Abstract
The objective of this investigation was to study whether Nigella Sativa and Trigonella Foenum-graecum, could modulate the losartan pharmacodynamic (PD) and pharmacokinetic (PK) in experimental L-NAME induced hypertensive rats. For in vivo study, the systolic blood pressure (SBP) of rats was measured by the “tail-cuff system” after the treatment of rats with herb alone and herb + losartan in hypertensive rats. The SBP of rats treated with L-NAME + losartan also recorded. For the PK study, blood samples were obtained for up to 12 h to determine the concentrations of the drug, and various PK parameters were calculated. The data displayed that the SBP was significantly (p < 0.05) decreased in the rats when administered with L-NAME + N. Sativa or L-NAME + T. Foenum-graecum in contrast to the rats administered with L-NAME alone. A more prominent decline (p < 0.05) in SBP was detected in rats administered with L-NAME + N. Sativa + losartan and L-NAME + T. Foenum-graecum + losartan. In a PK study, higher losartan Cmax and AUC0-t were noted in rats treated with N. Sativa + losartan and T. Foenum-graecum + losartan, although the difference was not significant in contrast to the control group. This study proposed that the interaction between N. Sativa & losartan and T. Foenum-graecum & losartan could take place on concurrent administration; consequently, the dose of losartan may need to be accustomed when they are utilized simultaneously.
Keywords: L-NAME, Losartan, Nigella Sativa, Pharmacodynamic, Pharmacokinetic, Trigonella Foenum-graecum
1. Introduction
Hypertension is well characterized by a rise in systolic blood pressure (SBP) of 140 mm Hg or more and/or diastolic blood pressure of 90 mm Hg or more. Hypertension is one of the main threating aspects for other complications, for instance, renal, cardiovascular, and cerebrovascular ailments (Jaarin et al., 2015). The angiotensin II receptor blockers (ARBs) present a more novel category of antihypertensive agents (Ahad et al., 2016a). The ARBs were introduced in the market to overwhelmed many deficiencies associated with angiotensin-converting enzyme inhibitors. ARBs could provide more complete angiotensin II inhibition by selectively acting on the receptor site (Burnier and Brunner, 2000). All seven ARBs either alone or in combination with other drugs are sanctioned by the Food and Drug Administration for the management of hypertension (Barreras and Gurk-Turner, 2003).
Losartan is an ARB indicated in hypertension and diabetic nephropathy (Liu et al., 2019). It can be absorbed rapidly after oral administration (Rincon et al., 2015). Since losartan has a good blood pressure-lowering effect, it has been one of the most frequently prescribed antihypertensive drugs for the management of hypertension (Ahad et al., 2016a). It was reported that the losartan is substantially accepted by the cases. While head ache (14.1%), infection of upper respiratory tract (6.5%), lethargy (3.8%) and coughing (3.1%) have been noted during the monotherapy of losartan potassium (Al-Majed et al., 2015, Goa and Wagstaff, 1996). Dizziness occurred in around 4%; first-dose hypotension noted in 0.4% of cases getting losartan 50 mg every day and 2.2% taking in 100 mg every day (Burrell, 1997). Hypotension and postural effects are uncommon. There have been occasional reports of cases with angioedema, migraine or ageusia (loss of taste functions of the tongue) throughout the therapy of losartan (Goa and Wagstaff, 1996). Losartan is generally metabolized by cytochrome P450 (CYP) enzymes particularly by CYP3A4 and CYP2C9, and hence, induction or inhibition of these enzymes may have substantial alteration in the pharmacokinetic (PK) of losartan (Li et al., 2009, Wang et al., 2016).
The usage of medicinal plants for the management of hypertension has also been enhancing for many years. Nigella sativa, (also named as black seed and black cumin; Family; Ranunculaceae), has been revealed to have antihypertensive properties in humans as well as in animals (Ahmad et al., 2013, Fallah Huseini et al., 2013, Khattab and Nagi, 2007). Black seed has been utilized for long times for medicative and cooking purposes all over India, Middle East, and Northern Africa (Albassam et al., 2018). Thymoquinone and polyphenols are the main chemical constituent found in N. Sativa seed oil (Jaarin et al., 2015).
Trigonella Foenum-graecum (Fabaceae), frequently acknowledged as fenugreek, is an originated from the eastern Mediterranean to Central Asia and Ethiopia and is harvested in, China, India, and Pakistan (Morton 1990). Fenugreek seeds contain the trigonelline which is an alkaloid with mucilage, diosgenin, gitogenin, a trace of trigogenin. Other constituents presents are fixed and volatile oils, yellow coloring matter, and tannic acid (Balaraman et al., 2006). Fenugreek is utilized for its antidiabetic, antiulcer, antihypertensive properties, aphrodisiac, hypocholesterolemic, and hypoglycemic activities (Ahmad et al., 2016, Balaraman et al., 2006, Basch et al., 2003, Nagulapalli Venkata et al., 2017). In spite of the usage of medicinal herbs since the earliest times, a prominent rise in their consumption all over the world has occurred in the last many years. Ever since phytochemicals are treated in the body by the similar type of mechanisms involved in drug bio-disposition, there is an increased possibility for herb-drug interactions (Rodrigues et al., 2014).
Since losartan is also a substrate of both CYP enzymes and P-gp (Yang et al., 2011), alteration of CYP and P-gp activities could cause noteworthy variations in the PK summary of losartan (Li et al., 2016). In view of several reports published regarding the many herb-drug interactions, causing by concomitant administration of herbal medicines with drugs. Hence, it is a critical requirement to examine the potential herb-drug interaction among losartan and black seed & fenugreek to circumvent the harmful incidences. Based on the grid search, it was observed that there is a dearth of information on the pharmacodynamic (PD) and PK interaction between black seed, fenugreek, and losartan. Hence the goal of the present research work was to ascertain the influence of these herbs on the PK and PD of losartan in Wistar rats.
2. Materials and methods
Sortiva (losartan potassium, 50 mg) was bought from “SPIMACO, Al-Qassim, Saudi Arab. N-nitro l-arginine methyl ester (L-NAME) was obtained from “Carbosynth limited, Berkshire, UK”. N. Sativa seeds and T. Foenum-graecum seeds were procured from “7 spices Trading establishment, Riyadh, Saudi Arab”. Eprosartan mesylate (internal standard, IS) were purchased from “BASF, Germany”. Methanol and acetonitrile (HPLC grade) were bought from “Sigma Aldrich and Fisher Scientific, U.K”.
2.1. Procurement and training of rats
Albino Wistar rats (approx. 250 g) were received once the complete experimental protocol was sanctioned by the “Research Ethics Committee at King Saud University with approval number KSU-SE-18-27”. They were marked on tail for easy identification. To lessen the deviations in SBP, animals were trained to accommodate in rat holder for 5 days before the commencement of the experiment, so that the rats acquired to remain in the holder without aggressiveness and were become acquainted with the experimental conditions (Ahad et al., 2017, Ahad et al., 2016b, Ahad et al., 2018).
2.2. Recording of animals blood pressure
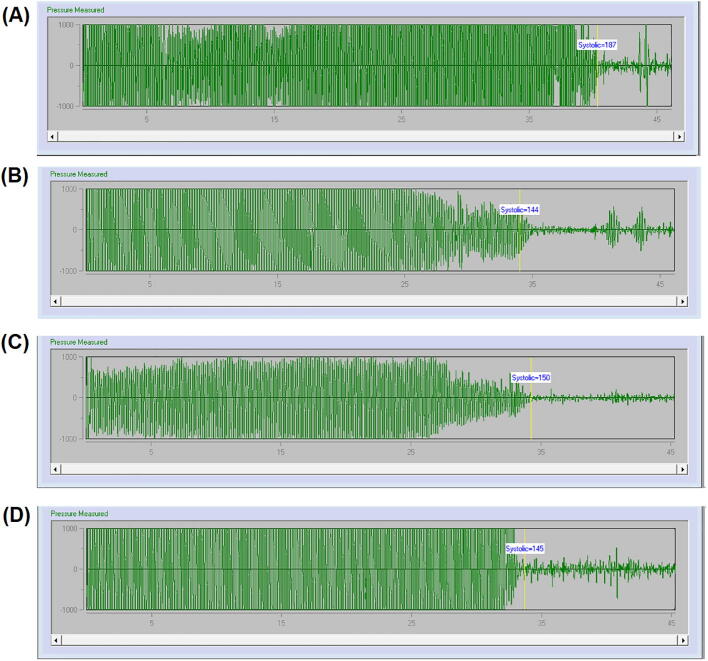
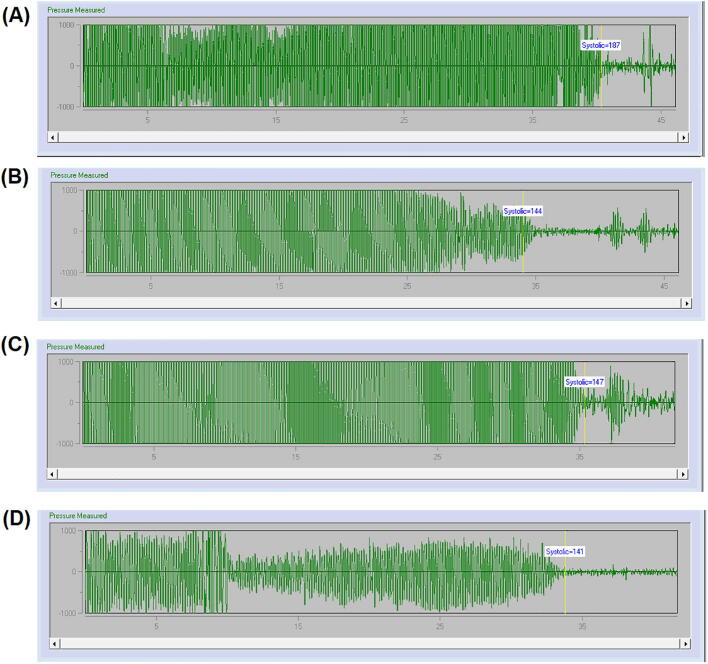
The rats were kept in the holders before the starting of blood pressure monitoring by “tail-cuff system (Visitech, BP-2000 series II, USA)”. The rat holders having circular small opening near the animal's nose which allow them for comfortable breathing. It is very essential to hold the rats softly in order to restrain them as calm as possible. The “Visitech tail-cuff system” consists of rat holders, rat platform, the control unit, and the computer. The rat holders were placed on rat platform which was heated by automatic temperature controller to gently warm the animals to establish well blood flow to the tail, and hence to develop good pulse signals from rat tail for improved detection by the sensor. The tail was protruded through the tail cuff and passing by the V-shaped furrow in the sensor. The intensified signals were recorded by the control unit during the automatic inflation and deflation of the tail cuff and these signals were analyzed by the control unit and displayed the blood pressure curve in the attached computer. Plot of tail-cuff pressure measured from a rat of different treated group using “Visitech tail-cuff system” demonstrated in Figs. 1 and 3.
Fig. 1.
Tracing of tail-cuff pressure from a rat of (A) L-NAME alone, (B) L-NAME + losartan, and (C) L-NAME + N. Sativa and (D) L-NAME + N. Sativa + losartan treated group using the Visitech blood pressure monitor system.
Fig. 3.
Tracing of tail-cuff pressure from a rat of (A) L-NAME alone, (B) L-NAME + losartan, and (C) L-NAME + T. Foenum-graecum and (D) L-NAME + T. Foenum-graecum + losartan treated group using the Visitech blood pressure monitor system.
2.3. Herbal medicine preparation
The fresh seeds of N. Sativa and T. Foenum-graecum were crushed in an electric grinding machine to obtain a fine powder. The calculated fine powered was then mixed well with small amount of normal saline by mortar and pestle and slowly remaining part of normal saline was added. The prepared aqueous saline suspension of each herb was then sonicated for 10–15 min. The calculated volume of suspension was then administered to rats as per their body weight with the help rats oral feeding needle and syringe (Al-Jenoobi et al., 2014a, Al-Jenoobi et al., 2014b, Korashy et al., 2015).
2.4. Herbs- losartan PD and PK interaction study
Wistar rats (n = 5) were randomly divided into 3 groups. All groups of animals given L-NAME (40 mg/kg daily) by oral route for the induction of hypertension. (Adaramoye et al., 2012, Sung et al., 2013). Group 1, rats received L-NAME daily for 2 weeks and on 14th day, systolic blood pressure of each rat was measured at 0, 1, 2, 4, 8 and 12 h by “tail-cuff system (Visitech, BP-2000 series II, USA)” and on 15th day, losartan (10 mg/kg, orally) (Ahad et al., 2020, Diogo et al., 2015, Kohzuki et al., 1994, Li et al., 2016) was given as single dose and systolic blood pressure was monitored at 0, 1, 2, 4, 8 and 12 h. Three days later (washout period) losartan (10 mg/kg, orally) was again given as single dose and blood samples were taken out from the animals at 0, 1, 2, 4, 8 and 12 h and plasma samples were analyzed by UPLC-MS-MS method (Alam et al., 2019).
Group 2 rats were received L-NAME + N. Sativa (300 mg/kg) (Al-Jenoobi et al., 2014b, Korashy et al., 2015) for 2 weeks and on 14th day, systolic blood pressure of each rat was measured at 0, 1, 2, 4, 8 and 12 h and on 15th day, losartan (10 mg/kg, orally) was given as single dose and systolic blood pressure was monitored 0, 1, 2, 4, 8 and 12 h. Three days later (washout period), losartan (10 mg/kg, orally) was again given as single dose and blood samples were taken out from the rats at 0, 0.5, 1, 2, 4, 8 and 12 h and plasma samples were detected for drug content by UPLC-MS-MS method (Alam et al., 2019). Similarly, in Group 3, animals were received L-NAME + T. Foenum-graecum (300 mg/kg) (Al-Jenoobi et al., 2014a, Al-Jenoobi et al., 2014b, Korashy et al., 2015) for 2 weeks and on 14th day, systolic blood pressure of each rat was measured at 0, 1, 2, 4, 8 and 12 h and on 15th day, losartan (10 mg/kg, orally) was given as single dose and systolic blood pressure was monitored at 0, 1, 2, 4, 8 and 12 h. After three days of washout period, losartan (10 mg/kg, orally) again given as single dose and blood samples were taken out from the animals at 0, 0.5, 1, 2, 4, 8 and 12 h and biological samples were evaluated for losartan content by UPLC-MS-MS method (Alam et al., 2019).
2.5. Sample preparations and analysis conditions
For the preparation of samples, each rat plasma (200 µl) sample was placed into Eppendorf tubes and then IS solution (20 μl of 1.0 μl/ml) was added to the plasma sample and mixed for 10 sec. Subsequently, methanol (620 μl) was added for protein precipitation to each sample and once again, the sample was vortexed for 25 sec to consistently mix in the sample. Later, samples were centrifuged for 6 min at 12000 rpm. The supernatant (160 µl) was pipetted out and was transferred to the glass insert for analysis.
The samples were examined by a “Waters® Acquity H-Class UPLC®-tandem quadrupole mass spectrometer (Waters, Milford, USA)”. For mass spectrometer analysis, protonated adducts of losartan and IS were monitored in MRM mode; the mass spectrometer was operated in electrospray ionization positive mode. The daughter species of losartan were obtained at m/z 423.19 > 179.93 and 423.19 > 207.00 and the daughter species of IS were obtained at m/z 425.11 > 106.97 and 425.11 > 134.99.
The mobile phase was the mixture of (A) water (containing formic acid 0.1%) and (B) acetonitrile (containing formic acid 0.1%). The mobile phase A and B was pumped in gradient mode at the flow rate of 250 μl/min under the following conditions: 0–0.60 min, 80:20; 0.61–0.95 min, 00:100; 0.96–3.0 min, 80:20. The column (Acquity UPLC® BEH C18 2.1 mm × 50 mm, 1.7 μm) was maintained at a temperature of 40 ± 5 °C, and the temperature of auto-sampler was set at 18 ± 3 °C (Alam et al., 2019).
2.6. Statistical analysis
“Unpaired t-test was implemented to evaluate which treatment groups showed a significant difference from the control group”. “GraphPad Prism 6.00 (GraphPad Software, Inc, CA, USA) was used as a tool for statistical analysis”. “P value less than 0.05 were considered as significant”.
3. Results and discussion
3.1. PD interaction of investigated herbs with losartan
Tracing of tail-cuff pressure measured from a rat by Visitech blood pressure monitor system presented in Figs. 1 and 3. In present study, hypertension was successfully induced in Group 1 to Group 3 animals by daily oral administration of L-NAME for two weeks.
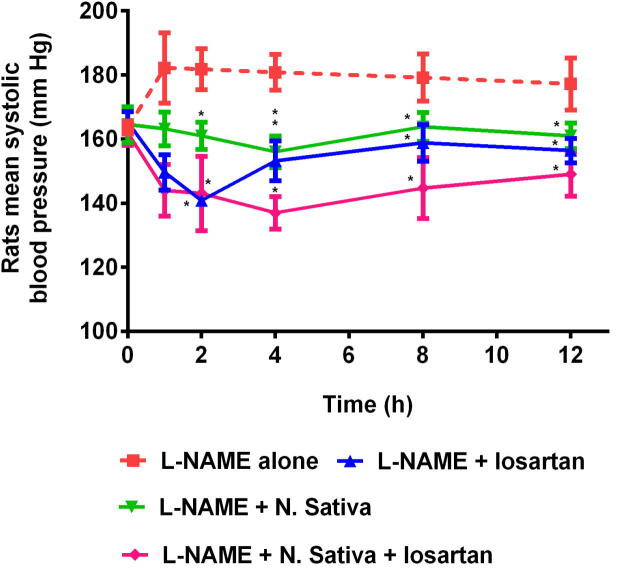
The SBP of L-NAME alone treated rats was considerably increased in two weeks (Figs. 2 and 4), and showed the SBP in the range of 154 mm Hg to 196 mm Hg. The SBP of L-NAME alone treated rats reached to 182.20 ± 10.99 mm Hg at 1 h (Ahad et al., 2020). The SBP of these rats stayed more than 160 mm Hg during the course of the study and a slow decreased in the SBP of L-NAME alone treated rats was observed at 12 h and the SBP was gradually reached to 177.20 ± 8.14 mm Hg (Ahad et al., 2020).
Fig. 2.
Rats systolic blood pressure following oral administration of L-NAME alone, L-NAME + losartan, L-NAME + N. Sativa, and L-NAME + N. Sativa + losartan (n = 5, mean ± SD). *p < 0.05 with respect to L-NAME alone group.
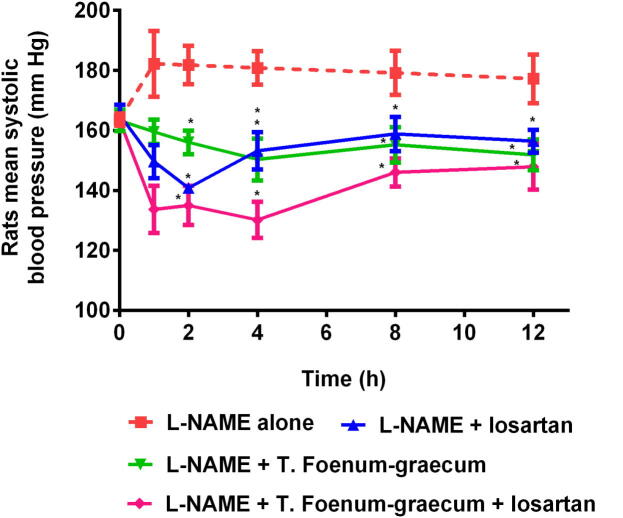
Fig. 4.
Rats systolic blood pressure following oral administration of L-NAME alone, L-NAME + losartan, L-NAME + T. Foenum-graecum, and L-NAME + T. Foenum-graecum + losartan (n = 5, mean ± SD). *p < 0.05 with respect to L-NAME alone group.
The “L-NAME is a nitric oxide synthase inhibitor, accordingly, it impedes nitric oxide synthesis from its precursor L-arginine which has been shown to be the active principle of the endothelium-derived relaxing factor leading to vasoconstriction and hypertension” (Nakamura et al., 1997).
In L-NAME + losartan (10 mg/kg, oral) treated group, a most prominent decreased in SBP of hypertensive rats by approx. 14.7% was observed at 2 h (SBP 140.80 ± 1.48 mm Hg) in comparison to the 0 h reading of 165.20 ± 3.35 mm Hg and about 22.55% reduction (p < 0.05) in the rats SBP was observed in comparison to the maximum value of SBP of 181.80 ± 6.42 mm Hg at 2 h of reading of L-NAME alone treated group. After 2 h, the SBP of animals received L-NAME + losartan begins to rise increasingly and reach to 156.40 ± 3.78 mm Hg at 12 h. This could be due to the half-life of losartan in rats (Li et al., 2016). Administration of L-NAME + losartan attenuated the SBP in the rats by 11.7% (p < 0.05) at 12 h, as correlated to the SBP reading (12 h reading) of animals those received L-NAME alone (Figs. 2 and 4).
Further, in the study, the data displayed that the SBP was decreased in the animals administered with L-NAME + N. Sativa as correlated to the animals administered with L-NAME alone (Figs. 1 and 2). The maximum decreased of SBP in animals administered with L-NAME + N. Sativa was found to be 5.17% at 4 h (156.00 ± 4.94 mm Hg) in contrast to the SBP value of 164.50 ± 5.54 mm Hg noted at 0 h. On later time point, the SBP value of animals from 156.00 ± 4.94 mm Hg start rising moderately and reached to 161.00 ± 4.05 mm Hg at 12 h, that was considerably lower in contrast to the SBP value of rats administered with L-NAME alone (177.20 ± 8.14 mm Hg at 12 h). Administration of N. Sativa daily contributed to lessening the elevated SBP in the L-NAME-treated rats by 9.14% in two weeks. This can be demonstrated by correlating the SBP value (161.00 ± 4.05 mm Hg at 12 h) of rats administered with N. Sativa with the SBP value of rats (177.20 ± 8.14 mm Hg at 12 h) administered with L-NAME alone (Fig. 2).
A more prominent decrement in rats SBP was noted when treated on L-NAME + N. Sativa + losartan. The maximum decreased by 15.35% in rats SBP was noticed at 4 h time point (p < 0.05, 137.00 ± 5.10 mm Hg) in contrast to the SBP value of 161.83 ± 3.76 mm Hg at 0 h time point, when they were administered with L-NAME + N. Sativa + losartan. Treatment with L-NAME + N. Sativa + losartan mitigate the SBP in hypertensive rats by 15.91% (p < 0.05, 149.00 ± 6.81 mm Hg) at 12 h, as compared with the SBP (at 12 h time point) of rats administered with L-NAME alone (Fig. 2).
It was observed that the losartan antihypertensive action was higher in rats administered with L-NAME + N. Sativa + losartan (Fig. 2) in comparison to the rats administered only with L-NAME + losartan or L-NAME + N. Sativa. It can be concluded here that the herb, for instance, N. Sativa potentiates the antihypertensive action of losartan in rats.
Similarly, L-NAME + T. Foenum-graecum treated rats showed 8% decrement in the SBP (p < 0.05, 150.33 ± 6.98 mm Hg) at 4 h and 7% reduction in the rats SBP was noticed at 12 h, in comparison to the rats SBP value of 163.33 ± 3.56 mm Hg at 0 h time point.
It was determined that the administration of T. Foenum-graecum daily contribute to lessening the elevated SBP in the L-NAME-treated rats by 14.32% in two weeks, this can be detected by corresponding the SBP value (151.83 ± 5.12 mm Hg at 12 h, p < 0.05) of rats administered with T. Foenum-graecum with SBP value (177.20 ± 8.14 mm Hg at 12 h) of rats administered with L-NAME alone (Fig. 4).
More obvious decreased in SBP was detected in animals on treatment with L-NAME + T. Foenum-graecum + losartan, in contrast to the animals administered with L-NAME + T. Foenum-graecum only. The SBP was gradually dropped to 130.17 ± 5.98 mm Hg (p < 0.05) at 4 h. The decreased was noted about 20.31% in SBP at 4 h of the study. On the other hand, the decreased was only 8% when rats were treated only with L-NAME + T. Foenum-graecum. The treatment of rats with L-NAME + T. Foenum-graecum + losartan decreased the rat's SBP to 147.83 ± 7.60 mm Hg at 12 h (p < 0.05) and about 9.5% decreased in SBP was observed. Administration of L-NAME + T. Foenum-graecum + losartan diminished the raised SBP in the L-NAME-treated rats by 16.57% (p < 0.05, 147.83 ± 7.60 mm Hg) at 12 h, as compared with the SBP of L-NAME alone treated rats at 12 h (Fig. 4). It was clearly observed from here that the administration of T. Foenum-graecum in animals augments the antihypertensive potential of losartan.
In the present report, we have concluded that L-NAME administration to the rats successfully developed hypertension in the rats and the induced high blood pressure in rats was successfully attenuated by losartan alone, herb alone and co-administration of losartan with the herb. The outcomes of the present study exhibited a blood pressure-lowering effect of both investigated herbs such as N. Sativa and T. Foenum-graecum in hypertensive rats.
3.2. PK interaction of investigated herbs with losartan
Antecedently Spanakis et al. investigated the effect of the herbal medicinal product of Rhodiola Rosea on the PK of losartan in experimental rabbits. The author reported that the co-administration of the herbal product with drugs leads to an increased in the bioavailability of losartan (2-fold) in rabbits (Spanakis et al., 2013). In another study, Yuan et al. reported that the compound danshen tablet produced substantial alteration in the PK of losartan and its metabolite (Yuan et al., 2013). Li et al. investigated the consequence of berberine on the losartan PK; the authors have concluded that the berberine could upsurge the losartan concentration and decreased its metabolite concentration in plasma by impeding the CYP3A4 or CYP2C9 enzymes activity (Li et al., 2016).
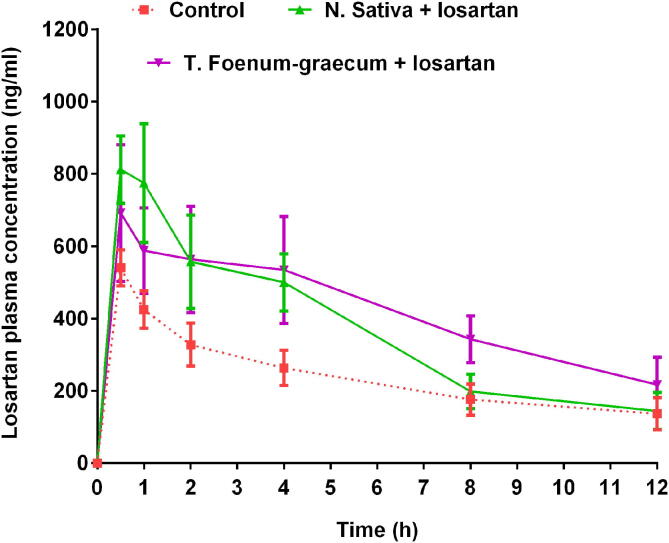
In this study, the PK parameters of losartan alone and losartan with the herb in rats were calculated and are shown in Table 1. The plasma concentration–time profiles of losartan after oral administration of losartan alone or in combination with N. Sativa and T. Foenum-graecum was presented in Fig. 5. The lower limit of quantitation for losartan was ascertained as 3 ng/ml and the linearity range of calibration curve was found in the concentration range of 3–800 ng/ml (Alam et al., 2019). Administration of losartan (10 mg/kg, oral) in rats presented the Tmax of 0.50 ± 0.00 h (Ahad et al., 2020). While the rats treated with N. Sativa + losartan presented a Tmax of 0.60 ± 0.10. Similarly, the higher Tmax (0.60 ± 0.11 h) of losartan was observed when rats treated with T. Foenum-graecum + losartan. It was detected that losartan Tmax was increased by 20% (p > 0.05) in experimental rats administered with N. Sativa and T. Foenum-graecum in contrast to control group.
Table 1.
PK parameters of losartan following an oral administration in rats before and after pretreatment with N. Sativa and T. Foenum-graecum (n = 5. mean ± SE).
| Parameters | Control | N. Sativa + losartan | T. Foenum-graecum + losartan |
|---|---|---|---|
| aTmax (h) | 0.50 ± 0.00 | 0.60 ± 0.10 | 0.60 ± 0.10 |
| bCmax (ng/ml) | 540.84 ± 49.62 | 866.96 ± 144.39 | 709.97 ± 181.55 |
| cAUC0→t (ng.h/ml) | 2426.68 ± 495.63 | 4202.19 ± 834.97 | 3658.60 ± 633.46 |
| dt1/2 (h) | 4.56 ± 0.59 | 4.48 ± 0.50 | 10.17 ± 2.00* |
| eKe (h−1) | 0.158 ± 0.02 | 0.161 ± 0.02 | 0.073 ± 0.01* |
Time to reach maximum plasma concentration.
Maximum plasma concentration.
Area under the concentration–time curve until 12 h.
Half-life.
Elimination rate constant.
p < 0.05 with respect to control group.
Fig. 5.
Plasma concentration–time profiles of losartan after oral administration of losartan alone or in combination with N. Sativa or T. Foenum-graecum (n = 5, mean ± SE).
Further, as depicted in Table 1, the Cmax and AUC0-t of losartan in the N. Sativa + losartan and T. Foenum-graecum + losartan treated groups were found higher as compared to the control group (losartan alone), although the increment was not significant, this could be due to the high variation in the data. The Cmax increased by 60.30% for N. Sativa + losartan and 31.27% for T. Foenum-graecum + losartan treated group; while the AUC0-t increased by 73.17% for N. Sativa + losartan and 50.77% for T. Foenum-graecum + losartan treated group; although the difference was not significant as compared to control group. The outcomes of the study presented that the t1/2 of losartan was comparable in the case of rats treated with N. Sativa + losartan while the t1/2 of losartan was significantly increased in rats when treated with T. Foenum-graecum + losartan (Table 1).
The present investigation advocated that herbs like N. Sativa and T. Foenum-graecum could modulate the PK performance while they are co-administered with losartan in rats. These data proposed that the herb-drug interaction between N. Sativa & losartan and T. Foenum-graecum & losartan may take place on concurrent administration. As observed that the plasma concentration of losartan was augmented when concurrently administered with investigated herbs, which advised that the pharmacological actions of losartan could be stimulated, and consequently, the dose of losartan ought to be accustomed when they are utilized simultaneously.
Earlier studies have described that herbs like N. Sativa and T. Foenum-graecum possibly will inhibit the activity of CYP3A (Albassam et al., 2018, Al-Jenoobi et al., 2014a, Al-Jenoobi et al., 2014b, Alkharfy et al., 2013, Korashy et al., 2015). The losartan in general metabolized by CYP3A4 and CYP2C9, and hence, we proposed that the herb-losartan interaction could happen owing to the effects of investigated herbs on the activity of these enzymes.
4. Conclusion
In conclusion, the consequences of the existing investigation demonstrated that herbs like N. Sativa and T. Foenum-graecum possibly will affect the PD and PK behavior of losartan when they are concurrently administered intentionally or unintentionally. Investigated herbs possibly will decelerate the metabolism of losartan and this could affect the PD and PK parameters when these herbs were concurrently administered with losartan.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no (RG-1435-041).
Footnotes
Peer review under responsibility of King Saud University.
References
- Adaramoye O.A., Nwosu I.O., Farombi E.O. Sub-acute effect of N(G)-nitro-l-arginine methyl-ester (L-NAME) on biochemical indices in rats: protective effects of Kolaviron and extract of Curcuma longa L. Pharmacognosy. Res. 2012;4:127–133. doi: 10.4103/0974-8490.99071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahad A., Al-Mohizea A.M., Al-Jenoobi F.I., Aqil M. Transdermal delivery of angiotensin II receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEIs) and others for management of hypertension. Drug Deliv. 2016;23:579–590. doi: 10.3109/10717544.2014.942444. [DOI] [PubMed] [Google Scholar]
- Ahad A., Al-Saleh A.A., Al-Mohizea A.M., Al-Jenoobi F.I., Raish M., Yassin A.E.B., Alam M.A. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol((R)) gel under Dermaroller((R)) on rats with methyl prednisolone acetate-induced hypertension. Biomed. Pharmacother. 2017;89:177–184. doi: 10.1016/j.biopha.2017.01.164. [DOI] [PubMed] [Google Scholar]
- Ahad A., Aqil M., Kohli K., Sultana Y., Mujeeb M. Nano vesicular lipid carriers of angiotensin II receptor blocker: anti-hypertensive and skin toxicity study in focus. Artif. Cells Nanomed. Biotechnol. 2016;44:1002–1007. doi: 10.3109/21691401.2015.1008509. [DOI] [PubMed] [Google Scholar]
- Ahad A., Raish M., Al-Jenoobi F.I., Al-Mohizea A.M. Sorbitane monostearate and cholesterol based niosomes for oral delivery of telmisartan. Curr. Drug Deliv. 2018;15:260–266. doi: 10.2174/1567201814666170518131934. [DOI] [PubMed] [Google Scholar]
- Ahad A., Raish M., Bin Jardan Y.A., Alam M.A., Al-Mohizea A.M., Al-Jenoobi F.I. Effect of Hibiscus sabdariffa and Zingiber officinale on the antihypertensive activity and pharmacokinetic of losartan in hypertensive rats. Xenobiotica. 2020:1–11. doi: 10.1080/00498254.2020.1729446. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Alghamdi S.S., Mahmood K., Afzal M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 2016;23:300–310. doi: 10.1016/j.sjbs.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Damanhouri Z.A., Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac. J. Trop. Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.A., Abou Obaid N.I., Ibrahim M.A., Raish M., Al-Jenoobi F.I. A validated ultra-performance liquid chromatography tandem triple quadrupole mass spectrometric method for fast determination of losartan in rabbit plasma. J. Chromatogr. Sci. 2019;57:323–330. doi: 10.1093/chromsci/bmy114. [DOI] [PubMed] [Google Scholar]
- Albassam A.A., Ahad A., Alsultan A., Al-Jenoobi F.I. Inhibition of cytochrome P450 enzymes by thymoquinone in human liver microsomes. Saudi Pharm. J. 2018;26:673–677. doi: 10.1016/j.jsps.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jenoobi F.I., Alam M.A., Alkharfy K.M., Al-Suwayeh S.A., Korashy H.M., Al-Mohizea A.M., Iqbal M., Ahad A., Raish M. Pharmacokinetic interaction studies of fenugreek with CYP3A substrates cyclosporine and carbamazepine. Eur. J. Drug Metab. Pharmacokinet. 2014;39:147–153. doi: 10.1007/s13318-013-0149-6. [DOI] [PubMed] [Google Scholar]
- Al-Jenoobi F.I., Korashy H.M., Ahad A., Raish M., Al-Mohizea A.M., Alam M.A., Al-Suwayeh S.A., Alkharfy K.M. Potential inhibitory effect of herbal medicines on rat hepatic cytochrome P450 2D gene expression and metabolic activity. Pharmazie. 2014;69:799–803. [PubMed] [Google Scholar]
- Alkharfy K.M., Al-Jenoobi F.I., Al-Mohizea A.M., Al-Suwayeh S.A., Khan R.M., Ahmad A. Effects of Lepidium sativum, Nigella sativa and Trigonella foenum-graceum on phenytoin pharmacokinetics in beagle dogs. Phytother. Res. 2013;27:1800–1804. doi: 10.1002/ptr.4947. [DOI] [PubMed] [Google Scholar]
- Al-Majed A.R., Assiri E., Khalil N.Y., Abdel-Aziz H.A. Losartan: comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2015;40:159–194. doi: 10.1016/bs.podrm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Balaraman R., Dangwal S., Mohan M. Antihypertensive effect of trigonella foenumgreacum. seeds in experimentally induced hypertension in rats. Pharm. Biol. 2006;44:568–575. [Google Scholar]
- Barreras A., Gurk-Turner C. Angiotensin II receptor blockers. Proc. (Bayl. Univ. Med. Cent.) 2003;16:123–126. doi: 10.1080/08998280.2003.11927893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch E., Ulbricht C., Kuo G., Szapary P., Smith M. Therapeutic applications of fenugreek. Altern. Med. Rev. 2003;8:20–27. [PubMed] [Google Scholar]
- Burnier M., Brunner H.R. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. doi: 10.1016/s0140-6736(99)10365-9. [DOI] [PubMed] [Google Scholar]
- Burrell L.M. A risk-benefit assessment of losartan potassium in the treatment of hypertension. Drug Saf. 1997;16:56–65. doi: 10.2165/00002018-199716010-00004. [DOI] [PubMed] [Google Scholar]
- Diogo L.N., Faustino I.V., Afonso R.A., Pereira S.A., Monteiro E.C., Santos A.I. Voluntary oral administration of losartan in rats. J. Am. Assoc. Lab. Anim. Sci. 2015;54:549–556. [PMC free article] [PubMed] [Google Scholar]
- Fallah Huseini H., Amini M., Mohtashami R., Ghamarchehre M.E., Sadeqhi Z., Kianbakht S., Fallah Huseini A. Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: a randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2013;27:1849–1853. doi: 10.1002/ptr.4944. [DOI] [PubMed] [Google Scholar]
- Goa K.L., Wagstaff A.J. Losartan potassium: a review of its pharmacology, clinical efficacy and tolerability in the management of hypertension. Drugs. 1996;51:820–845. doi: 10.2165/00003495-199651050-00008. [DOI] [PubMed] [Google Scholar]
- Jaarin K., Foong W.D., Yeoh M.H., Kamarul Z.Y., Qodriyah H.M., Azman A., Zuhair J.S., Juliana A.H., Kamisah Y. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics (Sao Paulo) 2015;70:751–757. doi: 10.6061/clinics/2015(11)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab M.M., Nagi M.N. Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytother. Res. 2007;21:410–414. doi: 10.1002/ptr.2083. [DOI] [PubMed] [Google Scholar]
- Kohzuki M., Yasujima M., Yoshida K., Kanazawa M., Abe K. Antihypertensive and antiproteinuretic losartan in spontaneously hypertensive chronic renal failure. Hypertens. Res. 1994;17:173–178. [Google Scholar]
- Korashy H.M., Al-Jenoobi F.I., Raish M., Ahad A., Al-Mohizea A.M., Alam M.A., Alkharfy K.M., Al-Suwayeh S.A. Impact of Herbal Medicines like Nigella sativa, Trigonella foenum-graecum, and Ferula asafoetida, on Cytochrome P450 2C11 Gene Expression in Rat Liver. Drug Res (Stuttg) 2015;65:366–372. doi: 10.1055/s-0034-1384604. [DOI] [PubMed] [Google Scholar]
- Li H., Liu L., Xie L., Gan D., Jiang X. Effects of berberine on the pharmacokinetics of losartan and its metabolite EXP3174 in rats and its mechanism. Pharm. Biol. 2016;54:2886–2894. doi: 10.1080/13880209.2016.1190762. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang G., Wang L.S., Zhang W., Tan Z.R., Fan L., Chen B.L., Li Q., Liu J., Tu J.H., Hu D.L., Liu Z.Q., Zhou H.H. Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica. 2009;39:788–793. doi: 10.1080/00498250903134435. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li L., Qiu M., Tan L., Zhang M., Li J., Zhu H., Jiang S., Su X., Li A. Renal and cerebral RAS interaction contributes to diabetic kidney disease. Am. J. Transl. Res. 2019;11:2925–2939. [PMC free article] [PubMed] [Google Scholar]
- Morton J.F. Mucilaginous plants and their uses in medicine. J. Ethnopharmacol. 1990;29:245–266. doi: 10.1016/0378-8741(90)90036-s. [DOI] [PubMed] [Google Scholar]
- Nagulapalli Venkata K.C., Swaroop A., Bagchi D., Bishayee A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201600950. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ohyama Y., Masuda H., Kurashina T., Saito Y., Kato T., Sumino H., Sato K., Sakamaki T., Sasaki A., Nagai R. Chronic blockade of nitric oxide synthesis increases urinary endothelin-1 excretion. J. Hypertens. 1997;15:373–381. doi: 10.1097/00004872-199715040-00008. [DOI] [PubMed] [Google Scholar]
- Rincon J., Correia D., Arcaya J.L., Finol E., Fernandez A., Perez M., Yaguas K., Talavera E., Chavez M., Summer R., Romero F. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015;124:81–90. doi: 10.1016/j.lfs.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M., Alves G., Francisco J., Fortuna A., Falcao A. Herb-drug pharmacokinetic interaction between carica papaya extract and amiodarone in rats. J. Pharm. Pharm. Sci. 2014;17:302–315. doi: 10.18433/j3559n. [DOI] [PubMed] [Google Scholar]
- Spanakis M., Vizirianakis I.S., Batzias G., Niopas I. Pharmacokinetic interaction between losartan and Rhodiola rosea in rabbits. Pharmacology. 2013;91:112–116. doi: 10.1159/000345929. [DOI] [PubMed] [Google Scholar]
- Sung J.H., Jo Y.S., Kim S.J., Ryu J.S., Kim M.C., Ko H.J., Sim S.S. Effect of lutein on L-NAME-induced hypertensive rats. Korean J. Physiol. Pharmacol. 2013;17:339–345. doi: 10.4196/kjpp.2013.17.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhang H., Wang Y., Yu X., Yuan Y. Effects of salvianolic acid B and tanshinone IIA on the pharmacokinetics of losartan in rats by regulating the activities and expression of CYP3A4 and CYP2C9. J. Ethnopharmacol. 2016;180:87–96. doi: 10.1016/j.jep.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Yang S.H., Choi J.S., Choi D.H. Effects of HMG-CoA reductase inhibitors on the pharmacokinetics of losartan and its main metabolite EXP-3174 in rats: possible role of CYP3A4 and P-gp inhibition by HMG-CoA reductase inhibitors. Pharmacology. 2011;88:1–9. doi: 10.1159/000328773. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang H., Ma W., Sun S., Wang B., Zhao L., Zhang G., Chai Y. Influence of compound danshen tablet on the pharmacokinetics of losartan and its metabolite EXP3174 by liquid chromatography coupled with mass spectrometry. Biomed. Chromatogr. 2013;27:1219–1224. doi: 10.1002/bmc.2930. [DOI] [PubMed] [Google Scholar]