Summary
Multipotent ΔNp63-positive cells maintain all epithelial cell lineages of the embryonic and adult salivary gland (SG). However, the molecular mechanisms by which ΔNp63 regulates stem/progenitor (SP) cell populations in the SG remains elusive. To understand the role of ΔNp63 in directing cell fate choices in this gland, we have generated ΔNp63-deleted adult mice and primary salivary cell cultures to probe alterations in SP cell differentiation and function. In parallel, we have leveraged RNA-seq and ChIP-seq-based characterization of the ΔNp63-driven cistrome and scRNA-seq analysis to molecularly interrogate altered SG cellular identities and differentiation states dependent on ΔNp63. Our studies reveal that ablation of ΔNp63 results in a loss of the SP cell population and skewed differentiation that is mediated by Follistatin-dependent dysregulated TGF-β/Activin signaling. These findings offer new revelations into the SP cell gene regulatory networks that are likely to be relevant for normal or diseased SG states.
Subject Areas: Cell Biology, Developmental Biology, Stem Cells Research
Graphical Abstract

Highlights
-
•
Loss of ΔNp63 expression results in a block in salivary gland cell differentiation
-
•
p63 maintains salivary gland stem and progenitor cell proliferation
-
•
p63 acts upstream of TGF-β/Activin signaling by directly regulating Fst expression
Cell Biology; Developmental Biology; Stem Cells Research
Introduction
Salivary glands (SGs) are exocrine glands whose primary function is to secrete saliva and thereby ensure sufficient lubrication to the oral cavity, which is necessary for proper speech, swallowing, mastication, and digestion of food and for maintaining overall oral health. In humans and rodents, saliva is generated by three major pairs of glands, the parotid (PG), sublingual (SLG), and the submandibular glands (SMG). All three glands share a common structural architecture and comprise secretary units composed of acinar cells, which produce and secrete the saliva into the oral cavity via an intricate and extensive ductal network (Amano et al., 2012). Surrounding the acini and intermingled within the basal cells are myoepithelial cells, a specialized cell type with contractile properties (Tucker, 2007). Irreversible loss of salivary secretion, also known as hyposalivation, is commonly associated with autoimmune diseases such as Sjögren's syndrome, from γ-irradiation therapy used to treat patients with oral cancers, aging, and developmental disorders. Indeed, patients suffering from hyposalivation, which leads to dry mouth, are predisposed to oral infections and have difficulty in speaking, chewing, and swallowing food, all of which can reduce their overall quality of life (Harunaga et al., 2011). Currently, there are no curative treatment options for these patients. This has spurred renewed interest in developing new strategies aimed at restoring SG function using stem cell-based approaches (Pringle et al., 2016; Nanduri et al., 2014). However, our current understanding of the basic physiological mechanisms and the transcriptional networks regulating stem/progenitor (SP) cell self-renewal, proliferation, and differentiation programs in the SG remains elusive.
p63, specifically the ΔNp63 isoform, is a lineage-specific master transcription factor that is highly expressed in epithelial-rich tissues and organs where it is plays important roles in stem cell self-renewal, cell fate decisions and lineage commitment, morphogenesis, and directing differentiation programs (Romano et al., 2012; Fan et al., 2018; Chakravarti et al., 2014; Chakrabarti et al., 2014; Senoo et al., 2007; Packard et al., 2011; Blanpain and Fuchs, 2007; Kumar et al., 2019). Interestingly, these studies have unearthed important p63-driven signaling pathways that direct stem cell fate choices in various stratified epithelial tissues and glandular secretory organs. Indeed, p63 has been shown to act upstream of a number of key signaling pathways including Wnt, Notch, and Egf in both the skin and mammary gland (Forster et al., 2014; Chakrabarti et al., 2014; Romano et al., 2012; Fan et al., 2018). In the SG, ΔNp63 is expressed in the epithelial cells of the developing placode, where it plays a critical role as ΔNp63-null animals display a complete block in gland morphogenesis (Romano et al., 2012). The contribution of ΔNp63 in actively directing SP cell fate choices in the SG is further highlighted by recent genetic lineage tracing studies, which demonstrated that p63+ve cells give rise to and maintain all the epithelial cell lineages in both embryonic and adult glands (Song et al., 2018). Although the role of ΔNp63 in SG development has been established, our understanding of the molecular function of this regulator in directing the intrinsic signals that define SG SP cell identity, self-renewal, maintenance, and differentiation are not well understood.
Transforming growth factor β (TGF-β) superfamily signaling has been implicated in driving developmental programs, cellular proliferation, and differentiation (Wu and Hill, 2009; Massague, 2012). Signaling by this superfamily has also been shown to play a prominent role in directing SP cell fate choices important for tissue homeostasis and regeneration (Lepletier et al., 2019; Mou et al., 2016; Suzuki et al., 2017). TGF-β signaling comprises TGF-β, bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), Activins, Nodal, and anti-Mullerian hormone (AMH) (Oshimori and Fuchs, 2012). Activins, considered evolutionarily to be the most ancient of the TGF-β signaling family members, signal through the type II Activin receptors and exert their function through the downstream effectors, phosphorylated Smad2 and Smad3. Moreover, in stratified epithelia including that of the skin, airway, and mammary gland, TGF-β/BMP/SMAD pathway signaling has been shown to be active in the differentiated luminal and suprabasal cells while being suppressed in the undifferentiated basal cells of these tissues (Mou et al., 2016; Suzuki et al., 2017). Although the role of TGF-β as a multifunctional cytokine that influences branching morphogenesis and extracellular matrix (ECM) deposition in the SG is well established, its role in directing SP cell fate choices and the identity of key upstream transcriptional regulators remains elusive.
To better investigate the function of p63 in directing cell fate choices in the murine submandibular salivary gland (SMG), we have generated animals with targeted deletion of ΔNp63 in adult glands. We demonstrate that ablation of ΔNp63 results in aberrant epithelial differentiation, which is accompanied by a dramatic loss to the SP cell population. To dissect the distinct p63-driven regulatory circuitry through which this transcription factor functions to maintain this important cell type, we have performed transcriptomic (RNA sequencing) and single cell RNA sequencing (scRNA-seq) of ΔNp63-null SGs. Integrated analysis of our transcriptomic studies reveals that ΔNp63 maintains the proliferative capacity and differentiation potential of SG epithelial SP cells by regulating TGF-β signaling. Moreover, by combining our transcriptomic and epigenomic (ChIP-seq) studies, we find that ΔNp63 directly regulates the expression of Follistatin, a key modulator of the Activin/SMAD signaling network, to maintain the proliferative state of SG SP cells. Taken together, our data demonstrate that the transcription factor ΔNp63 functions as an upstream regulator of the TGF-β signaling pathway governing the proliferative and differentiation programs in SG epithelial SP cells.
Results
ΔNp63 Deletion Results in Alterations to the Salivary Gland Epithelial Cell Differentiation Program
In the SG, ΔNp63 expression is restricted to the basal and myoepithelial cells where ΔNp63+ve cells have been shown to give rise to and maintain all the epithelial cell lineages in embryonic and adult glands (Song et al., 2018). To examine the role of ΔNp63 in adult SG maintenance, we crossed ΔNp63fl/fl mice to a transgenic strain that ubiquitously expresses Cre-recombinase fused to the estrogen-ligand binding domain ERT2 (UBCCreERT2). This inducible conditional mouse model system allowed for overcoming the perinatal lethality associated with loss of p63 and also for the excision of ΔNp63 in both the basal and myoepithelial cell populations as shown in other organs such as the skin and mammary glands (Kumar et al., 2019; Chakravarti et al., 2014). Tamoxifen (TAM) was administered to adult ΔNp63fl/fl (control) and UBCCreERT2;ΔNp63fl/fl (ΔNp63KO) mice, and SGs were harvested 8–10 days post TAM administration and analyzed. This time line was chosen since the ΔNp63KO animals appeared slightly smaller and leaner and exhibited some hair loss compared with control mice (Figure 1A). Loss of ΔNp63 expression in the SG was verified at both the protein and mRNA levels (Figures 1B and 1C). We next assessed for gross effects of the loss of ΔNp63 on the SMG by measuring salivary gland weight. Interestingly, we found a reduction in the weights of both male and female knockout glands compared with the controls (Figure 1D). Histological analysis of hematoxylin and eosin (H&E)-stained paraffin-embedded SMGs in both male and female mice revealed a dramatic reduction in ductal size in the SMGs of ΔNp63KO mice when compared with control and ΔNp63 heterozygous (ΔNp63Het) animals (Figures 1E and S1A). The observed phenotype in the ducts was accompanied by alterations to the acinar cells, which appeared enlarged in the ΔNp63KO as compared with control and ΔNp63Het mice (Figures 1E and S1A). Indeed, further quantification analysis comparing the duct and acini cell areas confirmed our findings (Figures S2A and S2B). To better define the overall cellular nature of the phenotypic changes resulting from the loss of ΔNp63, we performed immunofluorescence studies and examined both male and female KO SMGs utilizing a battery of well-established epithelial cell markers. Evaluation of the progenitor cell markers Keratin 5 (K5) and K14, which are restricted to the basal and myoepithelial cell populations in control mice, revealed a dramatic reduction in protein expression levels in SMGs of the ΔNp63KO mice, suggesting a loss to the progenitor cell populations (Figures 1F and S1B). In addition, in the ΔNp63KO mice, we observed reduced protein expression levels of α-smooth muscle actin (Sma), which is primarily expressed in the myoepithelial cells of the SG (Figures S3A and S3B). In agreement with our histological analysis, we observed reduced expression levels of the water channel protein aquaporin 5 (Aqp5) and the salivary enzyme amylase 1 (Amy1), in the ΔNp63KO glands compared with the control (Figures 1F and S1B). Interestingly, we did not observe any differences in the expression of Na+/K+/2Cl− co-transporter (Nkcc1), mucin10 (Muc10), or the transcription factor Mist1, all of which are specifically and uniquely enriched in the acinar cells (Figures S3A and S3B). Similarly, we did not detect alterations to the expression pattern of the granular convoluted ductal markers mucin13 (Muc13) or K7 in the glands of the ΔNp63KO mice (Figures 1F and S1B) (Amano et al., 2012). However, K19 expression, which is typically localized to the striated and excretory ducts, was dramatically reduced in the mutant glands compared with the control (Figures 1F and S1B). Since our immunofluorescence analysis did not reveal any significant alterations to the ductal cell differentiation program that could account for the dramatic decrease in overall duct size and individual ductal cell size in the ΔNp63KO mice (Figures 1F, S1B, S2, and S4A), we assessed whether these changes were driven by proliferation defects. Although the KO glands revealed a modest reduction in proliferation based on expression of the proliferation marker Ki67, we did observe a significant increase in apoptosis as demonstrated by the elevated numbers of ductal cells that stained positive for the apoptosis marker cleaved caspase-3 (Casp3) as compared with control glands (Figures S4B and S4C). Interestingly, this was accompanied by reduced salivary production in the KO glands (Figure S4D). As expected, we did not detect expression of ΔNp63 in the SMGs of the KO mice, confirming deletion of the ΔNp63 allele (Figure S1 and S3). To confirm our findings in an independent mouse model system, and to ensure that the phenotype we observed was not due to the broad effects of UBC-Cre-ERT2, inefficient Cre-mediated recombination, or secondary effects resulting from the global loss of ΔNp63, we crossed ΔNp63fl/fl mice to a transgenic strain expressing Cre-ERT2 under the control of the Trp63 (Trp63CreERT2) gene locus (Lee et al., 2014). Analogous to the phenotype observed in the UBCCreERT2;ΔNp63fl/fl KO mice, the Trp63CreERT2;ΔNp63fl/fl KO animals demonstrated similar histological and cellular alterations to the SG differentiation program (Figure S3C). Given that two independently generated ΔNp63KO strains displayed similar phenotypes regardless of sex, all subsequent studies were performed with UBCCreERT2;ΔNp63fl/fl animals.
Figure 1.
Histological and Immunochemical Analysis of Submandibular Salivary Glands of Mice with Targeted Deletion of ΔNp63
(A) Gross morphology of ΔNp63KO and control animals.
(B) Western blot analysis demonstrates reduced protein expression levels of ΔNp63 in SMGs of ΔNp63KO mice compared with controls.
(C) Quantitative RT-PCR analysis showing ΔNp63 mRNA expression levels in the SMGs of control and KO mice.
(D) Weight of submandibular salivary glands from control and ΔNp63KO male and female mice.
(E) H&E staining of control, ΔNp63 heterozygous (ΔNp63Het), and ΔNp63KO male SMGs. Compared with control and ΔNp63Het glands, KO mice show reduced ductal size along with enlarged acini. Scale bar: 200 μm.
(F) Immunofluorescence staining of male control and ΔNp63KO glands confirm the loss of ΔNp63 expression as well as a dramatic reduction in the K5 and K14 stem/progenitor cell population. Alterations to the acinar cell differentiation program is also observed in the KO glands as evident by the loss of Amy1 expression. K7 and Muc13 expression levels appear unchanged in the ducts of the mutant glands, whereas K19 expression is reduced. Scale bar: 37.5μm.
Data are represented as mean ± SD (n = 3). ∗∗∗p < 0.001. See also Figures S1–S4.
Perturbations in Transcriptional and Signaling Networks upon the Loss of ΔNp63
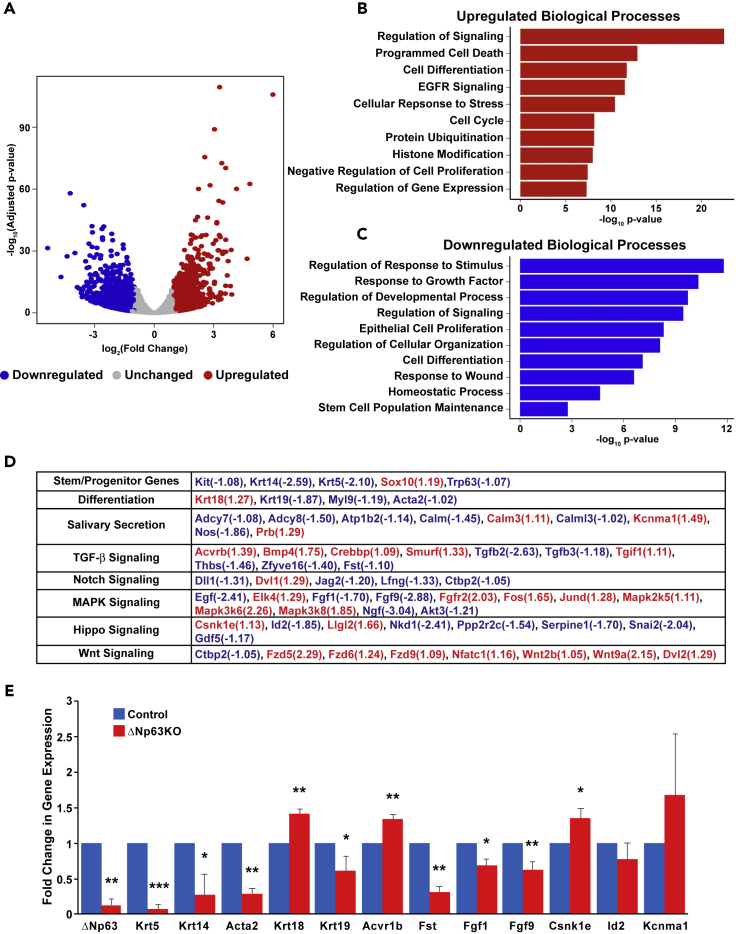
To probe the effects of the loss of ΔNp63 in adult SGs, next we compared the transcriptomic profiles of the control and ΔNp63KO SMGs by RNA-seq. Our analysis identified ∼2,800 differentially expressed genes (DEGs) between the control and KO glands, with 1,285 genes upregulated and 1,555 genes showing downregulation (Figure 2A and Table S1). Gene ontology analysis of the DEGs revealed enrichment of genes associated with Regulation of Signaling, Programmed Cell Death, and Cell Cycle (Figure 2B). In contrast, downregulated genes were associated with Epithelial Cell Proliferation, Cell Differentiation, and Stem Cell Population Maintenance (Figure 2C). Indeed, genes commonly associated with SP cell function were downregulated, as were genes involved in SG differentiation and salivary secretion (Figure 2D). Moreover, crucial players involved in the TGF-β, Notch, MAPK, Hippo, and Wnt signaling were also significantly altered upon the loss of ΔNp63 (Figure 2D). These findings are in line with other studies of gene expression changes that are unleashed upon the loss of p63 (Fan et al., 2018; Chakrabarti et al., 2014; Romano et al., 2012). We further confirmed the expression of a subset of key genes that were altered upon the loss of ΔNp63 expression by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR), thereby validating our RNA-seq results (Figure 2E). Taken together, these results reveal the global changes in the transcriptome, as well as alterations to various signaling pathways accompanying the loss of ΔNp63 in SMGs.
Figure 2.
ΔNp63-Driven Transcriptomic Changes in the Adult Salivary Gland
(A–C) (A) Volcano plot shows the distribution of statistically significant DEGs in the SMG upon deletion of ΔNp63. Upregulated genes are depicted in red and downregulated genes are shown in blue. Bar plots highlighting the top enriched upregulated (B) and downregulated (C) biological processes.
(D) Transcriptomic changes in control as compared with ΔNp63KO SMG. Red indicates upregulated genes and blue represents downregulated genes.
(E) Quantitative RT-PCR validation of selected genes in control and ΔNp63KO SMGs. Values were normalized to the housekeeping gene Hprt. Data are represented as mean ± SD (n = 3).∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
ΔNp63 Maintains Stem/Progenitor Cell Activity
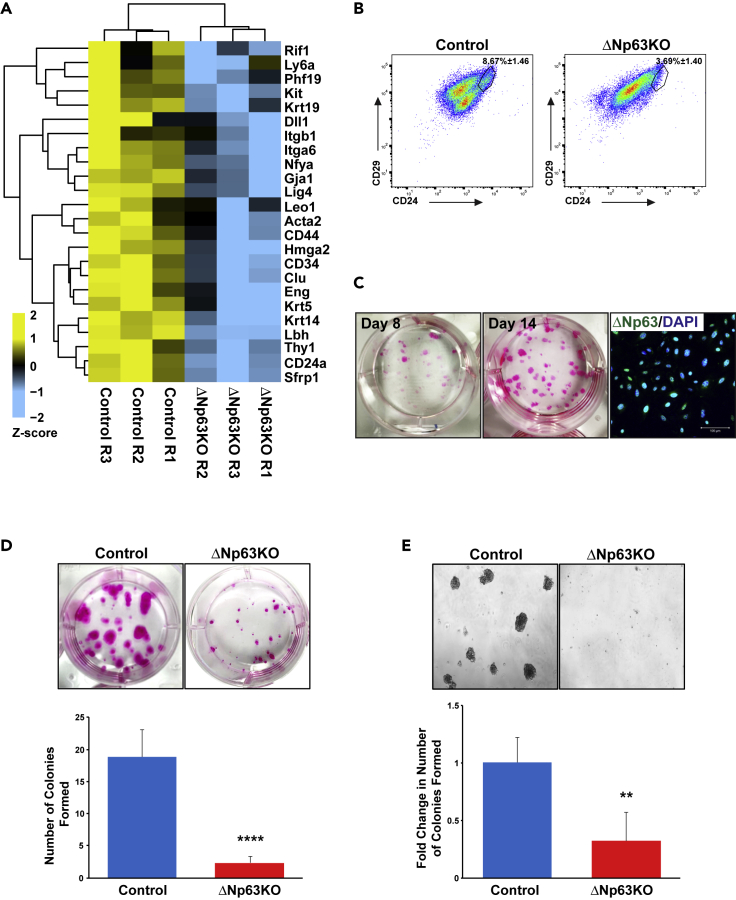
Given that our transcriptomic analysis revealed downregulation of genes involved in stem cell biology in the KO glands compared with the control, we next sought to explore the functional importance of ΔNp63 in SP cell activity. Indeed, mining of our RNA-seq datasets revealed downregulation of a number of well-established SG SP cell markers (Figure 3A) (Pringle et al., 2013). To further investigate the status of the SP cell populations, we performed fluorescence-activated cell sorting (FACS). As expected, our FACS analysis revealed a dramatic loss to the CD24hi/CD29hi SP cell population (Figure 3B) in the ΔNp63KO glands compared with the control animals (Nanduri et al., 2011, 2014). We next sought to examine the function of ΔNp63 in maintaining the clonogenic properties in salivary gland epithelial progenitor cells. In order to address this, we first established a system to support the clonal growth of salivary gland epithelial progenitor cells. Toward this end, primary salivary gland epithelial cells isolated from adult wild-type mice were grown in CnT-PR media, a commercially available basal media commonly used to grow progenitor cells from diverse epithelial tissues (Suzuki et al., 2017). Indeed, we found that, after 2 weeks of culture, primary salivary gland epithelial cells readily formed clones (Figure 3C). Importantly, a large percentage of the cells within the epithelial clones expressed high levels of ΔNp63 despite being derived from adult salivary glands in which only 5%–6% of the total epithelial cells are ΔNp63+ve (Figure 3C) (Song et al., 2018). Having established this clonogenic assay for primary salivary gland epithelial progenitor cells (pSEPCs) we next investigated the effects of loss of ΔNp63 on clonogenic potential. Toward this end, pSEPCs isolated from control and ΔNp63KO mice were grown in culture and clonal growth was assessed based on the number of colonies formed over time (Suzuki et al., 2017). Indeed, after 14 days we observed a dramatic loss in the clonogenic capabilities of pSEPCs isolated from the ΔNp63KO mice, compared with control animals (Figure 3D). In parallel we also compared the ability of isolated pSEPCs to form colonies as three-dimensional spheroid structures when grown on a 3D Matrigel-based matrix. By day 14, control pSEPCs formed larger salispheres consisting of tightly packed cells, whereas ΔNp63KO cells yielded significantly fewer and smaller sized spheres (Figure 3E). Overall, these results are consistent with a primary role for ΔNp63 in maintaining the proliferative potential of primary salivary gland epithelial progenitor cells, similar to what has been reported in progenitor cells from other epithelial tissues (Senoo et al., 2007; Chakrabarti et al., 2014).
Figure 3.
ΔNp63 Maintains the Proliferative Potential of Salivary Gland Epithelial Progenitor Cells
(A) Heatmap visualization of selected DEGs corresponding to a panel of well-established salivary gland stem/progenitor cell markers in control and KO SMGs (Pringle et al., 2013).
(B) Representative FACs profiles of CD24hi/CD29hi SP cells from control and ΔNp63KO mice.
(C) Representative images of Rhodamine B staining of pSEPCs clones grown at indicated time points. Right panel shows 14-day clones stained with an anti-ΔNp63 antibody showing enrichment of ΔNp63+ve epithelial cells in the clones. Scale bar: 100 μm.
(D) Representative images of Rhodamine B staining of pSEPCs clones grown for 14 days from control and KO animals. Bottom panel is a quantification of the number of colonies formed in control as compared with ΔNp63KO mice.
(E) Representative images of control and ΔNp63KO pSEPCs 3D-spheroids grown on matrigel.
Data are represented as mean ± SD (n = 4). ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Single-Cell Analysis Reveals the Requirement of ΔNp63 in Maintaining the Stem/Progenitor Cell Population in the Salivary Gland
To obtain a more detailed understanding of the cellular states and further interrogate the role of ΔNp63 in the adult SG at single-cell resolution, we performed scRNA-seq analysis of control and ΔNp63KO adult SMGs. We first established the global structure of the dataset by performing unsupervised clustering with affinity propagation based on the expression of high variance genes in the control animals (Frey and Dueck, 2007). This analysis identified seven distinct groups of epithelial cells, which we visualized via uniform manifold approximation and projection (UMAP) (Figure 4A). Cell type assignments were made based on the expression of known/validated marker genes similar to what we and others have previously reported in both the SMG and PG (Table S2) (Song et al., 2018; Oyelakin et al., 2019; Sekiguchi et al., 2020). Integrated analysis of control and ΔNp63KO datasets revealed striking differences between the various cell populations (Figure 4B). For example, compared with the control cells, ΔNp63KO cells revealed reduced numbers of cells that comprised the acinar clusters (C2 and C6) (Figure 4B). Although this finding was surprising given that ΔNp63 expression was not detected in the acinar cells, it does correlate well with the reduced Amy1 protein expression levels we observed in our immunofluorescence analysis, confirming alterations to this cell lineage in the absence of ΔNp63 (Figures 1F and S1B). Conversely, in the KO glands we observed increased numbers of cells in cluster C1, representing ductal cells, and cluster C5, which represent mixed basal and ductal cell lineages (Figure 4B). These differences are further highlighted in Figure 4C in which the top 100 DEGs across all clusters between the control and KO glands are shown (Figure 4C and Table S3). A subset of DEGs are shown in Figure 4D. Our analysis also revealed reduced numbers of basal cells represented in C3 and C7 in the ΔNp63KO glands, which is in good agreement with previous studies demonstrating a role for p63 in directing basal specific keratin gene expression and in directing epidermal cell fate specification in the skin (Figures 4B and 4D) (Romano et al., 2009; Fan et al., 2018).
Figure 4.
Single-Cell RNA Sequencing Analysis Reveals ΔNp63-Dependent Cellular States
(A) UMAP visualization of the various epithelial cell populations in control adult mouse SMG.
(B) UMAP projection overlays of epithelial cell populations from the control and ΔNp63KO adult SMG.
(C) Heatmap depicts the top 100 differentially expressed genes in each cluster as compared with all clusters between control and KO SMGs. Upper bars represent cluster assignments.
(D) Dot plot of proportion of cells in each cluster expressing each marker (dot size), and average expression (color scale) of known basal (Trp63, Krt5, Krt14 and Fst), ductal (Krt17, Krt7, Krt8, Muc13), and acinar (Aqp5, Bhlha15, Prol1) genes in control mice compared with KO. C, cluster; UMAP, uniform manifold approximation and projection.
In order to probe further into the ΔNp63-driven mechanisms underlying the cellular changes in the SG, we focused on cluster 7 of the control mice for further analysis. We reasoned that, since this cell population demonstrated the highest expression levels of Trp63 mRNA as compared with C3, it likely represented the SP cell population (Figure 4D). This notion is based on previous studies that have shown that p63 expression is highest in epithelial cells with high clonogenic potential, whereas low levels of p63 expression is associated with reduced proliferative capacity (Senoo et al., 2007; Pellegrini et al., 2001). With this in mind, we mined our scRNA-seq dataset and performed Gene Ontology (GO) analysis using the genes enriched in cluster C7 of the control glands (Figures 4A and Table S2). Indeed, our analysis identified overrepresentation of genes associated with biological pathways that included negative regulation of epithelial cell differentiation, gland morphogenesis, and regulation of epithelial cell proliferation (Figure S5). We also observed specific enrichment in somatic stem cell population maintenance and tissue regeneration, pointing to the possibility that this cluster may represent an SP cell population (Figure S5). Having identified negative regulation of cellular response to TGF-β stimulus as one of the top enriched biological pathways, we decided to focus our attention on the TGF-β signaling pathway. This was spurred by previous reports demonstrating that inhibition of TGF-β signaling leads to increased proliferative capacity of p63+ve epidermal progenitor cells (Mou et al., 2016; Suzuki et al., 2017). Interestingly, further mining of the bulk RNA-seq datasets revealed elevated mRNA expression levels of a number of genes involved in TGF-β signaling in the ΔNp63KO mice, suggesting that the loss of ΔNp63 expression results in activation of this signaling pathway (Figure S6). A possible explanation that may account for the aberrant activation of TGF-β signaling in the KO mice may be due to altered expression levels of several molecular players important in Activin signaling including activin receptors Acrv1 and Acrv1b as well as Activin downstream effectors Smad2 and Smad3 (Figure S6). Follistatin (Fst) is a soluble extracellular protein that acts as a key inhibitor of Activin signaling. Fst exerts its function by binding to circulating activin and preventing it from binding to its receptor and activating a downstream signaling cascade that results in phosphorylation and activation of Smad2/3. Indeed, we identified Fst as one of the top downregulated genes in the KO glands, similar to what we have previously reported in the skin epidermis of ΔNp63KO mice (Figure S6) (Fan et al., 2018). Although Fst has been shown to play key roles in skin and tooth morphogenesis and in maintaining thymic epithelial progenitor cells in an undifferentiated state, its role in the SMG has not been investigated (Lepletier et al., 2019; Matzuk et al., 1995; Nakamura et al., 2003).
ΔNp63 Mediates Salivary Gland Stem/Progenitor Cell Function by Directly Regulating Follistatin Expression
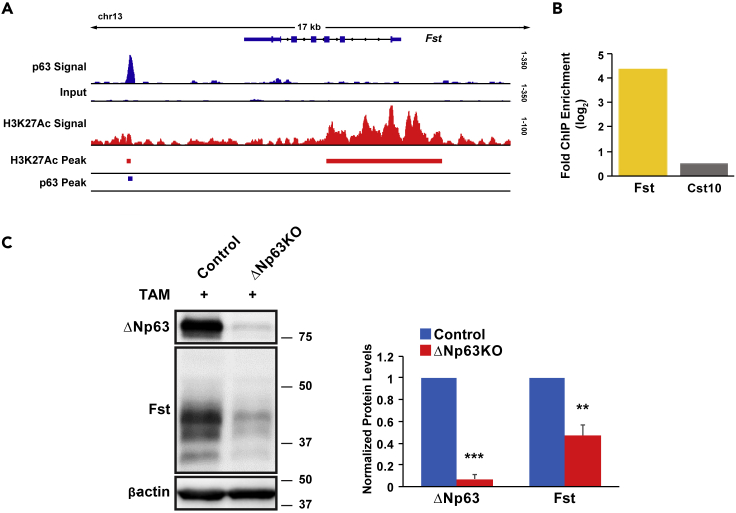
The dramatic downregulation of Fst mRNA gene expression evident from bulk RNA-seq and scRNA-seq data (Figures S6 and 4D), and confirmed by qRT-PCR (Figure 2E), prompted us to examine the in vivo expression profile of ΔNp63 and Fst in the SG and to test if Fst might be a direct transcriptional target of ΔNp63. Toward this end, we first sought to determine whether these two factors were co-expressed in the SMG, by performing RNAscope analysis. As expected, we found Trp63 and Fst mRNA to be co-expressed in the basal cells of the adult mouse SMG, similar to what has been reported in the murine skin epidermis (Figure S7) (Nakamura et al., 2003). This was further supported by our scRNA-seq studies, which revealed specific enrichment of Trp63 and Fst in cluster C7 (Figure 4D). Having established the co-localization of Trp63 and Fst, we next performed ChIP-seq experiments in pSEPCs with anti-p63 and anti-H3K27Ac antibodies to identify global ΔNp63-binding events and enhancers, respectively. Our ChIP-seq studies identified a large cohort of ∼4,000 ΔNp63 target genes and importantly revealed a ΔNp63-binding region downstream of the Fst genomic locus, suggesting that Fst is a direct transcriptional target of ΔNp63 (Figure 5A). Moreover, the ΔNp63-bound regulatory region represented an active enhancer site as demonstrated by enriched H3K27Ac peaks (Figure 5A). To confirm our ChIP-seq results, we performed an independent ChIP on mouse pSEPCs followed by quantitative-polymerase chain reaction (qPCR) quantification. To ensure the validity and specificity of our ChIP studies, we included an intragenic region within the Cst10 gene, which does not contain a p63 response element, to serve as a negative control. Indeed, our qPCR results demonstrated selective enrichment of the putative p63 response element in the Fst genomic locus relative to the control region (Figure 5B). Next, to test the functional consequences of p63 binding to this potential regulatory element we performed p63 knockdown (KD) experiments. Primary salivary gland epithelial cells isolated from control and ΔNp63KO mice were treated with activated TAM to specifically KD ΔNp63 expression. Indeed, western blot analysis revealed reduced protein expression levels of ΔNp63 and Fst in the ΔNp63KO cells as compared with control cells (Figure 5C). Taken together, these data strongly suggest that ΔNp63 can directly regulate the expression of Fst, which may account for the aberrant activation of Activin signaling leading to reduced proliferative potential of SG SP cells upon the loss of p63 expression.
Figure 5.
Direct Regulation of Fst Expression by ΔNp63
(A) Visualization of ΔNp63 interaction with the target gene Fst (Integrated Genomics Viewer). Top two lines display p63 ChIP signal enrichment, relative to Input, located at an enhancer near the Fst gene locus in pSEPCs. Third line displays H3K27Ac ChIP signal around the Fst genomic locus. Bottom two lines depict the p63 binding site (black box) and active enhancers (red boxes) within the pSEPCs.
(B) Independent ChIP qPCR results using a ΔNp63 antibody in pSEPCs confirms specific binding of p63 to the Fst genomic locus. Cst serves as a negative control. Values represent mean fold enrichment over the random genomic locus (Cst).
(C) Representative western blot analysis of control and ΔNp63KO pSEPCs treated with TAM reveals robust knockdown of ΔNp63 and Fst protein expression levels in the KO cells compared with control. ΔNp63 and Fst protein expression were normalized to β-actin. Quantification is shown on the right. Data are represented as mean ± SD (n = 3). ∗∗p < 0.01, ∗∗∗p < 0.001.
See also Figure S7.
Prior studies have demonstrated a role for Fst in maintaining thymic epithelial progenitor cells in an immature state (Lepletier et al., 2019). To test if Fst functions similarly in the SP cells of the SG we performed clonogenic assays in primary pSEPCs treated with increasing concentrations of Fst. Interestingly, addition of Fst resulted in increased clonogenic potential compared with control non-Fst treated cells (Figure S8). Having established a role for Fst in maintaining clonogenicity in pSEPCs, we next wondered if the addition of Fst could rescue the reduced clonogenic properties observed upon the loss of ΔNp63. To address this, we performed clonogenic assays on pSEPCs isolated from ΔNp63KO mice that were treated with TAM in the presence or absence of Fst. Indeed, compared with TAM-treated cells alone, pSEPCs treated with both TAM and Fst resulted in increased clonogenic capabilities suggesting that Fst is able to compensate for the loss of ΔNp63 expression and maintain the proliferative potential of SG SP cells (Figure 6A).
Figure 6.
Fst is a Key Modulator of Activin Signaling in Salivary Gland Epithelial Progenitor Cells
(A) Representative images of Rhodamine B staining of ΔNp63KO pSEPCs clones treated with TAM to KD ΔNp63 expression in the absence or presence of Fst. Clones were grown for 14 days. Fst is able to rescue the clonogenic defects observed upon the loss of ΔNp63 expression.
(B) Western blot showing the effects of Fst on Activin signaling in pSEPCs. Treatment with Activin A results in Smad2 phosphorylation, whereas addition of Fst attenuates phosphorylation (compare lanes 2 and 3). Smad 2, Smad 3, and pSmad2 protein expression levels were normalized to β-actin. A quantification is shown on the right.
Data are represented as mean ± SD (n = 3) ∗p < 0.05. See also Figures S8 and S9.
The Fst/Activin/Smad Signaling Axis Maintains the Clonogenic Potential of Salivary Gland Stem/Progenitor Cells
Having established the effects of Fst on the clonogenic properties of the SP cells in the SG, we next sought to investigate the mechanism through which Fst exerts its downstream effects. We first investigated the phosphorylation state of the various Smad proteins as a readout of BMP and activin signaling in the pSEPCs. Interestingly, western blot analysis of p-Smad1/5/8 revealed that BMP signaling was not active in these cells under normal growth conditions (Figure S9A). However, when pSEPCs were treated with increasing concentrations of BMP, increased Smad1/5/8 phosphorylation was observed (Figure S9A). In contrast, Activin signaling was constitutively active since we observed robust expression of pSmad2, which increased when pSEPCs were treated with increasing concentrations of Activin A (Figure S9B). In contrast, we did not detect expression of pSmad3. In light of these results, we focused our attention on Activin signaling. In order to assess the functional impact of Fst on Activin signaling, we treated pSEPCs with Activin A, which activated the pathway as demonstrated by increased pSmad2 expression levels as compared with untreated cells (Figure 6B left panel, compare lane 1 and lane 2). We next treated the cells with Activin A in the presence of Fst and determined the effects of Fst on Activin signaling. Indeed, the addition of Fst resulted in the attenuation of Activin A signaling as shown by reduced pSmad2 protein expression levels (Figure 6B left panel, compare lane 2 and lane 3). Overall, these data suggest a role for Fst in driving the clonogenic potential of SG SP cells by modulating Activin A signaling.
Discussion
ΔNp63 is highly enriched in the stem/progenitor cell populations of epithelial-rich tissues including the SG, where it plays indispensable roles in maintaining proliferative potential as well as directing lineage commitment and cell fate choices (Blanpain and Fuchs, 2007; Senoo et al., 2007). Although previous work has focused on epithelial tissues including those of the skin, mammary gland, and prostate, mechanistic studies on the SMG have been lacking. Here we have provided a comprehensive analysis of the effects of ablation of the transcription factor ΔNp63 in the SMG of adult mice. We find that loss of ΔNp63 expression in the basal/myoepithelial cells of adult mice resulted in wide-ranging effects including altered ductal and acinar cell differentiation and impaired SP cell function leading to the eventual loss of this important cell population.
One surprising yet interesting observation was the broad differentiation defects in the SG due to ΔNp63 loss such as those of the acinar and ductal cells, which do not express ΔNp63. These far-reaching effects of ΔNp63 fit well with results from our recent genetic lineage tracing experiments, which demonstrated that ΔNp63+ve basal cells serve as predecessor stem cells to several different cellular subpopulations. Remarkably, the cell fate transition process of ΔNp63+ve basal cells under homeostatic conditions is relatively rapid as the ΔNp63-derived cells are detected in the ductal luminal cells of the SMG in as little as 1 week (Song et al., 2018). Our findings are in line with genetic lineage tracing experiments performed in salivary glands of adult mice showing that the transition of actively proliferating K14+ basal cells to both the ductal intercalated and excretory luminal cells of the gland occurs fairly quickly—within 14 days (Kwak et al., 2016; Kwak and Ghazizadeh, 2015). Moreover, the wide-spread defects observed upon the loss of ΔNp63 in the SG are also in good agreement with similar studies reported in the mammary gland (Forster et al., 2014; Kumar et al., 2019). Interestingly, conditional deletion of ΔNp63 in the pubertal mammary gland results in aberrant ductal formation presumably owing to altered stem cell differentiation (Kumar et al., 2019). Moreover, ΔNp63 has also been shown to be important during pregnancy as ΔNp63KO mice showed defects in luminal progenitor cell proliferation and differentiation (Forster et al., 2014). These animals displayed a complete block in the development of milk-producing alveolar cells, which are functionally similar to the saliva-secreting acinar cells of the SG. The mechanism through which p63 directs luminal progenitor cell differentiation and alveologenesis in the mammary gland involves paracrine signaling and activation of the Nrg1/Erbb4/Stat5a signaling axis, whereas this is unlikely to be the case in the SG as components of this signaling pathway are not expressed in this organ (Table S1). The physiological defects that stem from maturation deficiency of the alveolar cells of the mammary gland and the acinar cells of the SG suggests that ΔNp63 may play a broader and pervasive role in modulating the secretory cell outputs of glandular tissues. Given the importance of acinar cells and the effects of loss of salivary secretion in diseases such as Sjögren's syndrome or due to off-target effects of γ-irradiation therapy in patients with oral cancers, identifying the molecular mechanisms through which p63 acts on this cell population warrants further investigation.
Although our analysis has revealed a number of significant alterations to acinar and ductal cell differentiation, one of the most striking observations were the dramatic effects to the SP cells, which included not only reduced proliferative and clonogenic potentials but also a distinct loss of this important cell population. Although these results are in good agreement with similar observations that have been reported in the thymus, skin, and mammary gland, where p63 has been shown to maintain stem cell “stemness,” the underlying molecular mechanisms through which ΔNp63 maintains the proliferative and self-renewal states of this cell type has been lacking. Recent studies have suggested a link between p63 and the TGF-β superfamily signaling pathway in maintaining the proliferative potential of epithelial progenitor cells. Indeed, Mou et al. have shown that TGF-β/BMP/SMAD signaling is activated specifically in the luminal and suprabasal cells of stratified epithelium where it promotes cellular differentiation while being suppressed in the basal cells where p63 is highly expressed (Mou et al., 2016). This notion is further supported by in vitro studies demonstrating that inhibition of TGF-β signaling results in the increased proliferative potential of diverse epithelial progenitor cells (Suzuki et al., 2017). Interestingly, TGF-β inhibition results in the enrichment and expansion of p63+ve progenitor cells. Although these studies collectively support the possibility of p63 functioning as an upstream regulator of the TGF-β signaling axis in maintaining stem progenitor cell immature states, the molecular mechanism through which p63 and TGF-β coordinate to direct these processes has not been elucidated (Mou et al., 2016; Suzuki et al., 2017). Based on our integrated analysis of transcriptomic (RNA-seq, scRNA-seq) and genomic (ΔNp63 ChIP-seq) studies we have identified Follistatin (Fst) as a putative candidate in maintaining the clonogenic potential of pSEPCs by inhibiting Activin signaling, similar to what has been reported in thymic epithelial progenitor cells (Lepletier et al., 2019). Moreover, our data demonstrate that Fst can rescue the clonogenic defects associated with the loss of ΔNp63 suggesting an intrinsic link between p63 and Fst in directing differentiation programs in SP cells. Taken together, our studies have identified Fst as an important molecular link in the genetic circuitry through which p63 coordinates with the Activin/Smad signaling pathway in maintaining the proliferative potential of pSEPCs (Figure 7). We posit that the intimate regulatory circuitry between Fst/Activin/p63 that underlies stem cell specification as described here might also be in play in other tissues and organs as evident by a recent report of this signaling axis as a mediator of telomere dysfunction in the mouse skin epidermis (Liu et al., 2019). Long term, unearthing the molecular mechanisms regulating the differentiation and self-renewal programs of SP cells has important implications for both regenerative medicine and understanding how these programs contribute to tumorigenesis.
Figure 7.
ΔNp63+ve Basal/Myoepithelial Cells Maintain the Proliferative Potential of Salivary Gland Epithelial Progenitor Cells Through Activation of the Follistatin/Activin/Smad Signaling Axis
(A) p63 directly regulates the expression of Fst, which functions as a potent antagonist of Activin signaling. Fst maintains the proliferative potential of pSEPCs by sequestering Activin A and preventing it from binding to its receptor and acting on its downstream effectors, phosphorylated Smad2/3.
(B) Ablation of p63 results in the loss of Fst expression and activation of Activin signaling resulting in the loss of salivary gland stem/progenitor cell proliferative potential. This is accompanied by alterations in ductal and acinar cell differentiation resulting in reduced ductal cell size and enlarged acinar cells, respectively.
Limitations of the Study
In the present study, we have leveraged genetic models and genomic interrogation techniques to uncover a critical role for p63 as a regulator of the Follistatin/Activin/Smad signaling axis to maintain stem/progenitor cell function. Our in vitro studies have focused on the role of p63 in the epithelial stem/progenitor cells in regulating Activin/Smad signaling; a limitation to this model system is that it does not examine the possibility of cross talk between the epithelium and the mesenchyme, in this process. Additionally, the inherent limitations associated with scRNA sequencing coupled with the lack of reliable markers have limited our ability to identify the various cellular populations that comprise the SMG.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Rose-Anne Romano (rromano2@buffalo.edu).
Materials Availability
This study did not generate new materials.
Data and Code Availability
Bulk RNA-seq, ChIP-seq, and scRNA-seq files have been uploaded to the Gene Expression Omnibus (GSE accession number GSE145268).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research (NIH/NIDCR) grants DE025889 and DE027660 to R.-A.R. A.O., E.-A.C.S., and S.M. were supported by the State University of New York at Buffalo, School of Dental Medicine, Department of Oral Biology training grant (NIH/NIDCR) DE023526.
Author Contributions
S.M., S.S., and R.-A.R. designed the experiments. S.M., A.O., E.-A.C.S., K.S., and R.-A.R. performed experiments. E.F. provided essential tools. S.M., C.G., A.O., J.E.B., E.-A.C.S., K.S., M.C., and R.-A.R. analyzed the data. S.M., S.S., and R.-A.R. wrote the paper. All authors reviewed and edited the drafts and approved the final version.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101524.
Supplemental Information
References
- Amano O., Mizobe K., Bando Y., Sakiyama K. Anatomy and histology of rodent and human major salivary glands: overview of the Japan salivary gland society-sponsored workshop. Acta Histochem. Cytochem. 2012;45:241–250. doi: 10.1267/ahc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Fuchs E. p63: revving up epithelial stem-cell potential. Nat. Cell Biol. 2007;9:731–733. doi: 10.1038/ncb0707-731. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R., Wei Y., Hwang J., Hang X., Andres Blanco M., Choudhury A., Tiede B., Romano R.A., Decoste C., Mercatali L. DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat. Cell Biol. 2014;16:1004–1015. doi: 10.1038/ncb3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti D., Su X., Cho M.S., Bui N.H., Coarfa C., Venkatanarayan A., Benham A.L., Flores Gonzalez R.E., Alana J., Xiao W. Induced multipotency in adult keratinocytes through down-regulation of DeltaNp63 or DGCR8. Proc. Natl. Acad. Sci. U S A. 2014;111:E572–E581. doi: 10.1073/pnas.1319743111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Wang D., Burgmaier J.E., Teng Y., Romano R.A., Sinha S., Yi R. Single cell and open chromatin analysis reveals molecular origin of epidermal cells of the skin. Dev. Cell. 2018;47:21–37 e5. doi: 10.1016/j.devcel.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster N., Saladi S.V., Van Bragt M., Sfondouris M.E., Jones F.E., Li Z., Ellisen L.W. Basal cell signaling by p63 controls luminal progenitor function and lactation via NRG1. Dev. Cell. 2014;28:147–160. doi: 10.1016/j.devcel.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B.J., Dueck D. Clustering by passing messages between data points. Science. 2007;315:972–976. doi: 10.1126/science.1136800. [DOI] [PubMed] [Google Scholar]
- Harunaga J., Hsu J.C., Yamada K.M. Dynamics of salivary gland morphogenesis. J. Dent. Res. 2011;90:1070–1077. doi: 10.1177/0022034511405330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Nandi A., Mahesh A., Sinha S., Flores E., Chakrabarti R. Inducible knockout of Np63 alters cell polarity and metabolism during pubertal mammary gland development. FEBS Lett. 2019;594:973–985. doi: 10.1002/1873-3468.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M., Alston N., Ghazizadeh S. Identification of stem cells in the secretory complex of salivary glands. J. Dent. Res. 2016;95:776–783. doi: 10.1177/0022034516634664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M., Ghazizadeh S. Analysis of histone H2BGFP retention in mouse submandibular gland reveals actively dividing stem cell populations. Stem Cells Dev. 2015;24:565–574. doi: 10.1089/scd.2014.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.K., Liu Y., Liao L., Wang F., Xu J. The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice. Int. J. Biol. Sci. 2014;10:1007–1017. doi: 10.7150/ijbs.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepletier A., Hun M.L., Hammett M.V., Wong K., Naeem H., Hedger M., Loveland K., Chidgey A.P. Interplay between follistatin, activin A, and BMP4 signaling regulates postnatal thymic epithelial progenitor cell differentiation during aging. Cell Rep. 2019;27:3887–3901.e4. doi: 10.1016/j.celrep.2019.05.045. [DOI] [PubMed] [Google Scholar]
- Liu N., Yin Y., Wang H., Zhou Z., Sheng X., Fu H., Guo R., Wang H., Yang J., Gong P. Telomere dysfunction impairs epidermal stem cell specification and differentiation by disrupting BMP/pSmad/P63 signaling. PLoS Genet. 2019;15:e1008368. doi: 10.1371/journal.pgen.1008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M.M., Lu N., Vogel H., Sellheyer K., Roop D.R., Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- Mou H., Vinarsky V., Tata P.R., Brazauskas K., Choi S.H., Crooke A.K., Zhang B., Solomon G.M., Turner B., Bihler H. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 2016;19:217–231. doi: 10.1016/j.stem.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Matzuk M.M., Gerstmayer B., Bosio A., Lauster R., Miyachi Y., Werner S., Paus R. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J. 2003;17:497–499. doi: 10.1096/fj.02-0247fje. [DOI] [PubMed] [Google Scholar]
- Nanduri L.S., Baanstra M., Faber H., Rocchi C., Zwart E., De Haan G., Van Os R., Coppes R.P. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep. 2014;3:957–964. doi: 10.1016/j.stemcr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri L.S., Maimets M., Pringle S.A., Van Der Zwaag M., Van Os R.P., Coppes R.P. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother. Oncol. 2011;99:367–372. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- Oshimori N., Fuchs E. The harmonies played by TGF-beta in stem cell biology. Cell Stem Cell. 2012;11:751–764. doi: 10.1016/j.stem.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelakin A., Song E.A.C., Min S., Bard J.E., Kann J.V., Horeth E., Smalley K., Kramer J.M., Sinha S., Romano R.A. Transcriptomic and single-cell analysis of the murine parotid gland. J. Dent. Res. 2019;98:1539–1547. doi: 10.1177/0022034519882355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A., Schnittke N., Romano R.A., Sinha S., Schwob J.E. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. J. Neurosci. 2011;31:8748–8759. doi: 10.1523/JNEUROSCI.0681-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G., Dellambra E., Golisano O., Martinelli E., Fantozzi I., Bondanza S., Ponzin D., Mckeon F., De Luca M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle S., Maimets M., Van Der Zwaag M., Stokman M.A., Van Gosliga D., Zwart E., Witjes M.J., De Haan G., Van Os R., Coppes R.P. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells. 2016;34:640–652. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- Pringle S., Van Os R., Coppes R.P. Concise review: adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells. 2013;31:613–619. doi: 10.1002/stem.1327. [DOI] [PubMed] [Google Scholar]
- Romano R.A., Ortt K., Birkaya B., Smalley K., Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One. 2009;4:e5623. doi: 10.1371/journal.pone.0005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano R.A., Smalley K., Magraw C., Serna V.A., Kurita T., Raghavan S., Sinha S. {Delta}Np63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139:772–782. doi: 10.1242/dev.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi R., Martin D., Genomics, Computational Biology, Core. Yamada K.M. Single-cell RNA-seq identifies cell diversity in embryonic salivary glands. J. Dent. Res. 2020;99:69–78. doi: 10.1177/0022034519883888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M., Pinto F., Crum C.P., McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Song E.C., Min s., Oyelakin a., Smalley K., Bard J.E., Liao L., Xu J., Romano R.A. Genetic and scRNA-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci. Rep. 2018;8:14043. doi: 10.1038/s41598-018-32343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki d., Pinto f., Senoo M. Inhibition of TGF-beta signaling supports high proliferative potential of diverse p63(+) mouse epithelial progenitor cells in vitro. Sci. Rep. 2017;7:6089. doi: 10.1038/s41598-017-06470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.S. Salivary gland development. Semin. Cell Dev. Biol. 2007;18:237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Wu M.Y., Hill C.S. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bulk RNA-seq, ChIP-seq, and scRNA-seq files have been uploaded to the Gene Expression Omnibus (GSE accession number GSE145268).