Highlights
-
•
Descriptive anatomy is clinically defined as a descriptive form of pre-deformation.
-
•
Practical anatomy is clinically defined as an intraoperative form of post-deformation.
-
•
A new practical anatomy is inspired by distinguishing between the cardinal and transverse cervical ligaments.
-
•
Practical anatomy leads to less-extirpating and nerve-sparing radical hysterectomy.
-
•
Radical hysterectomy of the 21st century has been built on the basis of practical anatomy.
Keywords: Radical hysterectomy, Nerve-sparing, Descriptive anatomy, Practical anatomy, Lateral parametrium, Terminologia Anatomica
Abstract
The resulting characteristics of 20th century radical hysterectomy demonstrated that, in the quest for a radical cure, its surgical procedure became extended. The consequences of this were increased difficulty in the procedure with risk of massive hemorrhage and vesical and anorectal dysfunction. Moreover, the cardinal and transverse cervical ligaments, which were individually recognized during the 19th century, became regarded as synonymous during the middle of the 20th century. Because of this, traditional surgical procedures such as Wertheim and Latzko’s were not precisely followed, further delaying the advent of a new idea(s) for radical hysterectomy. The desired goal for 21st radical hysterectomy should be an anatomy and operative procedure that clarify and rectify these aforementioned negative surgical outcomes. In 1998, International Federation of Associations of Anatomists (IFAA) introduced clinical terminology in Terminologia Anatomica. With the IFAA’s decision in mind, the author focused, out of the many theories and surgical procedures for cancer of the uterine cervix, on three noteworthy proposals. From these proposals, the possibility for a new idea(s) on theories and surgical procedures was explored through the fusion of anatomy and clinical practice. The idea for a new procedure(s) required a morphological distinction between description in textbooks and intraoperative findings, that is: a) a traditional anatomy that was based on gross/systemic anatomy; and b) an anatomy in which part of an artifact resulting from applying an artificial maneuver to a living body could be regarded as surgical anatomy. The author has tentatively called the former descriptive anatomy and the latter practical anatomy. The development of this practical anatomy led to the idea for and practical application of a less-extirpating and nerve-sparing operation, which improved patient’s outcome. Radical hysterectomy of the 21st century should be conducted following this new anatomy that is concentrated around practical anatomy and independent from descriptive anatomy.
1. Introduction
Historically, surgery for cervical cancer has evolved from new procedures coming into existence based on new anatomic theories. However, since the publication on Mackenrodt’s transverse cervical ligament (1895)regarding clinical anatomy and Latzko & Schiffmann’ method (1919) or Meigs’ the so-called Wertheim operation (1945, 1950) regarding surgical procedure for cervical cancer, there was no remarkable development apparent until the 1990′s (Yabuki et al.,1991, Yabuki et al., 1996). Thus, creation of any new theory was delayed. However, intraoperative hemorrhage was controlled barely with progress in surgical technique, which was one of the objectives of 20th century surgery. But no further theoretical and technical progress was made in the preservation of bladder and rectal function (Yabuki et al.,1991, Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005). One of the reasons for this delay was that gynecologists did not posess, except for gross/systemic anatomy (descriptive anatomy), a means of expressing artifacts emanating from intraoperative maneuvers. Consequently, discrepancy arose between theory and practice, leading to obstacle of thought (Yabuki et al., 1991). Another reason was a contradiction in the cardinal and transverse cervical ligaments being regarded as synonymous. This contradiction made the distinction between Wertheim and Latzko procedures vague, leading to a delay in theoretical and surgical response regarding hemorrhage and bladder paralysis (Yabuki et al.,1991, Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005, Yabuki, 1997, Yabuki, 2007)
In 1998, International Federation of Associations of Anatomists (IFAA) introduced clinical terminology into the text of Terminologia Anatomica (1998). Although many interpretations exist on this action, it led the author to believe that IFAA had recognized the necessity for clinical terminology to be included in anatomic terms.
In this article, a review was conducted on surgical procedures based on traditional descriptive anatomy and practical anatomy, the latter an anatomy in which part of an artifact results from applying an artificial maneuver to a living body. Ultimately, from the information gathered, a proposal was put forward to solve this contradiction that emanated from the two anatomies as well as to carry out a 21st century radical hysterectomy that was consistent in both anatomy and surgical procedure.
2. Definition
The following two anatomies, descriptive and practical, are clearly distinctive.
2.1. Descriptive anatomy is defined as one in which a living body is clearly seen as it is depicted.
2.2. Practical anatomy is defined as a field to express a surgically created artifact (Yabuki et al., 2011, Yabuki, 2016).
3. Terminology
3.1. Position and direction for practical anatomy
The terms describing position and direction for practical anatomy were determined from a dorsal position (Yabuki, 2016). From a surgical viewpoint, direction is divided into medio-lateral, cranio-caudal and dorso-ventral directions.
3.2. Cardinal ligament, transverse cervical ligament and paracervix
For the cardinal and transverse cervical ligaments (CL and TCL), the author has adopted the assertion made by Savage, 1880, Savage, 1870 and Mackenrodt (1895) as the paracervix being anatomically distinguishable from CL and TCL.
3.3. Abbreviations used in this article are presented as follows
CL: cardinal ligament, IFFA: International Federation of Associations of Anatomists, IHP: inferior hypogastric plexus, L&S: Latzko and Schiffmann, LP: lateral parametrium, LUL: lateral umbilical ligament, NSRH: nerve-sparing radical hysterectomy, P&A: Peham-Amreich, PSN: pelvic splanchnic nerve, Q&M: Querleu and Morrow, RH: radical hysterectomy, TA: Terminologia Anatomica, TCL: transverse cervical ligament, TMMR: total mesometrial resection, VUL: vesicouterine ligament.
3.4. Change of terminology
The superficial and deep layers of the vesicouterine ligament, which were the author’s original coined terms in previous articles, appear to be misleading and have led to misunderstandings, so the terms bladder pillar and anterior mesoureter have been used in this article, the former of which is equivalent to the superficial layer of the vesicouterine ligament and the latter the deep layer of the vesicouterine ligament (Yabuki et al., 1996). This resulted in using the term posterior mesoureter instead of the mesoureter.
3.5. CL (of Mackenrodt)
In the mid-twentieth century, the term cardinal ligament (of Mackenrodt) began to appear in articles written by Uhlenhuth et al., 1948, Reiffenstuhl, 1982 and Clemente (1985). To conform to this, CL and TCL were made synonymous by TA.
CL was Savage’s concept (1870, 1880) translated from the German term by Kocks (1880), which indicated a bundle on the medial aspect to the ureter. In 1895 Mackenrodt described TCL as ‘Fiber bundles extend from the pelvic fascia and attach to the posterior wall of the cervix’ (Speert 1996). Thus, these two ligaments cannot be regarded as synonymous.
4. Traditional lateral parametrium
Conventional pelvic ligaments are regarded as standard morphology as depicted by Peham and Amreich, 1930, Peham and Amreich, 1934, Netter, 2006. They are horizontally multilayered consisting of three plates: the lateral paracystium, lateral parametrium, and lateral paraproctium (Yabuki et al.,1991, Yabuki, 2019). For this reason, the principle of RH has been inherited from the procedure in which a space is created by dissecting the areolar tissue on the ventral and dorsal aspects of the lateral parametrium followed by its sagittal resection along the pelvic sidewall. However, this technique and its associated anatomy can only be applied to the resection of CL. Therefore, if applied to resection of TCL, massive hemorrhage and bladder/rectum dysfunction will ensue (Yabuki et al.,1991, Yabuki et al., 1996, Yabuki et al., 2011; Yabuki, 2016). The lateral parametrium depicted by Peham-Amreich and Netter is an image based on descriptive anatomy, which is a concept that is applied to Wertheim surgery (Fig. 1A). The space created by Latzko surgery is demonstrably different from that created by Wertheim surgery, so that a more clinically oriented description is required (Fig. 1B).
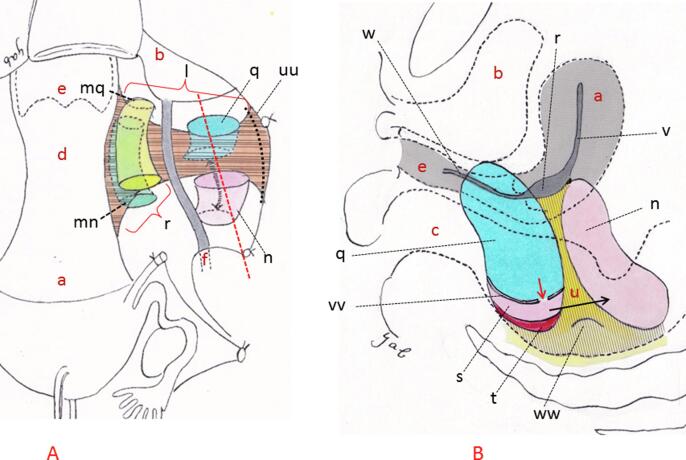
Fig. 1.
Paracervical resection in Wertheim and Latzko & Schiffmann procedures. Shown are schematic illustrations of an LP excision. The drawings are based on Wertheim and Latzko methods described in Operative Gynecology by Peham-Amreich (1934; original German publication in 1930). The Wertheim method involved excising the bladder pillar and uterorectal ligament, lateralizing the ureter and exposing the CL, followed by its excision on the medial aspect to the ureter (A). Latzko method entailed excavation of the paravesical and pararectal spaces on the lateral aspect to the ureter, followed by excision of the separated TCL (dotted black line in A). However, unlike the Wertheim method, as perpendicular excavation of both the paravesical and pararectal spaces progressed, this excavation terminated at the iliococcygeus muscle and on the sacral aspect, i.e. in the vicinity of S2-3 (vertical cylinder in A) (Yabuki et al.,1991, Höckel et al., 2003). A septum, i.e. the Mackenrodt ligament, then appears between the two aforementioned spaces (B). A perspective view of the sagittally sliced septum is shown in B, which is demonstrated by a dotted red line on A. To solve this ‘dead end’ problem caused by appearance of the septum, L&S created a new artificial space or the lower portion of the pararectal space between the capsule and levator ani muscle by incising this capsule that forms the base of the paravesical space (B). This capsule corresponds to the horizontal connective tissue ground bundle of P&A (1934) or the deep investing fascia (TA, 1998) or superior fascia of the pelvic diaphragm (TA, 1998). They then unified this new space and upper portion of the aforementioned pararectal space by breaking the septum with the fingertip at the level of the sacrospinous ligament (double-headed black arrow in A and black arrow in B) (P&A, 1934). They concluded that longitudinal clamping and excision of the origin of Mackenrodt ligament could be successfully carried out. a; uterus, b; bladder, c; rectum, d; uterine cervix, e; vagina, f; ureter, l; TCL, n; Latzko’s pararectal space (upper portion), q; paravesical space, r; CL, s; pararectal space (lower portion), t; levator ani muscle, u; septum or Mackenrodt ligament or perpendicular connective tissue ground bundle, v; uterine broad ligament, w; paracolpium, uu; pelvic sidewall, vv; capsule or deep investing fascia or horizontal connective tissue ground bundle, ww; sacrospinous ligament, mn; Wertheim’s dorsal space, mq; Wertheim’s ventral space.
4.1. CL resected by Clark’s and Wertheim’s methods
Clark (1895) and Wertheim, 1911, Wertheim, 1912 developed the ventral aspect of CL by separating the bladder followed by severance of the bladder pillar. They then resected the sacrouterine ligament and created a space behind CL, resecting CL at the medial aspect of the ureter (Figs. 1A and 2A). Thus, the term CL was passed down as a ligament that attaches parallel to the lateral aspect of the uterus (Fig. 2A).
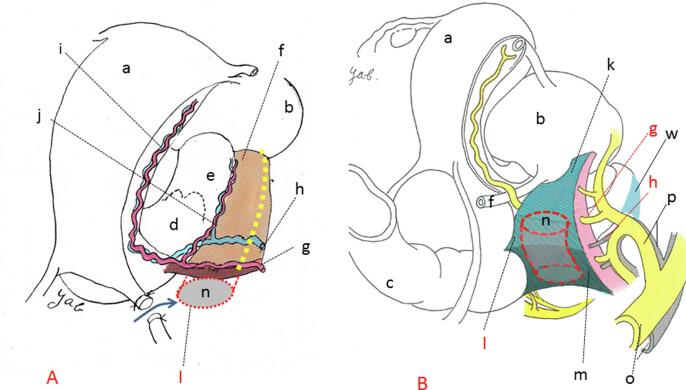
Fig. 2.
Two forms of the lateral parametrium. A denotes a P&A and Netter’ schematic illustration of the descriptive form of post-deformation of LP. The ventral aspect of LP, which horizontally connects the lateral aspect of the uterus and pelvic sidewall, is exposed by an incision to the anterior leaf of the broad ligament and mobilization of the bladder. This was followed by creation of the pararectal space by excision of areolar connective tissue along the dorsal aspect of LP. B is a modified version of a schematic illustration in an article by Yabuki, et al., 1996; 62: 370-8 showing a practical form of post-deformation of LP. Spontaneous and perpendicular excavation of the pararectal space takes place on the lateral aspect to the ureter. Perpendicular excavation of the paravesical space is also carried out, though it is not depicted. The relationship between the uterus and LP is perpendicular. a; uterus, b; bladder, c; rectum, d; uterine cervix, e; vagina, f; ureter, g; uterine artery, h; deep uterine vein, i; ascending branch of the uterine vessels, j; descending branch of the uterine vessels, k; vesicohypogastric fascia, l; TCL, m; lateral ligament of the rectum, n; Latzko’s pararectal space (upper portion), o; common iliac vessels, p; internal iliac vessels, w; paracolpium.
4.2. TCL resected by L&S’ method
L&S excavated the paravesical and pararectal spaces on the lateral aspect of the ureter and separated the septum. They stated that this septum was the Mackenrodt ligament (Mackenrodt, 1895) (Fig. 1A and B). As seen in Fig. 1B and 2B, the Mackenrodt ligament extends in a dorsal direction (never a caudal direction) and reaches the sacrospinous ligament. Finally, a hole was pierced in this ligament followed by its separation (black arrow in Fig. 1B). However, in P&A’s textbook, TCL was initially depicted perpendicularly to the uterus. But, at the time of its resection, it was depicted longitudinally at its origin. In other words, it was clamped horizontally to the uterine axis en masse followed by its resection (P&A, 1934). Thereby, TCL was judged to be the ligament that horizontally connects the lateral aspect of the uterus/vagina and pelvic sidewall, reaching the vicinity of the urogenital hiatus. The contradiction shown in these two diagrams has never been questioned even till the present time.
4.3. Contradiction with resection method for the lateral parametrium
In his article published in 1912, Wertheim stated as follows: Mackenrodt criticises us claiming that by the application of these clamps (Wertheim’s clamps) more parametrium is left on the pelvic floor. We cannot agree to this as happily, these clamps can be very closely applied on the pelvic floor. This indicates that CL of Wertheim does not reach the pelvic sidewall but continues to the pelvic floor. This traditional controversy continues to the present day from a viewpoint in which the lateral rectal ligament or lateral ligament of rectum is included within Cl or TCL. As a result, the pararectal space is excavated to the sacral aspect, which causes damage to the middle rectal vessels and sacral venous plexus, leading to hemorrhage (Yabuki, 1991, Possover et al., 2000).
Furthermore, the ligament theory for CL continues to exist through modification of the Wertheim operation by Meigs and Höckel and the statement by Q&M the part of the cardinal ligament that is medial to the ureter is mainly fibrous, whereas the part that is lateral to the ureter is non-fibrous (Meigs, 1945; Piver et al., 1974; Höckel et al., 1998, Querleu and Morrow, 2008). However, since the 1930′s, there have been negative opinions on LP being a ligament (Bastian and Lassau, 1982, Berglas and Rubin, 1953, Koster, 1933, Fritsch, 1992, Range and Woodburne, 1964, Ricci et al. 1947). Besides, one must not ignore the fact that LP is the main route for the lymphochannel of the uterine cervix. Concurrently, the rate of carcinoma extension is few in the avascular areolar connective tissue surrounding LP. Moreover, the view of CL and TCL as being synonymous has led to a state of confusion of whether hysterectomy should be radical or semi-radical.
5. Classification of surgical anatomy
In traditional RH, gynecologists did not possess a means to describe artifacts that were created by surgical maneuvers other than describing them through the means of descriptive anatomy. To make one’s procedure consistent under this condition, discrepancy between theory (anatomy) and clinical practice (surgery) ensued, an example being L&S’ surgical procedure (Latzko method). Further, resolution of this anatomic contradiction in CL and TCL that have been made synonymous has become a necessity. To resect an organ without damage to another, surgeons implicitly understand that an approach is required through knowledge of practical anatomy, which is specific to surgery, rather than one acquired in laboratory dissection.
In this article, a distinction in surgical anatomy has been made between the descriptive form of pre-deformation and practical form of post-deformation (Fig. 2A and B), the former being defined as preoperative morphology that closely resembled descriptive anatomy, and mainly applies to CL or in Wertheim surgery (Fig. 2A). The latter indicated initial surgical morphology following application of a surgical maneuver, specifically corresponding to the stage in which the pararectal and paravesical spaces were excavated followed by separation of TCL. This mainly applied to TCL or Latzko method and its modification (Fig. 2B).
5.1. Surgical theory based on descriptive form of pre-deformation.
5.1.1. Ontogenetic anatomy and total mesometrial resection advocated by Höckel et al.
A unique anatomic idea with excellent results has been reported (Höckel et al., 1998, Höckel et al., 2003, Höckel et al., 2005). These reports have consistently ignored any description of the lateral rectal ligament since publication of an article by them in 1998. The reason for this is inferred from the fact that the relationship between the lateral parametrium and lateral paraproctium is based on descriptive anatomy in which they are always separated horizontally, hence no description of the vaginal vein (deep uterine vein) in their reports. This is the reason why TMMR is defined as a modification of the Wertheim operation. The fact that the uterosacral/rectouterine ligaments were resected at the level of the superior margin of IHP meant that the boundary of traditional descriptive anatomy: c.f. between the mesometrium and mesorectum, could be left intact. Moreover, there is no contradiction in extended resection of the mesorectum by Höckel, et al. even if it is an extension of Wertheim surgery. However, the pararectal space was excavated to the presacral fascia and the paravesical space to the superior fascia of the levator ani or pelvic diaphragm with the uterine vessels resected at their origin. At this point the author had the impression that TMMR resembled L&S method. In addition, Höckel et al. (1998) must have thoroughly cleared the surrounding tissues of the vaginal vein by liposuction. These surgical procedures deviate from the boundary of descriptive anatomy. In spite of exposing PSN, no description has been found on innervation of IHP to its periphery. Exposure of PSN alone causes bladder dysfunction and hemorrhage. The bladder mesentery advocated by Höckel et al. corresponds to a combination of the bladder pillar and anterior mesoureter (Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005). From the author’s experience, invasion and metastasis to the bladder mesentery is low, so that the author agrees, in part, with the opinion of not resecting the anterior mesoureter of Höckel et al. However, considering the lymphochannel to the pelvic sidewall, the author disagrees that every case can be managed through the Wertheim procedure.
5.1.2. Q&M classification and RH
Cibula et al.’s description of type C RH on Q&M classification is as follows: 1) ‘C1-C2: the lateral border is identical for both types, formed by the medial aspect of the internal iliac vein and artery; 2) C1: the deep parametrial resection margin is formed by the vaginal vein, thus the caudal part of the lateral parametria containing the splanchnic nerves is preserved; and 3) C2: the resection line continues alongside the medial aspect of the internal iliac vessel and pudendal vessels caudally up to the pelvic floor.’ (Cibula et al., 2011; Querleu et al., 2017; Cibula et al., 2018).
The author’s view on each description is as follows: 1) The uterine, inferior vesical and middle rectal vessels bifurcate from the anterior trunk of the internal iliac vessels (Fig. 3A and 3D) (Yabuki et al., 2005), from which the author cannot clinically image their pathway; 2) The fact that PSN exists caudal to the vaginal vein means that the paracolpium covers the ventral aspect of PSN, which is anatomically abnormal (Fig. 3D) (Williams and Warwick, ed., 1980, Standring ed., 2005, Drake et al. ed., 2005). A quality-of-life-based NSRH is not for preserving PSN but preservation of IHP and its vesical nerve branch (Fig. 3D) (Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005). In the first place, excision of PSN belongs to FIGO stage III; and 3) It is doubtful that the parametrial limit should be expressed as ‘caudal’ or ‘caudally’. Needless to say, the anterior trunk of the internal iliac vessels extends dorsally, passing the greater sciatic foramen under the piriformis muscle and exiting the pelvic cavity. Furthermore, PSN originate from S2 to S4 (Fig. 3D). The direction of the internal iliac vessels’ passage cannot be expressed as ‘caudal’ or ‘caudally’ in the traditional anatomic position based on standing, or even in the dorsal position. Moreover, the pelvic floor refers to an area of the pelvic diaphragm, which should be distinguished from the presacral area (Williams and Warwick, ed., 1980, Standring ed., 2005, Drake et al. ed., 2005, Magrina et al., 2011). Q&M must have determined that the internal iliac and pudendal vessels pass within the inner aspect of the pelvis toward the urogenital hiatus. The idea advocated by Q&M places an emphasis on descriptive anatomy in which LP has a horizontal relationship with the lateral aspect of the uterus/vagina (Querleu and Morrow, 2008, Ramanah et al., 2012). However, practical anatomy is interspersed in Q&M’s idea, causing confusion and lack of theoretical unity.
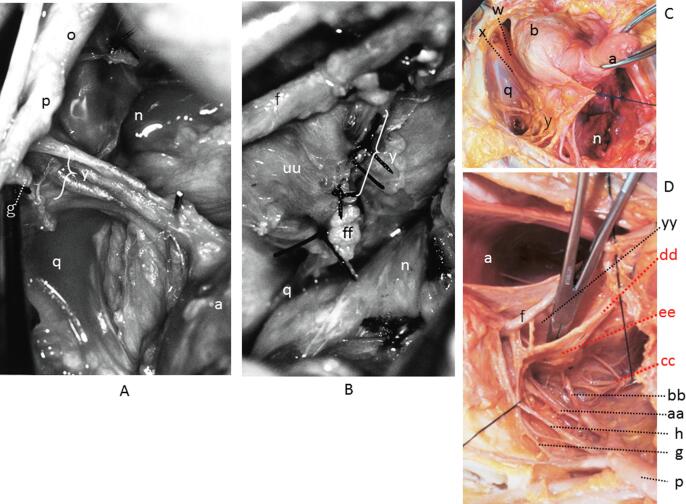
Fig. 3.
The supporting system. A shows the vessels of the supporting system following development of the paravesical and pararectal spaces with the uterine artery excised. B shows a severed end of the supporting system on the pelvic wall. The vesicohypogastric fascia, TCL and lateral rectal ligament constitute one lamina (Figs. A and C). Shown in C is the supporting system that is viewed from the pubic aspect following excavation of the paravesical space. Shown in D is also the supporting system viewed from a sacral aspect following excavation of the pararectal space. A and B show modified versions of original figures from an article by Yabuki, et al. (1991) and C and D from an article by Yabuki, et al. (2005). a; uterus, b; bladder, f; ureter, g; uterine artery, h; deep uterine vein, n; Latzko’s pararectal space (upper portion), o; common iliac vessels, p; internal iliac vessels, q; paravesical space, w; paracolpium, x; tendinous arc of the pelvic fascia, y; supporting system, aa; middle vesical vessels, bb; middle rectal vessels, cc; PSN, dd; hypogastric nerve, ee; IHP, ff; lateral stump of the tendinous arc of the pelvic fascia and sacrospinous ligament complex, uu; pelvic side wall.
5.2. Surgical theory based on practical form of post-deformation.
5.2.1. The vesicouterine ligament of Okabayashi
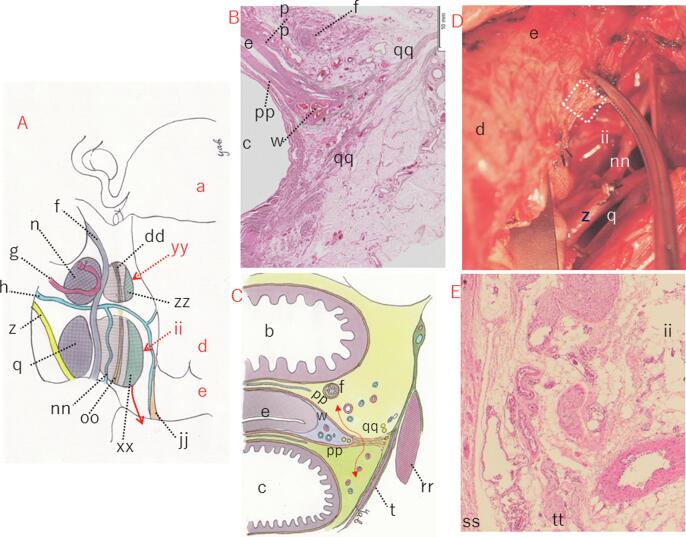
VUL has been devided into the anterior and posterior leaves (Fig. 4A and D) (Okabayashi, 1921, Okabayashi, 1952). The paravaginal space was excavated to separate the posterior leaf of VUL (Fig. 4D). Resection of the posterior leaf separated the bladder and TCL, exposing the paracolpium. Yabuki, et al. divided Okabayashi’s anterior leaf of VUL into the bladder pillar consisting of fascia and the roof of the ureteric tunnel (roughly corresponding to CL; dotted red triangle in Fig. 4C) that is equivalent to the neurovascular bundle (Fig. 4A, B, and C). In addition, morphology and function of Okabayashi’s posterior leaf of VUL (Fig. 4D) were elucidated, naming the extensive area the anterior mesoureter (Figs. 4E and 5A) (Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005). Then, to carry out simultaneous resection of the roof of the ureteric tunnel and excavation of Okabayashi’s paravaginal space, the fourth space was created (Fig. 4A, B, E, and 5A). Furthermore, the conception of the fourth space led to an idea of new semi-radical hysterectomy (double-headed white arrow in Fig. 4E).
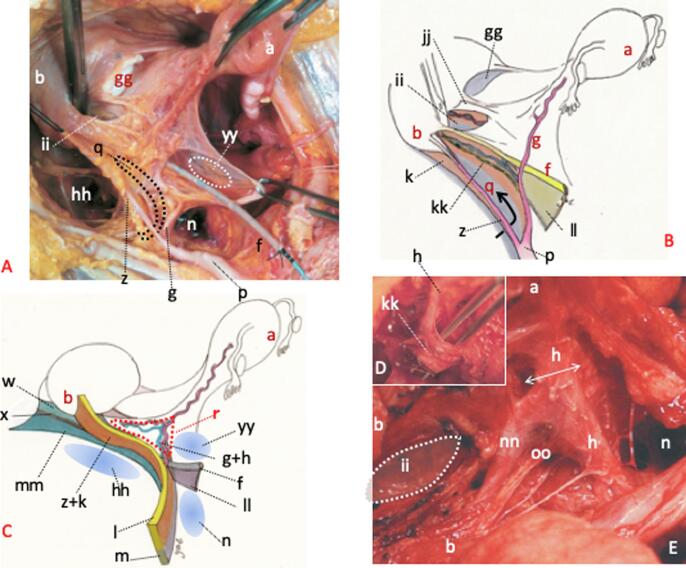
Fig. 4.
Anatomy of the lateral uterine ligament. Shown in A is a mock RH using a fresh cadaver. The vesicouterine, fourth, retroperitoneal and pararectal spaces are observed. The paravesical space is to be excavated within the dotted oval black circle. B is a schematic illustration of A. The paravesical space is developed, and the exposed anterior mesoureter is depicted. C denotes the structural component of the paracervix. The dotted red triangle denotes CL. D denotes the separated posterior leaf of VUL of Okabayashi. The superior vesical vein is seen to be transparent (Yabuki et al., 1996). E shows separation of the superior vesical vein and vesical nerve branch following excavation of the fourth space (Yabuki, 2019). a; uterus, b; bladder, f; ureter, g; uterine artery, h; deep uterine vein, k; vesicohypogastric fascia, l; TCL, m; lateral ligament of the rectum, n; Latzko’s pararectal space (upper portion), p; internal iliac vessels, q; paravesical space, r; CL, w; paracolpium, z; LUL, gg; vesicouterine space, hh; retroperitoneal space, ii; fourth space, jj; blader pillar (superficial layer of VUL), kk; anterior mesoureter, ll; posterior mesoureter, mm; superior fascia of the pelvic diaphragm, nn; superior vesical vein, oo; vesical nerve branch, yy; Okabayashi’s pararectal space.
Fig. 5.
The structure of the paracolpium and its excision. Shown in A is excavation of the four spaces in the lateral parametrium and depiction of the related tissues. B shows a frontally sectioned HE stained tissue specimen of fairly tough anterior and posterior vaginal fasciae from the superior most part of the vagina. These fasciae merge on the lateral aspect of the vagina and connect, via denser connective tissue fiber, to the parietal endopelvic fascia or the superior fascia of the pelvic diaphragm. This denser connective tissue fiber is considered to be the tendinous arc of the pelvic fascia. The area surrounded by a dotted green line in B denotes the paracolpium. Double-headed red arrow in C indicates the area where the fourth space is created. D shows separation of the paracolpium following extension of the fourth space within which the Kelly forceps can be seen clamped. E denotes a tissue specimen taken from within the dotted white frame in D. a; uterus, b; bladder, c; rectum, d; uterine cervix, e; vagina, f; ureter, g; uterine artery, h; deep uterine vein, n; Latzko’s pararectal space, q; paravesical space, w; paracolpium, z; LUL, dd; hypogastric nerve, ii; fourth space, jj; bladder pillar, nn; superior vesical vein, oo; vesical nerve branch, pp; vaginal fascia, qq; parietal fascia, rr; obturator internus muscle, ss; vaginal muscle, tt; vaginal nerve plexus, xx; superior fascia of the pelvic diaphragm or deep investing fascia, yy; Okabayashi’s pararectal space, zz; pre-sacral parietal fascia of the pelvis.
5.2.2. Yabuki’s lateral parametrium
Fig. 3A and B show intraoperative findings and Fig. 3C and D anatomy of a fresh cadaver. The main points on the practical form of post-deformation concerning TCL of Yabuki and his associates’ (Yabuki et al.,1991, Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005, Yabuki, 2019) are as follows: 1) The bundle (Fig. 3A, C and D), which appears when the paravesical and paprarectal spaces are excavated at the lateral aspect to the ureter, is a continuum consisting of the vesicohypogastric fascia, TCL and lateral rectal ligament (Fig. 2B). Yabuki et al. named this continuum the supporting system, for the relationship became perpendicular with the pelvic organs (Yabuki et al.,1991, Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005, Yabuki, 2019); 2) Fig. 3A shows intraoperative finding during development of the paravesical and pararectal spaces with vessels of the supporting system exposed. These vessels contain not only those of the uterus, but also the visceral branches of the internal iliac vessels of the bladder and rectum (Fig. 3D); 3) Seen in 3B are vessels shown in 3A, which have been individually separated and severed at their respective origin. The dorsal limit of the supporting system is the lateral stump of the sacrospinous ligament; and 4) Fig. 3D shows a cranial view of the supporting system following development of the pararectal space.
A summary of findings on practical anatomy of LP is as follows: (1) The visceral branches of the internal iliac vessels form a plate on a perpendicular plane and lie parallel in a ventral to dorsal direction; (2) PSN and hypogastric nerve are linked to each other, forming IHP, and represented on a sagittal plane; (3) As a result of the development of the pararectal space, the supporting system and aggregation of nerves are centered at their origins and separated into a V-shape; and (4) The perpendicular supporting system, which is dissected during surgery, is different from horizontal LP in its direction and morphology depicted by P&A and Netter (Fig. 2).
5.2.3. Yabuki’s paracervix and anatomy of the paracolpium
The uterine cervix is anatomically divided into the supravaginal and vaginal parts (TA, 1998), and Yabuki has described each of their lateral supports as follows: 1) The lateral support fuses with the supravaginal part roughly corresponding to the area of TCL (Fig. 4A and C). A part of TCL and CL can be seen overlapped on the medial aspect to the ureter as shown by a dotted red triangle (Fig. 4C). Furthermore, the dorsal aspect of TCL joins the lateral rectal ligament, and the LUL/vesicohypogastric fascia bifurcates from its ventral aspect (Fig. 4C); 2) The lateral support to the vaginal part consists of the following tissues from a medial to lateral aspect: bladder pillar, CL or the roof of the ureteral tunnel, ureter/anterior mesoureter, LUL/vesicohypogastric fascia, and retroperitoneal tissue (Fig. 4A and C). Between these ligaments exists areolar connective tissue, and spaces are surgically created from a medial to lateral part: vesicouterine, fourth, paravesical and retroperitoneal spaces (Figs. 4A and 5A). Only the bladder pillar and CL are true related tissues of the uterus. Considering these facts, the paracervix was identified as a complex consisting of the bladder pillar, TCL, CL, ureter/anterior mesoureter and LUL/vesicohypogastric fascia (Fig. 4B and C) (Yabuki, 2019). Furthermore, this complex possessed a three-dimensional structure where horizontal and perpendicular planes were intricately combined (Fig. 4B and C) (Yabuki et al.,1991, Yabuki et al., 1996, Yabuki et al.,2000, Yabuki et al., 2005).
There is a popular saying ‘the greater the amount of the vaginal excision, the worse the bladder paralysis,’ As the truth of this popular saying cannot be ascertained, there remain many unresolved issues concerning anatomy and dissection of the vagina and paracolpium. Mackenrodt (1895) stated the following regarding the paracolpium: ‘Short fibrous bundles arise from the fascia of the levator ani as a sort of inferior continuation of the transverse cervical ligament and attach to the side of the connective tissue sheath surrounding the vagina,…’ (Speert, 1996, in English). This fascia can be interpreted as the deep investing fascia or superior fascia of the pelvic diaphragm. These short fibrous bundles are excised in an Latzko colpectomy (Peham and Amreich, 1934). In contrast, Okabayashi excised the bundle recognizing it as the paracolpium on the lateral aspect of the vagina, which appears when the posterior leaf of VUL (Fig. 4D) is incised (Okabayashi, 1952). Q&M have stated that ‘the paracervix including the paracolpos of the upper third of the vagina’ (Q&M, 2008). Furthermore, the extended fourth space to the lateral vaginal ligament could, then, be excavated between the perivaginal tissue and superior vesical vein (vesical nerve branch is hidden underneath it). Many vaginal nerves, which have ganglia along the muscular layers, can be observed in the histological tissue specimen (Fig. 5E) of perivaginal tissue within the white dotted frame (Fig. 5D). In synthesizing the findings shown in Fig. 4, Fig. 5, the paracolpium was identified to be a neurovascular bundle that was covered with vaginal fascia. In addition, CL and the paracolpium including the descending uterine vessels and nerves were identified as a continuum. The histological specimen (Fig. 5B and E) and schematic illustration (Fig. 5C) show the pelvic organs severed at the superior most of the vagina (Yabuki, 2019). The area surrounded by a dotted green line indicates the paracolpium (Fig. 5B) and dotted double red arrow indicates the area where the fourth space is created (Fig. 5C). In principle, the length of vaginal resection is irrelevant to the bladder function.
6. Twenty-first century RH.
An issue of RH during the 20th century was confusion of descriptive and practical anatomies which emanated from CL and TCL being regarded as synonymous. This issue was solved by examination of Wertheim and Latzko methods from the viewpoint of practical anatomy, resulting in less-extirpating RH, followed by NSRH (Yabuki et al.,1991, Yabuki et al., 1996, Yabuki et al.,2000). Specifically, as shown in Fig. 5A, TCL has been skeletonized by excavation of the paravesical, fourth, Latzko’s pararectal and Okabayashi’s paravesical spaces transversally, separating the continuum consisting of the anterior and posterior mesoureters longitudinally (Fig. 5A). The utilization of this structure in 1991 led to the development of a less-extirpating RH, in which only TCL was selectively resected from the complex (supporting system) consisting of the vesicohypogastric fascia, TCL and rectal lateral ligament. Further, the anterior mesoureter was divided into the vascular and neural parts with only the former being resected. This procedure resulted in a marked decrease in bladder complication, establishing NSRH (Yabuki et al., 1996, Yabuki et al.,2000). Twentieth century RH, which is based on practical anatomy with its description centered around less-extirpating RH and NSRH, is summarized as follows. In addition, this procedure can be applied not only to abdominal open RH but also to laparoscopic or robot-assisted RH (Hoshiba et al., 2000, Kanao et al., 2018).
-
1.
Abdominal incision.
-
2.
Division of the round ligament of the uterus.
-
3.
Excavation of the retroperitoneal space.
-
4.
Pelvic lymphadenectomy.
-
5.
Excavation of the lateral or Latzko’s pararectal space: The vascular sheath of the internal iliac vessels and posterior fascia of TCL are separated from their respective tissues. The ureter remains attached to the reverse side of the posterior leaf of the broad ligament together with the separated vascular sheath. However, the sacral plexus/piriformis fascia/PSN are not exposed (Fig. 4A and 4C) (Yabuki, et al., 1996).
-
6.
Excavation of the medial or Okabayashi’s pararectal space and separation of the anterior portion of the ureter and posterior mesoureter: Okabayashi’s pararectal space is developed between the posterior leaf of the broad ligament and its subserous fascia (Yabuki, et al., 1996).
-
7.
Mobilization of the bladder
-
8.
Excavation of the paravesical space: the vesicohypogastric fascia is pierced between the uterine artery and superior vesical artery (P&A, 1934). The space is then developed by separating the uterine artery and bladder.
-
9.
Excavation of the fourth space: This space is excavated by the dissection of the CL or the roof of the ureteric tunnel and a retrograde incision, i.e. cephalically cutting, of the residual ureteric roof (Fig. 4A and B) (Yabuki et al., 1996, Yabuki et al.,2000).
-
10.
Separation of the anterior mesoureter: The ureter/neurovascular bundle is separated between the paravesical and fourth spaces.
-
11.
Denudation of CL/TCL by the excision of the fascia of LP.
-
12.
Separation and severance of the uterine artery and superficial vein.
-
13.
Separation and ligature of the deep uterine vein (Fig. 4E).
-
14.
Incision of the peritoneum of pouch of Douglas and excision of the sacrouterine fold: Resection of the rectouterine ligament at the ventral aspect of IHP (Fig. 3D) (Yabuki et al., 1996, Yabuki et al.,2000, Höckel et al., 2003).
-
15.
Resection of the infundibulopelvic ligament.
-
16.
Separation of the anterior and middle portions of the ureter from the anterior mesoureter.
-
17.
Separation of the superior vesical vein from the vesical nerve branch and its excision.
-
18.
Resection of the deep uterine vein at its origin. When requiring further less-extirpating surgery, the deep uterine vein is severed on its medial aspect where the superior vesical vein joins it (double-headed white arrow in Fig. 4E; author’s semi-RH).
-
19.
Separation of the paracolpium: Caudal extension of the fourth space and denudation of the paracolpium, severance of the vesicovaginal and rectovaginal ligaments.
-
20.
Severance of the paracolpium.
-
21.
Severance of the vagina and its closure.
-
22.
Abdominal closure.
7. Conclusion
The greatest issues concerning surgery for cervical cancer during the 20th century were prevention of massive intraoperative hemorrhage and functional preservation of bladder and rectum. These problems were solved by: a) development of a new anatomy, i.e. practical anatomy, that is precisely perceived and described as morphologic changes produced by surgical manipulation of a living body; and b) creation of a new surgical procedure, i.e. less extirpating and nerve-sparing RH based on this practical anatomy.
Author Contribution section
The author conducted the cadaver dissections, surgical procedures and writing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The author is deeply appreciative of Gen Murakami, a former Professor of Anatomy, Sapporo Medical College, Hokkaido, Japan, for his guidance and cooperation in cadaver dissection. The author thanks Dr. Shimpachiro Ogiwara, former Professor at the University of Kanazawa, and Mrs. Sandra M. Ogiwara, CSP (UK), BScPT (C), for English editing of the report. This study was self-funded and not supported by any other grants.
References
- Bastian D., Lassau J.P. The suspensory mechanism of the uterus, anatomical basis for statics and dynamics of the normal uterus. Anat. Clin. 1982;4:147–160. [Google Scholar]
- Berglas B., Rubin I.C. Histologic study of the pelvic connective tissue. Surg. Gynec. Obstet. 1953;97:277–287. PMID: 13090052. [PubMed] [Google Scholar]
- Cibula D., Abu-Rustum N.R., Benedetti-Panici P., Köhler C., Raspagliesi F., Querleu D., Morrow C.P. New classification system of radical hysterectomy: Emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol. Oncol. 2011;122:264–268. doi: 10.1016/j.ygyno.2011.04.029. Epub 2011 May 17. PMID: 21592548. [DOI] [PubMed] [Google Scholar]
- Clark J.G. A more radical method of performing hysterectomy for cancer of the uterus. Johns Hopkins Hosp. Bull. 1895;6:120–124. [Google Scholar]
- Clemente, C.D., ed. Anatomy of the Human Body by Henry Gray, 30th American ed. Philadelphia, Lea & Febiger; 1985, 1, 1574–1577.
- Drake, R. L., Vogl, W., Mitchell, A.W.M., ed. Gray’ Anatomy for Students. Elsevier Churchill Livingstone, Philadelphia 2005.
- Fritsch, H., 1992. The connective tissue sheath of uterus and vagina in the human female fetus. Ann. Anat. 174, 261–266. PMID: 1503247. http://doi.org/10.1016/s0940-9602(11)80366-3. [DOI] [PubMed]
- Höckel, M., Kondering, M.A., Heuel, C.P., 1998. Liposuction-assisted nerve-sparing extended radical hysterectomy: Oncologic rationale, surgical anatomy, and feasibility study. Am. J. Obstet. Gynecol. 178, 971–976. PMID: 9609569. http://doi.org/10.1016/s0002-9378(98)70533-2. [DOI] [PubMed]
- Höckel, M., Horn, L-C., Hentschel, B., Höckel, S., Naumanns, G., 2003. Total mesorectal resection: High resolution nerve-sparing radical hysterectomy based on developmentally defined surgical anatomy. Int. J. Gynecol. Cancer 13, 791–803. PMID: 14675316. http://doi.org/10.1111/j.1525-1438.2003.13608.x. [DOI] [PubMed]
- Höckel, M., Horn, L-C., Fritsch, H., 2005. Association between the mesenchymal compartment of uterovaginal organogenesis and local tumor spread in stage IB-IIB cervical carcinoma: a prospective study. Lancet Oncol. 6, 751–756. PMID: 16198980. http://doi.org/10.1016/S1470-2045(05)70324-7. [DOI] [PubMed]
- Hoshiba, T., Yabuki, Y., Asamoto, A., Nishimoto, H., Yagihara, A., Nishikawa, Y., 2000. Our laporoscopic radical hysterectomy. Gynecol. Obstet. Surg. 11, 93–100. (in Japanese).
- Kanao, H., Aoki, Y., takeshima, N., 2018. Unexpected result of minimally invasive surgery for cervical cancer. J. Gynecol. Oncol. 29, e73. http://doi.org/10.382/jgo.2018.29.e73. [DOI] [PMC free article] [PubMed]
- Kocks, J., 1880. Die normale und pathologische Lage und Gestalt des uterus sowie deren Mechanik. Bonn: Max Cohen & Sohn.
- Koster H. On supports of the uterus. Am. J. Obstet. Gynecol. 1933;25:67–74. [Google Scholar]
- Latzko, W., Schiffmann, J., 1919. Klinisches und Anatomisches des zur Radikaloperation des Gebarmutterkrebses (nach einem am 24. Juni 1919 gehaltenen Vortrag). Diskussinsbemerkungen, Weibel W und Wertheim E. Zbl Gynäk 43, 715–719.
- Mackenrodt A. Ueber die Ursachen der normalen und pathologischen Lagen des Uterus. Arch F Gynäk. 1895;48:393–421. [PubMed] [Google Scholar]
- Magrina J.F., Pawlina W., Kho R.M., Magtiby P.M. Robotic nerve-sparing radical hysterectomy: Feasibility and technique. Gynecol. Oncol. 2011;121:605–609. doi: 10.1016/j.ygyno.2011.11.006. Epub 2011 Nov 10. No abstract available. PMID: 22079679. [DOI] [PubMed] [Google Scholar]
- Meigs J.V. Wertheim Operation for carcinoma of cervix. Am. J. Obstet. Gynecol. 1945;49:542–553. [Google Scholar]
- Meigs, J.V., 1950. Radical hysterectomy for cancer of the cervix with bilateral pelvic lymphadenectomy (the so-called Wertheim Operation). In: Meigs J.V. (Ed.), Progress in Gynecology, Vol. 1. New York: Grune Stratton, pp. 540–560.
- Netter, F.H., 2006. Atlas of Human Anatomy 4th Ed. Sanders, an imprint of Elsevier Inc.
- Okabayashi H. Radical abdominal hysterectomy for cancer of cervix uteri. Surg. Gynecol. Obstet. 1921;33:335–341. [Google Scholar]
- Okabayashi, H., 1952. Radical abdominal hysterectomy for cancer of cervix uteri. Tokyo, Kanehara. (in Japanese).
- Peham, H.V., Amreich, J., 1930. Gynäkologische Operationslehre. Berlin/Basel: Verlag S. Karger.
- Peham, H.V., Amreich, J., 1934. Operative Gynecology, vol. 2 (translated by Ferguson LK). Philadelphia: JB Lippincott, 333–366.
- Piver M.S., Rutledge F., Smith J.P. Five classes of extended hysterectomy for women with cervical cancer. Obstet. Gynecol. 1974;44:265–271. PMID: 4417035. [PubMed] [Google Scholar]
- Possover, M., Stober, S., Plaul, K., Schneider, A., 2000. Identification and preservation of the motoric innervation of the bladder in radical hysterectomy. Gynecol. Oncol. 79, 154–157. PMID: 11063637. http://doi.org/10.1006/gyno.2000.5919. [DOI] [PubMed]
- Cibula, D., Potter, R., Planchamp, F., Avall-Lundqvist, E., Fischerrova, D., Meder, C.H., Köhler, C., Londoni, F., Lax, S., Lindegaard, J.C., Mahantshetty, U., Mathevet, P., McCluggage, W.G., McCormack, M., Naik, R., Nout, R., pignata, S., Ponce, J., Querleu, D., Raspagliesi, F., Rodolakis, A., Tamussino, K., Wimberger, P., Raspollini, M.R., 2018. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patient with cervical cancer. Int. J. Gynecol. Cancer. 28, 641-655. PMID: 29688967. http://doi.org/10.1097/IGC.0000000000001216.
- Querleu, D., Morrow, C.P., 2008. Classification of radical hysterectomy. Lancet Oncol. 9, 297–303. PMID:18308255. http://doi.org/10.1016/S1470-2045(08)70074-3. [DOI] [PubMed]
- Querleu, D., Cibula, D., Abu-Rustum, N.R., 2017. Update on the Querleu-Morrow classification of radical hysterectomy. Ann. Surg. Oncol. 24, 3406–3412. PMID: 28785898 PMCID: PMC6093205. http://doi.org/10.1245/s10434-017-6031-z. [DOI] [PMC free article] [PubMed]
- Ramanah, R., Berger, M.B, Parratte, B.M., DeLancy, J.O., 2012. Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligament. Int. Urogynecol. J. 23, 1483–1494. PMID: 22618209 PMCID: PMC4258694. http://doi.org/10.1007/s00192-012-1819-7. [DOI] [PMC free article] [PubMed]
- Range, R.L., Woodburne, R.T., 1964. The gross and microscopic anatomy of the transverse cervical ligament. Am. J. Obstet. Gynecol. 90, 460–467. PMID: 14217646. http://doi.org/10.1016/0002-9378(64)90802-6. [DOI] [PubMed]
- Reiffenstuhl G. The clinical significance of the connective tissue planes and spaces. Clin. Obstet. Gynecol. 1982;25:812–820. doi: 10.1097/00003081-198212000-00016. [DOI] [PubMed] [Google Scholar]
- Ricci, J.V., Lisa, J.R., Thom, C.H., Kron, W.L., 1947. The relationship of the vagina to adjacent organs in reconstructive surgery. Am. J. Surg. 74, 387–410. PMID: 20266018. http://doi.org/10.1016/0002-9610(47)90131-1. [DOI] [PubMed]
- Savage, H., 1870. The surgery, surgical pathology, and surgical anatomy of the female pelvic organ, ed.3. London: J & A Churchill & son, 76.
- Savage, H., 1880. The surgery, surgical pathology, and surgical anatomy of the female pelvic organ, ed.3. New York: William Wood & Company.
- Standring, S. ed., 2005. Gray’ Anatomy; the anatomical basis of clinical practice, 39th edition. Philadelphia, Elsevier Churchill Livingstone.
- Speert, H., 1996. Obstetrics and Gynecologic Milestones Illustrated. New York: Parthenon, 138–141.
- Terminologia Anatomica, 1998. International Anatomical Terminology / Federative Committee on Anatomical Terminology. Stuttgart / New York, Thieme.
- Uhlenhuth E., Day D.E., Smith R.D., Middleton E.B. The visceral endopelvic fascia and the hypogastric sheath. Surg. Gynecol. Obstet. 1948;86:9–28. PMID: 18920874. [PubMed] [Google Scholar]
- Williams, P.L., Warwick, R., ed. Gray’ Anatomy, 36th edition. London, Churchill Livingstone 1980.
- Wertheim, E., 1911. Die erweiterte abdominale Operation bei Carcinoma colli uteri (auf Grund von 500 Fällen). Urban & Schwarzenberg, Berlin.
- Wertheim E. The extended abdominal operation for carcinoma uteri (Based on 500 operative cases) Am. J. Obstet. Dis. Women Child. 1912;66:169–232. [Google Scholar]
- Yabuki, Y., Asamoto, A., Hoshiba, T., Nishimoto, H., Kitamura, S., 1991. Dissection of the cardinal ligament in radical hysterectomy for cervical cancer with emphasis on the lateral ligament. Am. J. Obstet. Gynecol. 164, 7–14. PMID: 1986630. http://doi.org/10.1016/0002-9378(91)90614-w. [DOI] [PubMed]
- Yabuki, Y., Asamoto, A., Hoshiba, T., Nishimoto, H., Satou, N., 1996. A new proposal for radical hysterectomy. Gynecol. Oncol. 62, 370–378. PMID: 8812535. http://doi.org/10.1006/gyno.1996.0251. [DOI] [PubMed]
- Yabuki Y. Cardinal ligament dissection basd on a new theory. CEM J. Gynecol. Oncol. 1997;2:278–287. [Google Scholar]
- Yabuki, Y., Asamoto, A., Hoshiba, T., Nishimoto, H., Nishikawa, Y., Nakajima, T., 2000. Radical hysterectomy: an anatomic evaluation of parametrial dissection. Gynecol. Oncol. 77, 155–163. PMID: 10739705. http://doi.org/10.1006/gyno.1999.5723. [DOI] [PubMed]
- Yabuki, Y., Sasaki, H., Hatakeyama, N., Murakami, G., 2005. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am. J. Obstet. Gynecol. 193, 7–15. PMID: 16021052. http://doi.org/10.1016/j.ajog.2005.02.108. [DOI] [PubMed]
- Yabuki, Y., 2007. Anatomy of the pelvis for radical hysterectomy; Does extensive resection of the vagina result in severer bladder dysfunction? The 16th Annual Review Course on Gynecologic Oncology and Pathology. Kyoto, Japan.
- Yabuki, Y., Murakami, G., Hoshiba, T., Sasaki, H., Hatakeyama, N, Asamoto, A., 2011. Redefinition of the pelvic connective tissue: in-situ histologic examination. Female Pelvic Med. & Reconstr. Surg.17, 60–66. PMID: 22453689. http://doi.org/10.1097/SPV.0b013e31820e9dbd. [DOI] [PubMed]
- Yabuki, Y., 2016. Clinical anatomy of the subserous layer: An amalgamation of gross and clinical anatomy. Clin. Anat. 29, 508–515. PMID: 26621479. http://doi.org/10.1002/ca.22579. [DOI] [PubMed]
- Yabuki, Y., 2019. New Radical Hysterectomy for Uterine Cancer, 3rd ed., Theory and Technique for Nerve-Sparing Operation. Tokyo: Medical View, 2019. (in Japanese with figure legend in English).