Abstract
Diabetes is one of the most prevalent metabolic disorders and is estimated to affect 400 million of 4.4% of population worldwide in the next 20 year. In diabetes, risk to develop vascular diseases is two-to four-fold increased. Ischemic tissue injury, such as refractory wounds and critical ischemic limb (CLI) are major ischemic vascular complications in diabetic patients where oxygen supplement is insufficient due to impaired angiogenesis/neovascularization. In spite of intensive studies, the underlying mechanisms of diabetes-impaired ischemic tissue injury remain incompletely understood. Hydrogen sulfide (H2S) has been considered as a third gasotransmitter regulating angiogenesis under physiological and ischemic conditions. Here, the underlying mechanisms of insufficient H2S-impaired angiogenesis and ischemic tissue repair in diabetes are discussed. We will primarily focuses on the signaling pathways of H2S in controlling endothelial function/biology, angiogenesis and ischemic tissue repair in diabetic animal models. We summarized that H2S plays an important role in maintaining endothelial function/biology and angiogenic property in diabetes. We demonstrated that exogenous H2S may be a theraputic agent for endothelial dysfunction and impaired ischemic tissue repair in diabetes.
Keywords: Hydrogen sulfide, EDHF-Mediated endothelium-dependent vascular relaxation, Angiogenesis, Ischemic tissue repair, Diabetes
1. Introduction
Diabetes is one of the most prevalent metabolic disorders and is estimated to affect 400 million of 4.4% of population worldwide in the next 20 year [1]. Diabetes has a 2- to 4-fold increased risk to develop vascular diseases compared to non-diabetes [2]. Refractory wounds and critical ischemic limb (CLI) are two of major ischemic vascular complications in diabetic patients where oxygen supplement is insufficient due to impaired angiogenesis/neovascularization. Accumulative studies demonstrated that impaired biology/function and angiogenic property of endothelial cells (ECs) play a critical role in diabetes-induced deficiency of angiogenesis/neovascularization [[3], [4], [5]]. However, the underlying mechanisms remain incompletely understood.
Insufficient gaso-transmitters, including nitric oxide (NO) and1) carbon monoxide (CO) have been considered as crucial regulators of EC dysfunction in diabetes [6]. In the last decades, hydrogen sulfide (H2S), a “toxic gas with strong odor of rotten eggs”, has been found in biological system and considered as the third gaso-transmitters regulating many biological functions [7]. Especially, insufficient production of H2S has been linked to different pathogenesis of cardiovascular diseases such as hypertension, endothelial dysfunction, myocardial infarction and ischemic limb injury [4,5,[8], [9], [10]], and emerged as an important regulator in diabetes-impaired endothelial cell (EC) function/biology, angiogenesis, wound healing and ischemic lamb repair [4,5]. Administration of H2S rescued endothelial dysfunction in diabetic animal models [11]. Recently, we provided strong evidence that insufficient production of H2S plays a crucial role in diabetes-impaired microvascular endothelial function and angiogenesis in ischemic hindlimb (IHL) of db/db mice [4,5]. In this review, we will summarize the current understanding of the role of H2S in diabetes-impaired EC function, angiogenesis, wound and IHL repair.
2. Endogenous H2S biosynthesis
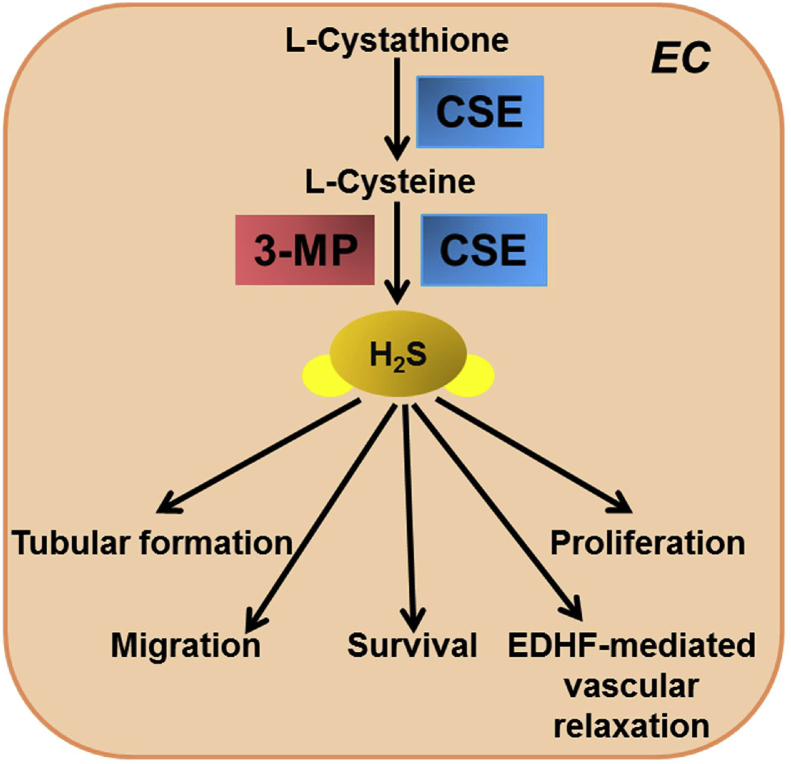
In mammalian tissues, H2S is mainly produced by three pyridoxal-5′-phosphate (PLP)-dependent enzymes, cystathionin-β-synthase (CBS), cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST) in the transulfuration pathway of homocysteine metabolism [[12], [13], [14], [15]]. CBS is predominantly expressed in the central nervous system whereas CSE is abundantly expressed in cardiovascular system [16]. Protein expression of CSE was detected in cultured bovine aortic ECs and human umbilical vein ECs, and endothelial layer of vascular tissues in wild-type mice [4,5,17]. H2S is produced in both vascular ECs and VSMCs mainly by activation of CSE [5,[17], [18], [19], [20], [21]]. Recent studies have shown 3-MST, along with cysteine aminotransferase (CAT), which produces H2S in the brain as well as in the vascular endothelium (Fig. 1) [15,22]. Physiological level of H2S is in wide range between 15 nM and 300 μM depending on detection methods and the tissues analyzed [[23], [24], [25], [26], [27], [28], [29], [30], [31]]. In fact, the concentration of free H2S in vascular tissues is around 100 times greater than that in the other tissues [26,32], implying that H2S is critical in the regulation of vascular homeostasis, endothelial function and angiogenic property of ECs.
Fig. 1.
H2S synthesis and function in vascular endothelial cells. In vascular endothelial cells (ECs), H2S is mainly generated by CSE. H2S plays important role in maintenance of vascular homeostasis, endothelial function and reparative property of ECs. H2S: hydrogen sulfide; CSE: cystathioine--lyase; 3-MST: mercaptopyruvate sulfurtransferase.
In addition to enzymatic synthesis pathways, endogenous H2S can also be produced through non-enzymatic processes which have not been well understood or characterized. Studies have reported that many factors are involved in non-enzymatic production of H2S, including glucose, glutathione, inorganic and organic polysulfides and elemental sulfur [33]. H2S can be generated from glucose either via glycoclysis or from phosphoglyconate via NADPH oxidase or reacting with sulfur-containing amino acid methionine, homocysteine or cystenine [34]. H2S can be also produced by direct reduction of glutathione and elemental sulfur through reducing equivalents of the glucose oxidation pathway [35]. Moreover, thiosulfate results from a reduction reaction involving pyruvate is an intermediate of sulfur metabolism from cysteine and a metabolite of H2S that can also lead to the production of H2S [35].
H2S can be enzymatically oxidized to sulfur atom in mitochondria by sulfide:quinone oxidoreductase [36]. This sulfur atom is then transferred to reduced glutathione to form glutathione persulfide (GSSH) and then may be either oxidized to sulfite (SO32−) by sulfur dioxygenase (persulfide dioxygenase, protein deficient in ethylmalonic encephalopathy, ETHE1) or transferred to sulfite by thiosulfate:cyanide sulfurtransferase (TST, rhodanese) to form thiosulfate (SSO32−) [37,38]. Sulfite is further oxidized to sulfate (SO42−) by sulfite oxidase. It was suggested that physiological concentration of H2S is maintained by its efficient oxidation [39]. However, under low oxygen supply (oxidative stress), H2S metabolism is compromised. Pathophysiological role of SO42− remain unclear.
3. H2S signaling mechanisms
H2S exerts its biological effects mainly via three routes: 1, Reactive oxygen species (ROS)/reactive nitrogen species (RNS) scavenging; 2, Metal center interaction; 3, S-persulfidation [40]. Compared to the first two routes, S-persulfidation, normally called S-sulfhydration, is the key process for H2S-mediated biological effects. S-sulfhydration is the process in which a thiol (R–SH) is converted into a perthiol (R–SSH). S-sulfhydration modulates the biological activity of proteins by decreasing the pKa and increase in nucleophilicity of perthiols with respect to thiols [41]. S-sulfhydration modification as a new post-translational modification is involved in many pathophysiological process including cell survival/death, differentiation, proliferation/hypertrophy, metabolism, endoplasmic reticulum (ER) stress, vascular relaxation, inflammation, oxidative stress [42]. Recently, insufficient Keap1 S-sulfhydration in ECs has been suggested to be involved in pathogenesis of atherosclerosis in STZ-induced diabetic low-density lipoprotein receptor (LDLr)−/− mice [43]. S-sulfhydration modification of targeted proteins from H2S may provide novel targets for therapeutic/prevention of ischemic tissue repair in diabetes.
4. H2S in diabetes
The synthesis of H2S is known to be reduced in diabetic patients and animal models [43,44]. Plasma H2S was significantly decreased in high fat diet-induced diabetic LDLR−/- mice [43]. In streptozotocin (STZ)-induced diabetic rats, blood H2S level were lower compared with age-matched non-diabetic rats [45]. Endogenous H2S production was also found to be reduced in type 2 diabetic animals [5,46,47]. Plasma H2S levels were significantly reduced in non-obese diabetic mice [46]. Renal CSE and CBS expression and H2S production are markedly lowered in spontaneous diabetic Akita mice [44]. Deficient CSE delayed the onset of STZ-induced type 1 diabetes, and diabetes was accompanied by increased pancreatic CSE and CBS protein expression [48]. Recently, we examined H2S production in small mesenteric arteries of type 2 diabetic db/db mice and non-diabetic db/+ mice with two different methodologies [5]. Using a florescent probe, we found that H2S production in small mesenteric arteries was decreased to 65% in db/db mice compared with that in db/+ mice. By RP-HPLC detection, we also found that free sulfide levels were reduced to 63.7% (45.9 pM/mg) in the small mesenteric arteries of db/db mice compared with that of db/+ mice [5]. By gas chromatography chemiluminescence and florescent probe, we found that H2S production was markedly reduced in bone marrow cells (BMCs), plasma and thigh skeletal muscles from db/db mice [4]. We also found that high glucose dose-dependently reduced H2S production in human microvascular ECs [4]. It has been demonstrated that the negative association between type 2 diabetes and H2S is reinforced by decreased plasma H2S levels [45,49]. Understanding the mechanisms of insufficient H2S-impaired EC function may provide therapeutic targets for ischemic tissue injury in diabetes.
One of the underlying mechanisms of diabetes-impaired H2S production is suggested to be related to hyperglycemia-enhanced H2S consumption through mitochondria-derived reactive oxygen species (ROS) signaling pathways in ECs [50]. ROS scavenger or mitochondrial uncoupling agents prevented H2S consumption in high glucose-treated ECs [50]. Another underlying mechanism may be related to downregulation/inactivation of H2S synthesis enzymes [5,44,47,48]. In good accordance with above studies, we also provide strong evidence that superoxide generation was enhanced whereas H2S production was reduced in the lung ECs and/or small mesenteric arteries of db/db mice (Table 1) [5]. Moreover, we demonstrated that decreased H2S production in microvascular ECs is also related to downregulation of CSE levels although studies to understand the underlying mechanisms of diabetes-downregulated CSE level are warranted [5]. Taken together, diabetes is associated with increased ROS-linked H2S consumption and CSE-mediated H2S reduction which might be related to increased cardiovascular risk in human and experimental models. The underlying mechanisms of diabetes-impaired H2S production are still needed to be explored.
Table 1.
H2S levels in diabetes.
| Diabetic patients/animals/cells | Method for H2S assay | Sources of H2S/free sulfide | H2S in diabetes vs. non-diabetes | Ref. # |
|---|---|---|---|---|
| db/db mice | Gas chromatography | Bone marrow cells | ↓ | 4 |
| Plasma | ↓ | |||
| Medial tight muscles | ↓ | |||
| High glucose-treated human microvascular ECs | Florescent dye sulfidefluor 7AM | ECs | ↓ | 4 |
| db/db mice | Florescent dye sulfidefluor 7AM | Mesenteric artery | ↓ | 5 |
| db/db mice | RP-HPLC | Mesenteric artery | ↓ | 5 |
| Akita mice | Spectrophotometer | kidney | ↓ | [44] |
| High glucose-treated mouse glomerular ECs | Spectrophotometer | ECs | ↓ | [44] |
| High fat diet-induced diabetic LDLR−/- mice | Spectrophotometer | Plasma | ↓ | [43] |
| Type 2 diabetic patients and STZ-treated diabetic rats | Spectrophotometer | Plasma | ↓ | [45] |
| Non-obese/Ltj diabetic mice | Spectrophotometer | Plasma | ↓ | [46] |
| Aorta | ↓ | |||
| STZ-treated mice | Spectrophotometer | Pancreas | ↓ | [48] |
| Type 2 diabetic patients | Spectrophotometer | Plasma | ↓ | [49] |
| High glucose-treated microvascular ECs (bEnd3) | Spectrophotometer | ECs | ↓ | [50] |
| Women with Gestational diabetes mellitus | Sulfide-sensitive electrode (PXS-270) | Plasma | ↓ | [69] |
| High fat diet-induced diabetic mice | Gas chromatography | Heart tissue | ↔ | [70] |
| Plasma | ↔ | |||
| Type 2 diabetic patients | Spectrophotometry (blood) | Blood | ↔ | [71] |
| Methylene blue (plasma) | Plasma | ↔ | ||
| STZ-treated diabetic rats | Spectrophotometer | Vascular smooth muscle cells of thoracic aorta | ↓ | [72] |
| STZ-treated diabetic rats | Spectrophotometer | Plasma | ↓ | [73] |
| Fructose-fed diabetic rats | Methylene blue | Adipose tissue | ↑ | [74] |
| STZ-treated diabetic rats | – | Liver | ↓ | [75] |
| Pancreas | ↓ | |||
| High glucose treated pancreatic islets | Methylene blue | Pancreatic islets | ↓ | [76] |
| STZ-treated diabetic rats | Spectrophotometer | Plasma, liver, pancreas and kidney | Plasma: ↔ Liver: ↑ Pancreas: ↑ Kidney: ↔ |
[75] |
5. H2S as ROS modulator in diabetic ECs
Reactive oxygen species (ROS) generated from ECs is a key factor in the pathogenesis of diabetic complications [51]. H2S was found to be beneficial against oxidative stress via 2 distinct mechanisms: 1) acts as a direct scavenger of ROS; 2) upregulates antioxidant defense system. Accumulative evidence show that H2S prevents diabetes-impaired EC biology/function via inhibition of oxidative stress. H2S reduced ROS formation, improved function and metabolic state of ECs under hyperglycemic condition [50]. H2S decreased ROS production and apoptosis in hyperglycemic ECs [52], and alleviated vascular abnormality in the aorta of diabetic rats via attenuating NADPH oxidase activity [53]. H2S decreased high glucose-induced autophagy in ECs though the nuclear factor erythroid2-reated factor 2 (Nrf2)-ROS-5′ adenosine monophosphate-activated protein kinase (AMPK) signaling pathway [54]. Administration of sodium hydrosulfide (NaHS) protected excessive autophagy in arterial ECs of db/db mice via enhancing superoxide dismutase/catalase activity [54]. NaHS improved endothelial function and decreased oxidative stress in STZ-treated diabetic mice [55]. Recently, study also provided an evidence that mitochondria-specific H2S donor AP123 and AP39 prevented hyperglycemia-triggered oxidative stress and metabolic abnormalities in microvascular ECs [56].
6. H2S affects metabolism in diabetes and ECs
Although numerous studies reported that H2S production is reduced in diabetes, the role of H2S in the metabolism in diabetes remains controversial. Studies have reported that H2S is essential for maintaining insulin bioactivity and glucose uptake. H2S was required for vitamin D-induced GLUT4 translocation and glucose uptake [57]. H2S reduced production of inflammatory cytokine, a known causal factor for the development of insulin resistance in adipose and other metabolic tissues, from resident adipose macrophages [58]. Endogenous glucose production was reduced in CSE KO mice [59]. H2S may directly stimulate gluconeogenesis via sulfhydration of pyruvate carboxylase, a key enzyme in gluconeogenesis [60]. Mitochondria plays a critical role in cell survival and death by regulating ATP synthesis through lipid and glucose metabolism. Increasing evidence indicate that mitochondrial function changes are implicated in diabetes-impaired EC function [61]. It was found that H2S protected the vascular endothelium in hyperglycemia by preserving mitochondria function [50]. Mitochondria-targeted H2S donors AP123 and AP39 increased the electron transport at respiratory complex III and improved the cellular metabolism in high glucose-treated microvascular ECs [56].
7. H2S is an endothelium-derived hyperpolarizing factor (EDHF)
Endothelium is a single layer of squamous ECs that lines the interior surface of blood vessel lumen. Endothelium is the first target of pathological stimuli from blood. Endothelium mediates blood flow homeostasis and fulfills tissue metabolic demands by supplying nutrients and oxygen. Endothelium regulates vasomotor tone and organ perfusion by releasing by releasing vasodilator substances such as nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF), and vasoconstrictor substances, such as angiotensin II, endothelin-1, thromboxane A2 and prostaglandin H2, in response to pathophysiological stimulation [62,63]. EC impairment thus endothelial dysfunction (ED) which is characterized by an impairment of endothelium-dependent vascular relaxation is an early event in the development of cardiovascular disease. EDHF-mediated vascular relaxation is defined by endothelium-dependent relaxation when production of NO and PGI2 is blocked. It is generally accepted that NO predominantly controls relaxation of macro-vasculature, whereas EDHF primarily controls relaxation of micro-vasculature and becomes more important when vessel diameter decreases [[64], [65], [66]]. Although extensively studied, the nature of EDHF remains unclear. Many EDHF candidates have been reported including NO, CO, H2O2, C-type natriuretic peptide, PGI2, EETs, trihydroxyeicosatrienoic acid [20,65,67,68]. Current studies support the concept that EDHF-mediated.
Vascular relaxation is elicited by the opening of Ca2+-activated potassium channels (KCa) in both ECs and vascular smooth muscle cells (VSMCs) [5,18,19,77].
Several lines of evidence indicate that H2S has a role in the regulation of vascular tone. H2S induced vascular relaxation in aorta [[78], [79], [80]], gastric artery [81], mesenteric artery [5,82], and internal mammary artery [83]. Accumulative evidence indicate that H2S is an EDHF [5,[17], [18], [19], [20],82,[84], [85], [86]]. H2S induced cell hyperpolarization by patch clamp [86]. By myograph, H2S induced endothelium-dependent vascular relaxation in the presence of eNOS inhibitor and cyclooxygenase inhibitor [5,[18], [19], [20],85]. Moreover, H2S induced vascular relaxation in the presence of eNOS and cyclooxygenase (COX) inhibitor to a greater extent in the rat mesenteric artery than that in the rat aorta [[17], [18], [19]]. Removal of endothelium completely abolished the H2S-induced relaxation in human and rat mesenteric arteries [18,82]. Endothelium-dependent vascular relaxation to acetylcholine in small mesenteric arteries was virtually abolished in the presence of eNOS and COX inhibitor in CSE knockout mice [20,85], suggesting H2S is a EDHF. Moreover, studies also found that the vascular relaxation in response to H2S is achieved via activation of ATP-sensitive potassium channel (KATP) in smooth muscle cells [19] and endothelial and/or muscular small- (SKCa), intermediate- (IKCa) and big- (BKCa) conductance calcium-activated and voltage-sensitive (Kv) potassium channel [[87], [88], [89], [90], [91]].
8. H2S in diabetes-impaired vascular relaxation
H2S exerts protective effects against hyperglycemic stress in the vascular endothelium and exhibits protective effects in animal models of diabetic complication [7,45,49,50,55,[92], [93], [94]]. We and others reported that H2S deficiency plays an important role in the pathogenesis of cardiovascular complications in diabetes [3,5,45,49,50,55,[92], [93], [94], [95]]. Low levels of H2S in the blood of diabetes and overweight patients and STZ-treated rats cause vascular inflammation [45,49]. Exogenous H2S is beneficial for prevention/therapeutics of diabetes-induced cardiovascular complications, such as rescued oxidative stress, endothelial dysfunction and cardiomyopathy in STZ-induced diabetic mice/rats [55,95]; reduced blood pressure and prevented the progression of diabetic nephropathy in spontaneously hypertensive rats [92]; decreased reactive oxygen species (ROS) and vascular inflammation markers in high glucoses-treated human monocytes [93]; improved metabolic state and mitochondria function of ECs under hyperglycemia condition [50].
Although many studies reported that H2S production was decreased in diabetes, vascular relaxation response to H2S was enhanced in thoracic aorta, mesenteric, pulmonary and carotid arteries of STZ- or alloxan-induced diabetic animal models [[96], [97], [98]]. Diabetes-enhanced vascular relaxation response to H2S has been suggested to be a compensatory mechanism overcome insufficient H2S production and impaired endothelial function by increasing sensitivity of vasculature to H2S [5,96,99]. However, the molecular/signaling pathways of enhanced EDHF-mediated endothelium-dependent vascular relaxation in response to H2S remain unclear. It has been showed that glibenclamide (KATP channel blocker) decreased maximum relaxation response to NaHS in the thoracic aorta, pulmonary and mesenteric arteries isolated from either 4- or 12-week-STZ-induced diabetic rats [96]. Moreover, vascular relaxation of rabbit carotid artery in response to NaHS was diminished by charybdotoxin (BKCa/IKCa blocker), 4-amynopiridine (Kv channel blocker) and glibenclamide in the carotid arteries of alloxan-induced diabetic rabbits but not in that of non-diabetic rabbits [97]. However, 4-amynopiridine did not modify the relaxant response to H2S in the control rat thoracic aorta [19]. In line with above findings, activity and expression of Kv channels were enhanced in the smooth muscle cells of mesenteric artery of OLETF diabetic rats [98].
Recently, we studied the role of H2S in diabetes-induced ED in the small mesenteric artery of db/db mice [5]. We were the first demonstrating that H2S is an effective EDHF in the mesenteric arteries of db/+ and db/db mice [5], because that l-cysteine and NaHS, precursor and donor of H2S, induced endothelium-dependent response in the presence of l-NAME (eNOS inhibitor) plus indomethacin (COX inhibitor), respectively [5]. Moreover, in good accordance with previous studies that diabetes enhanced vascular relaxation in response to H2S [[96], [97], [98]], we also provided strong evidence that EDHF-mediated vascular relaxation to NaHS was enhanced in the small mesenteric artery of db/db mice [100]. Finally we found that KATP blocker, but not any of KCa blockers, blocked enhanced relaxation to NaHS in the small mesenteric arteries of db/db mice [5]. Our study implicated that increased EDHF-mediated vascular relaxation to H2S in the small mesenteric artery of db/db mice is via, at least partially, activation of KATP but not KCa. Exogenous H2S and/or KATP openers may be beneficial for prevention/treatment of micro-vascular complication in diabetic patients. Further studies to examine the therapeutic effects of H2S and/or KATP activators on microvascular endothelial function in db/db mice are warranted.
Taken together, in general, the EDHF-mediated endothelium-dependent vascular relaxation to H2S is enhanced under diabetic condition. The diabetes-enhanced EDHF-mediated vascular relaxation in response to H2S is suggested mainly by sensitizing/overexpression of different potassium channels, including KCa, KATP and Kv. Enhanced endothelium-dependent vascular relaxation to H2S may be complementary effects to overcome insufficient H2S production and endothelial dysfunction in diabetes. The differences on the role of KCa, KATP and Kv in H2S-mediated endothelium-dependent vascular relaxation in diabetes may be related to the size and type of vessels, type and severity of diabetes, and species. Whether this complementary effects dependent on the species of animal model and severity/stage of diabetes remain unclear.
9. Low concentration of H2S mediate vascular constriction
It is noteworthy that low concentration of H2S has been considered as an endothelium-dependent vascular constriction factor (see review [101,102]). H2S relaxed phenylephrine-precontracted human internal mammary artery at higher concentrations but induced contraction at lower concentrations [83]. Intravenous infusion of a low concentration of NaHS into the anaesthetized rats significantly increased mean arterial blood pressure [103]. Low concentrations of NaHS induced contraction of aorta from mice/rats [104]. The molecular pathways involved in H2S-induced vascular contraction were found to be related to decreasing nitric oxide (NO) bioavailability by converting NO to an inactive nitrosothiol and N-nitrosohydroxylamine-N-sulfonate or directly reducing NO production [83,[103], [104], [105]], or down-regulation of cAMP endothelium-independently [106]. The pathophysiological importance of low concentration of H2S-mediated vascular constriction has been suggested for further investigation [102]. As diabetes markedly reduced H2S production (Table 1), it would be interesting to study if insufficient H2S-induced vascular contraction has an impact on impaired blood perfusion in diabetic wound and ischemic hindlimb via restriction of blood/nutrition supply in microvasculature.
10. H2S in diabetes-impaired angiogenesis
Angiogenesis is defined as a physiological process through which new blood vessels are sprouted from the preexisting blood vessels [107,108]. Angiogenesis is required for delivering nutrients and waste and supplying immune surveillance [100]. The rate of new blood vessels formation varies with biological age and differs between tissues in healthy organisms [109]. Organisms exhibit rapid angiogenesis during embryonic development [110]. In adults, angiogenesis is observed only in limited number of physiological responses and in tissue-specific manners. Under ischemic conditions, angiogenesis is a critical event for improving blood flow and oxygen/nutrients supply [111]. Under pathological stimulation, ECs process an angiogenic program to contribute wound healing and tissue remodeling [107]. Enhancing EC function and angiogenesis thereby improving blood perfusion has been suggested as an alternative approach in the treatment of ischemic tissue injury. The process of angiogenesis is complex which contains a series of cellular and molecular events which includes three steps: 1) enzymatic degradation of capillary basement membrane; 2) EC proliferation, migration and 3) tubulogenesis and the formation of capillaries [[112], [113], [114]], which involves the interaction between pro- and anti-angiogenic factors, growth mediators, cytokines, cells and extracellular matrix [115]. Recent studies demonstrated that rather than the amount of single angiogenic factor, balance between endogenous pro- and anti-angiogenic factor plays an important role in the regulation of angiogenesis [116]. Defects in the angiogenic balance may cause a shift towards either excessive or deficient angiogenesis.
Impaired angiogenesis is one of the hallmarks of diabetes which is involved in the pathogenesis of nephropathy, wound and critical limb ischemia in diabetes [4,[117], [118], [119]]. The risk factors involved in diabetes-impaired angiogenesis are suggested to be related to many factors, including hypoxia, chronic inflammation, oxidative stress, hyperglycemia/advanced glycation end products (AGE), connective tissue growth factors, lipoxidation and its products [120]. The underlying mechanisms of diabetes-impaired angiogenesis remain incompletely understood.
Emerging data support the role of H2S in the regulation of angiogenesis. H2S promotes EC proliferation, migration, survival, formation of tubular structure and networks [100,107,[121], [122], [123], [124], [125], [126], [127], [128], [129]]. The sufficient concentrations of H2S to induce angiogenesis are physiological levels [125,130]. H2S in a dose-dependent manner promoted human umbilical vein endothelial cells (HUVECs) growth, adhesion, migration, tube formation, and in vivo angiogenesis measured by matrigel plug assay [124,125,129]. Silencing of CSE in HUVECs decreased cell proliferation and tube formation [131]. Administration of H2S increased proliferation and migration in microvascular ECs and recovered microvessel sprouting in CSE-silenced rat aorta [122]. In addition, administration of 3-mercaptopyruvate (3-MP), the 3-MST substrate reserved increased Matrigel plug angiogenesis/neovascularization in mice [132]. Many molecules have been suggested to be involved in H2S-mediated angiogenesis. Most studies focused on the VEGF signaling in angiogenic response [125,133,134]. The pro-angiogenic effect of vascular endothelial growth factor (VEGF) is mediated by the endogenous production of H2S [125]. Silencing of CSE by siRNA attenuated VEGF-induced angiogenesis [125]. In addition, H2S stimulated angiogenesis by increasing the activity of endothelial nitric oxide synthesis (eNOS) [121,135], phosphatidylinositol 3 (PI3)-kinase/protein kinase B (AKT) [130], p38/mitogen-activated protein kinase (MAPK) [125], KATP,127 signal transducer and activator of transcription 3 (STAT3) [124], and sirtuin 1(SIRT1)/VEGF/cyclic guanosine 5′-monophosphate (cGMP) cascade [123].
Angiogenesis is impaired in diabetes [4,117]. However, the underlying mechanisms of diabetes-impaired angiogenesis remain unclear. A series of multiple mechanisms, including impaired EC biology/function and growth factor response, were suggested to lead to diminished peripheral blood perfusion and decreased angiogenesis in diabetes. H2S rescued hyperglycemia-impaired migration of HUVECs by upregulating miR-126–3p [136]. Hyperglycemia/diabetes-impaired angiogenesis is associated with an impairment of proangiogenic and bio-energetic effects of 3-MP [132]. NaHS promoted angiogenesis in ob/ob mice is related to decreasing infiltration of neutrophil and macrophage, production of TNF-α, interleukin (IL-6) [137]. H2S donor diallyl trisulfide (DATS) improved revascularization in STZ-induced diabetic mice via increasing NO bioavailability [138]. H2S promoted angiogenesis in db/db mice via improving VEGFR and PDGFR, and Angiopoietin-1 (Ang I) [118,128]. Recently we reported that diabetes impairs angiogenesis in db/db mice via eNOS-pT495-mediated reduction of NO [4].
11. H2S in diabetes-impaired wound healing
It is well known that in the physiological condition (before injury), the vasculature is quiescence in which blood vessel are adequately perfused to deliver sufficient oxygen/nutrients to the tissue [139,140]. There is a proper balance of pro-and anti-angiogenic factors are expressed to maintain a functional vascular network that neither proliferating nor diminishing [139,140]. However, this homeostasis is interrupted leading to an ischemic/hypoxic state when tissue is injured. Under post-injury, numerous proangiogenic factors are produced for formation of angiogenesis in wounds, such as VEGF and transcriptional activator hypoxia-inducible factor (HIF) which promotes angiogenesis by upregulating target genes such as VEGF-A [141].
Refractory wound is defined by slow healing of wound which is one of major microvascular complications in diabetes. In the wound healing process, impaired EC function/angiogenesis contribute to defects in the oxygen/nutrients supply, thus inhibiting normal healing process [142]. In the process of wound healing, angiogenesis is a crucial step which starts at the 3rd days after wounding [140,143]. Dysfunction of angiogenesis has been linked to impaired wound healing in diabetes [4,117,118,128]. For instance, the prevalence of foot ulcers due to impaired wound healing is predicted to be 25% in diabetic patients [144,145]. Many insufficient angiogenic factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF-2) and platelet-derived growth factor (PDGF) are involved in diabetes-impaired angiogenesis/wound healing [[146], [147], [148]]. Moreover, several studies also demonstrated that modulation of anti-angiogenic factors and capillary maturation factors are also involved in the pathogenesis of diabetes-impaired wound healing. For example, circulating levels of anti-angiogenic factor pigment epithelium-derived factor (PEDF) were significantly enhanced in patients with diabetic foot ulcers (DFU) [149], suggesting that enhanced PEDF could negatively impact wound healing in diabetes. Local administration of neutralizing antibodies to Ang I on wounded skin reduced maturation of capillary formation in STZ-induced diabetic mice [150]. In addition, several miRNAs (miRs) have also been shown to be involved in diabetic wound healing. miR-27b accelerated the wound healing process in db/db mice [128]. Inhibition of miR-155 reduced oxidative stress, increased angiogenesis and wound healing in STZ-induced diabetic rats [151]. Local administration of let-7b-containing exosomes, nanoscale membrane extracellular vesicles (30–100 nm), from LPS-primed mesenchymal stromal cells decreased inflammatory response and increased wound healing in STZ-induced diabetic mice [150]. miR-191 and -200b expression were increased in diabetic patients with PAD and chronic wounds and positively correlated with higher levels of inflammation associated markers [152,153].
H2S has been reported to accelerate gastric ulcer and skin burn wound healing [125,130,154]. Topical administration of H2S improved the recovery from burn wounds in wild-type rats and that genetic ablation of CSE delayed healing in mice [125]. H2S improves angiogenesis and wound healing in db/db mice via increasing transcription of VEGF, EGF, HIF-1α and eNOS, and protein expression of VEGF, PDGF, and phosphorylated VEGFR and PDGFR [128]. H2S accelerates wound healing in STZ-induced diabetic mice associated with formation of granulation, anti-inflammation, and the increased level of VEGF [155]. H2S improved diabetic wound healing in ob/ob mcie via attenuating inflammation [137]. H2S premised wound to close through inhibition of neutrophil extracellular traps (NET) release-coupled neutrophil death (NETosis) in db/db mice [117]. H2S improved wound healing via restoration of endothelial progenitor cell functions and activation of Ang 1 in db/db mice [118]. Cysteine/cysteine-rich un-denatured whey protein supplement improved pressure ulcer recovery in diabetic patients [156]. Accumulative evidence indicated that H2S regulates miR expression (i.e. miR-1, -221, −21, and −133a) [[157], [158], [159], [160]], however, the interaction between H2S and miRs in diabetic wound healing remain unclear. A recent study suggested that H2S may rescue diabetes-impaired wound healing via DNMT1 inhibition and consequently enhanced miR-126-3 production in ECs [161]. Further studies to understand the role of H2S in miRs-mediated EC dysfunction will provide novel insights for prevention/therapeutics ischemic tissue injury in diabetes.
12. H2S in diabetes-impaired critical limb ischemia
The morbidity of critical limb ischemia (CLI) in patients with diabetes, which affects 178 million US adults [1], is extremely high (up to 76% in some studies [162]). Patients with CLI suffer from consequent rest pain, ulcers, cool limbs, and even amputation. Disease severity at the time of presentation and progression of CLI in diabetes has also been noted to be worse [163]. Angiogenesis is a promising target for the treatment of ischemic limbs by providing extra blood for the ischemic region. Pro-angiogenic factors, such as VEGF and basic fibroblast growth factor (bFGF), plays an important role in promoting angiogenesis accompanied with enhanced blood perfusion in ischemic limbs of patients [164]. It is also reported that transplantation of bone marrow- or placenta-derived mesenchymal stem cells (MSCs) significantly improved capillary density and blood flow in ischemic hindlimbs of animal models [165,166]. Pro-angiogenic factors released from MSCs could promote angiogenic differentiation, leading to the stimulation of resident progenitor cells (EPCs) to migrate, proliferate, and differentiate into incorporated ECs [167].
In clinics, H2S levels in gastrocnemius tissue were attenuated in the setting of CLI in patients [168]. Administration of H2S was beneficial for ischemic hindlimb (IHL) repair in animal model. S-propargyl-cysteine, a novel water-soluble modulator of endogenous H2S promoted angiogenesis in IHL of mice via signal transducer and activator of transcription 3 (STAT3)/VEGF signaling [124]. Intraperitoneal injection of low dose, but not high dose, of NaHS significantly improved capillary density, angiographic scores, blood perfusion in IHL which may be mediated by interaction between the upregulated VEGF in the skeletal muscle cells and the VEGFR2 as well as its downstream signaling element AKT in the ECs [129]. H2S improved angiogenesis and blood perfusion in IHL of CSE−/- mice via peroxidsome proliferator-activated receptor-γ (PPAR-γ)/VEGF axis [169]. DATS enhanced angiogenesis and blood flow in IHL of wild-type mice via stimulation of AKT-eNOS signaling pathway [170]. H2S also limited cellular damage in myotubes and skeletal muscles of ischemic hindlimbs [171].
We examined role of H2S in diabetes-impaired biology/function of bone marrow cell (BMC) and IHL repair in db/db mice [4]. We found that H2S production and CSE expression was significantly reduced in BMCs from db/db mice. Systemic administration of DATS or local transplantation of DATS-treated or CSE-overexpressed BMCs improved capillary density, cell survival and blood perfusion in the IHL of db/db mice [4]. Mechanistically we found that DATS restored NO production and decreased eNOS-pT495 levels in human microvascular ECs. We demonstrated that H2S and overexpression of CSE in diabetic BMCs may rescue their dysfunction and open novel avenues for cell-based therapeutic of CLI in diabetic patients [4]. Consistent with the effects of DATS in IHL of db/db mice, recent study also revealed that administration of DATS improved angiogenesis and IHL repair in STZ-treated mice via NO signaling pathway [138]. However, the underlying mechanisms of downregulation of CSE expression/activity and insufficient H2S-induced eNOS-pT495 in diabetic ECs remain incompletely understood.
13. Conclusion
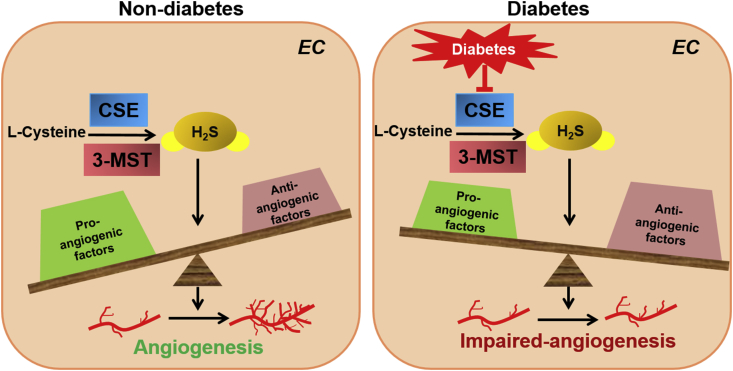
H2S is an endogenous gas with important physiological function. H2S plays an important role in maintenance of vasculature homeostasis. The evidence presented above shows that H2S, mainly synthesized by CSE or 3-MST, stimulates angiogenetic property of ECs. H2S-induced EDHF-mediated vascular relaxation is enhanced in micro-vasculature in diabetes which may play compensatory effects for overcoming H2S reduction-induced endothelial dysfunction. Diabetes enhances H2S-induced EDHF-mediated vascular relaxation in micro-vasculature by opening potassium channels, including KCa, KATP and Kv, which varies by type of vessels, diameter of vessels, severity of diabetes and species. Physiological doses of H2S increase angiogenesis by promoting EC growth, survival migration and tube formation. H2S production is reduced in plasma/blood, ECs and bone marrow cells in diabetes. Insufficient H2S production in diabetes impairs angiogenic property and ischemic tissue injury via, at least partially, interrupting the balance between pro- and anti-angiogenic factors (Fig. 2). Although studies showed that administration of H2S or overexpressing CSE improves EC biology/function, angiogenesis and IHL repair in diabetes, how H2S impacts on the metabolism of EC and contributes to it angiogenic properties in diabetes remain unclear. In addition, studies for significance of translational medicine of H2S on diabetes-impaired angiogenesis and ischemic tissue repair in patients are warranted.
Fig. 2.
Mechanistic scheme of H2S in non-diabetes-(left panel) and diabetic-mediated(right panel) angiogenic property of ECs in response to ischemic tissue injury. H2S plays an important role in maintaining physiological balance in ECs in response to ischemic tissue injury. Under diabetic condition, insufficient H2S production impaired angiogenetic property of ECs via disturbing the balance of pro-and anti-angiogenic factors. CSE: cystathionine--lase-lyase; 3-MST: mercaptopyruvate sulfurtransferase.
14. Declaration of completing interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported in part by the NIH grants HL091983, HL143892, and HL134608 (R.K.) and American Heart Association grant 17GRNT33660941 (Z.C.).
Contributor Information
Zhongjian Cheng, Email: zjcheng@temple.edu.
Raj Kishore, Email: tuf51785@temple.edu.
References
- 1.Gregg E.W., Li Y., Wang J. Changes in diabetes-related complications in the United States, 1990-2010. N. Engl. J. Med. 2014;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Fox C.S., Coady S., Sorlie P.D. Trends in cardiovascular complications of diabetes. J. Am. Med. Assoc. 2004;292(20):2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 3.van den Born J.C., Hammes H.P., Greffrath W., van Goor H., Hillebrands J.L., DFG GRK International Research Training Group 1874 Diabetic Microvascular Complications (DIAMICOM) Gasotransmitters in vascular complications of diabetes. Diabetes. 2016;65(2):331–345. doi: 10.2337/db15-1003. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z., Garikipati V.N., Nickoloff E. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation. 2016;134(19):1467–1483. doi: 10.1161/CIRCULATIONAHA.116.022967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Z., Shen X., Jiang X. Hyperhomocysteinemia potentiates diabetes-impaired EDHF-induced vascular relaxation: role of insufficient hydrogen sulfide. Redox Biol. 2018;16:215–225. doi: 10.1016/j.redox.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low Wang C.C., Hess C.N., Hiatt W.R., Goldfine A.B. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 8.Donertas Ayaz B., Zubcevic J. Gut microbiota and neuroinflammation in pathogenesis of hypertension: a potential role for hydrogen sulfide. Pharmacol. Res. 2020;153:104677. doi: 10.1016/j.phrs.2020.104677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnarumma E., Ali M.J., Rushing A.M. Zofenopril protects against myocardial ischemia-reperfusion injury by increasing nitric oxide and hydrogen sulfide bioavailability. J Am Heart Assoc. 2016;5(7) doi: 10.1161/JAHA.116.003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polhemus D.J., Calvert J.W., Butler J., Lefer D.J. The cardioprotective actions of hydrogen sulfide in acute myocardial infarction and heart failure. Sci. Tech. Rep. 2014;2014:768607. doi: 10.1155/2014/768607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durante W. Hydrogen sulfide therapy in diabetes-accelerated atherosclerosis: a whiff of success. Diabetes. 2016;65(10):2832–2834. doi: 10.2337/dbi16-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y.H., Yan C.D., Bian J.S. Hydrogen sulfide: a novel signaling molecule in the vascular system. J. Cardiovasc. Pharmacol. 2011;58(6):560–569. doi: 10.1097/FJC.0b013e31820eb7a1. [DOI] [PubMed] [Google Scholar]
- 13.Gadalla M.M., Snyder S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010;113(1):14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behera J., George A.K., Voor M.J., Tyagi S.C., Tyagi N. Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice. Bone. 2018;114:90–108. doi: 10.1016/j.bone.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibuya N., Mikami Y., Kimura Y., Nagahara N., Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009;146(5):623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 16.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114(4):730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G., Wu L., Jiang B. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y., Ndisang J.F., Tang G., Cao K., Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004;287(5):H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang G., Yang G., Jiang B., Ju Y., Wu L., Wang R.H. (2)S is an endothelium-derived hyperpolarizing factor. Antioxidants Redox Signal. 2013;19(14):1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 21.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxidants Redox Signal. 2003;5(4):493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 22.Shibuya N., Tanaka M., Yoshida M. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants Redox Signal. 2009;11(4):703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 23.Shen X., Peter E.A., Bir S., Wang R., Kevil C.G. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic. Biol. Med. 2012;52(11–12):2276–2283. doi: 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishigami M., Hiraki K., Umemura K., Ogasawara Y., Ishii K., Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxidants Redox Signal. 2009;11(2):205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 25.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitt M.D., Abdel-Rehim M.S., Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxidants Redox Signal. 2011;15(2):373–378. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y.H., Lu M., Hu L.F., Wong P.T., Webb G.D., Bian J.S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxidants Redox Signal. 2012;17(1):141–185. doi: 10.1089/ars.2011.4005. [DOI] [PubMed] [Google Scholar]
- 28.Ogasawara Y., Ishii K., Togawa T., Tanabe S. Determination of bound sulfur in serum by gas dialysis/high-performance liquid chromatography. Anal. Biochem. 1993;215(1):73–81. doi: 10.1006/abio.1993.1556. [DOI] [PubMed] [Google Scholar]
- 29.Togawa T., Ogawa M., Nawata M., Ogasawara Y., Kawanabe K., Tanabe S. High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre-column fluorescence derivatization with monobromobimane. Chem. Pharm. Bull. (Tokyo) 1992;40(11):3000–3004. doi: 10.1248/cpb.40.3000. [DOI] [PubMed] [Google Scholar]
- 30.Whitfield N.L., Kreimier E.L., Verdial F.C., Skovgaard N., Olson K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(6):R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 31.Wintner E.A., Deckwerth T.L., Langston W. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 2010;160(4):941–957. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long L.H., Liu J., Liu R.L. Differential effects of methionine and cysteine oxidation on [Ca2+] i in cultured hippocampal neurons. Cell. Mol. Neurobiol. 2009;29(1):7–15. doi: 10.1007/s10571-008-9289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolluru G.K., Shen X., Bir S.C., Kevil C.G. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Searcy D.G., Lee S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998;282(3):310–322. doi: 10.1002/(sici)1097-010x(19981015)282:3<310::aid-jez4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 35.Olson K.R., Deleon E.R., Gao Y. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305(6):R592–R603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- 36.Hildebrandt T.M., Grieshaber M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275(13):3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 37.Olson K.R. Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling. J. Comp. Physiol. B. 2012;182(7):881–897. doi: 10.1007/s00360-012-0654-y. [DOI] [PubMed] [Google Scholar]
- 38.Libiad M., Yadav P.K., Vitvitsky V., Martinov M., Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014;289(45):30901–30910. doi: 10.1074/jbc.M114.602664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beltowski J. Humana Press: Springer Protocols; 2007. Vascular Effects of Hyrdrogen Sulfide: Methods and Protocols. [Google Scholar]
- 40.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono K., Akaike T., Sawa T. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014;77:82–94. doi: 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D., Du J., Tang C., Huang Y., Jin H. H2S-Induced sulfhydration: biological function and detection methodology. Front. Pharmacol. 2017;8:608. doi: 10.3389/fphar.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie L., Gu Y., Wen M. Hydrogen sulfide induces Keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes. 2016;65(10):3171–3184. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kundu S., Pushpakumar S.B., Tyagi A., Coley D., Sen U. Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am. J. Physiol. Endocrinol. Metab. 2013;304(12):E1365–E1378. doi: 10.1152/ajpendo.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain S.K., Bull R., Rains J.L. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxidants Redox Signal. 2010;12(11):1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brancaleone V., Roviezzo F., Vellecco V., De Gruttola L., Bucci M., Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br. J. Pharmacol. 2008;155(5):673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Z.J., Vaskonen T., Tikkanen I. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension. 2001;37(2 Pt 2):433–439. doi: 10.1161/01.hyp.37.2.433. [DOI] [PubMed] [Google Scholar]
- 48.Yang G., Tang G., Zhang L., Wu L., Wang R. The pathogenic role of cystathionine gamma-lyase/hydrogen sulfide in streptozotocin-induced diabetes in mice. Am. J. Pathol. 2011;179(2):869–879. doi: 10.1016/j.ajpath.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiteman M., Gooding K.M., Whatmore J.L. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53(8):1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K., Olah G., Modis K. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 2011;108(33):13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin J., Chen M., Liu D. Exogenous hydrogen sulfide protects human umbilical vein endothelial cells against high glucoseinduced injury by inhibiting the necroptosis pathway. Int. J. Mol. Med. 2018;41(3):1477–1486. doi: 10.3892/ijmm.2017.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y.F., Dai D.Z., Dai Y. NaHS ameliorates diabetic vascular injury by correcting depressed connexin 43 and 40 in the vasculature in streptozotocin-injected rats. J. Pharm. Pharmacol. 2010;62(5):615–621. doi: 10.1211/jpp.62.05.0009. [DOI] [PubMed] [Google Scholar]
- 54.Liu J., Wu J., Sun A. Hydrogen sulfide decreases high glucose/palmitate-induced autophagy in endothelial cells by the Nrf2-ROS-AMPK signaling pathway. Cell Biosci. 2016;6:33. doi: 10.1186/s13578-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng H.H., Yildiz G.S., Ku J.M., Miller A.A., Woodman O.L., Hart J.L. Chronic NaHS treatment decreases oxidative stress and improves endothelial function in diabetic mice. Diabetes Vasc. Dis. Res. 2017;14(3):246–253. doi: 10.1177/1479164117692766. [DOI] [PubMed] [Google Scholar]
- 56.Gero D., Torregrossa R., Perry A. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016;113(Pt A):186–198. doi: 10.1016/j.phrs.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manna P., Jain S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-gamma-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 2012;287(50):42324–42332. doi: 10.1074/jbc.M112.407833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenberg A.S., Obin M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006;83(2):461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Yang G., Untereiner A., Ju Y., Wu L., Wang R. Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology. 2013;154(1):114–126. doi: 10.1210/en.2012-1658. [DOI] [PubMed] [Google Scholar]
- 60.Ju Y., Untereiner A., Wu L., Yang G. H2S-induced S-sulfhydration of pyruvate carboxylase contributes to gluconeogenesis in liver cells. Biochim. Biophys. Acta. 2015;1850(11):2293–2303. doi: 10.1016/j.bbagen.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Pangare M., Makino A. Mitochondrial function in vascular endothelial cell in diabetes. J. Smooth Muscle Res. 2012;48(1):1–26. doi: 10.1540/jsmr.48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng Z., Yang X., Wang H. Hyperhomocysteinemia and endothelial dysfunction. Curr. Hypertens. Rev. 2009;5(2):158–165. doi: 10.2174/157340209788166940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 64.Feletou M., Vanhoutte P.M. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler. Thromb. Vasc. Biol. 2006;26(6):1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 65.Triggle C.R., Ding H. Endothelium-derived hyperpolarizing factor: is there a novel chemical mediator? Clin. Exp. Pharmacol. Physiol. 2002;29(3):153–160. doi: 10.1046/j.1440-1681.2002.03632.x. [DOI] [PubMed] [Google Scholar]
- 66.Kohler R., Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int. 2007;72(2):145–150. doi: 10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- 67.Triggle C.R., Howarth A., Cheng Z.J., Ding H. Twenty-five years since the discovery of endothelium-derived relaxing factor (EDRF): does a dysfunctional endothelium contribute to the development of type 2 diabetes? Can. J. Physiol. Pharmacol. 2005;83(8–9):681–700. doi: 10.1139/y05-069. [DOI] [PubMed] [Google Scholar]
- 68.McGuire J.J., Ding H., Triggle C.R. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s) Can. J. Physiol. Pharmacol. 2001;79(6):443–470. [PubMed] [Google Scholar]
- 69.Teng Y., Xuan S., Jiang M., Tian L., Tian J., Chang Q. Expression of H2S in gestational diabetes mellitus and correlation analysis with inflammatory markers IL-6 and TNF-alpha. J Diabetes Res. 2020;2020 doi: 10.1155/2020/3085840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kar S., Shahshahan H.R., Hackfort B.T. Exercise training promotes cardiac hydrogen sulfide biosynthesis and mitigates pyroptosis to prevent high-fat diet-induced diabetic cardiomyopathy. Antioxidants. 2019;8(12) doi: 10.3390/antiox8120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grabowska-Polanowska B., Skowron M., Miarka P., Pietrzycka A., Sliwka I. The application of chromatographic breath analysis in the search of volatile biomarkers of chronic kidney disease and coexisting type 2 diabetes mellitus. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1060:103–110. doi: 10.1016/j.jchromb.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 72.Zhong X., Wang Y., Wu J. Calcium sensing receptor regulating smooth muscle cells proliferation through initiating cystathionine-gamma-lyase/hydrogen sulfide pathway in diabetic rat. Cell. Physiol. Biochem. 2015;35(4):1582–1598. doi: 10.1159/000373973. [DOI] [PubMed] [Google Scholar]
- 73.Dutta M., Biswas U.K., Chakraborty R., Banerjee P., Raychaudhuri U., Kumar A. Evaluation of plasma H2S levels and H2S synthesis in streptozotocin induced Type-2 diabetes-an experimental study based on Swietenia macrophylla seeds. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S483–S487. doi: 10.12980/APJTB.4.201414B58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng X., Chen Y., Zhao J., Tang C., Jiang Z., Geng B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem. Biophys. Res. Commun. 2009;380(1):153–159. doi: 10.1016/j.bbrc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 75.Yusuf M., Kwong Huat B.T., Hsu A., Whiteman M., Bhatia M., Moore P.K. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem. Biophys. Res. Commun. 2005;333(4):1146–1152. doi: 10.1016/j.bbrc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Kaneko Y., Kimura Y., Kimura H., Niki I. L-cysteine inhibits insulin release from the pancreatic beta-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes. 2006;55(5):1391–1397. doi: 10.2337/db05-1082. [DOI] [PubMed] [Google Scholar]
- 77.Ji C., Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J. Gastroenterol. 2004;10(12):1699–1708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Possomato-Vieira J.S., Chimini J.S., da Silva M.L.S., Dias-Junior C.A. Increases in placental nitric oxide, but not nitric oxide-mediated relaxation, underlie the improvement in placental efficiency and antihypertensive effects of hydrogen sulphide donor in hypertensive pregnancy. Clin. Exp. Pharmacol. Physiol. 2018;45(11):1118–1127. doi: 10.1111/1440-1681.13000. [DOI] [PubMed] [Google Scholar]
- 79.Yetik-Anacak G., Sevin G., Ozzayim O., Dereli M.V., Ahmed A. Hydrogen sulfide: a novel mechanism for the vascular protection by resveratrol under oxidative stress in mouse aorta. Vasc. Pharmacol. 2016;87:76–82. doi: 10.1016/j.vph.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 80.Liu Z., Han Y., Li L. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(-/-) mice. Br. J. Pharmacol. 2013;169(8):1795–1809. doi: 10.1111/bph.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kubo S., Kajiwara M., Kawabata A. Dual modulation of the tension of isolated gastric artery and gastric mucosal circulation by hydrogen sulfide in rats. Inflammopharmacology. 2007;15(6):288–292. doi: 10.1007/s10787-007-1590-4. [DOI] [PubMed] [Google Scholar]
- 82.Materazzi S., Zagli G., Nassini R. Vasodilator activity of hydrogen sulfide (H2S) in human mesenteric arteries. Microvasc. Res. 2017;109:38–44. doi: 10.1016/j.mvr.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Webb G.D., Lim L.H., Oh V.M. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J. Pharmacol. Exp. Therapeut. 2008;324(2):876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- 84.Wang R. Hydrogen sulfide: a new EDRF. Kidney Int. 2009;76(7):700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- 85.Mustafa A.K., Sikka G., Gazi S.K. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109(11):1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mustafina A.N., Yakovlev A.V., Gaifullina A., Weiger T.M., Hermann A., Sitdikova G.F. Hydrogen sulfide induces hyperpolarization and decreases the exocytosis of secretory granules of rat GH3 pituitary tumor cells. Biochem. Biophys. Res. Commun. 2015;465(4):825–831. doi: 10.1016/j.bbrc.2015.08.095. [DOI] [PubMed] [Google Scholar]
- 87.Dongo E., Beliczai-Marosi G., Dybvig A.S., Kiss L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide. 2018;81:75–87. doi: 10.1016/j.niox.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Holwerda K.M., Karumanchi S.A., Lely A.T. Hydrogen sulfide: role in vascular physiology and pathology. Curr. Opin. Nephrol. Hypertens. 2015;24(2):170–176. doi: 10.1097/MNH.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 89.Tang G., Wu L., Wang R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010;37(7):753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- 90.Wang R., Szabo C., Ichinose F., Ahmed A., Whiteman M., Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol. Sci. 2015;36(9):568–578. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han J., Chen Z.W., He G.W. Acetylcholine- and sodium hydrosulfide-induced endothelium-dependent relaxation and hyperpolarization in cerebral vessels of global cerebral ischemia-reperfusion rat. J. Pharmacol. Sci. 2013;121(4):318–326. doi: 10.1254/jphs.12277fp. [DOI] [PubMed] [Google Scholar]
- 92.Ahmad F.U., Sattar M.A., Rathore H.A. Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Ren. Fail. 2012;34(2):203–210. doi: 10.3109/0886022X.2011.643365. [DOI] [PubMed] [Google Scholar]
- 93.Manna P., Jain S.K. L-cysteine and hydrogen sulfide increase PIP3 and AMPK/PPARgamma expression and decrease ROS and vascular inflammation markers in high glucose treated human U937 monocytes. J. Cell. Biochem. 2013;114(10):2334–2345. doi: 10.1002/jcb.24578. [DOI] [PubMed] [Google Scholar]
- 94.Wei W.B., Hu X., Zhuang X.D., Liao L.Z., Li W.D. GYY4137, a novel hydrogen sulfide-releasing molecule, likely protects against high glucose-induced cytotoxicity by activation of the AMPK/mTOR signal pathway in H9c2 cells. Mol. Cell. Biochem. 2014;389(1–2):249–256. doi: 10.1007/s11010-013-1946-6. [DOI] [PubMed] [Google Scholar]
- 95.Zhou X., An G., Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin. Sci. (Lond.) 2015;128(5):325–335. doi: 10.1042/CS20140460. [DOI] [PubMed] [Google Scholar]
- 96.Denizalti M., Bozkurt T.E., Akpulat U., Sahin-Erdemli I., Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011;383(5):509–517. doi: 10.1007/s00210-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 97.Centeno J.M., Lopez-Morales M.A., Aliena-Valero A. Potassium channels contribute to the increased sensitivity of the rabbit carotid artery to hydrogen sulfide in diabetes. Eur. J. Pharmacol. 2019;853:33–40. doi: 10.1016/j.ejphar.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Hong da H., Li H., Kim H.W. Alterations of voltage-dependent K(+) channels in the mesenteric artery during the early and chronic phases of diabetes. Clin. Exp. Pharmacol. Physiol. 2016;43(9):808–817. doi: 10.1111/1440-1681.12599. [DOI] [PubMed] [Google Scholar]
- 99.Beltowski J., Wojcicka G., Jamroz-Wisniewska A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: implications for the pathogenesis and treatment of diabetes mellitus. Biochem. Pharmacol. 2018;149:60–76. doi: 10.1016/j.bcp.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 100.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 101.Olson K.R. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301(2):R297–R312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- 102.Yuan S., Shen X., Kevil C.G. Beyond a gasotransmitter: hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxidants Redox Signal. 2017;27(10):634–653. doi: 10.1089/ars.2017.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ali M.Y., Ping C.Y., Mok Y.Y. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006;149(6):625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kubo S., Doe I., Kurokawa Y., Nishikawa H., Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007;232(1–2):138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 105.Cortese-Krott M.M., Kuhnle G.G., Dyson A. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. U. S. A. 2015;112(34):E4651–E4660. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim J.J., Liu Y.H., Khin E.S., Bian J.S. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008;295(5):C1261–C1270. doi: 10.1152/ajpcell.00195.2008. [DOI] [PubMed] [Google Scholar]
- 107.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8(6):464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 108.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 109.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 110.Flamme I., Frolich T., Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J. Cell. Physiol. 1997;173(2):206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 111.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 112.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 113.Karamysheva A.F. Mechanisms of angiogenesis. Biochemistry (Mosc.) 2008;73(7):751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 114.Risau W., Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 115.Kuwano M., Fukushi J., Okamoto M. Angiogenesis factors. Intern. Med. 2001;40(7):565–572. doi: 10.2169/internalmedicine.40.565. [DOI] [PubMed] [Google Scholar]
- 116.Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 117.Yang C.T., Chen L., Chen W.L. Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis. Mol. Cell. Endocrinol. 2019;480:74–82. doi: 10.1016/j.mce.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 118.Liu F., Chen D.D., Sun X. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014;63(5):1763–1778. doi: 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feliers D., Lee H.J., Kasinath B.S. Hydrogen sulfide in renal physiology and disease. Antioxidants Redox Signal. 2016;25(13):720–731. doi: 10.1089/ars.2015.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Costa P.Z., Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92(22):1037–1045. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 121.Bir S.C., Kolluru G.K., McCarthy P. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc. 2012;1(5) doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coletta C., Papapetropoulos A., Erdelyi K. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U. S. A. 2012;109(23):9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu Q., Wu D., Ma F. Novel angiogenic activity and molecular mechanisms of ZYZ-803, a slow-releasing hydrogen sulfide-nitric oxide hybrid molecule. Antioxidants Redox Signal. 2016;25(8):498–514. doi: 10.1089/ars.2015.6607. [DOI] [PubMed] [Google Scholar]
- 124.Kan J., Guo W., Huang C., Bao G., Zhu Y., Zhu Y.Z. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxidants Redox Signal. 2014;20(15):2303–2316. doi: 10.1089/ars.2013.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papapetropoulos A., Pyriochou A., Altaany Z. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2009;106(51):21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Polhemus D., Kondo K., Bhushan S. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail. 2013;6(5):1077–1086. doi: 10.1161/CIRCHEARTFAILURE.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szabo C., Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol. 2011;164(3):853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang G.G., Li W. Hydrogen sulfide improves vessel formation of the ischemic adductor muscle and wound healing in diabetic db/db mice. Iran J Basic Med Sci. 2019;22(10):1192–1197. doi: 10.22038/ijbms.2019.36551.8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang M.J., Cai W.J., Li N., Ding Y.J., Chen Y., Zhu Y.C. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxidants Redox Signal. 2010;12(9):1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 130.Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76(1):29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 131.Saha S., Chakraborty P.K., Xiong X. Cystathionine beta-synthase regulates endothelial function via protein S-sulfhydration. Faseb. J. 2016;30(1):441–456. doi: 10.1096/fj.15-278648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coletta C., Modis K., Szczesny B. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by DL-alpha-lipoic acid. Mol. Med. 2015;21:1–14. doi: 10.2119/molmed.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 135.Kolluru G.K., Bir S.C., Yuan S. Cystathionine gamma-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc. Res. 2015;107(4):590–600. doi: 10.1093/cvr/cvv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xue W.L., Chen R.Q., Zhang Q.Q. Hydrogen sulfide rescues high glucose-induced migration dysfunction in HUVECs by upregulating miR-126-3p. Am. J. Physiol. Cell Physiol. 2020 May 1;318(5):C857–C869. doi: 10.1152/ajpcell.00406.2019. [DOI] [PubMed] [Google Scholar]
- 137.Zhao H., Lu S., Chai J. Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J. Diabet. Complicat. 2017;31(9):1363–1369. doi: 10.1016/j.jdiacomp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 138.Yang H.B., Liu H.M., Yan J.C., Lu Z.Y. Effect of diallyl trisulfide on ischemic tissue injury and revascularization in a diabetic mouse model. J. Cardiovasc. Pharmacol. 2018;71(6):367–374. doi: 10.1097/FJC.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Demidova-Rice T.N., Durham J.T., Herman I.M. Wound healing angiogenesis: innovations and challenges in acute and chronic wound healing. Adv. Wound Care. 2012;1(1):17–22. doi: 10.1089/wound.2011.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tonnesen M.G., Feng X., Clark R.A. Angiogenesis in wound healing. J. Invest. Dermatol. Symp. Proc. 2000;5(1):40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 141.Liu L., Marti G.P., Wei X. Age-dependent impairment of HIF-1alpha expression in diabetic mice: correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J. Cell. Physiol. 2008;217(2):319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shyng Y.C., Devlin H., Sloan P. The effect of streptozotocin-induced experimental diabetes mellitus on calvarial defect healing and bone turnover in the rat. Int. J. Oral Maxillofac. Surg. 2001;30(1):70–74. doi: 10.1054/ijom.2000.0004. [DOI] [PubMed] [Google Scholar]
- 143.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., Brem H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009;153(2):347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lavery L.A., Armstrong D.G., Wunderlich R.P., Tredwell J., Boulton A.J. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003;26(5):1435–1438. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- 145.Singh N., Armstrong D.G., Lipsky B.A. Preventing foot ulcers in patients with diabetes. J. Am. Med. Assoc. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 146.Galiano R.D., Tepper O.M., Pelo C.R. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004;164(6):1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brem H., Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest. 2007;117(5):1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng R., Ma J.X. Angiogenesis in diabetes and obesity. Rev. Endocr. Metab. Disord. 2015;16(1):67–75. doi: 10.1007/s11154-015-9310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qi W., Yang C., Dai Z. High levels of pigment epithelium-derived factor in diabetes impair wound healing through suppression of Wnt signaling. Diabetes. 2015;64(4):1407–1419. doi: 10.2337/db14-1111. [DOI] [PubMed] [Google Scholar]
- 150.Li C., Yu T., Liu Y., Chen X., Zhang X. Topical application of insulin accelerates vessel maturation of wounds by regulating angiopoietin-1 in diabetic mice. Int. J. Low. Extrem. Wounds. 2015;14(4):353–364. doi: 10.1177/1534734615600590. [DOI] [PubMed] [Google Scholar]
- 151.Ye J., Kang Y., Sun X., Ni P., Wu M., Lu S. MicroRNA-155 inhibition promoted wound healing in diabetic rats. Int. J. Low. Extrem. Wounds. 2017;16(2):74–84. doi: 10.1177/1534734617706636. [DOI] [PubMed] [Google Scholar]
- 152.Dangwal S., Stratmann B., Bang C. Impairment of wound healing in patients with type 2 diabetes mellitus influences circulating MicroRNA patterns via inflammatory cytokines. Arterioscler. Thromb. Vasc. Biol. 2015;35(6):1480–1488. doi: 10.1161/ATVBAHA.114.305048. [DOI] [PubMed] [Google Scholar]
- 153.Nagpal N., Kulshreshtha R. miR-191: an emerging player in disease biology. Front. Genet. 2014;5:99. doi: 10.3389/fgene.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wallace J.L., Dicay M., McKnight W., Martin G.R. Hydrogen sulfide enhances ulcer healing in rats. Faseb. J. 2007;21(14):4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 155.Wang G., Li W., Chen Q., Jiang Y., Lu X., Zhao X. Hydrogen sulfide accelerates wound healing in diabetic rats. Int. J. Clin. Exp. Pathol. 2015;8(5):5097–5104. [PMC free article] [PubMed] [Google Scholar]
- 156.Gutman J.B.L., Kongshavn P.A.L. Cysteine/cystine-rich undenatured whey protein supplement in patients' pressure ulcers outcomes: an open label study. J. Wound Care. 2019;28(Sup7):S16–S23. doi: 10.12968/jowc.2019.28.Sup7.S16. [DOI] [PubMed] [Google Scholar]
- 157.Kang B., Hong J., Xiao J. Involvement of miR-1 in the protective effect of hydrogen sulfide against cardiomyocyte apoptosis induced by ischemia/reperfusion. Mol. Biol. Rep. 2014;41(10):6845–6853. doi: 10.1007/s11033-014-3570-2. [DOI] [PubMed] [Google Scholar]
- 158.Kesherwani V., Nandi S.S., Sharawat S.K., Shahshahan H.R., Mishra P.K. Hydrogen sulfide mitigates homocysteine-mediated pathological remodeling by inducing miR-133a in cardiomyocytes. Mol. Cell. Biochem. 2015;404(1–2):241–250. doi: 10.1007/s11010-015-2383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu J., Hao D.D., Zhang J.S., Zhu Y.C. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem. Biophys. Res. Commun. 2011;413(2):342–347. doi: 10.1016/j.bbrc.2011.08.101. [DOI] [PubMed] [Google Scholar]
- 160.Hackfort B.T., Mishra P.K. Emerging role of hydrogen sulfide-microRNA crosstalk in cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2016;310(7):H802–H812. doi: 10.1152/ajpheart.00660.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xue W.L., Chen R.Q., Zhang Q.Q. Hydrogen sulfide rescues high glucose-induced migration dysfunction in HUVECs by upregulating miR-126-3p. Am. J. Physiol. Cell Physiol. 2020;318(5):C857–C869. doi: 10.1152/ajpcell.00406.2019. [DOI] [PubMed] [Google Scholar]
- 162.Dick F., Diehm N., Galimanis A., Husmann M., Schmidli J., Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: influence of diabetes mellitus on clinical outcome. J. Vasc. Surg. 2007;45(4):751–761. doi: 10.1016/j.jvs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 163.Kullo I.J., Bailey K.R., Kardia S.L., Mosley T.H., Jr., Boerwinkle E., Turner S.T. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc. Med. 2003;8(4):237–242. doi: 10.1191/1358863x03vm511oa. [DOI] [PubMed] [Google Scholar]
- 164.Oladipupo S., Hu S., Kovalski J. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. U. S. A. 2011;108(32):13264–13269. doi: 10.1073/pnas.1101321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu J., Hao H., Xia L. Hypoxia pretreatment of bone marrow mesenchymal stem cells facilitates angiogenesis by improving the function of endothelial cells in diabetic rats with lower ischemia. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0126715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xie N., Li Z., Adesanya T.M. Transplantation of placenta-derived mesenchymal stem cells enhances angiogenesis after ischemic limb injury in mice. J. Cell Mol. Med. 2016;20(1):29–37. doi: 10.1111/jcmm.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu F., Zhao X., Wu J. MSCs transfected with hepatocyte growth factor or vascular endothelial growth factor improve cardiac function in the infarcted porcine heart by increasing angiogenesis and reducing fibrosis. Int. J. Cardiol. 2013;167(6):2524–2532. doi: 10.1016/j.ijcard.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 168.Islam K.N., Polhemus D.J., Donnarumma E., Brewster L.P., Lefer D.J. Hydrogen sulfide levels and nuclear factor-erythroid 2-related factor 2 (NRF2) activity are attenuated in the setting of critical limb ischemia (CLI) J Am Heart Assoc. 2015;4(5) doi: 10.1161/JAHA.115.001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Majumder A., Singh M., George A.K., Behera J., Tyagi N., Tyagi S.C. Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-beta-synthase mutant mice via PPAR-gamma/VEGF axis. Phys. Rep. 2018;6(17) doi: 10.14814/phy2.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hayashida R., Kondo K., Morita S. Diallyl trisulfide augments ischemia-induced angiogenesis via an endothelial nitric oxide synthase-dependent mechanism. Circ. J. 2017;81(6):870–878. doi: 10.1253/circj.CJ-16-1097. [DOI] [PubMed] [Google Scholar]
- 171.Henderson P.W., Jimenez N., Ruffino J. Therapeutic delivery of hydrogen sulfide for salvage of ischemic skeletal muscle after the onset of critical ischemia. J. Vasc. Surg. 2011;53(3):785–791. doi: 10.1016/j.jvs.2010.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]