Abstract
Background
Nanotechnology is gaining emerging interest in advanced drug discovery therapeutics due to their tremendous properties including enhanced delivery of therapeutic payload, extensive surface to volume ratio, high permeability, retention behaviors, etc. The gold nanoparticles (AuNPs) are favored due to their advanced features, such as biogenic, tunable physiochemical response, ease in synthesis, and wide range of biomedical applications. The phytochemicals have been focused to design Au nano-carrier-based conjugation for active-targeting drug delivery due to their nano conjugation ability.
Aim
The present study describes the facile synthesis of 20nm spherical AuNPs and their conjugation with reported anti-cancer phytocompound Withanolide-A (1).
Methods
The 20nm sAuNPs were synthesized chemically and characterized their phytochemical gold nanoconjugates through UV-visible spectroscopy, dynamic light scattering (DLS) and transmission electron microscopy (TEM) imaging techniques. The anti-cancer therapeutic potentials were tested with both nanoconjugates and pure WithanolideA (1) by using SKBR3 breast cancer cells line.
Results
The synthesized sAuNPs showed significant conjugation with Withanolide-A and showed stability. Furthermore, these Au nanoconjugates with Withanolide-A (1) significantly induce blockage of SKBR3 cell growth at half maximal active concentration that compared to pure Withanolide-A (1).
Conclusion
Our findings provide a foundation to further progress how they can overcome cancer drug resistance by conjugating active drugs in combination with AuNPs through optimizing the effective drug concentration and removing the surface barrier.
Keywords: Withania somnifera, Withanolide-A, gold nanoparticle; AuNP, breast cancer, anti-cancer and incucyte
Introduction
Nanotechnology has received a lot of attention in recent years because of its enhanced payload to targeted therapeutics sites, modifying cellular permeability, uptake or pharmacokinetic profile.1–3 Among the diverse range of nanoparticle carriers (iron oxide, silicone material and quantum dots) the AuNPs are favored due to their high biocompatibility, quenching efficiencies, facile synthesis, multiple functionality and tunable optical nature.4–7 Several studies8–11 reported a mechanism of action of AuNPs as an active carrier of drug payload to targeted site. Their interaction modifies the cell and surface protein interaction kinetics profile; however, their action varies with size and charge factor. The previously reported study describes through ultra-high-resolution imaging atomic force revealed that AuNPs carrier have tendency to alter membrane roughness of cell, which enhanced their cellular uptakes.12
Breast cancer is frequently diagnosed among women worldwide, with the highest rate in Australian women, diagnosed more than other types of cancer.13,14 The accelerated rate of mortalities of around 73% in the metastatic disease is usually linked with various genetic and epigenetic factors.13,15,16 The conventional therapies have a number of flaws: poor accessibility to tumor tissues, non-selectivity, severe side-effects – hair loss, diarrhea, high dose, less efficacy, and safety profile concerns – which always demand novel and more effective treatment methods for this type of cancer. In this context, we prepared AuNPs combinational strategy with a reported anticancer compound (Withanolide-A) is being using to overcome disease burden and more effective potential.17
The phytochemical-based remedies have been reported over thousands of years ago to treat multiple disorders in traditional medicinal system and many of them approved by Food and Drug Administration (FDA) clinically.18 In previous years Withania somnifera extract passed clinical trial phase one (ID number: NCT00817752) against cancer disorders in 2010 and powder extracts passed phase and second clinical trial (ID number: NCT00689195) against osteosarcoma in 2011. The withanolide compounds from the medicinal plant Withania somnifera have been extracted and tested in vitro and in vivo against a number of disorders; including as an anticancer, neuromodulators, antidiabetic, adaptogenic, cardioprotective, and neuroprotective agent, etc.19–21
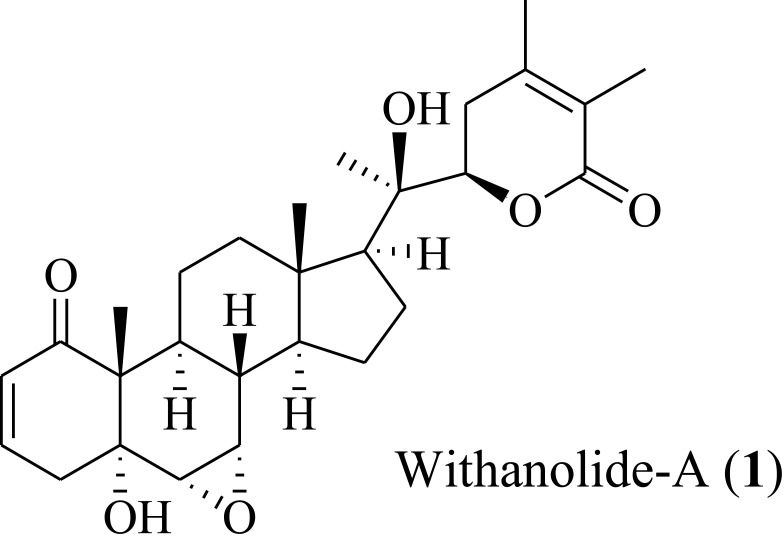
However, there are numbers of issues raised related to the use of Withanolide-A (1), so previous raised issues should be addressed; either concern with their low solubility or lesser bioavailability (rapid passage to type I, II type metabolism) and improved pharmacokinetics profile with enhanced targeted cellular uptake and delivery, which may modify its unique functions. Figure 1 showed the structural formula of tested compound 1.
Figure 1.
Structural formula of Withanolide-A (1). A phytocompound from Withania somnifera.
The application of nanobiotechnology along with the combination therapies is discussed in a number of studies, where nanoparticles conjugate with phytochemical for enhanced drug delivery mechanismresearchers.22–25 The current study was designed to synthesize 20nm sAuNPs and their conjugation with the previously reported anticancer compound Withanolide-A (1),26,27 which was extracted from the Withania somnifera plant and purified to test cytotoxicity against the SK-BR3 breast cancer cells line. To the best of our knowledge only limited work has evaluated this before, so we believe this is first time there has been a comprehensive study of 20nm sAuNPs+1 conjugation and cytotoxicity study against the breast cancer SKBR3 cell line.
Experimental Design
Chemical Materials
The Withanolide-A (1) (CAS: 32911–62-9) was purchased from ChromaDex. HAuCl4. 3H2O (99.99%); tri-sodium citrate (Na3 citrate), tannic acid and K2CO3 (Potassium carbonate) were purchased from Sigma-Aldrich (Australia). The phosphate buffer saline (PBS) was procured from Gymea, NSW, Australia. Formvar/carbon coated, square 200 mesh size copper grids and Millex-GV syringe filters (0.22 μm, PVDF, Cat. No. SLGV033RS) were purchased from Sigma-Aldrich (Australia). The RPMIM (Roswell Park Memorial Institute Medium), MTT reagent and DMSO (Me2SO) were purchased from Life Sci. Technology, Australia. The deionized H2O (electric resistivity >18 MΩ.cm) was supplied by Milli-Q.
Cell Culture
The SKBR-3 cell line was a kind gift from Dr. Thomas Grewal at the University of Sydney. The cell line procured from the American Type Culture Collection (ATCC). The Cell line was authenticated via short tandem repeat (STR) polymorphism, fingerprint analyses and single nucleotide polymorphism (SNP), passaged for less than 6 months, and cultured as formerly described.28
Instrument Used
The UV-Vis spectrophotometer (Shimadzu 2600, Japan), Ultracentrifugation SorvallTM WX Floor (Thermo scientific USA), DLS (Nano ZS, Malvern Instruments, UK), transmission electron microscopy (TEM; JEOL-JEM-1400, Tokyo, Japan), Incucyte, Multi-plate reader (Perkin-Elmer victor X4, US), Incucyte (Incucyte® ZOOM Live-cell Analysis System ESSEN, UK) were used during study.
Synthesis of Multifunctional and Spherical 20nm Au Nanoparticles (sAuNPs) and Conjugation with Withanolide-A (1) Phytochemical
Preparation of Different Working Solutions
The AuCl3 solution (12.5 mM or 0.05%) was prepared by the addition of 49.2 mg of HAuCl4.3H2O in sterile Milli-Q H2O (10 mL). The Na3 citrate (1% TSC) solution was prepared by dissolving 100 mg of Na3 citrate in sterile Milli-Q H2O (10 mL). A 2.5 mM tannic acid (TA) solution was prepared by dissolving 21.2 mg of it in 1% TSC solution (5 mL). The aq. K2CO3 solution (150 mM) was prepared by dissolving of 103.6 mg anhydrous K2CO3 in sterile Milli-Q H2O (5 mL). A 10 mM or 1 × PBS storage buffer (pH 7.4) was prepared by dissolving 2 tablets in 200 mL of sterile Milli-Q H2O and was filtered through 0.22 μm filter paper to obtain a 10 mM or 1× PBS phosphate buffer solution (pH 7.4 at 25 °C).
Colloidal sAuNPs Synthesis and Conjugation with Withanolide-A (1)
The synthesis of 20nm colloidal mono-dispersed sAuNPs solution was prepared following a protocol reported by Turkevich et al (2006), Frens et al (1973)29,30 and Jordi Piella et al (2016).31 The 12.5 mM (0.05%) AuCl3 solution in Milli-Q H2O, 1% TSC in Milli-Q H2O, 2.5 mM TA dissolved in 1% TSC and 150 mM aq. K2CO3 in Milli-Q water were prepared. The 0.05% AuCl3 solution (0.6 mL) was diluted to 25 mL with Milli-Q H2O and stirred for two minutes. Then, 1% TSC (150 µL), 2.5 mM TA (40 µL) and 150 mM aq. K2CO3 (40 µL) were added into a previously mixed solution and stirred slowly for 5 minutes at room temperature. The color of the mixed solution changed from light yellow to ash black. The wrapped glass container was charged with magnetic bar at 60 °C for 5 minutes without stirring. The mixture changed the color from dark to wine red and finally purple within 1–2 minutes due to presence of high reacting citrate-tannic acid complex. The heated Au nanoparticles colloidal solution was kept at 40 °C for 10 minutes followed by cooling to ambient temperature and finally stored at 4 °C. After cooling, the prepared sample was diluted around 1.0 OD (Optical Density) value.32 For conjugation, 10 μg of Withanolide-A (1) was dissolved in 1 mL of sAuNPs solution followed by overnight incubation in cold room with gentle shaking. For stability analysis, 10µL of 10% NaCl solution was added into conjugated and control samples for 15 minutes to check the color change as well as UV/Vis, TEM and Zetasizer measurements.33
Conjugation Purification
The number of moles in 1 mL gold solution was calculated and various molar excess compounds were tried to cover the gold particle surface. Interestingly, we found a nice surface covering with 10 µg/mL compound concentration that was checked with UV shift. There was no absorption of compound detected in supernatant. All the compounds might be bound to nanoparticles according to reports by Farooq et al (2018).1 Abs of conjugated compound-unconjugated compound in supernatant/Abs conjugated compound*100.
Characterization of Bare sAuNPs and Phyto-Conjugates
UV-Vis Spectroscopy
To obtain absorption spectra phyto-conjugate (70 μL of bare sAuNPs) solution and Withanolide-A (1), the solution was measured from 200 to 800 nm with blank subtraction of media and distil H2O.
Dynamic Light Scattering (DLS)
Certain physical parameters of conjugated nanoparticle such as polydispersity index (PDI), average particle hydrodynamic diameter and surface charge were measured with DLS. The sample was diluted to1:10 and 1:2000 with Milli-Q H2O for size and zeta-potential analysis respectively. All the measurements were done in triplicate and reported as average of ± standard deviation (SD).
Transmission Electron Microscopy (TEM)
To check the particle core image before and after conjugation, TEM analysis was performed by using small metal grid. Briefly, 10 μL of 1:4 diluted sample solution with and without conjugation were applied on Cu grid having C coating. Next, the grid was air dried overnight and analysis was performed at 120 kv.
Cell Culture and Cytotoxic Evaluation
The SKBR3 breast cancer cells were grown into RPMI media that was supplemented with 10% fetal bovine serum (FBS) and 1% suspension of penicillin-streptomycin. The flask mixture could grow under standard condition in incubator, which was humidified with 5.0% CO2 level at 37 °C. To check the Au-phytoconjugates cytotoxicity, 5000 cells/well were seeded for 24 hours. The next day’s cells were treated into collagen-coated Thermanox plastic well with corresponding concentrations of the tested sample 10, 20, 40 µg/mL and replenished with fresh media containing negative DMSO 1% and AuNPs 40µL/mL with positive control 10 µg/mL (Cisplatin drug) in triplicates. After specific time intervals (0, 24, 48, and 72 h) image collection of the well was done with 10× magnification using Incucyte zoom. After 72 hours, the percentage of confluence/time was obtained by using v2016A incucyte software to generate cell growth curves over time. The cell viability assay was performed by using MTT reagent (20 μL/100 mL) and cell viability count was done according to following equation:
% cell viability = (Average of treated cell absorbance-BLANK/Average of non-treated cell absorbance-BLANK) × 100
Where, blank is MTT reagent and non-treated cells are negative control such as media and AuNPs correspond concentration in media.34
Statistical Analysis
All the experiment was performed in triplicate to obtained the mean value and SD. For data processing SPSS software 19 and Microsoft Excel were used. The difference in activities of various group in term of mean was checked by Tukey post-hoc analysis where p<0.05 is an indication of statistically significant.3
Results and Discussion
Synthesis of Au-Phyto-Nanconjugate and Characterization
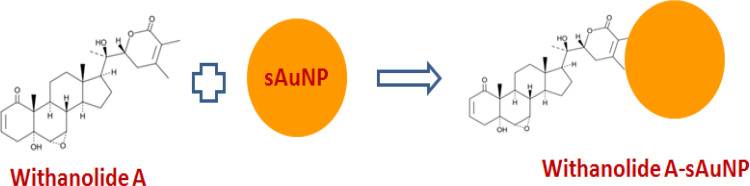
The AuNPs can be synthesized by various in vitro and in situ methods but uniform spherical particle only acquired by limited strategies. For ease of method working procedure and apparatus used chemical reduction considered as one of favorite method for synthesis of these spherical AuNPs synthesis, which involves reduction and stabilization. In this study, chemical synthesis method was used to prepare spherical AuNPS. It is already known that phytochemical withanolides possess strong hydrophobic hydrogen and electrostatic interactions. Herein, we conjugate Withanolide-A (1) 10μg/mL with spherical AuNP solution according to the same strategy as outlined in26 Figure 2.
Figure 2.
Conjugation of Withanolide-A with 20nm sAuNPs.
After conjugation the phytonanoconjugte of sAuNP+1 subjected to ultracentrifugation to obtained purified red loose pellets for 40min at 17,000 rpm at 10°C. The pellet was used to perform further analysis.
UV-Vis
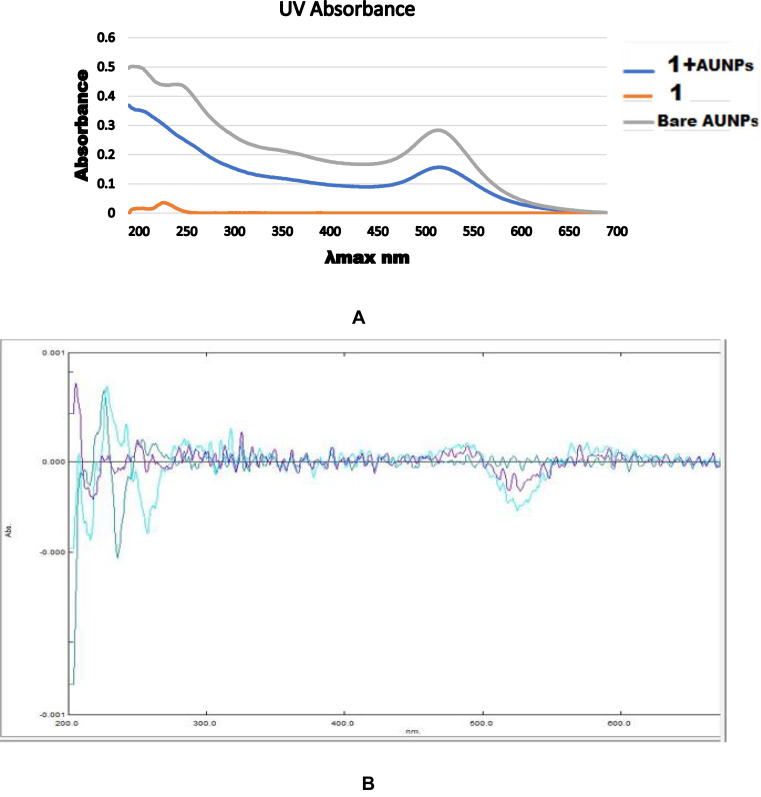
The UV–Vis spectroscopy is a sensitive method for detecting colloidal metal, due to exhibiting a characteristic absorption peak at about 490–600 nm, which is attributed to surface plasmon excitation. The absorption band maximum for bare sAuNPs and phytoconjugates were observed at λmax 523.5 and 525.5 nm respectively (a little bit shift is mentioned in Table 1 and shown in Figure 3) (supplemental Figure 1).
Table 1.
Phyto-Nanoconjugate Size, Zeta Potential and UV-Absorption Maximum and TEM Characterization
| Sample | Z-Avg Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | λmax (nm) | TEM (nm) |
|---|---|---|---|---|---|
| Bare sAuNP | 25.35±0.610 | 0.285±0.020 | −44.3±0.860 | 523.5 | 20 |
| Phyto-nanoconjugate AuNPs | 29.73±0.650 | 0.3±0.009 | −20±0.100 | 525.5 | 20 |
Note: Values are means ± SD (n=3) of three separate experiments.
Abbreviations: TEM, transmission electron microscopy; UV, ultraviolet.
Figure 3.
Image (A) UV absorbance of 20 nm Bare spherical Au nanoparticles (sAuNPs), Withanolide-A (1) and 1+sAuNPs. Image (B). Secondary derivative green line corresponds to compound 1 absorbance followed by blue line Au nanoparticles and purple 1 +sAuNPs absorbance.
These shifts indicate the formation of adsorbed layers around AuNPs. Aggregation of AuNPs is accompanied with a change of color: optical absorption spectroscopy quantifies this process. We did not noticed any color change in our tested samples before and after conjugation, which is similar reliability parameter as reported in previous finding.35,36 These sAuNPs showed λmax at 523.5 nm, which is quite close in agreement with Shafiq et al (2018)37 work who reported that 20nm particle is for λmax 522 nm. Furthermore, secondary derivatives were recorded which ensure us confidence on binding between two moieties with stability Figure 3.
Dynamic Laser Scattering DLS
Characteristic features, such as hydrodynamic size; PDI and surface charge were measured with DLS before and after conjugation with average corresponding value (Table 1). Before and after conjugation, Zeta potential value showed from −44.3±0.86 mV to −20±0.1 mV, which was a significant difference due to the presence of a slightly positively charged hydrogen atom of 1 on the Au surface. The PDI changed a little bit from 0.285±0.02 to 0.3±0.009, which was less than 0.3. It indicates that the particles were not aggregated and homogenous in size hydrodynamic size was less increased from 25.35±0.61nm to 29.73±0.65nm and authenticates that 1 molecule attached on Au surface.13 The results are presented in Table 1, before and after conjugation showed stability of this nanoconjugate. (See detail Supplemental Figure 2).
Transmission Electron Microscopy (TEM)
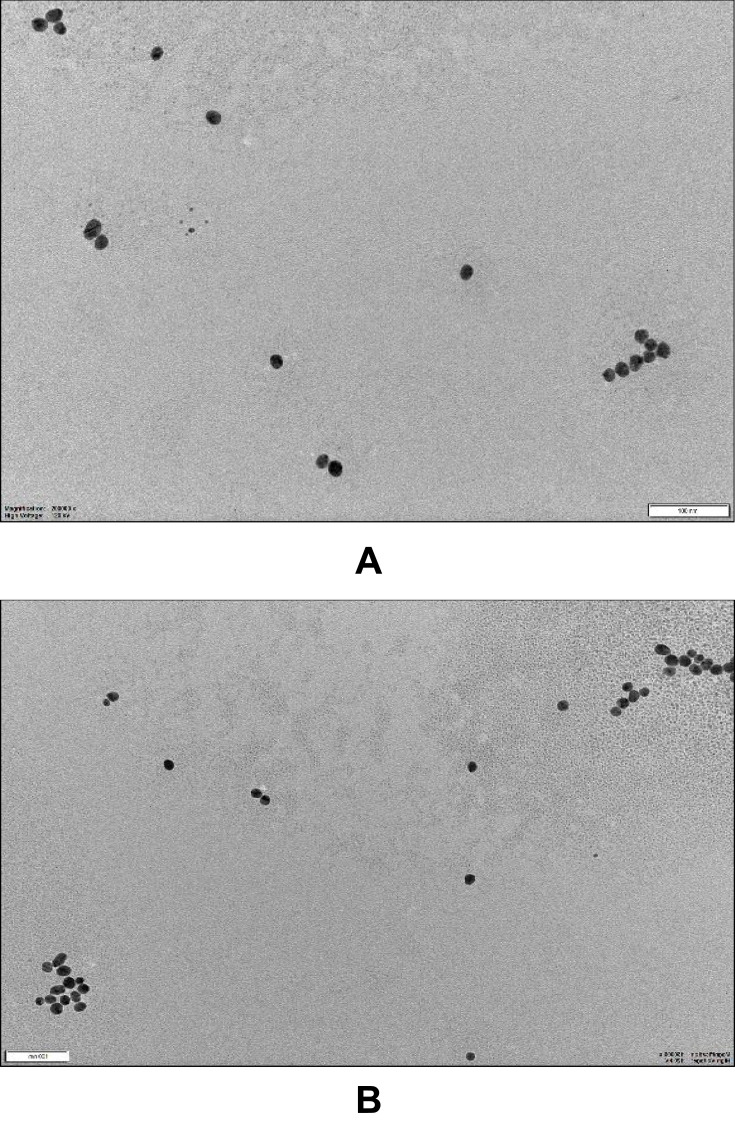
Characterization of bare and conjugated nano formulation through TEM displayed a uniform structural morphology and distribution. The size and size distribution of synthesized AuNPs were determined using TEM Figure 4. The average size 20 nm was recorded on micrograph reported in (Table 1) before and after conjugation (see more images in supplemental Figure 3).
Figure 4.
Image (A) is for Bare spherical Au nanoparticles and image (B) is for spherical Au nanoparticles + Withanolide-A at 100nm scale bar.
Determination of Cytotoxicity
We compared free compound 1 anticancer activity and phytonanconjugate with 20 nm sAuNPs against SKBR3 breast cancer cell lines. The standard MTT assay38 were used to determine the level of cell viability coupled with % confluence analysis of incucyte 10× magnification images results software generated growth curve/time.39 The anticancer compound 1 with its varying concentration 40, 20, 10 µg/mL along with the same nanoconjugate concentration applied as triplicate.
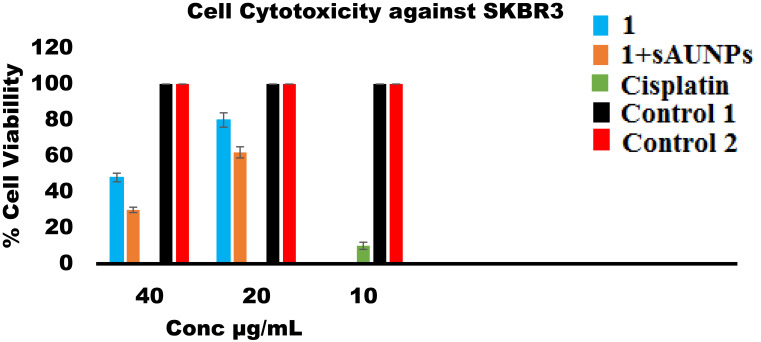
In the MTT assay results a dose dependent cytotoxicity response was observed. Interestingly, we found that our phytonanoconjugate shows higher antiproliferative effects and showed cell viability of 30% compared to 1 alone of 45% at the concentration of 40µg/mL followed by 60% and 80% at 20µg/mL, which might be due to higher uptake of complex mixture in nucleus or cytoplasm functionalized Au nanoparticle carrier. The results followed the same behavior towards cell viability a we observed in Vijayakumar (2019)40 studies, the Au nanoconjugate showed a higher antiproliferative profile than with conjugation.
As has already been proven in several studies (see, for example, Agarwalla et al, 2016;41 Wing Lee et al, 2016;13 and Lara et al, 2019),8 Au nanoparticle conjugated with phytochemical enhanced their payload and cellular uptake. However, in our findings we noticed slightly higher cell growth inhibition activity at concentration 40 µg/mL with 1 and 20 µg/mL in Au nanoconjugate sAuNP+1 compared to previous literature as shown in Figure 5 (supplemental Figure 4). There was no cell growth inhibition observed with corresponding negative control, while standard cisplatin drug behaves better than 1 and phytonanconjugate.
Figure 5.
Anticancer activity of Withanolide-A, Phyto-nanoconjugate at different concentration against SKBR3 Cell line. While control 1 and 2 correspond to AuNPs and media with corresponding concentration of DMSO (1% used to prepared sample Withanolide-A). Values indicate mean ±SD of three independent experiment. While, activity-based differences among various concentration found significant having p<0.05.
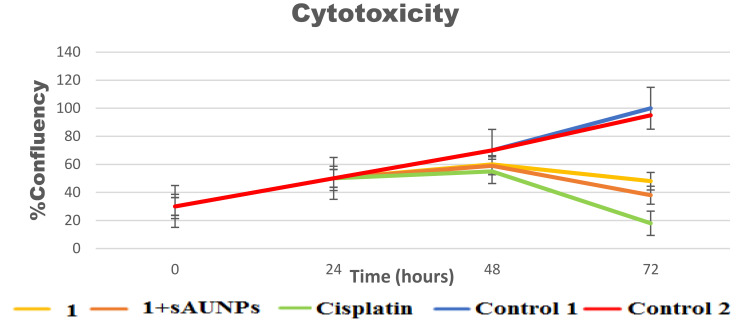
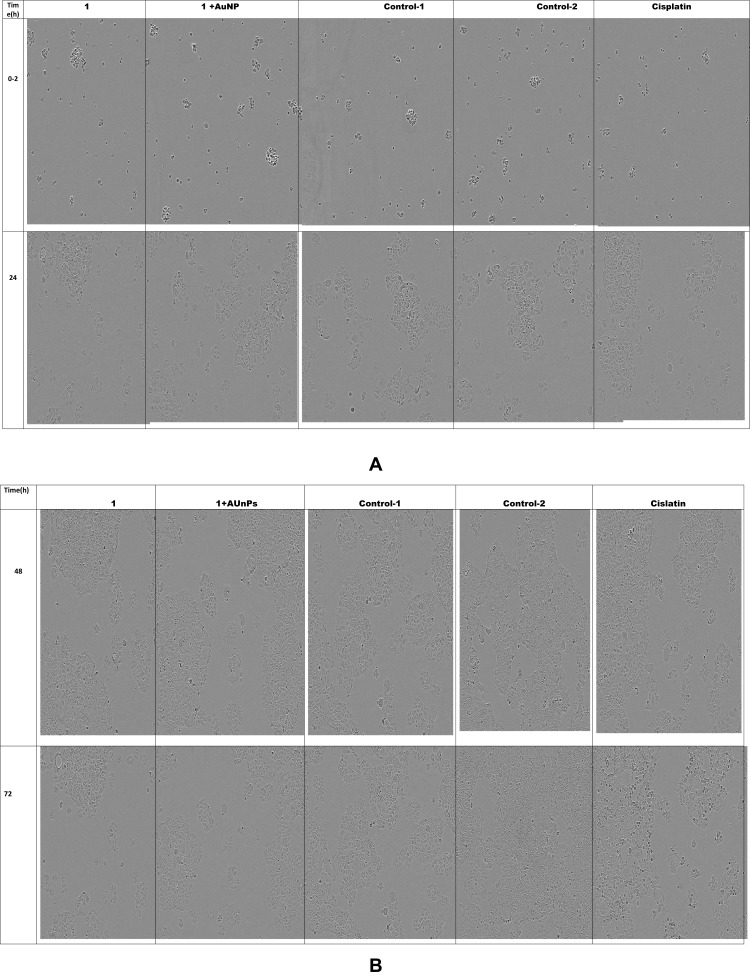
A similar trend has been seen with incucyte studies result where a minimum percentage of live cell confluency (number of cells) observed with Cisplatin (10 µg/mL) > (40 µg/mL 1 + 20 nm sAuNPs) > (40 µg/mL 1) > (20 µg/mL 1 + 20 nm sAuNPs) > (20 µg/mL 1) > (10 µg/mL 1 + 20 nm sAuNPs) > (10 µg/mL 1) > Media > AuNPs (Figures 6 and 7 (A, B).
Figure 6.
Percentage of Confluency (number of cell) growth curve/time against SKBR3 breast cancer cell line. There was significant difference found with specific interval of time having p<0.05.
Figure 7.
Image collection of cell growth inhibition assay after specific interval of time. (A) Images at time 0–2 (h). (B) Images at time 48.72(h).
Due to the higher uptake and active carrier delivery through AuNPs based targeted drug delivery it is possible to destroy the cancer cells by combining phytochemical with minimal effective cytotoxic concentration due to higher safety profile. Therefore, it is worthwhile exploring the biosynthesized nanoparticles as a possible source of novel anticancer drugs.
Conclusion
In conclusion this study has reported the effective synthesis of 20 nm sAuNPs and their conjugation with phytochemical anticancer compound 1, which was previously reported safe to normal cell line. Later characterization was done with UV-Vis, DLS and TEM techniques. Three important points observed as valuable findings. The synthesized phytonanconjugate proved as a stable moiety, tested nanoconjugate showed higher toxicity than normal compound and values of cytotoxic concentration seem to be higher. However, the stability and better performance of these phytonanconjugate towards in vitro SKBR3 cells make it an active target to explore for other applications in future.
Acknowledgment
We would like to thank School of Pharmacy, Madsen Building, Bosch Institute, University of Sydney (Australia) for sharing their instrument with us to complete this study. We would like thank to Dr. Veysel Kayser for funding and all support to complete this research work.
Abbreviations
Withanolide-A, ATCC, American Type Culture Collection; Au, gold; AuNPs, gold nanoparticles; DLS, dynamic light scattering; DLS, dynamic light scattering; DMSO, dimethyl sulfoxide; HAuCl4, chlorauric acid or tetrachloroauric acid; nm, nano meter; PBS, phosphate buffer saline; PDI, polydispersity index; PVDF, polyvinylidene fluoride; sAuNPs, spherical Au nanoparticles; sAuNPs, spherical Au nanoparticles; SKBR3, Sloan Kettering-breast cancer; STR, short tandem repeat; TA, tannic acid; TEM, transmission electron microscope; TEM, transmission electron microscopy.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Farooq MU, Novosad V, Rozhkova EA, et al. Nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa Cells. Sci Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-21331-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Patil MP, Jin X, Simeon NC, et al. Anticancer activity of Sasa borealis leaf extract-mediated gold nanoparticles. Artif Cells Nanomedi Biotechnol. 2018;46(1):82–88. doi: 10.1080/21691401.2017.1293675 [DOI] [PubMed] [Google Scholar]
- 3.Lee WH, Loo CYHX, Ong D, Traini P, Young M, Rohanizadeh R. Synthesis and characterization of inhalable flavonoid nanoparticle for lung cancer cell targeting. J Biomed Nanotechnol. 2016;12(2):371–386. doi: 10.1166/jbn.2016.2162 [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):1–12. doi: 10.1038/natrevmats.2016.14 [DOI] [Google Scholar]
- 5.Balakrishnan S, Bhat FA, Raja Singh P, et al. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016;49(6):678–697. doi: 10.1111/cpr.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyriazi ME, Giust D, El-Sagheer AH, et al. Multiplexed mRNA sensing and combinatorial-targeted drug delivery using DNA-gold nanoparticle dimers. ACS Nano. 2018;12(4):3333–3340. doi: 10.1021/acsnano.7b08620 [DOI] [PubMed] [Google Scholar]
- 7.Lukianova-Hleb EY, Ren X, Sawant RR, Wu X, Torchilin VP, Lapotko DO. “On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat Med. 2014;20(7):778–784. doi: 10.1038/nm.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lara-Cruz C, Jiménez-Salazar JE, Arteaga M, et al. Gold nanoparticle uptake is enhanced by estradiol in MCF-7 breast cancer cells. Int J Nanomedicine. 2019;14:2705. doi: 10.2147/IJN.S196683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan G, Onur MA. Cellular localization and biological effects of 20nm-gold nanoparticles. J Biomed Mater Res. 2018;106(6):1708–1721. doi: 10.1002/jbm.a.36373 [DOI] [PubMed] [Google Scholar]
- 10.Almeida JPM, Lin AY, Figueroa ER, Foster AE, Drezek RA. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small. 2015;1(12):1453–1459. doi: 10.1002/smll.201402179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan JA, Overton KW, Speight ME, et al. Cellular uptake of gold nanoparticles passivated with bsa−sv40 large t antigen conjugates. Anal Chem. 2007;79(23):9150–9159. doi: 10.1021/ac0715524 [DOI] [PubMed] [Google Scholar]
- 12.Lee CW, Jang, Pan HJ, Chen YR, Chen CC, Lee CH, Lee C-H. Membrane roughness as a sensitive parameter reflecting the status of neuronal cells in response to chemical and nanoparticle treatments. J Nanobiotechnology. 2016;14(1). doi: 10.1186/s12951-016-0161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Naydenov NG, Dozmorov MG, Koblinski JE, Ivanov AI. Anillin regulates breast cancer cell migration, growth, and metastasis by non-canonical mechanisms involving control of cell stemness and differentiation. Breast Cancer Res. 2020;22(1). doi: 10.1186/s13058-019-1241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anonymous, “Breast cancer in Australia statistics,” 2015Available: https://breast-cancer.canceraustralia.gov.au/statistics. Accessed August12, 2020.
- 15.Arjonen A, Kaukonen R, Ivaska J. Filopodia and adhesion in cancer cell motility. Cell Adhes Migr. 2011;5(5):421–430. doi: 10.4161/cam.5.5.17723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang P, Enomoto A, Takahashi M. Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton. Cancer Lett. 2019;284(2):122–130. doi: 10.1016/j.canlet.2009.02.034 [DOI] [PubMed] [Google Scholar]
- 17.Khan H, Ullah H, Martorell M, et al. Flavonoids nanoparticles in cancer: treatment, prevention and clinical prospects. Semin Cancer Biol. 2019. doi: 10.1016/j.semcancer.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 18.Sayed N, Khurana A, Godugu C. Pharmaceutical perspective on the translational hurdles of phytoconstituents and strategies to overcome. J Drug Deliv Sci Technol. 2019;53:101201. doi: 10.1016/j.jddst.2019.101201 [DOI] [Google Scholar]
- 19.Lee IC, Choi BY. Withaferin-A—A Natural anticancer agent with pleitropic mechanisms of action. Int J Mol Sci. 2016;17:3. doi: 10.3390/ijms17030290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhar N, Razdan S, S R, Bhat WW, Vishwakarma R, Lattoo SK. A decade of molecular understanding of withanolide biosynthesis and in vitro studies in withania somnifera (l.) dunal: prospects and perspectives for pathway engineering. Front Plant Sci. 2015;6. doi: 10.3389/fpls.2015.01031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey A, Chatterjee SS, Kumar V. Triethylene glycol-like effects of Ashwagandha (Withania somnifera (L.) Dunal) root extract devoid of withanolides in stressed mice. AYU Int Q J Res Ayurveda. 2018;39(4). doi: 10.4103/ayu.AYU_219_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thipe VC, Amiri KP, Bloebaum P, et al. Development of resveratrol-conjugated gold nanoparticles: interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int J Nanomedicine. 2019;14:4413–4428. doi: 10.2147/IJN.S204443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan T, Gurav P. PhytoNanotechnology: enhancing Delivery of Plant Based Anti-Cancer Drugs. Front Pharmacol. 2018;8. doi: 10.3389/fphar.2017.01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Ballesta M, Gil-Izquierdo Á, García-Viguera C, Domínguez-Perles R. Nanoparticles and controlled delivery for bioactive compounds: outlining challenges for new ‘smart-foods’ for health. Foods. 2018;7(5):72. doi: 10.3390/foods7050072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoobchandani, M. Targeted phytochemical-conjugated gold nanoparticles in cancer treatment: environmental issues in logistics and manufacturing. Biotech Prod Everyday Life. 2019;37–52. doi: 10.1007/978-3-319-92399-4_3 [DOI] [Google Scholar]
- 26.Yadav DK, Kumar S, Saloni S, et al. Molecular docking, QSAR and ADMET studies of withanolide analogs against breast cancer. Drug Des Devel Ther. 2017;11:1859–1870. doi: 10.2147/DDDT.S130601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nile SH, Nile GE, Baskar V, Kai G, Kai G. Subcritical water extraction of withanosides and withanolides from ashwagandha (Withania somnifera L) and their biological activities. Food Chem Toxicol in J Publ Br Ind Biol Re Assoc. 2019;132:110659. doi: 10.1016/j.fct.2019.110659 [DOI] [PubMed] [Google Scholar]
- 28.Bennett HL, Brummer T, Jeanes A, Yap AS, Daly RJ. Gab2 and Src co-operate in human mammary epithelial cells to promote growth factor independence and disruption of acinar morphogenesis. Oncogene. 2008;27(19):2693–2704. doi: 10.1038/sj.onc.1210928 [DOI] [PubMed] [Google Scholar]
- 29.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem. 2006;110(32):15700–15707. doi: 10.1021/jp061667w [DOI] [PubMed] [Google Scholar]
- 30.Frens G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat Phys Sci. 1973;241(105):20–22. doi: 10.1038/physci241020a0 [DOI] [Google Scholar]
- 31.Piella J, Bastús NG, Puntes V. Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem Mater. 2016;28(4):1066–1075. doi: 10.1021/acs.chemmater.5b04406 [DOI] [Google Scholar]
- 32.Perrault SD, Chan WCW. Synthesis and surface modification of highly monodispersed, spherical gold nanoparticles of 50−200 nm. J Am Chem Soc. 2009;131(47):17042–17043. doi: 10.1021/ja907069u [DOI] [PubMed] [Google Scholar]
- 33.Trouiller AJ, et al. Biocompatible spherical gold nanoparticles synthesis in aqueous tetraethylene oxide solution and their cellular uptake. J of Nanosci. 2019. doi: 10.1166/jnn.2019.16304 [DOI] [PubMed] [Google Scholar]
- 34.Romero-Benavides JC, Ortega-Torres GC, Villacis J, Vivanco-Jaramillo SL, Galarza-Urgilés KI, Bailon-Moscoso N. Phytochemical Study and Evaluation of the Cytotoxic Properties of Methanolic Extract from Baccharis obtusifolia. Int J Med Chem. 2018;2018:1–5. doi: 10.1155/2018/8908435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aslan K, Pérez-Luna VH. Surface Modification of Colloidal Gold by Chemisorption of Alkanethiols in the Presence of a Nonionic Surfactant. Langmuir. 2002;18(16):6059–6065. doi: 10.1021/la025795x [DOI] [Google Scholar]
- 36.Rycenga M, Cobley CM, Zeng J, et al. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem Rev. 2011;111(6):3669–3712. doi: 10.1021/cr100275d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafiqa AR, Abdul Aziz A, Mehrdel B. Aziz AA and Mehrdel B. Nanoparticle optical properties: size dependence of a single gold spherical nanoparticle. J Phys Conf Ser. 2018;1083:012040. doi: 10.1088/1742-6596/1083/1/012040 [DOI] [Google Scholar]
- 38.González ML, Joray MB, Laiolo J, et al. Cytotoxic Activity of Extracts from Plants of Central Argentina on Sensitive and Multidrug-Resistant Leukemia Cells: isolation of an Active Principle from Gaillardia megapotamica. Evid Based Complement Alter Med. 2018;2018:1–13. doi:10.1155/2018/9185935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz E, Kayser V. Synthesis and enhanced cellular uptake in vitro of anti-her2 multifunctional gold nanoparticles. Cancers. 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vijayakumar S. Eco-friendly synthesis of gold nanoparticles using fruit extracts and in vitro anticancer studies. J Saudi Chem Soc. 2019;23(6):753–761. doi: 10.1016/j.jscs.2018.12.002 [DOI] [Google Scholar]
- 41.Agarwalla P, Mukherjee S, Sreedhar B, Banerjee R. Glucocorticoid receptor-mediated delivery of nano gold-withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomed. 2016;11(19):2529–2546. doi: 10.2217/nnm-2016-0224 [DOI] [PubMed] [Google Scholar]