Abstract
Background:
Despite the scarcity of data based on randomized trials, FOLFIRINOX is widely used in the management of borderline resectable pancreatic cancer (BRPC) and locally advanced unresectable pancreatic cancer (LAPC). We investigated the clinical outcomes of neoadjuvant FOLFIRINOX in patients with BRPC and LAPC.
Methods:
This single-center retrospective analysis included a total of 199 consecutive patients with BRPC or LAPC who received conventional or modified FOLFIRINOX between February 2013 and January 2017. An independent radiologist reviewed all baseline computed tomography or magnetic resonance imaging scans were reviewed for vascular invasion status.
Results:
With median follow-up duration of 40.3 months [95% confidence interval (CI), 36.7–43.8] in surviving patients, median progression-free survival (PFS) and overall survival (OS) were 10.6 (95% CI, 9.5–11.7) and 18.1 (95% CI, 16.0–20.3) months, respectively. The 1-year PFS rate was 66.0% (95% CI, 65.3–66.7%), and the 2-year OS rate was 37.2% (95% CI, 36.5–37.9%). PFS and OS did not differ between BRPC and LAPC groups [median PFS, 11.1 months (95% CI, 8.8–13.5) versus 10.1 months (95% CI, 8.4–11.8), p = 0.47; median OS, 18.4 months (95% CI, 16.1–20.8) versus 17.1 months (95% CI, 13.2–20.9), p = 0.50]. Curative-intent conversion surgery (R0/R1) was performed in 63 patients (31.7%). C•A 19-9 response, objective tumor response to FOLFIRINOX, and conversion surgery were independent prognostic factors for OS.
Conclusion:
FOLFIRINOX was effective for management of BRPC and LAPC. Given the potential for cure, a significant proportion of patients can undergo conversion curative-intent surgery following FOLFIRINOX.
Keywords: borderline resectable, FOLFIRINOX, locally advanced, neoadjuvant chemotherapy, pancreatic cancer
Introduction
Pancreatic cancer has a dismal prognosis and a 5-year survival rate of less than 10%; it is the one of leading causes of cancer-related death globally and in Korea.1,2 While surgery remains the only curative treatment option for resectable disease, 5-year survival rates after resection remain poor at 15–30%.3–5
Because approximately 25–30% of newly diagnosed pancreatic cancers present as non-metastatic borderline resectable pancreatic cancer (BRPC) and locally advanced unresectable pancreatic cancer (LAPC),6 management of such patients is important. BRPC and LAPC are anatomically defined by the involvement extent of major vessels.7 As patients categorized to these disease groups often have positive resection margins that portend a poor prognosis,8 neoadjuvant therapy has been investigated to improve R0 resection rates and survival outcomes. Unlike in resected or metastatic pancreatic cancer, however, only a few randomized trials have investigated patients with BRPC and LAPC.9–11
There have been recent improvements in the efficacy of first-line chemotherapy in metastatic pancreatic cancer, such as FOLFIRINOX and nab-paclitaxel plus gemcitabine.12,13 Although there is no data based on randomized phase III trials investigating BRPC or LAPC, the proven efficacy in metastatic pancreatic cancer has led to wide incorporation of these regimens in managing patients with BRPC or LAPC.14–16
Conventional or modified FOLFIRINOX is a current standard neoadjuvant therapy for patients with BRPC and LAPC. However, previous studies of FOLFIRINOX in these patient groups were mostly based on small retrospective or prospective studies and meta-analyses. Many questions remain regarding overall clinical course after neoadjuvant chemotherapy, chances of conversion surgery, postoperative outcomes, and prognostic factors. Herein, we performed a retrospective analysis to investigate the efficacy and safety of FOLFIRINOX as upfront therapy in patients with BRPC or LAPC.
Materials and method
Patients
This study included patients with histologically or cytologically confirmed pancreatic ductal adenocarcinoma (PDAC) who received upfront conventional or modified FOLFIRINOX for management of BRPC or LAPC as defined by National Comprehensive Cancer Network (NCCN) criteria.7 Patients with resectable disease or other histology, such as adenosquamous carcinoma, acinar cell carcinoma, or neuroendocrine carcinoma, were excluded from this study. Between February 2013 and January 2017, a total of 204 consecutive patients with non-metastatic PDAC received conventional or modified FOLFIRINOX at Asan Medical Center, Seoul, Korea. All baseline computed tomography (CT) or magnetic resonance imaging (MRI) images were collected in a blinded manner and reviewed centrally by an academic radiologist (JHB) for disease extent according to the NCCN criteria.7 BRPC was radiologically defined as a pancreas head/uncinate process tumor contacting (a) the common hepatic artery without extension to the celiac axis (CA); (b) ⩽180° of the superior mesenteric artery (SMA); (c) >180°of the superior mesenteric vein (SMV) or portal vein (PV) or ⩽180° of these with contour irregularity or thrombosis; or (d) the inferior vena cava. For pancreas body/tail tumors, BRPC was defined as having contact with (a) ⩽180° of the CA; or (b) >180° of the CA without involvement of the aorta and with intact and uninvolved gastroduodenal artery. LAPC was defined as tumors (a) contacting >180° of the SMA; (b) contacting >180°of the CA; (c) contacting the CA and having aortic involvement; or (d) with unreconstructible SMV/PV involvement. Excluding five patients with resectable disease, 199 patients were classified as having BRPC or LAPC and included in this study. Clinical data regarding baseline patient characteristics, treatment history, and survival outcomes were retrospectively obtained by reviewing medical records. This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (Approval number 2017-0442) and was performed in accordance with the ethical standards of the institutional research committee and the latest Declaration of Helsinki. The IRB waived the need for informed consent for this study because regulations in Korea do not require consent in retrospective analyses.
Treatment and response assessment
Conventional FOLFIRINOX consisted of a 2-hour intravenous infusion of oxaliplatin 85 mg/m2 followed by a 90-min intravenous infusion of irinotecan 180 mg/m2 and a 2-hour infusion of leucovorin 400 mg/m2, followed by an intravenous bolus of 5-FU 400 mg/m2 and a 46-hour continuous infusion of 5-FU 2,400 mg/m2 administered every 2 weeks, as described in the PRODIGE-4 trial.12 Modified FOLFIRINOX was also used with reduced dose of irinotecan (150 mg/m2) and omission of 5-FU bolus at the discretion of attending physicians. Tumor response was assessed every 6–8 weeks using CT and was graded according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Surgery
Following FOLFIRINOX treatment, a multidisciplinary team including pancreatobiliary surgeons, gastroenterologists, radiologists, medical oncologists, and radiation oncologists reviewed imaging findings (CT, MRI, or 18F-fluoro-2-deoxyglucose positron emission tomography CT) for surgical resectability. Surgery was performed 4–6 weeks after the last dose of FOLFIRINOX for patients considered to have resectable tumors after the review. The extent of surgical resection was decided by attending surgeons. Pathologic findings, including margin status and nodal status, were graded by institutional standards following guidelines of the American Joint Committee on Cancer, 8th Edition. Pathologic response was graded according to the College of American Pathology (CAP) criteria: complete response, Score 0 (no viable cancer cells); near complete response, Score 1 (single/rare groups of cancer cells); partial response, Score 2 (residual cancer with regression); poor/no response, Score 3 (no tumor regression).17 Postoperative treatment was determined at the discretion of attending physicians. Postoperative 90-day complications were graded according to Clavien–Dindo classification,18 and postoperative pancreatic fistulae were assessed using guidelines of the International Study Group on Pancreatic Surgery (ISGPS).19
Statistical analysis
Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Overall survival (OS) was defined as length of time from the start of FOLFIRINOX to the date of death from any cause. Progression-free survival (PFS) was defined as length of time from the start of FOLFIRINOX to the date of tumor progression or death from any cause, whichever occurred first. Categorical variables were compared using Chi-square or Fisher’s exact tests, as appropriate. Univariate and multivariate analyses using a Cox proportional hazards regression model were performed to determine prognostic factors. Variables that showed potential association in univariate analysis (p < 0.2) were tested in multivariate analyses. All applicable tests were two-tailed, and p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 23.0 (IBM Corp, Armonk, NY, USA).
Result
Patient characteristics
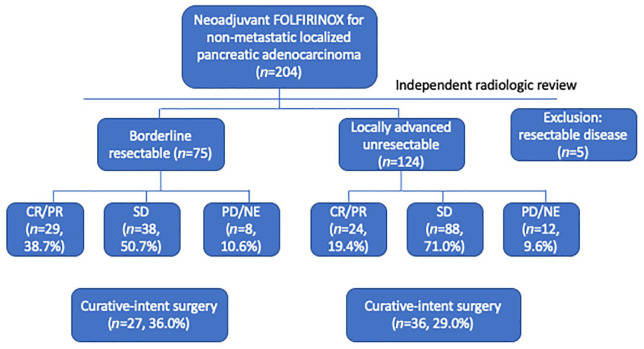
Baseline characteristics of 199 patients are listed in Table 1. According to blinded image review by an academic radiologist, 75 (37.7%) and 124 (62.3%) patients were classified as having BRPC and LAPC, respectively. Treatment flow for these patients is depicted in Figure 1. Median age was 60 years (range, 33–79), and 62.3% of patients were male. Pancreas head/uncinate (n = 119, 59.8%) was the most common primary tumor site, followed by body/tail (n = 74, 37.2%) and multifocal (n = 6, 3.0%). Most patients (99.0%) had Easter Cooperative Oncology Group performance status 0–1 when FOLFIRINOX was started. A median of 7 cycles (range, 1–41) of FOLFIRINOX were administered. Original and modified versions of FOLFIRINOX were given in 93 (46.7%) and 106 (53.3%) patients, respectively.
Table 1.
Baseline patient characteristics.
| Total (n = 199) | |
|---|---|
| Age (years) | |
| Median (range) | 60 (33–79) |
| <65 years | 144 (72.4%) |
| ⩾65 years | 55 (27.6%) |
| Sex | |
| Male | 124 (62.3%) |
| Female | 75 (37.7%) |
| Primary tumor site | |
| Head/Uncinate | 119 (59.8%) |
| Body/Tail | 74 (37.2%) |
| Multifocal | 6 (3.0%) |
| ECOG performance status | |
| 0–1 | 197 (99.0%) |
| Baseline serum CA 19-9 level | |
| Within normal range | 62 (31.2%) |
| Elevated | 137 (68.8%) |
| Median (range), U/mL | 126 (0.6–125000.0) |
| Disease extent by blinded central review | |
| Borderline resectable | 75 (37.7%) |
| Locally advanced unresectable | 124 (62.3%) |
ECOG, Eastern Cooperative Oncology Group.
Figure 1.
Patient flow chart.
CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Effectiveness outcomes
According to RECIST v1.1, 1 patient (0.5%) showed complete response (CR) and 52 patients (26.1%) achieved partial response (PR), indicating overall response rate (ORR) of 26.6% (Table 2). A total of 126 (63.3%) and 14 (7.0%) patients had stable disease (SD) and progressive disease (PD), respectively. Response evaluation was not available in six (3.1%) patients due to early loss to follow up. Disease control rate (CR + PR + SD) was 89.9%. ORR was higher in the BRPC group than in the LAPC group (38.7% versus 19.4%; p = 0.005).
Table 2.
Effectiveness outcomes.
| Total (n = 199) | |
|---|---|
| Best response to FOLFIRINOX | |
| Complete response | 1 (0.5%) |
| Partial response | 52 (26.1%) |
| Stable disease | 126 (63.3%) |
| Progressive disease | 14 (7.0%) |
| Not evaluable | 6 (3.1%) |
| Median PFS (95% CI) | 10.6 months (9.5–11.7) |
| Median OS (95% CI) | 18.1 months (15.9–20.3) |
| Curative-intent surgery | 63 (31.7%) |
| R0 resection | 49 (69.5%) |
| R1 resection | 14 (18.2%) |
CI, confidence interval; OS, overall survival; PFS, progression-free survival.
With median follow-up duration of 40.3 months (95% CI, 36.7–43.8) in surviving patients, median PFS and OS were 10.6 (95% CI, 9.5–11.7) and 18.1 (95% CI, 16.0–20.3) months, respectively (Figure 2). The 1- and 2-year PFS rates were 42.5% (95% CI, 35.6–49.4%) and 12.8% (95% CI 8.1–17.5%), respectively. The 1- and 2-year OS rates were 73.9% (95% CI, 67.8–80.0%) and 37.2% (95% CI, 36.5–37.9%), respectively. PFS and OS did not differ between BRPC and LAPC groups [median PFS, 11.1 months (95% CI, 8.8–13.5) versus 10.1 months (95% CI, 8.4–11.8), p = 0.47; median OS, 18.4 months (95% CI, 16.1–20.8) versus 17.1 months (95% CI, 13.2–20.9), p = 0.50] (Figure 3).
Figure 2.
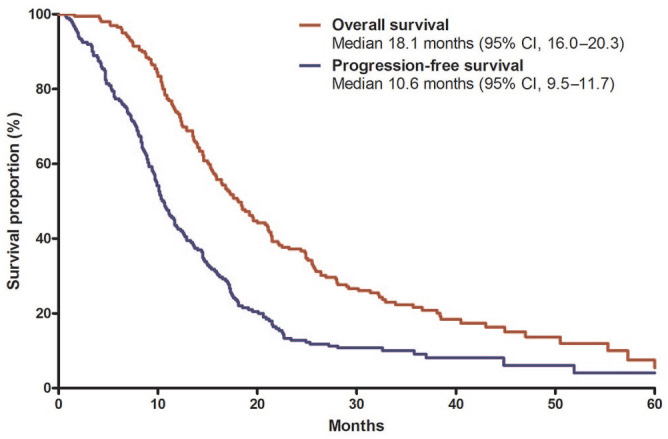
Progression-free survival and overall survival in all patients.
CI, confidence interval.
Figure 3.
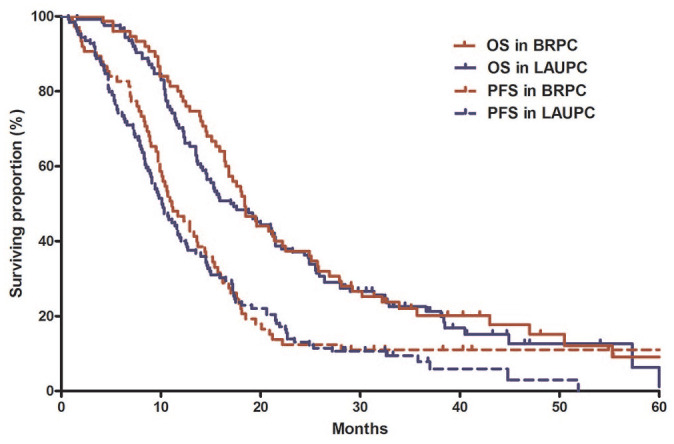
Survival outcomes between borderline resectable and locally advanced unresectable pancreatic cancer.
BRPC, borderline resectable pancreatic cancer; LAPC, locally advanced unresectable pancreatic cancer; PFS, progression-free survival; OS, overall survival.
Surgery
Following FOLFIRINOX chemotherapy, 71 patients (35.7%) underwent exploratory surgery, and 63 patients (31.7%) underwent curative-intent surgery (R0/R1). Three patients (1.5%) were determined to have resectable disease after multidisciplinary review but refused surgery. Prior to surgery, patients received a median of six cycles of FOLFIRINOX (range, 4–8). No patients received preoperative radiotherapy. Conversion surgery was performed more frequently in patients who achieved CR or PR (42 of 53, 79.2%) than in those with SD or PD (19 of 140, 13.6%; p < 0.001). In particular, no patient with PD underwent conversion surgery. Despite the higher rate of conversion surgery in the BRPC group (36.0%, 27 of 75) than the LAPC group (29.0%, 36 of 124), the difference was not significant (p = 0.35). Among patients who underwent curative-intent surgery, 49 patients (69.5%) and 14 patients (18.2%) had R0 and R1 resection, respectively. Details of the surgical profiles of patients who underwent conversion surgery are listed in Table 3. Pathologic treatment response graded by CAP criteria was as follows: complete response for two patients (3.2%), near complete response for two patients (3.2%), partial response for 39 patients (61.9%), and poor/no response for 20 patients (31.7%). There were no cases of 90-day postoperative mortality. Four patients (6.3%) and eight patients (12.7%) had major postoperative complications (Clavien–Dindo grade 3–4) and clinically relevant postoperative pancreatic fistulae, respectively. In patients who underwent arterial resection (n = 18), major complications and pancreatic fistulae were reported in two (11.1%) and three (16.7%) patients, respectively.
Table 3.
Details of patients who underwent curative-intent surgery.
| Total (n = 63) | |
|---|---|
| Pathological tumor size, Median (range), cm | 2.8 (0.1–7.5) |
| Type of surgery | |
| Pancreaticoduodenectomy | 35 (55.6%) |
| Distal pancreatectomy | 19 (30.2%) |
| Total pancreatectomy | 9 (14.2%) |
| Major artery resection | 18 (28.6%) |
| Major vein resection | 34 (53.9%) |
| Pathologic stage (AJCC 8th) | |
| I | 23 (36.5%) |
| II | 30 (47.6%) |
| III | 10 (15.9%) |
| Lymphovascular invasion | 24 (38.1%) |
| Perineural invasion | 53 (84.1%) |
| Postoperative complication | |
| No | 49 (77.8%) |
| Yes | 14 (22.2%) |
| Major (Grade 3 or greater) | 4 (6.3%) |
| Postoperative pancreatic fistula (Grade B/C) | |
| No | 55 (87.3%) |
| Yes | 8 (12.7%) |
AJCC, American Joint Committee on Cancer.
Postoperative anti-cancer therapy was given in 44 patients (69.8%) and comprised postoperative chemotherapy (n = 38, 60.3%), concurrent chemoradiation (n = 3, 4.8%), and concurrent chemoradiation and chemotherapy (n = 3, 4.8%). In patients who underwent conversion surgery, median disease-free survival (DFS) from surgery was 10.0 months (95% CI, 7.9–12.1), and median OS from surgery was 25.2 months (95% CI, 20.2–30.2). Median DFS and OS from surgery did not differ according to resection margin status (R0 versus R1) (DFS, 10.0 months (95% CI, 7.3–12.7) versus 9.0 months (95% CI, 3.7–14.3), p = 0.94; OS, 25.4 months (95% CI, 12.8–38.0) versus 18.1 months (95% CI, 14.4–21.8), p = 0.23).
Safety profiles
Adverse events with FOLFIRINOX are listed in Supplemental Table 1. There were no treatment-related deaths. Grade 3–4 neutropenia (n = 121, 60.8%) was the most frequent severe adverse event, followed by thrombocytopenia (n = 16, 8.0%) and nausea (n = 12, 6.0%). Although primary granulocyte-colony stimulating factor prophylaxis was not given in this patient population, febrile neutropenia occurred in only three patients (1.5%). FOLFIRINOX doses were reduced in 110 (55.3%) patients and interrupted in 55 (27.6%) patients.
Prognostic factor analysis
In univariate analyses, median OS showed a relationship with age, CA 19-9 response, tumor response to FOLFIRINOX, and curative-intent surgery (Table 4). Median OS was longer in patients who underwent curative-intent conversion surgery [29.2 months (95% CI, 21.4–36.9)] than in those who did not [15.0 months (95% CI, 13.3–16.7); p < 0.001; Figure 4]. In patients with elevated baseline CA 19-9 levels, patients with normalized CA 19-9 levels after FOLFIRINOX had better OS than those without normalized CA 19-9 levels [median 25.7 months (95% CI, 22.7–28.7) versus 14.2 months (95% CI, 12.9–15.5); Figure 5]. Tumor site, baseline CA 19-9 levels, type of FOLFIRINOX, and disease extent were not associated with OS. In the multivariate analysis, which included age, sex, CA 19-9 response, tumor response to FOLFIRINOX, and curative-intent surgery, CA 19-9 response [not normalized versus normalized; hazard ratio (HR)=2.02 (95% CI, 1.27–3.19); p = 0.003), objective response to FOLFIRINOX [CR/PR versus SD/PD/not evaluable; HR=0.37 (95% CI, 0.25–0.55); p < 0.001], and curative-intent surgery [yes versus no; HR=0.64 (95% CI, 0.41–0.97); p = 0.04] were independent prognostic factors.
Table 4.
Prognostic factor analysis for overall survival.
| Variables | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Age | ⩾ versus <65 years | 1.53 | 1.09–2.15 | 0.01 | 1.35 | 0.95–1.92 | 0.09 |
| Sex | Male versus Female | 1.36 | 0.99–1.88 | 0.06 | 0.71 | 0.51–1.00 | 0.05 |
| Tumor site | Other versus Head | 0.88 | 0.65–1.21 | 0.44 | |||
| Baseline CA 19-9 levels | ⩾Median versus < Median | 1.24 | 0.91–1.69 | 1.24 | |||
| CA 19-9 response | Normalized | Ref. | Ref. | ||||
| Not normalized | 2.85 | 1.89–4.29 | <0.001 | 2.02 | 1.27–3.19 | 0.003 | |
| Non-secretor | 1.56 | 0.83–1.92 | 0.28 | 0.96 | 0.61–1.52 | 0.87 | |
| Type of FOLFIRINOX | Modified versus Conventional | 1.06 | 0.78–1.45 | 0.70 | |||
| Tumor response to FOLFIRINOX | CR/PR versus SD/PD/NE | 0.44 | 0.30–0.63 | <0.001 | 0.63 | 0.40–0.99 | 0.04 |
| Disease extent | LAPC versus BRPC | 1.11 | 0.81–1.53 | 0.50 | |||
| Curative-intent surgery | Yes versus No | 0.40 | 0.28–0.56 | <0.001 | 0.63 | 0.41–0.97 | 0.04 |
BRPC, borderline resectable pancreatic cancer; CI, confidence interval; CR, complete response; HR, Hazard ratio; LAPC, locally advanced unresectable pancreatic cancer; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 4.
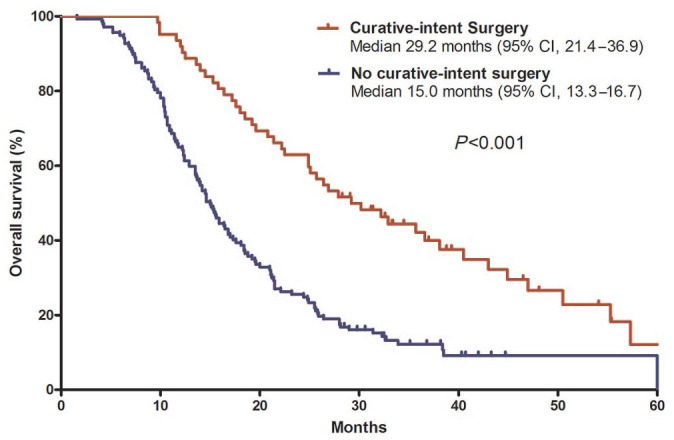
Overall survival according to curative-intent conversion surgery.
CI, confidence interval.
Figure 5.
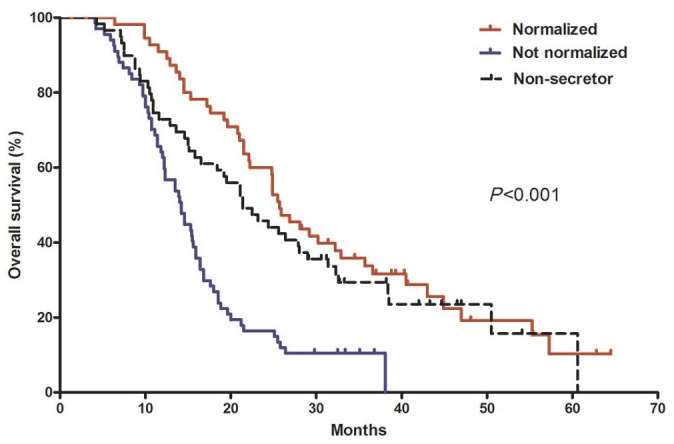
Overall survival according to CA 19-9 response.
Median overall survival was 25.7 months [95% confidence interval (CI), 22.7–28.7] in the normalized group, 14.2 months (95% CI, 12.9–15.5) in the non-normalized group, and 21.4 months (95% CI, 16.2–26.7) in the non-secretor group.
Discussion
The current study of 199 patients evaluated clinical outcomes and prognostic factors of BRPC and LAPC treated with FOLFIRINOX. In patients with BRPC and LAPC, median PFS was 11.1 and 10.1 months and median OS was 18.4 and 17.1 months, respectively. The rates of conversion surgery with BRPC and LAPC were 36.0% and 29.0%, respectively.
Efficacy outcomes of neoadjuvant FOLFIRINOX in the current study are in line with the results of previous studies.20–22 In patient-level meta-analyses for neoadjuvant FOLFIRINOX, median PFS and OS were 18.0 months and 22.2 months in patients with BRPC, respectively, while median PFS and OS were 15.0 and 24.2 months in patients with LAPC, respectively.21,22 Although our data seems somewhat inferior to the outcomes of these meta-analyses, direct data comparison should be performed with caution due to the heterogeneity that exists even within BRPC and LAPC. This fact is supported by the findings that median PFS and OS ranged from 3.0–20.4 months to 10.0–32.7 months, respectively, among studies included in the meta-analysis.22 The conversion surgery rate after neoadjuvant FOLFIRINOX in patients with LAPC in our study was 29.0%, which is comparable with 28% shown in the prior meta-analysis.22 However, the conversion surgery rate was only 36.0% for patients with BRPC in our study. This was inferior to the rate of 67.8% in the prior meta-analysis and 60–85% in previous prospective trials and retrospective study.20,21,23–25 This difference might be partially due to selection of the patient population for neoadjuvant FOLFIRINOX in our institution during the study period (before 2017). Because a significant proportion of patients with BRPC underwent upfront surgery with vascular reconstruction in our institution during the study period,26 more patients with extensive vascular involvement within the BRPC category could be included in the current analysis. In our recent prospective phase II trial of neoadjuvant mFOLFIRINOX for patients with BRPC, the conversion surgery rate was 62.9%,27 which is more in line with previous literature.
Objective tumor response (i.e. CR or PR by RECIST v1.1), CA 19-9 response (i.e. normalization) to FOLFIRINOX, and conversion surgery were positive prognostic factors for OS. Favorable outcomes in patients who achieved CA 19-9 response and conversion surgery were in line with the results of prior studies.26,28,29 Tumor response and CA 19-9 response to FOLFIRINOX may be reliable interim indicators for survival outcomes. In contrast to our findings, tumor response was not associated with OS in a previous study including 194 patients.28 This discrepancy may be due to different study populations. The previous study included only patients who underwent surgical resection following neoadjuvant therapy, and it is possible that patients with poor tumor response with limited surgery options were excluded. Conversely, our study population included all patients who started FOLFIRINOX regardless of conversion surgery. Interestingly, there was no difference in survival outcomes between BRPC and LAPC, in line with the results of patient-level meta-analysis.21 This may indicate that tumor biology, rather than anatomic involvement of major vessels, may be important in patients with BRPC or LAPC treated with FOLFIRINOX.
Toxicities of FOLFIRINOX were consistent with the results from previous studies, and there was no new safety signal related to FOLFIRINOX.5,12 Recently, modified FOLFIRINOX has been widely used to enhance tolerability, but this did not have any impact on survival outcomes in the multivariate analysis compared with conventional FOLFIRINOX. In patients who underwent conversion surgery following FOLFIRINOX, the incidence of major postoperative complications (Clavien–Dindo grade 3–4) was 12.7%. No cases had 90-day postoperative mortality. Our findings are in line with the results of previous studies and suggest that neoadjuvant FOLFIRINOX does not increase postoperative complications in patients with BRPC and LAPC.26,30
Despite recent advances in the management of BRPC and LAPC using neoadjuvant FOLFIRINOX, further studies are warranted to define the optimal duration of preoperative chemotherapy, postoperative chemotherapy regimens for patients who undergo conversion surgery, and biomarkers predicting prognosis. Although the role of radiotherapy in BRPC or LAPC has not been fully elucidated, modern radiotherapy techniques such as stereotactic body radiation therapy have shown promising results.31 Future studies are needed to evaluate the role of radiotherapy for BRPC or LAPC in the era of modern chemotherapy.
In conclusion, FOLFIRINOX was effective for the management of BRPC and LAPC. Given the potential for cure, a significant proportion of patients may undergo conversion curative-intent surgery following FOLFIRINOX. Future studies are required to enhance the efficacy of neoadjuvant therapy in BRPC and LAPC.
Supplemental Material
Supplemental material, Supplementary_Table for FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma by Changhoon Yoo, Inhwan Hwang, Tae Jun Song, Sang Soo Lee, Jae Ho Jeong, Do Hyun Park, Dong Wan Seo, Sung Koo Lee, Myung-Hwan Kim, Jae Ho Byun, Jin-hong Park, Dae Wook Hwang, Ki Byung Song, Jae Hoon Lee, Woohyung Lee, Heung-Moon Chang, Kyu-pyo Kim, Song Cheol Kim and Baek-Yeol Ryoo in Therapeutic Advances in Medical Oncology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported in part by the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI14C2640). The funding sources had no role in study design, collection and analysis of data, or writing of the report.
ORCID iD: Changhoon Yoo  https://orcid.org/0000-0002-1451-8455
https://orcid.org/0000-0002-1451-8455
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Changhoon Yoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Inhwan Hwang, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Tae Jun Song, Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Sang Soo Lee, Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jae Ho Jeong, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Do Hyun Park, Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Dong Wan Seo, Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Sung Koo Lee, Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Myung-Hwan Kim, Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jae Ho Byun, Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jin-hong Park, Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Dae Wook Hwang, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Ki Byung Song, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jae Hoon Lee, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Woohyung Lee, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Heung-Moon Chang, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Kyu-pyo Kim, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Song Cheol Kim, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Republic of Korea.
Baek-Yeol Ryoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, South Korea.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Jung K-W, Won Y-J, Kong H-J, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat 2018; 50: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer. JAMA 2013; 310: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 4. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; 389: 1011–1024. [DOI] [PubMed] [Google Scholar]
- 5. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018; 379: 2395–2406. [DOI] [PubMed] [Google Scholar]
- 6. Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol 2016; 34: 2654–2668. [DOI] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network. Pancreatic adenocarcinoma (version 1), http://qww.Nccn.org/Professionals/Physician_Gls/Pdf/Pancreatic.Pdf. (Accessed 14 May 2020) [DOI] [PubMed]
- 8. Katz MHG, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013; 20: 2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jang J-Y, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer. Ann Surg 2018; 268: 215–222. [DOI] [PubMed] [Google Scholar]
- 10. Kunzmann V, Algul H, Goekkurt E, et al. Conversion rate in locally advanced pancreatic cancer (LAPC) after nab-paclitaxel/gemcitabine- or FOLFIRINOX-based induction chemotherapy (NEOLAP): final results of a multicenter randomised phase II AIO trial. Ann Oncol 2019; 30: v253. [Google Scholar]
- 11. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 2020; 38: 1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 13. Hoff Von DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petrelli F, Coinu A, Borgonovo K, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 2015; 44: 515–521. [DOI] [PubMed] [Google Scholar]
- 15. Yoo C, Kang J, Kim K-P, et al. Efficacy and safety of neoadjuvant FOLFIRINOX for borderline resectable pancreatic adenocarcinoma: improved efficacy compared with gemcitabine-based regimen. Oncotarget 2017; 8: 46337–46347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol 2020; 5: 285–294. [DOI] [PubMed] [Google Scholar]
- 17. Kakar S, Shi C, Adsay V, et al. Protocol for the examination of specimens from patients with carcinoma of the pancreas 2017, www.CAP.org (Accessed 14 May 2018)
- 18. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017; 161: 584–591. [DOI] [PubMed] [Google Scholar]
- 20. Barenboim A, Lahat G, Geva R, et al. Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: an intention to treat analysis. Eur J Surg Oncol 2018; 44: 1619–1623. [DOI] [PubMed] [Google Scholar]
- 21. Janssen QP, Buettner S, Suker M, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst 2019; 111: 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016; 17: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol 2018; 4: 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol 2019; 5: 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khushman M, Dempsey N, Maldonado JC, et al. Full dose neoadjuvant FOLFIRINOX is associated with prolonged survival in patients with locally advanced pancreatic adenocarcinoma. Pancreatology 2015; 15: 667–673. [DOI] [PubMed] [Google Scholar]
- 26. Yoo C, Shin S, Kim K-P, et al. Clinical outcomes of conversion surgery after neoadjuvant chemotherapy in patients with borderline resectable and locally advanced unresectable pancreatic cancer: a single-center, retrospective analysis. Cancers 2019; 11: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoo C, Lee SS, Song K-B, et al. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a phase 2 study for clinical and biomarker analysis. Br J Cancer 2020; 123: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. Epub ahead of print 5 April 2019. DOI: 10.1097/SLA.0000000000003284. [DOI] [PubMed] [Google Scholar]
- 29. Rangelova E, Wefer A, Persson S, et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer. Ann Surg. Epub ahead of print 5 April 2019. DOI: 10.1097/SLA.0000000000003301. [DOI] [PubMed] [Google Scholar]
- 30. Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015; 261: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jung J, Yoon SM, Park J-H, et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS One 2019; 14: e0214970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table for FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma by Changhoon Yoo, Inhwan Hwang, Tae Jun Song, Sang Soo Lee, Jae Ho Jeong, Do Hyun Park, Dong Wan Seo, Sung Koo Lee, Myung-Hwan Kim, Jae Ho Byun, Jin-hong Park, Dae Wook Hwang, Ki Byung Song, Jae Hoon Lee, Woohyung Lee, Heung-Moon Chang, Kyu-pyo Kim, Song Cheol Kim and Baek-Yeol Ryoo in Therapeutic Advances in Medical Oncology