Abstract
The probiotic potential of Pediococcus acidilactici isolated from Wara, a Nigerian unripened soft cheese from cow milk was investigated in this study. The strain was evaluated for tolerance to low pH, bile salts, high osmotic pressure, exopolysaccharide production, auto-aggregation, microbial adhesion to solvent, survival in simulated gastro-intestinal juice and antimicrobial properties. The strain showed resistance to high acid and bile conditions surviving at pH 2 and 1.5% bile salt concentration. The strain survived at 8% Sodium chloride and produced exopolysaccharide. P. acidilactici possessed high auto-aggregative ability and hydrophobicity (>70%). Furthermore, the strain did not show hemolytic activity and survived in the presence of simulated gastric juice at pH 2 and simulated intestinal juice. The strain exhibited a broad spectrum inhibition against pathogens. The study concluded that P. acidilactici strain isolated from wara could be a useful probiotic for the development of functional food products.
Keywords: Biotechnology, Microbiology, Nutrition, Gastrointestinal system, Probiotic, Pediococcus acidilactici, Wara, Antimicrobial
Biotechnology; Microbiology; Nutrition; Gastrointestinal system; Probiotic; Pediococcus acidilactici; Wara; Antimicrobial
1. Introduction
Probiotic is generally defined as a live microorganism that confers beneficial effects on the health of a host when administered in adequate amount (FAO/WHO, 2002). Commercially, strains of Lactic acid bacteria (LAB) are mostly used as probiotics in functional foods (Ng et al., 2015). Lactic acid bacteria are a group of Gram positive, non-sporulating cocci or bacilli which produce lactic acid as major end product from fermentation of carbohydrates (Halder et al., 2017).
In order for dietary probiotics to survive in the gut and elicit beneficial effects on the host, their tolerance to acidic pH and the bile salts in the stomach and intestines is very essential (Villena and Kitazawa, 2017). The gastric juice contains hydrochloric acid thus making the pH of human stomach range from around 2.5 and 3.5 (Thakur et al., 2016). It is important therefore, that probiotics survive in the acidic gastric environment if they are to reach the small intestine and colonize the host (Villena and Kitazawa, 2017). Furthermore, probiotics are expected to adhere well enough to the mucosal layer in the gastrointestinal tract (GIT) for adequate competition with other pathogens for nutrient uptake (Shyamala et al., 2016). Other significant criterion for selecting a probiotic organism include the production of antimicrobial properties such as organic acids, hydrogen peroxide and bacteriocins as this is important for inhibiting (pathogen exclusion) the pathogenic intestinal microbiota (Mokoena, 2017).
Lactic acid bacteria isolated from various fermented foods have been screened for their probiotic potentials and reported to be effective in conferring various health benefits to both human and animals (Pieniz et al., 2014).
Fermented dairy products with active bacterial cultures are one of the most common sources of probiotics. They are a good source of protein, fat and major minerals which serve as daily diet especially for children and the aged and they are beneficial for gastrointestinal and digestive conditions (Gasmalla et al., 2017). The probiotic potentials of different strains Pediococcus acidilactici have been evaluated in recent studies (Gupta and Sharma, 2017; Abbasiliasi et al., 2017; Akmal et al., 2019). Wara is a Nigerian unripen soft cheese curd locally made by curdling fresh cow milk with juice extract of Sodom apple (Calostropis procera). The solid part (proteins and fats) is separated from the liquid part (water and whey) after the curdling process and pressed together. It is usually eaten alone, as a snack once fried (this is known as ‘Beske’ in Nigeria) or as an addition to various cuisines. It is often sold and consumed within 24 h of manufacture due to its poor shelf life (Sahingoz and Sahin, 2009). Wara is rich in fat, protein and a little amount of carbohydrate, minerals (calcium, Vitamin B12, Phosphorus, Selenium, Zinc, Sodium) and vitamins (Riboflavin, Vitamin A, Vitamin K2) (Sawhney et al., 2007) making it a suitable growth medium for microorganisms and a good energy source. It has been reported to contain organisms which are able to regulate the microbiota in the gut (Ayeni et al., 2014).
The aim of this study however was to screen the strain of P. acidilactici previously isolated from wara for probiotic potentials for possible application in Nigerian fermented foods.
2. Materials and methods
2.1. Bacteria strain and culture conditions
Lactic acid bacteria used in this study was previously isolated from Wara. The strain was stored and maintained on de Man Rogosa and Sharpe Agar (MRSA) slant (Peptone, Yeast extract, Meat extract, Glucose, Dipotassium phosphate, Triammonium citrate, Sodium acetate trihydrate, Magnesium sulphate, Manganese sulphate, Tween 80, Agar) at low temperature (4 °C). The strain was re-suspended in de Man Rogosa and Sharpe Broth (MRSB) (Peptone, Yeast extract, Meat extract, Glucose, Dipotassium phosphate, Triammonium citrate, Sodium acetate trihydrate, Magnesium sulphate, Manganese sulphate, Tween 80) and sub-cultured on MRSA plates. The pure colonies were subjected to Gram staining and catalase test as preliminary check to ensure it is lactic acid bacteria before the probiotic screening.
2.2. Identification of the test strain
The strain was identified by 16S rRNA gene sequence analysis. The nucleic acid was extracted using the methods described by Trindade et al. (2007) with slight modifications. The DNA fragments were amplified using universal primer 16SF: GTGCCAGCAGCCGCGCTAA and reverse primer: 16SR: AGACCCGGGAACGTATTCAC (Barghouthi, 2011). The PCR product was detected using gel electrophoresis at 80V in horizontal gels containing 1.5% agarose stained with ethidium bromide and purified with the addition of 70% ethanol and centrifugation at 9000 rpm before the sequencing. The purified products was sequenced using the ABI 3130xL Genetic Analyzer (Applied Biosystems, California, USA). The generated sequence was subjected to alignment using the Basic Local Alignment and Search Tool (BLAST) program to compare with the sequences deposited in the GenBank database.
2.3. Tolerance of Pediococcus acidilactici to acidic pH
The ability of the test organism to tolerate highly acidic environments was determined as previously described (Hassanzadazar et al., 2012) with slight modifications. Briefly, sterile MRSB that had been adjusted separately to pH 2, 3, 4 and 6 using 1M HCl was inoculated with standardized cell suspension (1% v/v) of P. acidilactici. The inoculated broth was incubated aerobically at 37 °C for 6 h during which period bacterial growth was monitored at 600 nm wavelength using a spectrophotometer (Vis Spectrophotometer 721D Life Assistance Scientific and Medical Institute, UK) at hourly intervals. The absorbance values obtained was plotted against the incubation time. Culture medium adjusted to pH 6 served as control.
2.4. Bile tolerance of Pediococcus acidilactici
The bile tolerance of the test organism was determined using the method of El-Naggar (2010) with slight modifications. Sterile MRSB was supplemented with different concentrations (0.5%, 1.0% and 1.5%) of bile salts (Oxoid LP0055 Basingstoke, England). The medium was separately inoculated with standardized cell suspension (1% v/v) and incubated aerobically at 37 °C for 6 h during which period bacterial growth was monitored hourly at 600 nm wavelength using a spectrophotometer (Vis Spectrophotometer 721D Life Assistance Scientific and Medical Institute, UK). The absorbance values obtained was plotted against the incubation time. Culture medium with no bile served as control.
2.5. Tolerance of Pediococcus acidilactici to high osmotic pressure
The ability of the test organism to tolerate high osmotic pressure was tested following the method of Collado and Sanz (2007). MRS broth was supplemented with different concentrations of NaCl (2%, 4%, 6% and 8%). Five millilitres of the medium was separately inoculated with standardized cell suspension (1% v/v) of the organism and incubated aerobically at 37 °C for 48 h during which period culture turbidity was monitored every 6 h at 600 nm wavelength using a spectrophotometer (Vis Spectrophotometer 721D Life Assistance Scientific and Medical Institute, UK). The absorbance values obtained was plotted against the incubation time. Culture medium with no NaCl served as control.
2.6. Auto-aggregation ability of Pediococcus acidilactici
The auto-aggregation of the test organism was determined as previously described (Bao et al., 2010). The organism was inoculated into Phosphate Buffered Saline (PBS) with the optical density 0.25 at 600 nm wavelength using a spectrophotometer. The suspension was then incubated at 37 °C for 20 h after which the absorbance was taken and recorded. The percentage auto-aggregation (A%) was calculated using the formula:
| (Ao - At)/Ao x100 |
where,
Ao = absorbance at time 0
At = absorbance at 20 h
2.7. Adhesion of Pediococcus acidilactici to solvent
Bacterial adhesion to the hydrocarbon assay, which determines the bacterial cell surface hydrophobicity was performed according to the method of Canzi et al. (2005). The standardized cell suspension of the test organism was inoculated (1% v/v) into Phosphate Buffered Saline (PBS) and the same volume of n-hexadecane was added and incubated at room temperature for an hour. The absorbance of the aqueous phase was measured at 600 nm after 1 h and the adhesion percentage was calculated according to the formula:
| (Ao - A)/Ao x 100 |
where,
Ao = absorbance before extraction
A = absorbance after extraction with n-hexadecane
2.8. Exopolysaccharides production by Pediococcus acidilactici
Exactly 1.5 mL of the 24 h old culture of the test organism in MRS-sucrose broth was centrifuged at 5000 rpm for 10 min and 1.0 mL of the supernatant was dispensed into a glass test tube and an equal volume of chilled ethanol (95%) was added. The formation of an opaque link at the interface of the cell supernatant and ethanol confirms the presence of EPS.
2.9. Hemolytic activity of Pediococcus acidilactici
Hemolytic activity of test organism was determined as described by Foulquié-Moreno et al. (2003). Overnight (18–24 h) culture of the organism was streaked on Columbia blood agar base (Oxoid CM0331) (Peptone, Starch, Sodium chloride, Agar) supplemented with 7% v/v human blood and incubated at 37 °C for 48 h. After incubation, the plates were observed for zone of inhibition. The production of green-hued zones around the colony was recorded as α-hemolysis while non production of any effect on the blood agar plates was recorded as γ-hemolysis. Lyses of blood around the colony of the test organism was classified as hemolytic (β-hemolysis).
2.10. Antibacterial activity of Pediococcus acidilactici
The test organism was screened for antibacterial activity against indicator organisms (Escherichia coli O157:H7, E. coli NCIB 86, Staphylococcus aureus NCIB 8588 and Klebsiella pneumoniae KP617) using the modified agar well diffusion method as previously described (Haghshenas et al., 2016). Exactly 5 mL of overnight (18–24 h) culture of the test organism in MRS broth medium incubated at 37 °C was centrifuged (15000 rpm for 10mins) and then filtered through 0.2 μm filter to obtain sterile cell free supernatant (CFS). The indicator organisms were suspended in normal saline and standardized to 0.1 at 540 nm wavelength using a spectrophotometer (Vis Spectrophotometer 721D Life Assistance Scientific and Medical Institute, UK). Exactly 100 μL of the indicator organisms were seeded separately in 15 mL Mueller Hinton agar plates (Beef infusion solids, Starch, Casein hydrolysate, Agar), swirled gently but properly, allowed to set and incubated for 4 h. Wells (7 mm) were made on the set plates and 50 μL of the CFS was aseptically dispensed into the wells. Wells in which sterile MRSB was dispensed served as control. The plates were then incubated at 37 °C for 24–48 h. Clear zones around each well was measured and considered as positive antibacterial activity.
2.11. Survival of Pediococcus acidilactici in simulated gastrointestinal tract
The tolerance of the test organism to gastrointestinal tract condition (simulated gastric and intestinal juices) was determined as previously described (Bao et al., 2010) with slight modifications. Survival of organism in gastric conditions was investigated by inoculating simulated gastric juice (0.35 % Pepsin, pH 2.0 and 3.0) with standardized cell suspension (1% v/v) and the inoculated medium was incubated at 37 °C for 3 h during which total viable count was determined hourly. The medium was then transferred aseptically with a sterile micropipette into simulated intestinal juice (0.1% Trypsin, pH 8) and was incubated at 37 °C for 12 h during which total viable count was determined every 3 h. LAB strain inoculated in sterile PBS served as control.
2.12. Statistical analysis
Data are expressed as mean ± standard error of mean (SEM) of three independent replications. The results were analyzed using one-way analysis of variance (one-way ANOVA) with GraphPad prism version 6.0. Statistical significance was set at p-value equal to or less than 0.05 (P ≤ 0.05).
3. Results
The strain was identified after the 16s rRNA gene sequencing as Pediococcus acidilactici with a similarity value of 99.16% with strain present in the GenBank sequence database of the National Center for Biotechnology Information (NCBI).
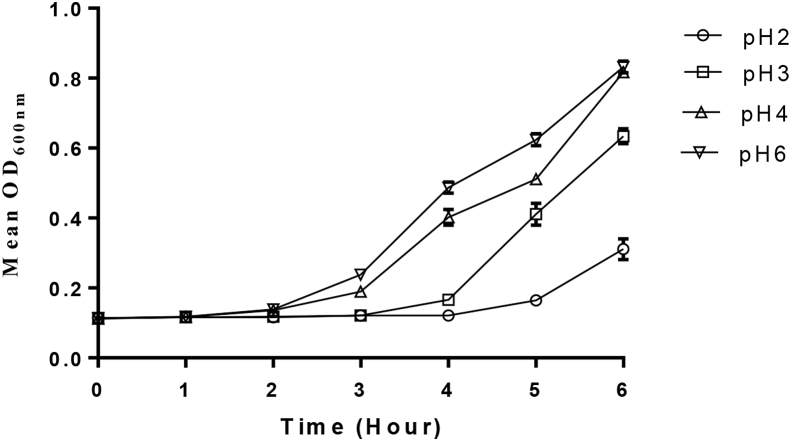
P. acidilactici exhibited excellent tolerance to acidic conditions. The strain compared favorably in the OD values obtained at pH 3 and pH 4 when compared to the values obtained at the control condition (pH 7) after 6 h of incubation while at pH 2, there was a significant increase in the OD values obtained at 6 h when compared to the OD value at 0 and 1hr (see Figure 1).
Figure 1.
Tolerance of P. acidilactici to acidic pH.
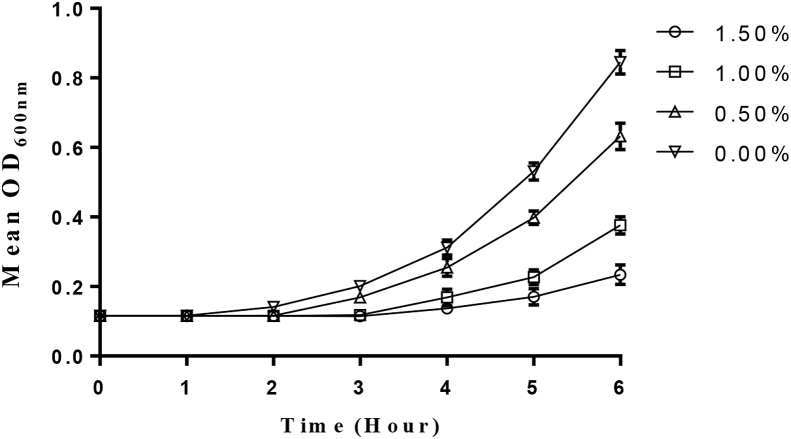
The result obtained for the tolerance of P. acidilactici to different concentrations of bile salts indicated that it was able to tolerate very high concentration of bile salts (1.5%) within the time of incubation (Figure 2). There was no significant difference in the OD values obtained at 0.5% and 1.0% bile salts when compared to the control (no bile salts) while at 1.5% bile salts concentration, there was a significant increase in the OD values at 6 h incubation period.
Figure 2.
Tolerance of P. acidilactici to bile salts concentrations.
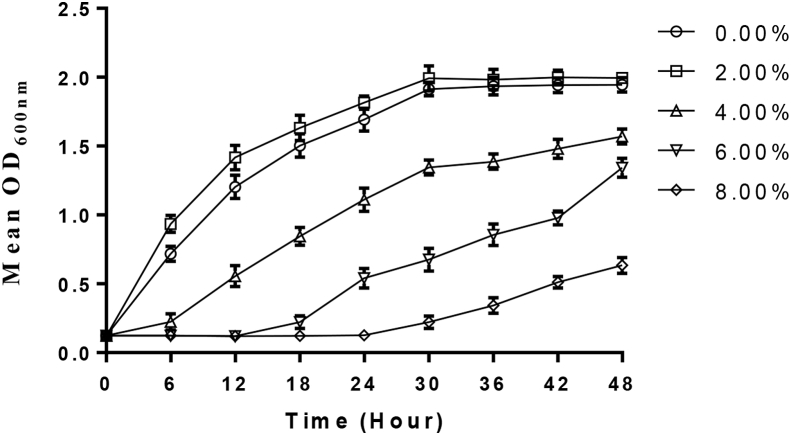
P. acidilactici survived favourably in MRS broth at high conditions of osmotic pressure (4–6% NaCl). However, the strain suffered a marked decrease (206%) in growth rate at 8% NaCl when compared with the control after 48 h of incubation (Figure 3).
Figure 3.
Tolerance of P. acidilactici to high osmotic pressure.
The P. acidilactici strain exhibited a high auto-aggregation ability with a percentage of 70.3% and its adherence ability evaluated by MATH method showed that it displayed high hydrophobicity percentage (71.6%) after the extraction period.
The result obtained on exopolysaccharide production showed that P. acidilactici is an excellent exopolysaccharide producer with the formation of large opaque level after extraction with ethanol. Furthermore, the organism did not exhibit any hemolytic activity (α and/or β) on Columbia blood agar base after 48 h of incubation.
The cell free supernatant of P. acidilactici exhibited antimicrobial effects against E. coli O157:H7, E. coli NCIB 86, Staphylococcus aureus NCIB 8588 and Klebsiella pneumoniae KP617 with inhibition zones ranging from 7.6mm to 10.4mm (Table 1). The highest inhibitory activity was observed against S. aureus NCIB 8588 while the lowest inhibitory activity was observed against E. coli O157:H7.
Table 1.
Antimicrobial activity of cell free supernatant of P. acidilactici against indicator microorganisms.
| Indicator microorganism | Diameter of zone of inhibition (mm) |
|---|---|
| E. coli O157:H7 | 7.6 ± 0.3 |
| E. coli NCIB 86 | 8.1 ± 0.2 |
| Staphylococcus aureus NCIB 8588 | 10.4 ± 0.3 |
| Klebsiella pneumoniae KP617 | 8.3 ± 0.4 |
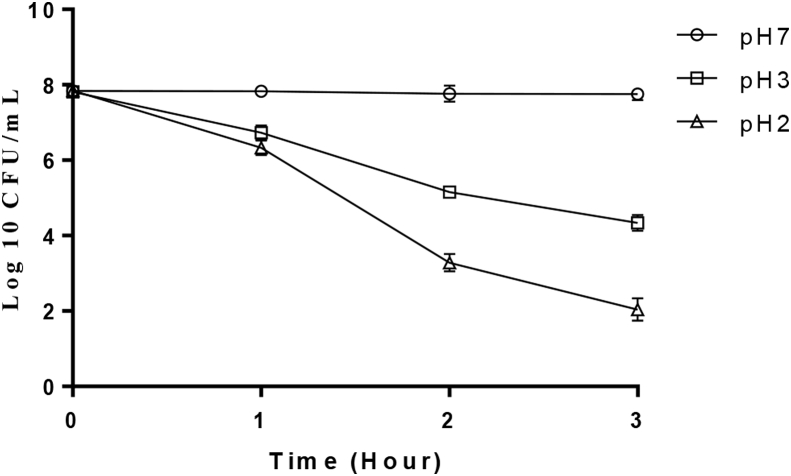
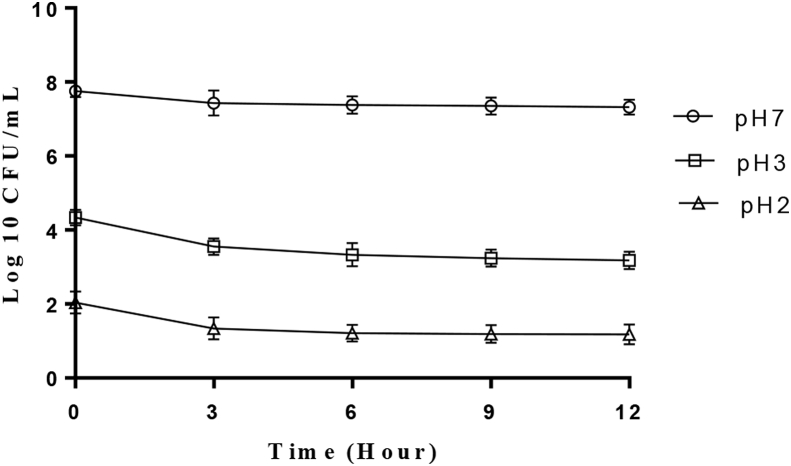
The ability of the test organism to survive in the presence of simulated gastric juice for 3 h at 37 °C is shown in Figure 4. At pH 2, the organism was relatively stable after 1 h of incubation but the viable cell count value decreased after 2 h though growth was observed after 3 h indicating its ability to survive simulated gastric juice at pH 2. However at pH 3, there was no significant difference (P ≤ 0.05) in the growth rate of the organism ranging between 7.65 log CFU/mL and 4.34 log CFU/mL when compared with the control after the incubation period.
Figure 4.
Tolerance of P. acidilactici to gastric juice.
The survivability of the test organism in intestinal juice (pH 8) when transferred from simulated gastric juice at pH 2 showed that the viable cell count of the organism reduced after 3 h but did not further decrease during the other evaluation times (6–12 h). Meanwhile, the test organism transferred from simulated gastric juice at pH 3 to intestinal juice (pH 8) gave a stable viable cell count with no significant difference in the values obtained compared to the control indicating the ability of the isolate to survive simulated intestinal juice (Figure 5).
Figure 5.
Survivability of P. acidilactici in intestinal juice (pH8) after exposure to simulated gastric juice for 3 h.
4. Discussion
Milk based products such as yoghurt and cheese have been found to be an ideal carrier for delivering probiotics to the human gastrointestinal tract as such products may improve gut microbiota and maintain overall health (Haghshenas et al., 2016). The selection of a probiotic organism depends on its ability to survive high acidic pH and bile salts in the gastrointestinal tract. The ability of the organism to adhere to mucosal wall is also important to elicit probiotic effect on the host and also, production of certain antimicrobial substances which can eliminate pathogenic organisms in the GIT. In the present study, the results obtained indicated that P. acidilactici strain isolated from wara; a Nigerian milk product possess acid and bile tolerance ability surviving exposure to pH 2.0 and 1.5% bile salt concentration. Generally, death in acidic environment occur when certain proteins and DNA are damaged due to the inability of the acid regulatory mechanisms of the LAB to maintain intracellular pH and the internal acidification had reduced the activity of H+-ATPase, an enzyme responsible for the regulation of the internal and external H+ concentrations (Ng et al., 2015). According to the reports of Liong and Shah (2005), the survival of LAB strains at pH 3.0 after exposure for 2–3 h is acceptable as one of the requirements for the bacteria to be considered as probiotics.
Stress adaptation mechanisms triggered by acidic environments can result in bile salt resistance which can be unpredictable and higher than the resistance to acidic conditions (Haghshenas et al., 2016). The ability of organisms to tolerate bile salt concentration could be due to special bilayer structures that enable organisms to tolerate adverse conditions (Madhusudan et al., 2017) as well as the activity of bile salt hydrolase which catalyzes the hydrolysis of glycine or taurine conjugated bile salts into amino acid residue (Yin et al., 2011). However, bile tolerance is strain-specific and cannot be generalized due to the variability in bile tolerance abilities found within species and genus of LAB (Nagyzbekkyzy et al., 2016).
According to Ibourahema et al. (2008), high salt concentration affects the organism through the loss of tugor pressure as water moves from the cell to the outside consequently damaging bacterial physiology and the activities of certain enzymes and metabolism. It is believed that osmo-tolerance is species specific and the difference in osmotic resistance of different bacterial strains are due to the distinct composition of their membrane phospholipids or the action of ATP-dependent glycine betaine transporter QacT (Wasko et al., 2013). LAB strains with high tolerance to osmotic pressure would be an excellent candidate for production of lactic acid on a large scale (Adnan-Mohd and Tan, 2007).
The auto-aggregation percentage of the P. acidilactici strain indicated that it possess an excellent auto-aggregative properties. Although there is no standard percentage auto-aggregation a probiotic organism should possess, it has recently been reported that probiotic isolates with less than 40% auto-aggregation possess weak auto-aggregative properties (Wang et al., 2010). The auto-aggregative property is beneficial for probiotics as it facilitates the adhesion of the strain to intestinal walls through its surface layer protein (Ng et al., 2015). Microbial adhesion to hydrocarbons (MATH) has been employed to determine the cell surface hydrophobicity of probiotics (Duary et al., 2011). It is believed that hydrophobicity in microorganisms is associated with the presence of fibrillar structures on the cell surface and specific cell wall proteins. This property is suggested to offer a competitive advantage, which is vital for bacterial maintenance in the gastrointestinal tract of the host (Maragkoudakis et al., 2006). Cell hydrophobicity is one of the physicochemical properties of LAB which play a crucial role in the interaction of probiotic with the host tissue. Furthermore, hydrophobicity has been reported to vary between species and are capable of changing with variations in physiological state of cells or expression of variable surface-associated proteins between strains (Anwar et al., 2014).
Exopolysaccharide (EPS) production is an important attribute which enhances the ability of probiotic to elicit beneficial effect in their host. EPS is secreted into the extracellular matrix via electrostatic interactions forming a slime layer (Zeidan et al., 2017). Along with several health benefits, EPS producing LAB are able to withstand technological stress and survive harsh conditions of the gastrointestinal tract compared to non-producing bacteria (Stack et al., 2010). The ability of LAB strains to produce EPS to a large extent, depend on the presence of EPS genes which encode the proteins required for EPS synthesis (Mendo et al., 2016).
Hemolytic microorganisms are organisms which have the ability to lyse or breakdown human red blood cell (Ray et al., 2004) and organisms with such ability are not safe as probiotics (FAO/WHO, 2002). The absence of hemolytic activity by P. acidilactici strain used in this study further support its selection as a probiotic candidate. Similar observations were reported in the study of Abbasiliasi et al. (2017) for P. acidilactici strain isolated from a dairy product which showed no hemolytic activity when investigated for its potential use in the food industry.
The formation of beneficial compounds such as carbondioxide, hydrogen peroxide organic acids and bacteriocins which serve as antimicrobial substances is a very crucial mechanism probiotics use in eliciting beneficial effect on the host (Arimah and Ogunlowo, 2014). Thus, it is crucial that an organism is able to inhibit the growth of pathogenic organisms without producing any metabolites that could be harmful to the host before it is considered as a probiotic. It has been reported recently that harsh living conditions accompanied with reduced physical activity, stress, inadequate nutrition and unhealthy lifestyle have increased human susceptibility to infectious diseases whereas, the incessant use of synthetic antimicrobial agent such as antibiotics has increased the resistance of infectious agents (Karami et al., 2018), creating serious health problems and future medicinal concerns thus making natural antimicrobials such as probiotics very essential. The ability of P. acidilactici to inhibit different pathogens in this study is probably due to the production of lactic acid and other organic acids or metabolites that are able to increase the acidic condition of the medium thus causing damage to the membrane proteins making it unbearable for pathogens to survive. Furthermore, the isolate may have produced antimicrobial peptides such as bacteriocins which are able to act primarily against closely related organisms (Zacharof and Lovitt, 2012).
Survival of probiotics when passing through the gastrointestinal tract is an essential feature in order for probiotics to preserve and exert their health promoting benefits (Sagheddu et al., 2018). The resistance of P. acidilactici indicated its excellent ability to survive gastric transit and deliver health benefits. The result obtained is similar to that of Guo et al. (2009) who reported that certain LAB strains survived the simulated gastric juice at pH 2.5. The viability of P. acidilactici in gastric juice at pH 3.0 indicated the efficiency of the organism's buffering mechanism in the gastric juice. On the other hand, the susceptibility P. acidilactici in gastric juice (pH 2) does not necessarily mean the strain is not applicable as probiotics as it has been reported that the stomach gastric juice condition at pH 2.0 is a rare phenomenon except in conditions of fasting. The pH of the stomach is usually elevated to between 3.0 and 6.0 when food is present (Sagheddu et al., 2018).
Finally, the viability of P. acidilactici strain in simulated intestinal juice (trypsin) shows that trypsin (intestinal enzyme) or other alkaline conditions may not significantly affect its ability to deliver probiotic benefits in the host. In a similar investigation, Wang et al. (2009) reported that about 95% of LAB species remained viable after exposure to intestinal juice.
In conclusion, the results obtained in this study indicated that P. acidilactici isolated from wara posseses desirable probiotic properties in vitro with the inhibition of pathogens and high adhesion abilities. Although further investigation may be necessary to elucidate the probiotic properties of the strain in a living host, P. acidilactici could be a useful probiotic for the development of functional food products.
Declarations
Author contribution statement
Temidayo Emmanuel Olajugbagbe: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Bridget Okiemute Omafuvbe: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Oluwatosin Esther Elugbadebo: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to appreciate the staff of the General Laboratory, Department of Microbiology, Obafemi Awolowo University, Ile-Ife for the assistance rendered during the study.
References
- Abbasiliasi S., Tan J.S., Bashokouh F., Ibrahim T.A., Mustafa S., Vakhshiteh F., Sivasamboo S., Ariff A. In vitro assessment of Pediococcus acidilactici kp10 for its potential use in the food industry. BMC Microbiol. 2017;17(121):1–11. doi: 10.1186/s12866-017-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan-Mohd A.F., Tan I.K. Isolation of lactic acid bacteria from Malaysian foods and assessment of the isolates for industrial potential. Bioresour. Technol. 2007;98:1380–1385. doi: 10.1016/j.biortech.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Akmal U., Subhani M.M., Usman S., Abbasi S.S., Sonyia Ghori I., Ghazanfar S., Arif I. Isolation and identification of probiotic to improve nutritional value of diet supplements. Acta Sci. Nutr. Health. 2019;3(8):139–143. [Google Scholar]
- Anwar A.A., Thirkra A.A., Saeed A.M. Adhesion, autoaggregation and hydrophobicity of six Lactobacillus strains. Br. Microbiol. Res. J. 2014;4:381–391. [Google Scholar]
- Arimah B.D., Ogunlowo O.P. Identification of lactic acid bacteria isolated from Nigerian foods: medical importance and comparison of their bacteriocins activities. J. Nat. Sci. Res. 2014;4(23):76–86. [Google Scholar]
- Ayeni A.O., Adeeyo O.A., Oresegun O.M. The production of Wara cheese from locally sourced coagulants and its nutritional evaluation. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014;8(10):2319–2402. [Google Scholar]
- Bao Y., Zhang Y., Zhang Y., Liu Y., Wang S., Dong X. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Contr. 2010;21:695–701. [Google Scholar]
- Barghouthi S.A. A universal method for the identification of bacteria based on general PCR primers. Indian J. Microbiol. 2011;51(4):430–444. doi: 10.1007/s12088-011-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzi E., Guglielmetti S., Mora D., Tamagnini I., Parini C. Conditions affecting cell surface properties of human intestinal bifidobacteria. Antonie Leeuwenhoek. 2005;88:207–219. doi: 10.1007/s10482-005-6501-3. [DOI] [PubMed] [Google Scholar]
- Collado M.C., Sanz Y. Induction of acid resistance in Bifidobacterium: a mechanism for improving desirable traits of potentially probiotic strains. J. Appl. Microbiol. 2007;103:1147–1157. doi: 10.1111/j.1365-2672.2007.03342.x. [DOI] [PubMed] [Google Scholar]
- Duary R.K., Raiput Y.S., Batish V.K., Grover S. Assessing the adhesion of putative indigenous probiotic Lactobacilli to human colonic epithelial cells. Indian J. Med. Res. 2011;134(5):664–671. doi: 10.4103/0971-5916.90992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar M.Y.M. Comparative study of probiotic cultures to control the growth of Escherichia coli O157:H7 and Salmonella typhymurium. Biotechnology. 2004;3(2):173–180. [Google Scholar]
- Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO) 2002. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; pp. 1–11. London, Ontario, Canada. [Google Scholar]
- Foulquié-Moreno M.R., Callewaert R., Devreese B., Van Beeumen J., De Vuyst L. Isolation and biochemical characterisation of enterocins produced by Enterococci from different sources. J. Appl. Microbiol. 2003;94:214–229. doi: 10.1046/j.1365-2672.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- Gasmalla M.A., Tessema H.A., Salaheldin A., Kamal- Alahmad, Hassanin H.A., Aboshora W. Health benefits of milk and functional dairy products. MOJ Food Process. Technol. 2017;4(4):108–111. [Google Scholar]
- Guo Z., Wang J., Yan L., Chen W., Liu X.M., Zhang H.P. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT-Food Sci.Technol. 2009;42:1640–1646. [Google Scholar]
- Gupta A., Sharma N. Characterization of potential probiotic lactic acid bacteria Pediococcus acidilactici Ch-2, isolated from Chuli- A traditional apricot product of Himalayan region for the production of novel bioactive compounds with special therapeutic properties. J. Food Microbiol. Saf. Hygene. 2017;2:119–123. [Google Scholar]
- Haghshenas B., Haghshenas M., Nami1 Y., Khosroushahi A.Y., Abdullah N., Barzegari A., Rosli1 R., Hejazi M.S. Probiotic assessment of Lactobacillus plantarum 15HN and Enterococcus mundtii 50H isolated from traditional dairies microbiota. Adv. Pharmaceut. Bull. 2016;6(1):37–47. doi: 10.15171/apb.2016.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder D., Mandal M., Chatterjee S., Pal N., Mandal S. Indigenous probiotic Lactobacillus isolates presenting antibiotic like activity against human pathogenic bacteria. Biomedicines. 2017;5:31–38. doi: 10.3390/biomedicines5020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanzadazar H., Ehsani A., Mardani K., Hesari J. Investigation of antibacterial, acid and bile tolerance properties of Lactobacilli isolated from Koozeh cheese. Vet. Res. Forum. 2012;3(3):181–185. [PMC free article] [PubMed] [Google Scholar]
- Ibourahema C., Dauphin R.D., Jacqueline D., Thonart P. Characterization of lactic acid bacteria isolated from poultry farms in Senegal. Afr. J. Biotechnol. 2008;7:2006–2012. [Google Scholar]
- Karami S., Rashidian E., Birjandi M., Mahmood L. Antagonistic effect of isolated probiotic bacteria from natural sources against intestinal Escherichia coli pathotypes. Electron. Phys. 2018;10(3):6534–6539. doi: 10.19082/6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liong M.T., Shah N.P. Acid and bile tolerance and cholesterol removal ability of Lactobacilli strains. J. Dairy Sci. 2005;88:55–66. doi: 10.3168/jds.S0022-0302(05)72662-X. [DOI] [PubMed] [Google Scholar]
- Madhusudan N.M., Manjunath H., Dhavalagi P. A study on isolation of acid and bile tolerance on lactic acid bacteria (LAB) in dairy product (Dahi) of Bengaluru in Karnataka. IOSR J. Environ. Sci. Toxicol. Food Technol. 2017;11(6):61–65. [Google Scholar]
- Maragkoudakis P.A., Zoumpopoulou G., Miaris C., Kalantzopoulos G., Pot B., Tsakalidou E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006;16:189–199. [Google Scholar]
- Mendo S., Rohm H., Jaros D. Influence of exopolysaccharides on the structure, texture, stability and sensory properties of yoghurt and related products. Int. Dairy J. 2016;52:57–71. [Google Scholar]
- Mokoena M.P. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules. 2017;22:1255. doi: 10.3390/molecules22081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagyzbekkyzy E., Abitayeva G., Anuarbekova D., Shaikhina D., Li K., Shaikhin S., Almagambetov K., Abzhalelov A., Saduakhassova S., Kushugulova A., Marotta F. Investigation of acid and bile tolerance, Antimicrobial Activity and Antibiotic Resistance of Lactobacillus strains isolated from Kazakh dairy foods. Asian J. Appl. Sci. 2016;9(4):143–158. [Google Scholar]
- Ng S.Y., Koon S.S., Padam B.S., Chye F.Y. Evaluation of probiotic potential of lactic acid bacteria isolated from traditional Malaysian fermented Bambangan (Mangifera pajang) CyTA - J. Food. 2015;13(4):563–572. [Google Scholar]
- Pieniz S., Andreazza R., Anghinoni T., Camargo F., Brandelli A. Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Contr. 2014;37:251–256. [Google Scholar]
- Ray C.G., Ryan K.J., Keneth R. fourth ed. McGraw Hill; 2004. Sheris Medical Microbiology: an Introduction of Infectious Disease; p. 237. [Google Scholar]
- Sagheddu V., Elli M., Biolchi C., Lucido J., Morelli L. Impact of mode of assumption and food matrix on probiotic viability. J. Food Microbiol. 2018;2(2):1 6. [Google Scholar]
- Sahingoz S.A., Sahin H. Consumer awareness and food poisoning. Pakistan J. Nutr. 2009;8(8):1218–1223. [Google Scholar]
- Sawhney I.K., Sarkar B.C., Patil G.R. International Conference on Traditional Dairy Foods (ICTDF). Karnal. India. Nov 14–17, 2007. 2007. Moisture adsorption in dried casein from buffalo skim milk. [Google Scholar]
- Shyamala G.R., Meenambigai P., Prabhavathi P., Raja R., Yesudoss L. Probiotics and its effects on human health- A review. Int. J. Curr. Microbiol. Appl. Sci. 2016;5(4):384–392. [Google Scholar]
- Stack H.M., Kearney N., Stanton C., Fitzgerald G.F., Ross R.P. Association of beta-glucan endogenous production with increased stress tolerance of intestinal Lactobacilli. Appl. Environ. Microbiol. 2010;76:500–507. doi: 10.1128/AEM.01524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur N., Rokana N., Panwar H. Probiotics: selection criteria, safety and role in health and disease. J. Innov. Biol. 2016;3(1):259–270. [Google Scholar]
- Trindade L.C., Marques E., Lopes D.B., Ferreira M.A.S. Development of a molecular method for detection and identification of Xanthomonas campestris pv. viticola. Summa Phytopathol. 2007;33(1):16–23. [Google Scholar]
- Villena J., Kitazawa H. Probiotic microorganisms; A closer look. Microorganisms. 2017;5:17–28. doi: 10.3390/microorganisms5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Guo Z., Zhang Q., Yan L., Chen W., Liu X.-M., Zhang H.P. Fermentation characteristics and transit tolerance of probiotic Lactobacillus casei Zhang in soymilk and bovine milk during storage. J. Dairy Sci. 2009;92:2468–2476. doi: 10.3168/jds.2008-1849. [DOI] [PubMed] [Google Scholar]
- Wang L.Q., Meng X.C., Zhang B.R., Wang Y., Shang Y.L. Influence of cell surface properties on adhesion ability of Bifidobacteria. World J. Microbiol. Biotechnol. 2010;26:1999–2007. [Google Scholar]
- Wasko A., Polak-Berecka M., Gustaw W. Increased viability of probiotic Lactobacillus rhamnosus after osmotic stress. Acta Aliment. 2013;42(4):520–528. [Google Scholar]
- Yin S., Zhai Z., Wang G., An H., Luo Y., Hao Y. A novel vector for lactic acid bacteria that uses a bile hydrolase gene as a potential food-grade selection marker. J. Biotechnol. 2011;152:49–53. doi: 10.1016/j.jbiotec.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Zacharof M.P., Lovitt R.W. Bacteriocins produced by lactic acid bacteria: a review article. APCBEE Procedia. 2012;2:50–56. [Google Scholar]
- Zeidan A.A., Poulsen V.K., Janzen T., Buldo P., Patrick M.F., Derkx P., Øregaard G., Neves A.R. Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol. Rev. 2017;41:168–200. doi: 10.1093/femsre/fux017. [DOI] [PubMed] [Google Scholar]