Abstract
Coriander seeds essential oil is used in food preparation, perfume, cosmetics and pharmaceuticals. In this study, extraction of essential oil from coriander seeds was done by hydrodistillation (HD) and microwave assisted hydrodistillation (MAHD) methods. Chemical composition, total phenol contents, antimicrobial and antioxidant properties of essential oils were measured and the results were compared between HD and MAHD methods. Scanning electron microscopy (SEM) results showed that the essential oils had inhibitory effect on the bacterial membrane and cell wall. Results showed that total phenol contents and antioxidant activity increased under heat and microwave conditions. Coriander seeds essential oil had a very strong effect on Candida albicans. Gram-positive bacteria were more sensitive to the essential oil of coriander seeds than Gram-negative bacteria. The essential oil extracted by MAHD showed better antimicrobial activity, higher phenols yield and antioxidant activity. According to the results of GC-MS, linalool was the most common constituent of both essential oils.
Keywords: Food science, Coriander seeds, DPPH assay, Antimicrobial activity, OSI, GC-MS, SEM
Food science; Coriander seeds; DPPH assay; Antimicrobial activity; OSI GC-MS; SEM.
1. Introduction
Essential oils are complex mixtures of volatile, aromatic, low molecular weight and hydrophobic compounds present in various parts of aromatic plants, including leaves, flowers, seeds, sprouts and shoots (Khalil et al., 2018). These compounds are secondary plant metabolites with well-known antimicrobial and antioxidant properties (Rivera Calo et al., 2015). One of the most important properties of essential oils is their antimicrobial property. Essential oils are a good source of several bioactive compounds, which have antioxidative and antimicrobial properties (Tongnuanchan and Bnjakul, 2014). Essential oils contain a number of compounds and metabolites that can inhibit a wide range of microorganisms such as bacteria, molds and yeasts (Nazzaro et al., 2013). Due to its antioxidant and antimicrobial properties, coriander seeds essential oil has a role in preventing food spoilage and food preservation (Mandal and Mandal, 2015). Essential oils are plant-based antimicrobial compounds that have therapeutic potential. They are not only effective in the treatment of infectious diseases, but also reduce the many side effects that are often caused by synthetic antimicrobial compounds (Zare Zardini et al., 2012).
Coriander (Coriandrum sativum L.) is an herbaceous annual plant, aromatic, and belongs to the family Apiaceae. It has a long history of nutritional and therapeutic uses and is used in the preparation of various foods as spices. It is endemic to the mediterranean region and grows extensively in India, Bangladesh, Russia, Morocco and central Europe (Mandal and Mandal, 2015). Coriander has many therapeutic properties including anti-inflammatory, analgesic, anticonvulsant, blood pressure lowering, cholesterol lowering, indigestible and sedative (Rajeshwari and Andallu, 2011; Asgharpanah and Kazemivash, 2012; Laribi et al., 2015). Coriander leaves and seeds are relatively good sources of essential oil (Mandal and Mandal, 2015). The content of essential oils is generally affected by coriander cultivar, weather conditions, stage of maturity and geographical location of the plant growth area (Laribi et al., 2015). Linalool is the greatest (40–70%) and most important constituent of the essential oil of coriander seeds. Linalool is a monoterpene alcohol found in many plants and has an antimicrobial effect on a wide range of bacteria (Mandal and Mandal, 2015). It's anti-inflammatory, anticoagulant, antinociceptive, anticonvulsant and antioxidant properties have also been known (Peana and Moretti, 2008; Laribi et al., 2015).
The essential oils of plants are usually obtained by water or steam distillation and solvent extraction. Disadvantages of these methods are long extraction time, low efficiency, toxicity of solvents, loss of volatile compounds and decomposition of unsaturated compounds due to heat (Ebrahimzadeh et al., 2003). Nowadays methods such as microwave, supercritical fluid, ultrasonic and pressure solvent extractions are used to increase extraction efficiency, shorten extraction time, improve the quality of extracted compounds and reduce process costs (Golmakani and Rezaei, 2008). Microwave assisted hydrodistillation (MAHD) is a new and advanced method for extracting essential oils from plants (Golmakani and Rezaei, 2008). In this method, the microwave waves are converted to thermal energy, resulting in the heating of the solvent and sample in the microwave oven, and ultimately the extraction process is performed more rapidly. In the MAHD method, heat and warmth are specifically transferred to the test sample and no significant energy loss to the environment. The most important advantages of this method include more efficient heat flow, reduced extraction time, lower solvent utilization, higher quality of extracted products, and more environmentally friendly method (Nitthiyah et al., 2017). MAHD process, from published studies, successfully used microwaves to extract essential oils from different aromatic plants (Golmakani and Rezaei, 2008; Wu Wang et al., 2010; Karakaya et al., 2012; Khanavi et al., 2013).
Golmakani and Rezaei (2008) used MAHD method with 990 W power to extract essential oil from thyme. In a study published by Wu Wang et al. (2010), with 800 W power, MAHD method was successfully used to extract essential oil from mango flowers. Also, Karakaya et al. (2012) obtained essential oil from rosemary with 622 W power, and in year 2013 Khanavi et al. extracted essential oil from thyme with 225 W power using MAHD method.
The aim of this study was to investigate the effects of microwave radiation on chemical compounds, antimicrobial properties (Staphylococcus aureus, Pseudomonas aeruginosa, Aspergillus niger and Candida albicans), antioxidant activity, extraction efficiency, phenolic compounds yield and oxidative stability of coriander seeds essential oils, and compare it with the classic distillation method.
2. Materials and methods
2.1. Plant material
Coriander is a tropical plant that needs a cool, relatively dry and frost-free climate, especially during flowering and seed formation. The ideal season for sowing coriander seeds is last week of October to first week of November. After 90–135 days, the coriander crop matures. At harvest time, the color of the fruit is light brown and its moisture content is 20%, which is reduced to 9–10%. It is stored in a cool, dry place during storage (Meena et al., 2013). Coriander seeds were purchased from the grocery in Mashhad, Iran. Essential oil yield (%) was measured using the following formula Eq. (1) (Tohidi et al., 2017).
| (1) |
2.2. Hydrodistillation (HD) using the conventional Clevenger apparatus
In this method, the Clevenger apparatus was used. 200 g of dried coriander seeds was grinded then poured into a 2000 ml balloon and added to a 1500 ml of distilled water (240 min). Moisture-free essential oils were collected and stored at -18 °C (Salehi Sourmaghi et al., 2014).
2.3. Microwave-assisted hydrodistillation (MAHD)
MAHD was performed at less than 100 °C and atmospheric pressure by a microwave equipment (MicRosynth, Milestone, USA). 200 g of grinded coriander seeds was poured into a 2000 ml flask and added to a 1500 ml of distilled water, then placed in a microwave oven. A condenser was placed above (outside the oven) to collect the obtained essential oil. First for 10 min at 800 W power reaching less than 100 °C and then after the boiling temperature was extracted for 60 min with 500 W power. The final 10 min was considered for ventilation (Kosar et al., 2005).
2.4. Physical constants
For both methods, specific gravity, color and refractive index (ATAGO, Rx-5000α, Japan) of the essential oils extracted from the coriander seeds were measured according to the method by Karakaya et al. (2012). Refractive index was measured at 20 °C and specific gravity was measured at 25 °C.
2.5. Total phenolics content
The amount of total phenolic compounds was measured using Folin–Ciocalteu reagent (FCR). The basis of the work was to reduce the FCR by phenolic compounds in the alkaline medium, and to create a blue complex. In this test dilution of 10 mg/ml was prepared for both essential oils and methanol was used as solvent for essential oils. To determine the amount of total phenolic compounds, 0.5 ml of the essential oil was thoroughly mixed with 2.5 ml of 10% FCR and 2 ml of 7.5% Na2CO3 (w/v), then was placed in a dark place for 30 min. The absorbance of the solution was read using UV-Visible spectrophotometer (UV-160 A, SHIMADZU, Japan) at 765 nm. The standard curve (y = 0.0088x + 0.0412, R2 = 0.9976) was plotted in gallic acid (0–0.2 mg/ml). Results were expressed as mg of gallic acid equivalent (GAE) per 100 g dry weight of sample, i.e. mg GAE/100 g (Shaddel et al., 2014).
2.6. Radical scavenging ability (DPPH assay)
The basis of the radical scavenging method is reduction of DPPH free radical by antioxidants which results in the discoloration of the DPPH methanol solution from purple to yellow. Both essential oils 5 and 10 mg/ml dilutions in methanol as solvent were prepared and BHT (butylated hydroxyl toluene), synthetic antioxidant, was used as positive control. To this end, 1 ml of methanol solution of DPPH (0.5 mM) was added to 4 ml of the dilution which was prepared from the essential oil solution and was placed in a dark place at room temperature for 30 min. The absorbance was then read at 517 nm. Finally, the percentage of DPPH radical scavenging (RSA, %) was calculated as follows Eq. (2):
| (2) |
Where, Abscontrol expresses the absorbance of the control and Abssample is the absorbance of the sample. To compare the radical scavenging power of the essential oils, IC50, which represents an effective concentration of the samples with a 50% inhibitory capacity of DPPH was used (Kukic et al., 2008).
2.7. Oxidative stability index (OSI)
The rancimat test measures the secondary and volatile products resulting from the oxidation of oils and fats (including aldehydes, ketones and acids) (Shahidi and Zhong, 2005). In this test, volatile compounds are trapped in deionized water and increase the conductivity of deionized water. The induction period is actually the time taken to reach the turning point in the conductivity versus time and is defined as OSI. In this test, 0.3 mg/ml dilution of the essential oils in methanol as solvent was prepared and added to 5 g of purified, deodorized and bleached soybean oil without antioxidant. BHT was used as positive control and soybean oil without antioxidant as negative control. The thermal resistance of the specimens was measured using a rancimeter (Metrohm 743, Switzerland) at temperature of 110 °C and air flow rate of 15–20 L/h (Issaoui et al., 2010; Najafi et al., 2015).
2.8. Peroxide value (PV) analysis
Hydroperoxides are the primary products of the oxidation of fats, which are unstable compounds, and during oxidation, convert to secondary compounds such as acids, alkanes, alcohols and aldehydes. Hydroperoxides are measured using a peroxide index (Singh et al., 2006).
IDF (International Dairy Federation) method is a fast, sensitive, spectrophotometric-colorimetric method used to measure peroxide index (Hornero-Méndez et al., 2001). Peroxide index was measured at zero time and after fourth and eighth days. For this purpose, the essential oils and synthetic antioxidant BHT (positive control) were diluted (0.2 mg/ml) and added to 2 g of purified soybean oil without antioxidant, and the samples were heated to 60 °C. Purified soybean oil without antioxidant was used as negative control. According to the IDF method, we weighed 0.01–0.3 g of the samples and added 9.8 ml of chloroform-methanol solution (7:3, v/v), and the sample was mixed on a vortex mixer for 2–4 s. Ammonium thiocyanate solution (50 μl) was added and the sample was mixed on a vortex mixer for 2–4 s, then added 50 μl of iron (II) solution and the sample was mixed on a vortex mixer for 2–4 s. After 5 min incubation at room temperature, the absorbance of the samples and absorption of control sample (containing all compounds except sample) at 500 nm were read. The standard curve (y = 0.0016x + 0.388, R2 = 0.9626) was obtained using dilutions made of iron (III) chloride solution (Mehta et al., 2015).
2.9. Antimicrobial activity
2.9.1. Microbial strains and growth conditions
The microbial strains used in this study were obtained from the Faculty of Pharmacy, Mashhad University of Medical Sciences, Iran. These strains included one Gram-positive bacterium, Staphylococcus aureus (ATCC 25923), one Gram-negative strain, Pseudomonas aeruginosa (PTCC1707), and one mold including Aspergillus niger (PTCC 5010) and one yeast Candida albicans (PTCC 5027). Bacterial strains were cultured at 37 °C for 24 h at Muller hinton agar (Merck, Germany) medium, and fungai were cultured at Potato dextrose agar (Merck, Germany) for 72 h at 25 °C.
2.9.2. Determination of MIC
Dilution method was used to obtain the minimal inhibitory concentration (MIC) of essential oil. Sample dilution was performed in a 96 well microplate, filled with mueller hinton broth (MHB) for bacterias and potato dextrose broth (PDB) for fungai. Sample dilutions were prepared from 0.25-128 mg/ml with the aid of 1.5 ml of Tween 80 and culture medium. Dilution was performed by microdilution. In the first well, 150 μl of 128 mg/ml dilution was added, then in all wells (except the first well) 50 μl of the added culture medium was then transferred from the first well 64 mg/ml to the second well, and dilution was corrected. The rest of the dilutions were made the same way. Lastly, 10 μl of microbial suspension (1.5 × 108 cfu/ml) was added to each well, and the microplate was incubated for the bacteria at 37 °C for 24 h and for the fungai at 25 °C for 72 h. The turbidity was read by spectrophotometer using the Elisa Reader (BioTek, ELx 808, USA) at 630 nm and the lowest turbidity well was reported as MIC (Moghimi et al., 2016; Nazari et al., 2016).
2.9.3. Determination of MBC and MFC
To determine the minimal bactericidal concentration (MBC) and the minimal fungicidal concentration (MFC), 10 μl of all wells without microbial growth in the MIC test was placed on MHA medium for bacteria and PDA medium for the fungai was cultured on the surface and incubated under appropriate conditions with microorganisms (37 °C for 24 h for bacteria and 25 °C for 72 h for fungai). A competition was selected as MBC and MFC with no growth observed (Silveira et al., 2009; Moghimi et al., 2016).
2.9.4. Disc diffusion agar assay (DD)
In this test, 15 ml of MHA medium for the bacterium and PDA for the fungai were poured into the plate under sterile conditions and solidified at room temperature. Then, microbial suspension, which was adsorbed to 0.5 McFarland (1.5 × 108 cfu/ml), was cultured in 10 μl. Sterilized blank disks (6 mm in diameter) were placed in the selected dilutions of essential oils, 150 and 300 mg/ml for 10 min. In this test, vancomycin, gentamicin and amphotericin B (antibiotics) were used as positive control for Gram-positive, Gram-negative bacteria and fungai, and sterile distilled water and Tween 80, as negative control, respectively. Finally, the bacterial plate was incubated at 37 °C h for 24 h and incubated for fungai at 25 °C for 72 h. The size of the inhibition zone formed in millimeters was reported (Goñi et al., 2009).
2.9.5. Well diffusion agar assay (WD)
For this purpose, 15 ml of MHA medium for bacteria and PDA for fungai were sterilized on a plate to solidify at room temperature. The wells were cultured with a cork borer of 9 mm diameter and 10 μl of microbial suspension equivalent to 0.5 McFarland (1.5 × 108 cfu/ml). 100 μl of dilutions of 150 and 300 mg/ml of the essential oil were poured into each well and incubated at 37 °C for 24 h and incubated for fungai at 25 °C for 72 h. The size of the inhibition zone formed in millimeters was reported (El-Sayed et al., 2016).
2.10. Gas chromatography-mass spectrometry (GC-MS)
Gas chromatography-mass spectrometer (Konik, HRGC 5000c, Spain) was used with quadrature detector and DB-5 capillary column (30 m × 0.25 mm, 0.25 μm stationary phase). The column temperature was increased from 40 °C to 250 °C with the heating rate of 2.5 °C/min. The sample (0.1 μl) was injected to the gas chromatograph, and helium gas was used as carrier gas (with flow rate 1 ml/min). The heating rate was 5 °C/min. In the mass spectrometer, the ionization energy was 70 eV. Alkanes outflows were considered as reference points in the calculation of relative inhibition indices (Salehi Sourmaghi et al., 2014).
2.11. Scanning electron microscopy (SEM)
2.11.1. SEM of coriander seeds
Coriander seeds were used as untreated samples treated with MAHD (60 min) and HD (240 min) to investigate the microscopic structure of coriander seeds using SEM. The seeds were placed on a stub and were coated with Sputter Coater (SC7620) with palladium-gold for 180s. Subsequently, the imaging was performed by SEM (LEO 1450vp, Germany) with 205 nm resolution under vacuum and 20 kV voltage conditions at various magnifications (Golmakani and Rezaei, 2008).
2.11.2. SEM of bacteries
Scanning electron microscopy was used to investigate the effect of essential oil on the cell wall of Staphylococcus aureus and Pseudomonas aeruginosa. In order to activate the bacteria, it was incubated in MHB medium for 24 h at 37 °C. Essential oil emulsion was prepared in MIC dilution of bacteria (HD - S. aureus: 16 mg/ml, HD - P. aeruginosa: 128 mg/ml and MAHD - S. aureus: 32 mg/ml, MAHD - P. aeruginosa: 64 mg/ml) and was added to the microbial suspension at a ratio of 1:1 and for 24 h the other was incubated at 37 °C. 2 ml of the microbial + essential oil suspension was poured into the microtube and centrifuged at 4500 rpm for 5 min. The liquid onto the microtube was removed, and the existing pellet was fixed with the glutaraldehyde (2.5%, v/v, in sodium cacodylate buffer) fixator for 2 h and was cooled at 4 °C. In the next step, the sample was washed three times with sodium cacodylate buffer 0.1 M (pH 7.4), any times for 15 min. Then for dehydration with alcohol, different percentages of alcohol were prepared (30%, 50%, 70%, 80%, 90% and 100%), and each was centrifuged 3 times for 5 min. In the end, a drop of the sample was coated with Sputter Coater (Model SC7620) with palladium-gold for 180 s. The imaging was performed by SEM (LEO 1450vp, Germany) with 205 nm resolution under vacuum and 20 kV voltage conditions at various magnifications (Moghimi et al., 2016).
2.12. Statistical analysis
The results were analyzed using ANOVA in completely randomized design with factorial arrangement and significance level of α < 0.05. Duncan test was used to compare the means. SPSS software version 16.0 was used for statistical analysis. All experiments were performed in two replicates.
3. Results and discussion
3.1. Essential oil yield
The content of coriander seeds essential oil was (v/w), 0.325% and 0.31% for MAHD and HD, respectively (Table 1). Although there was no significant difference between extraction efficiency of HD and MAHD methods (P > 0.05), according to the shorter MAHD extraction process (60 min), higher and more efficient heat flow, indoor extraction process and no energy loss (Golmakani and Rezaei, 2008; Nitthiyah et al., 2017) compared with longer extraction time in classical HD (240 min) method, energy dissipation and diffusion to the surrounding environment and loss of some volatile compounds (Ebrahimzadeh et al., 2003), MAHD was a better method for separation essential oils. For MAHD and HD methods, the extraction was started at the boiling point of water (less than 100 °C). MAHD reached this temperature after 15 min and HD method after 45 min. The energy requirement to perform the distillation based on the maximum power consumption of the electromantle for microwave oven for MAHD and HD were 0.63 and 2.52 kWh, respectively (Golmakani and Rezaei, 2008). Therefore, in terms of energy consumption and cost, MAHD method was also more economical.
Table 1.
Yield, TPC, DPPH and OSI values of coriander seeds of essential oil.
| Samples | Essential oil content (%, v/w) | TPC (mg GAE/100 g) | DPPH (mg/ml) | OSI (h) |
|---|---|---|---|---|
| HD | 0.31 ± 0.014a | 0.164 ± 0.004b | 31.875 ± 0.009a | 5.20 ± 0.028c |
| MAHD | 0.325 ± 0.021a | 0.1895 ± 0.002a | 26.973 ± 0.011b | 5.345 ± 0.021b |
| BHT | _ | _ | 0.410 ± 0.002c | 6.10 ± 0.042a |
| Soyben oil | _ | _ | _ | 4.60 ± 0.282d |
Values in the same column with different letters (a-d) are significantly different at P < 0.05.
3.2. Physical properties
The results of refractive index, specific gravity and color of essential oil obtained by HD and MAHD are shown in Table 2. No significant differences were observed between treatments (P > 0.05). The only difference in the color of the MAHD essential oil was compared with the HD treatment, which was completely colorless. Karakaya et al. (2012) obtained the specific gravity of rosemary essential oil extracted by HD and MAHD methods, 0.875 and 0.879, respectively. The refractive indexes of both essential oils were identical (1.465).
Table 2.
Physical properties of coriander seeds essential oil.
| Physical properties | HD | MAHD |
|---|---|---|
| Specific gravity | 0.910 ± 0.004a | 0.911 ± 0.002a |
| Refractive index | 1.4608 ± 0.0002a | 1.4610 ± 0.0004a |
| Color | Pale yellow | Colorless |
3.3. Total phenolics contents (TPC)
The results of TPC for both essential oils are shown in Table 1. The total phenolic contents of coriander seeds essential oil obtained by MAHD and HD methods were 0.1895 and 0.164 mg GAE/100 g, respectively. It seems that simultaneous application of heat treatment and microwave irradiation in MAHD method compared with heat treatment alone in HD method resulted in higher extraction efficiency of phenolic compounds than classical HD method. This may be due to the more efficient heat flow of the microwave (Golmakani and Rezaei, 2008), the faster sample temperature rise (Chumyam et al., 2013), and the simultaneous application of heat and microwave wave combined phenolic compounds free form has been attributed to the release of these compounds into the intracellular fluid and to increase their extraction efficiency (Chumyam et al., 2013). The low solubility of phenolic compounds in essential oils and the compounds combined with seed material, may explain the low TPC values in coriander seeds essential oil (Lutterodt et al., 2011). In the study of Neffati et al. (2010) the total phenol contents for the methanolic extract of Tunisian coriander was reported 1.04 mg GAE/g DW. In a 2004 study by Wangensteen et al., phenolic compounds extracted from coriander seeds ethyl acetate extract were reported 1.09 g GAE/100 g DW.
3.4. Antioxidant activity (DPPH assay)
The IC50 values for MAHD and HD essential oils were 26.973 and 31.875 mg/ml, respectively. The antioxidant BHT showed a higher radical scavenging activity than that of the coriander seeds essential oil (0.41 mg/ml). As the concentration of essential oil increased, its radical scavenging power increased. The essential oil obtained by MAHD method had higher radical scavenging activity than HD essential oil. This is attributed to the higher total phenolics content of MAHD essential oil compared with HD essential oil. It seems that due to the sensitivity of the antioxidant compounds to the heat and due to the shorter heating time in the MAHD method than the HD method, the MAHD essential oil had a higher radical scavenging ability. Phenolic compounds have an important antioxidant role as a good barrier against oxidative reactions. As shown by many studies, there was a positive correlation between antioxidant activity and phenolics content of natural resources (plants) (Kukic et al., 2008; Nanasombat and Wimuttigosol, 2011; Zeljković et al., 2017; Tohidi et al., 2017). Sriti et al. (2011) reported that the DPPH scavenging ability of Canadian and Tunisian coriander seeds essential oil were 60000 and 61000 μg/mL, respectively.
3.5. Oxidative stability index (OSI)
The oxidative stabilities of the essential oils obtained from MAHD, HD and BHT were measured as 5.345, 5.20 and 6.1 h, respectively. The control sample had the lowest induction time (4.6 h), and the synthetic antioxidant BHT had the highest induction time (6.1 h). Regarding DPPH radical inhibitory power, MAHD essential oil showed higher inhibitory effect than HD essential oil, which was similar to oil oxidative stability (MAHD essential oil was more stable than HD essential oil). The oxidative stability of the essential oil seems to follow the mechanism of free radical inhibition.
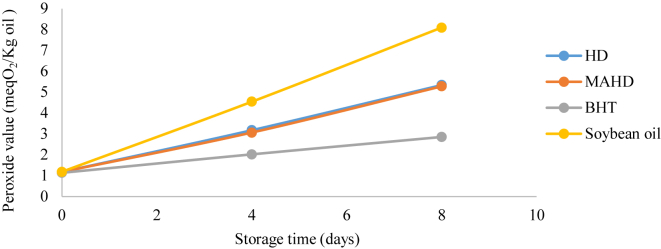
3.6. Peroxide value (PV)
The peroxide index increased in all samples over the 8 days of storage at 60 °C, due to the presence of unstable compounds in the oil that are susceptible to oxidation. The antioxidant-free control peroxide index reached its highest level till the end of the 8th day. The lowest amount of peroxide was observed for synthetic BHT antioxidant. There was no significant difference between the index of soybean oil treated with 2 essential oils of coriander seeds obtained by MAHD and HD methods (P > 0.05) (see Table 3; Figure 1).
Table 3.
Peroxide values of coriander seeds essential oil.
| Peroxide values |
|||
|---|---|---|---|
| 0 | 4 | 8 | |
| HD | 1.174785 ± 0.013a | 3.170965 ± 0.067b | 5.334285 ± 0.037b |
| MAHD | 1.174785 ± 0.006a | 3.061125 ± 0.027b | 5.274595 ± 0.020b |
| BHT | 1.131805 ± 0.013b | 2.015281 ± 0.020c | 2.848615 ± 0.006c |
| Soybean oil | 1.181948 ± 0.070a | 4.536771 ± 0.008a | 8.092168 ± 0.080a |
Values in the same column with different letters (a-c) are significantly different at P < 0.05.
Figure 1.
Peroxide values of HD and MAHD essential oils.
3.7. Antimicrobial activity
The antimicrobial activity of coriander seeds essential oils against four microorganisms causing foodborne infection and poisoning is presented in Tables 4.1 and 4.2. The essential oil showed good inhibitory effect against all the microorganisms under investigation with different degrees of inhibition. According to the results of MIC, MBC and MFC tests and the comparison between Gram-positive and Gram-negative bacteria and fungai under investigation, the highest activity of HD and MAHD essential oils against Staphylococcus aureus and Candida albicans, with the lowest MIC values and MBC (MFC) was seen. The highest values of MIC and MBC (MFC) were Pseudomonas aeruginosa and Aspergillus niger, indicating low essential oil activity against these two microorganisms and in fact their resistance to coriander seeds essential oil. The largest inhibition zones in disk diffusion test (disk diameter 6 mm) were obtained for S. aureus and C. albicans, indicating the high susceptibility of these microorganisms to coriander seeds oil. In addition, the smallest inhibition zones, which showed high resistance of the microorganism to the essential oil, were measured for P. aeruginosa and A. niger. In the well diffusion test (well diameter 9 mm), both Gram-positive and Gram-negative bacteria showed the same sensitivity to essential oil. Although, C. albicans showed more sensitivity than A. niger among the fungai. The analysis of the results indicated that the essential oil of coriander seeds obtained by both MAHD and HD method had a very strong effect on C. albicans so that in all antimicrobial tests the lowest MIC dilutions and the largest inhibition zones were obtained (p < 0.05). Coriander seeds oil also has more activity against Gram-positive bacteria than Gram-negative bacteria, which is attributed to differences in cell wall structure of Gram-positive and Gram-negative bacteria. This was consistent with the research results (Ijaz Hussain et al., 2008; Karakaya et al., 2012; Goldbeck et al., 2014). The outer membrane of Gram-negative bacteria has a highly hydrophilic surface that prevents the penetration of hydrophobic compounds (Rivera Calo et al., 2015). However, the lipophilic ends of the lipoteichoic acids of the cell membrane of Gram-positive bacteria facilitates the penetration of hydrophobic compounds such as essential oils, which lead to greater susceptibility to the bacteria (Karakaya et al., 2012). The analysis of Tables 4.1 and 4.2 shows that the essential oil obtained by MAHD method had higher antimicrobial activity compared with HD method. Changes in the antimicrobial activity of the essential oils depend on their different chemical constituents. Therefore, the higher antimicrobial activity of essential oil is attributed to higher levels of linalool and other oxygenated compounds in MAHD (Ijaz Hussain et al., 2008). The major groups responsible for the antimicrobial activities of plants include phenols, phenolic acids, quinones, flavonoids, tannins, coumarines, alkaloids and terpenoids. Changes in the structure and chemical composition of these components lead to differences in the antimicrobial activity of the essential oils (Gyawali and Ibrahim, 2014). The mode of action of essential oils against bacteria is primarily the destruction of the cell wall and cell membrane integrity, which in turn increases membrane permeability and results in disruption of cellular activities such as energy production and membrane transport. Damage to the cell membrane causes changes in critical processes such as nutrient processing, synthesis of structural macromolecules, and growth regulators (Swamy et al., 2016). The antifungal mechanism of the essential oils is similar to bacteria and their main purpose is the cell membrane of the fungi, which leads to increase the membrane permeability and loss its integrity (Krisch et al., 2011). The essential oil breaks down the mitochondrial membranes by penetrating the cytoplasmic membrane. In molds, it affects the growth of hyphae and their sporulation. Inactivation of conidia by essential oil is a key inhibition process because it is difficult to remove conidia due to their heat and chemical constituents (Laranjo et al., 2017).
Table 4.1.
Antimicrobial activity of HD essential oil.
| HD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microorganisms | MIC | MBC (MFC) | DD |
WD |
Antibiotics |
||||
| 150 | 300 | 150 | 300 | Vancomycin | Gentamicin | Amphotericin B | |||
| S. aureus | 16 | 32 | _ | 10 | 11 | 15 | 16 | _ | _ |
| P. aeruginosa | 128 | 128 | _ | 10 | _ | 15 | _ | 14 | _ |
| C. albicans | 2 | 4 | 15 | 24 | 26 | 29 | _ | _ | 25 |
| A. niger | 16 | 32 | 10 | 14 | 11 | 13 | _ | _ | 25 |
MIC, MBC and MFC (mg/ml).
DD and WD Inhibition zones in diameter (mm).
(-): no antimicrobial activity.
Table 4.2.
Antimicrobial activity of MAHD essential oil.
| MAHD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microorganisms | MIC | MBC (MFC) | DD |
WD |
Antibiotics |
||||
| 150 | 300 | 150 | 300 | Vancomycin | Gentamicin | Amphotericin B | |||
| S. aureus | 32 | 64 | _ | 11 | 12 | 15 | 16 | _ | _ |
| P. aeruginosa | 64 | 128 | _ | 10 | _ | 14 | _ | 14 | _ |
| C. albicans | 4 | 8 | 20 | 24 | 27 | 29 | _ | _ | 25 |
| A. niger | 8 | 16 | 12 | 14 | 12 | 16 | _ | _ | 25 |
MIC, MBC and MFC (mg/ml).
DD and WD Inhibition zones in diameter (mm).
(-): no antimicrobial activity.
3.8. Chemical composition of coriander seeds essential oil
The chemical composition of coriander seeds essential oils are shown in Table 5. In total, 50 compounds were identified in the essential oil (HD 98.2% and MAHD 98.7%). Most of the compounds identified in HD essential oil were also present in the MAHD essential oil. The dominant compound of both essential oils was linalool in agreement with the results of the studies by Kosar et al. (2005) and Salehi Sourmaghi et al. (2014). There was a difference between the relative percentage of linalool in the essential oil of MAHD (52.6%) compared with the HD essential oil (49%). The diversity of the identified compounds was lower in MAHD essential oil, but the relative percent of oxygenated monoterpene in MAHD method (54.8%) was higher than HD essential oil (51.9%). The antioxidant activity of the essential oil has been attributed to the amount of phenolic and terpenoid compounds in the essential oils such as linalool, p-cymene, γ-terpinene, terpin-4-ol, α-pinene, β-pinene and borneol (Ruberto and T.Baratta, 2000). Antimicrobial activity and changes in the antimicrobial activity of the essential oils depend on their chemical composition (Ijaz Hussain et al., 2008). Hence phenolic compounds, radical scavenging activity and higher antimicrobial activity of MAHD essential oil are justified.
Table 5.
Chemical composition of coriander seeds essential oil of HD and MAHD.
| No. | Compounds | RT∗ (HD) | RT∗(MAHD) | %HD | %MAHD |
|---|---|---|---|---|---|
| 1 | cis-1-Ethyl-3-methyl-cyclopentane | 4.09 | 4.06 | 0.8 | 1.6 |
| 2 | Octane | 4.33 | 4.29 | 6.7 | 10.3 |
| 3 | 3-Chlorohexane | 4.40 | 4.40 | 0.1 | 0.2 |
| 4 | Propyl-cyclopentane | 4.90 | 4.91 | 0.4 | 0.4 |
| 5 | Ethyl-cyclohexane | 4.98 | 4.99 | 0.2 | 0.2 |
| 6 | 2,5-Dimethyl-Heptane | 5.67 | _ | 0.1 | _ |
| 7 | p-Xylene | _ | 6.40 | _ | 0.9 |
| 8 | Heptanal | 6.50 | 6.64 | 0.2 | 0.1 |
| 9 | 3-ethyl-2-methyl-heptane | 6.73 | _ | 0.1 | _ |
| 10 | β-Thujene | 7.09 | 7.26 | 0.2 | 0.1 |
| 11 | α-Pinene | 7.36 | 7.53 | 6.8 | 5.9 |
| 12 | Camphene | 7.74 | _ | 0.5 | _ |
| 13 | 5-Methyl-nonane | 7.87 | 8.15 | 0.4 | 0.5 |
| 14 | 3-Methyl-nonane | 8.21 | 8.52 | 0.6 | 0.8 |
| 15 | 3-Ethyl-octane | _ | 8.39 | _ | 0.1 |
| 16 | β-Phellandrene | 8.35 | 8.67 | 0.7 | 0.4 |
| 17 | β-Pinene | 8.50 | 8.83 | 1.5 | 1 |
| 18 | β-Myrcene | 8.74 | _ | 0.6 | _ |
| 19 | Decane | 9.07 | 9.49 | 5 | 7.3 |
| 20 | Octanal | 9.20 | _ | 0.2 | _ |
| 21 | 3-Carene | 9.32 | _ | 0.2 | _ |
| 22 | α-Terpinene | 9.52 | 10.01 | 0.1 | 0.1 |
| 23 | m-Cymene | 9.78 | 10.28 | 1.8 | 1.5 |
| 24 | D-limonene | 9.87 | 10.39 | 0.5 | 0.3 |
| 25 | Terpinolene | 10.73 | _ | 7 | _ |
| 26 | 1-Octanol | 11.41 | 11.89 | 1 | 1 |
| 27 | Linalool | 11.83 | 12.66 | 49 | 52.6 |
| 28 | 5-Methyl-undecane | 13.11 | _ | 0.1 | _ |
| 29 | 3-Methyl-undecane | 13.51 | 14.63 | 0.1 | 0.2 |
| 30 | 1-Nonanol | 13.60 | 14.74 | 0.1 | 0.1 |
| 31 | Borneol | 13.76 | 14.89 | 0.1 | 0.1 |
| 32 | Terpinen-4-ol | 13.96 | 15.12 | 0.3 | 0.3 |
| 33 | Butyl-cyclohexane | 14.10 | _ | 0.2 | _ |
| 34 | Dodecane | 14.27 | 15.54 | 1.9 | 2.7 |
| 35 | Decanal | 14.52 | 15.82 | 1.5 | 1 |
| 36 | Citronellol | 14.97 | 16.37 | 0.5 | 0.4 |
| 37 | Geraniol | 15.58 | 17.08 | 1.5 | 1.1 |
| 38 | (E)-2-Decenal | 15.90 | 17.45 | 0.8 | 0.6 |
| 39 | 2-Butyl-cyclohexanol | 15.96 | _ | 0.1 | _ |
| 40 | 1-Decanol | 16.05 | 17.66 | 0.3 | 0.2 |
| 41 | Estragole | 16.52 | _ | 0.2 | _ |
| 42 | Undecanal | 16.92 | 18.69 | 0.2 | 0.1 |
| 43 | Myrtenyl acetate | 17.32 | _ | 0.1 | _ |
| 44 | Citronellyl valerate | 17.82 | _ | 0.1 | _ |
| 45 | Anethole | _ | 18.19 | _ | 0.1 |
| 46 | 2-Undecenal | 18.24 | _ | 0.1 | _ |
| 47 | Geranyl acetate | 18.53 | 20.60 | 3 | 2.6 |
| 48 | Tetradecane | 18.92 | 21.09 | 0.4 | 0.7 |
| 49 | Dodecanal | 19.21 | 21.43 | 0.2 | 0.1 |
| 50 | Caryophyllene | 19.55 | 21.77 | 0.2 | 0.2 |
| 51 | 2-Dodecenal | 20.50 | 22.95 | 1.3 | 1 |
| 52 | Hexadecane | 23.08 | 26.06 | 0.1 | 0.2 |
| 53 | (E)-2-Tetradecenal | 24.58 | 27.83 | 0.1 | 0.1 |
| 54 | (Z)-9,17-Octadecadienal | _ | 34.11 | _ | 0.4 |
| 55 | Linoleyl methyl ketone | _ | 35.81 | _ | 0.1 |
| 56 | Hexadecanoic acid | _ | 36.54 | _ | 1 |
| 57 | octadecamethyl-Cyclononasiloxane | _ | 38.15 | _ | 0.8 |
| 58 | (Z)-9-octadecen-4-olide | _ | 39.83 | _ | 0.5 |
| Hydrocarbon monoterpenes | 10.3 | 8.6 | |||
| Oxygenated monoterpenes | 51.9 | 54.8 | |||
| Hydrocarbon sesquiterpenes | 0.2 | 0.2 | |||
| Total | 98.2 | 98.7 |
∗RT- Retention time (min) and (-): not identified.
3.9. SEM
3.9.1. SEM of coriander seeds
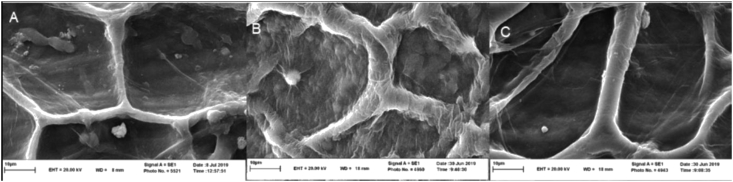
The images of untreated coriander seeds (Figure 2 A) treated with HD (Figure 2 B) and MAHD treatment (Figure 2 C) are shown together in Figure 2. Coriander seeds images were observed without treatment of completely defined and intact cells and cell walls. Both HD and MAHD treatments altered the appearance and appearance properties of coriander seeds. In the images taken from MAHD treatment compared with untreated coriander seeds and HD treatment, the cell damage was higher. In some parts, the cell wall was ruptured and the structure of cell wall was destroyed. This appears to be caused by a better and more efficient diffusion of heat in the MAHD treatment. In the HD treatment compared with MAHD treatment and untreated coriander seeds, the major changes by HD treatment were in appearance and appearance properties of the seeds.
Figure 2.
SEM of coriander seeds. A: Untreated. B: HD treatment. C: MAHD treatment.
3.9.2. SEM of bacteries
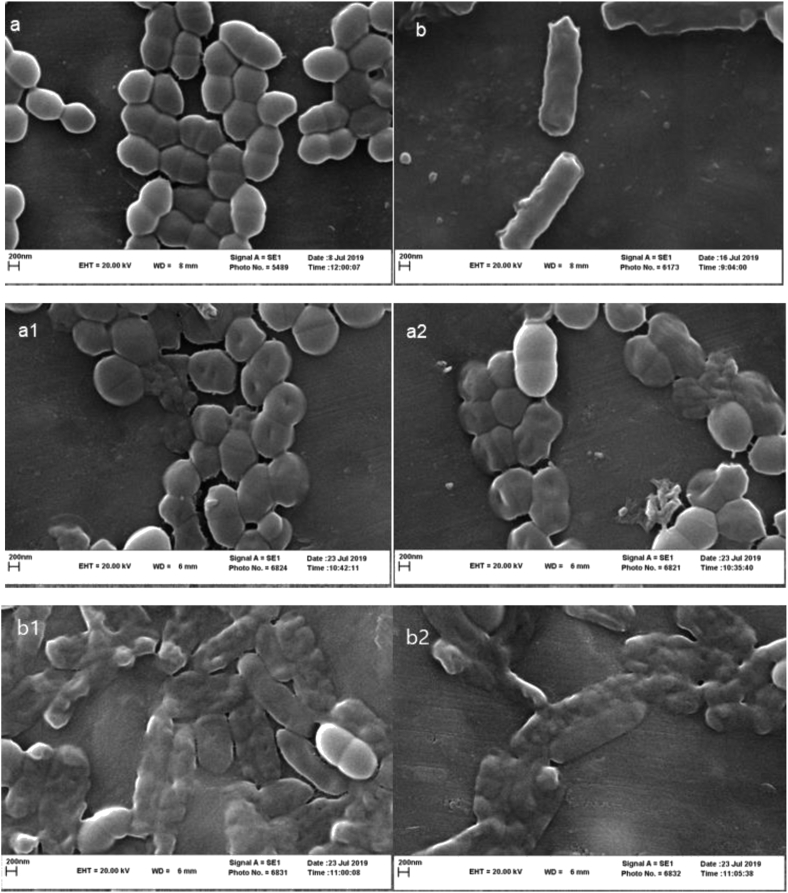
Scanning electron microscopy was used to obtain information about on bacterial morphology changes after essential oil treatment compared with S. aureus and P. aeruginosa untreated. The images in Figure 3 (a: Intact S. aureus, b: Intact P. aeruginosa, a1: HD and S. aureus, a2: MAHD and S. aureus, b1: HD and P. aeruginosa, b2: MAHD and P. aeruginosa) have been shown. Untreated bacterial samples with essential oil, rod-shaped P. aeruginosa and spherical-shaped S. aureus were observed. Significant morphological changes were observed in the treated samples compared with the untreated samples. The images taken of S. aureus under both HD and MAHD treatments showed truncations on the surface of the bacteria. Some of these bacteria were more severely damaged as their cell walls ruptured and the inside content leaked out of the cell. P. aeruginosa showed a different degree of deformation and degradation. Bacteria were largely destroyed and the integrity of the cell wall and their appearance were almost completely destroyed. Many researchers have reported that the possible mechanisms of the essential oils and their derivatives include cell wall destruction, cytoplasmic membrane damage, damage to membrane proteins, intracellular content leakage, and cytoplasmic coagulation (Goldbeck et al., 2014; Swamy et al., 2016). Based on the analysis of SEM images, it seems that the cell wall and the membrane of pathogenic bacteria are the main targets of the essential oils.
Figure 3.
SEM of bacteries (a: Intact S. aureus, b: Intact P. aeruginosa, a1: HD and S. aureus, a2: MAHD and S. aureus, b1: HD and P. aeruginosa, b2: MAHD and P. aeruginosa).
4. Conclusion
MAHD method is a new and advanced method of essential oils production, which is recognized as a green method due to the lower extraction time, lower energy consumption resulting in lower cost, environmental friendliness, more efficient heat flow and less solvent consumption. Accordingly, the usual extraction methods can be replaced by MAHD method. Although the extraction yield obtained by MAHD and HD methods were approximately similar, the extraction time of MAHD method was significantly shorter than HD method. The investigation of microbial and the antioxidant test data showed better results of essential oil extracted by MAHD method. Coriander seeds essential oil had a higher inhibitory effect against Gram-posetive bacteria than Gram-negative bacteria. SEM images showed the inhibitory effect of coriander seeds essential oil against bacteria on their cell walls.
Declarations
Author contribution statement
Negin Ghazanfari: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Seyed A. Mortazavi, Farideh T. Yazdi: Conceived and designed the experiments; Analyzed and interpreted the data.
Morteza Mohammadi: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Ferdowsi University of Mashhad, Iran (48977).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to the Seh Gol Khorasan vegetable oil factory and Research Institute of Food Science and Technology (RIFST), Mashhad, Iran, for providing laboratory facilities to carry out this study.
References
- Asgharpanah J., Kazemivash N. Phytochemistry, pharmacology and medicinal properties of Coriandrum sativum L. Afr. J. Pharm. Pharmacol. 2012;6:2340–2345. [Google Scholar]
- Chumyam A., Whangchai K., Jungklang J., Faiyue B., Saengnil K. Effects of heat treatments on antioxidant capacity and total phenolic content of four cultivars of purple skin eggplants. Sci. Asia. 2013;39:246–251. [Google Scholar]
- Ebrahimzadeh H., Yamini Y., Sefidkon F., Chaloosi M., Pourmortazavi S.M. Chemical composition of the essential oil and supercritical CO2 extracts of Zataria multiflora Boiss. Food Chem. 2003;83:357–361. [Google Scholar]
- El-Sayed H.S., Chizzola R., Ramadan A.A., Edris A.E. Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and selfmicroemulsifying water based delivery systems. Food Chem. 2016;221:196–204. doi: 10.1016/j.foodchem.2016.10.052. [DOI] [PubMed] [Google Scholar]
- Goldbeck J.C., Novack Victoria F., Motta A., Savegnago L., Jacob Raquel G., Perin G., Joao Lenardao E., Padilha da Silva W. Bioactivity and morphological changes of bacterial cells after exposure to 3-(p-chlorophenyl) thio citronellal. LWT - Food Sci. Technol. 2014;59:813–819. [Google Scholar]
- Golmakani M.T., Rezaei K. Comparison of microwave-assisted hydrodistillation with the traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem. 2008;109:925–930. doi: 10.1016/j.foodchem.2007.12.084. [DOI] [PubMed] [Google Scholar]
- Goni P., Lopez P., Sanchez C., Gomez-Lus R., Becerril R., Nerin C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009;116:982–989. [Google Scholar]
- Gyawali A., Ibrahim A. Natural products as antimicrobial agents. Food Contr. 2014;46:412–429. [Google Scholar]
- Hornero-Méndez D., Pérez-Gálvez A., Mínguez-Mosquera M.I. A rapid spectrophotometric method for the determination of peroxide value in food lipids with high carotenoid content. J. Am. Oil Chem. Soc. 2001;78:1151–1155. [Google Scholar]
- Ijaz Hussain A., Anwar F., Hussain Sherazi S.T., Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Issaoui M., Flamini G., Brahmi F., Dabbou S., Ben Hassine K., Taamali A., Chehab H., Ellouz M., Zarrouk M., Hammami M. Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chem. 2010;119:220–225. [Google Scholar]
- Karakaya S., Nehir El S., Karagozlu N., Sahin S., Sumnu G., Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J. Food Sci. Technol. 2012;51:1056–1065. doi: 10.1007/s13197-011-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N., Ashour M., Fikry S., Naser Singab A., Salama O. Chemical composition and antimicrobial activity of the essential oils of selected Apiaceous fruits. J. Pharmaceut. Sci. 2018;4:88–92. [Google Scholar]
- Khanavi M., Hajimehdipoor H., Emadi F., Kalantari Khandani N. Essential oil compositions of Thymus kotschyanus Boiss. obtained by hydrodistillation and microwave oven distillation. J. Essent. Oil Bearing Plants. 2013;16:117–122. [Google Scholar]
- Kosar M., Ozek T., Goger V., Kurkcuoglu M., Can Baser H. Comparison of microwave-assisted hydrodistillation and hydrodistillation methods for the analysis of volatile secondary metabolites. Pharmaceut. Biol. 2005;43:491–495. [Google Scholar]
- Krisch J., Tserennadmid R., Vágvölgyi C. Essential oils against yeasts and moulds causing food spoilage. In: Mendez-Vilas A., editor. Science against Microbial Pathogens: Communicating Current Research and Technological advance. Badajoz: Formatex; 2011. pp. 1135–1142. [Google Scholar]
- Kukic J., Popovic V., Petrovic S., Mucaji P., Ciric A., Stojkovic D., Sokovic M. Antioxidant and antimicrobial activity of Cynara cardunculus extracts. Food Chem. 2008;107:861–868. [Google Scholar]
- Laranjo M., Fernández-Léon A.M., Potes M.A., Agulheiro-Santos A.C., Elias M. Use of essential oils in food preservation. In: Mendez-Vilas A., editor. Antimicrobial Research: Novel Bioknowledge and Educational Programs. Badajoz: Formatex; 2017. pp. 177–188. [Google Scholar]
- Laribi B., Kouki K., M'Hamdi M., Bettaieb T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia. 2015;103:9–26. doi: 10.1016/j.fitote.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Lutterodt H., Slavin M., Whent M., Turner E., Yu L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011;128:391–399. doi: 10.1016/j.foodchem.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Mandal Sh., Mandal M. Coriander (Coriandrum sativum L.) essential oil: chemistry and biological activity. Asian Pac. J. Tropical Biomed. 2015;5:421–428. [Google Scholar]
- Meena S.S., Singh B., Singh D., Ranjan J.K., Meena R.D. Pre and post harvest factors effecting yield and quality of seed spices: a review. Int. J. Seed Spices. 2013;3:1–11. [Google Scholar]
- Mehta B.M., Darji V.B., Aparnathi K.D. Comparison of five analytical methods for the determination of peroxide value in oxidized ghee. Food Chem. 2015;185:449–453. doi: 10.1016/j.foodchem.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Moghimi R., Ghaderi L., Rafati H., Aliahmadi A., McClements D.J. Superior antibacterial activity of nanoemulsion of thymus daenensis essential oil against E. coli. Food Chem. 2016;194:410–415. doi: 10.1016/j.foodchem.2015.07.139. [DOI] [PubMed] [Google Scholar]
- Najafi V., Barzegar M., Sahari M.A. Physicochemical properties and oxidative stability of some virgin and processed olive oils. J. Agric. Sci. Technol. 2015;17:847–858. [Google Scholar]
- Nanasombat S., Wimuttigosol P. Antimicrobial and antioxidant activity of spice essential oils. Food Sci. Biotechnol. 2011;20:45–53. [Google Scholar]
- Nazari H., Yavarmanesh M., Haddad Khodaparast M.H. In vitro study to evaluate the antibacterial effect of pistacia khinjuk stocks oil as compared with olive oil on food borne pathogenic bacteria (Staphylococcus aureus, Escherichia coli, Listeria monocytogenes) J. Essent. Oil Bearing Plants. 2016;19:125–133. [Google Scholar]
- Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neffati M., Sriti J., Hamdaoui G., Kchouk M.E., Marzouk B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 2010;124:221–225. [Google Scholar]
- Nitthiyah J., Hamid Nour A., Kantasamy R., Akindoyo J.O. Microwave assisted hydrodistillation – an overview of mechanism and heating properties. Aust. J. Basic Appl. Sci. 2017;11:22–29. [Google Scholar]
- Peana A.T., Moretti M.D.L. Linalool in essential plant oils:pharmacological effects. Bot. Med. Clin. Pract. 2008;79:716–724. [Google Scholar]
- Rajeshwari U., Andallu B. Medicinal benefits of coriander (Coriandrum sativum L.) Spatula DD. 2011;1:51–58. [Google Scholar]
- Rivera Calo J., Crandall P.G., Bryan C.A.O.’, Ricke S.C. Essential oils as antimicrobials in food systems– A Review. Food Contr. 2015;54:111–119. [Google Scholar]
- Ruberto G., Baratta M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. [Google Scholar]
- Salehi Sourmaghi M.H., Kiaee G., Golfakhrabadi F., Jamalifar H., Khanavi M. Comparison of essential oil composition and antimicrobial activity of Coriandrum sativum L. extracted by hydrodistillation and microwave-assisted hydrodistillation. J. Food Sci. Technol. 2014;52:2452–2457. doi: 10.1007/s13197-014-1286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddel R., Maskooki A., Haddad-Khodaparast M., Azadmard-Damirchi S., Mohamadi M., Fathi-Achachlouei B. Optimization of extraction process of bioactive compounds from bene hull using subcritical water. Food Sci. Biotechnol. 2014;23:1459–1468. [Google Scholar]
- Shahidi F., Zhong Y. sixth ed. Six Volume Set. Memorial University of Newfoundland; Canada: 2005. Lipid Oxidation: Measurement Methods, Bailey’s Industrial Oil and Fat Products. [Google Scholar]
- Silveira C.P., Torres-Rodrı´guez J., Alvarado-Ramı´rez E., Murciano-Gonzalo F., Dolande M., Panizo M., Reviakina V. MICs and minimum fungicidal concentrations of amphotericin B, itraconazole, posaconazole and terbinafine in Sporothrix schenckii. J. Med. Microbiol. 2009;58:1607–1610. doi: 10.1099/jmm.0.007609-0. [DOI] [PubMed] [Google Scholar]
- Singh G., Maurya S., Lampasona M.P., Catalan C. Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Contr. 2006;17:745–752. [Google Scholar]
- Sriti J., Aidi Wannes W., Talou T., Vilarem G., Marzouk B. Chemical composition and antioxidant activities of Tunisian and Canadian coriander (Coriandrum sativum L.) fruit. J. Essent. Oil Res. 2011;23:7–15. [Google Scholar]
- Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an Updated Review. Evid. base Compl. Alternative Med. 2016;2016:1–21. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidi B., Rahimmalek M., Arzani A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017;220:153–161. doi: 10.1016/j.foodchem.2016.09.203. [DOI] [PubMed] [Google Scholar]
- Tongnuanchan P., Bnjakul S. Essential oils: extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014;79:1231–1249. doi: 10.1111/1750-3841.12492. [DOI] [PubMed] [Google Scholar]
- Wangensteen H., Berit Samuelsen A., Egil Malterud K. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–297. [Google Scholar]
- Wu Wang H., Qing Liu Y., Lian Wei S., Jun Yan Z., Lu k. Comparison of microwave-assisted and conventional hydrodistillation in the extraction of essential oils from mango (Mangifera indica L.) flowers. Molecules. 2010:7715–7723. doi: 10.3390/molecules15117715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare Zardini H., Tolueinia B., Momeni Z., Hasani Z., Hasani M. Analysis of antibacterial and antifungal activity of crude extracts from seeds of Coriandrum sativum L. Gomal J. Med. Sci. 2012;10:167–171. [Google Scholar]
- Zeljković S.C., Tan K., Siljak-Yakovlev S., Maksimović M. Essential oil profile, phenolic content and antioxidant activity of Geranium kikianum. Nat. Prod. Commun. 2017;12:273–276. [PubMed] [Google Scholar]