Abstract
Ethnopharmacological relevance
The consumption of khat (Catha Edulis, Forsk) is on the rise despite the much publicized associated deleterious health effects. How chemicals present in khat, affect various physiological and biochemical processes requires further scrutiny. A clear understanding of these processes will provide an avenue for countering khat-driven negative effects using appropriate pharmacological and/or nutritional interventions.
Aim of the study
The current study investigated the effect of khat on vital physiological and biochemical processes such as oxidative stress, inflammation and immune responses and the role of Coenzyme-Q10 (CoQ10), a potent antioxidant and anti-inflammatory, in modulating any negative effects due to khat exposure.
Methodology
Three (3) weeks old forty (40) Swiss albino mice were randomly assigned into four treatment groups (n = 10). The first group was the control that was not administered with khat or CoQ10. The second group received 200 mg/kg body weight (b/w) of CoQ10, while the third group received 1500 mg/kg b/w of khat extract and finally the forth group was co-treated with 200 mg/kg b/w of CoQ10 and 1500 mg/kg b/w of khat extract. The experiment was conducted for 90 days after which samples were collected for physiological and biochemical analyses.
Results
The effects of khat and CoQ10 on the weights of brain, liver, kidney and spleen was determined. Administration of khat decreased the levels of RBCs and its subtypes (MCV, MCH, RDW-SD and RDW-CV), a clear indicator of khat-induced normochromic microcytic anemia. White blood cells (lymphocytes, monocytes, neutrophils and eosinophil) which are vital in responding to infections were markedly elevated by khat. Moreover, these results provide evidence for khat-induced liver and kidney injury as shown by increased biomarkers; AST, ALT, GGT and creatinine respectively. Standard histopathological analysis confirmed this finding for khat-driven liver and kidney injury. Further studies showed evidence for khat-induced inflammation and oxidative stress as depicted by increased levels of the pro-inflammatory cytokine TNF-alpha and elevation of GSH in the brain, liver and spleen. Remarkably, this is the first study to demonstrate the potential of CoQ10 in ameliorating khat-induced negative effects as outlined. CoQ10 supplementation restored the khat-induced reduction in RBC subtypes, and was protective against liver and kidney injury as shown by the appropriate biomarkers and standard histopathology analysis. The other significant finding was the CoQ10-driven normalization of GSH and TNF-α levels, indicating a protective effect from khat-driven oxidative stress and inflammation respectively.
Conclusion
From this study, we conclude that CoQ10 may be useful in nullifying khat-driven deleterious events among chronic khat users.
Keywords: Khat toxicity, Coenzyme-Q10, Oxidative stress, Inflammation, Immune system, Khat-induced anemia, Biological sciences, Neuroscience, Pharmaceutical science, Biochemistry, Toxicology, Health sciences, Alternative medicine
Khat toxicity; Coenzyme-Q10; Oxidative stress; Inflammation; Immune system; Khat-induced anemia; Biological sciences; Neuroscience; Pharmaceutical science; Biochemistry; Toxicology; Health sciences; Alternative medicine
1. Introduction
Khat (Catha Edulis, Forsk) is an ever green shrub widely grown in East Africa and the horn of Africa. The shrub is mostly chewed for recreational purposes (Al-habeshi and Skaug, 2005; Balint et al., 2009). Three alkaloids, namely cathinone, cathine and norephedrine are responsible for the effects due to khat (Feyissa and Kelly, 2008). Cathinone is the most potent of these alkaloids with the highest concentration present in the leaves (Bogale et al., 2016; Patel, 2015). In Kenya, the leaves of the young shoots are the most preferred over the traditional sticks due to enhanced capacity to induce euphoria. Cathinone and cathine are structurally related to amphetamine with similar pharmacodynamics (Graziani et al., 2008). Hence, the higher prevalence rates associated with consumption of this herb may be attributed to the two cathedulins potential for addiction similar to that of amphetamines and their analogs (Lemieux et al., 2015). Essentially, various studies in animal models of addiction using khat have validated this assumption (Riley et al., 2020). The two cathedulins act by elevating levels of dopamine, serotonin and noradrenalin. Cathinone acts directly on catecholaminergic synapses by increasing the levels of dopamine, preventing reuptake while enhancing release in the striatum and nucleus accumbens (Banjaw et al., 2006; Feyissa and Kelly, 2008; Riley et al., 2020). The euphoric effect following consumption of this herb is attributed to the cathinone-induced increase in the levels of dopamine at the neuronal terminals (Feyissa and Kelly, 2008; Geresu, 2015). And as such, there has been a spike in the production of cathinone analogs, also known as “novel psychoactive substances” (NPS) in many western nations (Lemieux et al., 2015; Riley et al., 2020).
Repeated exposure to the psycho-stimulant has been associated with a myriad of deleterious effects on the central nervous system (CNS) and the peripheral organs. Oxidative stress is among the main channels leading to khat-induced injury. During active oxidative stress, the endogenous antioxidant defense systems fail to curtail the khat-induced production of reactive oxygen species (ROS) (Kryston et al., 2011). The mitochondria is among the most affected cellular organelles by ROS production (Eftekhari et al., 2018a; Lu et al., 2017). The organophosphates (OP) present in pesticides used during cultivation of khat interfere with mitochondrial pathways releasing ROS (Al-Akwa et al., 2009; Engidawork, 2017). Some of the khat-induced ROS generation in the kidney and the liver may be attributed to the pesticide and herbicide components such as glyphosate, paraquat, 1, 2-dibromo-3-chloroporopan (DBCP). Exposure to these results in kidney necrosis which leads to acute and chronic kidney failure in addition (Petejova et al., 2019). Paraquat is also known to induce neurotoxicity by inhibiting acetylcholinesterase (Al-Akwa et al., 2009).
As mentioned earlier, khat consumption increases dopamine release in the striatal and nucleus accumbens regions of the brain. Oxidative metabolism of dopamine may trigger generation of free radicals with subsequent increase in reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Melo et al., 2011), thereby contributing to the etiologies in brain cell degeneration. Additionally, studies on human khat chewers have shown that khat potentially reduces the total antioxidant capacity in serum and saliva samples (Abdelwahab et al., 2018; Al-Akwa et al., 2009; Al-Zubairi et al., 2003).
Genotoxic effects have also been associated with khat use. In this regard, it has been shown recently that human khat chewers produce higher levels of 8-hydroxydeoxyguanosine with significant elevations and suppressions in glutathione reductase (GR) and superoxide dismutase (SOD) respectively (Alsanosy et al., 2020). Other than demonstrating the oxidative damage potential of khat, the perturbations in GR and SOD additionally demonstrate the DNA-damage-potential of khat, given that 8-hydroxydeoxyguanosine is a marker of nucleic acid damage due to oxidative stress (Rosy and Goyal, 2016). This finding may have direct implication in carcinogenesis and could partly be associated to the incidences of khat-induced cancers in humans (Alsanosy et al., 2020; Eftekhari et al., 2018b; Kryston et al., 2011).
Khat has been shown to interfere with the immune system. Cytokines serve a critical function in the host immune surveillance system. Notably, an elevation in the levels of pro-inflammatory cytokines is an indicator of underlying tissue damage (Abdelwahab et al., 2018; Ali et al., 2015). A study by Lukandu et al. (2010) demonstrated khat's capacity to trigger abnormal buccal epithelial cell differentiation, a process that is associated with increased incidences of oral cancer recorded in long term khat chewers (Soufi et al., 1991). Additionally, khat-induced depletion of the inflammatory cytokines TNF-alpha and interleukin-6 (IL-6) with concomitant elevation of IFN-gamma, IL-2 and IL-10 has been demonstrated (Ali et al., 2015); hence, the inflammation-driven histopathological changes in the buccal mucosa of long term khat chewers and the increased susceptibility to diseases. A double insult on the antioxidant and immune system begs the question as to whether an antioxidant with anti-inflammatory effect might benefit chronic khat users. This hypothesis formed the basis for the current study.
Hematological derangements have been used as a major test in assessing the toxicity of plant extracts (Ismaeel et al., 2014). Experimental studies in rodents and primates have demonstrated that khat and its components reduce the red blood cells (RBCs) and consequently affect its indices leading to anemic conditions (Alsalahi et al., 2012; Ismaeel et al., 2014). Moreover, khat affects White Blood Cells (WBCs) and its indices in a similar fashion (Alsalahi et al., 2012; Ketema et al., 2015). Such hematopoietic and cellular inflammatory disturbances eventually compromise the host's immune system and subsequent cell death (Hogg, 1987). Despite the widespread information on the toxicity attributed to khat, there's still an increasing use with limited efforts geared towards finding approaches to reduce khat driven pathology for addicted chronic khat users.
In the present study, the role of Co-enzyme Q10 (CoQ10) in modulating khat-driven oxidative stress, inflammation, immune-modulation and alteration of hematological indices was investigated. CoQ10 (2,3 dimethoxy-5-methyl -6-decaprenyl benzoquinone) is a powerful endogenous antioxidant with anti-inflammatory and immune-modulatory properties that acts as an essential cofactor in the electron transport chain (Dos Santos et al., 2009; Mancuso et al., 2009). Levels of this useful antioxidant have been shown to decline with age in the tissues and brain of humans and animals (Belardinelli et al., 2008; Shi et al., 2013). CoQ10 has been shown to be neuroprotective in in vitro models of neurotoxicity and is potent against iron-induced neuronal apoptosis in mice models and oxidative stress in neuronal cell models (Xing et al., 2004). Other studies have strongly recommended use of CoQ10 in neurodegenerative diseases (Mancuso et al., 2009; Monsef et al., 2019).
One of the most recent studies involving nanoparticle formulated CoQ10 demonstrated putative roles of CoQ10 against ROS formation, lipid peroxidation, cell apoptosis and organophosphate-induced liver disease (Eftekhari et al., 2018a). These findings demonstrate the significance of CoQ10 nanoparticle formulations to enhance bioavailability, biocompatibility and protection from degradative enzymes (Eftekhari et al., 2018b). Supplementation of 200 mg/kg b/w of CoQ10 to Swiss white mice protected them from Melasoprol-induced lipid peroxidation and oxidative stress in a study by Nyariki et al. (2014). In the same study, CoQ10 supplementation was beneficial against T. b. rhodesiense-induced leukocytosis in mice. Collectively, the outcomes of Nyariki's study show that CoQ10 may partly impart neuroprotection from toxic chemicals by quenching free radicals and regulating pathways vital to inflammatory processes.
Previous studies have also shown the ability of CoQ10 to decrease the production of the pro-inflammatory cytokines TNF-α and IL-6, by inhibiting the NF-κB gene expression (Schmelzer et al., 2008). These studies provide compelling evidence that CoQ10 may nullify some of the negative effects due to khat. In the present study, we demonstrate that CoQ10 administration prevented khat-induced deleterious events in mice. These novel observations in respect to the anti-oxidant and anti-inflammatory properties of CoQ10 might be useful in broadening therapeutic options following khat-induced toxicity.
2. Methods
2.1. Ethical statement
Experimental procedures and protocols involving the use of mice were approved by the Institute of Primate Research (IPR) ethics committee (approval number IRC/03/16). A lot of effort was made to ensure comfort for the mice used in this study. This research was conducted in accordance with the internationally accepted principles for laboratory animal use and care, as stipulated in the United States guidelines (NIH publication #2-35, revised in 2011).
2.2. Experimental animals
Forty healthy male Swiss albino mice aged 3–4 weeks old, with body weights of 23–26 g were purchased from the Kenya Medical Research Institute (KEMRI) and transported to the Technical University of Kenya research animal facility. They were housed in clean plastic cages under a controlled room temperature of 23–25 °C and a 12 h light/dark cycle and allowed to acclimatize for one week before the commencement of the experiments. The mice were fed on pellets (Unga feeds) and had access to clean water ad libitum.
2.3. Preparation of Co-enzyme Q10
CoQ10 solution (Now Foods, Bloomingdale, IL, USA) was prepared by dissolving the powder supplement in olive oil. Fresh CoQ10 concentrations were always prepared immediately before administration and were protected from the light before administration to the animals. A dose of 200 mg/kg was selected for CoQ10 treatment in the current study. The choice of the dosage was informed by a previous finding that 200 mg/kg is sufficient to cross the blood brain barrier and offer neuroprotection (Matthews et al., 1998). Additionally, this dose has been demonstrated to be an effective dose against pathology mediated by oxidative stress and inflammation (Mirmalek et al., 2016).
2.4. Preparation of khat extract
Fresh khat leaves were purchased from a local market in Nairobi County. The leaves were washed with distilled and deionized water and 30gms of carefully selected fresh leaves blended using an electric blender (Sayona electric blender, USA). They were soaked in 10 mL of distilled water for 2 h to make a concentration of 3000 mg/mL. Next, 0.2 mL (concentration of 1500 mg/kg body weight) of the filtrate was used for gavage intra-gastric administration into each mouse under khat treatment for 90 days. The choice of this dose (1500 mg/kg body weight) was informed by the average quantity chewers consume and dosages used in similar studies (Al-Zubairi et al., 2008; Alsalahi et al., 2012; Gitonga et al., 2017; Ismaeel et al., 2014).
2.5. Mice grouping and treatment
The current study utilized 40 male Swiss Albino mice randomly divided into four groups. Group one served as the control group and had free access to mice pellets and clean water ad-libitum only; group two was given CoQ10 at a dose of 200 mg/kg body weight, group three was administered with 1500 mg/kg body weight of crude khat extract and group four received 1500 mg/kg body weight of khat extract and 200 mg/kg body weight of CoQ10. The groups receiving CoQ10 were given 200 mg/kg body daily for two weeks prior to the start of the experiment as this pretreatment has been demonstrated to be effective in boosting CoQ10 concentration in the various organs (Nyariki et al., 2014). For group four that received both khat and CoQ10, CoQ10 was administered one hour before administration of khat throughout the experimentation period.
2.6. Sample collection
Twelve hours after termination of the experiment, mice were partially anesthetized with Ketamine injection followed by prilumbal surgical sectioning to draw blood via cardiac puncture. The blood samples were used for hematological and serological analysis. For serological analysis, the blood samples were allowed to settle at room temperature for half an hour then centrifuged for 5 min at 10,000 rpm and 4 °C (Centurion Scientific Ltd K240R, UK). The serum obtained was stored at -22 °C for serological and immunological analysis. Liver, kidney, spleen and brain samples were extracted for reduced glutathione (GSH) assay and histopathological analysis. For GSH assay, the organs were homogenized in ice-cold conditions in a homogenizing buffer (0.5 ml of 0.25M sucrose, 5mM Hepes-Tris pH 7.4 with protease inhibitor) and aliquoted into 0.5 cryovial tubes. The homogenates were kept at -80 °C until further analyses.
2.7. Determination of organ weights
The mice body weights were taken after every 15 days. The weights of brain, liver, kidney and spleen samples were measured at the end of the experiment. Subsequently, the relative organ weights were determined. Individual organ weight divided by the respective body weight at 90 days post treatment (90dpt) multiplied by 100 provided the relative organ weight. All the weight measurements were done using an analytical electronic balance (Mettler PM34, DoltaRange®).
2.8. Hematological and biochemical assays
The blood samples were collected into heparinized tubes for full hemogram analysis using automated Benchman Coulter counter (Benchman, Indianapolis, USA). Serum Alanine Amino Transferase (ALT), Gamma-glutamyl transferase (GGT), Aspartate Amino Transferase (AST), Direct Bilirubin (DBIL) and Total Bilirubin (TBIL) were measured using automated analyzer (COBAS Integra-400 plus analyzer, Roche, Basel, Switzerland).
2.9. Cytokine assays
Serum cytokine levels of IL-10, IFN-γ and TNF-α were measured by sand-witch enzyme linked immune sorbent assay (sand-witch ELISA) using cytokine specific kit from Invitrogen (Thermofischer Scientific, California, USA) according to the manufacturer's instructions. Measurement was done using ELISA optical reader (Multiskan ex-355, Thermo electron corporation, Waltham, Massachusetts, USA) with absorbance set at 450nm.
2.10. Determination of GSH levels
Reduced glutathione (GSH) assay is often used as a marker for oxidative stress in the tissues. The assay was used in the present study to evaluate the potential of CoQ10 to protect mice against Khat driven-oxidative stress. For this test, 50μl of the organ homogenates (brain, liver, spleen, kidney) were mixed with 50μl of solution A (Sulphosalicyclic acid (5%w/v), 0.25mM ethylene diamine tetra acetic acid (EDTA), and the mixture centrifuged at 8000 x g for 10 min at 4 °C). 200μmol of GSH standard solution was prepared in 0.5% sulphosalicylic acid (SSA) and serial dilutions made using the same solution (0.5% SSA) to final concentrations of 100, 50, 25, 12.5, 6.25, 3.13 and 1.56μmol of 5.5′ Dithiobis (2-nitrobenzoic acid (DTNB). Ellman's reagent was prepared by dissolving 0.1M potassium phosphate buffer with 5mM EDTA disodium salt, pH 7.5 (KPE buffer) to a final concentration of 0.6 mg/ml 25μl of each standard was loaded on a 96-well microtitre plate wells (first two rows), followed by 25μl of the sample to the remaining wells in triplicate. To each well, 100μl of freshly prepared DTNB was added and the absorbance measured at 405 nm at intervals of 30 s using a multi-detection microtitre plate reader (R&D Systems, Minneapolis, MN).
2.11. Histopathological analysis
The harvested liver, kidney, spleen and brain samples were fixed in 4% formalin, dehydrated in absolute ethanol and finally embedded in paraffin. The prepared tissues were then sectioned at 5μ with subsequent staining using hematoxylin and eosin for microscopic examination. The sections were then examined for pathology and images taken under a light microscope (Zeis Axio scope).
2.12. Statistical analysis
Statistical analysis was done using GraphPad prism software package (Version 5.0). One way ANOVA was done to compare the treatment groups with controls. For internal comparisons, Turkey's post-hoc test was used. The results were given as a mean ± with significance set at p < 0.05.
3. Results
3.1. Organ weights
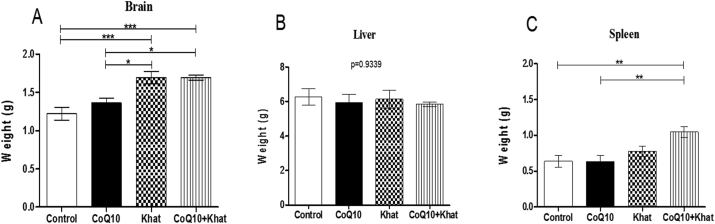
Based on data on internal organs, there was significant increase (p < 0.0001) in the weight of the brain (Figure 1A) and a marginal increase in the weight of the spleen (Figure 1C) in the khat-exposed groups. The liver weight was unaffected by khat exposure (Figure 1B).
Figure 1.
Comparison of the effects of Khat and Coenzyme Q10 administration on organ weight (g) in male Swiss albino mice. The figures shows the change in organ weight of the brain (Figure A), liver (Figure B) and spleen (Figure C). Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons between various groups were done by one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001∗∗∗P ≤ 0.0001). n = 10. Bars represent mean ± SEM.
3.2. Oral Coenzyme-Q10 supplementation restored khat-induced RBC and HCT suppression
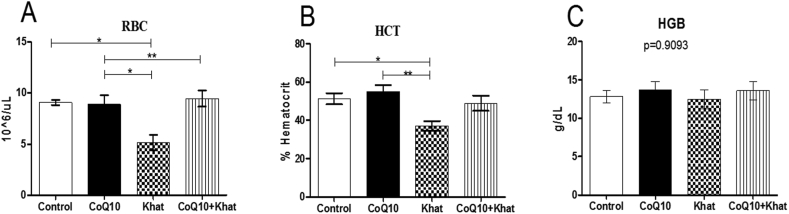
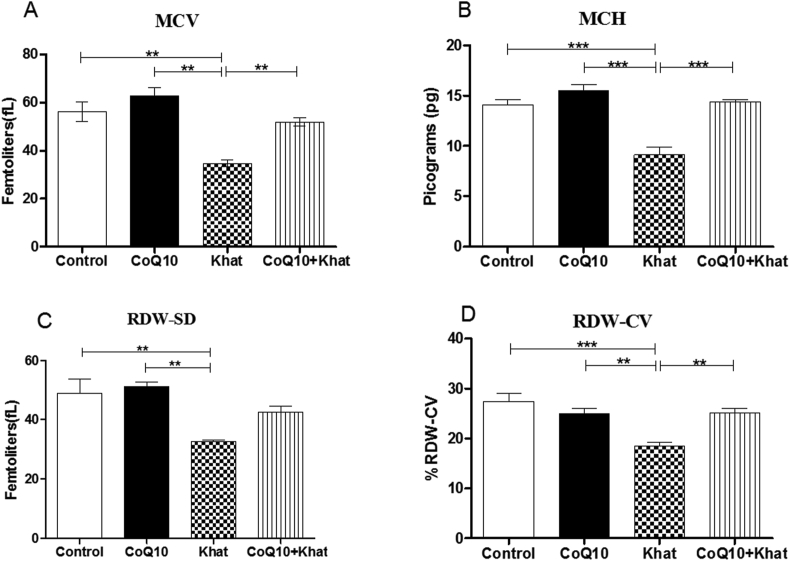
Khat significantly depleted the levels of RBC (Figure 2A) with concomitant decrease in the levels of hematocrit (HCT) (Figure 2B) with no change in hemoglobin (HGB) (Figure 2C); a clear indication of anemia. Analysis of RBC mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), red cell distribution width-standard deviation (RDW-SD) and red cell distribution width-coefficient variation (RDW-CV) was used to determine the type of anemia induced by khat. The results predominantly demonstrated presence of khat-induced microcytic anemia as depicted by the significant reduction in the levels of MCV, MCH, RDW-CV and RDW-SD (Figures 3A–D respectively). Quite remarkably, CoQ10 supplementation significantly restored the levels of MCV, MCH and RDW-CV.
Figure 2.
Mean number of RBC (Figure A), HCT (Figure C) and HGB (Figure C) from blood of male Swiss albino mice. Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. The levels of each RBC subtype were compared by One way ANOVA with Turkey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001∗∗∗P ≤ 0.0001). n = 10. Bars represent mean ± SEM. u/L: microliters per Liter, g/dL: grams per deciliter.
Figure 3.
Mean comparison of red blood cell indices following CoQ10 and khat administration in male Swiss albino mice. The figures shows MCV (mean corpuscular volume) (Figure A), MCH (mean corpuscular hemoglobin) (Figure B), RDW-SD (red cell distribution width –standard deviation) (Figure C) and RDW-CV (red cell distribution width –coefficient of variation) (Figure D). Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Red Blood Cell subtype levels from each group were compared by One way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001∗∗∗P ≤ 0.0001). n = 10. Bars represent mean ± SEM.
3.3. Coenzyme Q10 down-regulated khat-induced elevations of lymphocytes, monocytes, neutrophils and eosinophils
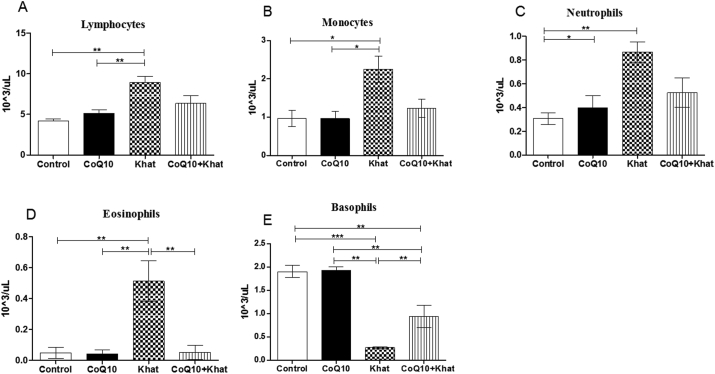
There was no significant difference in the level of white blood cells (WBC) across all groups (Figure 4). However, WBC subtypes that include lymphocytes, monocytes, neutrophils and eosinophils showed significant increase (Figure 5A–D). However, an opposite effect was observed for basophils which showed a significant khat-induced decrease (Figure 5E). It is noteworthy that, CoQ10 showed the ability to prevent the khat-induced elevation of lymphocytes, monocytes, neutrophils and eosinophils. Additionally, CoQ10 was also able to prevent khat-driven down-regulation of basophils.
Figure 4.
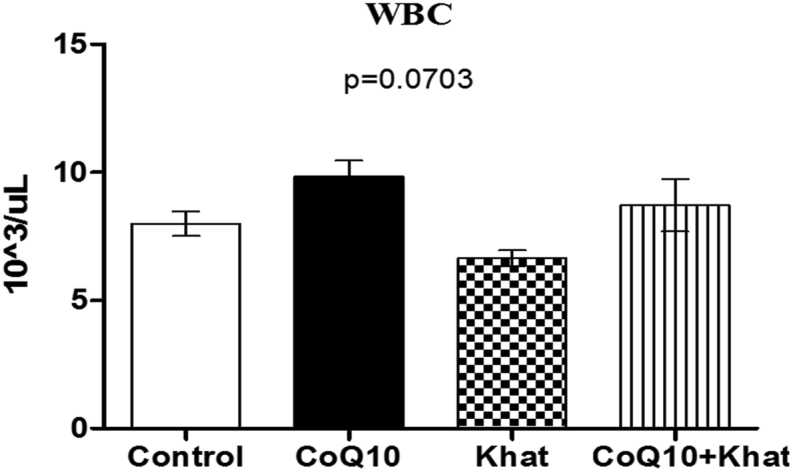
Comparison of the effect of khat and or CoQ10 supplementation on the WBC in male Swiss albino mice. Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons between various groups were done using one way ANOVA with Tukey multiple comparisons post hoc test. Bars represent mean ± SEM.
Figure 5.
Effect of khat and or CoQ10 supplementation on WBC subtypes in male Swiss albino mice. Figure A: Effect on lymphocytes, B: Monocytes, C: Neutrophils, D: Eosinophils and E: Basophils. Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons were done with one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001 ∗∗∗P ≤ 0.0001). uL: microliters. n = 10. Bars represent mean ± SEM.
3.4. Coenzyme-Q10 supplementation restored khat-induced suppression of platelets
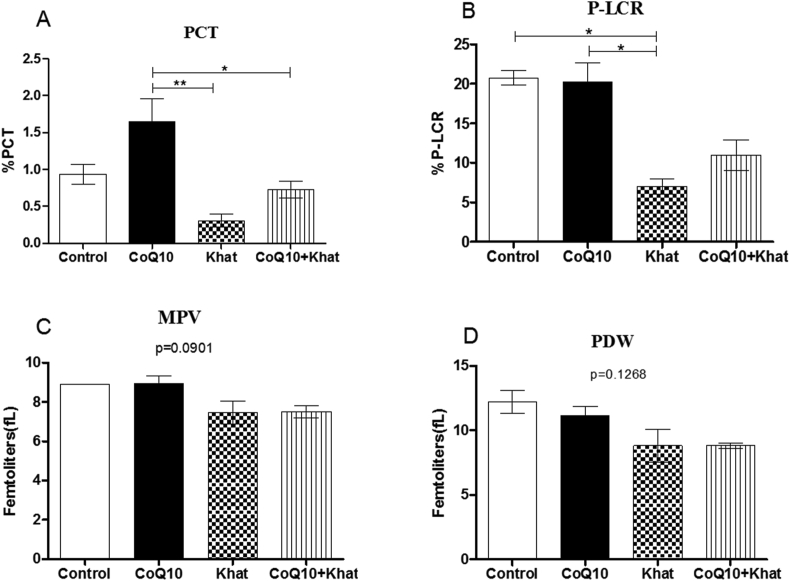
Khat-induced thrombocytopenia was evident through suppression of platelet levels. However, CoQ10 appeared to aid the recovery of platelet levels (Figure 6). Further analysis of the platelet indices showed down-regulation of the plateletcrit (PCT) (Figure 7A). A significant reduction in the levels of platelet large cell ratio (P-LCR) (Figure 7B) was noted. Remarkably, CoQ10 showed the tendency to reverse the khat-driven reduction in PCT and P-LCR levels. Additionally, khat significantly down-regulated the mean platelet volume (MPV) and the platelet distribution width (PDW) in a comparable manner (Figure 7C, D).
Figure 6.
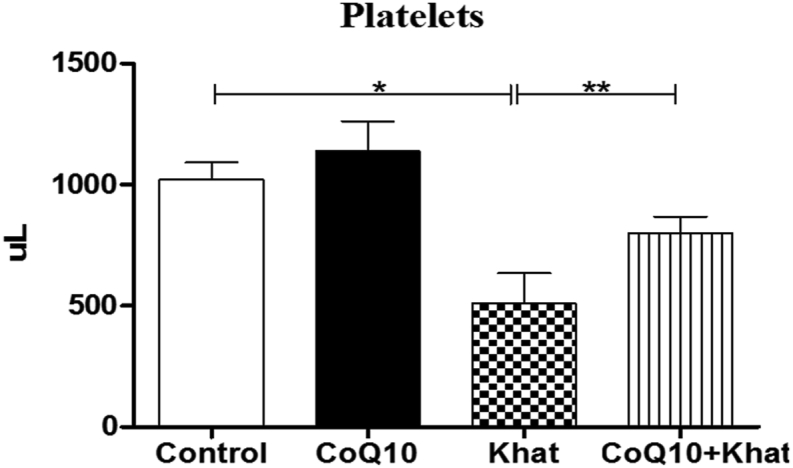
Comparison of the effect of khat and or CoQ10 supplementation on the platelets in male Swiss albino mice. Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons between various groups were done by one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001∗∗∗P ≤ 0.0001). n = 10 mice per group). Bars represent mean ± SEM. uL: Microliters.
Figure 7.
Comparison of the effect of khat and or CoQ10 supplementation on platelet subtypes in male Swiss albino mice. Figure A: Effect on PCT (plateletcrit), B: P-LCR (platelet large cell ratio), C: MPV (mean platelet volume) and D: PDW (platelet distribution width). Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. The platelet subtype levels from each group were compared by one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001∗∗∗P ≤ 0.0001) n = 10. Bars represent mean ± SEM.
3.5. CoQ10 protected the liver from khat-induced hepatotoxicity
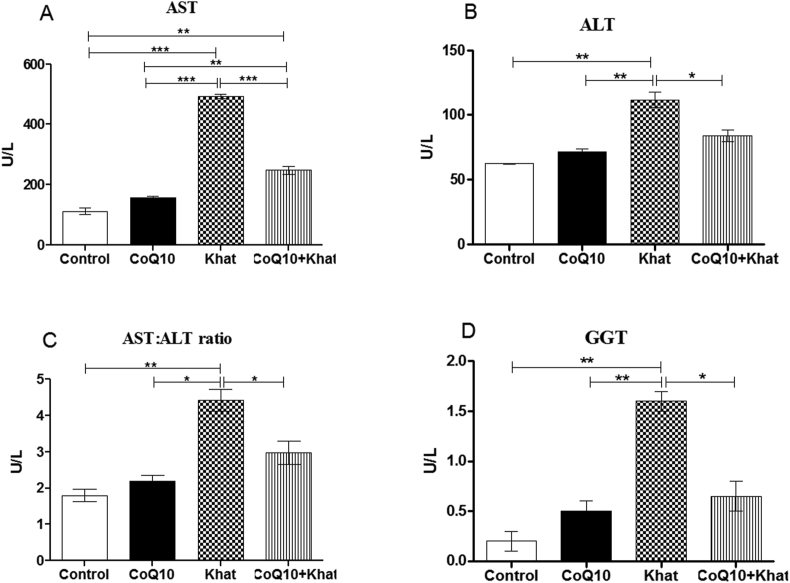
Administration of khat resulted in an increase of the levels of the liver enzymes; aspartate aminotransferase (AST) (P < 0.001), alanine aminotransferase (ALT) (P < 0.001) and gamma glutamyl transpeptidase (GGT) (p < 0.001) (Figure 8A, B, D respectively). The rise in the levels of liver enzymes is a sign of khat-induced hepatotoxicity. The rise in the ratio of AST: ALT in the present study further confirmed the presence of liver injury induced by khat (Figure 8C). CoQ10 supplementation restored the levels of AST (p < 0.0001), ALT (p < 0.05) and GGT (p < 0.05); a clear demonstration of a protective effect by CoQ10 against khat-induced liver damage and/or hepatotoxicity.
Figure 8.
Effects of CoQ10 and Khat supplementation on the liver transaminases enzymes in male Swiss albino mice. Figure 8A–D shows changes in the activity of A: AST (asparte aminotransferase), B: ALT (Alanine aminotransferase), C: Ratio AST: ALT and D: GGT (Gamma-glutamyl transferase) respectively. Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons between various groups were done by one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤0.001∗∗∗P ≤ 0.0001). n = 10. U/L: Units per liter. Bars represent mean ± SEM.
3.6. Coenzyme-Q10 supplementation blocked khat-induced increase in bilirubin and creatinine
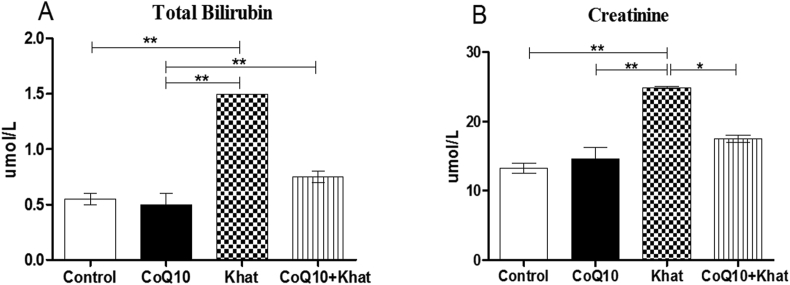
A rise in the total bilirubin level was recorded in the khat-treated group (p < 0.001) while co-supplementation with CoQ10 resulted in normal levels of bilirubin (Figure 9A).
Figure 9.
Comparison of the effect of CoQ10 and Khat on the serum levels of bilirubin and creatinine in male Swiss albino mice. The figure shows the effect of khat and/or CoQ10 on total bilirubin (Figure A) and creatinine (Figure B). Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons between the various groups were done by one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001; ∗∗∗P ≤ 0.0001). n = 10. Bars represent mean ± SEM. umol/L: micromole per liter.
Khat induced a significant increment in the level of creatinine (P < 0.001). The CoQ10 supplemented group had normal levels of creatinine (Figure 9B).
3.7. Co-Q10 supplementation reduced khat-induced oxidative stress in the brain, liver and spleen
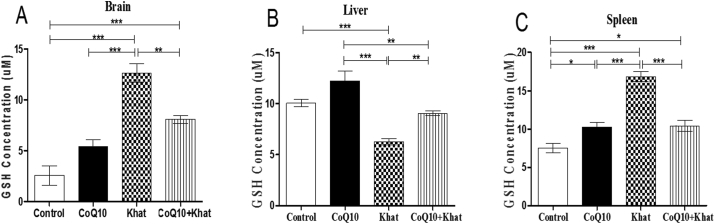
Reduced Glutathione (GSH) is critical in maintaining the cell's antioxidant capacity. Elevation and consequently depletion of GSH is a clear indicator of induction of oxidative stress or active oxidative stress. The levels of GSH were determined on the brain, liver and spleen samples from all treatment groups respectively. In the khat-treated group, there was significant increase in the level of GSH in the brain (p < 0.0001) (Figure 10A), and spleen (p < 0.0001) (Figure 10C). On the contrary, a significant reduction (p < 0.0001) was observed in the liver (Figure 10B). Supplementation with CoQ10 resulted in a marginal increase in the level of GSH in the brain and liver (Figure 10A, B respectively). However, the CoQ10-induced increase in the spleen GSH was significant (p < 0.05) (Figure 10C), when compared to the control group. Co-administration of CoQ10 and khat, resulted in decreased levels of GSH in the brain (p < 0.001); as well as spleen (p < 0.0001), and significantly increased the GSH in the liver (p < 0.001) (Figure 10A–C respectively).
Figure 10.
Comparison of the effect of khat and or CoQ10 supplementation on cellular GSH concentration in male Swiss albino mice. Coenzyme-Q10 supplementation modulated khat-induced oxidative stress in the brain (Figure A), liver (Figure B) and spleen (Figure C). Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons in GSH levels in the brain, liver and kidney from each group was done by one way ANOVA with Turkey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001; ∗∗∗P ≤ 0.0001). Bars represent mean ± SEM. GSH units expressed in micromoles/g (umol/g).
3.8. Oral Co-enzyme-Q10 modulates khat-induced inflammatory response
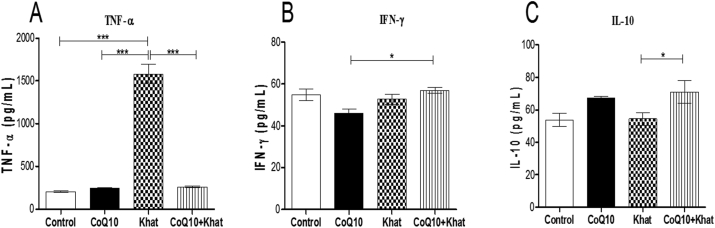
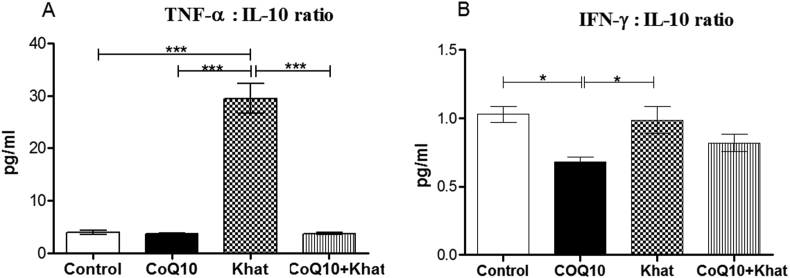
Cytokines have the capacity to modulate inflammatory responses in the peripheral and central nervous systems. In the present study, the levels of the pro-inflammatory cytokines tumor necrotic factor-alpha (TNF-α) and interferon gamma (IFN-γ), as well as the anti-inflammatory cytokine interleukin-10 (IL-10), were determined to assess the capacity of khat to induce inflammation. Khat induced a remarkable rise in the expression of TNF-α (P < 0.0001). In the presence CoQ10, such induction of serum TNF-α levels was abrogated (P < 0.0001) (Figure 11A). Khat did not significantly alter IFN-γ or IL10 (Figure 11B, C respectively). Dynamic cytokine balance between pro-inflammatory and anti-inflammatory are very critical in determining the extent of inflammation. In this case, our analysis showed a net imbalance between the pro-inflammatory and anti-inflammatory immune responses induced by khat. Notably, this imbalance was significant (P < 0.05) in TNF-α:IL-10 ratio and unaffected in the IFN-γ:IL-10 ratio (Figure 12A, B respectively). Notably, CoQ10 supplementation abrogated the khat-induced TNF-α:IL-10 imbalance.
Figure 11.
The effect of CoQ10 and Khat supplementation on the levels of the cytokines Tumor Necrosis Factor-alpha (TNF-α) (Figure A), Interferon gamma (IFN-γ) (Figure B) and Interleukin-10 (IL-10) (Figure C). Units pg/mL: Picograms per milliliter. Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons between the various groups were done by one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001; ∗∗∗P ≤ 0.0001). n = 10. Bars represent mean ± SEM.
Figure 12.
Comparison of the effect of CoQ10 and Khat supplementation on the levels of the ratios of cytokines Tumor Necrosis Factor-alpha (TNF-α): Interleukin-10 (IL-10) (Figure A) and Interferon gamma (IFN-γ): Interleukin-10 (IL-10) (Figure B). Wild type naïve (Control) group, CoQ10 only administered group, Khat only administered group and Khat-CoQ10 co-administered group. Comparisons between various groups were done by one way ANOVA with Tukey multiple comparisons post hoc test. (Indicated level of significance: ∗P ≤ 0.05; ∗∗P ≤ 0.001; ∗∗∗P ≤ 0.0001). n = 10. Bars represent mean ± SEM.
3.9. Co-enzyme-Q10 supplementation attenuated khat-induced liver and kidney damage
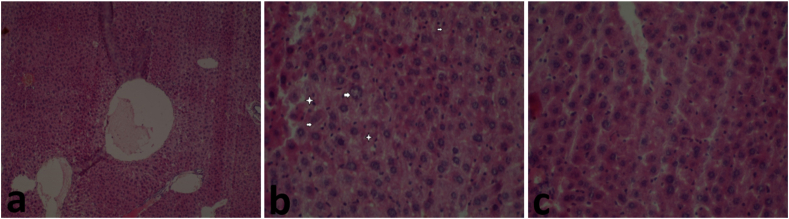
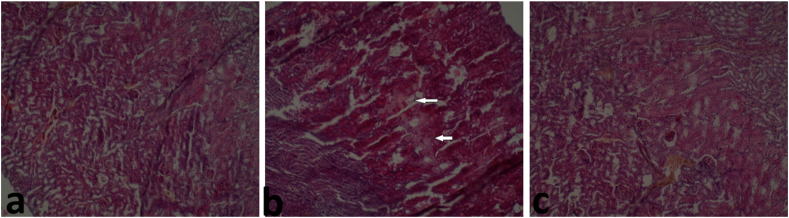
Histological examination of liver sections from mice treated with khat revealed multifocal areas of hepatic necrosis (shown by the arrows) and hepatocyte cytoplasm vacuolation (shown by the stars) (Figure 13B). The liver tissue sections from the control group revealed normal hepatocytes (Figure 13A); the same was observed in mice co-treated with CoQ10 and khat (Figure 13C). In the Kidney, there was tubular necrosis and tubular epithelium injury characterized by the vacuolar degeneration of the tubular epithelial cells (shown by the arrows) (Figure 14B). On the other hand, kidney sections of the control and the group co-treated with CoQ10 and khat showed normal kidney cellular structure (Figure 14A, C respectively).
Figure 13.
Comparison of the effect of khat and/or CoQ10 on the liver tissue in male Swiss albino mice. Mice orally administered with Coenzyme Q10 show mild liver damage as shown. (a) Normal liver section from control group, (b) Liver section from mice that received 1500 mg/kg bwt of Khat orally showing focal hepatocyte necrosis (arrow) and hepatocyte cytoplasm vacuolation (star) (x100, H&E). (c)Normal Liver from mice treated with 200 mg/kg of CoQ10 and 1500 mg/kg of body weight of khat (×100, H&E). The results clearly demonstrate that khat induced liver damage; and CoQ10 protected from such injury.
Figure 14.
Assessment of the effect of khat and/or CoQ10 on the kidney tissue in male Swiss albino mice. (a) Normal Kidney of mice from control group (b) Focal areas of tubular necrosis in mice administered with Khat administered (arrows) and (c) Normal kidney of ice treated with 200 mg/kg of CoQ10 and 1500 mg/kg of body weight of Khat. (Magnification: × 100, H&E). The results clearly show that khat extract induced kidney injury; and CoQ10 provided some protection from such damage.
4. Discussion
From this study, it is remarkably clear that oral administration of crude khat extract resulted in a myriad of negative physiological and biochemical changes in mice. Most importantly, some of the negative effects were assuaged by CoQ10, which is a powerful antioxidant and anti-inflammatory. There was marked increase in the brain weight which may be attributed to the edema-inducing potential of khat. Other studies have previously noted this phenomenon in New Zealand rabbits (Abdul-Mughni et al., 2018). Increase in spleen weight was noted and could be attributed to B-lymphocytes proliferation as a result of khat toxicity. The levels of lymphocytes were evidently increased (Figure 5A). Notably, previous studies have also reported that cathinone mediates T and B lymphocyte proliferation in a dose dependent manner (Ketema, 2015).
Khat-induced derangement of blood components has been observed in previous studies (Alsalahi et al., 2012; Ismaeel et al., 2014); such derangements potentially lead to blood related syndromes. Khat had remarkable effects on the red blood cells. Notably, it induced anemia as depicted by the depletion of RBC and HCT (Figure 2) in concert with findings in other studies on khat (Ismaeel et al., 2014; Ketema et al., 2015). These results suggest the possibility of khat-induced RBC hemolysis and/or khat induced impairment of liver iron recycling function due to increased total bilirubin levels (Figure 9) and histopathological findings (Figure 13). Exposure to khat resulted in microcytic anemia as depicted by the decreased levels of HCT (Figure 2B) and RBC indices (MCV, MCH, RDW-SD and RDW-CV) in Figure 3. Previously, administration of khat resulted increased RBC subtypes indicating khat-induced macrocytic anemia (Ismaeel et al., 2014). Our study is the first to demonstrate that co-administration of khat with CoQ10 significantly restored the khat-induced effects on the levels of RBCs and its subtypes. Given the known antioxidant potential of CoQ10, this may validate the assumption that khat-induced hemolysis could be mediated by the increased cellular oxidative stress burden. This finding is not surprising, given that erythrocytes enriched with exogenous CoQ10 have been shown to be more resistant to oxidative damage (Belardinelli et al., 2008). Notably, RBCs are rich in higher amounts of membrane phospholipids and lack mitochondria as well as mitochondrial antioxidant enzymes; this makes them vulnerable to lipid peroxidation and auto-oxidation (Fibach and Rachmilewitz, 2008).
Further findings from this study indicate that the total WBCs did not significantly change due to khat-induced cytotoxicity. However, the WBC subtypes (lymphocytes, monocytes, neutrophils and eosinophils) were significantly elevated, with the exception of basophils. The khat-induced elevations of neutrophils and eosinophils could account for the earlier observed splenomegaly as a result splenic sequestration and clonal expansion of these granulocytes. Previous studies have shown that proliferation of monocytes and neutrophils trigger the release of the pro-inflammatory cytokines IL-8, TNF-α, IL-6, IFN-γ subsequently resulting in inflammation linked diseases condition (Das et al., 2015; Hicks and Cooper, 2008), hence, this could explain the role of khat in exacerbating inflammation and pathologies. The effect of khat on WBC subtypes might have negative impact on diagnosis for infections and some types of cancers that rely on WBC indices for diagnosis. The implication is that chronic khat use can compromise diagnostic tests for disease processes and cancer. This phenomenon is of profound importance and requires further scrutiny. A remarkable finding here is that co-supplementation of CoQ10 with khat nullified khat-induced increases in WBC subtypes. Therefore, this observation suggests that CoQ10 protects from khat-induced elevation of lymphocytes, monocytes, neutrophils and eosinophils (Figure 5A–D).
The observed low platelet count in the khat group (Figure 6) could be attributed to the hemolytic anemia as earlier indicated by the khat-induced RBC decrease accompanied by elevation of bilirubin levels (Figure 9). The observed effect of khat on platelets could predispose khat chewers to blood clotting abnormalities. It could also have far reaching implications on people on blood thinning pharmaceutical prescriptions. The khat-induced low platelet count was accompanied by decreases in platelet subtypes (PCT, P-LCR) (Figure 7). Previous studies have shown similar khat-induced patterns in platelets of mice (Ketema et al., 2015; Ismaeel et al., 2014). Remarkably, co-supplementation of khat with CoQ10 appeared to significantly restore the depleted platelet count and its subtypes.
Reduced glutathione (GSH) is a key player in the cellular antioxidant system (Yabuki and Fukunaga, 2013). The present study sought to determine the levels of GSH in selected organs (brain, liver and spleen) to assess the putative ability of CoQ10 to protect from khat-mediated oxidative burden in the organs. As observed in the findings (Figure 10), the significant elevation of GSH in the brain and spleen of mice on khat, depict an increased oxidative burden in the respective organs. The significant elevation of GSH in the brain and spleen of mice on khat, has been interpreted to imply induction of oxidative stress via increase in GSH, in response to the khat challenge. However, what was noted in the liver of mice exposed to khat, was complete depletion of GSH; a clear indicator of a severely overwhelmed antioxidant capacity in hepatocytes. This is a characteristic finding in liver toxicity due to chemical xenobiotics, including acetaminophen toxicity (Fu et al., 2018). Note that in instances of extreme toxicity to the liver, GSH is characteristically depleted. Hence, the khat-induced GSH depletion may be a true reflection of underlying oxidative damage initiated by exposure to khat. Oxidative stress induction by khat has been determined in a number of studies. What has never been investigated is whether CoQ10 can assuage such oxidative stress. Exposure to khat extract increases reactive oxygen species (ROS) during metabolism of its alkaloid cathinone or through breakdown of organophosphate pesticides used during cultivation of khat (Al-Akwa et al., 2009). However, co-supplementation with CoQ10 appears to rescue the organs from oxidative damage. This is attributable to the clearly established role of CoQ10 as a potent antioxidant (Mirmalek et al., 2016; Rashid et al., 2014). Other studies have shown that CoQ9 is more abundant in mice brain when compared to (Bentinger et al., 2003), our study showed that oral CoQ10 supplementation dramatically down-regulated the khat-induced GSH spike in the brain homogenates (Figure 10 A). However, other studies have demonstrated that oral CoQ10 supplementation increases brain CoQ10 concentrations in mice (Kwong et al., 2002; Matthews et al., 1998). Further, outcomes of previous experiments have shown a direct link between oral CoQ10 supplementation and oxidative damage protection, neuroprotection and immunomodulation (Nyariki et al., 2019; Rashid et al., 2014).
Histopathological analysis revealed an abnormal distribution of hepatocytes characterized by spotty necrosis in the liver and kidney tubular necrosis (Figures 13 and 14 respectively). In addition, the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma glutamyl transpeptidase (GGT) were significantly elevated indicating liver damage. There were also elevated levels of total bilirubin indicating the inability of the liver to process bilirubin by the khat group. In addition, elevated levels of creatinine were signs of structural and functional abnormalities in the kidney. The observed pathological events in both the liver and the kidney are as a result of the toxic effects from chemicals found in khat such as cathine and cathinone. Remarkably, CoQ10 co-supplementation with khat was able to restore the liver hepatocytes (Figure 13c) and kidney architecture (Figure 14c), and attenuated abnormal rise in liver and kidney damage biomarkers.
Khat-induced increase in the pro-inflammatory cytokine TNF-α, with a marginal increase in another pro-inflammatory cytokine IFN-γ (Figure 12) was recorded. The marked increase in the level of pro-inflammatory cytokine TNF-α, could be attributed to the ability of khat to trigger apoptosis through TNF-α activation of caspases (Ismaeel et al., 2014). Our study demonstrated that co-treatment of CoQ10 with khat attenuated the khat-induced increase in TNF-α. This finding is in support of previous findings on the immunomodulatory capacity of CoQ10 on TNF-α (Neyrinck et al., 2015; Nyariki et al., 2019; Zhai et al., 2017). The levels of anti-inflammatory cytokine interleukin-10 (IL-10), were not affected by khat intake. At this point, it is not clear if higher doses of CoQ10 may trigger anti-inflammatory cytokines. We observed systemic imbalance of pro- and anti-inflammatory response due to khat administration that may be associated with central pathophysiological development including inflammation. Intriguingly, mice orally administered with CoQ10 showed equilibrium between the pro- and anti-inflammatory cytokines, thus this balance can be ascribed to the anti-inflammatory properties of CoQ10 which has been observed previously (Schmelzer et al., 2008).
Overall, our results provide reasonable evidence to conclude that khat deranges vital physiological and biochemical processes central to the normal functioning of the cell such as oxidative stress, immunological status, and inflammation. Furthermore, the results provide evidence that khat negatively affects erythropoiesis, with tremendous implications for blood clotting cascades and the ability to fight infections. The impact of khat on the liver and kidney clearly shows the potential for serious risk for khat chewing patients, who may be prescribed drugs that are extensively metabolized in respective organs; hence, heightened potential for toxicity cannot be ruled out.
Perhaps, the most important finding here is that CoQ10 can modulate positively any potential effect on the oxidative stress, immune response and inflammation. Most importantly, it can protect vital organs, namely, brain, liver and kidney.
5. Conclusion
Chronic exposure to khat, leads to a decrease in body weight and a plethora of pathological effects in Swiss albino mice including derangement of the hematological profile and damage to the liver and kidney. These deleterious physical and physiological effects are however rescued upon supplementation with Coenzyme Q10. The outcome of this study show that CoQ10 may be beneficial when considered as a therapeutic strategy in reducing khat-induced deleterious effects in chronic khat users.
Declarations
Author contribution statement
A.O. Isaac: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
C.W. Kennedy: Analyzed and interpreted the data; Wrote the paper.
J.N. Nyariki: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
P. Okanya: Conceived and designed the experiments.
P. Amwayi: Contributed reagents, materials, analysis tools or data.
N. Jillani: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by The World Academy of Sciences (TWAS) [RG/BIO/AF/AG_G-FR324028702]; National Commission for Science, Technology and Innovation (NACOSTI) [NACOSTI/RCD/ST&I/7TH CALL/PHD/298].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdelwahab S.I., Alsanosy R., Mohamed Elhassan Taha M., Mohan S. Khat induced toxicity: role on its modulating effects on inflammation and oxidative stability. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/5896041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Mughni A.S., El-Nahla S.M., Hassan S.A., Ali A.A.D. Teratogenic effects of Khat (Catha edulis) in New Zealand rabbit. J. Adv. Vet. Anim. Res. 2018;5:25–36. [Google Scholar]
- Al-Akwa A.A., Shaher M., Al-Akwa S., Aleryani S.L. Free radicals are present in human serum of Catha edulis Forsk (Khat) abusers. J. Ethnopharmacol. 2009;125:471–473. doi: 10.1016/j.jep.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Al-habeshi N.N., Skaug N. Khat ( Catha edulis )— an updated review. Addiction Biol. 2005;10(4):299–307. doi: 10.1080/13556210500353020. [DOI] [PubMed] [Google Scholar]
- Al-Zubairi A., Al-Habori M., Al-Geiry A. Effect of Catha edulis (khat) chewing on plasma lipid peroxidation. J. Ethnopharmacol. 2003;87:3–9. doi: 10.1016/s0378-8741(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Al-Zubairi A., Ismail P., Pei Pei C., Rahmat A. Genotoxic effect of Catha edulis (khat) crude extract after sub-chronic administration in rats. Environ. Toxicol. Pharmacol. 2008;25:298–303. doi: 10.1016/j.etap.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Ali E.H.A., Hegazy H.G., Mosaad R.M. Interaction between pro-inflammatory cytokines and brain oxidative stress biomarkers of khat, cathinone and pseudoephedrine hydrochloride intoxication in male mice. 2015;9:585–594. [Google Scholar]
- Alsalahi A., Abdulla M.A., Al-Mamary M., Noordin M.I., Abdelwahab S.I., Alabsi A.M., Shwter A., Alshawsh M.A. Toxicological features of Catha edulis (Khat) on livers and kidneys of male and female Sprague-Dawley rats: a subchronic study. Evidence-based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/829401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsanosy R., Alhazmi H.A., Sultana S., Abdalla A.N., Ibrahim Y., Al Bratty M., Banji D., Khardali I., Khalid A. Phytochemical screening and cytotoxic properties of ethanolic extract of young and mature khat leaves. J. Chem. 2020;2020:1–9. [Google Scholar]
- Balint E.E., Falkay G., Balint G.A. Khat - a controversial plant. Wien Klin Wochenschr. 2009;121(19–20):604–614. doi: 10.1007/s00508-009-1259-7. [DOI] [PubMed] [Google Scholar]
- Banjaw M.Y., Miczek K., Schmidt W.J. Repeated Catha edulis oral administration enhances the baseline aggressive behavior in isolated rats. J. Neural. Transm. 2006;113:543–556. doi: 10.1007/s00702-005-0356-7. [DOI] [PubMed] [Google Scholar]
- Belardinelli R., Tiano L., Littarru G.P. Oxidative stress, endothelial function and coenzyme Q10. Biofactors. 2008;32:129–133. doi: 10.1002/biof.5520320115. [DOI] [PubMed] [Google Scholar]
- Bentinger M., Dallner G., Chojnacki T., Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic. Biol. Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- Bogale T., Engidawork E., Yisma E. Subchronic oral administration of crude khat extract (Catha edulis forsk) induces schizophernic-like symptoms in mice. BMC Compl. Altern. Med. 2016;16 doi: 10.1186/s12906-016-1145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C.K., Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos G.C., Antunes L.M.G., Dos Santos A.C., Bianchi M.D.L.P. Coenzyme Q10 and its effects in the treatment of neurodegenerative diseases. Brazilian J. Pharm. Sci. 2009;45:607–618. [Google Scholar]
- Eftekhari A., Ahmadian E., Azami A., Johari-Ahar M., Eghbal M.A. Protective effects of coenzyme Q10 nanoparticles on dichlorvos-induced hepatotoxicity and mitochondrial/lysosomal injury. Environ. Toxicol. 2018;33:167–177. doi: 10.1002/tox.22505. [DOI] [PubMed] [Google Scholar]
- Eftekhari A., Dizaj S.M., Chodari L., Sunar S., Hasanzadeh A., Ahmadian E., Hasanzadeh M. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 2018;103:1018–1027. doi: 10.1016/j.biopha.2018.04.126. [DOI] [PubMed] [Google Scholar]
- Engidawork E. Pharmacological and toxicological effects of Catha edulis F. (Khat) Phyther. Res. 2017 doi: 10.1002/ptr.5832. [DOI] [PubMed] [Google Scholar]
- Feyissa A.M., Kelly J.P. Vol. 32. 2008. Review Article A Review of the Neuropharmacological Properties of Khat; pp. 1147–1166. [DOI] [PubMed] [Google Scholar]
- Fibach E., Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr. Mol. Med. 2008;8:609–619. doi: 10.2174/156652408786241384. [DOI] [PubMed] [Google Scholar]
- Fu C. lin, Liu Y., Leng J., Zhang J., He Y. fang, Chen C., Wang Z., Li W. Platycodin D protects acetaminophen-induced hepatotoxicity by inhibiting hepatocyte MAPK pathway and apoptosis in C57BL/6J mice. Biomed. Pharmacother. 2018;107:867–877. doi: 10.1016/j.biopha.2018.08.082. [DOI] [PubMed] [Google Scholar]
- Geresu B. Khat (Catha edulis F.) and cannabinoids: parallel and contrasting behavioral effects in preclinical and clinical studies. Pharmacol. Biochem. Behav. 2015 doi: 10.1016/j.pbb.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Gitonga G.M., Ngeranwa J., King A., Muthee D.G., Kimutai R., Gitonga A.W. Nephrotoxicity effects of Khat (Catha edulis) on mice when administered orally. 2017;6:27–33. [Google Scholar]
- Graziani M., Milella M.S., Nencini P. Khat chewing from the pharmacological point of view: an update. Subst. Use Misuse. 2008;43:762–783. doi: 10.1080/10826080701738992. [DOI] [PubMed] [Google Scholar]
- Hicks P., Cooper D.J. The surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Resuscitation J. Australas. Acad. Criti. Care Med. 2008 [PubMed] [Google Scholar]
- Hogg J.C. Neutrophil kinetics and lung injury. Physiol. Rev. 1987;67(4):1295. doi: 10.1152/physrev.1987.67.4.1249. [DOI] [PubMed] [Google Scholar]
- Ismaeel B.-J., Mohammad A.D., Fahaid H.A.-H., Luke O.N., Hussein F.S., Abdul-Moneim J., Mahmoud A.-K. Derangement of hemopoiesis and hematological indices in Khat (Catha edulis) - treated rats. Afr. J. Biotechnol. 2014;13:349–355. [Google Scholar]
- Ketema T. 2015. Evaluation of Immunomodulatory Activities of Methanolic Extract of Khat ( Catha Edulis , Forsk ) and Cathinone in Swiss Albino Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketema T., Yohannes M., Alemayehu E., Ambelu A. Evaluation of immunomodulatory activities of methanolic extract of khat (Catha edulis, Forsk) and cathinone in Swiss albino mice. BMC Immunol. 2015:1–11. doi: 10.1186/s12865-015-0072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryston T.B., Georgiev A.B., Pissis P., Georgakilas A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. Fund Mol. Mech. Mutagen. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Kwong Linda, Kamzalov Sergey, Igor Rebrin A.C. Original contribution effects of coenzyme q 10 administration on its tissue. Science (80- ) 2002;33:627–638. doi: 10.1016/s0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Lemieux A.M., Li B., Al’Absi M. Khat use and appetite: an overview and comparison of amphetamine, khat and cathinone. J. Ethnopharmacol. 2015 doi: 10.1016/j.jep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Li Y., Xiang M., Zhou J., Chen J. Khat promotes human breast cancer MDA-MB-231 cell apoptosis via mitochondria and MAPK-associated pathways. Oncol. Lett. 2017;14 doi: 10.3892/ol.2017.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukandu O.M., Neppelberg E, Vintermyr O.K., Johannessen A.C., Costea D.E. Khat alters the phenotype of in vitro-reconstructed human oral mucosa. J. Dent. Res. 2010;89(3):270–275. doi: 10.1177/0022034509354980. [DOI] [PubMed] [Google Scholar]
- Mancuso M., Orsucci D., Volpi L., Calsolaro V., Siciliano G., Spinelli A., Correale C., Szabo H., Montorsi M. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr. Drug Targets. 2009;11:111–121. doi: 10.2174/138945010790031018. [DOI] [PubMed] [Google Scholar]
- Matthews R.T., Yang L., Browne S., Baik M., Beal M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo A., Monteiro L., Lima R.M.F., De Oliveira D.M., De Cerqueira M.D., El-Bachá R.S. Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longev. 2011;2011 doi: 10.1155/2011/467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmalek S.A., Gholamrezaei Boushehrinejad A., Yavari H., Kardeh B., Parsa Y., Salimi-Tabatabaee S.A., Yadollah-Damavandi S., Parsa T., Shahverdi E., Jangholi E. Antioxidant and anti-inflammatory effects of coenzyme Q10 on L-arginine-induced acute pancreatitis in rat. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/5818479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsef A., Shahidi S., Komaki A. Influence of chronic coenzyme Q10 supplementation on cognitive function, learning, and memory in healthy and diabetic middle-aged rats. Neuropsychobiology. 2019;77:92–100. doi: 10.1159/000495520. [DOI] [PubMed] [Google Scholar]
- Neyrinck A.M., Catry E., Sohet F.M., Cani P.D., Pachikian B.D., Bindels L.B., Delzenne N.M. Lack of anti-inflammatory effect of coenzyme Q10 supplementation in the liver of rodents after lipopolysaccharide challenge. Clin. Nutr. Exp. 2015;1:10–18. [Google Scholar]
- Nyariki J.N., Thuita J.K., Nyambati G.K., Isaac A.O. Coenzyme Q10 prevented full blown splenomegaly and decreased melarsoprol-induced reactive encephalopathy in mice infected with Trypanosoma brucei rhodesiense. J. Coast. Life Med. 2014;2(3):230–238. [Google Scholar]
- Nyariki J.N., Ochola L.A., Jillani N.E., Nyamweya N.O., Amwayi P.E., Yole D.S., Azonvide L., Isaac A.O. Oral administration of Coenzyme Q 10 protects mice against oxidative stress and neuro-inflammation during experimental cerebral malaria. Parasitol. Int. 2019;71:106–120. doi: 10.1016/j.parint.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Patel N.B. International Review of Neurobiology. 2015. “Natural amphetamine” Khat: a cultural tradition or a drug of abuse? [DOI] [PubMed] [Google Scholar]
- Petejova N., Martinek A., Zadrazil J., Teplan V. Acute toxic kidney injury. Ren. Fail. 2019;41:576–594. doi: 10.1080/0886022X.2019.1628780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid K., Wachira F.N., Nyariki J.N., Isaac A.O. Kenyan purple tea anthocyanins and coenzyme-Q10 ameliorate post treatment reactive encephalopathy associated with cerebral human African trypanosomiasis in murine model. Parasitol. Int. 2014;63:417–426. doi: 10.1016/j.parint.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Riley A.L., Nelson K.H., To P., López-Arnau R., Xu P., Wang D., Wang Y., Shen H. wei, Kuhn D.M., Angoa-Perez M., Anneken J.H., Muskiewicz D., Hall F.S. Abuse potential and toxicity of the synthetic cathinones (i.e., “Bath salts”) Neurosci. Biobehav. Rev. 2020;110:150–173. doi: 10.1016/j.neubiorev.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosy, Goyal R.N. Determination of 8-Hydroxydeoxyguanosine: a potential biomarker of oxidative stress, using carbon-allotropic nanomaterials modified glassy carbon sensor. Talanta. 2016;161:735–742. doi: 10.1016/j.talanta.2016.09.038. [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Lindner I., Rimbach G., Niklowitz P., Menke T., Döring F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–183. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- Shi T.-J.S., Zhang M.-D., Zeberg H., Nilsson J., Grunler J., Liu S.-X., Xiang Q., Persson J., Fried K.J., Catrina S.B., Watanabe M., Arhem P., Brismar K., Hokfelt T.G.M. Coenzyme Q10 prevents peripheral neuropathy and attenuates neuron loss in the db-/db- mouse, a type 2 diabetes model. Proc. Natl. Acad. Sci. 2013;110:690–695. doi: 10.1073/pnas.1220794110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi H.E., Kameswaran M., Malatani T. Khat and oral cancer. J. Laryngol. Otol. 1991;105:643–645. doi: 10.1017/s0022215100116913. [DOI] [PubMed] [Google Scholar]
- Xing L.W., Rainwater D.L., Mahaney M.C., Stocker R. Cosupplementation with vitamin E and coenzyme Q10 reduces circulating markers of inflammation in baboons. Am. J. Clin. Nutr. 2004;80:649–655. doi: 10.1093/ajcn/80.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuki Y., Fukunaga K. Oral administration of glutathione improves memory deficits following transient brain ischemia by reducing brain oxidative stress. Neuroscience. 2013;250:394–407. doi: 10.1016/j.neuroscience.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Zhai J., Bo Y., Lu Y., Liu C., Zhang L. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. PloS One. 2017;12:1–11. doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]