Highlights
-
•
MicroRNAs have been linked to redox sensitive cellular processes.
-
•
MicroRNAs associated control of mitochondrial function, includes regulation of reactive oxygen species.
-
•
Exercise-associated redox- sensitive microRNAs regulation is an important element for exercise-mediated adaption.
Keywords: Adaptation, Exercise, MicroRNA, Oxidative damage, Reactive oxygen species, Redox regulation
Abstract
MicroRNAs (miRs) are small regulatory RNA transcripts capable of post-transcriptional silencing of mRNA messages by entering a cellular bimolecular apparatus called RNA-induced silencing complex. miRs are involved in the regulation of cellular processes producing, eliminating or repairing the damage caused by reactive oxygen species, and they are active players in redox homeostasis. Increased mitochondrial biogenesis, function and hypertrophy of skeletal muscle are important adaptive responses to regular exercise. In the present review, we highlight some of the redox-sensitive regulatory roles of miRs.
Graphical abstract
1. Introduction
MicroRNAs (miRs) are a unique subset of noncoding RNA, whose primary function is to post-transcriptionally modulate gene expression. The first miRs were discovered in the model organism Caenorhabidtitis elegans, a popular organism in genetic studies.1 The majority of miRs are transcribed from the nuclear DNA similarly to other mRNAs: by the polymerase II enzyme. After transcription, the so-called Pri-micro RNA undergoes a maturation process with multiple stages.2 First, the characteristic “hairpin” structure forms: the transcript base pairs with itself, leaving single-stranded overhangs at the 5′ and 3′ end of the hairpin. miRs may be transcribed individually or in a cluster, and they may have their own promoter. There are examples that originate from an intronic sequence, like miR-499 of the myosin heavy chain 14 (MYH14) gene3 and there are a few miRs even from protein coding exonic regions.4 Subsequent enzymatic processes, executed by Drosha and DiGeorge syndrome chromosomal region 8 (DGCR8), cleave the overhanging parts, leaving only the hairpin (pre-miR), which is exported to the cytoplasm across the exportin-5 complex. In the cytoplasm, miRs are subjected to another enzymatic processing step where the Dicer ribonuclease (RNase) cleaves the stem-loop, leaving the 18–23 base pair (bp) long, double-stranded RNA, with 2-bp overhang at the 3′ end.4 In the following sections, principles of miR-dependent gene silencing will be discussed along with their implications in skeletal muscle adaptation and their role in redox biology.
2. Mechanism of action and tissue specificity
Mature miRs, as mentioned above, are processed in a consecutive manner to form double-stranded polynucleotides. However, during their silencing function (usually), only one strand is actively used. The active strand, which is termed “the guide strand”, is loaded to the RNA-induced silencing complex (RISC).5 The other so-called passenger strand can be subject to enzymatic decay.6 In some cases, the passenger and the guide strand have similar probability of getting accepted by the silencing apparatus.7 In the RISC complex, the first 2–8 base, called the miR seed region, of the loaded miR 3′ end can formulate base pairing with mRNA messages. Usually the complementary sequence is at the 3′ untranslated region (3′ UTR) of the mRNA, and the miR–mRNA association results in decreased mRNA levels or message translatability. It is important to note that base pairing is not necessarily limited to the seed region, as other “non-canonical” forms exist. For a more comprehensive review of seed matching and related mechanisms, the reader is directed to Bartel's excellent publication.8
The accurate number of endogenous miRs in the human body is still a matter of debate. In mammals, it is believed that approximately 50% of the protein-coding RNAs are directly affected by miRs.9 However, a recent study highlighted that the computational approaches are more likely to produce false positive results, and even results of more rigid algorithms can find connections with very little or no biological significance.10 There are 1881 human miRs according to the latest GENCODE release (Version 33.0) (https://www.gencodegenes.org/human/stats.html); mirBase contains 1917 annotated hairpin precursors,11 and the canonical human “bona fide” miR pool includes around 519 miRs.12 Not surprisingly, the expression level of the individual RNA species is not homogeneous in different tissues.13, 14, 15 Moreover, studies in some cases have demonstrated obligate tissue specificity, such as the heart-specific miR-20816 and the skeletal muscle-specific miR-206.17 In the following sections, described miRs refer to microRNAs in tissue unless they are claimed to be circulating or secreted.
3. miR involvement in myocellular pathways and the connection with oxidative stress
There are many different attributes for miRs, with distinct intracellular processes being involved, including hypoxamirs18 or redoxomirs, and attributes that refer to tissue-specific myomiRs.19,20 However, the fact is that miRs do not exclusively act on a single mRNA, but can down-regulate many mRNAs, thus making it difficult to characterize miR-related pathways without overlap. One mRNA may be affected by many miRs, and that is why—somehow unexpectedly in some cases—genetic ablation of an miR or miRs does not result in a significant phenotype. One possible explanation for this phenomenon is functional redundancy, since many miRs share a common seed sequence,21 or the phenomenon can reflect the nature of some miR-related genes expression control where the suppression fine tunes the expression profile(s) rather than executes robust programs.22 As an example, knocking-out the muscle-specific miR-206 and miR-133b23 does not present with significant alteration in development or in muscle phenotype in unchallenged conditions. Similarly, the loss of the miR-23–27–24 cluster24 does not affect muscle development or exercise tolerance significantly. Although miR-125 and miR-133a26 have been shown to be substantial in cardiac development, miR-206, miR-133a, and miR-133b expression are believed to play a role in skeletal muscle differentiation and possibly in muscle growth.27 With genetic ablation, some of these miRs produce abnormal phenotypes when the genetically engineered animals are exposed to exercise.28 In line with this, if miR-206 is overexpressed, as in a mouse model for Duchenne muscular dystrophy, the progression of the disease is substantially delayed.29 Moreover, miR-206 helps to slow down denervation-induced muscle atrophy in rats.30 Other studies have demonstrated the importance of miR in myoblast fate determination31,32 and muscle regeneration.33 Paired-like homeodomain transcription factor 2 gene (Pitx2) and Pitx3, 2 transcription factors involved in paired box 7 (Pax7)-mediated muscle differentiation, have been shown to be associated with redox regulation during myogenesis, because double-conditional Pitx2/3 mouse mutants accumulated abnormal levels of reactive oxygen species (ROS).34 Furthermore, Lozano-Velasco et al.35 found that Pitx2c overexpression increases myoblast proliferation with concomitant decreases in miR-targeting cyclins, namely, 15b (miR-15b), miR-23b, miR-106b, and miR-503.
The relationship between neuromuscular disorders and ROS signaling has been established by studies in amyotrophic lateral sclerosis (ALS) models. A common experimental setup for ALS is the G93A super oxide dismutase 1 (SOD1) transgenic mouse,36 where multiple copies of the mutant DNA are inserted into the 12th chromosome.37 Animals harboring the human G93A SOD1 with a functional mutation die around the ∼150th postnatal day.38 During their shortened lifetime, accumulation of carbonylated SOD1, translationally controlled tumor protein (TCTP), ubiquitin carboxyl-terminal hydrolase-L1 (UCH-L1), and, possibly, alpha B-crystallin can be detected.39 ALS mice gripping strength was 70% less than that of the B6SJL mice wild type, and there was a decrease in total sulfhydryl groups in the skeletal muscle of symptomatic ALS mice.40 Indeed, during ALS progression, the loss of motoneurons leads to skeletal muscle atrophy and concurrent muscle weakness. Williams and colleagues41 showed that miR-206 is up-regulated both in G93A SOD1-related ALS and in surgical denervation of skeletal muscle. According to their hypothesis, muscle innervation controls miR-206 expression with myoblast determination protein (MyoD) and fibroblast growth factors (FGF)-dependent mechanisms. During denervation, MyoD is upregulated and enhances miR 206 expression. Furthermore, miR-206 targets histone deacetylases 4 (HDAC4) mRNA, the product of which may down-regulate fibroblast growth factor-binding protein (FGFBP), a potential activator of skeletal muscle reinnervation. In the normal state, the feedback mechanism will eventually down-regulate itself (reinnervation blocks MyoD activity) and a dynamic balance occurs. But in pathological situations, runaway expression of miR-206 may take place. In accordance with this idea, another study showed that MyoD promoted myogenesis by down-regulating Twist-1 by miR-206 induction.42 It is important to note that, similar to ALS, denervation is associated with increased muscle mitochondrial ROS production.43 There is a possibility that miR-206, along with other myomiRs, indirectly modulate ROS defense in skeletal muscle by helping to tune the tissue-specific gene expression environment. In fact, if myomiR miR-133a-1 and miR-133a-2 are knocked out from the mouse genome, impaired exercise tolerance occurs, and the peroxisome proliferator-activated receptor γ coactivator-1α/Mt transcription factor A (PGC-1α-mtTFA) pathway activity decreases and decreased citrate synthase activity occurs.28
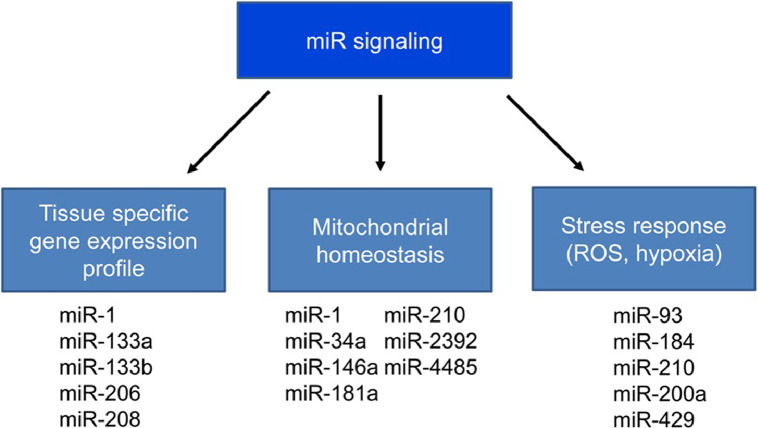
An interesting concept in miR biology is that miRs do not execute robust transcriptional programs. Instead, they change the expressional topology in respect to the tissue or other biochemical environment. The miR functional/physiological significance—meaning how severely the miR mutates or whether there is complete loss and how this affects the organism—is likely to depend on the process it modulates and on the target sensitivity or redundancy (Fig. 1).
Fig. 1.
The tissue specificity of miR production. MicroRNAs have complex regulatory roles but show tissue specificity as well. miR = microRNA; ROS = reactive oxygen species.
4. Oxidative stress-related miRs
A number of miRs have been linked to processes associated with oxidative damage. In an intestinal ischemic-reperfusion model, Hu and colleagues44 showed that miR-351-5p promotes oxidative injury, presumably by targeting sirtuin 6 (SIRT6) and mitogen-activated protein kinase 13 (MAPK13). Findings from the same group strengthen the SIRT6 MAPK13 interaction, because dioscin administration reduces the damage derived from hypoxia reoxygenation with co-occurring ROS reduction and miR-351-5p down-regulation.45 In a similar ischemia/reperfusion mouse model, Wang and coworkers46 found that significant miR-34a-5p and miR-495-3p increases aligned with ROS levels, but only with intestinal injury attenuated by miR-34a-5p inhibition. The authors suggest that this prophylactic effect is mediated by SIRT1-dependent ROS reduction, since wild-type SIRT1 mRNA contains an miR-34a-5p target site at the 3′ UTR, and mutation of that site decreases the luciferase reporter signal in Caco-2 cells. Sirtuin family member SIRT4 is also involved in ROS balance, since its translation is regulated by miR-15b. miR-15b inhibition leads to elevation of both SIRT4 mRNA and protein, which eventually has a negative impact on ROS generation and mitochondrial membrane potential.47
Oxidative damage is a major player in the pathomechanism of cardiovascular events48,49 and related inflammatory50 signaling. In normoxic conditions, the endogenous free radical scavenging system can effectively neutralize the ROS. In the case of ischemia/reperfusion, there is an oxidative overflow with potential deleterious effects. miR-210 is known as a hypoxia-induced miR,51, 52, 53 and it seems to have a tight connection with the hypoxic transcription factor-1α (HIF-1α). miR-210 is induced by HIF-1α. Under hypoxic conditions, HIF-1α is stabilized and binds to the miR-210 promoter, thus inducing its expression. Conversely, miR-210 can act as an HIF-1α enhancer by targeting glycerol-3-phosphate dehydrogenase 1-like (GPD1L), an enzyme that promotes HIF-1α proline hydroxylation54; thus, miR-210 indirectly limits the proteasomal degradation of HIF-1α. In contrast, miR-429, another member of the hypoxamir family, acts through a negative feedback loop where its expression induced by HIF-1α decreases HIF-1α stability.55
Iron-sulfur cluster scaffold homolog (ISCU) and cytochrome c oxidase assembly factor heme A (COX 10) are merged as potential mRNA targets of miR-210.56 The protein product of these genes can contribute to ROS accumulation by down-regulating mitochondrial respiration and up-regulating glycogenic processes. Also, miR-210 is known to target the succinate dehydrogenase complex, subunit D (SDHD), another member of the electron transport chain57 in A549 cells. In miR-210–transfected A549 cells, the SDHD-fused luciferase reporter is decreased upon transfection and complex II subunit (SDHA) is also down-regulated, but without significant change of the complex I subunit (NDUFA9). Enlarged mitochondria exhibiting altered cristae organization are prevalent upon miR-210 induction.57,58 In contrast, in the ischemic heart model, miR-210 overexpression is associated with better progression,59 as indicated by reduced apoptosis and higher ventricular fractional shortening.
There is great deal of interest in the role of miRs-mediated regulation of ROS and redox signaling in the brain. miR-210 increases microvascular density60 in the stroke model, where, interestingly, the brain-derived neurotrophic factor (BDNF) mRNA is found to be a direct target of miR-210. Somewhat conversely, transfected animals have higher Hexaribonucleotide Binding Protein-3 doublecortin (NeuN+/DCX+) ratios, indicating promoted neurogenesis in the hippocampal and subventricular areas. The authors of the cited study suggest an explanation according to which the pro-BDNF/mature BDNF balance is the main regulator in focal angiogenesis and neurogenesis, since in their experimental conditions, the reduction mainly occurred in the pro-BDNF pool of the ipsilateral hemisphere. In a similar transfection-based study on lentiviral vector carrying miR-210,61 it was found that overexpression of miR-210 results in up-regulation of vascular endothelial growth factor (VEGF) along with endothelial cell proliferation. HIF-1 is involved in VEGF up-regulation, and miR-210–mediated VEGF induction has recently been confirmed by novel in vivo and in vitro experiments using neural62,63 and myocardial64 cells. Ischemia/reperfusion brain injury resulted in decreased SOD activity in male C57BL/6J mice, but intra-cerebroventricular miR-93 antagomir injection increased SOD activity even above the control levels and reduced the infarct volumes.65 Moreover, miR-93 antagomir inhibited primary cortical neuron death after H2O2 treatment.
Nuclear factor erythroid-derived 2-like 2 (NFE2L2) has a pinnacle importance in governing endogenous antioxidant defense. Triggered by oxidative stress, NFE2L2 protein (complex) is stabilized and translocated into the nucleus where it binds to antioxidant response element (ARE) cis acting elements.66 NFE2L22 is a member of the cap “n” collar (CNC) transcription factor family, and includes NFE2L1, NFE2L3, Bach1, and Bach267 proteins. NFE2L1 knockout mice embryos die at a late stage of the embryonic development;68,69 loss of NFE2L2 seems to be more tolerable, but the NFE2L2-null mice are sensitive to oxidative stress70,71 and develop autoimmune diseases and die prematurely.72,73 Data suggest that miR-155 can directly influence the expression of NFE2L2.74 NFE2L2 can form hetero dimers with small musculoaponeurotic fibrosarcoma (Maf) proteins and activate gene transcription related to oxidative stress.75,76 Maf family member v-Maf avian Maf oncogene homolog G (MAFG) is repressed by miR-128 and impaired NFE2L2 activity.77 MiR-128 also promotes ROS increase78 by polycomb complex protein (Bmi-1) inhibition; and during hypoxia, miR-128 levels decrease, with an increase in MAFG and heme oxygenase 1 (HMOX-1)77 protein levels in C2C12 cells and mouse hind-limb adductor muscles.
Guanine is the most susceptible to oxidative damage among the 4 DNA bases. In contact with ROS, guanine may convert to 8-oxogunine or 8-hidroxyguanine, which can lead to DNA mutation if left uncorrected. There is a significant body of literature on ROS-related DNA modification. Importantly, a recent study showed that RNA can be also a target of ROS. One miR-related example is the oxidative modification of miR-184 and its role in apoptosis.79 In H9c2 cells, oxidative stress increased the abundance of 8-oxo-7, 8-dihydroguanosine in miR-184 and, strikingly, the oxidized form was able to reduce Bcl-w and Bcl-xl protein levels and subsequently promote apoptosis. This mechanism may serve as an emergency switch: if the cellular environment is critical in terms of redox balance, the induction of apoptosis can tone down the detrimental effect at the organismal level by hijacking the more deleterious necrosis or excessive DNA mutation.
Caloric restriction (CR) is a potent modulator of health in laboratory model organisms. In fact, CR has the capacity to increase non-selective metabolic stress resistance, including oxidative challenges. Caloric or dietary restriction can positively change the redox balance and ROS-associated molecular damage,80 but CR seems to follow a hormetic dose trajectory, since severe CR increases oxidative damage and decreases antioxidant capacity of hepatocytes.81 Interestingly, aging increases 45 known miRs species levels in the mice circulation, and CR has the potential to reduce the rising trajectory.82 There are a number of rodent studies demonstrating the CR effect on miR expression profile in different tissues, including mouse breast tissue,83 mouse liver,84,85 mouse heart,86 intrauterine rat pancreas,87 rat cerebro-microvascular endothelia,88 and rat cerebral cortex.89 In a primate model, CR has reduced the age-related s-t of miR-181b, miR-451, and miR-14490 in skeletal muscle. These studies mainly demonstrated the positive effects of CR in mitochondrial related pathways.91 In a recent study, 12 weeks of CR (first week at 20% and then 11 weeks at 40%) significantly induced mitochondrial miRs in mouse liver.92 In that experimental setup, miR-122 increase was the highest during CR and miR induction was concomitant with enhanced production of mtDNA-encoded proteins. There is a connection between CR and nuclear respiratory factor 1 (NRF-1) activity, since NRF-1, PGC-1α, and cytochrome c oxidase subunit 4 (COXIV) protein levels keep their middle-age levels during long-term CR in rats.93 Moreover, NRFs are involved in mitochondrial transport because it regulates the gene expression of TOM genes.94,95 With respect to redox balance, mitochondrial-enriched SIRT3 helped to reduce ROS levels by activating SOD2 through deacetylation96 in CR mice and, consequently, the age-associated accumulation of cellular oxidative damage may be mitigated by CR interventions. The miRs that have been associated with the above-mentioned molecular pathways are listed in Table 1.
Table 1.
MicroRNAs target various cellular signaling pathways.
| miR | mRNA target | Tissue/cell line | Seed location in 3′ UTR (hsa) | PMID | Human reference target mRNA | miR sequence |
|---|---|---|---|---|---|---|
| miR-28-5p | NFE2L2 | MCF-12A, MCF-7 and HEK293T cells | 55 | 21638050 | NM_001145413 | AAGGAGCUCACAGUCUAUUGAG |
| miR-93-5p | NFE2L2 | mouse N2A cells | 186 | 27300700 | NM_001145412 | CAAAGUGCUGUUCGUGCAGGUAG |
| miR-93-5p | NFE2L2 | MCF-10A and T47D human breast cancer cell line | 186 | 23492819 | NM_001145412 | CAAAGUGCUGUUCGUGCAGGUAG |
| miR-153-3p | NFE2L2 | SH-SY5Y neuronal cells | 98 | 23236440 | NM_001145412 | UUGCAUAGUCACAAAAGUGAUC |
| miR-27a-3p | NFE2L2 | SH-SY5Y neuronal cells | 62 | 23236440 | NM_001145412 | UUCACAGUGGCUAAGUUCCGC |
| miR-142-5p | NFE2L2 | SH-SY5Y neuronal cells | 83 | 23236440 | NM_006164 | CAUAAAGUAGAAAGCACUACU |
| miR-144-3p | NFE2L2 | SH-SY5Y neuronal cells | 265, 370 | 23236440 | NM_006164 | UACAGUAUAGAUGAUGUACU |
| miR-200a-3p | KEAP-1 | Rat hepatic stellate cell | 131 | 25049078 | NM_203500 | UAACACUGUCUGGUAACGAUGU |
| miR-200a-3p | KEAP-1 | MDA-MB-231 and Hs578T cells | 131 | 21926171 | NM_012289 | UAACACUGUCUGGUAACGAUGU |
| miR-200a-3p | KEAP-1 | Mahlavu and HuH7 cells | 131 | 23857252 | NM_203500 | UAACACUGUCUGGUAACGAUGU |
| miR-196a-5p | Bach1 | mouse embryonic fibroblast cells | 2161, 2280 | 27343195 | NM_001186 | UAGGUAGUUUCAUGUUGUUGGG |
| miR-196a-5p | Bach1 | 9-13 human hepatoma cells | 2161, 2280 | 20127796 | NM_001186 | UAGGUAGUUUCAUGUUGUUGGG |
| miR-155-5p | C/EBPβ | human and mouse mesenchymal stem cells | 554 | 28967703 | NM_001285878 | UUAAUGCUAAUCGUGAUAGGGGUU |
| miR-128-3p | MAFG | HEK293 and C2C12 cells | 1564 | 29138682 | NM_032711.4 | UCACAGUGAACCGGUCUCUUU |
| miR-195-3p | HIF-1α | ATDC 5 and HEK293T cells | 803 | 25753868 | NM_001243084 | CCAAUAUUGGCUGUGCUGCUCC |
| miR-199a-5p | HIF-1α | A2780 cells | 177 | 24706848 | NM_181054 | CCCAGUGUUCAGACUACCUGUUC |
| miR-217-5p | PGC-1α | MCF-7, MDA-MB-231 and HEK-293T cells | 3746 | 27916422 | NM_013261 | UACUGCAUCAGGAACUGAUUGGA |
| miR-494-3p | PGC-1α | 3T3-L1 white and beige adipocytes | 3788 | 30305668 | NM_013261 | UGAAACAUACACGGGAAACCUC |
| miR-34a-5p | SIRT1 | primary rat hepatocytes | 891, 1434 | 24421392 | NM_001142498 | UGGCAGUGUCUUAGCUGGUUGU |
| miR-34a-5p | SIRT1 | HCT116 cells | 891, 1435 | 18755897 | NM_001142498 | UGGCAGUGUCUUAGCUGGUUGU |
| miR-133a-3p | SIRT1 | H9c2 cells | 403 | 29487709 | NM_001142498 | UUUGGUCCCCUUCAACCAGCUG |
| miR-504-5p | NRF-1 | CNE2 and HEK 293 cells | 506 | 26201446 | NM_001040110 | AGACCCUGGUCUGCACUCUAUC |
| miR-494-3p | SIRT3 | SH-SY5Y neuronal cells | 1618 | 29567426 | NM_012239.6 | UGAAACAUACACGGGAAACCUC |
Abbreviations: Bach1 = transcription regulator protein BACH1; C/EBPβ = CCAAT/enhancer-binding protein β; HEK = human embryonic kidney; HIF-1α = hypoxia-inducible factor 1-α; KEAP-1 = Kelch-like ECH-associated protein 1; Maf = musculoaponeurotic fibrosarcoma; MAFG = v-Maf avian Maf oncogene homolog G; miR = microRNA; NFE2L2 = nuclear factor erythroid 2-related factor 2; NRF-1 = nuclear respiratory factor 1; p = point mutation; PGC-1α = peroxisome proliferator-activated receptor gamma coactivator 1-α; PMID = PubMed Unique Identifier; SIRT = sirtuin; UTR = untranslated region.
5. Can miR regulate mitochondrial function and ROS production?
Mitochondria are organelles that play a fundamental role in oxidative metabolism and cellular redox balance. Therefore, it is not surprising that any perturbation of the organellar gene expression—whether it is of nuclear or mitochondrial origin—has a major impact on cellular homeostasis. In skeletal or cardiac muscle, the mitochondrion can be a significant source of ROS production. After extracting high-energy electrons from the upstream metabolic processes, the harvested electrons progressively enter lower energy states, and this process drives the maintenance of mitochondrial membrane potential. But during metabolic stress (exercise, ischemic-reperfusion event) or in disease-related conditions, elevated numbers of electrons can escape from the system and form ROS. High-intensity interval training results in impaired oxygen supply during exercise bouts, while at the resting periods the blood supply is normalized; hence, this type of exercise training can create a situation similar to ischemia/reperfusion in the skeletal muscle.
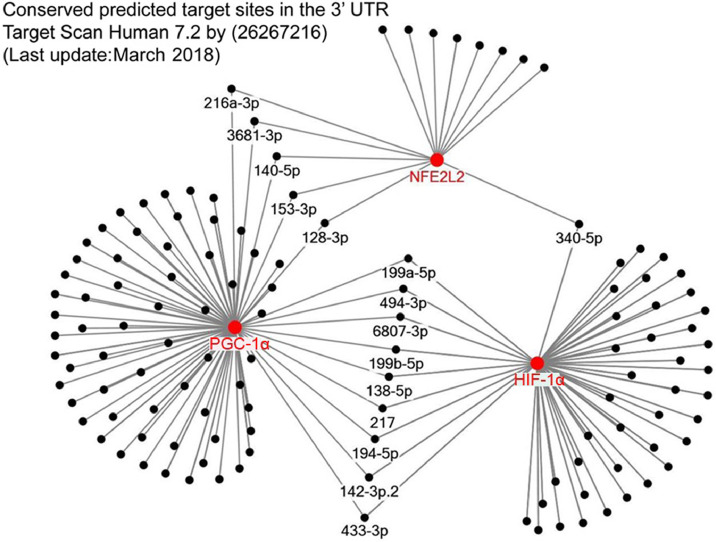
It has been well demonstrated that endurance capacity is strongly related to mitochondrial biogenesis.97 Overexpression of PGC-1α in mice leads to switch to slow type of muscle fibers and greater resistance to fatigue.98 PGC-1α is often referred to as the master regulator of mitochondrial biogenesis. Indeed, there is a great deal of literature about the involvement of PGC-1α99 in the induction of mitochondrial respiratory genes along with mitochondrial transcriptional factors like mtTFA and mtTFB, the 2 proteins controlling mtDNA replication. This mechanism depends on NRFs (NRF1 and NRF2) and downstream targets of PGC-1α. PGC-1α can be post-translationally modified by 5′ AMP-activated protein kinase (APMK)100,101 and SIRT1.102,103 Furthermore, PGC-1α-mediated pathways are activated during physical exercise and CR in AMPK104 and SIRT1-dependent105 pathways. However, SIRT1 involvement in CR cell physiology is not equivocal in all publications.106 Both SIRT1 and PGC-1α mRNA can be miR targets (Fig. 2).
Fig. 2.
MicroRNA-mediated regulation of NFE2L2, PGC-1α, and HIF-1α. The suggested microRNA-associated regulation of 3 important signaling proteins in the cell. HIF-1α = hypoxia-inducable factor 1-α; NFE2L2 = nuclear factor erythroid 2-related factor 2; PGC-1α = peroxisome proliferator-activated receptor gamma coactivator 1-α; UTR = untranslated region; p = point mutation.
It has been shown that miR-696 increased by 4 weeks of exercise training and decreased by 5 days of immobilization, and this was paralleled by the changes of PGC-1α content.107 The related cell culture studies confirmed that miR-696 is one of the regulators of PGC-1α.107
Because mitochondria play a major role in energy production during skeletal muscle contraction, it is plausible that during exercise adaptation, miRs may affect nuclear and even mitochondrial encoded transcripts. A major obstacle of the regulation of mt-transcripts by miRs is that the RNA-induced silencing complex (RISK) complex processes mRNA in the cytoplasm, and in order to implement an intra-mitochondrial effect, RISK elements, along with effector miRs, need to be transported to the mitochondrial compartment. There are some miRs that have been found to be spatially associated with mitochondria.108
It has been suggested that miR-4485 regulates mitochondrial ribosomal RNA processing, and its abundance increases after H2O2 treatment.109 Transfection with miR-4485 mimetic decreased the activity of mitochondrial respiratory complex I. However, ROS levels and membrane potential are decreased in breast cancer-derived mitochondria. Conversely, miR-4485 inhibitor also increases ROS levels, along with complex I activity and membrane potential. In fact, miR-4485 negatively affected most of the 13 protein-coding transcripts in human embryonic kidney 293 (HEK293) cells, which suggests transcriptional inhibition.109
Drawing general conclusions from studies dealing with miR subcellular location should be done with caution. Sometimes the discrepancies between the published mitomiR pathways and the reported spatial ambiguities may not always have robust biological meaning. Rather, this finding highlights the technical difficulties inherent in isolating pure cellular compartments. Contamination in miR studies can be an issue, but using cytoplasmic-enriched controls like miR-145110 aids in filtering out false detections. Subjecting the isolated mitochondria to RNase A109 digestion may help to avoid cytoplasmic or outer-membrane-bound RNA contamination.
6. miR and exercise
During physical activity the metabolic state of the working skeletal muscle changes significantly: ATP production and demand increases,111 and the tissue is subjected to greater mechanical112 and oxidative stress.113,114 High-volume or high-intensity exercise may perturb the intramuscular homeostasis at such a level that the metabolic processes can be shifted to mainly anaerobic energy production. This phenomenon results in excessive lactate accumulation,115,116 and decreased oxygen tension leads to the activation of the hypoxia-sensitive genes, like the already mentioned HIF-1α.117,118 The acute and chronic metabolic changes can turn on and off miR expression processes (like the HIF-1α–miR-210 pathway), which is likely to contribute to exercise-induced skeletal muscle adaptation.
There are numerous studies published on how various exercise stimuli affect the miR levels in the circulation,119, 120, 121 but almost always the connection is correlative, without providing information about the nature of causality. Indeed, miR-1, miR-133a, and miR-206 circulatory levels have been shown to be increased after a half-marathon run,122 but it has not been shown whether this is always a controlled secretion process with distinct physiological function. A vast number of miRs have been shown to increase in blood due to different types of exercise stimuli.119 However, the appearance of miR in the plasma can be an outcome of temporal loss of sarcolemma integrity during exercise and is not necessarily accountable to increased miR expression and/or secretion.
It has been reported that acute high-intensity resistance training changed the levels of miR-23a, miR-133a, miR-146a, miR-206, miR-378b, and miR-486 in the biopsy samples of skeletal muscle, and a relationship was not found between muscular and circulating miR levels.123 Yin and co-workers124 examined the effects of uphill and downhill running on myo-miRs (miR-1, miR-133a, miR-133b, miR-206, miR-208a, and miR-499) in quadriceps, gastrocnemius, soleus, and cardiac muscle and in the blood (exosomes and freely circulating). Results revealed that the exercise modality is one of the key factors that affects myo-miR levels. Moreover, the changes of the same miRs in the skeletal muscles, cardiac muscles, exosomes, and circulation are not significantly related.124
Some studies, on the other hand, have shown that miRs were released from microvascular compartments into the circulation due to exercise training.125,126
When the muscle biopsy samples of master athletes and age-matched control subjects were studied, it was demonstrated that aged, untrained skeletal muscle contains elevated levels of miR-7.127 Because miR-7 has been linked to impaired cellular repair and inflammation, this result suggests that 4–5 decades of training by master athletes has provided protection against sarcopenia.127 Five myo-miRs—miR-1, miR-34a, miR-133a, miR-133b, and miR-206—were measured from the biopsy samples that were taken 2 h after the acute training, with and without blood flow restriction, during the rest periods of high-intensity resistance training.128 Only the level of miR-206 was altered (i.e., significantly decreased in the leg), which was subjected to blood flow restriction. Because inhibition of miR-206 can readily enhance satellite cell proliferation and increase Pax7 protein levels, it was suggested that down-regulation of miR-206 could be important to cope with blood-flow restriction and training-caused damage and might be a necessary part of the adaptive response. One of the consequences of adaptation induced by high-intensity resistance training is muscle hypertrophy, although it is not easy to induce muscle hypertrophy in animal models using weight or anaerobic training. Therefore, we selected the very reliable compensatory hypertrophy model to study the possible role of miR in hypertrophy. Our data revealed that 2 weeks of compensatory hypertrophy resulted in a 40% increase in muscle mass and a significant increase in SIRT1 content and activity.129 It is well documented that sirtuins are redox-sensitive proteins.130 Compensatory hypertrophy was associated with decreased levels of miR-133a, which has binding sites for the SIRT1 mRNA 3′ UTR region; hence, it can down-regulate SIRT1 through its mRNA.131 Thus, it is suggested that decreased level of miR-133a was important to the upregulation of SIRT1 in the muscle hypertrophy model. Not only was miR-133a changed with compensatory hypertrophy, but the levels of miR-1 also decreased. Previously it has been demonstrated that miR-1 levels increased during muscle atrophy, so the down-regulation of miR-1 during hypertrophy is in accordance with earlier findings. We found in the hypertrophy model that the miR-214 levels increased nearly 10-fold. It has been reported that miR-214 can decrease superoxide production via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4).132 This fits nicely with our results in that we found a negative correlation between miR-214 and 2′-,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescence (r2 = –0.816, p < 0.05) and NADH levels (r2 = –0.837, p < 0.05), suggesting that miR-214 can modulate the generation of various oxidants during muscle hypertrophy.129
7. Conclusion
Our knowledge of miR-regulated redox metabolism signaling is expanding day by day. Despite the complex overlapping effects of various microRNAs, it is clear that they play an important role in adaptive responses to oxidative challenge. miRs are involved in ROS production and in the regulation of antioxidant systems and repair as well. The miR-associated control of mitochondrial function, including ROS production, is also well established. Exercise-associated redox-sensitive miR regulation is an important element for exercise-mediated adaption on redox homeostasis.
Acknowledgments
Acknowledgments
This study was supported by OTKA (112810) and National Excellence Program (126823) Grants awarded to ZR.
Authors’ contributions
FT, ZG, MJ, IB, MT, TM, and FG contributed by searching for finding and discussing the importance of all relevant literature and contributed to the manuscript writing; ZR contributed by searching for finding and discussing the importance of all relevant literature, drafted the final version of the paper, and contributed to the manuscript writing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix. Supplementary materials
References
- 1.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Bhuiyan S.S., Kinoshita S., Wongwarangkana C., Asaduzzaman M., Asakawa S., Watabe S. Evolution of the myosin heavy chain gene MYH14 and its intronic microRNA miR-499: muscle-specific miR-499 expression persists in the absence of the ancestral host gene. BMC Evol Biol. 2013;13:142. doi: 10.1186/1471-2148-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki S., Kobayashi M., Yoda M., Sakaguchi Y., Katsuma S., Suzuki T. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol Cell. 2010;39:292–299. doi: 10.1016/j.molcel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Shin C. Cleavage of the star strand facilitates assembly of some microRNAs into Ago2-containing silencing complexes in mammals. Mol Cells. 2008;26:308–313. [PubMed] [Google Scholar]
- 7.Kawamata T., Tomari Y. Making RISC. Trends Biochem Sci. 2010;35:368–376. doi: 10.1016/j.tibs.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 10.Pinzon N., Li B., Martinez L., Sergeeva A., Presumey J., Apparailly F. microRNA target prediction programs predict many false positives. Genome Res. 2017;27:234–245. doi: 10.1101/gr.205146.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozomara A., Birgaoanu M., Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47:D155–D162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fromm B., Billipp T., Peck L.E., Johansen M., Tarver J.E., King B.L. A uniform system for the annotation of vertebrate microRNA genes and the evolution of the Human microRNAome. Annu Rev Genet. 2015;49:213–242. doi: 10.1146/annurev-genet-120213-092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig N., Leidinger P., Becker K., Backes C., Fehlmann T., Pallasch C. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865–3877. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shingara J., Keiger K., Shelton J., Laosinchai-Wolf W., Powers P., Conrad R. An optimized isolation and labeling platform for accurate microRNA expression profiling. RNA. 2005;11:1461–1470. doi: 10.1261/rna.2610405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Y., Ridzon D., Wong L., Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction) J Mol Cell Cardiol. 2016;94:107–121. doi: 10.1016/j.yjmcc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy J.J. MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta. 2008;1779:682–691. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertero T., Rezzonico R., Pottier N., Mari B. Impact of microRNAs in the cellular response to hypoxia. Int Rev Cell Mol Biol. 2017;333:91–158. doi: 10.1016/bs.ircmb.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Wang X.H. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care. 2013;16:258–266. doi: 10.1097/MCO.0b013e32835f81b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy J.J. The MyomiR network in skeletal muscle plasticity. Exerc Sport Sci Rev. 2011;39:150–154. doi: 10.1097/JES.0b013e31821c01e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamanu T.K., Radovanovic A., Archer J.A., Bajic V.B. Exploration of miRNA families for hypotheses generation. Sci Rep. 2013;3:2940. doi: 10.1038/srep02940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celic T., Metzinger-Le Meuth V., Six I., Massy Z.A., Metzinger L. The mir-221/222 cluster is a key player in vascular biology via the fine-tuning of endothelial cell physiology. Curr Vasc Pharmacol. 2017;15:40–46. doi: 10.2174/1570161114666160914175149. [DOI] [PubMed] [Google Scholar]
- 23.Boettger T., Wust S., Nolte H., Braun T. The miR-206/133b cluster is dispensable for development, survival and regeneration of skeletal muscle. Skelet Muscle. 2014;4:23. doi: 10.1186/s13395-014-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M., Wada S., Oikawa S., Suzuki K., Ushida T., Akimoto T. Loss of microRNA-23-27-24 clusters in skeletal muscle is not influential in skeletal muscle development and exercise-induced muscle adaptation. Sci Rep. 2019;9:1092. doi: 10.1038/s41598-018-37765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y., Peng S., Wu M., Sachidanandam R., Tu Z., Zhang S. Multifaceted roles of miR-1s in repressing the fetal gene program in the heart. Cell Res. 2014;24:278–292. doi: 10.1038/cr.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu N., Bezprozvannaya S., Williams A.H., Qi X., Richardson J.A., Bassel-Duby R. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirby T.J., McCarthy J.J. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic Biol Med. 2013;64:95–105. doi: 10.1016/j.freeradbiomed.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie Y., Sato Y., Wang C., Yue F., Kuang S., Gavin T.P. Impaired exercise tolerance, mitochondrial biogenesis, and muscle fiber maintenance in miR-133a-deficient mice. FASEB J. 2016;30:3745–3758. doi: 10.1096/fj.201600529R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu N., Williams A.H., Maxeiner J.M., Bezprozvannaya S., Shelton J.M., Richardson J.A. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J Clin Invest. 2012;122:2054–2065. doi: 10.1172/JCI62656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Q.K., Qiao H.Y., Fu M.H., Li G., Li W.B., Chen Z. MiR-206 attenuates denervation-induced skeletal muscle atrophy in rats through regulation of satellite cell differentiation via TGF-beta1, Smad3, and HDAC4 signaling. Med Sci Monit. 2016;22:1161–1170. doi: 10.12659/MSM.897909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goljanek-Whysall K., Sweetman D., Abu-Elmagd M., Chapnik E., Dalmay T., Hornstein E. MicroRNA regulation of the paired-box transcription factor Pax3 confers robustness to developmental timing of myogenesis. Proc Natl Acad Sci U S A. 2011;108:11936–11941. doi: 10.1073/pnas.1105362108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg M.I., Georges S.A., Asawachaicharn A., Analau E., Tapscott S.J. MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J Cell Biol. 2006;175:77–85. doi: 10.1083/jcb.200603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maciotta S., Meregalli M., Cassinelli L., Parolini D., Farini A., Fraro G.D. Hmgb3 is regulated by microRNA-206 during muscle regeneration. PLoS One. 2012;7:e43464. doi: 10.1371/journal.pone.0043464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.L'honoré A., Commère P.H., Ouimette J.F., Montarras D., Drouin J., Buckingham M. Redox regulation by Pitx2 and Pitx3 is critical for fetal myogenesis. Dev Cell. 2014;29:392–405. doi: 10.1016/j.devcel.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Lozano-Velasco E., Vallejo D., Esteban F.J., Doherty C., Hernandez-Torres F., Franco D. A Pitx2-MicroRNA pathway modulates cell proliferation in myoblasts and skeletal-muscle satellite cells and promotes their commitment to a myogenic cell fate. Mol Cell Biol. 2015;35:2892–2909. doi: 10.1128/MCB.00536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 37.Achilli F., Boyle S., Kieran D., Chia R., Hafezparast M., Martin J.E. The SOD1 transgene in the G93A mouse model of amyotrophic lateral sclerosis lies on distal mouse chromosome 12. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:111–114. doi: 10.1080/14660820510035351. [DOI] [PubMed] [Google Scholar]
- 38.Pitzer C., Krüger C., Plaas C., Kirsch F., Dittgen T., Muller R. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 2008;131:3335–3347. doi: 10.1093/brain/awn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poon H.F., Hensley K., Thongboonkerd V., Merchant M.L., Lynn B.C., Pierce W.M. Redox proteomics analysis of oxidatively modified proteins in G93A-SOD1 transgenic mice–a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;39:453–462. doi: 10.1016/j.freeradbiomed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Flis D.J., Dzik K., Kaczor J.J., Cieminski K., Halon-Golabek M., Antosiewicz J. Swim training modulates mouse skeletal muscle energy metabolism and ameliorates reduction in grip strength in a mouse model of amyotrophic lateral sclerosis. Int J Mol Sci. 2019;20:E233. doi: 10.3390/ijms20020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams A.H., Valdez G., Moresi V., Qi X., McAnally J., Elliott J.L. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koutalianos D., Koutsoulidou A., Mastroyiannopoulos N.P., Furling D., Phylactou L.A. MyoD transcription factor induces myogenesis by inhibiting Twist-1 through miR-206. J Cell Sci. 2015;128:3631–3645. doi: 10.1242/jcs.172288. [DOI] [PubMed] [Google Scholar]
- 43.Muller F.L., Song W., Jang Y.C., Liu Y., Sabia M., Richardson A. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1159–R1168. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y., Tao X., Han X., Xu L., Yin L., Sun H. MicroRNA-351-5p aggravates intestinal ischaemia/reperfusion injury through the targeting of MAPK13 and Sirtuin-6. Br J Pharmacol. 2018;175:3594–3609. doi: 10.1111/bph.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Y., Mao Z., Xu L., Yin L., Tao X., Tang Z. Protective effect of dioscin against intestinal ischemia/reperfusion injury via adjusting miR-351-5p-mediated oxidative stress. Pharmacol Res. 2018;137:56–63. doi: 10.1016/j.phrs.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Wang G., Yao J., Li Z., Zu G., Feng D., Shan W. miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid Redox Signal. 2016;24:961–973. doi: 10.1089/ars.2015.6492. [DOI] [PubMed] [Google Scholar]
- 47.Lang A., Grether-Beck S., Singh M., Kuck F., Jakob S., Kefalas A. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging (Albany NY) 2016;8:484–505. doi: 10.18632/aging.100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 49.Brown D.I., Griendling K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015;116:531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutharasan R.K., Nagpal V., Ichikawa Y., Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol Heart Circ Physiol. 2011;301:H1519–H1530. doi: 10.1152/ajpheart.01080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivan M., Huang X. miR-210: fine-tuning the hypoxic response. Adv Exp Med Biol. 2014;772:205–227. doi: 10.1007/978-1-4614-5915-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X., Ding L., Bennewith K.L., Tong R.T., Welford S.M., Ang K.K. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–867. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly T.J., Souza A.L., Clish C.B., Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol. 2011;31:2696–2706. doi: 10.1128/MCB.01242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartoszewska S., Kochan K., Piotrowski A., Kamysz W., Ochocka R.J., Collawn J.F. The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1alpha expression in human endothelial cells through a negative feedback loop. FASEB J. 2015;29:1467–1479. doi: 10.1096/fj.14-267054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z., Li Y., Zhang H., Huang P., Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362–4368. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 57.Puisségur M.P., Mazure N.M., Bertero T., Pradelli L., Grosso S., Robbe-Sermesant K. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–478. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grosso S., Doyen J., Parks S.K., Bertero T., Paye A., Cardinaud B. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544. doi: 10.1038/cddis.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu S., Huang M., Li Z., Jia F., Ghosh Z., Lijkwan M.A. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122(Suppl. 11):S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng L.L., He X.S., Liu J.R., Zheng C.B., Wang Y.T., Yang G.Y. Lentivirus-mediated overexpression of microRNA-210 improves long-term outcomes after focal cerebral ischemia in Mice. CNS Neurosci Ther. 2016;22:961–969. doi: 10.1111/cns.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng L., He X., Wang Y., Tang Y., Zheng C., Cai H. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 2014;21:37–43. doi: 10.1038/gt.2013.55. [DOI] [PubMed] [Google Scholar]
- 62.Meng Z.Y., Kang H.L., Duan W., Zheng J., Li Q.N., Zhou Z.J. MicroRNA-210 promotes accumulation of neural precursor cells around ischemic foci after cerebral ischemia by regulating the SOCS1-STAT3-VEGF-C pathway. J Am Heart Assoc. 2018;7:e005052. doi: 10.1161/JAHA.116.005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H., Wu J., Wu J., Fan Q., Zhou J., Wu J. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology. 2019;17:29. doi: 10.1186/s12951-019-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arif M., Pandey R., Alam P., Jiang S., Sadayappan S., Paul A. MicroRNA-210-mediated proliferation, survival, and angiogenesis promote cardiac repair post myocardial infarction in rodents. J Mol Med (Berl) 2017;95:1369–1385. doi: 10.1007/s00109-017-1591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P., Liang X., Lu Y., Zhao X., Liang J. MicroRNA-93 downregulation ameliorates cerebral ischemic injury through the Nrf2/HO-1 defense pathway. Neurochem Res. 2016;41:2627–2635. doi: 10.1007/s11064-016-1975-0. [DOI] [PubMed] [Google Scholar]
- 66.Mitsuishi Y., Motohashi H., Yamamoto M. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol. 2012;2:200. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sykiotis G.P., Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal. 2010;3 doi: 10.1126/scisignal.3112re3. re3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan J.Y., Kwong M., Lu R., Chang J., Wang B., Yen T.S. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17:1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Kwong M., Lu R., Ginzinger D., Lee C., Leung L. Nrf1 is critical for redox balance and survival of liver cells during development. Mol Cell Biol. 2003;23:4673–4686. doi: 10.1128/MCB.23.13.4673-4686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu K.C., Liu J., Klaassen C.D. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol Appl Pharmacol. 2012;262:321–329. doi: 10.1016/j.taap.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y.K., Wu K.C., Klaassen C.D. Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice. PLoS One. 2013;8:e59122. doi: 10.1371/journal.pone.0059122. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Q., Battelli L., Hubbs A.F. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am J Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoh K., Itoh K., Enomoto A., Hirayama A., Yamaguchi N., Kobayashi M. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 74.Onodera Y., Teramura T., Takehara T., Obora K., Mori T., Fukuda K. miR-155 induces ROS generation through downregulation of antioxidation-related genes in mesenchymal stem cells. Aging Cell. 2017;16:1369–1380. doi: 10.1111/acel.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katsuoka F., Yamamoto M. Small Maf proteins (MafF, MafG, MafK): history, structure and function. Gene. 2016;586:197–205. doi: 10.1016/j.gene.2016.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katsuoka F., Motohashi H., Ishii T., Aburatani H., Engel J.D., Yamamoto M. Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol Cell Biol. 2005;25:8044–8051. doi: 10.1128/MCB.25.18.8044-8051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caggiano R., Cattaneo F., Moltedo O., Esposito G., Perrino C., Trimarco B. miR-128 is implicated in stress responses by targeting MAFG in skeletal muscle cells. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9308310. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venkataraman S., Alimova I., Fan R., Harris P., Foreman N., Vibhakar R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS One. 2010;5:e10748. doi: 10.1371/journal.pone.0010748. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J.X., Gao J., Ding S.L., Wang K., Jiao J.Q., Wang Y. Oxidative modification of miR-184 enables it to target Bcl-xL and Bcl-w. Mol Cell. 2015;59:50–61. doi: 10.1016/j.molcel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Vucevic D., Mladenovic D., Ninkovic M., Aleksic V., Stankovic M.N., Stankovic M. The effects of caloric restriction against ethanol-induced oxidative and nitrosative cardiotoxicity and plasma lipids in rats. Exp Biol Med (Maywood) 2013;238:1396–1405. doi: 10.1177/1535370213506806. [DOI] [PubMed] [Google Scholar]
- 81.Stankovic M., Mladenovic D., Ninkovic M., Vucevic D., Tomasevic T., Radosavljevic T. Effects of caloric restriction on oxidative stress parameters. Gen Physiol Biophys. 2013;32:277–283. doi: 10.4149/gpb_2013027. [DOI] [PubMed] [Google Scholar]
- 82.Dhahbi J.M., Spindler S.R., Atamna H., Yamakawa A., Guerrero N., Boffelli D. Deep sequencing identifies circulating mouse miRNAs that are functionally implicated in manifestations of aging and responsive to calorie restriction. Aging (Albany NY) 2013;5:130–141. doi: 10.18632/aging.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ørom U.A., Lim M.K., Savage J.E., Jin L., Saleh A.D., Lisanti M.P. MicroRNA-203 regulates caveolin-1 in breast tissue during caloric restriction. Cell Cycle. 2012;11:1291–1295. doi: 10.4161/cc.19704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makwana K., Patel S.A., Velingkaar N., Ebron J.S., Shukla G.C., Kondratov R. Aging and calorie restriction regulate the expression of miR-125a-5p and its target genes Stat3, Casp2 and Stard13. Aging (Albany NY) 2017;9:1825–1843. doi: 10.18632/aging.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kulkarni S.R., Armstrong L.E., Slitt A.L. Caloric restriction-mediated induction of lipid metabolism gene expression in liver is enhanced by Keap1-knockdown. Pharm Res. 2013;30:2221–2231. doi: 10.1007/s11095-013-1138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noyan H., El-Mounayri O., Isserlin R., Arab S., Momen A., Cheng H.S. Cardioprotective signature of short-term caloric restriction. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang L., Chen W., Dai Y., Zhu Z., Liu Q. Detection of expressional changes induced by intrauterine growth restriction in the developing rat pancreas. Exp Biol Med (Maywood) 2016;241:1446–1456. doi: 10.1177/1535370216638771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Csiszar A., Gautam T., Sosnowska D., Tarantini S., Banki E., Tucsek Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wood S.H., van Dam S., Craig T., Tacutu R., O'Toole A., Merry B.J. Transcriptome analysis in calorie-restricted rats implicates epigenetic and post-translational mechanisms in neuroprotection and aging. Genome Biol. 2015;16:285. doi: 10.1186/s13059-015-0847-2. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mercken E.M., Majounie E., Ding J., Guo R., Kim J., Bernier M. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany NY) 2013;5:692–703. doi: 10.18632/aging.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walsh M.E., Shi Y., Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med. 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang R., Wang X., Qu J.H., Liu B., Zhang P., Zhang T. Caloric restriction induces microRNAs to improve mitochondrial proteostasis. iScience. 2019;17:155–166. doi: 10.1016/j.isci.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Picca A., Pesce V., Fracasso F., Joseph A.M., Leeuwenburgh C., Lezza A.M. Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver. PLoS One. 2013;8:e74644. doi: 10.1371/journal.pone.0074644. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blesa J.R., Prieto-Ruiz J.A., Abraham B.A., Harrison B.L., Hegde A.A., Hernández-Yago J. NRF-1 is the major transcription factor regulating the expression of the human TOMM34 gene. Biochem Cell Biol. 2008;86:46–56. doi: 10.1139/o07-151. [DOI] [PubMed] [Google Scholar]
- 95.Blesa J.R., Prieto-Ruiz J.A., Hernández J.M., Hernández-Yago J. NRF-2 transcription factor is required for human TOMM20 gene expression. Gene. 2007;391:198–208. doi: 10.1016/j.gene.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 96.Qiu X., Brown K., Hirschey M.D., Verdin E., Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 97.Pilegaard H., Saltin B., Neufer P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin J., Wu H., Tarr P.T., Zhang C.Y., Wu Z., Boss O. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 99.Spiegelman B.M. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–63. [PubMed] [Google Scholar]
- 100.Vaughan R.A., Mermier C.M., Bisoffi M., Trujillo K.A., Conn C.A. Dietary stimulators of the PGC-1 superfamily and mitochondrial biosynthesis in skeletal muscle. A mini-review. J Physiol Biochem. 2014;70:271–284. doi: 10.1007/s13105-013-0301-4. [DOI] [PubMed] [Google Scholar]
- 101.Jäger S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{α} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 103.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 104.Burkewitz K., Zhang Y., Mair W.B. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menzies K.J., Singh K., Saleem A., Hood D.A. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013;288:6968–6979. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Higashida K., Kim S.H., Jung S.R., Asaka M., Holloszy J.O., Han D.H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001603. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aoi W., Naito Y., Mizushima K., Takanami Y., Kawai Y., Ichikawa H. The microRNA miR-696 regulates PGC-1{α} in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab. 2010;298:E799–E806. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- 108.Sripada L., Tomar D., Singh R. Mitochondria: one of the destinations of miRNAs. Mitochondrion. 2012;12:593–599. doi: 10.1016/j.mito.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 109.Sripada L., Singh K., Lipatova A.V., Singh A., Prajapati P., Tomar D. hsa-miR-4485 regulates mitochondrial functions and inhibits the tumorigenicity of breast cancer cells. J Mol Med (Berl) 2017;95:641–651. doi: 10.1007/s00109-017-1517-5. [DOI] [PubMed] [Google Scholar]
- 110.Sripada L., Tomar D., Prajapati P., Singh R., Singh A.K., Singh R. Systematic analysis of small RNAs associated with human mitochondria by deep sequencing: detailed analysis of mitochondrial associated miRNA. PLoS One. 2012;7:e44873. doi: 10.1371/journal.pone.0044873. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gaitanos G.C., Williams C., Boobis L.H., Brooks S. Human muscle metabolism during intermittent maximal exercise. J Appl Physiol (1985) 1993;75:712–719. doi: 10.1152/jappl.1993.75.2.712. [DOI] [PubMed] [Google Scholar]
- 112.Olsen L.A., Nicoll J.X., Fry A.C. The skeletal muscle fiber: a mechanically sensitive cell. Eur J Appl Physiol. 2019;119:333–349. doi: 10.1007/s00421-018-04061-x. [DOI] [PubMed] [Google Scholar]
- 113.Nemes R., Koltai E., Taylor A.W., Suzuki K., Gyori F., Radak Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants (Basel) 2018;7:E85. doi: 10.3390/antiox7070085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Powers S.K., Radak Z., Ji L.L. Exercise-induced oxidative stress: past, present and future. J Physiol. 2016;594:5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kristensen D.E., Albers P.H., Prats C., Baba O., Birk J.B., Wojtaszewski J.F. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J Physiol. 2015;593:2053–2069. doi: 10.1113/jphysiol.2014.283267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peake J.M., Tan S.J., Markworth J.F., Broadbent J.A., Skinner T.L., Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab. 2014;307:E539–E552. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 117.Favier F.B., Britto F.A., Freyssenet D.G., Bigard X.A., Benoit H. HIF-1-driven skeletal muscle adaptations to chronic hypoxia: molecular insights into muscle physiology. Cell Mol Life Sci. 2015;72:4681–4696. doi: 10.1007/s00018-015-2025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lindholm M.E., Rundqvist H. Skeletal muscle hypoxia-inducible factor-1 and exercise. Exp Physiol. 2016;101:28–32. doi: 10.1113/EP085318. [DOI] [PubMed] [Google Scholar]
- 119.Domańska-Senderowska D., Laguette M.N., Jegier A., Cieszczyk P., September A.V., Brzeziańska-Lasota E. MicroRNA profile and adaptive response to exercise training: a review. Int J Sports Med. 2019;40:227–235. doi: 10.1055/a-0824-4813. [DOI] [PubMed] [Google Scholar]
- 120.Fernández-Sanjurjo M., de Gonzalo-Calvo D., Fernández-García B., Díez-Robles S., Martínez-Canal Á, Olmedillas H. Circulating microRNA as emerging biomarkers of exercise. Exerc Sport Sci Rev. 2018;46:160–171. doi: 10.1249/JES.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 121.Polakovičová M., Musil P., Laczo E., Hamar D., Kyselovič J. Circulating microRNAs as potential biomarkers of exercise response. Int J Mol Sci. 2016;17:E1553. doi: 10.3390/ijms17101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gomes C.P., Oliveira G.P., Jr., Madrid B., Almeida J.A., Franco O.L., Pereira R.W. Circulating miR-1, miR-133a, and miR-206 levels are increased after a half-marathon run. Biomarkers. 2014;19:585–589. doi: 10.3109/1354750X.2014.952663. [DOI] [PubMed] [Google Scholar]
- 123.D'Souza R.F., Markworth J.F., Aasen K.M.M., Zeng N., Cameron-Smith D., Mitchell C.J. Acute resistance exercise modulates microRNA expression profiles: combined tissue and circulatory targeted analyses. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181594. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yin X., Zhao Y., Zheng Y.L., Wang J.Z., Li W., Lu Q.J. Time-course responses of muscle-specific microRNAs following acute uphill or downhill exercise in Sprague-Dawley rats. Front Physiol. 2019;10:1275. doi: 10.3389/fphys.2019.01275. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lovett J.A.C., Durcan P.J., Myburgh K.H. Investigation of circulating extracellular vesicle microRNA following two consecutive bouts of muscle-damaging exercise. Front Physiol. 2018;9:1149. doi: 10.3389/fphys.2018.01149. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma C., Wang J., Liu H., Chen Y., Ma X., Chen S. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med Sci Sports Exerc. 2018;50:2024–2032. doi: 10.1249/MSS.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 127.Koltai E., Bori Z., Osvath P., Ihasz F., Peter S., Toth G. Master athletes have higher miR-7, SIRT3 and SOD2 expression in skeletal muscle than age-matched sedentary controls. Redox Biol. 2018;19:46–51. doi: 10.1016/j.redox.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Torma F., Gombos Z., Fridvalszki M., Langmar G., Tarcza Z., Merkely B. Blood flow restriction in human skeletal muscle during rest periods after high-load resistance training down-regulates miR 206 and induces Pax7. J Sport Health Sci. 2019 doi: 10.1016/j.jshs.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koltai E., Bori Z., Chabert C., Dubouchaud H., Naito H., Machida S. SIRT1 may play a crucial role in overload-induced hypertrophy of skeletal muscle. J Physiol. 2017;595:3361–3376. doi: 10.1113/JP273774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Radak Z., Koltai E., Taylor A.W., Higuchi M., Kumagai S., Ohno H. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic Biol Med. 2013;58:87–97. doi: 10.1016/j.freeradbiomed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 131.Forterre A., Jalabert A., Chikh K., Pesenti S., Euthine V., Granjon A. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle. 2014;13:78–89. doi: 10.4161/cc.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ma W., Li J., Hu J., Cheng Y., Wang J., Zhang X. miR214-regulated p53-NOX4/p66shc pathway plays a crucial role in the protective effect of Ginkgolide B against cisplatin-induced cytotoxicity in HEI-OC1 cells. Chem Biol Interact. 2016;245:72–81. doi: 10.1016/j.cbi.2016.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.