Abstract
Treatment of psychosis in Parkinson’s disease (PD) is challenging; pharmacological options are limited, with clozapine considered most effective. The risk of agranulocytosis restricts the use of clozapine, but, where this occurs, cautious re-challenge with granulocyte stimulating factor can be successful. We present a unique case of a patient who developed early-onset PD on a background of antecedent treatment-resistant schizophrenia, who had been treated effectively with clozapine for over 15 years with no adverse events. However, during a hospital admission intended to optimise her Parkinsonian medications, she developed persistent neutropenia necessitating clozapine discontinuation. Numerous attempts to re-challenge with clozapine failed until augmentation with lithium and G-CSF was trialled. Two doses of G-CSF led to a sustained increase in the neutrophil count, allowing the continuation of clozapine therapy in the 1 year of follow up. This illustrates the potential for G-CSF to be used to facilitate clozapine use in a patient population not described previously. Neutrophil augmentation allowed the sustained continuation of this effective therapy, treating her psychotic symptoms without detriment to her movement disorder. We suggest that G-CSF might be considered as a treatment option in other cases where clozapine-associated neutropenia obstructs its use.
Keywords: agranulocytosis, clozapine, G-CSF, Parkinson’s, schizophrenia
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, severely affecting the lives of patients and their families. Neuropsychiatric symptoms are non-motor symptoms commonly occurring in its natural history, even at the early stages of disease onset. Their presentation is varied, but most commonly includes depression, anxiety, apathy, fatigue, and psychotic symptoms, all of which may occur independently but frequently overlap. 1 Depending on the definition used, psychotic symptoms may affect up to 50% of treated PD patients and typically manifest as visual hallucinations, illusions, passage hallucinations, and a feeling of presence beside or behind the patient.1,2 Auditory hallucinations can occur in the context of visual hallucinations, as can delusions. 1
Although psychosis in PD is associated most commonly with dopaminergic medications aimed at treating the motor symptoms, it can also be the complex outcome of several disease-related factors.3,4 However, in rare cases, the psychosis in PD can be associated with a primary psychotic illness such as schizophrenia.5–7 In a few case reports, co-existing idiopathic PD and schizophrenia has been confirmed post-mortem, although in vivo motor symptoms were often misattributed to side effects from neuroleptics, while in fact it may have been the combined effect of the former with the underlying PD.8,9 Nevertheless, this co-morbidity remains uncommon; the hypodopaminergic transmission inherent to PD is unlikely to coexist with the hyperdopaminergic transmission in the mesolimbic pathway that is hypothesized to mediate the positive symptoms of schizophrenia.10,11 Furthermore, the typical psychotic symptoms seen in PD often differ from the classic symptoms in schizophrenia, with a greater representation of visual hallucinations and retained insight. 12 It has been suggested that psychotic symptoms in PD arise due to a disruption in dopaminergic pathways leading to supersensitivity of striatal dopaminergic neurons, which causes imbalance in serotonin, acetylcholine and dopamine. 13 Other studies have suggested a common pathway between PD and psychosis development as illustrated by schizotypal traits often present in PD. 14
Pharmacological management of psychosis in PD with antipsychotics is complicated by their inhibitory effect on dopamine, which affects motor function, and hence they are used only when other options have failed. Double-blind placebo-controlled trials have evaluated the impact of a number of atypical antipsychotics in treating psychotic symptoms. Olanzapine use is not advised in PD; double-blind controlled trials have consistently shown it to be both ineffective in treating psychosis in PD while also causing a deterioration in motor function.12,15–17 In contrast, quetiapine has minimal effect on motor symptoms and is often used unlicensed in many European countries despite limited efficacy. 18
Clozapine is an atypical antipsychotic licensed in the United Kingdom (UK) for treatment of psychosis in PD. Its unique receptor profile as a 5-HT2A and D4 receptor antagonist with weak D2 blocking properties allows for effective control of psychotic symptoms without worsening motor function. 18 Its use is, however, limited by the risk of inducing neutropenia and agranulocytosis so requires strict monitoring. Neutropenia is defined as an absolute neutrophil count less than 1.5 × 109/l and agranulocytosis less than 0.5 × 109/l. Life-threatening agranulocytosis occurs in 0.68% of patients within the first year, decreasing by a factor of 10 thereafter.19,20 Approved clozapine monitoring systems, where they exist, limit relevant risk. Dose adjustments and/or cessation following a significant drop in neutrophil count may be recommended, yet adjunct pharmacological options aiming to boost neutrophil counts may also be explored when clinically relevant.
A small number of reports have been published dealing with the acute or long-term management of agranulocytosis during clozapine therapy. In particular, granulocyte colony stimulating factor (G-CSF) has been used with good effect to treat clozapine-induced agranulocytosis in schizophrenia. 21 However, to our knowledge, there is a single prior case report which describes the use of G-CSF for clozapine induced agranulocytosis in PD, clozapine being used in this context to treat the psychosis and tremor secondary to PD. 22
We present a second case where use of G-CSF allowed maintenance of clozapine therapy in PD, and the first case to trial this approach in a patient with comorbid schizophrenia and PD.
Case report
We present a middle-aged female with comorbid early onset PD on a background of treatment-resistant paranoid schizophrenia. She was diagnosed with paranoid schizophrenia in her early twenties, characterised by severe self-neglect, isolation, paranoid ideation, and thought disorder. She was initially commenced on risperidone but, due to extrapyramidal side effects, this was discontinued after a year. Multiple other oral agents, including olanzapine and trifluoperazine, were trialled before she was initiated on flupentixol decanoate depot due to partial response and non-adherence. She eventually suffered a relapse of her psychosis, and clozapine was commenced. For 15 years, her mental state remained stable on clozapine 500 mg and citalopram 40 mg daily, with no adverse events. In her early thirties, she developed early onset idiopathic PD, confirmed with dopamine active transporter (DaT) imaging and antipsychotic medication withdrawal and she was prescribed Stalevo (levodopa/carbidopa/entacapone).
In June 2019, she was admitted to general hospital due to a deterioration in her PD, characterised by reduced oral intake and decompensated swallow secondary to poor compliance with medication. Physical examination revealed profound rigidity, bilateral resting tremor, hypomimia and slow speech, with mental state examination noting paranoid ideation and command auditory hallucinations to self-harm. A drop in neutrophil count to 1.28 × 109/l resulted in clozapine being withheld for 48 h. Rechallenge with clozapine led to another drop in neutrophils from 2.88 × 109/l to 1.26 × 109/l. Clozapine was stopped following haematology advice and quetiapine was commenced.
A deterioration in swallow precipitated a switch to a rotigotine patch for 48 h before enteral co-careldopa could be resumed, and quetiapine was increased to 100 mg twice daily in early July the same year. However, her parkinsonism worsened and quetiapine was discontinued. While her rigidity consequently improved, her mental state deteriorated, with delusional beliefs and auditory hallucinations becoming apparent. This was further complicated by the acquisition of hospital-acquired infection, likely chest related due to an aspiration event, which required antibiotic treatment. A percutaneous endoscopic gastrostomy (PEG) was fitted for administration of nutrition and medications.
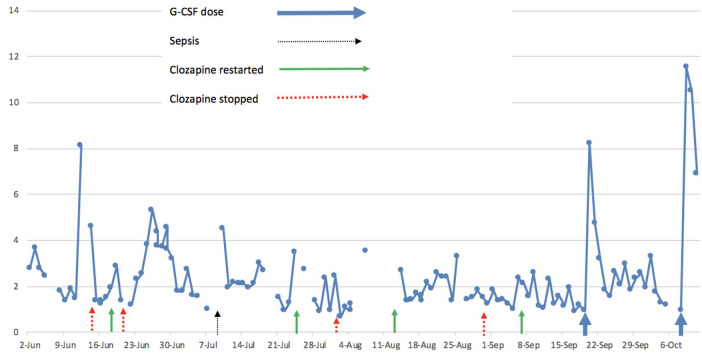
Clozapine retitration began at the end of July with daily full blood count monitoring. This precipitated another drop in neutrophil count to 0.67 × 109/l, requiring clozapine cessation with a detrimental effect on her mental state. Following multidisciplinary team discussion, clozapine was restarted at an initial dose of 25 mg daily alongside lithium carbonate 200 mg daily. Clozapine was increased progressively to a total dose of 125 mg daily with a positive impact on psychotic symptoms. However, further drops in neutrophil counts led to a reduction in clozapine dose, which was eventually stopped again when levels fell to 1.24 × 109/l. Figure 1 charts this fluctuation in neutrophil levels chronologically.
Figure 1.
Fluctuation of neutrophil counts (×109/l).
Following liaison with haematology colleagues in September, clozapine was restarted at 25 mg daily alongside lithium carbonate 200 mg and daily full blood count monitoring. Where neutrophil levels fell below a threshold of 1 × 109/l, it was agreed that G-CSF would be administered. Indeed, neutrophil levels soon dropped to 0.93 × 109/l, prompting administration of 300 µg of subcutaneous G-CSF, which boosted neutrophil counts to 8.23 × 109/l the following day. Clozapine was thus increased to 50 mg twice daily. A further drop in neutrophils led to a second dose of G-CSF being administered, again boosting neutrophil counts. This strategy allowed for safe uptitration of clozapine and successful treatment of her psychotic symptoms (see Figure 1).
The patient was eventually discharged from general hospital in October on clozapine 50 mg twice daily, lithium carbonate 300 mg twice daily and G-CSF as required. She was scheduled for weekly clozapine blood tests and community mental health team follow up. Since discharge, she has not required any further doses of G-CSF and neutrophil counts have remained stable.
Discussion
Clinicians are faced with a significant challenge when attempting to treat psychotic symptoms in PD. Whilst quetiapine has been a popular choice due to its lower side effect profile, clozapine has until recently remained the only antipsychotic with robust evidence for treating psychotic symptoms in PD without exacerbating motor symptoms.23,24 It has been further shown to be superior to other antipsychotics in controlling psychotic symptoms. 25 However, more recently, pimvanserin has emerged an effective treatment option for PD psychosis without motor function deterioration. 26
Clozapine therapy is limited by its potential to induce life-threatening agranulocytosis, requiring frequent blood monitoring, although relevant guidelines tend to differ between countries. 27 This is more common within the first 3 months of initiating therapy, in white men aged between 40 and 59 and certain HLA haplotypes.28,29 However, our case demonstrates the potential for agranulocytosis to occur many years after first commencing clozapine, highlighting the importance of continued blood monitoring. Interestingly, the only other case to use G-CSF to treat clozapine-induced agranulocytosis in a patient with PD also described a similar delayed neutropenia. 22 While the rate of agranulocytosis does not appear to be increased in PD patients treated with clozapine, further research is needed to explore a potential time-related effect.30,31
Although the mechanism of clozapine-induced agranulocytosis is unclear, evidence suggests that this may be immune-mediated rather than toxic. 29 Myeloid precursors and mature neutrophils may be affected, although given severe neutropenia is not dose-dependent, a direct toxic effect is unlikely. 32 Major histocompatibility complex antigens and heat shock protein variants are believed to be directly involved in determining individual response. 29
Current practice dictates immediate withdrawal of clozapine with the development of neutropenia, which presents limited alternative treatment options. Lithium has been shown to be effective as an adjunct treatment, augmenting short- and long-term neutrophil counts.3,33 Haematology specialist input may be required when considering further options, such as the use of G-CSF. This molecule endogenously stimulates bone marrow production and release of granulocytes and stem cells, and can be produced synthetically for exogenous use where neutrophil counts are low. 34
Clozapine-induced agranulocytosis has an absolute prevalence of approximately 1–2% and there is no current evidence to suggest that idiopathic PD or PD psychosis are independent risk factors for clozapine-induced neutropenia and agranulocytosis. 28 Although a prospective study is yet to occur, there are a number of reports in the literature of patients who received G-CSF to support clozapine rechallenge after an episode of neutropenia or agranulocytosis. A systematic review found that 75% of patients receiving adjunct G-CSF therapy for agranulocytosis were able to continue clozapine therapy for a minimum of 10 months. 35
In this case report, neutrophil counts initially dropped to a threshold requiring cessation of clozapine following an unexplained jump in neutrophil levels in the preceding days, during which the patient was clinically well. The only known clinical correlate at this time was insertion of a nasogastric tube. Whether this represents an acute inflammatory response that triggered an immune-mediated reaction resulting in clozapine induced neutropenia, remains unclear. Subsequent dips in neutrophils occurred proximally thereafter, including following an episode of hospital-acquired infection and sepsis. One could speculate that an immune mediated reaction may therefore have occurred in the context of a pro-inflammatory milieu. Lithium alone was insufficient to maintain neutrophil counts in the patient, but two doses of G-CSF allowed the continuation of clozapine therapy without subsequent neutropenia or further G-CSF requirement in 1 year of follow up.
In summary, we report the case of a patient with comorbid idiopathic PD and schizophrenia, who developed a neutropenia 15 years after being treated with clozapine without adverse event. The delay in this case highlights the importance of continual blood monitoring, while the effective use of adjunct lithium and G-CSF illustrates that clozapine therapy can be maintained despite these issues. Neutrophil augmentation allowed sustained continuation of clozapine, treating psychotic symptoms without detriment to the movement disorder. Given the efficacy of clozapine in managing psychosis in PD, G-CSF presents a valuable option for patients who develop life-threatening agranulocytosis in the context of clozapine use.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sotirios Posporelis  https://orcid.org/0000-0001-8779-1068
https://orcid.org/0000-0001-8779-1068
Contributor Information
Olivia Morrow, King’s College Hospital NHS Foundation Trust, London, UK.
Lucy Gibson, South London and Maudsley NHS Foundation Trust, London, UK; Institute of Psychiatry, Psychology and Neuroscience, London, UK.
Manraj Bhamra, South London and Maudsley NHS Foundation Trust, London, UK; Institute of Psychiatry, Psychology and Neuroscience, London, UK.
Anthony S. David, UCL Institute of Mental Health, London, UK
Sotirios Posporelis, South London and Maudsley NHS Foundation Trust, 1st Floor, Cheyne Wing, King’s College Hospital, London, SE5 9RS, UK; Institute of Psychiatry, Psychology and Neuroscience; King’s College Hospital NHS Foundation Trust, London, UK.
References
- 1. Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 2009; 24: 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graham JM, Grunewald RA, Sagar HJ. Hallucinosis in idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1997; 63: 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Small JG, Klapper MH, Malloy FW, et al. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol 2003; 23: 223–228. [DOI] [PubMed] [Google Scholar]
- 4. Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson’s disease and dementia with Lewy bodies. Am J Psychiatry 2007; 164: 1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Habermeyer B, Kneifel S, Kneifel Lotz-Blauer I, et al. Psychosis in a case of schizophrenia and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2008; 20: 373–375. [DOI] [PubMed] [Google Scholar]
- 6. Urban A. Comorbidity of parkinsonism and schizophrenia in a patient treated with clozapine. Eur Psychiatry 2003; 18: 258–259. [DOI] [PubMed] [Google Scholar]
- 7. Crow TJ, Johnstone EC, McClelland HA. The coincidence of schizophrenia and Parkinsonism: some neurochemical implications. Psychol Med 1976; 6: 227–233. [DOI] [PubMed] [Google Scholar]
- 8. Lam RW. Chronic schizophrenia and idiopathic Parkinson’s disease. Can J Psychiatry 1993; 38: 75–77. [DOI] [PubMed] [Google Scholar]
- 9. Friedman JH, Fernandez HH. Autopsy follow-up of a patient with schizophrenia and presumed idiopathic Parkinson’s disease. Clin Neuropharmacol 2001; 24: 120–121. [DOI] [PubMed] [Google Scholar]
- 10. Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol 2008; 119: 1459–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull 2009; 35: 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedman JH. Parkinson disease psychosis: update. Behav Neurol 2013; 27: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papapetropoulos S, Mash DC. Psychotic symptoms in Parkinson’s disease. From description to etiology. J Neurol 2005; 252: 753–764. [DOI] [PubMed] [Google Scholar]
- 14. Housden CR, O’Sullivan SS, Joyce EM, et al. Intact reward learning but elevated delay discounting in Parkinson’s disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology 2010; 35: 2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ondo WG, Levy Jk, Vuong KD, et al. Olanzapine treatment for dopaminergic-induced hallucinations. Mov Disord 2002; 17: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 16. Breier A, Sutton VK, Feldman PD, et al. Olanzapine in the treatment of dopamimetic-induced psychosis in patients with Parkinson’s disease. Biol Psychiatry 2002; 52: 438–445. [DOI] [PubMed] [Google Scholar]
- 17. Goetz CG, Blasucci LM, Leurgans S, et al. Olanzapine and clozapine: comparative effects on motor function in hallucinating PD patients. Neurology 2000; 55: 789–794. [DOI] [PubMed] [Google Scholar]
- 18. Shotbolt P, Samuel M, David A. Quetiapine in the treatment of psychosis in Parkinson’s disease. Ther Adv Neurol Disord 2010; 3: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schulte P. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother 2006; 40: 683–688. [DOI] [PubMed] [Google Scholar]
- 20. Munro J, O’Sullivan D, Andrews C, et al. Active monitoring of 12,760 clozapine recipients in the UK and Ireland. Beyond pharmacovigilance. Br J Psychiatry 1999; 175: 576–580. [DOI] [PubMed] [Google Scholar]
- 21. Silva E, Higgins M, Hammer B, et al. Clozapine rechallenge and initiation despite neutropenia- a practical, step-by-step guide. BMC Psychiatry 2020; 20: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedman J, Yeboah E, Hermenau M. Addition of filgrastim (neupogen) for clozapine rechallenge in the case of Parkinson disease patient. Clin Neuropharmacol 2017; 40: 233–234. [DOI] [PubMed] [Google Scholar]
- 23. Factor SA, Friedman JH, Lannon MC, et al. Clozapine for the treatment of drug-induced psychosis in Parkinson’s disease: results of the 12 week open label extension in the PSYCLOPS trial. Mov Disord 2001; 16: 135–139. [DOI] [PubMed] [Google Scholar]
- 24. Pollak P. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry 2004; 75: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taddei RN, Cankaya S, Dhaliwal S, et al. Management of psychosis in Parkinson’s disease: emphasizing clinical subtypes and pathophysiological mechanisms of the condition. Parkinsons Dis 2017; 2017: 3256542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014; 383: 533–540. [DOI] [PubMed] [Google Scholar]
- 27. Andres E, Mourot-Cottet R, Maloisel F, et al. Idiosyncratic drug-induced neutropenia & agranulocytosis. QJM 2017; 110: 299–305. [DOI] [PubMed] [Google Scholar]
- 28. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 1993; 329: 162–167. [DOI] [PubMed] [Google Scholar]
- 29. Wicinski M, Weclewicz MM. Clozapine-induced agranulocytosis/granulocytopenia: mechanisms and monitoring. Curr Opin Hematol 2018; 25: 22–28. [DOI] [PubMed] [Google Scholar]
- 30. Gomide L, Kummer A, Cardoso F, et al. Use of clozapine in Brazilian patients with Parkinson’s disease. Arq Neuropsiquiatr 2008; 66: 611–614. [DOI] [PubMed] [Google Scholar]
- 31. Thomas AA, Friedman JH. Current use of clozapine in Parkinson disease and related disorders. Clin Neuropharmacol 2010; 33: 14–16. [DOI] [PubMed] [Google Scholar]
- 32. Hummer M, Kurz M, Barnas C, et al. Clozapine-induced transient white blood count disorders. J Clin Psychiatry 1994; 55: 429–432. [PubMed] [Google Scholar]
- 33. Palominao A, Kukoyi O, Xiong GL, et al. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry 2010; 22: 205–206. [PubMed] [Google Scholar]
- 34. Dale DC. Colony-Stimulating factors for the management of neutropenia in cancer patients. Drugs 2002; 62: 1–15. [DOI] [PubMed] [Google Scholar]
- 35. Lally J, Malik S, Krivoy A, et al. The use of granulocyte colony-stimulating factor in clozapine rechallenge: a systematic review. J Clin Psychopharmacol 2017; 37: 600–604. [DOI] [PubMed] [Google Scholar]