton T.L., Zuo L., Klawitter Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Med. 1999;222:253–262. doi: 10.1046/j.1525-1373.1999.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 78.Powers S.K., Ji L.L., Kavazis A.N., Jackson M.J. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011;1:941–969. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reid M.B., Andrade F.H., Balke C.W., Esser K.A. Redox mechanisms of muscle dysfunction in inflammatory disease. Phys Med Rehabil Clin N Am. 2005;16:925–949. doi: 10.1016/j.pmr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 80.Reid M.B., Haack K.E., Franchek K.M., Valberg P.A., Kobzik L., West M.S. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol (1985) 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 81.Reid M.B., Khawli F.A., Moody M.R. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol (1985) 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- 82.Reid M.B., Stokić D.S., Koch S.M., Khawli F.A., Leis A.A. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest. 1994;94:2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Supinski G.S., Stofan D., Ciufo R., DiMarco A. N-acetylcysteine administration alters the response to inspiratory loading in oxygen-supplemented rats. J Appl Physiol (1985) 1997;82:1119–1125. doi: 10.1152/jappl.1997.82.4.1119. [DOI] [PubMed] [Google Scholar]
- 84.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moopanar T.R., Allen D.G. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37°C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng W., Liu G., Allen P.D., Pessah I.N. Transmembrane redox sensor of ryanodine receptor complex. J Biol Chem. 2000;275:35902–35907. doi: 10.1074/jbc.C000523200. [DOI] [PubMed] [Google Scholar]
- 87.Marengo J.J., Hidalgo C., Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Posterino G.S., Cellini M.A., Lamb G.D. Effects of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle. J Physiol. 2003;547:807–823. doi: 10.1113/jphysiol.2002.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun J., Xu L., Eu J.P., Stamler J.S., Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem. 2001;276:15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 90.Brotto M.A., Nosek T.M. Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. J Appl Physiol (1985) 1996;81:731–737. doi: 10.1152/jappl.1996.81.2.731. [DOI] [PubMed] [Google Scholar]
- 91.McKenna M.J., Medved I., Goodman C.A., Brown M.J., Bjorksten A.R., Murphy K.T. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Cabrera M.C., Domenech E., Romagnoli M., Arduini A., Borras C. Pallardo FV, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 93.Pastor R., Tur J.A. Antioxidant supplementation and adaptive response to training: a systematic review. Curr Pharm Des. 2019;25:1889–1912. doi: 10.2174/1381612825666190701164923. [DOI] [PubMed] [Google Scholar]
- 94.Ristow M., Zarse K., Oberbach A., Klöting N., Birringer M., Kiehntopf M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powers S.K., Duarte J., Kavazis A.N., Talbert E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Powers S.K., Talbert E.E., Adhihetty P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589:2129–2138. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smuder A.J., Min K., Hudson M.B., Kavazis A.N., Kwon O.S., Nelson W.B. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J Appl Physiol (1985) 2012;112:501–510. doi: 10.1152/japplphysiol.01086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vargas-Mendoza N., Morales-González Á., Madrigal-Santillán E.O., Madrigal-Bujaidar E., Álvarez-González I., García-Melo L.F. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants (Basel) 2019;8:E196. doi: 10.3390/antiox8060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ismaeel A., Holmes M., Papoutsi E., Panton L., Koutakis P. Resistance training, antioxidant status, and antioxidant supplementation. Int J Sport Nutr Exerc Metab. 2019;29:539–547. doi: 10.1123/ijsnem.2018-0339. [DOI] [PubMed] [Google Scholar]
- 101.Leslie N.R., Bennett D., Lindsay Y.E., Stewart H., Gray A., Downes C.P. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ito N., Ruegg U.T., Kudo A., Miyagoe-Suzuki Y., Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med. 2013;19:101–106. doi: 10.1038/nm.3019. [DOI] [PubMed] [Google Scholar]
- 103.de Sousa C.V., Sales M.M., Rosa T.S., Lewis J.E., de Andrade R.V., Simões H.G. The antioxidant effect of exercise: a systematic review and meta-analysis. Sports Med. 2017;47:277–293. doi: 10.1007/s40279-016-0566-1. [DOI] [PubMed] [Google Scholar]
- 104.Di Meo S., Napolitano G., Venditti P. Mediators of physical activity protection against ROS-linked skeletal muscle damage. Int J Mol Sci. 2019;20:E3024. doi: 10.3390/ijms20123024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nocella C., Cammisotto V., Pigozzi F., Borrione P., Fossati C., D'Amico A. Impairment between oxidant and antioxidant systems: short- and long-term implications for athletes' health. Nutrients. 2019;11:E1353. doi: 10.3390/nu11061353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Radak Z., Ishihara K., Tekus E., Varga C., Posa A., Balogh L. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017;12:285–290. doi: 10.1016/j.redox.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Calabrese E.J., Baldwin L.A. Chemical hormesis: its historical foundations as a biological hypothesis. Toxicol Pathol. 1999;27:195–216. doi: 10.1177/019262339902700207. [DOI] [PubMed] [Google Scholar]
- 108.Mattson M.P. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Radak Z., Chung H.Y., Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 110.Musci R.V., Hamilton K.L., Linden M.A. Exercise-induced mitohormesis for the maintenance of skeletal muscle and healthspan extension. Sports (Basel) 2019;7:E170. doi: 10.3390/sports7070170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korsager Larsen M., Matchkov V.V. Hypertension and physical exercise: role of oxidative stress. Medicina (Kaunas) 2016;52:19–27. doi: 10.1016/j.medici.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 112.Paillard T., Rolland Y., de Souto Barreto P. Protective effects of physical exercise in Alzheimer's disease and Parkinson's disease: a narrative review. J Clin Neurol. 2015;11:212–219. doi: 10.3988/jcn.2015.11.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hojman P., Gehl J., Christensen J.F., Pedersen B.K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27:10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 114.Moore S.C., Lee I.M., Weiderpass E., Campbell P.T., Sampson J.N., Kitahara C.M. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holmes M.D., Chen W.Y., Feskanich D., Kroenke C.H., Colditz G.A. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 116.Kenfield S.A., Stampfer M.J., Giovannucci E., Chan J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meyerhardt J.A., Giovannucci E.L., Holmes M.D., Chan A.T., Chan J.A., Colditz G.A. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 118.Thomas R.J., Kenfield S.A., Jimenez A. Exercise-induced biochemical changes and their potential influence on cancer: a scientific review. Br J Sports Med. 2017;51:640–644. doi: 10.1136/bjsports-2016-096343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lavie C.J., Arena R., Swift D.L., Johannsen N.M., Sui N.M., Lee D.C. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Foulds H.J., Bredin S.S., Charlesworth S.A., Ivey A.C., Warburton D.E. Exercise volume and intensity: a dose-response relationship with health benefits. Eur J Appl Physiol. 2014;114:1563–1571. doi: 10.1007/s00421-014-2887-9. [DOI] [PubMed] [Google Scholar]
- 121.Barnham K.J., Masters C.L., Bush C.L. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 122.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 123.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lucas S.J., Cotter J.D., Brassard P., Bailey D.M. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cereb Blood Flow Metab. 2015;35:902–911. doi: 10.1038/jcbfm.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blair S.N., Kampert J.B., Kohl H.W., 3rd., Barlow C.E., Macera C.A., Paffenbarger R.S., Jr. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 126.Harber M.P., Kaminsky L.A., Arena R., Blair S.N., Franklin B.A., Myers J. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog Cardiovasc Dis. 2017;60:11–20. doi: 10.1016/j.pcad.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 127.Lee I.M., Skerrett P.J. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(Suppl. 6):S459–S471. doi: 10.1097/00005768-200106001-00016. [DOI] [PubMed] [Google Scholar]