Abstract
Objectives
The Polaris™ loop ureteric stent reduces the severity of stent discomfort by minimising stent material in the bladder. Early impact of ureteral stenting on quality of life (QoL) within 1 week remains unclear. The usefulness of the patient-administered ureteral stent symptoms questionnaire (USSQ) during this period of stent insertion was assessed. In this pilot single-blinded prospective randomised study, we investigate 1) the presence of early (within the 1st week) stent discomfort via the visual analog scale (VAS); 2) determine the QoL of the loop stent against conventional stent.
Methods
Forty adults requiring retrograde unilateral ureteral stent placements were enrolled. Patients with single ureteric stone or benign stricture were selected. Patients were randomised in 1:1 ratio to the loop and pigtail arm. The USSQ was administered before placement (baseline), USSQ and VAS were administered on Day 3, 7, and 14.
Results
There were no significant differences between the USSQ scores. Median pain scores on Day 3 were lower in the loop stent group (2.9 vs. 4.0, p=0.047). There was a significant reduction in pain from Day 3–7 (0 vs. −1, p=0.016) in the pigtail group.
Conclusions
Our results suggest that peak stent discomfort occurs but resolves quickly within 1 week of post-stent insertion. The loop stent offers a better pain profile compared with conventional stents at Day 3 but no difference in QoL. The loop stent reduces early pain experience post-stent insertion and may have a role in the care of patients who experience significant stent discomfort previously.
Keywords: Quality of life, Stent discomfort, Ureteral stent symptoms questionnaire, Ureteric stents
1. Introduction
Ureteral stents have been used in the field of urology since 1967 [1]. Ureteral stent discomfort remains a significant cause of morbidity in patients after stent insertion. Up to 80% of patients experience some form of decreased quality of life (QoL) after stent insertion [2,3]. While the etiology of the stent discomfort is unknown, it has been postulated that stent discomfort symptoms are caused by irritation of the bladder trigone, smooth muscle spasm and the presence of ureteral stent material in the bladder [[4], [5], [6]].
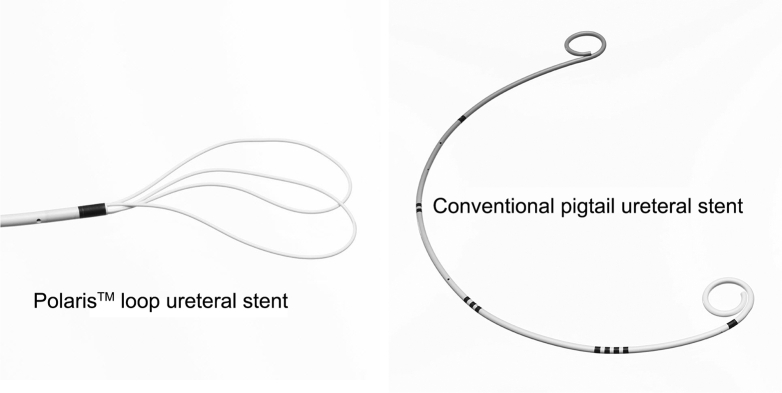
The Polaris™ (Boston Scientific, MA, USA) loop ureteric stent has a unique soft tail distal loop, which is hypothesised to reduce the severity of stent discomfort by minimising the amount of stent material in the bladder compared with conventional pigtail stent [5]. This minimises the bladder footprint, which may result in better quality of life for patients requiring stent insertion (Fig. 1).
Figure 1.
Polaris™ loop ureteral stent and conventional pigtail ureteral stent.
Early impact of ureteral stenting on QoL and pain remains unclear [5]. Clinical experience suggests that stent discomfort is most pronounced immediately after stent insertion, which tapers down significantly over the next 72 h. The majority of published literature, however, assesses stent symptoms beyond Day 4 by which meaningful differences may no longer be found [5,7,8].
Ureteral Stent Symptoms Questionnaire (USSQ) has been validated by the current literature to assess stent discomfort [3,9]. The questionnaire includes subdomains regarding urinary symptoms, body pain, general health, work performance, sexual health and additional problems to assess the overall QoL post-stent insertion. As pain remains the most significant morbidity experienced by patients, we separately analysed the visual analog scale (VAS) to assess the pain profile of our patients undergoing different stents.
In this pilot single-blinded prospective randomised study, we investigated the presence of early (defined as within the 1st week) stent discomfort experienced by the patient via the VAS. Our secondary endpoint is to determine the impact on the QoL of the loop stent compared with conventional pigtail stent as assessed through the USSQ.
2. Material and methods
In this Singapore General Hospital institutional research board approved study, 40 English speaking adults between 21 and 75 years old requiring unilateral retrograde ureteral stent placements for at least 10–14 days were enrolled from April 2014 to July 2015 at a single institution in a 1:1 ratio. A total of 60 patients were assessed for study eligibility between April 2014 and July 2015. There were 15 exclusions while five patients declined.
Study inclusion criteria include: Patients requiring unilateral ureteroscopy and laser lithotripsy for single ureteric stone or benign obstruction (e.g. tuberculosis or stricture from previous endoscopic procedures). Exclusion criteria include urinary obstruction from malignancies, history of ureteric stenting in the preceding year, intraoperative ureteric injury on day of randomisation requiring open repair, history of spinal cord injury, bilateral ureteric stenting, active urinary infection, antegrade stenting or chronic pain issues requiring long-term analgesia.
All patients provided signed informed consents before enrollment into the study.
The USSQ was administered before placement (baseline), on Day 3, 7, and 14. A separate VAS was applied on the same days to assess pain as a single modality.
All patients were instructed on how to fill in the USSQ before placement. These baseline USSQ scores were not used in the analysis, and all patients were presumed to be pain-free at the start of the study. USSQ and VAS questionnaires were returned to the study team at the point of stent removal and assessed for completeness.
All ureteric stents were placed in a retrograde fashion under fluoroscopic guidance. Stent sizes were either 6 Fr or 7 Fr. In our institution, we typically inserted size 6 Fr and 7 Fr ureteral stents for female and male patients respectively. The conventional double pigtail ureteral stents routinely used in our institution included Cook, Boston and Bard ureteral stents. The length of the stents deployed was decided by the attending surgeon according to patient body habitus.
Prior to the start of the trial, simple randomisation generated by a computer was performed. Results of the randomisation were placed sequentially into sealed envelopes which were opened when patients were recruited into the study intra-operatively.
At the end of the procedure, the attending surgeon would decide if a ureteric stent is required. If a stent is needed, the attending surgeon will open the sealed randomisation envelope to reveal the randomisation and the type of stent allocated to maintain single blindness. On discharge, only simple analgesia (paracetamol 1 g qds × 1 week) was prescribed for pain relief.
Two patients did not return the questionnaires. A total of 38 USSQ and VAS were available for final analysis. USSQ and VAS scores were tabulated and analysed using STATA version 13.0 (StataCorp, Texas, USA). Non-parametric test like Wilcoxon rank-sum test was used to compare the differences in scores between baseline and Day 3, 7, and 14 for the loop stent and control groups. Median and interquartile range were used. A difference in statistical significance was considered if the p-value was <0.05 (Fig. 2).
Figure 2.
Consort diagram of the study. LL, laser lithotripsy; URS, ureteroscopy; UTI, urinary tract infection; USSQ, ureteral stent symptoms questionnaire; VAS, visual analog scale.
Generalized estimating equation (GEE) was used for multivariable repeated measures analysis for two continuous outcome variables i) USSQ score ii) VAS score to study differences in change between loop stent and control groups over the three time points.
Subgroup analysis on the USSQ subdomains including urinary symptoms, body pain, general health, work performance, sexual health and additional problems was performed.
3. Results
Of the 40 patients that were randomised and received study questionnaires, two patients did not return their questionnaires despite persistent follow-up. These two patients were excluded from the study.
A total of 20 patients with the loop stent and 18 patients with conventional stents completed and returned the questionnaires, and the results for these patients were analysed.
There are no significant differences when comparing the baseline demographics between the two groups (Table 1). There were 14 males and six females vs. 14 males and four females in the loop and conventional group respectively. While there were more males than females in both groups, there were no significant differences in the ratio of male to female between the groups (p=0.719). Mean age was 50 and 52 years in the loop and conventional group respectively (p=0.867).
Table 1.
Baseline demographic.
| Loop stent (n=20) | Conventional stent (n=18) | p-Value | |
|---|---|---|---|
| Gender (male/female) | 14/6 | 14/4 | 0.719 |
| Mean age (range), year | 50 (29–70) | 52 (29–77) | 0.867 |
| Ureteroscopy and laser lithotripsy | 16 | 17 | 0.344 |
| Ureteroscopy | 0 | 1 | 0.474 |
| Retrograde intrarenal surgery | 2 | 0 | 0.488 |
| Previous ureteric stenting | 2 | 0 | 0.488 |
| Stent duration, mean±SD, day | 19±8.29 | 25±11.13 | 0.083 |
Of the 38 patients, mean stent duration was 19 vs. 25 days (p=0.083) in the loop and conventional group. One patient required ureteral stent removal on Day 7 due to severe stent symptoms. Thirty-three patients underwent ureteroscopy and laser lithotripsy for single ureteric stone; one patient had ureteroscopy; two patients had retrograde intrarenal surgery, and two underwent pre-stenting before definitive surgery. Two patients had ureteral stents inserted at least 1 year ago.
3.1. Pain scores
The VAS scores were tabulated (Table 2). Median VAS scores on Day 3 were significantly lower in the loop stent group (2.9 vs. 4.0, p=0.047) compared with the conventional group. There was a significant reduction in pain from Day 3–7 (0 vs. −1, p=0.016) in the conventional group. The improvement in pain score for both stent groups, however, was lost beyond Day 3 (Table 2).
Table 2.
VAS score.
| Loop (n=20) | Control (n=18) | p-Value | |
|---|---|---|---|
| Median VAS3 (interquartile range) | 2.9 (0.5–4) | 4 (3–6) | 0.047 |
| Median VAS7 (interquartile range) | 2.6 (1–4) | 2.6 (1.4–5) | 0.670 |
| Median VAS14 (interquartile range) | 2 (0–4) | 2.9 (1–4.8) | 0.422 |
| Median difference VAS7 & VAS3 (interquartile range) | 0 (−0.8–1.2) | −1 (−2–0) | 0.016 |
| Median difference VAS14 & VAS3 (interquartile range) | 0 (−1.4–0.1) | −1 (−2.4–0) | 0.139 |
| Median difference VAS14 & VAS7 (interquartile range) | −0.5 (−1–0) | 0 (−1–0) | 0.671 |
VAS, visual analog scale.
3.2. USSQ scores
The USSQ scores were tabulated (Table 3). Median USSQ scores were 82.0, 81.5 and 77.1 in the loop arm and 86.5, 81.0 and 81.2 in the conventional arm on Day 3, 7 and 14 respectively. There were no significant differences between the overall USSQ scores between the loop stent and conventional stent at Day 3, 7 and 14. There was also no detectable improvement in QoL when comparing within each group. Subgroup analysis of the domain in USSQ only showed improved sexual experience 2 weeks after stent insertion (p = 0.042). There was no significant difference in the domains of urinary symptoms, body pain, general health, work performance and additional problems between the different types of stent used.
Table 3.
USSQ scores.
| Loop (n=20) | Control (n=18) | p-Value | |
|---|---|---|---|
| Median USSQ3 (interquartile range) | 82 (65.2–94.1) | 86.5 (75.9–95) | 0.650 |
| Median USSQ7 (interquartile range) | 81.5 (61.5–98) | 81.0 (69–95) | 0.942 |
| Median USSQ14 (interquartile range) | 77.1 (58–93) | 81.2 (70–91) | 0.466 |
| Median differences USSQ7 & USSQ3 (interquartile range) | 2.2 (−10–13) | −3 (−9.8–0) | 0.357 |
| Median differences USSQ14 & USSQ3 (interquartile range) | −2 (−15–3) | −2.5 (−7.5–4) | 0.975 |
| Median differences USSQ14 & USSQ7 (interquartile range) | −4 (−11–3.8) | −1 (−7–0) | 0.456 |
USSQ, Ureteral Stent Symptoms Questionnaire.
Multivariate analysis was performed separately for USSQ and VAS scores against time and stent type variables. With regards to VAS, there was an improvement in pain score (p=0.018) over time regardless of which stent was used (Table 4). We found that patients with a higher baseline USSQ score result in a significantly higher USSQ scores at the end of the study (p=0.018).
Table 4.
Multivariate analyses of USSQ and VAS.
| Adjusted coefficient (95% CI) | p-Value | |
|---|---|---|
| USSQ | ||
| Stent type | −1.42 (−14.62 to 11.79) | 0.834 |
| Time | −0.29 (−0.66 to 0.09) | 0.133 |
| Baseline score | 0.32 (0.06 to 0.60) | 0.018 |
| VAS | ||
| Stent type | −0.64 (−2.16 to 0.88) | 0.408 |
| Time | −0.06 (−0.11 to -0.01) | 0.018 |
CI, confidence interval; USSQ, Ureteral Stent Symptoms Questionnaire; VAS, visual analog scale.
4. Discussion
4.1. Peak stent discomfort
Ureteral stents have been an integral part of Urology throughout the world to relieve obstruction, reduce pain and improve urinary flow. A broad spectrum of complication from stent migration, stent fracture to discomfort can occur. While the majority of complications are inconsequential, most patients experience some form of discomfort or pain following stent insertion which can result in unexpected return visits [10,11].
Recent efforts to reduce stent discomfort have been through the use of medication. Alpha-blockers and anticholinergic have been studied to minimise stent discomforts while other studies looked the position of the stent in the bladder [[12], [13], [14]]. Stent design advancements have mainly focused on the modifying the stent material or design to reduce the bladder footprint which is hypothesised to be a cause of stent discomfort [15].
4.2. Peak stent discomfort
Our primary aim was to investigate if stent discomfort peaks immediately after stent insertion, which draws us to highlight the two key points for discussion.
First, we believe that pain score peaks immediately after stent insertion, which corresponds to our findings which show that peak pain occurs at Day 3 regardless of the type of stent used. However, patients who underwent the loop ureteric stents insertion reported a lower baseline level of pain compared with conventional stents. This difference in pain level was statistically significant at Day 3 in the loop stent group (p=0.047) despite our small numbers. We believe that the body accommodates quickly to a baseline level with no discernible differences after that, which correlates with our findings which showed that pain level for both stents plateaued after 1 week and failed to show any significant decrease in pain level regardless of stent types. Lingeman et al. [5], in the largest study to date found no statistical difference in VAS score from baseline to Day 4 but pain medication usage peaked at Day 1. We believe our assessment at 72 h presents the earliest opportunity to explore pain level post insertion and any earlier evaluation (within 48 h) may be influenced by residual anesthetic effect. Conversely, pain assessment past this period may be too late as per published literature.
Second, we were able to show a difference in pain score using the VAS but not the USSQ pain subdomain. This could be due to different anatomical areas used to assess male and female region of discomfort in the USSQ and bring into question the sensitivity of USSQ to assess pain at the early interval. Similar discordance was reported in the Lingeman study in pain medication and pain scores [5].
To our knowledge, this is the first study that focused primarily on early stent discomfort. Park et al. [7] compared pain scores 7 days after stent placement while Davenport and colleagues [8] compared pain scores 2 weeks after stent insertion. Both studies did not show any difference in pain scores. This is in contrast to our multivariate analysis of pain score against time, which proved correlation with time from stenting regardless of which stent was used. This supports our belief that pain peaks immediately after ureteric stenting and that our understanding of patient's pain journey post ureteric stenting is incomplete. Our analysis showed that the USSQ did not detect difference in pain while the VAS did in the domain of pain. While it is out of the scope of this paper to discuss the suitability of the USSQ in near-term usage, the result does raise the issue of whether different subdomains of the USSQ should be applied at different time points to improve sensitivity further.
4.3. USSQ and QoL
Clinical experiences suggest that stent discomfort occurs very early and significant stent-related morbidity might not be captured by Day 7. The USSQ has been validated internationally for long-term comparison of stent symptoms in international studies; its role in assessing near-term stent symptoms (less than 1 week) is unclear. Our study design took into consideration residual anaesthetic effect and balance against delaying the capture of symptoms. As previously discussed, Day 3 was assessed to be the earliest possible implementation of the USSQ for objective assessment. Nevertheless, we failed to show any significant difference in USSQ scores between different stent types over the 2 week period similar to published studies.
Although not statistically significant, the USSQ trends reflect that QoL is maximally impacted right after stenting and quickly improves after that. Our small numbers could be a possible confounder on the lack of statistical significance.
4.4. USSQ subdomain
Except for improved sexual experience in favour of loop stent at Day 14, subgroup analysis of the various subdomains of the USSQ did not reveal any difference between the two groups. Similar results have been reported in USSQ subgroup analysis comparing conventional stent types, but it is difficult to interpret these effects due to the large difference in male and female ratio in our study and different stent designs. Also, there is significant heterogeneity in our study population, which further compounds our results.
There are several limitations in our study. Our population size is small compared to existing studies that may limit the ability to interpret small differences. Our patients were asked to fill out the USSQ at Day 14 and not after stent removal. This is due to the differences in stent duration by different surgeons. Also, the study was designed to focus on the immediate post-stenting period and thus post removal USSQ was omitted.
We assumed our patient to be pain-free on Day 0 which may be less than ideal as most of our patients experienced urolithiasis requiring surgical intervention. While we acknowledge this limitation, we believe that the trends score are more illuminating than the absolute value. We are also unaware of any existing instrument that can accurately quantify pain in pre-stented patients.
In this pilot single-blinded prospective randomised study, our primary aim was to investigate if stent discomfort peaks immediately after stent insertion. Our secondary endpoint is to determine the QoL of the loop stent compared with conventional pigtail stent as assessed through the USSQ.
We were able to show via pain score that peak stent discomforts occur immediately after stent insertion and resolve quickly within 1 week of post-stent insertion regardless of stent used. Our results suggest the USSQ should be used as early as Day 3 after stent insertion to detect a significant impact on QoL in larger studies.
Regarding comfort, the loop stent reduces pain in the immediate period following stent placement. This improvement disappears by Day 7 and no difference in pain profile is measurable after the 1st week. However, there were no significant differences in QoL score as assessed by the USSQ. Subgroup analysis of the various subdomains analysis showed no significant difference.
5. Conclusion
We conclude that peak stent discomfort occurs as early as 72 h immediately after stent insertion but improves quickly after that. The loop stent reduces peak pain experience after stent insertion and may have a role in the care of patients who experience significant stent discomfort previously. Our understanding of our patients' pain journey after ureteric stenting is incomplete. Larger studies and better assessment instruments may be required to answer these questions.
Author contributions
Study concept and design: Henry Sun Sien Ho, Allen Soon Phang Sim.
Data acquisition: Marcus Way Lunn Chow, Zhi Wei Law, Kheng Sit Lim.
Data analysis: Kheng Sit Lim.
Drafting of manuscript: Zhi Wei Law, Kheng Sit Lim.
Critical revision of the manuscript: Kheng Sit Lim.
Conflicts of interest
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript. This is an investigator-initiated study. Boston Scientific provided ureteric stent for the study but did not influence the design, analysis and writing of this manuscript.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Zimskind P.D., Fetter T.R., Wilkerson J.L. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97:840–844. doi: 10.1016/S0022-5347(17)63130-6. [DOI] [PubMed] [Google Scholar]
- 2.Byrne R.R., Auge B.K., Kourambas J., Munver R., Delvecchio F., Preminger G.M. Routine ureteral stenting is not necessary after ureteroscopy and ureteropyeloscopy: a randomized trial. J Endourol. 2002;16:9–13. doi: 10.1089/089277902753483646. [DOI] [PubMed] [Google Scholar]
- 3.Joshi H.B., Stainthorpe A., MacDonagh R.P., Keeley F.X., Jr., Timoney A.G., Barry M.J. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169:1065–1069. doi: 10.1097/01.ju.0000048980.33855.90. [DOI] [PubMed] [Google Scholar]
- 4.Rane A., Saleemi A., Cahill D., Sriprasad S., Shrotri N., Tiptaft R. Have stent-related symptoms anything to do with placement technique? J Endourol. 2001;15:741–745. doi: 10.1089/08927790152596352. [DOI] [PubMed] [Google Scholar]
- 5.Lingeman J.E., Preminger G.M., Goldfischer E.R., Krambeck A.E., Comfort Study Team Assessing the impact of ureteral stent design on patient comfort. J Urol. 2009;181:2581–2587. doi: 10.1016/j.juro.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez-Probst C.E., Fernandez A., Denstedt J.D. Current status of ureteral stent technologies: comfort and antimicrobial resistance. Curr Urol Rep. 2010;11:67–73. doi: 10.1007/s11934-010-0091-y. [DOI] [PubMed] [Google Scholar]
- 7.Park H.K., Paick S.H., Kim H.G., Lho Y.S., Bae S. The impact of ureteral stent type on patient symptoms as determined by the ureteral stent symptom questionnaire: a prospective, randomized, controlled study. J Endourol. 2015;29:367–371. doi: 10.1089/end.2014.0294. [DOI] [PubMed] [Google Scholar]
- 8.Davenport K., Kumar V., Collins J., Melotti R., Timoney A.G., Keeley F.X., Jr. New ureteral stent design does not improve patient quality of life: a randomized, controlled trial. J Urol. 2011;185:175–178. doi: 10.1016/j.juro.2010.08.089. [DOI] [PubMed] [Google Scholar]
- 9.Joshi H.B., Newns N., Stainthorpe A., MacDonagh R.P., Keeley F.X., Jr., Timoney A.G. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]
- 10.Leibovici D., Cooper A., Lindner A., Ostrowsky R., Kleinmann J., Velikanov S. Ureteral stents: morbidity and impact on quality of life. Isr Med Assoc J. 2005;7:491–494. [PubMed] [Google Scholar]
- 11.Joshi H.B., Okeke A., Newns N., Keeley F.X., Jr., Timoney A.G. Characterization of urinary symptoms in patients with ureteral stents. Urology. 2002;59:511–516. doi: 10.1016/s0090-4295(01)01644-2. [DOI] [PubMed] [Google Scholar]
- 12.Norris R.D., Sur R.L., Springhart W.P., Marguet C.G., Mathias B.J., Pietrow P.K. A prospective, randomized, double-blinded placebo-controlled comparison of extended release oxybutynin versus phenazopyridine for the management of postoperative ureteral stent discomfort. Urology. 2008;71:792–795. doi: 10.1016/j.urology.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Beddingfield R., Pedro R.N., Hinck B., Kreidberg C., Feia K., Monga M. Alfuzosin to relieve ureteral stent discomfort: a prospective, randomized, placebo controlled study. J Urol. 2009;181:170–176. doi: 10.1016/j.juro.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Al-Kandari A.M., Al-Shaiji T.F., Shaaban H., Ibrahim H.M., Elshebiny Y.H., Shokeir A.A. Effects of proximal and distal ends of double-J ureteral stent position on postprocedural symptoms and quality of life: a randomized clinical trial. J Endourol. 2007;21:698–702. doi: 10.1089/end.2007.9949. [DOI] [PubMed] [Google Scholar]
- 15.Lennon G.M., Thornhill J.A., Sweeney P.A., Grainger R., McDermott T.E., Butler M.R. ‘Firm’ versus ‘soft' double pigtail ureteric stents: a randomised blind comparative trial. Eur Urol. 1995;28:1–5. doi: 10.1159/000475010. [DOI] [PubMed] [Google Scholar]