The orders Cellvibrionales, Oceanospirillales, and Pseudomonadales, as three major orders of the largest bacterial class, Gammaproteobacteria, play important roles in various ecosystems as the keystone taxa of microbiomes, but their evolutionary relationship is currently polyphyletic and chaotic. Here, we constructed a bac120 tree and core-genome tree and calculated the amino acid identity (AAI) value to explore their intrinsic evolutionary history. In this study, we proposed two novel orders and three novel families. This evolution study vastly reconstructed the taxonomic framework of class Gammaproteobacteria and could provide a more distinct perspective on global distribution and evolutionary patterns of these environmental microorganisms.
KEYWORDS: class Gammaproteobacteria , core genome, Moraxellales ord. nov., Kangiellales ord. nov., Marinobacteraceae fam. nov., Zooshikellaceae fam. nov., Perlucidibacaceae fam. nov., Pseudomonadales ord. nov.
ABSTRACT
Orders Oceanospirillales and Pseudomonadales play important roles in various ecosystems as the keystone taxa of microbiomes. However, the two orders present a close evolutionary relationship, which might have caused taxonomic misinterpretation and resulted in an incorrect understanding of their evolutionary history. In this study, first, we used the 16S rRNA gene sequences of 2,049 species of Gammaproteobacteria to build a phylogenetic tree, which demonstrated that reports regarding the evolutionary relationship of orders Cellvibrionales, Oceanospirillales, and Pseudomonadales based on a single conserved gene with a poor resolution have been conflicting; in particular, the major families Moraxellaceae and Pseudomonadaceae of order Pseudomonadales were separated from orders Cellvibrionales and Oceanospirillales. Subsequently, we constructed the bac120 trees of all representative reference genomes of class Gammaproteobacteria based on 120 ubiquitous single-copy proteins from bacteria and a phylogenomic tree based on the 119 core genes of 257 reference genomes obtained from orders Cellvibrionales, Oceanospirillales, and Pseudomonadales to cross validate and infer their intrinsic evolutionary relationships. These results indicated that two novel orders, Moraxellales ord. nov. and Kangiellales ord. nov., and three novel families, Marinobacteraceae fam. nov., Perlucidibacaceae fam. nov., and Zooshikellaceae fam. nov., should be proposed. Additionally, orders Cellvibrionales and Oceanospirillales were merged into the order Pseudomonadales except for families Moraxellaceae and Kangiellaceae in class Gammaproteobacteria, which currently includes 18 families. Our work sheds some light on the evolutionary history of class Gammaproteobacteria, which could facilitate the detection and taxonomic analysis of natural communities.
IMPORTANCE The orders Cellvibrionales, Oceanospirillales, and Pseudomonadales, as three major orders of the largest bacterial class, Gammaproteobacteria, play important roles in various ecosystems as the keystone taxa of microbiomes, but their evolutionary relationship is currently polyphyletic and chaotic. Here, we constructed a bac120 tree and core-genome tree and calculated the amino acid identity (AAI) value to explore their intrinsic evolutionary history. In this study, we proposed two novel orders and three novel families. This evolution study vastly reconstructed the taxonomic framework of class Gammaproteobacteria and could provide a more distinct perspective on global distribution and evolutionary patterns of these environmental microorganisms.
INTRODUCTION
The tree of life is arguably the most important organizing principle in biology and perhaps the most widely understood depiction of the evolutionary process. It explains how we are related to other organisms and where we may have come from (1). With the continuous reduction in sequencing costs and new developments in biotechnology and bioinformatic tools, a multigene-based phylogenomic tree approach in which genomic data are used for phylogenomic analysis appears to be a better approach for defining genera or higher taxa than the use of 16S rRNA gene-derived phylogeny (2). In August 2018, Parks et al. (3) proposed a standardized bacterial taxonomy based on a genome phylogeny and substantially revised the tree of life. We believe that the new classification framework will provide important guidance for future reclassification studies.
Gammaproteobacteria spreads throughout global ecosystems, including marine, land, and sediment environments and animal hosts, and represents the largest bacterial class, including 19 orders, 58 families, and 381 genera according to LPSN and the website https://www.ezbiocloud.net/taxonomy?tn=Gammaproteobacteria&depth=3. To date, 78,338 Gammaproteobacteria genomes have been deposited in the National Center for Biotechnology Information (NCBI) database (October 2019); the representative genomes in the RefSeq category include 808 genomes. Therefore, phylogenomic tree construction based on all genomes of Gammaproteobacteria is difficult due to the restriction of computing resources; alternatively, the construction of an evolutionary tree based the representative genomes in the RefSeq category is relatively easy and accurate.
Cellvibrionales, Oceanospirillales, and Pseudomonadales are three major orders of Gammaproteobacteria that play important roles in various ecosystems as the keystone taxa of microbiomes (4). For instance, order Cellvibrionales has a putative important function in oligotrophic marine environments (5). The order Oceanospirillales shows remarkable potential for the natural attenuation of spilled oil in deep-sea surface sediments (6), and almost all species of family Endozoicomonadaceae have been isolated from marine animals, while most members of genus Zooshikella can produce prodigiosin, which is an effective proapoptotic agent that can be used against various cancer cell lines while showing little or no toxicity toward normal cell lines (7). The order Pseudomonadales plays an important role in contaminated soil remediation and plant-associated microbiota (4), and many members of order Pseudomonadales present clear associations with human health as pathogens, such as Acinetobacter baumannii, Moraxella catarrhalis, and Pseudomonas aeruginosa.
As of the writing of the manuscript, order Oceanospirillales includes 11 families (https://lpsn.dsmz.de/order/oceanospirillales), order Pseudomonadales includes 3 families (https://lpsn.dsmz.de/order/pseudomonadales), and order Cellvibrionales includes 5 families (5). In the last decade, based on rapid advances in phylogenetic and molecular analyses, several revisions have been carried out in the order Oceanospirillales, with numerous genera being split into separate families (5); for instance, family Endozoicomonadaceae was split from family Hahellaceae in 2018 (8). Additionally, genus Marinobacterium was reclassified into family Oceanospirillaceae (9), indicating that the systematic evolution of the order is still unclear due to the discovery of increasing numbers of species. In 2017, we discovered a novel genus, Mangrovitalea, which is closely related to genus Marinobacter, and we classified this new genus into order Alteromonadales (10). However, in this study, we found that genera Tamilnaduibacter (11), Mangrovitalea, and Marinobacter formed a robust clade in a phylogenetic tree and that they were distantly phylogenetically related to Alteromonadales; therefore, they should be allocated to higher taxonomic ranks. The major families Moraxellaceae and Pseudomonadaceae of order Pseudomonadales were separated by a branch containing orders Cellvibrionales and Oceanospirillales according to the 16S rRNA-based The All-Species Living Tree (LTP), release 132, which illustrated that the order Pseudomonadales had polyphyletic lineages. Intriguingly, in 2018, the Genome Taxonomy Database (GTDB) taxonomy proposed the transfer of the majority of the members of orders Oceanospirillales and Cellvibrionales to order Pseudomonadales; however, this classification has not been proposed anywhere in the literature, and thus, the intrinsic evolutionary relationship of orders Oceanospirillales, Cellvibrionales, and Pseudomonadales is still a question worth discussing.
RESULTS AND DISCUSSION
Phylogenetic tree based on small-subunit (SSU)-rRNA of Gammaproteobacteria.
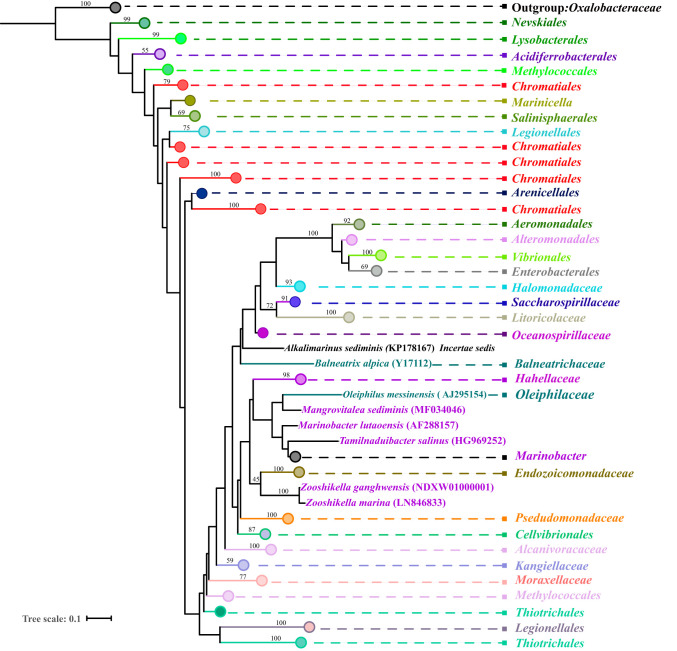
Figure 1 was generated based on 16S rRNA gene sequences, and 2,049 sequences representing 2,049 species of class Gammaproteobacteria with validly published names were downloaded from the SILVA Living Tree Project v128 database and the EzBioCloud database. The results indicated that genera Marinobacter, Mangrovitalea, and Tamilnaduibacter formed a monophyletic clade with family Oleiphilaceae (Fig. 1), which was also shown by the GTDB phylogeny reconstructed from 120 ubiquitous single-copy protein-coding genes (3), and these genera were distantly related to order Alteromonadales, implying that the monophyletic clade could represent a novel family. We refer to the clade as group 1 in the following text.
FIG 1.
Maximum-likelihood tree showing the phylogenetic evolution of class Gammaproteobacteria. The phylogeny was inferred from 16S rRNA gene sequences of almost all type strains of Gammaproteobacteria in a maximum-likelihood framework by using RAxML with the GTRGAMMA model. Family Oxalobacteraceae was used as the outgroup of the tree. Bootstrap values are based on 1,000 replicates, and only bootstrap values greater than 40 are shown.
According to the phylogenetic tree based on 16S rRNA genes in group 1, Zooshikella, Endozoicomonadaceae, Pseudomonadaceae, and Cellvibrionales presented an indication of sharing a relatively close ancestor, whereas Pseudomonadaceae and Moraxellaceae were separated on different branches (Fig. 1). However, the topological structure of the branch with low bootstrap values (Fig. 1) indicated that the tree was unstable; therefore, it was also unclear what order group 1 belongs to in the tree.
Evolutionary analysis based on the genomes.
We constructed a bac120 tree based on 120 concatenated ubiquitous single-copy proteins of bacteria (12) (Fig. 2; see also Fig. S1 in the supplemental material) from a total of 783 genomes (completeness >90% and contamination <5%) (Table S1) by using FastTree software according to the method described by Parks et al. (3). In 2018, the GTDB taxonomy proposed the transfer of the majority of the members of Oceanospirillales and Cellvibrionales and group 1 to Pseudomonadales, and Kangiellaceae was transferred to order Enterobacterales (3). Similarly, orders Pseudomonadales, Cellvibrionales, and Oceanospirillales and group 1 were also clustered on a branch with the support of the highest bootstrap value of 1.0, except for family Kangiellaceae in class Gammaproteobacteria, and according to Fig. 2a and Fig. S1, the family Kangiellaceae formed an independent branch at the order level that was different from GTDB taxonomy. In addition, the clade of family Moraxellaceae displayed the longest length in Fig. 2b and was located away from other families of order Pseudomonadales; Fig. 2b indicated they were partitioned by the branch of the order Cellvibrionales.
FIG 2.
The unrooted maximum-likelihood tree was constructed by using FastTree with the WAG+CAT model based on 120 concatenated protein amino acid sequences of the 783 genomes. Each tip represents a species. (a) A pruned subtree from the unrooted maximum-likelihood tree. The bootstrap value of the backbone is displayed with a number. (b) Bootstrap values (from 0.9 to 1) are shown with filled circles. The tree was modified and visualized using the Interactive Tree of Life (iTOL 4.3) (itol.embl.de/).
The uncollapsed tree from Fig. 2a provides all clade information. Download FIG S1, PDF file, 1.5 MB (1.5MB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Main information for the genome sequences used to reconstruct the bac120 tree. Download Table S1, XLSX file, 0.1 MB (144.9KB, xlsx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Then, we chose 257 reference genomes of Pseudomonadales, Cellvibrionales, Oceanospirillales, and group 1 for further analysis; the major information for these genomes is collected in Table S2. The pangenomes of the reference genomes were analyzed, and the results indicated that they shared 119 core genes, which were annotated according to the UniProt database (13); these proteins are mostly involved in DNA replication, transcription and translation, and ATP production. The sizes of the core and pangenomes were strongly dependent on the number of genomes analyzed, resulting in shrinking core genomes and expanding pangenomes with an increase in the depth of genome sampling (Fig. S2).
Core–pangenome size evolution of the 257 reference genomes. Pangenome size (green) is directly proportional to the number of genomes. Core-genome size (yellow) is inversely proportional to the number of genomes. Download FIG S2, DOCX file, 0.4 MB (439.8KB, docx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Main information for the 257 reference genome sequences used to reconstruct the core-genome tree. Download Table S2, XLSX file, 0.05 MB (52.5KB, xlsx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Subsequently, a core-genome-based phylogeny was reconstructed based on 119 concatenated single-copy core genes of the 257 genomes with optimal models by using the IQtree package. The results showed that the topological structure of the tree based on the core genome (Fig. 3 and Fig. S3) was highly similar to the bac120 tree (Fig. 2b and Fig. S1).
FIG 3.
Maximum-likelihood tree constructed by using IQtree with the optimal model based on the concatenated core-genome sequences of the 257 genomes. Bootstrap values (expressed as percentages of 1,000 replicates) greater than 90 are shown at branch points with filled circles, and the bootstrap value of the backbone is displayed with a number. The tree was modified and drawn using the Interactive Tree of Life (iTOL 4.3) (itol.embl.de/).
The uncollapsed tree from Fig. 3 provides all clade information. Download FIG S3, PDF file, 0.4 MB (456.7KB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
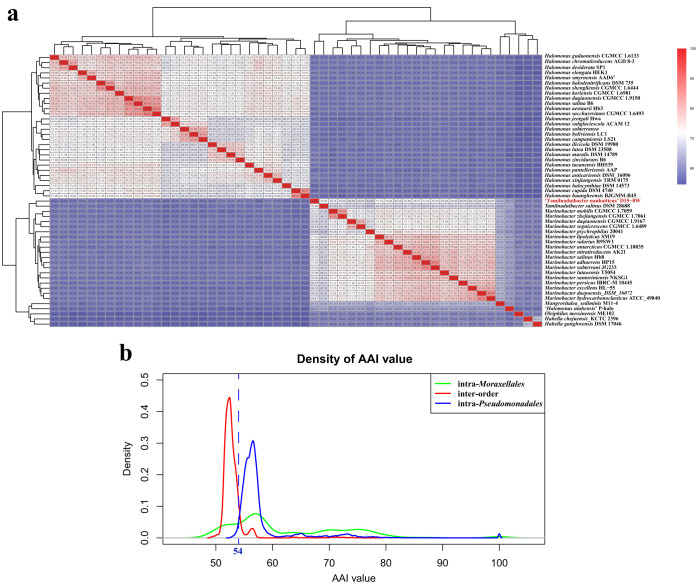
The amino acid identity (AAI) values between the 257 genomes were calculated as well because AAI values are used for prokaryotic taxonomic analyses (14). The AAI comparisons conducted by Luo et al. (15) indicated that related but different genera typically exhibit values ranging from 60% to 80%; thus, interfamilies typically exhibit values of less than 60%. In our study, the AAI values were clustered in a heatmap via the complete method of hclust (Fig. 4); we found that the interfamily AAI values were below 60% and that intrafamily AAI values were mostly greater than 60% (Fig. S4), consistent with the work of Luo et al. (15).
FIG 4.
(a) Heatmap showing the AAI values between genera Halomonas, Marinobacter, Mangrovitalea, and Tamilnaduibacter. One species in red, Marinobacter nanhaiticus, was renamed “Tamilnaduibacter nanhaiticus” in the present study, respectively. (b) Density of the AAI values of intra-Moraxellales (green line); the interorder (red line) between orders Moraxellales and Pseudomonadales; and intra-Pseudomonadales (blue line). The names of the orders were proposed in the study (blue line) (b).
Heatmap showing the AAI values between the 257 genomes. Download FIG S4, PDF file, 1.2 MB (1.2MB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The order Oceanospirillales was paraphyletic (Fig. 2a and Fig. 3). The type genus Kangiella (16) of family Kangiellaceae (17) formed a stably separate clade, was positioned away from order Oceanospirillales, and was distinct from closely related orders Aeromonadales (18) and Thiotrichales (19) of class Gammaproteobacteria based on the bac120 tree with bootstrap value 100 (Fig. 2a and Fig. S1). In terms of physiological phenotypic characteristics, the genomic G+C content of family Kangiellaceae ranged from 40.1 to 44.4%, whereas that of Oceanospirillales ranged from 43.1 to 68.6%, revealing a significant difference (Wilcoxon test; P < 0.01). Additionally, extracellular protein degradation and amino acid utilization are significant and prominent features of the type genus Kangiella of family Kangiellaceae due to the absence of a complete pathway for carbohydrate metabolism according to the description of Wang et al. (20); for instance, almost all members of genus Kangiella can hydrolyze casein and gelatin, while most of the members of order Oceanospirillales were negative for that (Table 1). In terms of fatty acid composition characteristics, the major fatty acid components of family Kangiellaceae are iso-C15:0, C16:0 10-methy, and iso-C11:0 3-OH (21), which are obviously different from almost all other members of order Oceanospirillales, in which C16:0, C16 : 1 ω7c, and/or C16 : 1 ω6c are the major fatty acid components, The major polar lipids of almost all members of family Kangiellaceae were phosphatidylglycerol (PG), phosphatidylethanolamine (PE), and phosphatidylmonomethylethanolamine (PME) (22), while those for the order Oceanospirillales were diphosphatidylglycerol (DPG), PE, and PG. Additionally, Q-8 was the predominant ubiquinone of family Kangiellaceae, while the predominant ubiquinone is Q-9 in all other members of order Oceanospirillales. A Manhattan-based principal-coordinate analysis (PCoA) of the gene presence and absence profile also showed that the members of the type genus Kangiella of family Kangiellaceae formed a cluster divided from other genera of order Oceanospirillales (Fig. S5). These evidences indicated that family Kangiellaceae should be reclassified as the novel order Kangiellales ord. nov., including the family Kangiellaceae, of which the type genus is Kangiella. Despite the GTDB classifying the order Kangiellales into the order Enterobacterales (23, 24), however, the order Enterobacterales was very large, including some clades with excessive branch length based on the bac120 tree (Fig. S1 and Fig. 2a). Additionally, the major fatty acids of almost all members of order Enterobacterales were C14:0, C16:0, C18:1 ω7c, and C17:0 (24), illustrating an obvious difference from order Kangiellales. Therefore, we inferred the classification was inaccurate in the GTDB.
TABLE 1.
Phenotypic characteristics of Kangiellales, Moraxellales, Pseudomonadales, Enterobacterales, and Perlucidibacaceaea
| Kangiellales | Moraxellales | Pseudomonadales | Enterobacterales | Perlucidibacaceae | |
|---|---|---|---|---|---|
| Cell shape | Rods | Short rods, coccoid,
or coccal |
Rods, spiral | Rods | Rods |
| G+C content (%) | 40.1–44.4 | 38–48 | 43.1–68.6 | 22–60 | 55–65 |
| Fatty acids | iso-C15:0, C16:0 10-methy,
and iso-C11:0 3-OH |
C18:1
ω9c, C18:0, C16:0, and C16:1 ω6c/C16:1 ω7c |
C16:0, C16 : 1
ω7c, and/or C16 : 1 ω6c |
C14:0, C16:0, C16:1 ω7c |
C16:0, C18:1
ω7c, C16 : 1
ω7c and/or C16 : 1 ω6c, and C12:0 3-OH |
| Ubiquinone | Q8 | Q8 | Q9 | NA | Q12 |
| Flagellation | + | − | + | + | + |
| Hydrolysis of: | |||||
| Casein | + | NA | V | NA | NA |
| Gelatin | + | NA | V | NA | NA |
The data are from original isolation papers and/or Bergey’s Manual. References are as follows: Kangiellales, 17; Moraxellales, 29; Pseudomonadales, 30, 36; Enterobacterales, 30. The names of the orders or family were proposed in the study. NA, not applicable; +, present/tested positive; −, absent/tested negative; V, variable among strains.
Manhattan distance-based principal-coordinate analysis (PCoA) showing the difference in the gene presence and absence profiles between different genera. Download FIG S5, PDF file, 0.7 MB (714.5KB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Intriguingly, the shared gene blocks of Pseudomonadaceae (including genera Azotobacter, Pseudomonas, and Oblitimonas) and Moraxellaceae (including genera Acinetobacter, Alkanindiges, Moraxella, Perlucidibaca [25], and Psychrobacter) displayed obvious distinction, and the dendrogram of heatmap rows revealed that they formed an independent branch that was consistent with the topology of the bac120 and core-genome tree (Fig. 3 and Fig. S3 and S6). Additionally, the type genus Ventosimonas of family Ventosimonadaceae formed a clade within family Pseudomonadaceae with a long branch length in the bac120 and core-genome tree (Fig. S1 and S5). We also observed that Alcanivoracaceae, Balneatrichaceae, Halomonadaceae, Hahellaceae, Oleiphilaceae, Oceanospirillaceae, Saccharospirillaceae, Zooshikellaceae, Pseudomonadaceae, Ventosimonadaceae, Cellvibrionaceae, Halieaceae, Microbulbiferaceae, Porticoccaceae, Spongiibacteraceae, and group 1 shared more genes with each other than they shared with Moraxellaceae (Fig. S6). A Manhattan-based principal-coordinate analysis (PCoA) of the gene presence and absence profile also showed that the members of the type genus Pseudomonas of family Pseudomonadeae and the type genus Moraxella of family Moraxellaceae clustered in different quadrants (Fig. S6). Additionally, the phenotypic characteristics between family Moraxellaceae and other families of order Pseudomonadales are notably different. First, almost all members of family Moraxellaceae contain C18:1 ω9c as a major fatty acid component (26, 27), while the component was not detected in other families of Pseudomonadales (28). Second, the cell shapes of family Moraxellaceae are short rods or coccoid or coccal or may exhibit a characteristic multicellular micromorphology, and cells usually occur in pairs or short chains (29); however, other families of order Pseudomonadales have just one cell form that is rod-shaped, and cells usually occur in singles (Table 1). Third, the cells of family Moraxellaceae are nonmotile in liquid media and do not exhibit flagellation, but the other families of order Pseudomonadales typically have polar flagella (Table 1) (30). Fourth, except for some strains of Acinetobacter and Psychrobacter, no acid is produced from carbohydrates in family Moraxellaceae; however, the other families of order Pseudomonadales can produce acid from glucose and so on (30). In addition, the genome size and G+C% between Pseudomonadaceae and Moraxellaceae present significant differences (Wilcoxon test; P < 0.0001) (Fig. S7). In light of these results, it is proposed that Moraxella be reclassified as the type genus of Moraxellales ord. nov.
Heatmap of the gene presence and absence profiles of the 257 reference genomes. The numeral 1 represents the presence of genes, and 0 represents the absence of genes. Download FIG S6, TIF file, 2.7 MB (2.8MB, tif) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomic G+C% of families Pseudomonadaceae and Moraxellaceae (a). Genome size of families Pseudomonadaceae and Moraxellaceae (b). Download FIG S7, DOCX file, 0.1 MB (136.6KB, docx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
However, we found the family Moraxellaceae was paraphyletic and formed three separate clades, and the three clades clustered with genera Moraxella and Psychrobacter, Alkanindiges and Acinetobacter, and Perlucidibaca in the bac120 and core-genome tree, respectively (Fig. 3 and Fig. S1 and S5). The genus Perlucidibaca was positioned away from the other two clades in the core-genome tree (Fig. 3 and Fig. S6). The comparison of the AAI values, and shared gene blocks of the pangenome of intrafamily Moraxellaceae, also indicated that genus Perlucidibaca apparently differed from genera Acinetobacter, Alkanindiges, Moraxella, and Psychrobacter (Fig. 5 and Fig. S4). In addition, the phenotypic synapomorphies of Perlucidibaca obviously differed from those of Acinetobacter, Alkanindiges, Moraxella (25), and Psychrobacter. For example, the original description of Perlucidibaca (25) indicated that the members of this taxon are facultatively aerobic and that their anaerobic growth is similar to aerobic growth, whereas genera Acinetobacter, Alkanindiges, Psychrobacter, and Moraxella are strictly aerobic bacteria (25). Besides, the major fatty acids of genus Perlucidibaca are C16:0, C18:1 ω7c, C16 : 1 ω7c, and/or C16 : 1 ω6c and C12:0 3-OH, while C18:1 ω9c is a minor component (31), the major respiratory quinone of genus Perlucidibaca is Q-12 (32), while that of the major member of family Moraxella is Q-8, and additionally, the cells of genus Perlucidibaca usually occur as singles (Table 1) (31). In light of these results, it is proposed that Perlucidibaca should be reclassified as the type genus of Perlucidibacaceae fam. nov., and it should be shifted out of the novel order Moraxellales and merged into order Pseudomonadales.
FIG 5.
Heatmap showing the AAI values between genera Acinetobacter, Alkanindiges, Moraxella, Psychrobacter, and Perlucidibaca.
As shown in Fig. 3, group 1 formed a robust lineage and presented a close relationship with families Hahellaceae and Oleiphilaceae in the bac120 and core-genome tree (Fig. 2 and 3), and the GTDB taxonomy classified the branch as belonging to family Oleiphilaceae; however, we found that the G+C content of the genera Marinobacter, Mangrovitalea, and Tamilnaduibacter ranged from 53.7 to 63.2% and that of family Oleiphilaceae ranged from 43.4 to 47.8%. Additionally, the AAI value between group 1, Hahellaceae, and family Oleiphilaceae was less than 60% (Fig. 4a). Furthermore, many species of group 1 can utilize various carbon sources including aliphatic and polycyclic aromatic hydrocarbons, acyclic isoprenoid compounds, and many sole carbon sources, while all strains of family Oleiphilaceae can use only aliphatic hydrocarbons and their derivatives as carbon sources for growth (33). Additionally, the cellular fatty acid patterns of most strains of group 1 were C16: 0, C18:1 ω9c, C16:1 ω7c, and/or C16 : 1 ω6c and C12:0 3-OH, while those of the Oleiphilaceae were C16: 0, C16:1 ω7c, and/or C16 : 1 ω6c and C16:1 ω9c. The major polar lipids of group 1 were diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), and phosphatidylglycerol (PG), and those of the family Oleiphilaceae were PE, PG, and phosphatidyldimethylethylamine (DME) (33); thus, these chemotaxonomic indices between group 1 and family Oleiphilaceae have certain differences. These results indicated that the two clades represented two different families, contradicting the GTDB taxonomy. Therefore, we designated the lineage as family Marinobacteraceae fam. nov. because the first valid name of the genus of this clade was Marinobacter, first proposed in 1992 (34); the family comprises four genera: Marinobacter, Mangrovitalea, Pseudohalomonas, and Tamilnaduibacter. In addition, the species “Marinobacter nanhaiticus” (35) was transferred from genus Marinobacter to Tamilnaduibacter and named “Tamilnaduibacter nanhaiticus” comb. nov., which was suggested because the species “Marinobacter nanhaiticus” always forms a robust clade with genus Tamilnaduibacter in the bac120 tree and the core-genome tree (Fig. 2 and 3), and the AAI value between Tamilnaduibacter salinus and “T. nanhaiticus” was 70.99 and higher than those of T. salinus and other Marinobacter members (Fig. 4a).
Oceanospirillaceae (36) appeared to be polyphyletic and formed five highly supported subgroups in the bac120 and core-genome tree, implying that it should be split into five novel families. However, Oceanospirillaceae has been found to include 21 genera (https://www.ezbiocloud.net/taxonomy?tn=Oceanospirillaceae&depth=2) thus far, whereas only 15 genera were obtained in the core-genome tree. Hence, additional genome sequences need to be made available if these new families are to be proposed.
We also observed that the family Endozoicomonadaceae and the genus Zooshikella formed a robust clade with family Pseudomonadaceae, indicating that the genus Zooshikella presents the closest evolutionary relationship with family Endozoicomonadaceae (Fig. 2 and 3 and Fig. S1). The family Endozoicomonadaceae and genus Zooshikella shared more genes with each other than they shared with other families (Fig. S6). Additionally, the phenotypical synapomorphies of Zooshikella and family Endozoicomonadaceae are not obviously different; for example, they have similar major fatty acid components including C16:0, C18 : 1 ω7c, C16:1 ω7c, and/or C16:1 ω6c, the major quinone was Q-9, and almost all members of genus Zooshikella and family Endozoicomonadaceae were mesophilic (37, 38), except that genus Endozoicomonas also included C10:0 3-OH as a major hydroxyl fatty acid component; further, PE, PG, phosphatidylserine (PS), and DPG are present in the polar lipid pattern of family Endozoicomonadaceae, while the genus Zooshikella shows DPG, PE, and PG, except that PS was not detected (Table 2) (8, 39). In light of these results, the family Endozoicomonadaceae should be transferred to a novel family and named Zooshikellaceae fam. nov. because the genus Zooshikella was first proposed in 2003 within the clade (37), and the family comprises four genera: Endozoicomonas, Kistimonas, Parendozoicomonas (8), and Zooshikella. The family name has been given already (https://gtdb.ecogenomic.org/searches?q=%25Zooshikella%25&s=al), but the classification is different from GTDB in that family Zooshikellaceae includes only the genus Zooshikella. This proposal does not conflict with the 16S rRNA gene tree provided in the initial description of the genus, despite the tree being poorly resolved.
TABLE 2.
Phenotypic characteristics of Marinobacteraceae fam. nov., Oleiphilaceae, Zooshikellaceae, and “Endozoicomonadaceae”a
| Marinobacteraceae | Oleiphilaceae | Zooshikellaceae | “Endozoicomonadaceae” | |
|---|---|---|---|---|
| Colony color | White | NA | Red | Beige |
| Fatty acids | C16: 0, C18:1
ω9c, C16:1 ω7c and/or C16 : 1 ω6c, and C12:0 3-OH |
C16: 0, C16:1
ω7c
and/or C16 : 1 ω6c, and C16:1 ω9c |
C16:0, C16:1
ω7c
and/or C16:1 ω6c, and C18 : 1 ω7c |
C16:0, C16:1
ω7c
and/or C16:1 ω6c, C18 : 1 ω7c, and C10:0 3-OH |
| Polar lipids | DPG, PE, PG | PE, PG, DME | DPG, PG, PE | PE, PG, PS, DPG |
| Ubiquinone | Q9 | Q9 | Q9 | Q9 |
| G+C content (%) | 53.7–63.2 | 43.4–47.8 | 40.2–41.3 | 47.0–51.0 |
| Genomic size (Mbp) | 3.4–5.3 | 6.4 | 5.8 | 5.4–6.7 |
The data are from original isolation papers and/or Bergey’s Manual. References are as follows: Marinobacteraceae fam. nov., 34; Oleiphilaceae, 33; Zooshikellaceae, 37; “Endozoicomonadaceae,” 8. The names of the families were proposed in the study, except “Endozoicomonadaceae.” NA, not applicable; +, present/tested positive; −, absent/tested negative; V, variable among strains.
As indicated by the results presented in Fig. S4 and Fig. 4b, the AAI values between Alcanivoracaceae, Balneatrichaceae, Halomonadaceae, Hahellaceae, Oleiphilaceae, Oceanospirillaceae, Saccharospirillaceae, Zooshikellaceae, Pseudomonadaceae, Perlucidibacaceae, Cellvibrionaceae, Halieaceae, Microbulbiferaceae, Porticoccaceae, Spongiibacteraceae, Ventosimonadaceae, and Marinobacteraceae were significantly higher than those between the above taxa and Moraxellaceae (Wilcox test P < 0.0001). Together, the results from this study indicated that 17 families shared a common ancestor at the order level; therefore, these families were merged into one order, Pseudomonadales, consistent with the designation of the GTDB (3) because the first species proposed was Pseudomonas aeruginosa in 1900. The closest order to Pseudomonadales is Moraxellales. A possible AAI threshold of 54 was proposed to differentiate among orders according to Fig. 4b, but it needs further study to be used in other complex phyla such as Firmicutes or Bacteroidetes.
DISCUSSION
Emendation of the order Pseudomonadales.
In this study, we proved that two novel families, Marinobacteraceae and Perlucidibacaceae; families Alcanivoracaceae (40), Balneatrichaceae, Halomonadaceae (41), Hahellaceae (42), Oleiphilaceae (33), Oceanospirillaceae, Saccharospirillaceae (43), and Zooshikellaceae of order Oceanospirillales; Pseudomonadaceae and Ventosimonadaceae (28) of order Pseudomonadales; and Cellvibrionaceae, Halieaceae, Microbulbiferaceae, Porticoccaceae, and Spongiibacteraceae (5) of order Cellvibrionales shared a relatively recent ancestor and formed a robust branch based on two typical phylogenomic tree and AAI values in class Gammaproteobacteria. The family Litoricolaceae should also be classified into order Pseudomonadales tentatively because it formed a stable clade with family Saccharospirillaceae (bootstrap value 72) based on the 16S rRNA tree, as no genome is available for Litoricolaceae. In addition, the family Natronospirillaceae was proposed by Kevbrin et al. (43), and when we submitted the article to the journal in 2020, the closest family to family Natronospirillaceae was Saccharospirillaceae according to the description by Kevbrin et al. (43); thus, the family Natronospirillaceae should be classified into order Pseudomonadales as well. Therefore, orders Oceanospirillales, Cellvibrionales, and Pseudomonadales were merged into the single order Pseudomonadales with the exception of families Moraxellaceae and Kangiellaceae, including 19 families in the partial taxonomic reconstruction of class Gammaproteobacteria.
Almost all members of order Pseudomonadales are mesophilic, the major fatty acid components are C16:0, C16:1 ω7c and/or C16:1 ω6c, and C18:1 ω7c, and the major respiratory quinone is Q-9.
Type genus: Pseudomonas; class: Gammaproteobacteria.
Description of Moraxellales ord. nov.
Moraxellales (Mo.ra.xel.la′les. N.L. fem. dim. n. Moraxella type genus of the order; suff. -ales, ending denoting an order; N.L. fem. pl. n. Moraxellales, the Moraxella order).
The description is the same as that for family Moraxellaceae (29). Type genus: Moraxella; class: Gammaproteobacteria.
Description of Kangiellales ord. nov.
Kangiellales (Kan.gi.el.la.les. N.L. fem. dim. n. Kangiella, type genus of the order; suff. -ales, ending denoting an order; N.L. fem. pl. n. Kangiellales, the Kangiella order).
The description is the same as that for family Kangiellaceae (17). Type genus: Kangiella; class: Gammaproteobacteria.
Description of Marinobacteraceae fam. nov.
Marinobacteraceae (Ma.ri.no.bac.te.ra′ce.ae. N.L. masc. n. Marinobacter, type genus of the family; -aceae, suff. ending denoting a family; N.L. fem. pl. n. Marinobacteraceae, the Marinobacter family).
The family belongs to order Oceanospirillales, class Gammaproteobacteria, and mainly consists of bacteria isolated from the sediments of marine environments. The cellular fatty acid patterns of most strains are C16: 0, C18:1 ω9c, summed features 3 and C12:0 3-OH. The G+C content of the genomic DNA ranges from 53.7 to 63.2%. At present, the family comprises genera Marinobacter, Mangrovitalea, Pseudohalomonas, and Tamilnaduibacter. The definition of the family relies mainly on the construction of phylogenetic relationships based on 16S rRNA gene sequences and phylogenomic relationships based on core genomes and concatenated 120 ubiquitous single-copy protein sequences.
Type genus: Marinobacter; order: Pseudomonadales.
Description of Perlucidibacaceae fam. nov.
Perlucidibacaceae (Per.lu.ci.di.ba.ca'ce.ae. N.L. fem. n. Perlucidibaca, type genus of the family; -aceae, suff. ending denoting a family; N.L. fem. pl. n. Perlucidibacaceae, the Perlucidibaca family).
The description is the same as for genus Perlucidibaca (25, 32).
Type genus: Perlucidibaca; order: Pseudomonadales.
Description of Zooshikellaceae fam. nov.
Zooshikellaceae (Zoo.shi′ke.lla′ce.ae. N.L. fem. dim. n. Zooshikella, type genus of the family; -aceae, suff. ending denoting a family; N.L. fem. pl. n. Zooshikellaceae, the Zooshikella family).
The major fatty acid components were C16:0, C16:1 ω7c and/or C16:1 ω6c, and C18:1 ω7c; the major quinone was Q-9; PE, PG, PS, and DPG are present in the major polar lipid pattern; and almost all members of the family were mesophilic. At present, the family comprises genera Endozoicomonas, Kistimonas, Parendozoicomonas, and Zooshikella. Members of this family form a stable clade in the reconstructed phylogenetic tree based on 16S rRNA gene sequences and the phylogenomic tree based on core genomes and concatenated 120 ubiquitous single-copy protein sequences. The type genus of the family is Zooshikella.
MATERIALS AND METHODS
SSU-rRNA-based phylogeny.
Reference sequences of class Gammaproteobacteria with valid published names were downloaded from the SILVA Living Tree Project v128 database and the EzBioCloud database. The package MAFFT v7.402 was used for sequence alignment, and identical sequences were deleted by using RAxML before constructing the tree. Phylogenetic trees based on data sets of 16S rRNA gene sequences were constructed using RAxML (44) by applying the -f a, -p 12345, -x 12345, -# 1,000 or 200, and -m GTRGAMMA parameters. The 16S rRNA gene identity values were obtained through a BLASTN all-versus-all sequence similarity search.
Reference genome of Gammaproteobacteria.
First, 808 reference genomes of Gammaproteobacteria out of 78,338 genomes were downloaded from the genome database of the NCBI on 19 October 2019. Then, the genomes of the species Mangrovitalea sediminis (PRJNA402051) and Tamilnaduibacter salinus (PRJNA442664) were added to the data set. Then, genome completeness and contamination were controlled by using CheckM, and the genomes exhibiting <90% completeness or >5% contamination were filtered out. Finally, 783 high-quality genomes were obtained for subsequent analysis. The major information for the genomes is listed in Table S1 in the supplemental material, and genome size ranged from 0.3 Mbp to 7.8 Mbp.
Phylogenetic analysis of 120 ubiquitous single-copy proteins.
The bac120 tree was inferred from the dereplicated data set by applying the WAG model (45) of protein evolution with gamma-distributed rate heterogeneity (46) (+GAMMA) in FastTree to a concatenated alignment of 120 ubiquitous single-copy proteins (12) with the GTDB -tk tool (3). These trees were modified and visualized using the Interactive Tree of Life (iTOL) (itol.embl.de/).
Pangenome and phylogenomic analysis.
The sequences were annotated using Prokka v1.12 (47). The pangenome was estimated using the rapid large-scale prokaryotic pangenome analysis (Roary v3.11.2) tool (48) with parameters -i 50 -cd 99. Briefly, the annotated genes from all 257 representative reference genomes of orders Oceanospirillales, Cellvibrionales, and Pseudomonadales and genera Tamilnaduibacter, Mangrovitalea, and Marinobacter (Table S2) were first filtered to remove partial sequences and iteratively preclustered with CD-HIT. These procedures resulted in a substantially reduced set of protein sequences. An all-against-all comparison of the reduced sequences with 50% sequence identity was performed with BLASTP. The sequences were then clustered with Markov clustering algorithm (MCL), and the preclustering results from CD-HIT were finally merged together with the results of MCL. Homologous clusters were divided into core, accessory, and unique genomes. The core genome comprised genes shared within at least 99% of the genomes. The cumulative sizes of the pangenome and core genome were calculated by selecting genomes with replacement in random order 500 times and then calculating the mean size of each sampling point.
A pangenome matrix was generated based on the presence or absence of all genetic loci in each individual genome produced by Roary. We selected the top 6,000 genes of the matrix to produce the heatmap with the pheatmap package, species were clustered, and PCoA was performed based on the presence and absence of orthologs according to the Manhattan distance by using hclust in R.
Phylogenomic analysis of 119 concatenated single-copy core genes.
The phylogenomic tree was generated based on the concatenated single-copy core genes. The core-genome sequences were accurately aligned with MAFFT v7.402. The resulting multiple sequence alignment length was 121,975 bp and retained 37,526 bp after trimming with Gblocks 0.91b (49) with default paraments. A phylogenomic tree was inferred using the IQtree package to search optimal models and further verify the morphologies and topologies of the phylogenomic tree (50) using the command -bb 1000 -m MFP+MERGE+R, and the RAxML program was applied with the parameters -f a, -p 12345, -x 12345, -# 200, and -m GTRGAMMAI (50), based on trimmed concatenated single-copy core genes.
Whole-genome relatedness indices.
The AAI is the mean amino acid identity of orthologous genes. To validate our taxonomic proposals, we performed AAI comparisons between these genomes. The AAI indices were deduced from pairwise conserved comparisons of coding proteins and calculated using CompareM v0.0.21 software (https://github.com/dparks1134/CompareM) (which employs DIAMOND v0.9.24 to obtain the best reciprocal hits [51]) with the default BLASTP parameters (i.e., 10−5 E value, 30% sequence identity cutoff, and ≥70% alignment length) to define the bidirectional best BLAST hits between genomes. The resulting AAI values were clustered by using hclust, and a heatmap was generated with the pheatmap package in R.
Data processing and availability.
All data (codes, other supplemental tables, and files) are available at the website https://github.com/liaohu1231/phylogenomic_analysis.
ACKNOWLEDGMENTS
We are very appreciative of Zhongjie Wang for his help with the bioinformatic analysis and Qiliang Lai for comments and suggestions.
This work was supported by the National Natural Science Foundation of China (no. 41676105).
We declare that we have no conflict of interest.
REFERENCES
- 1.Castelle CJ, Banfield JF. 2018. Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell 172:1181–1197. doi: 10.1016/j.cell.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu X-W, De Meyer S, Trujillo ME. 2018. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol 68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 3.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil P-A, Hugenholtz P. 2018. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Schlaeppi K, van der Heijden MGA. 2018. Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol 16:567–576. doi: 10.1038/s41579-018-0024-1. [DOI] [PubMed] [Google Scholar]
- 5.Spring S, Scheuner C, Göker M, Klenk H-P. 2015. A taxonomic framework for emerging groups of ecologically important marine gammaproteobacteria based on the reconstruction of evolutionary relationships using genome-scale data. Front Microbiol 6:281. doi: 10.3389/fmicb.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacosa HP, Erdner DL, Rosenheim BE, Shetty P, Seitz KW, Baker BJ, Liu Z. 2018. Hydrocarbon degradation and response of seafloor sediment bacterial community in the northern Gulf of Mexico to light Louisiana sweet crude oil. ISME J 12:2532–2543. doi: 10.1038/s41396-018-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darshan N, Manonmani HK. 2015. Prodigiosin and its potential applications. J Food Sci Technol 52:5393–5407. doi: 10.1007/s13197-015-1740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartz JO, Blom J, Busse HJ, Mvie JB, Hardt M, Schubert P, Wilke T, Goessmann A, Wilharm G, Bender J, Kampfer P, Glaeser SP. 2018. Parendozoicomonas haliclonae gen. nov. sp. nov. isolated from a marine sponge of the genus Haliclona and description of the family Endozoicomonadaceae fam. nov. comprising the genera Endozoicomonas, Parendozoicomonas, and Kistimonas. Syst Appl Microbiol 41:73–84. doi: 10.1016/j.syapm.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Satomi M, Fujii T. 2014. The family Oceanospirillaceae, p 491–527. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes: Gammaproteobacteria. Springer, Berlin, Germany. [Google Scholar]
- 10.Liao H, Li Y, Guo X, Lin X, Lai Q, Xu H, Zheng T, Tian Y. 2017. Mangrovitalea sediminis gen. nov., sp. nov., a member of the family Alteromonadaceae isolated from mangrove sediment. Int J Syst Evol Microbiol 67:5172–5178. doi: 10.1099/ijsem.0.002433. [DOI] [PubMed] [Google Scholar]
- 11.Verma A, Mual P, Mayilraj S, Krishnamurthi S. 2015. Tamilnaduibacter salinus gen. nov., sp. nov., a halotolerant gammaproteobacterium within the family Alteromonadaceae, isolated from a salt pan in Tamilnadu, India. Int J Syst Evol Microbiol 65:3248–3255. doi: 10.1099/ijsem.0.000401. [DOI] [PubMed] [Google Scholar]
- 12.Parks DH, Rinke C, Chuvochina M, Chaumeil P-A, Woodcroft BJ, Evans PN, Hugenholtz P, Tyson GW. 2017. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 2:1533–1542. doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Huang H, Wu CH. 2017. Protein bioinformatics databases and resources, p 3–39. In Wu CH, Arighi CN, Ross KE (ed), Protein bioinformatics: from protein modifications and networks to proteomics. Springer, New York, NY. [Google Scholar]
- 14.Rosselló-Mora R. 2005. Updating prokaryotic taxonomy. J Bacteriol 187:6255–6257. doi: 10.1128/JB.187.18.6255-6257.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo C, Rodriguez-R LM, Konstantinidis KT. 2014. MyTaxa: an advanced taxonomic classifier for genomic and metagenomic sequences. Nucleic Acids Res 42:e73. doi: 10.1093/nar/gku169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon J-H, Oh T-K, Park Y-H. 2004. Kangiella koreensis gen. nov., sp. nov. and Kangiella aquimarina sp. nov., isolated from a tidal flat of the Yellow Sea in Korea. Int J Syst Evol Microbiol 54:1829–1835. doi: 10.1099/ijs.0.63156-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Tang M, Wu H, Dai S, Li T, Chen C, He H, Fan J, Xiang W, Li X. 2015. Aliikangiella marina gen. nov., sp. nov., a marine bacterium from the culture broth of Picochlorum sp. 122, and proposal of Kangiellaceae fam. nov. in the order Oceanospirillales. Int J Syst Evol Microbiol 65:4488–4494. doi: 10.1099/ijsem.0.000601. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Carnahan A, Joseph SW. 2005. Aeromonadales ord. nov., p 556–587. In Brenner DJ, Krieg NR, Staley JT, Garrity GM, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H (ed), Bergey’s manual of systematic bacteriology, vol 2. The Proteobacteria, part B. The Gammaproteobacteria. Springer, Boston, MA. [Google Scholar]
- 19.Garrity GM, Bell JA, Lilburn T. 2005. Thiotrichales ord. nov., p 131–210. In Brenner DJ, Krieg NR, Staley JT, Garrity GM, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H (ed), Bergey’s manual of systematic bacteriology, vol 2. The Proteobacteria, part B. The Gammaproteobacteria. Springer, Boston, MA. [Google Scholar]
- 20.Wang J, Lu Y, Nawaz MZ, Xu J. 2018. Comparative genomics reveals evidence of genome reduction and high extracellular protein degradation potential in Kangiella. Front Microbiol 9:1224. doi: 10.3389/fmicb.2018.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu F-D, Li X-G, Xiao X, Xu J. 2015. Kangiella profundi sp. nov., isolated from deep-sea sediment. Int J Syst Evol Microbiol 65:2315–2319. doi: 10.1099/ijs.0.000257. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Park S, Oh TK, Yoon JH. 2013. Kangiella sediminilitoris sp. nov., isolated from a tidal flat sediment. Int J Syst Evol Microbiol 63:1001–1006. doi: 10.1099/ijs.0.040691-0. [DOI] [PubMed] [Google Scholar]
- 23.Adeolu M, Alnajar S, Naushad S, Gupta RS. 2016. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int J Syst Evol Microbiol 66:5575–5599. doi: 10.1099/ijsem.0.001485. [DOI] [PubMed] [Google Scholar]
- 24.Octavia S, Lan R. 2014. The family Enterobacteriaceae. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes. Springer, Berlin, Germany. [Google Scholar]
- 25.Song J, Choo Y-J, Cho J-C. 2008. Perlucidibaca piscinae gen. nov., sp. nov., a freshwater bacterium belonging to the family Moraxellaceae. Int J Syst Evol Microbiol 58:97–102. doi: 10.1099/ijs.0.65039-0. [DOI] [PubMed] [Google Scholar]
- 26.Bozal N, Montes MJ, Tudela E, Guinea J. 2003. Characterization of several Psychrobacter strains isolated from Antarctic environments and description of Psychrobacter luti sp. nov. and Psychrobacter fozii sp. nov. Int J Syst Evol Microbiol 53:1093–1100. doi: 10.1099/ijs.0.02457-0. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys GJ, Oates A, Ledder RG, McBain AJ. 2015. Faucicola mancuniensis gen. nov., sp. nov., isolated from the human oropharynx. Int J Syst Evol Microbiol 65:11–14. doi: 10.1099/ijs.0.066837-0. [DOI] [PubMed] [Google Scholar]
- 28.Lin JY, Hobson WJ, Wertz JT. 2016. Ventosimonas gracilis gen. nov., sp. nov., a member of the Gammaproteobacteria isolated from Cephalotes varians ant guts representing a new family, Ventosimonadaceae fam. nov., within the order ‘Pseudomonadales’. Int J Syst Evol Microbiol 66:2869–2875. doi: 10.1099/ijsem.0.001068. [DOI] [PubMed] [Google Scholar]
- 29.Rossau R, Van Landschoot A, Gillis M, De Ley J. 1991. Taxonomy of Moraxellaceae fam. nov., a new bacterial family to accommodate the genera Moraxella, Acinetobacter, and Psychrobacter and related organisms. Int J Syst Evol Microbiol 41:310–319. doi: 10.1099/00207713-41-2-310. [DOI] [Google Scholar]
- 30.Brown DR, Tasker S, Messick JB, Neimark H. 2010. Bergey’s manual of systematic bacteriology. Springer, New York, NY. [Google Scholar]
- 31.Baek K, Han J-H, Lee M-H. 2017. Perlucidibaca aquatica sp. nov., isolated from fresh water. Int J Syst Evol Microbiol 67:2296–2300. doi: 10.1099/ijsem.0.001940. [DOI] [PubMed] [Google Scholar]
- 32.França L, Albuquerque L, da Costa MS. 2015. Cavicella subterranea gen. nov., sp. nov., isolated from a deep mineral-water aquifer, and emended description of the species Perlucidibaca piscinae. Int J Syst Evol Microbiol 65:3812–3817. doi: 10.1099/ijsem.0.000493. [DOI] [PubMed] [Google Scholar]
- 33.Golyshin PN, Chernikova TN, Abraham W-R, Lünsdorf H, Timmis KN, Yakimov MM. 2002. Oleiphilaceae fam. nov., to include Oleiphilus messinensis gen. nov., sp. nov., a novel marine bacterium that obligately utilizes hydrocarbons. Int J Syst Evol Microbiol 52:901–911. doi: 10.1099/00207713-52-3-901. [DOI] [PubMed] [Google Scholar]
- 34.Gauthier MJ, Lafay B, Christen R, Fernandez L, Acquaviva M, Bonin P, Bertrand JC. 1992. Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. Int J Syst Bacteriol 42:568–576. doi: 10.1099/00207713-42-4-568. [DOI] [PubMed] [Google Scholar]
- 35.Gao W, Cui Z, Li Q, Xu G, Jia X, Zheng L. 2013. Marinobacter nanhaiticus sp. nov., polycyclic aromatic hydrocarbon-degrading bacterium isolated from the sediment of the South China Sea. Antonie Van Leeuwenhoek 103:485–491. doi: 10.1007/s10482-012-9830-z. [DOI] [PubMed] [Google Scholar]
- 36.Garrity GM, Bell JA, Lilburn T. 2005. Oceanospirillales ord. nov., p 270–323. In Brenner DJ, Krieg NR, Staley JT, Garrity GM, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H (ed), Bergey’s manual of systematic bacteriology, vol 2. The Proteobacteria, part B. The Gammaproteobacteria. Springer, Boston, MA. [Google Scholar]
- 37.Yi H, Chang Y-H, Oh HW, Bae KS, Chun J. 2003. Zooshikella ganghwensis gen. nov., sp. nov., isolated from tidal flat sediments. Int J Syst Evol Microbiol 53:1013–1018. doi: 10.1099/ijs.0.02521-0. [DOI] [PubMed] [Google Scholar]
- 38.Yang C-S, Chen M-H, Arun AB, Chen CA, Wang J-T, Chen W-M. 2010. Endozoicomonas montiporae sp. nov., isolated from the encrusting pore coral Montipora aequituberculata. Int J Syst Evol Microbiol 60:1158–1162. doi: 10.1099/ijs.0.014357-0. [DOI] [PubMed] [Google Scholar]
- 39.Ramaprasad EVV, Bharti D, Sasikala C, Ramana CV. 2015. Zooshikella marina sp. nov. a cycloprodigiosin- and prodigiosin-producing marine bacterium isolated from beach sand. Int J Syst Evol Microbiol 65:4669–4673. doi: 10.1099/ijsem.0.000630. [DOI] [PubMed] [Google Scholar]
- 40.Golyshin PN, Harayama S, Timmis KN, Yakimov MM. 2005. Family II. Alcanivoraceae fam. nov., p 295. In Brenner DJ, Krieg NR, Staley JT, Garrity GM, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H (ed), Bergey’s manual of systematic bacteriology, 2nd ed, vol 2. The Proteobacteria, part B. The Gammaproteobacteria. Springer, Boston, MA. [Google Scholar]
- 41.Franzmann PD, Wehmeyer U, Stackebrandt E. 1988. Halomonadaceae fam. nov., a new family of the class Proteobacteria to accommodate the genera Halomonas and Deleya. Syst Appl Microbiol 11:16–19. doi: 10.1016/S0723-2020(88)80043-2. [DOI] [Google Scholar]
- 42.Garrity GM, Bell JA, Lilburn T. 2015. Hahellaceae fam. nov. In Trujillo ME, Dedysh S, DeVos P, Hedlund B, Kämpfer P, Rainey FA, Whitman WB (ed), Bergey’s manual of systematics of archaea and bacteria. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 43.Kevbrin V, Boltyanskaya Y, Grouzdev D, Koziaeva V, Park M, Cho J-C. 2020. Natronospirillum operosum gen. nov., sp. nov., a haloalkaliphilic satellite isolated from decaying biomass of a laboratory culture of cyanobacterium Geitlerinema sp. and proposal of Natronospirillaceae fam. nov., Saccharospirillaceae fam. nov. and Gynuellaceae fam. nov. Int J Syst Evol Microbiol 70:511–521. doi: 10.1099/ijsem.0.003781. [DOI] [PubMed] [Google Scholar]
- 44.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 45.Whelan S, Goldman N. 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- 46.Yang Z. 1994. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol 39:306–314. doi: 10.1007/BF00160154. [DOI] [PubMed] [Google Scholar]
- 47.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 48.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchfink B, Xie C, Huson DH. 2015. Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The uncollapsed tree from Fig. 2a provides all clade information. Download FIG S1, PDF file, 1.5 MB (1.5MB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Main information for the genome sequences used to reconstruct the bac120 tree. Download Table S1, XLSX file, 0.1 MB (144.9KB, xlsx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Core–pangenome size evolution of the 257 reference genomes. Pangenome size (green) is directly proportional to the number of genomes. Core-genome size (yellow) is inversely proportional to the number of genomes. Download FIG S2, DOCX file, 0.4 MB (439.8KB, docx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Main information for the 257 reference genome sequences used to reconstruct the core-genome tree. Download Table S2, XLSX file, 0.05 MB (52.5KB, xlsx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The uncollapsed tree from Fig. 3 provides all clade information. Download FIG S3, PDF file, 0.4 MB (456.7KB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Heatmap showing the AAI values between the 257 genomes. Download FIG S4, PDF file, 1.2 MB (1.2MB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Manhattan distance-based principal-coordinate analysis (PCoA) showing the difference in the gene presence and absence profiles between different genera. Download FIG S5, PDF file, 0.7 MB (714.5KB, pdf) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Heatmap of the gene presence and absence profiles of the 257 reference genomes. The numeral 1 represents the presence of genes, and 0 represents the absence of genes. Download FIG S6, TIF file, 2.7 MB (2.8MB, tif) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomic G+C% of families Pseudomonadaceae and Moraxellaceae (a). Genome size of families Pseudomonadaceae and Moraxellaceae (b). Download FIG S7, DOCX file, 0.1 MB (136.6KB, docx) .
Copyright © 2020 Liao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.