Abstract
Background:
Palliative chemotherapy has been the mainstay treatment for patients with recurrent or metastatic nasopharyngeal carcinoma (R/M-NPC). However, little is known about the efficacy and toxicity of nimotuzumab (NTZ) – a monoclonal antibody drug targeting epidermal growth factor receptor – plus chemotherapy (CT) versus CT alone for these patients.
Methods:
The database at Cancer Hospital of Chinese Academy of Medical Sciences was queried for patients diagnosed with NPC who received CT with or without NTZ between 2004 and 2018. Treatment compliance, survival outcomes, and adverse effects were compared among these groups.
Results:
Records of 70 patients with R/M-NPC were reviewed: 21 (30%) received NTZ plus CT (NTZ+CT) and 49 (70%) received CT. CT regimens included gemcitabine plus platinum, taxane plus platinum (TP), and fluorouracil plus platinum. Comparing the CT group with NTZ+CT group, the median follow up was 62 months (range = 3–133) versus 59 months (range = 9–117); median progression free survival was 7.5 [95% confidence interval (CI) 6.552–8.381] months versus 8.5 (95% CI 6.091–10.976) months, p = 0.424; median overall survival (OS) was 25.6 (95% CI 18.888–32.379) months versus 48.6 (95% CI 35.619–61.581) months, p = 0.017, respectively. Multivariable analysis established treatment group (CT versus NTZ+CT) as an independent prognostic factor for OS (hazard ratio, 0.5; 95% CI 0.255–0.979; p = 0.043). No significant difference with regard to toxicities was observed between the two groups. Among them, a subgroup analysis was performed in 53 (75.7%) patients who received TP with or without NTZ, which showed similar results.
Conclusion:
Our findings suggested that NTZ+CT provides a novel treatment option and prolongs survival significantly for R/M-NPC.
Keywords: adverse effects, chemotherapy, distant metastasis, efficacy, nasopharyngeal carcinoma, nimotuzumab, recurrence
Introduction
Nasopharyngeal carcinoma (NPC) is an uncommon malignancy, accounting for 0.7% of new cancer cases and 0.8% of cancer deaths in global cancer statistics 2018.1 Unlike other cancer types, NPC is prevalent in South China, South-eastern Asia, and North Africa, with obvious geographical distribution. In China, the incidence and mortality of NPC was about 40% higher in males than in females in 2015.2 Despite its high sensitivity to radiotherapy (RT), retrospective study of patients treated with intensity modulated radiation therapy (IMRT) has provided strong evidence that 5–15% of patients will experience local recurrence, and 15–30% will develop metastasis3; hence, palliative chemotherapy (PCT) remains the treatment mainstay. Commonly used drugs in this setting include fluorouracil, platinum, gemcitabine, and taxane. PCT utilizing these agents produces tumor shrinkage, delays tumor progression, and prolongs survival. Phase II studies of platinum-based doublets have revealed that the median overall survival (OS) in the first-line setting ranged from 12 to 15 months.4,5 However, the benefits are far from satisfactory, and regimens integrating novel agents are needed.
Epidermal growth factor receptor [EGFR (HER-1, erbB1)] – a member of the ErbB receptor family – is associated with cell proliferation, migration, and differentiation.6 Leong et al. reported that EGFR was expressed in more than 82% of NPC, and its overexpression in NPC was strongly associated with aggressive local disease.7 That is to say, the majority of those with locoregional recurrence or distant metastasis were associated with EGFR over-expression. EGFR represents a promising target in NPC. Preclinical studies have revealed that combining the conventional cytotoxic drug cisplatin or 5-fluorouracil with the cetuximab (CTX) exerts a strong anti-tumor effect on NPC cells.8 Furthermore, the apoptosis rate of the combination treatment was increased significantly as compared with that of CTX or cisplatin treatment alone.9 In line with this, a phase II study reported that CTX in combination with carboplatin (CBP) demonstrates clinical activity and an acceptable safety profile in R/M-NPC.10
Nimotuzumab (NTZ; alternatively referred to as Tai Xin Sheng®, OSAG-101 or YMB-1000) is an IgG1 humanized anti-EGFR monoclonal antibody jointly developed by Cuba and China.11 Compared with CTX, NTZ has potential advantages. Its humanized structure allows for low immunogenicity, which may prevent the possible ineffectiveness caused by neutralizing antibodies, especially after a long-term drug exposure. In addition, its intermediate affinity against the EGFR contributes to milder toxicities than CTX,12 which may help improve patient compliance, especially when added to combination CT rather than the single agent. Recently, anti-EGFR monoclonal antibodies (CTX and NTZ) have been developed for the treatment of various epithelial tumors.13 However, compared with CT, there are few data reporting whether this drug, in combination with CT, has benefits in patients with R/M-NPC. Thus, we performed this retrospective study to evaluate the efficacy and safety of NTZ in combination with platinum-based CT in these patients.
Materials and methods
Patients and selection criteria
Patients with NPC were identified in the intelligence database at the Cancer Hospital of Chinese Academy of Medical Sciences between 2004 and 2018. Inclusion criteria were as follows: (i) histologically confirmed NPC; (ii) presented with recurrence or distant metastasis based on physical examination and radiologic imaging; (iii) treated with cisplatin-based double drug regimens, with or without NTZ; (iv) aged ⩾18 years; (v) Eastern Cooperative Oncology Group (ECOG) performance status score 0–2; and (vi) no evidence of other malignancies.
This study was approved by the ethics committee of Cancer Hospital of Chinese Academy of Medical Sciences (approval number, 20/101-2297), and informed consent was waived for its retrospective and non-interventional nature. Patient records were anonymized and de-identified prior to analysis.
Detection of EGFR expression
The expression of EGFR in tumor tissue specimens was graded as a score of 0, 1+, 2+, or 3+ by immunohistochemical examination (kits were purchased from ACCB Biotech, Beijing, China). It was scored as 0 for no staining or less than 10% staining of the tumor cells, 1+ for faint staining in more than 10% of tumor cells, 2+ for weak to moderate complete membrane staining in more than 10% of tumor cells, and 3+ for strong complete membrane staining in more than 10% of tumor cells.
Treatment protocol
The platinum-based regimens including gemcitabine plus platinum (GP), taxane plus platinum (TP), and fluorouracil plus platinum (PF) were administered as chemotherapy and repeated every 3 weeks. Platinum included cisplatin (DDP), nedaplatin (NDP), CBP, and oxaliplatin (L-OHP). Cisplatin 70–80 mg/m2 or NDP 80–100 mg/m2 was given as intravenous infusion on days 1–3, and CBP AUC 4–5 or oxaliplatin 85 mg/m2 was administered intravenously on day 1. Taxane included docetaxel, paclitaxel liposome, paclitaxel, and paclitaxel (albumin bound). Docetaxel 75 mg/m2 or paclitaxel/paclitaxel liposome 175 mg/m2 was administered intravenously on day 1, and paclitaxel (albumin bound) 260 mg/m2 or gemcitabine 1000 mg/m2 was administered intravenously on days 1 and 8. Fluorouracil 750–1000 mg/m2 was administered intravenously on days 1–4. NTZ was delivered at a dose of 200–400 mg every week, diluted in 250–500 ml saline, and infused intravenously over 1 h.
Assessments and follow up
According to institutional guidelines, patients received direct nasopharyngoscopy and histopathological review before treatment. Moreover, magnetic resonance imaging (MRI) of the head and neck; computed tomography of the thorax, abdomen, and pelvis; skeletal scintigraphy; and electrocardiogram were undertaken before, during and after treatment. Routine blood profiles included pre-treatment levels of lactate dehydrogenase (LDH), EGFR expression, copies of plasma Epstein-Barr virus deoxyribonucleic acid (EBV-DNA) copy number, early antigen-immunoglobulin A (EA-IgA), viral capsid antigen-immunoglobin A (VCA-IgA), complete blood cell count, and comprehensive biochemistry testing. Real-time fluorescence quantitative polymerase chain reaction (FQ-PCR) technology was used to detect plasma EBV-DNA (kits and instruments were purchased from DaAn Gene, Guangzhou, China). Serum EA-IgA and VCA-IgA in patients with NPC were detected using enzyme-linked immunosorbent assay (ELISA; kits were purchased from EUROIMMUN, Beijing, China). All missing data were classified as “unknown”.
All patients were restaged according to the 8th edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) manual based on their medical records and imaging examinations.
Tumor response was evaluated by computed tomography or MRI according to the RECIST v1.1, and classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). All adverse effects (AEs) were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 5.0 (NCI-CTCAE v5.0).
Clinical endpoints and statistical analysis
Primary end point of this study was OS (defined as time from treatment administration to the death from any case or the last date of follow up, whichever came first). Other study endpoints of this study included progression-free survival (PFS, defined as the time from treatment administration to disease progression or death from any cause, whichever came first); objective response rate (ORR, defined as the rate of CR and PR among all patients); disease control rate (DCR, defined as the rate of CR, PR and SD among all patients); and AEs.
Chi-squared-test or Fisher’s exact test was adopted to compare categorical variables, such as sex, smoking, family history, histology, previous therapy, treatment line, condition, VCA-IgA, EA-IgA, EBV-DNA, cycles, and subsequent treatment in the study population. The Mann–Whitney U test was used for continuous variables and hierarchical variables, such as ECOG, LDH, EGFR, number of metastatic organs, and clinical stage. Independent-sample t test was used for continuous variables which are distributed normally, such as age. Survival outcomes were calculated using the Kaplan–Meier method and compared by log-rank test in univariate analysis. The multivariate Cox proportional hazards model was used to estimate hazard ratios (HR), 95% confidence intervals (CI) and independent prognostic factors. The statistical analysis was conducted with IBM SPSS Statistics for Mac, version 25.0 (IBM Corp., Armonk, NY, USA), and the survival curves was performed using GraphPad Prism for Windows, version 6.0 (GraphPad Software, La Jolla, CA, USA, www.graphpad.com). Statistical tests were two-tailed, and a p < 0.05 was considered statistically significant.
Results
Patient baseline characteristics
In total, 70 patients were eligible for this study, including 49 and 21 that received either CT alone or NTZ+CT, respectively. Distant metastatic organs included the lung, bone, liver, and kidney. The baseline characteristics in both groups are shown in Table 1. The characteristics were comparable between the two groups, except for gender (p = 0.049). The pathological type of all patients is the non-keratinising subtype. The median follow-up time was 62 months (range = 3–133) in the CT group and 59 months (range = 9–117) in the NTZ+CT group. Among the 70 patients, 53 (75.7%) received the TP regimen, with 18 (34.0%) patients in the NTZ+TP group and 35 (66.0%) patients in the TP group. The baseline characteristics of patients were similar between the TP group and NTZ+TP group.
Table 1.
Baseline characteristics of the 70 patients with advanced recurrent or metastatic NPC in each treatment arm.
| Characteristics | CT (n = 49) no. (%) |
NTZ+CT (n = 21) no. (%) |
p value | |
|---|---|---|---|---|
| Sex | male | 40 (81.6) | 21 (100.0) | 0.049a |
| female | 9 (18.4) | 0 (0.0) | ||
| Agef, average | 49.08 ± 10.72 | 48.81 ± 13.32 | 0.928b | |
| ECOG score | 0 | 23 (46.9) | 14 (66.7) | 0.122c |
| 1 | 25 (51.0) | 7 (33.3) | ||
| 2 | 1 (2.1) | 0 (0.0) | ||
| Histology | Nonkeratinizing differentiated carcinoma | 26 (53.1) | 6 (28.6) | 0.059d |
| Nonkeratinizing Undifferentiated carcinoma | 23 (46.9) | 15 (71.4) | ||
| Smoking | YES | 25 (51.0) | 9 (42.9) | 0.531d |
| NO | 24 (49.0) | 12 (57.1) | ||
| Family history | YES | 12 (24.5) | 4 (19.0) | 0.761a |
| No | 37 (75.5) | 17 (81) | ||
| Previous therapy | None | 9 (18.4) | 6 (28.6) | 0.728d |
| RT | 6 (12.2) | 2 (9.5) | ||
| RT+CT | 33 (67.3) | 13 (61.9) | ||
| CT | 1 (2.0) | 0 (0.0) | ||
| treatment line | first-line treatment | 31 (63.3) | 13 (61.9) | 0.914d |
| Second-line treatment and above | 18 (36.7) | 8 (38.1) | ||
| Condition | Primary metastases | 45 (91.8) | 19 (90.5) | 0.806d |
| Recurrence with distant metastases | 3 (6.1) | 1 (4.8) | ||
| Local recurrence | 1 (2.0) | 1 (4.8) | ||
| LDHg, U/L (IQR) | 180 (147–215) | 227 (162–394.5) | 0.074c | |
| VCA-IgA (c/o) |
<1.1 | 25 (51.0) | 8 (38.1) | 0.321d |
| ⩾1.1 | 24 (49.0) | 13 (61.9) | ||
| EA-IgA (c/o) |
<1.1 | 37 (75.5) | 17 (81.0) | 0.761a |
| ⩾1.1 | 12 (24.5) | 4 (19.0) | ||
| EBV-DNA (copies/ml) | <500 | 20 (40.8) | 6 (28.6) | 0.331d |
| ⩾500 | 29 (59.2) | 15 (71.4) | ||
| EGFR | 1+ | 5 (10.2) | 2 (9.5) | 0.804c |
| 2+ | 5 (10.2) | 3 (14.3) | ||
| 3+ | 9 (18.4) | 5 (23.8) | ||
| Missing data | 30 (61.2) | 11 (52.4) | ||
| Num. of metastatic organ | 0 | 1 (2.0) | 1 (4.8) | 0.277c |
| 1 | 34 (69.4) | 11 (52.4) | ||
| 2 | 13 (26.5) | 7 (33.3) | ||
| ⩾3 | 1 (2.0) | 2 (9.5) | ||
| Clinical stagee | Stage I | 1 (2.0) | 0 (0.0) | 0.549c |
| Stage IVA | 0 (0.0) | 1 (4.8) | ||
| Stage IVB | 48 (98.0) | 20 (95.2) | ||
| Cycles | <5 | 24 (49.0) | 13 (61.9) | 0.321d |
| ⩾5 | 25 (51.0) | 8 (38.1) | ||
| Subsequent treatment | Systemic treatment | 18 (36.7) | 13 (61.9) | 0.106d |
| Local treatment | 9 (18.4) | 1 (4.8) | ||
| Missing data | 22 (44.9) | 7 (33.3) | ||
CT, chemotherapy treatment; EA-IgA, early antigen-immunoglobin A; EBV-DNA, Epstein-Barr virus deoxyribonucleic acid; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; LDH, lactate dehydrogenase; NTZ+CT, nimotuzumab plus chemotherapy; RT, radiotherapy; VCA-IgA, viral capsid antigen-immunoglobin A.
p value was calculated using Fisher’s exact test.
p value was calculated using independent-sample t test.
p value was calculated using the Mann–Whitney U test.
p value was calculated using the chi-square test.
Re-staged according to the 8th edition of UICC/AJCC staging system.
Normal distribution.
Non-normal distributions.
EGFR expression
Of the 70 patients, records of the immunohistochemical examination of EGFR expression in the original tumor tissue specimens were available in 19 patients in the CT group and 10 patients in the NTZ+CT group, In the CT arm, five, five, and nine patients were graded as 1+, 2+, and 3+ for EGFR expression, respectively. In the NTZ+CT arm, two, three, and five patients were graded as 1+, 2+, and 3+ for EGFR expression, respectively. No difference was found between the two groups in EGFR expression (Table 1). Of the 53 patients that received TP regimen, the EGFR expression was assessed in 14 (40%) in the TP group and 10 (55.5%) in the NTZ+TP group. In the TP arm, four, three, and seven patients were graded as 1+, 2+, and 3+, respectively. In the NTZ+TP arm, two, two, and six patients were graded as 1+, 2+, and 3+, respectively.
Treatment with chemotherapy and NTZ
In the CT group, there were 12 (24.5%), 35 (71.4%), and 2 (4.1%) patients who received GP, TP, and PF regimen, respectively. Median cycles were five cycles (range = 2–8), and the median cumulative dose intensity for platinum was 394 mg/m2 (range = 133–1437).
In the NTZ+CT group, two (9.5%), 18 (85.7%) and one (4.8%) patients received the NTZ+GP, NTZ+TP, and NTZ+PF regimens, respectively. Median cycles were four cycles (range = 2–8), with the median cumulative dose intensity for platinum of 353 mg/m2 (range = 142–2062). The median cumulative dose intensity for NTZ was 2339 mg/m2 (range = 226–5882), with a median infusion number of 12 (range = 3–31).
In the TP group, the median number of cycles was 4 (range = 2–8), and the median cumulative dose intensity for taxane and platinum were 684 mg/m2 (range = 226–1193) and 383 mg/m2 (range = 133–1437), respectively.
While, in the NTZ+TP group, median number of cycles was 4 (range = 2–8), and the median cumulative dose intensity for taxane, platinum and NTZ were 661.5 mg/m2 (range = 256–1237), 354 mg/m2 (range = 142–2062) and 2354 mg/m2 (range = 656–5882), respectively. Median dose intensity of NTZ was 203.5 mg/m2/week (range = 103–238). The median dosing number of NTZ was 13.5 (range = 6–31).
Response and survival analysis
In the CT group, 2 (4.10%), 27 (55.10%), 16 (32.70%), and 4 (8.20%) patients achieved CR, PR, SD, and PD, respectively; resulting in an ORR of 59.2% and a DCR of 91.8%. In the NTZ+CT group, no patients, 12 (57.10%) patients, 6 (28.60%) patients, and 3 (14.30%) patients achieved CR, PR, SD, and PD, respectively; the ORR and DCR were 57.1% and 85.7%, respectively. There was no obvious association between EGFR expression level and best response. Comparing between the CT and NTZ+CT groups, median PFS was 7.5 (95% CI 6.552–8.381) months versus 8.5 (95% CI 6.091–10.976) months, p = 0.424 (Figure 1); while the median OS was 25.6 (95% CI 18.888–32.379) months versus 48.6 (95% CI 35.619–61.581) months, p = 0.017 (Figure 2), respectively. Univariate analysis showed that the treatment modality was significantly correlated with OS. The addition of NTZ to CT was associated with longer median OS (48.6 versus 25.6 months, p = 0.017). Treatment modality and sex were included in multivariate analysis. The results suggested that patients with NTZ+CT had better OS (HR, 0.5; 95% CI, 0.255–0.979; p = 0.043), as shown in Table 2. The 3-year OS rates for the CT versus NTZ+CT group was 36.7% versus 76.2%, respectively. The 5-year OS rates for the CT versus NTZ+CT group was 24.5% versus 42.9%, respectively. No correlation was found between EGFR expression and PFS (p = 0.584) or OS (p = 0.160) in the two groups. There was no statistically significant difference between the patient’s subsequent treatment and OS (p = 0.400, Supplemental Table S1).
Figure 1.
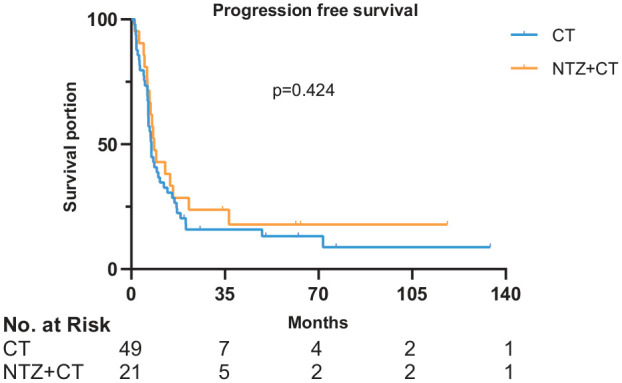
Survival curves of PFS in 70 patients.
x-axis, months; y-axis, cumulative survival; blue line, CT group; yellow line, NTZ+CT group.
CT, chemotherapy; NTZ, nimotuzumab; PFS, progression free survival.
Figure 2.
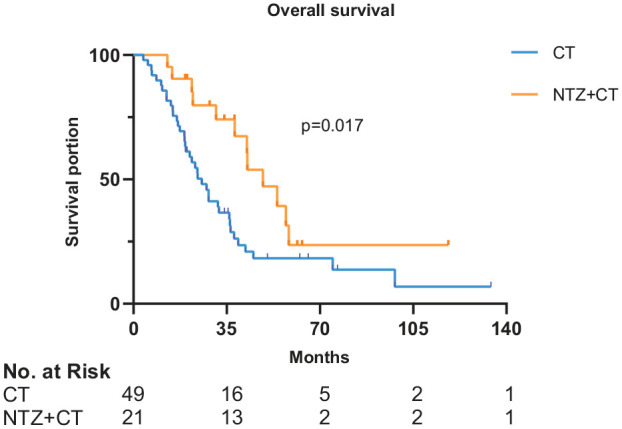
Survival curves of OS in 70 patients.
x-axis, months; y-axis, cumulative survival; blue line, CT group; yellow line, NTZ+CT group.
CT, chemotherapy; NTZ, nimotuzumab; OS, overall survival.
Table 2.
Multivariate regression analysis for prognostic factors.
| Endpoints | Group | Variable | HR (95% CI) | p-value |
|---|---|---|---|---|
| Overall survival | CT versus NTZ+CT | Treatment modality (CT versus NTZ+CT) |
0.500 (0.255–0.979) | 0.043 |
| sex (male versus female) | 1.693 (0.761–3.769) | 0.197 | ||
| TP versus NTZ+TP | Treatment modality (TP versus NTZ+TP) |
0.440 (0.195–0.990) | 0.047 | |
| sex (female versus male) | 1.760 (0.537–5.771) | 0.351 | ||
| Histology (Non-keratinizing differentiated carcinoma versus Non-keratinizing Undifferentiated carcinoma) |
1.439 (0.718–2.884) | 0.304 | ||
| EBV-DNA | 1.409 (0.677–2.933) | 0.359 |
p values were calculated using Cox proportional hazards model.
CI, confidence interval; CT, chemotherapy; EBV-DNA, Epstein-Barr virus deoxyribonucleic acid; HR, hazard ratio; NTZ+CT, nimotuzumab plus chemotherapy; NTZ+TP, nimotuzumab plus taxane plus platinum; TP, taxane plus platinum.
In the TP group, 2 (5.70%), 18 (51.40%), 11 (31.40%), 4 (11.50%) patients achieved CR, PR, SD, and PD, respectively. The ORR and DCR were 57.1% and 88.6%, respectively. In the NTZ+TP group, 0, 11 (61.10%), 4 (22.20%), 3 (16.70%) patients achieved CR, PR, SD, and PD, respectively. The ORR and DCR were 61.1% and 83.3%, respectively. There was no obvious association between the level of EGFR expression and best response. Comparing the TP group with the NTZ+TP group, the median PFS was 7.3 (95% CI 5.909–8.691) months versus 8.5 (95% CI 4.134–12.866) months, p = 0.743 (Figure 3); while median OS was 27.4 (95% CI 13.36–41.44) months versus 48.6 (95% CI 31.55–65.65) months, p = 0.047 (Figure 4), respectively. The results from the univariate analysis revealed that therapeutic modality (TP alone versus NTZ+TP, p = 0.047) and gender (male versus female, p = 0.042) were prognostic factors for OS. Multivariate analysis was also performed with OS as the endpoint, with therapeutic modality (TP versus NTZ+TP), sex, histology, and EBV-DNA status included. Results indicated that therapeutic modality (TP versus NTZ+TP: HR, 0.440; 95% CI, 0.195–0.990; p = 0.047) was independently associated with OS, as shown in Table 2. The 3-year OS rates for the TP versus NTZ+TP group was 40% versus 83.3%. The 5-year OS rates for the TP versus NTZ+TP group was 25.7% versus 44.4%. No correlation was found between EGFR expression and PFS (p = 0.157) or OS (p = 0.331) in the two groups.
Figure 3.
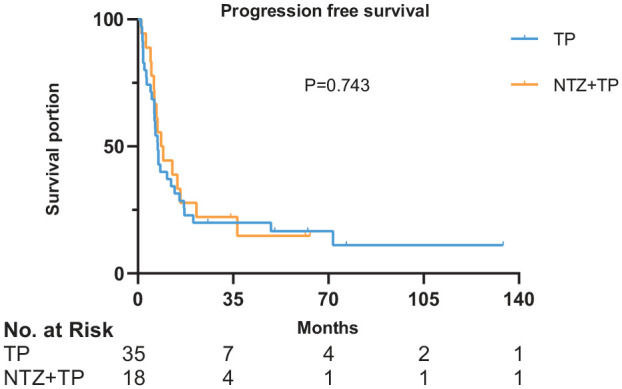
Survival curves of PFS in 53 patients.
x-axis, months; y-axis, cumulative survival; blue line, TP group; yellow line, NTZ+TP group.
NTZ, nimotuzumab; PFS, progression free survival; TP, taxane plus platinum.
Figure 4.
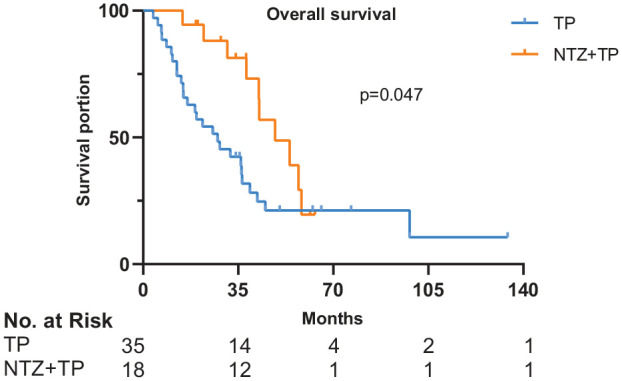
Survival curves of OS in 53 patients.
x-axis, months; y-axis, cumulative survival; blue line, TP group; yellow line, NTZ+TP group.
NTZ, nimotuzumab; OS, overall survival; TP, taxane plus platinum.
Adverse events
The detailed AEs are presented in Table 3. Hematological and gastrointestinal AEs were similar between the two groups (all rates, p > 0.05). No significant difference with regard to toxicities was observed between these two groups. Among the 21 patients who were infused NTZ, three patients were observed with AEs, which were associated with NTZ. One patient had an episode of fever peaking at 38.5°C after infusing the patient with 50 mg NTZ. After discontinuing with the infusion, the fever was relieved. An hour later, the remaining dose was safely administered with no additional symptoms. Also, mild nausea and fatigue were documented, each in two patients. No anti-EGFR treatment-associated skin rash or mucositis was observed with NTZ.
Table 3.
Acute toxicity profile during treatment between the two groups.
| Toxicity | CT group (n = 49, %) |
NTZ+CT group (n = 21, %) |
p-value |
|---|---|---|---|
| Hematological | |||
| Leucopenia | |||
| G0-2 | 27 (55.1) | 15 (71.4) | 0.201 |
| G3-4 | 22 (44.9) | 6 (28.6) | |
| Neutropenia | |||
| G0-2 | 28 (57.1) | 12 (57.1) | 1.000 |
| G3-4 | 21 (42.9) | 9 (42.9) | |
| Anemia | |||
| G0-2 | 46 (93.9) | 20 (95.2) | 0.822 |
| G3-4 | 3 (6.1) | 1 (4.8) | |
| Non-hematological | |||
| Nausea | |||
| G0-2 | 47 (95.9) | 20 (95.2) | 0.898 |
| G3-4 | 2 (4.1) | 1 (4.8) | |
| Vomiting | |||
| G0-2 | 47 (95.9) | 21 (100.0) | 0.348 |
| G3-4 | 2 (4.1) | 0 (0.0) | |
| Decreased appetite | |||
| G0-2 | 49 (100.0) | 20 (95.2) | 0.124 |
| G3-4 | 0 (0.0) | 1 (4.8) | |
| Alopecia | |||
| G0-2 | 44 (89.8) | 17 (81.0) | 0.311 |
| G3-4 | 5 (10.2) | 4 (19.0) | |
| Neuropathy | |||
| G0-2 | 48 (98.0) | 20 (95.2) | 0.531 |
| G3-4 | 1 (2.0) | 1 (4.8) | |
p value was calculated using the chi-square test.
Discussion
Systemic treatments have had a major role in improving survival and quality of life in R/M disease.14 Due to the unsatisfactory results of metastatic NPC, no optimum treatment strategy has been well established and treatment failure remains confusing to clinicians. What we hope to achieve is to extend the patient’s life as long as possible. Therefore, identification of an effective treatment is necessary. In this present study, the NTZ+CT combination offered significant OS benefit over CT alone in R/M-NPC patients, and was well tolerated without an increase in treatment-related toxicities. To the best of our knowledge, this work is the first study to evaluate the survival outcome of NTZ+TP versus TP alone in R/M-NPC.
In recent times, platinum-based CT is considered as the cornerstone of treatment, with response rates greater than 50%.15 Gemcitabine or fluorouracil or taxane is the most active agent often combined with platinum. Recently, Zhang et al. reported a superior ORR (64% versus 42%), DCR (90% versus 86%), and PFS (7.0 versus 5.6 months) in R/M-NPC patients with GP versus PF,16 which established GP as the standard first-line chemotherapeutic regimen in these patients. Relevant research indicated that R/M-NPC patients are likely to harbor platinum-resistant tumor clones, which may be associated with the EGFR signalling pathway.17 Furthermore, EGFR expression was significantly related to low OS and shorter time to progression.18 Thus, we have reason to speculate that suppressing the EGFR signalling pathway may make the tumor sensitive again to platinum-based CT. Fortunately, with the development of molecular-targeted therapy, EGFR represents a promising new therapeutic target in R/M-NPC. A clinical trial in recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) indicated that CTX combined with fluorouracil-cisplatin chemotherapy achieved significantly better DFS and OS compared with fluorouracil-cisplatin alone when given as first-line therapy.19 A randomized phase III trial compared NTZ plus cisplatin and RT versus cisplatin and RT in locally advanced HNSCC, and also showed that the addition of NTZ improved PFS, LRC, DFS, and had a trend toward improved OS.20 Both studies mentioned previously revealed that CTX or NTZ adds additional anti-tumor efficacy, thereby resulting in improved efficacy, which is similar to our findings. NTZ is biologically and molecularly different from CTX,21 although they have similar mechanisms, NTZ inhibits both ligand-dependent and ligand-independent signaling of the EGFR pathway.22 A meta-analysis found that, compared with CTX+RT/chemoradiotherapy (CRT), NTZ+RT/CRT may achieve higher CR rate or overall remission rate (ORR) of the primary tumor in the treatment of advanced NPC. However, no difference was noted in survival outcomes, and NTZ had a more advantageous in efficacy.23 Moreover, Zhao et al. conducted a prospective phase II study assessing the efficacy of NTZ combined with cisplatin and 5-fluorouracil in R/M NPC.24 The researchers found that this combination regimen demonstrates potential efficacy as first-line treatment, with ORR and DCR of 71.4% and 85.7%, respectively; similar to our results. These data provide the rationale for exploring the efficacy of NTZ+CT in the treatment of R/M-NPC. In our current study, we provided a new insight to improve survival outcomes by adding NTZ to TP.
In this study, we found that NTZ+CT prolonged survival significantly than CT alone in R/M-NPC patients with a median OS of 25.6 months in the CT group versus 48.6 months in the NTZ+CT group. Compared with platinum-based CT, the median PFS has improved by 1 month in the NTZ+CT group; however, this was not statistically significant. Since the TP regimen was used in most patients, in order to eliminate the impact of inconsistencies in chemotherapy regimens on survival outcomes, a further statistical analysis was performed on TP group versus NTZ+TP group. The subgroup analysis showed that, compared with the TP group, the median PFS improved non-significantly by 1.2 months in the NTZ+TP group, while the median OS significantly improved by 21.2 months in the NTZ+TP group. These results could provide strong evidence for the treatment of this population. A randomized trial of cetuximab plus carboplatin in the treatment of recurrent/metastatic nasopharyngeal carcinoma published in the Journal of Clinical Oncology,10 showed that cetuximab plus carboplatin, as second-line therapy, seems to be associated with a prolonged survival time compared with third-line therapy or higher. In our study, 61.9% of the patients in the NTZ+CT group were on first-line treatment; this may be the reason for the slightly higher survival rate.
The combination of NTZ-CT is well-tolerated; incremental toxicities with NTZ were minimal. Due to its humanized degree of over 90%, NTZ remarkably reduces human anti-mouse antibody and allergic reactions, unlike CTX toxicities, which include acne-like skin rash, itching, fever, nausea and so on.25 In our study, skin rash was not observed in the patients, and just three patients were observed with mild AEs that were associated with NTZ; these included fever, nausea, and fatigue. NTZ showed a greater advantage in terms of the lower number of toxicities, and this finding is consistent with that of previous studies.
Although the current study demonstrated encouraging survival outcome of NTZ plus platinum-based CT in R/M-NPC, limitations exist. Firstly, our study was retrospective and treatment regimens were not uniform for patients, and thus the potential of selection bias might have existed. Due to the different previous treatment processes for each patient, the choice of treatment regimen was at the discretion of the physicians. By performing the subgroup statistical analysis for the TP group versus NTZ+TP group, we reduced this potential bias as much as we could. Secondly, the sample size was small and the baseline characteristic, gender (p = 0.049), was not balanced between the CT and NTZ+CT groups. This was inevitable, because our study was not conducted in NPC endemic areas and the incidence of NPC is higher in males than in females. Meanwhile, NTZ+CT is not yet included in the medical insurance reimbursement of R/M-NPC patients, and many families can still not afford the high costs of medical NTZ. Thus, few cases of patients receiving NTZ have been identified in the decade since its launch. However, the follow-up duration was adequate and the OS endpoint was sufficiently long. Thirdly, owing to the missing data on EGFR expression in some of the patients, we could not determine whether expression of EGFR was related to the response. However, a phase II study of CTX plus CBP in R/M-NPC reported that there was no obvious association between the level of EGFR expression and best response.10 Perhaps, what is noteworthy is the fact that this retrospective study assessed the role of NTZ in association with CT for R/M-NPC in China.
In summary, NTZ in combination with TP may be a more effective treatment than TP alone in improving OS for patients with R/M-NPC. We believe that the results are important for decision making when choosing a first-line treatment in R/M-NPC, although these findings need to be validated in prospective studies.
Supplemental Material
Supplemental material, Supplementary_materials for Nimotuzumab plus platinum-based chemotherapy versus platinum-based chemotherapy alone in patients with recurrent or metastatic nasopharyngeal carcinoma by Yunshu Zhu, Sheng Yang, Shengyu Zhou, Jianliang Yang, Yan Qin, Lin Gui, Yuankai Shi and Xiaohui He in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to acknowledge the patients, their families, and the medical staff who participated in the study.
Footnotes
Author contributions: Yunshu Zhu: Substantial contributions to the concept and design of the work, acquisition, analysis and interpretation of the data, drafting of the manuscript or critical revision of the manuscript. Sheng Yang: Substantial contributions to the concept and design, analysis of the data and critical revision of the manuscript. Shengyu Zhou, Jianliang Yang, Yan Qin, Lin Gui, Yuankai Shi: Substantial contributions to the concept and design, final approval of the version to be published, and agreement to be accountable for all aspects of the work. Xiaohui He: Substantial contributions to the concept and design or analysis of the data, final approval of the version to be published, agreement to be accountable for all aspects of the work, full access to all the data in the study, and responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Cancer Institute and Hospital, Chinese Academy of Medical Sciences. The funding agency had no role in the design or conduct of the study, the collection, management, analysis, or interpretation of the data, the preparation, review, or approval of the article, or the decision to submit the article for publication.
ORCID iD: Yunshu Zhu  https://orcid.org/0000-0002-7135-969X
https://orcid.org/0000-0002-7135-969X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yunshu Zhu, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
Sheng Yang, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
Shengyu Zhou, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
Jianliang Yang, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
Yan Qin, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
Lin Gui, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
Yuankai Shi, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
Xiaohui He, Department of Medical Oncology, Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 3. Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 2015; 33: 3356–3364. [DOI] [PubMed] [Google Scholar]
- 4. Tan EH, Khoo KS, Wee J, et al. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol 1999; 10: 235–237. [DOI] [PubMed] [Google Scholar]
- 5. Ngan RK, Yiu HH, Lau WH, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol 2002; 13: 1252–1258. [DOI] [PubMed] [Google Scholar]
- 6. Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol 2007; 19: 124–134. [DOI] [PubMed] [Google Scholar]
- 7. Leong JL, Loh KS, Putti TC, et al. Epidermal growth factor receptor in undifferentiated carcinoma of the nasopharynx. Laryngoscope 2004; 114: 153–157. [DOI] [PubMed] [Google Scholar]
- 8. Sung FL, Poon TC, Hui EP, et al. Antitumor effect and enhancement of cytotoxic drug activity by cetuximab in nasopharyngeal carcinoma cells. In Vivo 2005; 19: 237–245. [PubMed] [Google Scholar]
- 9. Gu J, Yin L, Wu J, et al. Cetuximab and cisplatin show different combination effect in nasopharyngeal carcinoma cells lines via inactivation of EGFR/AKT signaling pathway. Biochem Res Int 2016; 2016: 7016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol 2005; 23: 3568–3576. [DOI] [PubMed] [Google Scholar]
- 11. Mazorra Z, Chao L, Lavastida A, et al. Nimotuzumab: beyond the EGFR signaling cascade inhibition. Semin Oncol 2018; 45: 18–26. [DOI] [PubMed] [Google Scholar]
- 12. Talavera A, Friemann R, Gomez-Puerta S, et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res 2009; 69: 5851–5859. [DOI] [PubMed] [Google Scholar]
- 13. Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs 2009; 1: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mesia R, Rivera F, Kawecki A, et al. Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol 2010; 21: 1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan AT, Teo PM, Leung TW, et al. The role of chemotherapy in the management of nasopharyngeal carcinoma. Cancer 1998; 82: 1003–1012. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016; 388: 1883–1892. [DOI] [PubMed] [Google Scholar]
- 17. Ma L, Zhang G, Miao XB, et al. Cancer stem-like cell properties are regulated by EGFR/AKT/beta-catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma. FEBS J 2013; 280: 2027–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma BB, Poon TC, To KF, et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma—a prospective study. Head Neck 2003; 25: 864–872. [DOI] [PubMed] [Google Scholar]
- 19. Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 20. Patil VM, Noronha V, Joshi A, et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer 2019; 125: 3184–3197. [DOI] [PubMed] [Google Scholar]
- 21. Garrido G, Tikhomirov IA, Rabasa A, et al. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther 2011; 11: 373–382. [DOI] [PubMed] [Google Scholar]
- 22. Berger C, Krengel U, Stang E, et al. Nimotuzumab and cetuximab block ligand-independent EGF receptor signaling efficiently at different concentrations. J Immunother 2011; 34: 550–555. [DOI] [PubMed] [Google Scholar]
- 23. Yuan C, Xu XH, Xu L, et al. Cetuximab versus nimotuzumab for the treatment of advanced nasopharyngeal carcinoma: a network meta-analysis. J BUON 2017; 22: 1004–1010. [PubMed] [Google Scholar]
- 24. Zhao C, Miao J, Shen G, et al. Anti-epidermal growth factor receptor (EGFR) monoclonal antibody combined with cisplatin and 5-fluorouracil in patients with metastatic nasopharyngeal carcinoma after radical radiotherapy: a multicentre, open-label, phase II clinical trial. Ann Oncol 2019; 30: 637–643. [DOI] [PubMed] [Google Scholar]
- 25. Fury MG, Sherman E, Lisa D, et al. A randomized phase II study of cetuximab every 2 weeks at either 500 or 750 mg/m2 for patients with recurrent or metastatic head and neck squamous cell cancer. J Natl Compr Canc Netw 2012; 10: 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_materials for Nimotuzumab plus platinum-based chemotherapy versus platinum-based chemotherapy alone in patients with recurrent or metastatic nasopharyngeal carcinoma by Yunshu Zhu, Sheng Yang, Shengyu Zhou, Jianliang Yang, Yan Qin, Lin Gui, Yuankai Shi and Xiaohui He in Therapeutic Advances in Medical Oncology


