Abstract
One reason for the unpopularity of ozonetherapy in the medical field is that toxicity of ozone is considered equal to that of ROS. In fact, there are substantial differences because ozonetherapy is occasional and can be controlled whereas endogenous ROS formation goes on unperturbed throughout life (Farber et al., 1990; Ames et al., 1993).
Keywords: Ozone Concentration, Epithelial Line Fluid, Paravertebral Muscle, Ozone Dose, Ozonation Process
One reason for the unpopularity of ozonetherapy in the medical field is that toxicity of ozone is considered equal to that of ROS. In fact, there are substantial differences because ozonetherapy is occasional and can be controlled whereas endogenous ROS formation goes on unperturbed throughout life (Farber et al., 1990; Ames et al., 1993).
The topography of formation of ROS is also quite different: mitochondria, which convert 95% of the inhaled oxygen to harmless water, are the main source of ROS since at least 3% of oxygen is converted to superoxide, O2 •– (Richter et al., 1988, 1995; Halliwell, 1994). Dismutation of superoxide by SODs (Fridovich, 1995; Carlsson et al., 1995) is the source of H2O2, which, in the presence of Fe2+, may generate the fearsome, non-specific hydroxyl radical, OH•. Halliwell (1994) estimated that a 70 kg human produces no less than 0.147 mol or 5 g/day of superoxide, whereas one AHT uses at most 18 mg of ozone, equivalent to less than 0.4% of the minimum daily production of superoxide!
Other small amounts of hydrogen peroxide are directly generated by oxidoreductases known as NADPH oxidases (NOXs). There is now consensus that the normal production of hydrogen peroxide is essential for the cell life and the revised concept is that “reactive species are not merely instruments of cellular torture but of normal cellular physiology” (Forman et al., 2008).
The formation of endogenous ROS in mitochondria, deeply immersed in the cell, explains the damage to mitochondrial DNA (Wiseman and Halliwell, 1996; Kowaltowski et al., 2009), which is oxidized about 10 times more than nuclear DNA (Richter et al., 1988) and remains persistently damaged (Yakes and Van Houten, 1997). Conversely, ozone acts from the outside on the plasma, which has a huge reservoir of antioxidants. Nonetheless, the ozone dose added to blood must reach a threshold level in order to generate sufficient H2O2, of which only 10% passes from the plasma into the blood cell cytoplasm where it triggers several biological effects. We do not hide the fact that for ozone to act, we have to induce a calculated, transitory, acute oxidative stress that is rapidly corrected by the antioxidant system. Thus, there is no doubt regarding the formation of peroxyl radicals, hydroxyaldehydes while traces of OH• and HOCl, if present, are promptly neutralized by a variety of antioxidants in the plasma. What is important to note is that all the vital cell compounds, such as enzymes, proteins, RNA and DNA (Van der Zee et al., 1987; Stadtman and Oliver, 1991; Ames et al., 1993), are spared during the extracellular ozone decomposition.
Particularly in the USA, ozonetherapy is regarded as a “barbaric” therapy and unscrupulous ozonetherapists and quacks have done their best to reinforce this concept. However, it is now time to clarify this issue; without prejudices, we must evaluate the merits and demerits and put an end to the confusion between the constant oxidative stress (COS) due to oxygen and the occasional acute stress due to ozone.
Knowing the importance of oxidative DNA lesions in ageing and cancer, I am not surprised when often asked: is ozone mutagenic? And does ozonetherapy accelerate ageing?
I have already discussed in details (Bocci, 1996b, 2002, 2004) a number of reports regarding these questions. Results have often been controversial because some Authors (Goldstein and Balchum, 1967; Freeman et al., 1979), working with saline-washed erythrocytes or with tissue cultures deprived of antioxidants, have observed a damage or mutagenic changes in cells exposed to ozone for a length of time. Once cells are washed in a protein-free saline solution, thus removing precious antioxidants, both oxygen and ozone become cytotoxic, as Halliwell (2003) and we (Larini et al., 2003; 2004) have re-emphasized. In a recent past, Galleano and Puntarulo (1995), Leist et al. (1996), Matos et al. (2000) and Dumaswala et al. (2000) have also shown that cell damage or genotoxicity induced by hydrogen peroxide or iron overload or prolonged storage can be checked if tissue culture media or plasma contain adequate amounts of antioxidants.
Victorin (1992), who has beautifully reviewed this topic, stated that “no cytogenetic effects have been reported for bone marrow cells or spermatocytes and the few experimental and epidemiological studies with human subjects do not allow a conclusion on the cytogenetic effects of ozone in human lymphocytes”. The latest study by Diaz et al. (1995) is important because it was specifically carried out in lymphocytes of eight Retinitis pigmentosa patients before and after 15 treatments of AHT. The results showed no significant differences in sister chromatid exchanges (SCE), micronuclei frequencies and proliferation index values between control and ozone-treated lymphocytes. On the other hand, Diaz-Llera et al. (2002) demonstrated that 1-h exposure of SALINE-DILUTED BLOOD to 5 mM ozone induces genotoxic effects on human leukocytes. However, during AHT, WHOLE BLOOD is exposed for only a few minutes to ozone concentrations between 0.21 and 1.68 mM that clearly explain why ozone is not mutagenic in practice. A careful study by Shinriki et al. (1998) has shown neither cell damage nor haemolysis of human blood exposed exactly with our technique to ozone concentrations up to 100 mcg/ml per ml of blood.
As far as induction of tumours is concerned, lung adenomas were induced in the sensitive strain A/J but not in Swiss-Webster male mice after 4.5 months of inhalation exposure to 0.8 ppm ozone (Last et al., 1987). Witschi et al. (1999) concluded that animal studies do not support the idea that ozone is a pulmonary carcinogen.
Trying to sum up this important topic, it appears that the lack of natural antioxidants is critical in allowing mutagenic changes in cells exposed to ozone in vitro for a length of time. After the removal of plasma, washing and resuspension in physiological media without or with only a small amount of antioxidants, erythrocytes and other cells (Larini and Bocci, 2004) become very sensitive to even very low ozone concentrations, as demonstrated by intense haemolysis or apoptosis. Instead of stigmatizing ozonetherapy as toxic, published papers (Goldstein and Balchum, 1967; Gooch et al., 1976; Freeman et al., 1979; Sato et al., 1999; Fukunaga et al., 1999), which have been performed with artificial conditions, ought to have pointed out the importance of antioxidants in preventing damage.
Another blunder has been made by several cell biologists by keeping cell cultures under constant ozone exposure (Merz et al., 1975; Tarkington et al., 1994) at extremely low levels, but for several hours or days. The conclusion that ozone is toxic even at minimal levels is misleading: firstly, the level of antioxidants in tissue culture media is far lower than in plasma and, more seriously, the authors have not taken into account the cumulative ozone dose. Although I have already mentioned this point, it is appropriate to remind the reader that ozone solubility is very high: according to Henry’s law, every second, ozone solubilizes into water, reacts and disappears, so that more ozone solubilizes and reacts, and this process goes on for days! Although minimal, all of these continuous reactions lead to increasing concentrations of H2O2, OH•, 4-HNE, etc., which go unquenched on account of the scarcity and consumption of antioxidants and thus become toxic. Therefore, with time, even the lowest ozone concentration becomes toxic.
In contrast, exposure of blood to oxygen-ozone is performed with ozone concentrations within the therapeutic window and is over after 1 min during EBOO and about 5 min during AHT. However, if the ozonetherapist uses either ozone concentrations above 100–160 mcg/ml or ozonated saline, he makes another blunder. A typical example is represented by the IV infusion of ozonated saline: Foksinski et al. (1999) infused into peripheral occlusive arterial disease (POAD) patients 500 ml of saline ozonated for 1 h (!), obviously without worrying about the high content of newly formed HOCl; they recorded a 450% increase of 8-hydroxy-2′deoxyguanosine (8-OHdG) in the lymphocyte DNA isolated from some of these unlucky patients. 8-OHdG is a marker indicating the occurrence of DNA oxidation. Thus Foksinski’s results should absolutely preclude (as clarified in 10.1007/978-90-481-9234-2_6) the use of ozonated saline. An interesting, but not unexpected, result of this study was that only 3 of 6 patients showed the appearance of this marker, suggesting a possible genetic sensitivity to oxidative agents. Kleeberger et al. (1997) were the first to show that a susceptible strain of mice presents different ozone sensitivity (see also Cho et al., 2001). Unfortunately, the state of the ozonetherapeutic art is still primordial to allow examination of the genetic pattern of antioxidant enzymes in putative patients. Nevertheless, it is necessary to check TAS levels in plasma and ascertain if patients have a G-6PD deficiency. It is also reminded that the normal antioxidant capacity of plasma presents an individual variability from about 1.3 up to 1.8 mM (Miller et al., 1993), that, nonetheless, amply protects blood cells during ozonation performed within the therapeutic range.
A reassuring fact is that after millions of AHT sessions performed in Germany, Austria, Switzerland and Italy, neither serious acute nor chronic side effects, nor an increased cancer incidence has been reported. Yet this does not absolve us from improving our controls by monitoring oxidative stress and lipid peroxidation in patients during and after ozonetherapy, e.g., by measuring F2-isoprostanes (F2-IsoPs), hydroperoxides and/or other parameters in plasma or urine. This is easier said than done, but I am hopeful that a specific and reliable assay for routine clinical use will soon become available. Furthermore, we must never lower our attention to the use of precise ozone generators and ozone doses that are biologically active but atoxic. If we work correctly, perhaps in due time, the scientific community will accept the concept that ozonetherapy is not comparable to life-long endogenous ROS toxicity.
In conclusion, I cannot avoid saying that ozone is potentially toxic and mutagenic (like all cytotoxic drugs!) but so far, our experimental data and clinical evidence has not shown any risk. Jacobs (1982) has carefully examined all the possible negative effects of ozonetherapy. In spite of the famous “toxicity” of ozone, it appears that the incidence is only 0.0007%, one of the lowest in medicine. Four deaths due to direct IV injection of the gas were included in his data, but since 1982 other deaths due to malpractice have occurred, of which at least three in Italy. Thus Jacobs’ data are valuable only with regard to side effects such as nausea, headache, tiredness and the like.
The reader will have to trust the Italian experience: at the Verona Congress (1999), Dr. Giuseppe Amato, who has always worked at the Hospital in Conegliano (Veneto) and is a very scrupulous ozonetherapist, reported only minor side effects and no sequelae in a thousand patients treated with AHT for several years. Our experience at the Siena University Hospital is also significant: since 1995, we have performed about 8,000 major AHT in ARMD patients and about 100 in patients with fibromyositis, as well as about 800 EBOO sessions, countless topical applications in chronic ulcers of the limbs, and either direct (intradisc) or indirect (chemical acupuncture with oxygen-ozone in the paravertebral muscles) applications in about 80 patients with backache.
Firstly, regarding side effects occurring during and after major AHT, we have to distinguish about 5,000 treatments performed between 1995 and June 2000, unfortunately using PVC autotransfusion bags. These contained 63 ml of CPD (up to 450 ml blood could be collected), but usually only 200–250 ml blood was withdrawn to treat ARMD patients. In order to avoid any contamination, the excess of CPD (about 30 ml) was not discarded and it was responsible for one of the following side effects. Moreover plastic autotransfusion bags had the following disadvantages:
Venous puncture was done with a venous fistula needle set (G17) and occasionally some patients fainted with fear. No case of lipothymia was observed, probably because, after blood collection during the ozonation process, a volume of about 100 ml saline was infused via the same needle.
Some patients (almost always women) reported a tingling sensation in the lips and tongue, most frequently towards the end of the reinfusion. This did not occur with very slow infusion, nor with the new atoxic system (sodium citrate solution well calibrated to the blood volume), nor with heparinized blood; hence this symptom has been attributed to an excessively rapid reinfusion with a transitory slight hypocalcaemia due to the excess of citrate.
During blood reinfusion, more frequently women (10–15%) have reported nausea, a feeling of stomach bloating and a strange metallic taste in their mouth, which could be due to Zn-stearate or Zn-2-ethyl hexanoate present as additives in PVC bags.
For about 1 day after the first 4–5 treatments, 20–30% of both male and female patients reported feeling tired. Another 10–20% had no symptoms, while 50% reported a feeling of wellness. It must be noted that in all of these patients (60–80 years old), the major AHT was performed with a constant ozone concentration of 65–70 mcg/ml per ml of blood, without scaling up the dosage. In retrospect, this was a mistake and particularly in aged patients we must begin with a low ozone dose (20 mcg/ml) and slowly scale up to 40–50 mcg/ml. Since 2001, we have adopted the strategy: “start low (10–20 mcg/ml), go slow” (up to 40–80 mcg/ml, if necessary) and no side effects have been noted.
After 4–12 AHT sessions, four women patients (one with the history of an episode of anaphylactic shock to a wasp-sting) had a sudden appearance of a diffuse erythematous skin rash, with itching, nausea, hot flushes and slight hypotension, at the end of a blood reinfusion. Intravenous infusion of 1 g methyl-prednisolone Na-succinate relieved the symptoms in about 2 h. Interestingly, before undergoing ozonetherapy, one of these patients had participated as a control and had received 12 oxygenated (no ozone present) AHT without any problem. These cases of definitive intolerance were attributed to progressive sensitization to an immunogen due to phthalates bound to lipoproteins or to other PVC-additive components released after ozone addition.
From June 2000 until March 2004, we have been using the new atoxic system (glass, etc.), a precise volume of 3.8% Na Citrate to blood (1:9 v/v or 25–225 ml or exceptionally 30–270 ml) and the slow scaling up of the ozone concentration (usually from 10 to 60 mcg/ml). ALL OF THE ABOVE-MENTIONED SIDE EFFECTS HAVE DISAPPEARED, AND NO OTHERS HAVE APPEARED. MOREOVER, NO ALLERGIC-LIKE INTOLERANCE HAS BEEN OBSERVED. BECAUSE THE GLASS BOTTLE IS UNDER VACUUM, BLOOD IS DRAWN EASILY AND QUICKLY WITH A SMALLER NEEDLE (G19). IN ANY CASE THE USE OF PVC BAGS HAS BEEN PROHIBITED BY THE MINISTRY OF HEALTH.
Today ozone is widely used in orthopaedics, particularly in the case of low back ache (10.1007/978-90-481-9234-2_9) and it has become fashionable to inject the gas mixture of oxygen-ozone into the trigger points detectable in the paravertebral muscles of patients. I defined this approach as “chemical acupuncture” (Bocci, 1998a) and a likely explanation is that ozone acts locally on nociceptors and evokes a rapid and effective (in about 2/3 of patients) antinociceptive response through chemical mediators. While direct intradisc injection of oxygen-ozone (to degrade the proteoglycans of the herniated disc) remains in the hands of orthopaedics and neurosurgeons, some physicians decide overnight to become ozonetherapists and, with the opportunistic encouragement of an ozone generator salesman, begin to practise the indirect method without knowing anything about ozone. This situation has some risks: in May 2001, one death in Naples was due to this therapy. Immediately after IM injection, ozone dissolves locally in the interstitial water and generates several ROS: if, at the first administration, the ozone concentration is 20–25 mcg/ml and the gas volume exceeds 10 ml, a very acute pain may occasionally cause vagal hypertone (inotropic and chronotropic negative effects), which may culminate in cardiac arrest. If the patient is lucky, he will recover or undergo only transitory lipothymia (bradycardia, hypotension, profuse perspiration, transitory loss of consciousness, etc.). Therefore, it is advisable to practise “chemical acupuncture” with the usual precaution and by injecting the gas very slowly. It is advisable to remind the patient of the aphorism “no pain, no gain” and that the pain will be bearable and will last only for a few minutes. In general, the improvement of backache outweighs the transitory therapeutic pain, so that the compliance is good. With a proper injection, the risk of oxygen embolism is nil and only one case of subcutaneous haematoma has been reported (Fabris et al., 2001). The direct intradisc injection may present very slight side effects and rare transitory cephalea. However, in the case of a herniated cervical disk in a young athlete, Alexandre et al. (1999) reported that the patient presented a bilateral amaurosis fugax after the injection, which fortunately reversed after 1 day. This serious complication can more likely be attributed to transitory ischaemia of the vertebral arteries due to an erroneous position of the head during ozonetherapy than to the ozone itself.
If ozonetherapy is performed correctly, it tends not to cause problems but the ozonetherapist must be able to overcome any emergency because a delayed intervention may end with death. He must know all the steps of basic life support (BLS) and have at hand the Ambu, medical oxygen, an automated defibrillator and a few ampoules of epinephrine, atropine and corticosteroids (Cummins, 1994).
On the other hand ozonetherapy procures positive effects: about 2/3 of patients, particularly those that feel depressed and asthenic, report a feeling of well being and euphoria after a few treatments. Whether this is due to the “staging” of the procedure or to ozone or to oxygen, or to all these factors, remains unknown. For a long time, I have wished to perform a kinetic study of the hormonal pattern (CRH, ACTH, Cortisol, DHEA, GH, β-endorphin, somatostatin plasma levels) after these types of treatment. Needless to say, such a study must be performed with appropriate controls and this, unfortunately, will imply the collection of many blood samples in volunteers. It will be more difficult to evaluate whether there is also a concomitant serotonin and/or dopamine upsurge.
An unresolved question is the optimal time of the day to perform the systemic approaches. On the basis of circadian rhythms of crucial hormones, I believe that the afternoon is the preferable period (Bocci, 1985b), but this is not always possible.
Can Ozonetherapy Interfere with Conventional Treatment?
Before endeavouring ozonetherapy, the physician must know all the medical history of the patient and the drugs in current use. Mattassi et al. (unpublished), have observed a sudden marked hypotension upon rapid reinfusion of ozonated blood in patients treated with ACE inhibitors. This effect may be due to the activation of the kallikrein-kininogen cascade, as reported by Shiba et al. (1997) and Abe et al. (1998). However plasma bradykinin is degraded within minutes and a very slow infusion reduces this adverse effect. We have confirmed Mattassi’s observations in two patients and I can suggest the following: firstly, warn the patient to omit taking the ACE inhibitor on the day of the AHT’s treatment; secondly, slow down the infusion of ozonated blood and thirdly, keep ready a vasopressive drug.
Are There Contraindications for Ozonetherapy?
This is particularly important for systemic therapy and the risk of ozonetherapy must be weighed against the clinical condition of the patient. Moreover, the following situations preclude or limit its use:
Patients with a significant deficit of G-6PD. Favism is a haemolytic disease observed in some people lacking the enzyme. This enzyme provides crucial reducing equivalents able to abolish excessive oxidation and intensive haemolysis (10.1007/978-90-481-9234-2_4). The problem of genetic susceptibility to ozone is surely appropriate (McDonnell, 1991; Prows et al., 1997; Kleeberger et al., 1997) and besides individual TAS levels, each patient has a different enzymatic profile, different absorption and metabolism of antioxidants and so on. However, Miller et al. (1993) after measuring the total antioxidant status in a great number of Europeans have found consistent values that exclude a significant depletion during ozonetherapy. That is the reason why in the frequent case that the biochemistry of the ozonation process cannot be measured (Hernandez, 2007) by private physicians, we can still cautiously perform ozonetherapy (Bocci, 2007a).
Pregnancy, particularly the early phase, to exclude any mutagenic risk, although it is unlikely.
Patients being treated with ACE inhibitors.
Abnormal situations with hyperthyroidism, thrombocytopenia and serious cardio-vascular instability.
Allergy to ozone has been claimed, but what is it? I reckon that the hypersensitivity of asthmatic patients breathing air polluted with ozone has created some confusion (McConnell et al., 2002a). Moreover the use of plastic bags may induce the release of an allergic components from the plastifying material.
Last but not least important, 4 years ago I felt absolutely necessary to clarify the controversial issue of ozone toxicity. The title of the paper was clear: “Is it true that ozone is always toxic? The end of the dogma” (Bocci, 2006b). The undoubtful strong reactivity of ozone and its toxicity for the respiratory system during prolonged exposure to polluted air have contributed to establish the dogma that ozone is always toxic and consequently it should never be used in medicine. However, during the last 15 years, a clear understanding of the action of ozone in biology and medicine has clarified that the dogma does not hold true. It appeared essential to compare the topography, anatomical and biochemical characteristics of the organs exposed to ozone versus the potent antioxidant capacity of blood exposed to a small and precisely calibrated dose of ozone only for a few minutes. It is enough to remind that the total surface of the human lungs is about 70 m2 and that the surface of the epithelial lining fluid (ELF) is only protected by a liquid film as thin as 0.1 μm, so that its total volume is only between 17 and 20 ml, absolutely insufficient to protect the alveoli from the continuous presence of air-ozone-contaminated. This gas does not penetrate into the cells but readily dissolves and reacts in the thin layer of water generating toxic molecules and causing inflammation, thus establishing a vicious circle with local and generalized damages. Indeed the ELF, by containing only a minor quantity of protective antioxidants is unable to neutralize ozone. (Table 7.1). In contrast, both blood and extravascular fluids are constituted by large liquid volumes and contain a wealth of antioxidants able to readily neutralize the small ozone dose. It is also instructive to examine Fig. 7.1, which shows how the respiratory system subjected to a chronic ozone inhalation steadily releases a huge amount of toxic compounds able to enter the circulation and cause multiorgan damages. It is therefore understandable how serious is ozone toxicity for the organism and why morbidity and mortality has increased in contaminated American cities (Bell et al., 2005; Ruidavets et al., 2005; Jerrett et al., 2009). This paper published in Toxicology and Applied Pharmacology has received many positive comments and it is hoped that the issue of ozone toxicity had been definitively clarified.
Table 7.1.
A comparison between the composition of ELF and blood of a normal 70 kg human showing the striking difference in antioxidant capacity of these two fluids
| ELF | Blood |
|---|---|
| Volume: 17–20 ml | Plasma volume: ~ 2.71 |
| Eruthrocytes: ~ 2.3 kg | |
| Total proteins: ~ 7 mg/ml | Total plasma proteins: ~ 75 mg/ml |
| (total: ~ 130 mg) | (total: ~ 202.5 g) |
| Albumin: ~ 3.5 mg/ml | Albumin: ~ 45 mg/ml |
| (total: ~ 63 mg) | (total: ~ 121.5 g) |
| Transferrin:~ 0.3 mg/ml | 2–4 mg/ml |
| Ceruloplasmin: ~ 25 μg/ml | 140–400 μg/ml |
| Lactoferrin: ~ 0.5 μg/ml | ? |
| GSH: 300–400 μM | In plasma: ~ 3 μM |
| In erythrocytes: ~ 2.2 mM | |
| Vitamin E: ~ 2 μg/ml | 10–20 μg/ml |
| Vitamin C: ~ 3.5 μg/ml | ~ 9 μg/ml |
| Uric acid: ~ 0.05 mg/ml | 0.04–0.07 mg/ml |
| Glucose: ~ 0.4 mg/ml | 0.7–1.0 mg/ml |
| Total Bililrubin: ? | ~ 1.0 mg/dl |
| Na: ~ 82; Cl: ~ 84; K: ~ 29 mM | Na: ~ 139; Cl: ~ 103; K: ~ 4 mM |
| pH 6.9 | pH 7.4 |
? refers ‘the value is unknown’
Fig. 7.1.
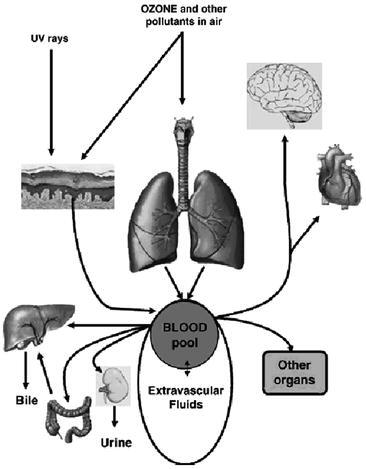
A chronic exposure of the pulmonary system and skin to ozone and UV radiation causes the formation of a great number of toxic compounds, which, in spite of the natural antioxidant system, flow continuously into the blood and reach vital organs complicating the pulmonary damage
Does Prolonged Use of Ozonetherapy Give Rise to Sequelae Such as Tumours, Degenerative Disease, ETC?
The question is theoretically appropriate because ozone induces ROS and these are at least partly responsible for many ailments and ageing. This is the sixth time that I propose that all national Health Authorities oblige all ozonetherapists (who ought to be physicians with appropriate specific training) to keep a medical register in which they should record all pathological events appearing in patients during and after ozonetherapy.
The following form may be useful:
Surname and Name … … … … … … … … … … … …
Sex … … … … … … … Age … … … … … … … … .
Address … … … … … … … … … … … … … … … .
Type of employment … … … … … … … … … … … . .
Diagnosis … … … … … … … … … … … … … … …
Type of O2-O3 treatment … … … … … … … … … … .
Period of treatment: from … … … to … … … … … … .
Clinical evolution … … … … … … … … … … … … .
Whenever possible, the patient should be followed during subsequent years and it should be noted if the disease improves or persists or worsens, as well as the possible appearance of new pathologies related to oxidative stress.
Great attention should be given to:
agranulocytosis, asthma, atherosclerosis, bone marrow dysplasia or atrophy, cataract, degenerative diseases, emphysema, fibrosis (paravertebral muscles), gastrointestinal diseases, hepatitis, hypertension, leukemia and other haematological neoplasias, multiple sclerosis, neurodegenerative diseases (Parkinson, dementias), renal sclerosis, rheumatoid arthritis, scleroderma, skin carcinomas, SLE, solid tumours, others.
Conclusions
As other medical approaches using potent drugs, ozonetherapy may present some risks, which can be avoided if the ozonetherapist is theoretically and practically well prepared. The use of judicious ozone doses related to the antioxidant capacity of tissues and body fluids excludes the risk of citotoxicity and mutagenicity. Adverse effects, noted with the use of PVC bags and an excess of citrate, are now totally avoided with the use of the optimized method using ozone-resistant glass bottles. Great care must be exercised when injecting the gas mixture directly into the paravertebral muscles: if this is done correctly, most patients comply well with the therapy. There are a few cases when ozonetherapy is contraindicated and, whenever possible, we must follow the patients during subsequent years and note any possible toxicity or new pathologies.
References
- Abe H., Ikebuchi K., Shimbo M., Sekiguchi S. Hypotensive reactions with a white cell-reduction filter: activation of kallikrein-kinin cascade in a patient. Transfusion. 1998;38:411–412. doi: 10.1046/j.1537-2995.1998.38498257383.x. [DOI] [PubMed] [Google Scholar]
- Alexandre A., Pentimalli L., Rigobello L., Corò N. Amaurosi fugax in un caso di discolisi cervicale mediante O2–O3. In: Ceccherelli F., Giron F., editors. L’Ozonoterapia nel 2000. Torino: Edizioni Libreria Cortina; 1999. pp. 141–144. [Google Scholar]
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M. L., Dominici F., Samet J. M. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci V. Administration of interferon at night may increase its therapeutic index. Cancer Drug Del. 1985;2:313–318. doi: 10.1089/cdd.1985.2.313. [DOI] [PubMed] [Google Scholar]
- Bocci V. Ozone as a bioregulator. Pharmacology and toxicology of ozonetherapy today. J. Biol. Regul. Homeost. Agents. 1996;10:31–53. [PubMed] [Google Scholar]
- Bocci V. Ipotetici meccanismi di azione dell’ozono nel trattamento del conflitto discoradicolare, in Lombalgie e lombosciatalgie. In: Ceccherelli F., Ricciardi A., editors. Criteri di diagnosi e cura. Torino: Edizioni Libreria Cortina; 1998. pp. 331–340. [Google Scholar]
- Bocci V. Oxygen-ozone therapy, a critical evaluation. Dordrecht: Kluwer Academic Publischer; 2002. [Google Scholar]
- Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators Inflamm. 2004;13:3–11. doi: 10.1080/0962935062000197083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci V. Is it true that ozone is always toxic? The end of the dogma. Toxicol. Appl. Pharmacol. 2006;216:493–504. doi: 10.1016/j.taap.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Bocci V. Can ozonetherapy be performed if the biochemistry of the process cannot be controlled? Arch. Med. Res. 2007;38:584–585. doi: 10.1016/j.arcmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Carlsson L. M., Jonsson J., Edlund T., Marklund S. L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. Y., Zhang L. Y., Kleeberger S. R. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L537–L546. doi: 10.1152/ajplung.2001.280.3.L537. [DOI] [PubMed] [Google Scholar]
- Cummins R. O. Textbook of advanced cardiac life support. Dallas, TX: Scientific Publishing American Heart Association; 1994. [Google Scholar]
- Diaz, S., Menendez, S., Eng, L., and Fernandez, I., 1995, No increase in sister chromatid exchanges and micronuclei frequencies in human lymphocytes exposed to ozone in vitro, in Proceedings Ozone in Medicine 12th World Congress of the International Ozone Association, 15th to 18th May 1995, Lille France (International Ozone Association, Ed.), Instaprint S.A., Tours, pp. 43–52.
- Diaz-Llera S., Gonzalez-Hernandez Y., Prieto-Gonzalez E. A., Azoy A. Genotoxic effect of ozone in human peripheral blood leukocytes. Mutat. Res. 2002;517:13–20. doi: 10.1016/s1383-5718(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Dumaswala U. J., Wilson M. J., Wu Y. L., Wykle J., Zhuo L., Douglass L. M., Daleke D. L. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic. Res. 2000;33:517–529. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- Fabris G., Tommasini G., Petralia B., Lavaroni A., De Nardi F., De Luca G., Biasizzo E., Iaiza F. L’ossigeno-ozono terapia intra-foraminale. Riv. Neuroradiol. 2001;14:61–66. [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Biology of disease. Mechanisms of cell injury by activated oxygen species. Lab. Invest. 1990;62:670–679. [PubMed] [Google Scholar]
- Foksinski M., Bialkowski K., Skiba M., Ponikowska I., Szmurlo W., Olinski R. Evaluation of 8-oxodeoxyguanosine, typical oxidative DNA damage, in lymphocytes of ozone-treated arteriosclerotic patients. Mutat. Res. 1999;438:23–27. doi: 10.1016/s1383-5718(98)00155-7. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fukuto J. M., Miller T., et al. The chemistry of cell signalling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch. Biochem. Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Miller B. E., Mudd J. B. Reaction of ozone with human erythrocytes. In: Lee S. D., Mudd J. B., editors. Assessing toxic effects of environmental pollutants. Ann Arbor, MI: Ann Arbor Science Publishers; 1979. pp. 151–171. [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Fukunaga K., Nakazono N., Suzuki T., Takama K. Mechanism of oxidative damage to fish red blood cells by ozone. IUBMB Life. 1999;48:631–634. doi: 10.1080/713803566. [DOI] [PubMed] [Google Scholar]
- Galleano M., Puntarulo S. Role of antioxidants on the erythrocytes resistance to lipid peroxidation after acute iron overload in rats. Biochim. Biophys. Acta. 1995;1271:321–326. doi: 10.1016/0925-4439(95)00049-a. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Balchum O. J. Effect of ozone on lipid peroxidation in the red blood cell. Proc. Soc. Exp. Biol. Med. 1967;126:356–359. doi: 10.3181/00379727-126-32444. [DOI] [PubMed] [Google Scholar]
- Gooch P. C., Creasia D. A., Brewen J. G. The cytogenetic effects of ozone: inhalation and in vitro exposures. Environ. Res. 1976;12:188–195. doi: 10.1016/0013-9351(76)90023-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants: a personal view. Nutr. Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 2003;540:3–6. doi: 10.1016/s0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- Jacobs M.-T. Untersuchung uber Zwischenfalle und typische Komplikationen in der Ozon-Sauerstoff-Therapie. OzoNachrichten. 1982;1:5. [Google Scholar]
- Jerrett M., Burnett R. T., Pope C. A., et al. Long-term ozone exposure and mortality. N. Engl. J. Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeberger S. R., Levitt R. C., Zhang L. Y., Longphre M., Harkema J., Jedlicka A., Eleff S. M., DiSilvestre D., Holroyd K. J. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat. Genet. 1997;17:475–478. doi: 10.1038/ng1297-475. [DOI] [PubMed] [Google Scholar]
- Kowaltowski, A. J., de Souza-Pinto, N. C., Castilho, R. F. et al., 2009, Mitochondria and reactive oxygen species, Free Radic. Biol. Med. in press, May 7. [DOI] [PubMed]
- Larini A., Bianchi L., Bocci V. The ozone tolerance: (I) Enhancement of antioxidant enzymes is ozone dose-dependent in Jurkat cells. Free Radic. Res. 2003;37:1163–1168. doi: 10.1080/10715760310001604170. [DOI] [PubMed] [Google Scholar]
- Larini A., Bianchi L., Bocci V. Effect of 4-hydroxynonenal on antioxidant capacity and apoptosis induction in Jurkat T cells. Free Radic. Res. 2004;38:509–516. doi: 10.1080/10715760410001684649. [DOI] [PubMed] [Google Scholar]
- Last J. A., Warren D. L., Pecquet-Goad E., Witschi H. Modification by ozone of lung tumor development in mice. J. Natl. Cancer Inst. 1987;78:149–154. doi: 10.1093/jnci/78.1.149. [DOI] [PubMed] [Google Scholar]
- Leist M., Raab B., Maurer S., Brigelius-Flohé R. Conventional cell culture media do not adequately supply cells with antioxidants and thus facilitate peroxide-induced genotoxicity. Free Radic. Biol. Med. 1996;21:297–306. doi: 10.1016/0891-5849(96)00045-7. [DOI] [PubMed] [Google Scholar]
- Matos H. R., Di Mascio P., Medeiros M. H. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch. Biochem. Biophys. 2000;383:56–59. doi: 10.1006/abbi.2000.2035. [DOI] [PubMed] [Google Scholar]
- McConnell R., Berhane K., Gilliland F., London S. J., Islam T., Gauderman W. J., Avol E., Margolis H. G., Peters J. M. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- McDonnell W. F. Intersubject variability in human acute ozone responsiveness. Pharmacogenetics. 1991;1:110–113. doi: 10.1097/00008571-199111000-00010. [DOI] [PubMed] [Google Scholar]
- Merz T., Bender M. A., Kerr H. D., Kulle T. J. Observations of aberrations in chromosomes of lymphocytes from human subjects exposed at a concentration of 0.5 ppm for 6 and 10 hours. Mutat. Res. 1975;3:299–302. doi: 10.1016/0165-1161(75)90095-3. [DOI] [PubMed] [Google Scholar]
- Miller N. J., Rice-Evans C., Davies M. J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Prows D. R., Shertzer H. G., Daly M. J., Sidman C. L., Leikauf G. D. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 1997;17:471–474. doi: 10.1038/ng1297-471. [DOI] [PubMed] [Google Scholar]
- Richter C., Gogvadze V., Laffranchi R., Schlapbach R., Schweizer M., Suter M., Walter P., Yaffee M. Oxidants in mitochondria: from physiology to diseases. Biochim. Biophys. Acta. 1995;1271:67–74. doi: 10.1016/0925-4439(95)00012-s. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. USA. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruidavets J. B., Cournot M., Cassadou S., et al. Ozone air pollution is associated with acute myocardial infarction. Circulation. 2005;111:563–569. doi: 10.1161/01.CIR.0000154546.32135.6E. [DOI] [PubMed] [Google Scholar]
- Sato Y., Sato K., Suzuki Y. Mechanisms of free radical-induced hemolysis of human erythrocytes: comparison of calculated rate constants for hemolysis with experimental rate constants. Arch. Biochem. Biophys. 1999;366:61–69. doi: 10.1006/abbi.1999.1205. [DOI] [PubMed] [Google Scholar]
- Shiba M., Tadokoro K., Sawanobori M., Nakajima K., Suzuki K., Juji T. Activation of the contact system by filtration of platelet concentrates with a negatively charged white cell-removal filter and measurement of venous blood bradykinin level in patients who received filtered platelets. Transfusion. 1997;37:457–462. doi: 10.1046/j.1537-2995.1997.37597293873.x. [DOI] [PubMed] [Google Scholar]
- Shinriki N., Suzuki T., Takama K., Fukunaga K., Ohgiya S., Kubota K., Miura T. Susceptibilities of plasma antioxidants and erythrocyte constituents to low levels of ozone. Haematologia. 1998;29:229–239. [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J. Biol. Chem. 1991;266:2005–2008. [PubMed] [Google Scholar]
- Tarkington B. K., Duvall T. R., Last J. A. Ozone exposure of cultured cells and tissues. Meth. Enzymol. 1994;234:257–265. doi: 10.1016/0076-6879(94)34093-5. [DOI] [PubMed] [Google Scholar]
- Van der Zee J., van Beek E., Dubbelman T. M. A. R., Van Steveninck J. Toxic effects of ozone on murine L929 fibroblasts. Biochem. J. 1987;247:69–72. doi: 10.1042/bj2470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorin K. Review of the genotoxicity of ozone. Mutat. Res. 1992;277:221–238. doi: 10.1016/0165-1110(92)90045-b. [DOI] [PubMed] [Google Scholar]
- Wiseman H., Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi H., Espiritu I., Pinkerton K. E., Murphy K., Maronpot R. R. Ozone carcinogenesis revisited. Toxicol. Sci. 1999;52:162–167. doi: 10.1093/toxsci/52.2.162. [DOI] [PubMed] [Google Scholar]
- Hernandez F.A. To what extent does ozone therapy need a real biochemical control system? assessment and importance of oxidative stress. Arch Med Res. 2007;38:571–578. doi: 10.1016/j.arcmed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Yakes F. M., Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]


