Abstract
Background
Imaging of novel coronavirus disease 2019 (COVID-19) has been described in adults, but children have milder forms of disease. Pediatric imaging descriptions are of asymptomatic children, raising the question of whether imaging is needed in this patient group.
Objective
To describe the utilization and imaging findings in children with COVID-19 along with the comorbidities, treatment and short-term outcomes.
Materials and methods
We retrospectively reviewed pediatric patients who had a confirmed positive test for COVID-19 during a 2-month period. We noted symptoms and presence of imaging at presentation. Comorbidities were recorded for children with imaging. Children were categorized as having multisystem inflammatory syndrome in children (MIS-C) if they met criteria for the disorder. For children who were admitted to the hospital, we documented length of hospital stay, need for intensive care unit care/ventilator support, and treatment regimen. We evaluated all imaging for acute/chronic abnormalities including chest radiographs for interstitial or alveolar opacities, distribution/symmetry of disease, zonal predominance, and pleural abnormalities. We performed descriptive statistics and compared children with MIS-C with the cohort using a Fisher exact test.
Results
During the study period, 5,969 children were tested for COVID-19, with 313 (5%) testing positive. Of these, 92/313 (29%) were asymptomatic and 55/313 (18%) had imaging and were admitted to the hospital for treatment. Forty-one of 55 patients (75%) with imaging had comorbidities. Chest radiographs were the most common examination (51/55 patients, or 93%) with most demonstrating no abnormality (34/51, or 67%). Children with MIS-C were more likely to have interstitial opacities and pleural effusions. US, CT or MRI was performed in 23/55 (42%) children, 9 of whom had MIS-C. Only one chest CT was performed.
Conclusion
In our study, most pediatric patients with COVID-19 did not require hospital admission or imaging. Most children with imaging had comorbidities but children with MIS-C were more likely to have no comorbidities. Children with imaging mostly had normal chest radiography. Advanced imaging (US, CT, MRI) was less common for the care of these children, particularly CT examination of the chest and for children without MIS-C.
Keywords: Chest, Children, Computed tomography, Coronavirus, COVID-19, Multisystem inflammatory syndrome in children, Radiography, Utilization
Introduction
Although it is a daily changing number, as of April 2, 2020, there were 149,760 laboratory confirmed cases of novel coronavirus disease 2019 (COVID-19) in the United States, of which 2,572 (1.7%) were in children younger than 18 years [1]. The first pediatric case of COVID-19 in the United States was reported on March 5, 2020 [1]. Severity of disease is variable in adults, ranging from asymptomatic to pneumonia to death, but children seem to have more mild to moderate or even silent forms of the disease [2–4]. A subset of children exposed to COVID-19 develop multisystem inflammatory syndrome in children (MIS-C), which presents with a spectrum of clinical findings such as an erythematous rash, lymphadenopathy and abdominal pain in addition to respiratory symptoms [5]. Imaging findings of COVID-19 have been described in adults [6, 7], but given that children are believed to have milder forms of the disease, there are fewer descriptions of imaging in pediatric patients. Li et al. [8] described chest CT findings in five children, four of whom were asymptomatic, and Kai et al. [9] reported on a cohort in which 10 of 15 children with chest CT examinations were asymptomatic. Additionally, a paper by Xia et al. [10] described chest CT findings in 20 pediatric patients with most children having no symptoms of pulmonary disease. Furthermore, when compared to adults, pediatric patients were less likely to have positive findings on chest CT examinations [11]. This raises the question of whether the exposure to ionizing radiation and infection involved with transporting these children to a radiology department is worth the risk with regard to treatment and outcomes in children.
In this study, we retrospectively reviewed a cohort of children who presented to a tertiary children’s hospital with confirmed cases of COVID-19. We describe the utilization and imaging findings in this patient cohort along with the comorbidities, treatment and short-term patient outcomes.
Materials and methods
The institutional review board at our tertiary children’s hospital approved the study with waiver for informed consent.
Study cohort
We retrospectively reviewed consecutive children younger than 18 years who tested positive for COVID-19 and were treated at the Children’s Hospital of Philadelphia or an affiliated hospital between March 17, 2020, and May 21, 2020.
All children were positive for COVID-19 using an in-house laboratory test with reverse transcriptase polymerase chain reaction (rRT-PCR) of respiratory secretions or confirmed testing of respiratory secretions performed at another facility. Although our testing criteria evolved over the study period, our current COVID-19 testing criteria include any patients with any of the following: fever greater than or equal to 100.4° Fahrenheit, cough, shortness of breath, or sore throat. Additionally, mildly ill children can be tested if their parents are health care workers or if the child has had a known exposure to a COVID-19-positive patient. Children admitted to the hospital or undergoing sedation are tested for COVID-19 by standard protocol regardless of symptoms. Children with incomplete medical records that could not definitively confirm a positive COVID-19 test were excluded.
For all children who had a confirmed positive test, we performed a chart review to determine presenting symptoms along with demographic information including gender and age and the presence or absence of imaging at presentation (within 7 days of the positive test). Imaging included radiologic diagnostic and procedural exams. For all children who had a confirmed positive test, comorbidities were recorded. Positive patients were also included if they met criteria for MIS-C per criteria from the Centers for Disease Control and Prevention, Royal College of Paediatrics and Child Health or the World Health Organization [12]. For those admitted, we recorded length of hospital stay, need for intensive care unit (ICU) care and ventilator support, and treatment regimen.
Imaging analysis
Available imaging performed at presentation was reviewed in consensus by two board-certified radiologists with fellowship training in pediatric radiology (D.M.B., with 10 years of experience; and J.B.R., with 3 years of experience) and echocardiography was reviewed by a single board-certified pediatric cardiologist (A.B., with 30 years of experience). Chest radiographs were evaluated for presence of interstitial or alveolar opacities. We also assessed distribution of disease (central, peripheral, scattered, diffuse), laterality or symmetry of disease, zonal predominance (upper, mid, lower or none), and presence of pleural effusions or pneumothorax. Evaluation for underlying chronic lung disease was also performed. Abdominal radiographs were evaluated for signs of ileus or obstruction. Ultrasound and cross-sectional imaging such as CT or MRI were evaluated for all pathology including acute or chronic disorders. Left and right ventricular function was evaluated with echocardiography. For children with greater than one chronological exam, we assessed the initial examination only.
Descriptive statistics were performed using SPSS (version 25; IBM, Armonk, NY). Continuous variables are presented as mean ± standard deviation and median (interquartile range). Categorical variables are presented as percentages and counts. Given that the clinical presentation and number of medical imaging studies available can vary according to age, demographic and clinical information was also presented according to the following age groups: ≤1 year, 2–5 years, 6–12 years and ≥13 years of age. Additionally, we performed a comparison between children diagnosed with MIS-C and those not diagnosed with MIS-C using a Fisher exact test for categorical values. P-values <0.05 were considered statistically significant.
Results
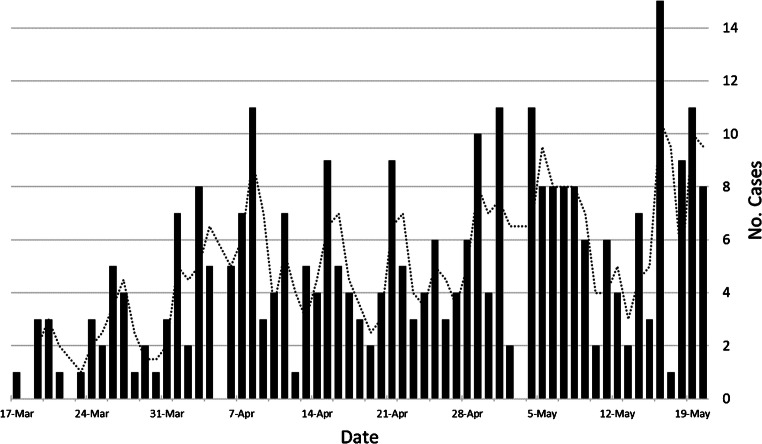
During the study period, a total of 6,005 tests for COVID-19 were administered, with 5,969 of these tests on unique pediatric patients. Among these patients, a total of 313 (5%) tested positive for COVID-19 (median age 8.6 years; interquartile range [IQR] 1.8–14.2 years) (Fig. 1). There were 164 boys (median age 6.6 years, IQR 1.5–13.4 years) and 149 girls (median age 9.4 years, IQR 3.1–14.7 years). Of the 313 children who tested positive for COVID-19, 92 (29%) were asymptomatic. Demographics and presenting symptoms of the children who tested positive for COVID-19 are listed in Tables 1 and 2. One child who transferred from an outside hospital had presented with a posterior cerebral artery stroke 5 weeks before testing.
Fig. 1.
Histogram demonstrates the number of positive cases of novel coronavirus disease 2019 (COVID-19) per day at our institution
Table 1.
Demographic and clinical information in children positive for novel coronavirus disease 2019 (COVID-19)
| Variables (n=313) | n (%) |
|---|---|
| Age, years, median (IQR) | 8.6 (12.4) |
| Gender | |
| Girls | 149 (48%) |
| Boys | 164 (52%) |
| Available imaging | 55 (18%) |
| PICU | 19 (6%) |
| Ventilation assistance | 14 (4%) |
| Asymptomatic | 92 (29%) |
| Signs and symptomsa | |
| Cough | 118 (38%) |
| Fever | 132 (42%) |
| Rhinorrhea | 54 (17%) |
| Headache | 35 (11%) |
| Dyspnea/shortness of breath | 35 (11%) |
| Sore throat | 26 (8%) |
| Myalgia | 14 (4%) |
| Decreased oral intake/loss of appetite | 21 (7%) |
| Vomiting | 24 (8%) |
| Chest pain/chest tightness | 15 (5%) |
| Loss of smell | 7 (2%) |
| Diarrhea/loose stools | 24 (8%) |
| Abdominal pain | 24 (8%) |
| Loss of taste | 6 (2%) |
| Irritability | 6 (2%) |
| Nausea | 5 (2%) |
| Wheezing/stridor | 4 (1%) |
| Lymphadenopathy | 2 (1%) |
IQR interquartile range, PICU pediatric intensive care unit
aSome children had multiple symptoms
Table 2.
Demographic and clinical information in children positive for novel coronavirus disease 2019 (COVID-19) per age group
| Variables | ≤1 year (n=82) |
2–5 years (n=53) |
6–12 years (n=76) |
≥13 years (n=102) |
|---|---|---|---|---|
| Age, years, median (IQR) | 0 (1) | 4 (2) | 9 (4) | 15 (3) |
| Gender | ||||
| Girls | 33 (40%) | 23 (43%) | 42 (55%) | 59 (58%) |
| Boys | 49 (60%) | 30 (57%) | 34 (45%) | 43 (42%) |
| Available images | 13 (16%) | 7 (13%) | 15 (20%) | 20 (20%) |
| PICU | 4 (5%) | 2 (4%) | 5 (7%) | 8 (8%) |
| Ventilation assistance | 2 (2%) | 1 (2%) | 5 (7%) | 6 (6%) |
| Asymptomatic | 17 (21%) | 16 (30%) | 19 (25%) | 40 (39%) |
| Signs and symptoms | ||||
| Cough | 37 (45%) | 18 (34%) | 28 (37%) | 35 (34%) |
| Fever | 42 (51%) | 26 (49%) | 36 (47%) | 28 (27%) |
| Rhinorrhea | 29 (35%) | 8 (15%) | 8 (11%) | 9 (9%) |
| Headache | 0 | 2 (4%) | 15 (20%) | 18 (18%) |
| Dyspnea/shortness of breath | 13 (16%) | 3 (6%) | 6 (8%) | 13 (13%) |
| Sore throat | 2 (2%) | 2 (4%) | 10 (13%) | 12 (12%) |
| Myalgia | 0 | 1 (2%) | 2 (3%) | 11 (11%) |
| Decreased oral intake/loss of appetite | 8 (10%) | 5 (9%) | 3 (4%) | 5 (5%) |
| Vomiting | 12 (15%) | 4 (8%) | 5 (7%) | 3 (3%) |
| Chest pain/chest tightness | 0 | 0 | 7 (9%) | 8 (8%) |
| Loss of smell | 0 | 0 | 2 (3%) | 5 (5%) |
| Diarrhea/loose stools | 10 (12%) | 5 (9%) | 6 (8%) | 3 (3%) |
| Abdominal pain | 3 (4%) | 4 (8%) | 11 (14%) | 6 (6%) |
| Loss of taste | 0 | 0 | 1 (1%) | 5 (5%) |
| Irritability | 3 (4%) | 1 (2%) | 2 (3%) | 0 |
| Nausea | 0 | 0 | 3 (4%) | 2 (2%) |
| Wheezing/stridor | 1 (1%) | 2 (4%) | 1 (1%) | 0 |
| Lymphadenopathy | 1 (1%) | 0 | 1 (1%) | 0 |
IQR interquartile range, PICU pediatric intensive care unit
A total of 55 children (median age 9.0 years, IQR 2.4–14.4 years) had imaging examinations. Of the children who had imaging, 29 were boys and 26 were girls. Forty-one of 55 children (75%) with imaging had additional comorbidities (Table 3). Ten of the 55 children (18%) with imaging met criteria for MIS-C but only 3 of these 10 children with MIS-C had comorbidities (neurologic disease, asthma, prematurity). When comparing these two groups, children with no comorbidities were more likely to have imaging if they were diagnosed with MIS-C (P=0.0013). Chest radiographs were the most common imaging examination (51/55 children, or 93%) with most performed as single-view anteroposterior (AP) radiographs (48/51, or 94%). The chest radiographs demonstrated no abnormality related to pneumonia in 34 of 51 patients (67%). Chronic findings were noted in four children, two of whom had chronic findings of neonatal chronic lung disease, one postoperative changes following congenital diaphragmatic hernia repair, and the last secondary to neuromuscular scoliosis.
Table 3.
Demographic and clinical information in children positive for novel coronavirus disease (COVID-19) who had medical imaging and comorbidities
| Variables (n=55) | n (%) |
|---|---|
| Age, years, median (IQR) | 9 (12) |
| Gender | |
| Girls | 29 (53%) |
| Boys | 26 (47%) |
| PICU | 17 (31%) |
| Ventilation assistance | 14 (25%) |
| Asymptomatic | 11 (20%) |
| Symptomatic | 44 (80%) |
| Number of comorbidities per patient | |
| None | 14 (25%) |
| One comorbidity | 13 (24%) |
| Two comorbidities | 4 (7%) |
| Three or more comorbidities | 26 (47%) |
| Comorbiditiesa | |
| Acute lymphoblastic anemia | 2 (4%) |
| Adrenal insufficiency | 1 (2%) |
| Anemia | 3 (5%) |
| Anxiety/depression | 4 (7%) |
| Asthma | 7 (13%) |
| Autism | 1 (2%) |
| Cardiomyopathy | 2 (4%) |
| Cerebral venous thrombosis | 1 (2%) |
| Chronic lung disease | 1 (2%) |
| Congenital diaphragmatic hernia | 1 (2%) |
| Congenital heart disease | 3 (5%) |
| Croup | 1 (2%) |
| Epilepsy | 3 (5%) |
| Gastroesophageal reflux disease | 3 (5%) |
| Gastrointestinal disease | 2 (4%) |
| Glucose-6-phosphate dehydrogenase deficiency | 2 (4%) |
| Gray matter heterotopia | 1 (2%) |
| G-tube | 4 (7%) |
| Hypertension | 1 (2%) |
| Mediastinal ganglioneuroblastoma | 1 (2%) |
| MRSA bacteremia | 1 (2%) |
| Neurofibromatosis Type 1 | 2 (4%) |
| Neurologic impairment/infection | 4 (7%) |
| Obesity | 2 (4%) |
| Osteomyelitis | 3 (5%) |
| Osteosarcoma | 1 (2%) |
| History of pulmonary embolism | 2 (4%) |
| Renal disease | 3 (5%) |
| Sepsis | 3 (5%) |
| Short gut syndrome | 1 (2%) |
| Sickle cell disease | 2 (4%) |
| Stroke | 1 (2%) |
| Tracheomalacia | 1 (2%) |
| Trisomy 21 | 1 (2%) |
| Type 2 diabetes mellitus | 2 (4%) |
| Valvulopathy | 1 (2%) |
G-tube gastrostomy tube, IQR interquartile range, PICU pediatric intensive care unit, MRSA methicillin-resistant Staphylococcus aureus
aNumber of children with the specific comorbidity; some children had more than one comorbidity
Imaging findings
Figures 2, 3 and 4 are examples of imaging findings on chest radiography. The most common acute finding was interstitial opacities (16 of 51 patients, or 31%), 8 of whom were diagnosed with MIS-C (8 of 10 children with MIS-C, or 80%). Interstitial opacities were predominately diffuse (10 of 16 children, or 63%). Alveolar opacities were noted in 14 of 51 children with radiography (27%), 2 of whom were diagnosed with MIS-C (2 of 10 children with MIS-C, or 20%). The distribution of alveolar opacity was predominately diffuse (4 of 14), followed by scattered (3 of 14), peripheral (3 of 14) and central (2 of 14). No well-defined pattern was seen in 2 of 14 cases. Pleural effusion was present in 5 of 51 cases (10%) but 4 of these cases were children diagnosed with MIS-C (4 of 10 with MIS-C, or 40%). Pneumothorax was not present on any chest radiographs. Compared to children who were positive for COVID-19 without MIS-C, children with MIS-C were statistically more likely to have pleural effusions (P=0.0038) and interstitial opacities (P=0.0001) on chest radiography.
Fig. 2.
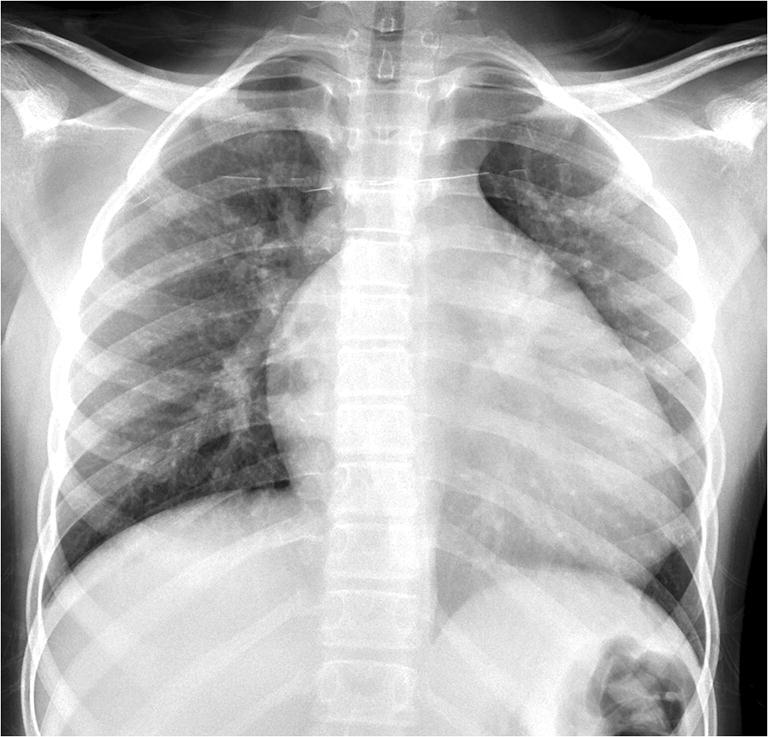
Radiography in a 14-year-old boy with a history of sickle cell disease who presented with dry cough. Anteroposterior chest radiograph demonstrates increased prominence of the interstitia of the lung bilaterally. The cardiac silhouette is enlarged, likely related to the boy’s underlying sickle cell disease
Fig. 3.
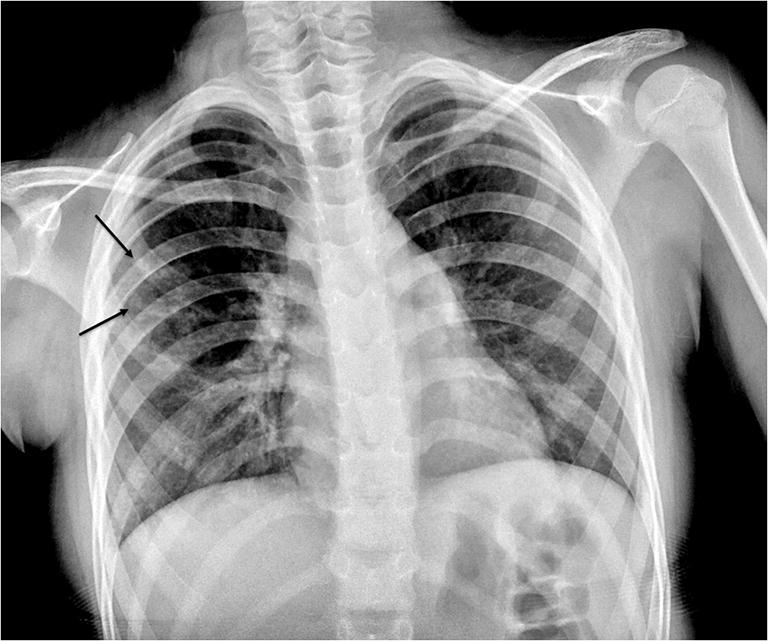
Radiography in a 9-year-old girl who presented with fever. Anteroposterior chest radiograph demonstrates asymmetrical opacities within the right lung (arrows)
Fig. 4.
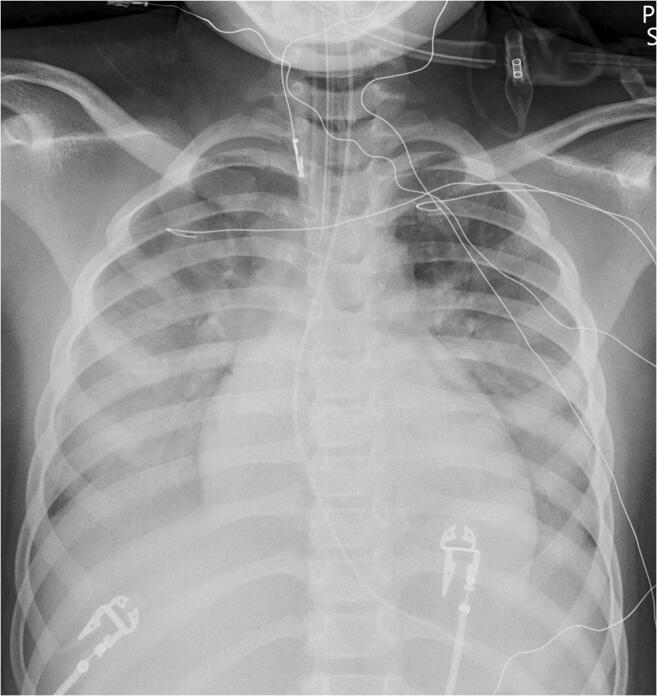
Radiography in a 6-year-old girl with multisystem inflammatory syndrome in children (MIS-C) who presented with fever, vomiting and altered mental status. Anteroposterior chest radiograph demonstrates patchy bilateral interstitial and alveolar opacities within the lung
Abdominal radiographs were obtained in five children; none of these demonstrated abnormalities. Three of these five children were diagnosed with MIS-C.
Advanced imaging was performed in a total of 23 children. Ten total US examinations were performed, seven of which were in children not diagnosed with MIS-C. Only one of the children without MIS-C had an acute abnormality demonstrating acute appendicitis. The remaining US examinations demonstrated no acute abnormalities, with one showing non-obstructing renal calculi.
Cross-sectional imaging CT or MR was performed in 15 children, 7 of whom were diagnosed with MIS-C. Only a single CT of the chest was performed; it showed no abnormalities. This was in a child who was asymptomatic who presented for follow-up for evaluation of Ewing sarcoma, and this child also had an MRI of the lumbar spine for tumor follow-up. Six of these children had head CT examinations, of which four were normal. The only child with an abnormality without MIS-C had a history of a stroke and a recent cardiac arrest. MRI was performed in seven children; two of the brain demonstrated chronic sinus thrombosis and acute osteomyelitis of the skull following craniopharyngioma resection. One child presented to MRI for follow-up for neurofibromatosis. An additional child with a fever was discovered to have left humeral osteomyelitis on a whole-body MRI, where peripheral-based nodules were also visualized. This child was also found to be positive for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. A child with MIS-C had an MRI to evaluate the appendix; this MRI demonstrated no evidence of acute appendicitis. An additional child with MIS-C had a cardiac MRI, which showed no findings consistent with myocarditis, including no evidence of myocardial edema and normal myocardial delayed enhancement and T1 mapping [13].
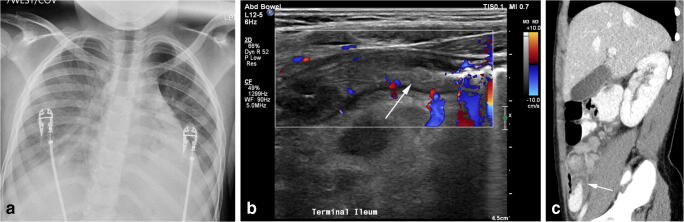
Of the 10 children with MIS-C, all had chest radiography and 9 of 10 had advanced imaging such as US (or echocardiography), CT or MRI (Table 4). Nine children had echocardiography (Table 5; [14–16]), which demonstrated low to low-normal left ventricular systolic function when measuring left ventricular ejection fraction and left ventricular fractional shortening, but decreased global left ventricular systolic function when measuring global longitudinal strain, which is used to detect subtle left ventricular dysfunction. Three children with MIS-C had additional abnormalities seen on more advanced imaging. One child had a US examination of the right lower quadrant and CT of the abdomen and pelvis demonstrating bowel wall thickening consistent with ileocolitis (Fig. 5). Another child had a head CT, which demonstrated cerebral edema, and the last had US exam of the right upper quadrant demonstrating pancreatic edema concerning for pancreatitis.
Table 4.
Demographic and clinical information in children who met criteria for multisystem inflammatory syndrome in children (MIS-C) who presented for medical imaging
| Patient # | M/F | Age at presentation | Presentation | Comorbidities | Imaging performed |
|---|---|---|---|---|---|
| 1 | F | 15 y 5 m | Fever, increased secretions, cough, respiratory distress | Type 2 neuronal ceroid lipofuscinosis | Chest radiograph |
| 2 | M | 5 y 3 m | Fever, emesis, diarrhea, abdominal pain | None | Chest radiograph, ultrasound RLQ, CT abdomen/pelvis echocardiography |
| 3 | F | 9 y 3 m | Fever, sore throat, decreased sense of smell, abdominal pain, diarrhea | None | Chest radiograph, ultrasound RLQ and RUQ, CT abdomen/pelvis echocardiography |
| 4 | F | 5 y 11 m | Fever, rash, diarrhea | None | Chest radiograph, CT head, echocardiography |
| 5 | F | 6 y 1 m | Fever, emesis, altered mental status, cardiogenic shock, respiratory failure | None | Chest radiograph, ultrasound kidneys, CT head, echocardiography |
| 6 | F | 7 y 8 m | Fever, diarrhea, abdominal pain | None | Chest and abdominal radiograph, ultrasound complete abdomen, CT abdomen/pelvis, echocardiography |
| 7 | F | 14 y 11 m | Fever, cough | Prematurity | Chest radiograph, ultrasound RUQ, echocardiography |
| 8 | M | 13 y 10 m | Fever, emesis, diarrhea, abdominal pain | Asthma | Chest and abdominal radiograph, echocardiography, cardiac MRI |
| 9 | F | 14 y 3 m | Fever, abdominal pain, lethargy, muscle aches, shortness of breath | None | Chest and abdominal radiograph, echocardiography |
| 10 | M | 9 y 11 m | Fever, rash, emesis, diarrhea, abdominal pain | None | Chest radiograph, ultrasound RLQ, echocardiography, MRI appendix |
F female, M male, m months, RLQ right lower quadrant, RUQ right upper quadrant, y years
Table 5.
Echocardiography findings in nine children with multisystem inflammatory syndrome in children (MIS-C) who presented for imaging with normal values
| Variable | Mean (range) | Abnormality threshold values [14–16] |
|---|---|---|
| LVEF (%) | 55.0 (42.0 to 58.0) | >55 |
| LVSF (%) | 30.0 (24.0 to 34.0) | >25 |
| GLS (%) | −15.5 (−18.0 to −12.5) | > −20.2 |
| TAPSE (cm) | 1.9 (1.4 to 2.0) | >1.7 |
| RVFWS (%) | −15.6 (−19.8 to −13.6) | > −27.2 |
GLS global longitudinal strain, LVEF left ventricular ejection fraction, LVSF left ventricular shortening fraction, RFSWS right ventricular free wall strain, TAPSE tricuspid annular plane systolic excursion
Fig. 5.
Multisystem inflammatory syndrome in children (MIS-C) in a 9-year-old girl who presented with fever, decreased sense of smell, abdominal pain and diarrhea. a Anteroposterior chest radiograph demonstrates bilateral interstitial opacities with consolidation within the lung bases. b Transverse US image of the right lower quadrant demonstrates thickening of the terminal ileum (arrow). c Sagittal reconstruction from a contrast-enhanced CT again shows the thickened terminal ileum (arrow)
Treatment, interventions and short-term outcomes
Of the 55 children who had imaging, treatment was most commonly guidance regarding self-isolation (18 of 55 children, or 33%) and use of broad-spectrum antibiotics (12 of 55 children, or 22%). Remdesivir treatment was only performed in two children. Forty-nine of 55 children (89%) required hospital admission with length of hospital stays ranging from 1 to greater than 76 days, with 1 child remaining hospitalized at the time of this writing. Seventeen of 55 children (31%) required intensive care treatment and 14 of 55 (25%) required ventilator support. Fifty-four of 55 children (96%) were alive at the time of this submission. The single deceased child did not meet criteria for MIS-C and had diffuse alveolar abnormalities on chest radiograph with pleural fluid but also had B cell acute lymphoblastic leukemia and developed Escherichia coli sepsis. No children without any imaging who tested positive for COVID-19 were admitted to the hospital.
All children diagnosed with MIS-C (n=10) were admitted to the hospital, with 9 of 10 requiring intensive care treatment and ventilator support. Length of hospital stay ranged from 3 days to greater than 76 days, with a single child remaining in the hospital at the time of this writing. Treatment in these children was most commonly intravenous immunoglobulin (IVIG; 7 of 10 children) and steroids (6 of 10 children), with 2 of these children receiving donated plasma antibodies. None of the children diagnosed with MIS-C had died at the time of this report.
Discussion
In this study we evaluated the imaging utilization of pediatric patients diagnosed with COVID-19 at a tertiary children’s hospital. Although 313 children were diagnosed with COVID-19, only 55 had imaging performed. All children admitted to the hospital were evaluated with imaging studies. Asthma was the most common comorbidity seen in pediatric patients who were evaluated with imaging. Most children who needed imaging had comorbidities; note that 47% had three or more comorbidities. Most of the imaging studies ordered for these children were chest radiographs, which were normal in greater than half of cases (34/51, or 67%). Abnormalities of both interstitial and alveolar opacities were seen on the remaining radiographs, with pleural effusion and interstitial opacities more commonly seen in children with MIS-C.
Although in adults chest CT is widely used, with evidence showing its role in risk stratification [7, 17–19], only a single chest CT was performed in our patient population and this was in an asymptomatic child who was in for routine oncological follow-up and demonstrated no abnormality. In the pediatric population in our study, the need for chest CT to evaluate COVID-19 was not apparent, which supports the pediatric consensus recommendations of only using CT in cases where there is concern for clinical progression, an alternative diagnosis, or poor clinical improvement [20]. Even in the small cohort of children diagnosed with MIS-C, chest CT was not used for management.
Ten total US examinations and 14 additional CT or MR examinations were performed, 7 of which were for children diagnosed with MIS-C. Head CT was the most common CT or MR examination and was performed in six of the children; four of these were normal, and one child with MIS-C demonstrated cerebral edema and the other had a recent posterior cerebral stroke that was present on the exam. Additionally, one child had sinus thrombosis on an MRI of the brain. Another had a whole-body MRI performed for a fever and demonstrated peripheral nodular opacities, but this child was also diagnosed with humeral osteomyelitis and MRSA bacteremia, and these were suspected to represent septic emboli. Given the reports of thromboembolism with COVID-19, a relationship between COVID-19 and these findings is possible [21, 22].
The small cohort of children diagnosed with MIS-C was less likely to have additional comorbidities when compared to the remainder of the cohort. Nine of these 10 children (90%) had advanced imaging such as US, CT, MRI or echocardiography for management and required intensive care, whereas only 8 of the remaining 45 children (18%) without MIS-C required intensive care. All children diagnosed with MIS-C remained alive at the time of this writing, with the only death in the cohort occurring in a child without MIS-C who had complications of bacterial sepsis.
The study is limited by its retrospective nature and selection bias resulting from referral to a tertiary children’s hospital. In this study, only the initial exam performed within 7 days and closest to the positive COVID-19 test were evaluated, even though some radiographic findings might develop later in the course of the disease. Additionally, a limited number of chest radiographs were evaluated and pneumonia can be difficult to diagnose in children [23].
Conclusion
Most pediatric patients with COVID-19 do not require hospital admission or imaging for evaluation. Children in our study who had imaging evaluation were predominately evaluated with chest radiography, which was usually normal. Interstitial opacities and pleural effusions were more commonly seen in children diagnosed with MIS-C. Most children with imaging had comorbidities, but children with MIS-C were more likely to have no comorbidities. At our tertiary children’s hospital, advanced imaging (US, CT, MRI) was less common for the care of these children, particularly chest CT and all advanced imaging in children without MIS-C.
Compliance with ethical standards
Conflicts of interest
None
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.(2020) Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. Morb Mortal Wkly Rep 69:1–8 [DOI] [PMC free article] [PubMed]
- 2.Paraluppi V, Pintus MC, Fanos V, Marcialis A. COVID-19 in newborns and in children: the state of the art. J Pediatr Neonatal Individ Med. 2020;9:1–13. [Google Scholar]
- 3.Lu Q, Shi Y. Coronavirus disease (COVID-19) and neonate: what neonatologist need [sic] to know. J Med Virol. 2020;92:564–567. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;2020:e2010369. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 7.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1,014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Cui H, Li K, et al. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020;50:796–799. doi: 10.1007/s00247-020-04656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kai F, Yongxing Y, Xianfeng W, et al. CT image features analysis of 15 cases of novel coronavirus infection in children. Zhonghua Er Ke Za Zhi. 2020;58:E007. doi: 10.3760/cma.j.issn.0578-1310.2020.0007. [DOI] [PubMed] [Google Scholar]
- 10.Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen A, Huang J, Liao Y, et al. Differences in clinical and imaging presentation of pediatric patients with COVID-19 in comparison with adults. Radiol Cardiothorac Imaging. 2020;2:e200117. doi: 10.1148/ryct.2020200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hameed S, Elbaaly H, Reid CEL, et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020;2020:202543. doi: 10.1148/radiol.2020202543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 14.Kantor PF, Lougheed J, Dancea A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian cardiovascular society guidelines. Can J Cardiol. 2013;29:1535–1552. doi: 10.1016/j.cjca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Levy PT, Sanchez A, Machefsky A, et al. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2014;27:549–560. doi: 10.1016/j.echo.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy PT, Machefsky A, Sanchez AA et al (2016) Reference ranges of left ventricular strain measures by two-dimensional speckle tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 29:209–225 [DOI] [PMC free article] [PubMed]
- 17.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. 2020;296:E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foust AM, Phillips GS, Chu WC et al (2020) International expert consensus statement on chest imaging in pediatric COVID-19 patient management: imaging findings, imaging study reporting and imaging study recommendations. Radiol Cardiothorac Imaging. https://pubs.rsna.org/doi/10.1148/ryct.2020200214. Accessed 12 Aug 2020 [DOI] [PMC free article] [PubMed]
- 21.Grillet F, Behr J, Calame P, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;2020:201544. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lushina N, Kuo JS, Shaikh HA. Pulmonary, cerebral, and renal thromboembolic disease associated with COVID-19 infection. Radiology. 2020;2020:201623. doi: 10.1148/radiol.2020201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiekara O, Korppi M, Tanska S, Soimakallio S. Radiological diagnosis of pneumonia in children. Ann Med. 1996;28:69–72. doi: 10.3109/07853899608999077. [DOI] [PubMed] [Google Scholar]