Abstract
Nanotechnology is evolving as a significant discipline of research with various applications. It includes the materials and their applications having one dimension in the range of 1–100 nm. Many chemical and physical protocol have been utilized for the nanoparticles (NPs) fabrication. These protocols are costly, hazardous and consumes high energy. Thus, researchers are inclined towards biological synthesis of NPs using plant and or herbal extract as these methods are simple, sustainable, ecofriendly and cost-effective. Flower is an important part of plants, and contained several phytochemicals such as flavonoids, terpenoids, coumarins, sterol and xanthones which acts as an important precursor for NPs synthesis. These compounds acted as reducing as well as stablishing agent during fabrication processes. They have been thoroughly characterized by various techniques. The fabricated NPs have shown potential antimicrobial activity against bacterial and fungal infections. They have been also used as potential therapeutic agent for human breast cancer, gastric adenocarcinoma cell, colorectal adenocarcinoma cell and pancreas ductal adenocarcinoma cells. Overall, the aim of this review article to facilitates the recent understanding of flower-mediated NPs fabrication (a sustainable and ecofriendly resource), their application in different disciplines and challenges.
Keywords: Flower extract, Green synthesis, Electron microscopy, Anticancer, Antimicrobial, Catalytic activities
1. Introduction
Nanotechnology is evolving as a significant discipline of science with its various applications such as in biomedicines, pharmaceutics, catalysis, sensors, cosmetics, agriculture, textile products, mechanics, optics, electronics, energy etc. (Boomi et al., 2019, Gour and Jain, 2019, Husen and Siddiqi, 2014a, Husen and Siddiqi, 2014b, Husen and Siddiqi, 2014c, Bachheti et al., 2019, Husen, 2019, Husen, 2019a, Husen et al., 2019, Husen, 2020a, Husen, 2020b, Joshi et al., 2019, Mishra et al., 2019, Singh and Husen, 2019). It includes the particles and their applications having one dimension in the range of 1–100 nm; and are called nanomaterials (NMs). Various chemical/physical protocol have been utilized for the NMs synthesis. These conventional methods used for NMs synthesis are considered as expensive, time consuming and hazardous to environment due to involvement of unsafe and toxic chemicals. Thus, researchers are inclined towards the biological route of nanoparticles (NPs) synthesis from the various plant parts as these procedures are simple, sustainable, ecofriendly and cost-effective (Husen and Siddiqi, 2014a, Husen and Siddiqi, 2014b, Husen and Siddiqi, 2014c, Siddiqi et al., 2018a, Siddiqi et al., 2018b, Bachheti et al., 2020a, Husen, 2020c, Painuli et al., 2020). Among the various NMs, metal and metal-oxide NPs are considered as most effective as these particles have shown significant biomedical and other applications due to their increased surface area to volume ratio. In recent past, the utilization of various plant species and herbal extract (obtained from different plant parts) worked as reducing and capping agents in the synthesis of NPs has evolved as a novel field of nanoscience. Initially, the entire plant part extracts without isolation of pure compounds were used in the green synthesis of NPs. Further, the isolated pure plant-based compounds such as cellulose, glucose, starch or the whole plants, plant dry mass or extracts were utilized in the synthesis of NPs (Alle et al., 2020a, Alle et al., 2020b, Alle et al., 2020c, Chandran et al., 2006, Song et al., 2008, Kasthuri et al., 2009, Husen and Iqbal, 2019). For example, leaf (Siddiqi et al., 2019, Khan et al., 2019), latex (Arsalani et al., 2018, Arsalani et al., 2019), seeds (Radini et al., 2018, Hussein et al., 2018), gums (Alle et al., 2020a, Alle et al., 2020b, Alle et al., 2020c, Alle et al., 2019), flowers (Abdallah et al., 2019, Johnson et al., 2018; fruits (Lakshmanan et al., 2018), peel (Vinay et al., 2018), stem (Azad et al., 2014), bark (Rao and Rao, 2016, Das and Smita, 2018), pulp (Pushkar and Sevak 2018) and root (Shaikh et al., 2019, Bachheti et al., 2020b) were used NPs synthesis. Additionally, some researcher isolated cellulose nanocrystals and nanofibrils from various forest wood and non-wood products (Alle et al., 2020a, Alle et al., 2020b, Alle et al., 2020c).
It has been already understood that the most of the plant parts are rich in terms phytochemical, and floral extract is one them. Flowers are the important part of human life and used by people to mark important event in their lives such as for decoration in marriages, birthday, celebration and many other events. Many flowers are used for cooking, drinks, as salad and to prepare cake (Kelley et al., 2001, Kelley et al., 2002). Flower are the source of vegetables (such as cauliflower, broccoli), spices (saffron-most expensive spices, cloves and capers) as flavoring agent for beer (Hops flowers), raw material for wine (dandelion and elder flower) and squash (Rhododendron arboreum flower). Flowers are easily available (a sustainable and ecofriendly resource), contain huge amount of phytochemical. They are rich in flavonoids (anthocyanins and catechins), coumarins, terpenoids, sterol and xanthones which can be used as precursor for NPs synthesis. Anthocyanins are plant pigments responsible for giving colour to different parts of plants especially flower in different species. It seems as red pigment in acidic condition, whereas blue in alkaline condition. It is responsible for imparting colour to most of flower for example, in red hibiscus, red rose, red pineapple sage, red clover, and pink blossom (Khoo et al., 2017). Anthocyanins have many biological activities for instance antioxidant, anticancer and anti-inflammatory features (Bowen-Forbes et al., 2010). Anthocyanins are water soluble (Wang et al., 2010) which make it suitable for NPs synthesis. Abbasi et al. (2019) fabricated, silver NPs (Ag-NPs) from anthocyanins extract of purple basil (Ocimum basilicum). Also, kaempferol (flavonoid) a phytochemical present in many flowers was used for gold NPs (Au-NPs) synthesis (Raghavan et al., 2015). Hussain et al., 2019, Mashwani et al., 2016 have also examined the role of flavonoids and terpenoids in NPs synthesis and their various applications, respectively.
Another phytochemical coumarins present in different part of plant but in large concentration in flower and fruits (Miranda and Cuéllar 2001) and it is reported to use for NP synthesis (Karthik et al., 2017). Five new xanthones, garciniacowones along with 14 known xanthones, were isolated from fruits and fresh flowers of Garcinia cowa (Sriyatep et al., 2015). Some xanthones are also reported for NPs synthesis (Aisha et al., 2015). Recent published paper showed that terpenoids obtained from the flower bud extract of Tussilago farfara were also used for Ag-NPs and Au-NPs synthesis (Lee et al., 2019).
Till date, there has been no review available on the involvement of flower extract in the biological synthesis of metal/metal-oxide NPs. The present review article highlights and elucidates the mechanistic role of flower constituents as reducing as well as capping agent in the NPs synthesis. Additionally, the present review also focuses the applications of synthesized NPs in various discipline of science.
2. Important phytochemicals of some flowers
As already reported, like other parts of plants, flowers are also important source of phytochemical and known for large number of biological activities. For instance, Punica granatum is a shrub found in Iran, China and Afghanistan (Flora Respublicae Popularis Sinicae, Tomus, 1983, Wang et al., 2006). The flower of Punica granatum was observed as an, astringent and haemostatic. In Unani and Ayurvedic medicine systems its flower was reported to use in diabetes while in traditional Chinese medicine it is used for injuries treatment, hair fall and greying of hair. Medicinal use of pomegranate flowers was reported in Ayurvedic, Unani and Chinese medicine system (Sivarajan and Balachandran, 1994, Wang et al., 2006). Phytochemical present in pomegranate flowers are polyphenols, gallic acid (Huang et al., 2005a), ellagic acid and ethyl brevifolin-carboxylate (Wang et al., 2006), triterpenes - oleanolic acid, ursolic acid (Huang et al., 2005b), maslinic acid and asiatic acids (Batta and Rangaswami, 1973).
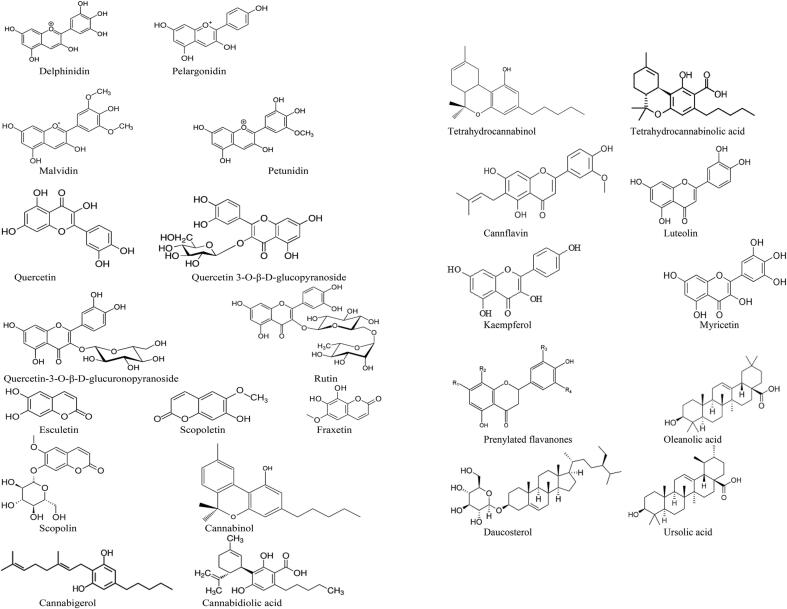
Also, tea flowers chemical compositions were reported similar to its leaves and contain good quantities of total catechins. (Lin et al., 2003, Su et al., 2000). Moreover, the tea flower extracts reported for antioxidant activity (Lin et al., 2003). Eight catechins, five flavonol glycosides were isolated from ethyl acetate-soluble fraction (EEA) from the tea flowers which showed antioxidant activity (Yang et al., 2009). Structure of isolated compounds were elucidated by mass spectrometry and nuclear magnetic resonance. The isolated compounds were Myricetin 3-O-β-D-galactopyranoside, Quercetin 3-O- β -D-galactopyranoside, Kaempferol 3-O- β -D-galactopyranoside, Kaempferol 3-O- β-D-glucopyranoside, Kaempferol 3-O-[α-L-rhamnopyranosyl-(1–6)- β -D-glucopyranoside. Four anthocyanins (1) delphinidin 3,5-di-O-(6-O-malonyl-β-d-glucoside) (ii) delphinidin 3-O-(6-O-malonyl-b-d-glucoside)-5-O-β-D-glucoside (iii)delphinidin 3-O- β -D-glucoside-5-O-(6-O-malonyl- β-D-glucoside) (iv) delphinidin 3,5-di-O- β -D-glucoside were isolated from flowers of Cichorium intybus (Nørbæk, et al., 2002). These anthocyanins are responsible for imparting color to flowers. Delphinidin, pelargonidin, peonidinis and petunidin are example of such anthocyanins (Katsumoto et al., 2007, Bąkowska-Barczak, 2005, Tanaka et al., 1998, Yabuya et al., 1997). Four prenylated flavanones (1) 5,7,4′-trihydroxy-8-prenylflavanone, (2) 5,4′-dihydroxy-7-methoxy-8-prenylflavanone (3) 5,7,4′-trihydroxy-3′,8-diprenylflavanone (4) and 5,7,4′-trihydroxy- 3′,5′-diprenylflavanone were isolated from the methanol extract of the flowers of Azadirachta indica (Nakahara et al., 2003). From the above information it is clear that flowers obtained from various plant species are rich source of different chemicals as presented in Fig. 1 and, thus these products or compounds can be used for reducing as well as stablishing agent in the process of green synthesis of NPs.
Fig. 1.
Important phytochemical obtained from various flowers.
3. Flower-based NMs synthesis and their characterization
3.1. Metal NMs
Petals aqueous extract of Rosa santana (rose) was used for Ag-NPs fabrication. An absorption peak at 438 nm in UV–vis confirmed the formation of the Ag-NPs. Further, they were examined by Fourier transform infrared spectroscopy (FTIR). FTIR characterization of flower extract showed 3447 cm−1 due to –O–H bonded, intramolecular hydrogen bond, 2926 cm -1 due –C–H saturated alkane (stretch), 1639 cm -1 due to alkenyl stretching (monosubstituted), 10623 cm−1 due to sulfoxide, 968 cm−1 due to alkenyl stretching (disubstituted (trans), 799 cm−1 due to alkenyl stretching (trisubstituted), 660 cm -1 due to halo compounds (C-X bond). These chemicals act as reducing as well as stablishing agent for Ag-NPs. The shape of NPs was observed to be spherical by TEM with size 6.52–25.24 nm with particle size of ~ 14.48 nm. The average zeta potential value was −26.50 mV for the Ag-NPs which shows long term stability of NPs (Jahan et al. 20019). Patil et al. (2019) reported Au-NPs synthesis from Lonicera japonica flower-extract. Change in color from light yellow to ruby red and absorbance ~530-580 nm in UV–vis spectroscopy confirmed the formation of Au-NPs. FTIR analysis of flower extract shows the presence of alcohols, phenols, 1° amines, aromatic amines, carboxylic acids, alkane and alkynes. Shape of most of synthesized Au-NPs were spherical, in addition, few were triangular, and hexagonal with size between 10 and 40 nm. Energy-dispersive X-ray (EDX) spectroscopy and X-ray diffraction (XRD) studies exhibited the crystalline nature of synthesized Au-NPs.
Alshehri et al. (2017) have investigated the fabrication of iron NPs (Fe-NPs) using aqueous flower extract of Hibiscus sabdariffa. TEM analysis exhibited the spherical shape of synthesized Fe-NPs with size 100 nm. FTIR study revealed the presence of anthocyanin compound in the extract of H. sabdariffa. Manjari et al. (2017) synthesized Ag-NPs and Au-NPs from flower extract o Aglaia elaeagnoidea. TEM studies exhibited spherical shape Ag-NPs and Au-NPs with size of 17 and 25 nm, respectively. It was claimed by FTIR that phenols, proteins, sugars, and other phytochemicals present in A. elaeagnoidea flower extract worked as reducing as well as stablishing agent. Mata et al. (2016) used flower extract of Plumeria alba for Au-NPs synthesis. TEM analysis showed that size of NPs varies between 28 ± 5.6 and 15.6 ± 3.4. Mata et al. (2015) also reported the Ag-NPs synthesis from same P. alba flower extract with size of 36.19 nm and spherical in shape. FTIR studies showed the presence of polyphenols in the flower extract. Caesalpinia pulcherrima flower extract was used by Nagaraj et al. (2012) for Au-NPs synthesis. TEM studies revealed that the synthesized NPs were spherical in shape and particles size were range from 10 to 50 nm.
Gnidia glauca plant extracts obtained from flower, leaf and stem were used for copper NPs (Cu-NPs) synthesis (Jamdade et al. 2019). Colour change from pale blue to yellow and finally to dark brown confirmed the formation of Cu-NPs. HR-TEM showed that 5 nm spherical nanoparticle were obtained when prepared from flower extract of Gnidia glauca. FTIR spectra showed sharp characteristic peak at ~3400–3420 cm−1 due to the presence hydroxyl group in alcoholic and phenolic compounds (Ogunyemi et al. 2019). Flower extract of Albizia lebbeck was used as reducing as well as capping agents for Ag-NPs synthesis (Gharpure et al. 2019). Obtained Ag-NPs, under HR-TEM investigation have shown the average particles size of 25 nm.
Fritillaria flower plant extract act as reducing and stabilizing agent for Ag-NPs synthesis (Hemmati et al., 2019). Absorption band at 430 nm in UV–Vis spectrum shows the formation of Ag-NPs. FTIR analysis of Fritillaria flower extract showed medium intense band at 1637 cm−1 was due to C = O stretching vibration, broad peak at 3421 cm−1 was due to presence of O-H stretching vibration. The two bands noted at 1387 cm−1 and 1087 cm−1 was assigned to the C-N stretching vibrations of aromatic and aliphatic amines. Both SEM/TEM studies revealed that Ag-NPs particles were spherical in shape with an average size of 10 nm. Further, Ag-NPs purity in the composite was noted to be 77.5 wt% by thermogravimetric analysis (TGA).
Ag-NPs were also prepared using an aqueous flower extract of Scrophularia striata (Mameneh et al. 2019). They were further examined by FE-SEM, XRD, UV–Vis and FTIR analysis. The peak at 440 nm in UV–Vis was corresponding to SPR band of Ag-NPs. Size of synthesized Ag-NPs was noted 8–12 nm. FTIR analysis of flower extract revealed the presence of hydroxyl, amine and carbonyl groups, which acts as reducing and stabilizing agents for Ag-NPs. Spherical shaped Ag-NPs were obtained from flower extract of Bauhinia variegata. The size of NPs was fond to be 5–15 nm when analyzed by TEM. FTIR analysis indicated the presence of phenols, flavonoids, benzophenones, nitro compounds, aromatics and aliphatic (Johnson et al., 2018). Mladenova et al. (2018) synthesized Ag-NPs from flower extract of Tilia cordata, Matricaria chamomilla Calendula officinalis and Lavandula angustifolia and investigated by UV–Vis, TEM and XRD. The shape of synthesized Ag-NPs was spherical and they were between 5 and 30 nm in size. XRD revealed the face-centered cubic (FCC) structure of Ag-NPs.
Ipomoea digitata flower extract was used for Ag-NPs synthesis (Varadavenkatesan et al. 2018). A peak of 412 nm in UV–Vis studies revealed the synthesis of Ag-NPs. SEM studies have shown the polydispersed nature of NP, and presence of elemental Ag in NPs which was confirmed by EDX. XRD studied showed face-centered cubic structure of NPs. The zeta potential was −25.1 mV which indicated the stability of the NPs. Au-NPs were prepared at room temperature from the aqueous flowers extract of Melastoma malabathricum (Krishnaprabha and Pattabi, 2019). Morphological, optical, and structural characterization of synthesized Au-NPs were performed by UV–Vis, FTIR spectroscopy, FESEM, TEM and XRD studies. UV–Vis investigation showed the fabrication of Au-NPs synthesis. FESEM and TEM studies showed spherical shape Au-NPs, and size ranged from 20 to 60 nm. Crystallinity of the synthesized Au-NP was examined by using XRD. FTIR studies of flower extract showed absorption band at 3331 cm−1, due to the –OH stretching which shifted to 3308 cm−1 thus showed the presence of –OH group in the reduction of Au3+ to Au. Karthik et al. (2019) produced Ag-NPs from flower of Calotropis gigantea and further characterized by UV–Vis, FTIR, FESEM, and XRD analysis. XRD revealed the crystalline and face centered structure of Ag-NPs with average size 50 nm (Table 1).
Table 1.
Flower-based metal NPs synthesis and their various applications.
| Metal NPs | Plant name (Family) | Synthesis condition | Size (nm) | Shape | Characterization techniques | Responsible phytochemical | Applications | Key reference |
|---|---|---|---|---|---|---|---|---|
| Ag |
Osmanthus fragrans (Oleaceae) |
@ temp of (25 °C, 40 °C and 60 °C) | 13.54- 18.17 |
Spherical | UV–Vis, SEM, FTIR, XRD, TGA and Zetasizer | Carboxylic acid, hydroxyl and methylene group containing compound | Waste water treatment, bio-medicals, medical textiles, wound dressing and antimicrobial activities | Chinyerenwa et al., 2018 |
| Ag |
Datura inoxia (Solanaceae) |
Reaction carried out at 37 °C | 15–73 | polygonal | UV–Vis, FTIR, EDX and XRD | Ketones, aromatics and aliphatic amines and alkyl halides |
Cytotoxic activity | Gajendran et al., 2019 |
| Ag |
Mangifera indica (Anacardiaceae) |
--- | 10–20 | Spherical | UV–Vis, FTIR, EDX and TEM | Alkaloids, flavonoids, amino acids and proteins | Antibacterial activity | Ameen et al., 2019 |
| Ag |
Catharanthus roseus (Apocynaceae) |
Incubated on a sand bath 60 0C for 10 min | 6–25 | Spherical | UV–Vis, FTIR and TEM | – | Antibacterial activity | Manisha et al., 2014 |
| Ag |
Bauhinia variegate (Fabaceae) |
@ room temp | 5–15 | Spherical | UV–Vis, FTIR, XRD, EDX and Zetasizer | Phenols, flavonoids, benzophenones, nitro compounds, aromatics and aliphatic amines | Antioxidant activity | Johnson et al., 2018 |
| Ag |
Bauhinia purpurea (Fabaceae) |
pH 7.0 and time 24 hrs | 20 | Spherical | UV–Vis, FTIR, TEM), SEM, EDS and XRD |
Alcohols, phenolic Compounds, carbonyl group |
Antibacterial activity |
Chinnappan et al., 2018 |
| Ag |
Fritillaria (Liliaceae) |
@ 30 0C | 5–10 | Spherical | TEM SEM, FTIR, XRD and EDX |
Hydroxyl, amid and carbonyl groups | Antibacterial activity | Hemmati et al., 2019 |
| Ag |
Ipomoea digitate (Convolvulaceae) |
Heated in a water bath (80 0C) for 10 min | 100 | Spherical | UV–Vis, SEM, EDX, XRD, DLS and FTIR |
Aromatic amines, amides, carbonyl groups, polyphenols, alcohols and proteins |
Catalytic and antibacterial activities | Varadavenkatesan et al., 2018 |
|
Ag |
Couroupita guianensis (Lecythidaceae) |
@ 70 0C for 15mins on water bath and incubated overnight | 15–57 | Spherical | TLC, UV–Vis, SEM, TEM, XRD and FTIR | Alcohol and phenol, amide linkages of the proteins | Antioxidant and antibacterial activities | Pandurangan et al., 2018 |
| Ag |
Allamanda cathartica (Apocynaceae) |
@ 37 0C for 15 min | 39 | Spherical | UV–Vis, FTIR, EDS, TEM and XRD | (E,E)-geranyl linalool, n-pentacosane, 1,8-cineole and n-tricosane. |
Antibacterial and antioxidant activities | Karunakaran et al., 2016 |
| Ag |
Millettia pinnata (Fabaceae) |
Heated with magnetic stirrer at 60 0C for 30 min | 16–38 | Spherical | UV–Vis, XRD, SEM, TEM and FTIR | Nitriles. alkenes and aromatic groups | Antibacterial, and cytotoxicity activities | Rajakumar et al., 2017 |
| Ag |
Scrophularia striata (Scrophulariaceae) |
Incubated for 24 h at 27 0C at 120 rpm | 8–12 | – | GC–MS, UV–Vis, FESEM, XRD and FTIR | Alcohols, phenols, alkanes, aromatic, aromatic amines, alcohols and carboxylic acids | Toxicity studies | Mameneh et al., 2019 |
| Ag |
Caesalpinia Pulcherrima (Fabaceae) |
Boiling timing was 5 min for flower extract, 1 mM silver nitrate con., pH 8, and reaction time was 24 h | 2–22 | Spherical | FTIR, XRD, TEM and TGA | Alkanes group and the aldehyde group, primary amines and carbonyl group | Antimicrobial, antioxidant and cytotoxic activities | Moteriya and Chanda, 2017 |
| Ag |
Spartium junceum (Fabaceae) |
Heated @ 80 0C and pH = 9 for 20 min | 15–25 | Nearly-spherical in shape | UV–Vis, FTIR, XRD, DLS and TEM | – | – | Nasseri et al., 2019 |
| Ag |
Albizia lebbeck (Fabaceae) |
@ room temperature | 25 | Spherical | UV–Vis, TEM, FTIR, GC–MS and NMR | Alcohols, amines and alkyls | Antibacterial and anticancer activities | Gharpure et al., 2019 |
| Ag | Tagetes erecta (Asteraceae) | @ room temperature | 10–90 | Spherical, hexagonal and irregular | UV–Vis, FTIR, SEM, TEM, SAED, EDX and zeta potential |
Amide, aromatic monosubstituted benzene and vinyl disubstituted alkenes | Antibacterial activity |
Padalia et al., 2015 |
| Ag |
Hydrangea paniculata (Hydrangeaceae) |
@ 25 0C | 36–75 | Spherical | UV–Vis, SEM, TEM, FTIR, XRD, EDX and SAED | Terpenoids, steroid, saponins, alkaloids, quinone, glycosides and flavonoid | Antioxidant potential and antibacterial activities | Karunakaran et al., 2017 |
| Ag |
Tilia cordata, (Malvaceae) Matricaria chamomilla (Asteraceae) Calendula officinalis (Asteraceae) and Lavandula angustifolia (Lamiaceae) |
@ 25 0C |
5–30 | Spherical | UV–Vis, TEM and XRD | – | – | Mladenova et al., 2018 |
| Ag |
Rosa santana (rose) Petals () |
Heated at 80 0C for 60 min | 6.5–25.2 | Nearly spherical | UV–Vis, FTIR, XRD, TEM, and Zeta-size analyzer | Functional group containing –O–H, C-H, –C = C and –S = O |
Antimicrobial activity, and cytotoxic effect | Jahan et al., 2019 |
| Ag |
Tussilago farfara (Asteraceae) |
80 0C dry oven for 4 h or 24 h. | 13.57 ± 3.26 | Spherical | UV–Vis, HR-XRD, FE-TEM, AFM and zeta Potential | Sesquiterpenoids | Antibacterial and anticancer activities |
Lee et al., 2019 |
| Au |
Tussilago farfara (Asteraceae)) |
80 0C dry oven for 4 h or 24 h. | 18.20 ± 4.11 | Spherical | UV–Vis, HR-XRD, FE-TEM, AFM and zeta Potential | Sesquiterpenoids | Antibacterial and anticancer activities |
Lee et al., 2019 |
| Au |
Gnidia glauca (Thymelaeaceae.) |
@ tem 50 0C, 20 min, with 0.7 mM of AuCl4 | ~10 | Spherical | UV–Vis, TEM, HR-TEM, XRD, EM and DLS | Hydroxyl group in alcoholic, phenolic and amine group | Catalytic | Ghosh et al., 2012 |
| Au |
Alhagi maurorum (Fabaceae) |
@35 0C for 15 min | 12–24 | Spherical | UV–Vis, TEM and FTIR | methyl, methylene and methoxy groups | Antimicrobial | Preeti et al., 2017 |
| Au |
Mangifera indica (Anacardiaceae) |
@ different temp (25, 30, 45, 60 0C) | 10–60 | Spherical, triangular, pentagons and hexagons |
UV–Vis, HRTEM, EDS, SAED, XRD and FTIR | Polyphenols or flavonoids such as mangiferin, quercetin and gallic acid | Catalytic | Nayan et al., 2018 |
| Au |
Mimosa pudica (Fabaceae) |
@100 0C and at 30 0C. | 24 | Spherical | UV–Vis, SEM, TEM, XRD, DLS, Zeta sizer and FTIR | Hydroxyl stretching group | Catalytic | Mapala and Pattabi, 2017 |
| Au |
Lonicera Japonica (Caprifoliaceae) |
Incubated @ 60 0C | 10–40 | Spherical and hexagonal | UV–Vis, EDX and XRD, FTIR and GC–MS | Alkaloids, phenolic, polyphenols, amino acids and vitamins | Anticancer activity | Patil et al., 2019 |
| Cd | Rose (Rosaceae) and marigold (Asteraceae) |
Room temperature | – | Spherical | UV–Vis, SEM and FTIR |
Tannins, flavonoids, alkaloids and carotenoids | Mosquito larvicidal activity | Hajra et al., 2016 |
| Cu |
Coccinia grandis (Cucurbitaceae) |
Heated at 60 0C for 10 min after 6 h stirring mixture at room temp | 18–20 | Spherical | UV–Vis, FTIR, XRD, SEM, TEM, and SAED | Alcohols, ester/ether and amine group | Catalysis | Devi and Aharuzzaman, 2018 |
| Mg |
Hydrangea paniculata (Hydrangeaceae) |
@ 25 0C | 56–107 | Spherical | UV–Vis, SEM, TEM, FTIR, XRD and EDX | Bis 3,5,5- tri methyl hexyl ether,1,2-diphenyl-1,2 dithiocyantolethane and phytol acetate | Antioxidant potential and antibacterial activities | Karunakaran et al., 2017 |
| Pd |
Moringa oleifera (Moringaceae) |
1 mM Pd acetae, 20 min | 10–50 | Spherical | UV–Vis, SEM with EDX, FTIR, TEM & DLS, GC–MS coupled with FTIR and NMR | Bis-phthalate compounds | Catalytic and antimicrobial activities | Anand et al., 2016 |
Hajra et al. (2016) used petal extracts of marigold for synthesis of cadmium NPs (Cd-NPs). Most fabricated particles were roughly in shape. Moringa oleifera flower extract mediated palladium nanoparticles (Pd-NPs) was synthesized by Anand et al. (2016). Further they were examined using SEM, EDX, FTIR, DLS and TEM. GC–MS was used to determine the chemical composition of crude flower extract which showed that palmitic acid, docosane, tricosane, tetracosane, pentacosane, Bis(2-ethylhexyl) phthalate, octacosane and hexacosane were major constituent of flower extract. TEM images revealed that size of NPs range between 10 and 50 nm. EDX showed the presence of elemental Pd in NPs. Nayan et al. (2018) used flower extract of Mangifera indica for Au-NPs synthesis and characterized by UV–Vis, FTIR, TEM, HRTEM, EDX spectroscopy, and NP tracking analysis (NTA), DLS. These particles were spherical and size varied from 10 to 60 nm by TEM studies and a modal size of 32 nm by NTA (Nayan et al. 2018).
Ghosh et al. (2012) used flower extract of Gnidia glauca for Au-NPs synthesis; and optimized condition for chloroauric acid concentration was 0.7 mM and at temperature 50 °C. EDX was use to check the presence elemental gold in synthesized Au-NPs. Average size of NPs was ~ 10 nm and shape were spherical.
Flower extract of Mangifera indica was used for Ag-NPs fabrication (Ameen et al., 2019). TEM showed spherical shape of NPs with size range between 10 and 20 nm. EDX studies revealed the presence of Ag in synthesized NPs. FTIR indicated the presence of phytochemical alkaloids, flavonoids, amino acids and proteins which acted as reducing and stabilizing agents Further, details of various investigations are presented and summarized in Table 1.
3.2. Metal-oxide NMs
Abdallah et al. (2019) used aqueous flower extract of Rosmarinus officinalis for fabrication of magnesium oxide NPs (MgO-NPs). Reaction conditions used were continuous stirring at 70∘C for 4 h to obtained MgO-NPs. These synthesized MgO-NPs were further analyzed by using UV–Vis, SEM, TEM, XRD and FTIR studies. UV–Vis shows the absorption peak at 250 nm, and exhibited the formation of MgO-NPs. Particles size were 8.8 nm. Elements present in synthesized NPs were confirmed by EDS which shows Mg 35.55% and O 64.45%.
Matricaria chamomilla (chamomile flower) flower extract of along with olive leave and tomato fruit were used for zinc oxide NPs (ZnO-NPs) synthesis. Further, UV–Vis, FTIR, XRD, SEM and TEM techniques were used for the characterization of synthesized ZnO-NPs (Mladenova et al. 2018). Average size of M. chamomilla flower extract synthesized ZnO-NPs was 51.2 ± 3.2 nm (Table 2).
Table 2.
. Flower-based metal-oxide NPs synthesis and their various applications.
| Metal-oxide NPs | Plant name (Family) | Synthesis condition | Size (nm) | Shape | Characterization techniques | Responsible phytochemical | Applications | Key reference |
|---|---|---|---|---|---|---|---|---|
| CdO | Cassia auriculta (Caesalpiniaceae) | Heated on magnetic stirrer at70 0C | – | – | – | Photocatalytic activity | Gurulakshmi et al., 2019 | |
| CdO |
Hibiscus Sabdariffa (Malvaceae) |
Room temp (25 °C) | 16–41 | Cuboid | HRSEM, HRTEM, EDX and XRD | Pectin and delphinidin | – | Thovhogi et al., 2016 |
| CeO2 |
Hibiscus Sabdariffa (Malvaceae) |
Solution was mixed and then thermal annealing at 500 °C (2 h) | 3.9 | Face centered cubic | HRTEM, EDX, ATR-FTIR, X-rays and photoemission spectroscopy | Quercetin, pectin, hibiscetin, hossypectin and delphinidin | – | Thovhogi et al., 2015 |
| Cr2O3 | Callistemon viminalis (Myrtaceae) | Synthesis was performed room temp. and product obtained was dried at 250 °C and heated at 500 °C (2 h) | ~92.2 | Cubic-like platelet with sharp edges | HRTEM, XRD, ATR/FT-IR, X-Ray and Raman spectroscopy | Flavonoids, monoterpenoids, tannins and triterpenoids | Antimicrobial activity | Sone et al., 2016 |
| FeO | Avicennia marina (Acanthaceae) | – | 30–100 | UV–Vis, SEM, FTIR, XRD and AFM | Aromatic and aliphatic C-H stretching | Electro catalytic | Karpagavinayagam and Vedhi, 2019 | |
| Fe3O4 | Polpala (Amaranthaceae) | Heated @ 60 0C until reduced | 38 | Irregular spherical | UV–Vis, FTIR, XRD and SEM | Alcohol, aldehydes and amine | Nano-catalyst | Clarina et al., 2018 |
| HgO | Callistemon viminalis (Myrtaceae) | Boiled for 10 min at 80 0C | UV–Vis and FTIR | Saponins, phenolic compounds and flavonoids | Antibacterial activity | Das et al., 2014 | ||
| MgO | Rosmarinus officinalis L. (Lamiaceae) | Heated at 600 rpm (70 0C) for 4 h using magnetic stirrer | ≤20 | Flower -shaped | UV–Vis, XRD, SEM, TEM and FTIR | Amine and alcohol group | Antibacterial activity | Abdallah et al., 2019 |
| ZnO | Chamomile (Asteraceae) Olive (Oleaceae) and Red tomato fruit (Solanaceae) | On water bath at 60–70 0C for 4 h | 40.5–124.0 | – | UV–Vis, FTIR, XRD, SEM, TEM, and EDS | Terpenes, saponins, alkaloids, flavonoids, tannins, glycosides and carbohydrates | Antibacterial activity | Ogunyemi et al., 2019 |
| ZnO | Peltophorum pterocarpum (Fabaceae) | Heated @ 80 0C until deep yellow paste | 50–100 | Spherical and irregular | UV–Vis, FTIR, XRD, XRD, SEM and TEM | Phenolic compounds, flavonoids, saponins, steroids,etc | Antimicrobial and cytotoxic activities | Khara et al., 2018 |
| ZnO | Trifolium pretense (Fabaceae) | Solution was stirred for 4 h (at 90 0C) | 60–70 | Spherical | UV–Vis, FTIR, XRD, XRD, SEM, TEM and total reflection X-ray fluorescence analysis | – | Antimicrobial activity | Dobrucka and Długaszewska (2016) |
| ZnO | Nyctanthes arbor-tristis (Oleaceae) | pH at 12 solution was stirred continuously for 2 h | 12–32 | – | UV–Vis, FTIR XRD, DLS and TEM | Amide, aromatic amine, aliphatic amine and alcohol group | Antifungal activity | Jamdagni et al., 2016 |
| ZnO | Bougainvillea (Nyctaginaceae) | Under dark, stirring conditions at room temperature overnight | 40 | – | UV–Vis, FTIR, DLS, SEM and EDX | OH functional group | Antimicrobial and anticancer activities | Rauf et al., 2019 |
Iron oxide NPs (FeO-NPs) were prepared using a flower extract of Avicennia marina (Karpagavinayagam and Vedhi 2019) and UV–Vis spectra of FeO-NPs showed absorption peak 295–301 nm. SEM image revealed that average size of synthesized NPs was in the range of 30–100 nm.
Kumar et al. (2014) fabricated titanium dioxide NPs (TiO2-NPs) from the flower of Hibiscus rosa-sinensis. Average size of fabricated NPs was 7 nm based on XRD data. SEM showed that NPs were monodispersed spherical with no agglomeration. FTIR spectra showed that the phytochemicals present in flower extract acted as a capping as well as stabilizing agents. Marimuthu et al. (2013) used the aqueous extract of the flower of Calotropis gigantean for TiO2-NPs synthesis. XRD revealed the average size of synthesized TiO2-NPs was 10.52 nm. SEM showed an aggregated sphere structure with a size of 160–220 nm. In another study zinc nitrate and flower extract of Aspalathus linearis were used for ZnO-NPs synthesis. The synthesis was performed by heating the solution at 80 °C for 2 h to yield 1–8.5 nm amorphous ZnO-NPs; and then hardened the sample at 300 °C for 2 h to yield crystallized ZnO NPs without changing the size (Diallo et al 2015). ZnO-NPs was fabricated using flower extract of Jacaranda mimosifolia. GCMS of flower extract showed that oleic acid was present as major phytochemical and act as stabilizing agent. The size of synthesized ZnO-NPs was 2 to 4 nm (Sharma et al., 2016). Dobrucka and Długaszewska (2016) fabricated ZnO-NPs using Trifolium pratense flower extract. The synthesized particles shape was spherical and size ranged from 60 to 70 nm when calculated from XRD while SEM studies revealed 100–190 nm in size.
Flower extract of Peltophorum pterocarpum was used for the synthesis of zinc oxide NPs (ZnO-NPs). Characterization was performed using UV–Vis, FTIR, XRD, SEM, zeta potential analysis and TGA. SEM analysis revealed that shape of NPs was spherical and irregular with average size 69.45 nm. TGA curve of ZnO-NPs indicated that synthesized NPs were stable between 200 and 800 0C temperature range. Surface charge of ZnO-NPs was measured by zeta potential fond to be 0.73 mV (Khara et al., 2018). Recently, ZnO-NPs were fabricated by using Bougainvillea flower extracts by Rauf et al. (2019). These biosynthesized ZnO-NPs were examined by UV–Vis, SEM, FTIR, DLS and EDX which showed that size of NPs was 40 nm. Further, details of various investigations are presented and summarized in Table 2.
4. Applications
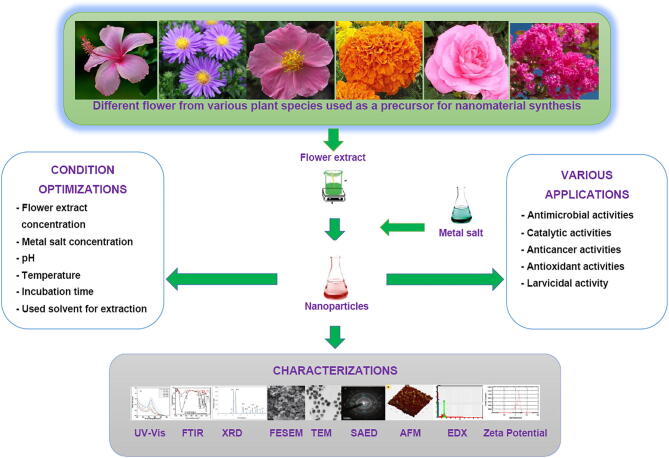
Overall, a summarized flower-based NMs fabrication and their various applications is presented in Fig. 2.
Fig. 2.
Flower-mediated NP fabrication, characterization and their applications.
4.1. Antimicrobial activities
Flower-mediated metal and metal-oxides NPs were studied and have shown better antimicrobial activities. The antibacterial potential of NPs could be verified using well diffusion method. Ipomoea digitata flower extract mediated Ag-NPs showed effective antimicrobial activity against both pathogenic gram-positive as well as gram-negative bacteria (Varadavenkatesan et al., 2018). Abdallah et al. (2019) biosynthesized MgO-NPs using Rosmarinus officinalis flower extract and tested them against the bacteria causing blight disease in rice. Result showed that MgO-NPs remarkedly reduced bacterial growth, biofilm formation, and motility of Xanthomonas oryzae pv. oryzae. Authors have reported that the bacterial cell death was due to the damage of cell integrity, and which leads to the leakage of intracellular content.
The antibacterial activities of the fabricated Ag-NPs from Rosa santana (rose) petals were tested against S. aureus (ATCC 25923) and E. coli (TCC 25922). The zone of inhibition was 11.73 ± 0.25 mm for S. aureus and in case of Escherichia coli it was 10.20 ± 0.36 mm. The cytotoxic effect of synthesized Ag-NPs tested on a mouse fibroblast cell line (L929) which showed that NPs were nontoxic to normal cell line at various doses (Jahan et al. 20019). ZnO-NPs synthesized by Matricaria chamomilla showed antibacterial activity against cultured Xoo strain GZ 0003 bacteria responsible for leaf blight diseases of rice (Ogunyemi et al. (2019). Ag-NPs@Fritillaria showed greater antibacterial activity than Ag-NPs and Fritillaria extract. Antibacterial activity of fabricated Ag-NPs@Fritillaria was examined on bacteria growth; and inhibitory zone was ranging from 10.2 ± 0.83 mm for P. mirabilis and 59 ± 1 mm in case of S. saprophyticus (Hemmati et al. 2019). Jacaranda mimosifolia flower extract fabricated ZnO-NPs and was examined for antibacterial activity by treating bacterial culture with varying doses of NPs (10–100 μg mL−1). The synthesized ZnO-NPs showed effective antibacterial activity against E. coli and E. faecium bacteria (Sharma et al., 2016). Karthik et al. (2019) fabricated the Ag-NPs using the flower extract of Calotropis gigantea which exhibited antibacterial activity in case of E. coli. Agar well diffusion method was used to examine the antimicrobial activity of fabricated ZnO-NPs from Peltophorum pterocarpum flower extract against four Gram positive bacteria (Bacillus cereus, Bacillus subtilis , Staphylococcus aureus, Corynebacterium rubrum) four Gram negative bacteria (E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhimurium) and three fungi (Cryptococcus neoformans, Candida albicans, Candida glabrata). Fabricated ZnO-NPs showed a remarkable antimicrobial activity in comparison to some antibiotics (Khara et al., 2018)
4.2. Antioxidant activities
Ag-NPs synthesized using Bauhinia variegata flower extract showed remarkable antioxidant and α-amylase enzyme activity inhibition (Johnson et al., 2018) and suggested as an effective nano-drug for the treatment of diabetic conditions. Pandurangan et al., (2018) studies a comparative observation on different biochemical compounds obtained from leaf, flower and fruit (Couroupita guianensis) and fabricated Ag-NPs using water, ethyl acetate, and chloroform crude extract. The result of the study showed significant antioxidant and antibacterial activity hence stating the presence of bioactive compounds. Further, flower extracts of Couroupita guianensis, Allamanda cathartica (Karunakaran et al., 2016) mediated Ag-NPs have shown potential antioxidant activities. Jamdade et al. (2019) reported that the novel Cu-NPs fabricated from Gnidia glauca and Plumbago zeylanica used as a promising antidiabetic agent; and have demonstrated that these particles are considered as a potential candidate in antidiabetic nanomedicine preparation.
4.3. Anticancer activities
Flower extract of Peltophorum pterocarpum used for ZnO-NPs fabrication; and further examined for their antimicrobial and cytotoxic activities; and reported as a remarkable application in treatment of some disease (Khara et al., 2018). Brine shrimp cytotoxic activities of plant extract determined their various pharmacological features (Hatano et al., 1989). Rajakumar et al. (2017) and studied for anti-cholinesterase, antibacterial and cytotoxic activities using Millettia pinnata flower extract mediated Ag-NPs and showed that synthesized NPs have potential cytotoxicity activities. Cytotoxic effects of NPs towards shrimp's larvae can linked with anticancer activity and the synthesized NPs could be alternative source of anticancer drugs. Gharpure et al. (2019) studies the non-antibacterial and non-anticancer activity of flower extract and its biosynthesized silver NPs. Synthesized Ag-NPs has not been shown remarkable toxicity even with increase in concentration, due to their biocompatible, hence can be a better candidate as the drug carrier. Further, Mameneh et al. (2019) also studied toxicity of Ag-NPs synthesized using aqueous flower extract of Scrophularia striata on MCF-7 human breast cancer cell line. Authors have demonstrated that Ag-NPs from S. striata flower extract as a potential therapeutic agent for human breast cancer treatment. One report is available on green synthesized of Au-NPs using flower-extract of Lonicera japonica were evaluated for the cytotoxic effect on normal embryonic kidney cells (HEK293) and cervix cancer (HeLa) cells. Result indicated that synthesized Au-NPs were safe to normal cell while inhibited the growth of cancer cells. Immunofluorescent staining studies of HeLa cells showed that condensation and fragmentation of nuclear material were observed that indicates apoptotic cell death (Patil et al. (2019). Another report for anticancer activity of flower extract of mediated NPs is given by Anand et al. (2016) in which biosynthesized Pd-NPs from Moringa oleifera flower extract anti-proliferative activity in A549. Tussilago farfara flower bud extract used for Ag-NPs and Au-NPs synthesis (Lee et al., 2019) and both (Ag and Au) synthesized NPs were tested for anticancer activity against gastric adenocarcinoma cell, human colorectal adenocarcinoma cell (HT-29) and human pancreas ductal adenocarcinoma cells. The cytotoxic activity of synthesized Au-NPs was found higher. Among these examined cells, the highest cytotoxicity of synthesized NPs was noticed in human pancreas ductal adenocarcinoma cells. In a recent report, ZnO-NPs prepared from Bougainvillea flower extracts showed the anticancer activity against the breast cancer cell line (MCF-7) whereas cytotoxicity was not observed against healthy kidney cells (HEK-293) and erythrocytes were established their biocompatible nature (Rauf et al., 2019).
4.4. Catalytic activities
Flower-mediated NPs also exhibited the catalytic activity and thus supported by many investigations. For instance, Mimosa pudica flowers extract synthesized Au-NPs showed good catalytic activity in the model reduction reaction of 4-nitrophenol to 4-aminophenol. (Mapala and Pattabi, 2017). Ipomoea digitata extract flower mediated Ag-NPs also displayed a noticeable catalytic reduction for methylene blue dye in the presence of NaBH4. It showed pseudo-first order kinetics with a rate constant of 0.1714 min−1 (Varadavenkatesan et al. 2018). Also, Ag-NPs prepared from flower of Saraca indica and have shown significant catalytic activity (Vidhu and Philip, 2014). Nayan et al. (2018) obtained AuNPs from flower extract of Mangifera indica displayed good catalytic property in the reduction of 4-nitrophenol to 4-aminophenol by NaBH4 in aqueous phase. And, hence these NPs are useful for waste-waters treatment and also the effluents containing nitroarene treatment, for example 4-nitrophenol. Anand et al. (2016) produced Pd-NPs using Moringa oleifera flower extract and reported the reduction of methylene blue using NaBH4 as a reducing agent. The dyes degradation by Pd-NPs was shown by the decolorization of the dye solution. Also, green synthesized Pd-NPs shown the catalytic reduction of p-nitrophenol to p-aminophenol by NaBH4 (Table 1). Cassia auriculta flower extract was used for synthesis of CdO-NPs and tested for their photocatalytic activity for the degradation of methyl orange and methylene blue dyes and results indicated that synthesized CdO-NPs act as good photo catalyst (Gurulakshmi et al. 2019).
4.5. Miscellaneous application
The redox potential and electrocatalytic performance of FeO-NPs (prepared from flower extract of Avicennia marina) were established by using an electrochemical workstation (Karpagavinayagam and Vedhi 2019). Cd-NPs were fabricated from marigold petal extracts and have shown remarkable larvicidal activity of mosquito (Hajra et al., 2016). It was reported that the marigold flower petal extract along with 10 ppm of Cd-NPs shown 100% mortality after 72 h of incubation.
5. Conclusion
Flower-based NPs synthesis is ecofriendly, nontoxic, have distinctive properties, and are synthesized in a cost-effective manner. It has been found that the flowers are enriched with bioactive molecules such as flavonoids (anthocyanins catechins), terpenoids, coumarins, sterol, xanthones etc. that have great potential ability in the reduction of metal ions. These compounds acted as reducing and stablishing agent in the process of flower based green synthesis of NPs. A number of reports shows that the flower-mediated NPs synthesis under basic condition in the range of 45 to 70 0C were spherical in shape and have higher stability. The spherical NPs were suggested to have several applications and stable for a long time. In the characterization of the flower-mediated NPs some of the techniques such as SEM, TEM, DLS, XRD, AFM, EDX, TGA and Zetasizer were used along with UV–Vis, and FTIR were used. Some important points related with the NPs shape, size and their stability; and the precise mechanisms involved in fabrication process is still remain unresolved or partially resolved. However, taken together, flower mediated-NPs has showed potential application as an antibacterial, antioxidant, anticancer, catalytic agents and so on in different investigations. It is therefore anticipated that the biogenic fabrication of nanomaterials from flower-based chemical compounds will brighten the future prospect and enhance our knowledge for the effective formulation and applications in different discipline of science and technology.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbasi B.H., Nazir M., Muhammad W., Hashmi S.S., Abbasi R., Rahman L., Hano C. A comparative evaluation of the antiproliferative activity against Hepg2 liver carcinoma cells of plant-derived silver nanoparticles from basil extracts with contrasting anthocyanin contents. Biomolecules. 2019;9:320–332. doi: 10.3390/biom9080320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah Y., Ogunyemi S.O., Abdelazez A., Zhang M., Hong X., Ibrahim E., Hossain A., Fouad H., Li B., Chen J. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L. (Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. oryzae. Biomed. Res. Int. 2019;17:5620989. doi: 10.1155/2019/5620989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisha A.F., Abdulmajid A.M.S., Ismail Z., Alrokayan S.A., Abu-Salah K.M. Development of polymeric nanoparticles of Garcinia mangostana xanthones in eudragit RL100/RS100 for anti-colon cancer drug delivery. J. Nanomater. 2015;16:385. [Google Scholar]
- Alle M., Lee S.H., Kim J.C. Ultrafast synthesis of gold nanoparticles on cellulose nanocrystals via microwave irradiation and their dyes-degradation catalytic activity. J Mater Sci Technol. 2020;41:168–177. [Google Scholar]
- Alle M., Kim T.H., Park S.H., Lee S.H., Kim J.C. Doxorubicin-carboxymethyl xanthan gum capped gold nanoparticles: Microwave synthesis, characterization, and anti-cancer activity. Carbohydr. Polym. 2020;229 doi: 10.1016/j.carbpol.2019.115511. [DOI] [PubMed] [Google Scholar]
- Alle, M., Bandi, R., Lee, S.H. and Kim, J.C., 2020. Recent trends in isolation of cellulose nanocrystals and nanofibrils from various forest wood and nonwood products and their application, in: Husen, A., Jawaid, M. (Eds.) Nanomaterials for Agriculture and Forestry Applications. Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp. 41–80.
- Alle, M., Reddy, G.B. and Krishana, I.M., 2019. Green synthesis of gold nanoparticles by using natural gums, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 111–134
- Alshehri A., Malik M.A., Khan Z., Al-Thabaiti S.A., Hasan N. Biofabrication of Fe nanoparticles in aqueous extract of Hibiscus sabdariffa with enhanced photocatalytic activities. RSC Adv. 2017;7:25149–25159. [Google Scholar]
- Ameen F., Srinivasan P., Selvankumar T., Kamala-Kannan S., Al Nadhari S., Almansob A., Dawoud T., Govarthanan M. Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg. Chem. 2019;88 doi: 10.1016/j.bioorg.2019.102970. [DOI] [PubMed] [Google Scholar]
- Anand C., Tiloke K., Phulukdaree A., Ranjan B., Chuturgoon A., Singh S., Gengan R.M. Biosynthesis of palladium nanoparticles by using M. oleifera flower extract and their catalytic and biological properties. J. Photochem. Photobiol. B. 2016;165:87–95. doi: 10.1016/j.jphotobiol.2016.09.039. [DOI] [PubMed] [Google Scholar]
- Arsalani S., Guidelli E.J., Silveira M.A., Salmon C.E.G., Araujo J.F.D.F., Bruno A.C., Baffa O. Magnetic Fe3O4 nanoparticles coated by natural rubber latex as MRI contrast agent. J. Magn. Magn. Mater. 2019;475:458–464. [Google Scholar]
- Arsalani, S., Guidelli., E.J., Araujo, J.F.D.F., Bruno, A.C., Baffa, O., 2018. Green synthesis and surface modification of iron oxide nanoparticles with enhanced magnetization using natural rubber Latex. ACS Sustain. Chem. Eng. 6, 13756–13765.
- Azad, B., Banerjee., A., 2014. Formulation of silver nanoparticles using methanolic extract of stem of plant Desmodium gangeticum, their characterization and antibacterial and anti-oxidant evaluation. Pharm. Innov. J. 3, 77–81.
- Bachheti, R.K., Konwarh, R., Gupta, V., Husen, A. and Joshi, A., 2019. Green synthesis of iron oxide nanoparticles: cutting edge technology and multifaceted applications, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 239–259
- Bachheti, R.K., Godebo, Y., Joshi, A., Yassin, M.O., Husen, A., 2020a. Root-based fabrication of metal and or metal-oxide nanomaterials and their various applications, in: Husen, A., Jawaid, M. (Eds.) Nanomaterials for Agriculture and Forestry Applications. Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp. 135–166.
- Bachheti, R.K., Sharma, A., Bachheti, A., Husen, A., Shanka, G.M., Pandey, D.P., 2020b. Nanomaterials from various forest tree species and their biomedical applications in: Husen, A., Jawaid, M. (Eds.) Nanomaterials for Agriculture and Forestry Applications. Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp. 81–106.
- Bąkowska-Barczak A. Acylated anthocyanins as stable, natural food colorants – A review. Pol. J. Food Nutr. Sci. 2005;14(55):107–116. [Google Scholar]
- Batta A.K., Rangaswami S. Crystalline chemical components of some vegetable drugs. Phytochemistry. 1973;12:214. [Google Scholar]
- Boomi P., Ganesan R.M., Poorani G., Prabu H.G., Ravikumar S., Jeyakanthan J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater. Sci. Eng. C. 2019;99:202–210. doi: 10.1016/j.msec.2019.01.105. [DOI] [PubMed] [Google Scholar]
- Bowen-Forbes C.S., Zhang Y., Nair M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010;23:554–560. [Google Scholar]
- Chandran S., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of Gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- Chinnappan S., Kandasamy S., Arumugam S., Seralathan K.K., Thangaswamy S., Muthusamy G. Biomimetic synthesis of silver nanoparticles using flower extract of Bauhinia purpurea and its antibacterial activity against clinical pathogens. Environ. Sci. Pollut. Res. Int. 2018;25:63–969. doi: 10.1007/s11356-017-0841-1. [DOI] [PubMed] [Google Scholar]
- Chinyerenwa A.C., Munna Md.K.H., Rahman S., Mia Md.R., Yousuf Md.A., Hasan J. Ecofriendly sweet scented Osmanthus leaf extract mediated synthesis of Ag-NPs. Int. J. Text. Sci. 2018;7:35–42. [Google Scholar]
- Clarina T., Flomina P.J., Thangeswari P., Rama V. Polpala flower extract mediated one step green synthesis and characterization of magnetite (Fe3O4) nanoparticles. AJRC. 2018;11:459–462. [Google Scholar]
- Das A.K., Marwal A., Sain D. One-step green synthesis and characterization of flower extract-mediated mercuric oxide (HgO) nanoparticles from Callistemon viminalis. RRJPNT. 2014;2:25–28. [Google Scholar]
- Das M., Smita S.S. Biosynthesis of silver nanoparticles using bark extracts of Butea monosperma (Lam.) Taub. and study of their antimicrobial activity. Appl. Nanosci. 2018;8:1059–1067. [Google Scholar]
- Devi Th.B., Aharuzzaman M. Removal of perilous nitro compound from aqueous phase using biogenic copper nanoparticles as catalyst. IJCT. 2018;25:561–564. [Google Scholar]
- Diallo A., Ngom B.D., Park E., Maaza M. Green synthesis of ZnO nanoparticles by Aspalathus linearis: structural & optical properties. J. Alloys Compd. 2015;646:425–430. [Google Scholar]
- Dobrucka R., Długaszewska J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol Sci. 2016;23:517–523. doi: 10.1016/j.sjbs.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora Respublicae Popularis Sinicae, Tomus., 1983. 52. Beijing: Science Press, p. 120.
- Gajendran B., Durai P., Varier K.M., Liu W., Li Y., Rajendran S., Nagarathnam R., Chinnasamy A. Green synthesis of silver nanoparticle from Datura inoxia flower extract and its cytotoxic activity. BioNanoScience. 2019;9:564–572. [Google Scholar]
- Gharpure S., Kirtiwar S., Palwe S., Akash A., Ankamwa B. Non-antibacterial as well as non-anticancer activity of flower extract and its biogenous silver nanoparticles. Nanotechnology. 2019;30:1–10. doi: 10.1088/1361-6528/ab011a. [DOI] [PubMed] [Google Scholar]
- Ghosh, S., Patil, S., Ahire, M., Kitture, R., Gurav, D.D., Jabgunde, A.M., Kale, S., Pardesi,K., Shinde, V., Bellare, J., Dhavale, D.D., Chopade, B.A., 2012. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J. Nanobiotechnol. 10, 17. [DOI] [PMC free article] [PubMed]
- Gour A., Jain N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019;47:844–851. doi: 10.1080/21691401.2019.1577878. [DOI] [PubMed] [Google Scholar]
- Gurulakshmi P., Duraiselvi P., Maheshwari U., Parkavi M. Photocatalytic activity of cadmium oxide nanoparticles. JETIR. 2019;6:439–442. [Google Scholar]
- Hajra A., Dutta S., Mondal N.K. Mosquito larvicidal activity of cadmium nanoparticles synthesized from petal extracts of marigold (Tagetes sp.) and rose (Rosa sp.) flower. J. Parasit. Dis. 2016;40:1519–1527. doi: 10.1007/s12639-015-0719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T., Edamatsu R., Hiramatsu M., Mori A., Fujita Y., Yasuhara T., Yoshida T., Okuda T. Effects of the interaction of tannins with co-existing substances. VI: effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-diphenyl-2- picrylhydrazyl radical. Chem. Pharm. Bull. 1989;37:2016–2021. [Google Scholar]
- Hemmati S., Rashtiani A., Zangeneh M.M., Mohammadi P., Zangeneh A., Veisi H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019;158:8–14. [Google Scholar]
- Huang T.H.W., Peng G., Kota B.P., Li G.Q., Yamahara J., Roufogalis B.D., Li Y. Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: role of lowering circulating lipids. Toxicol. Appl. Pharmacol. 2005;207:160–169. doi: 10.1038/sj.bjp.0706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.H.W., Peng G., Kota B.P., Li G.Q., Yamahara J., Roufogalis B.D., Li Y. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Br. J. Pharmaco. 2005;145:767–774. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Husen, A., 2019b. Medicinal plant-product based fabrication nanoparticles (Au and Ag) and their anticancer effect, in: Plants that Fight Cancer - Second Edition (Eds. Kintzios, S.E, Barberaki, M., Flampouri) Taylor & Francis/CRC Press, pp. 133–147.
- Husen, A., 2019a. Natural product-based fabrication of zinc-oxide nanoparticles and their applications, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 193–291.
- Husen, A., Iqbal, M., 2019. Nanomaterials and plant potential: an overview, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 3–29.
- Husen, A., Rahman, Q.I., Iqbal, M., Yassin, M.O., Bachheti, R.K., 2019. Plant-mediated fabrication of gold nanoparticles and their applications, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 71–110.
- Husen A., Siddiqi K.S. Carbon and fullerene nanomaterials in plant system. J. Nanobiotechnol. 2014;12:16. doi: 10.1186/1477-3155-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husen A., Siddiqi K.S. Phytosynthesis of nanoparticles: concept, controversy and application. Nano Res. Lett. 2014;9:229. doi: 10.1186/1556-276X-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husen A., Siddiqi K.S. Plants and microbes assisted selenium nanoparticles: characterization and application. J. Nanobiotechnol. 2014;12:28. doi: 10.1186/s12951-014-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husen, A., 2020a. Carbon-based nanomaterials and their interactions with agricultural crops, in: Husen, A., Jawaid, M. (Eds.) Nanomaterials for Agriculture and Forestry Applications. Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp. 199–218.
- Husen, A., 2020b. Interactions of metal and metal-oxide nanomaterials with agricultural crops: an overview, in: Husen, A., Jawaid, M. (Eds.) Nanomaterials for Agriculture and Forestry Applications. Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp. 167–197.
- Husen, A., 2020c. Introduction and techniques in nanomaterials formulation, in: Husen, A., Jawaid, M. (Eds.) Nanomaterials for Agriculture and Forestry Applications. Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp. 1–14.
- Hussain M., Raja N.I., Iqbal M., Aslam S. Applications of plant flavonoids in the green synthesis of colloidal silver nanoparticles and impacts on human health. Iran J. Sci. Technol. A. 2019;43:1381–1392. [Google Scholar]
- Hussein N.H., Shaarawy H.H., Hawash S.I., Abdel-Kader A.E. Green synthesis of silver nano particles using Fenugreek seeds extract. J. Eng. Appl. Sci. 2018;13:417–422. [Google Scholar]
- Jahan I., Erci F., Isildak I. Microwave-assisted green synthesis of non-cytotoxic silver nanoparticles using the aqueous extract of Rosa santana (rose) Petals and their antimicrobial activity. Anal. Lett. 2019;52:1860–1873. [Google Scholar]
- Jamdade, D.A., Rajpali, D., Joshi, K.A., Kitture, R., Kulkarni, A.S., Shinde, V.S., Bellare, J., Babiya, K.R., Ghosh, S., 2019. Gnidia glauca and Plumbago zeylanica Mediated synthesis of novel copper nanoparticles as promising antidiabetic agents. Adv. Pharmacol. Sci. https://doi.org/10.1155/2019/9080279. [DOI] [PMC free article] [PubMed]
- Jamdagni P., Khatri P., Rana J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. 2016;30:168–175. [Google Scholar]
- Johnson P., Krishnan V., Loganathan C., Govindhan K., Raji V., Sakayanathan P., Vijayan S., Sathish K.P., Palvannan T. Rapid biosynthesis of Bauhinia variegata flower extract-mediated silver nanoparticles: an effective antioxidant scavenger and a-amylase inhibitor. Artif. Cells Nanomed. Biotechnol. 2018;46:1488–1494. doi: 10.1080/21691401.2017.1374283. [DOI] [PubMed] [Google Scholar]
- Joshi, A., Sharma, A., Bachheti, R.K., Husen, A., Mishra, V.K., 2019. Plant-mediated synthesis of copper oxide nanoparticles and their biological applications, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 221–237.
- Karpagavinayagam P., Vedhi C. Green synthesis of iron oxide nanoparticles using Avicennia marina flower extract. Vacuum. 2019;160:286–292. [Google Scholar]
- Karthik C., Anand K., Leecanro M., Preethy K.R. Phytosynthesis of silver nanoparticles using Calotropis gigantea flower extract and its antibacterial activity. JoNSNEA. 2019;9:53–60. [Google Scholar]
- Karthik S., Jana A., Selvakumar M., Venkatesh Y., Paul A., Shah S.S., Singh N.P. Coumarin polycaprolactone polymeric nanoparticles: light and tumor microenvironment activated cocktail drug delivery. J. Mater. Chem. B. 2017;5:1734–1741. doi: 10.1039/c6tb02944b. [DOI] [PubMed] [Google Scholar]
- Karunakaran G., Jagathambal M., Gusev A., Kolesnikov E., Mandal A.R., Kuznetsov D. Allamanda cathartica flower’s aqueous extract-mediated green synthesis of Ag-NPs with excellent antioxidant and antibacterial potential for biomedical application. MRS Commun. 2016;6:41–46. doi: 10.1049/iet-nbt.2015.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran G., Jagathambal M., Venkatesh M., Kumar G.S., Kolesnikov E., Dmitry A., Gusev A., Kuznetsov D. Hydrangea paniculata flower extract-mediated green synthesis of MgNPs and Ag-NPs for health care applications. Powder Technol. 2017;305:488–494. [Google Scholar]
- Kasthuri J., Veerapandian S., Rajendiran N. Biological and synthesis of silver and gold nanoparticles using Apiin as reducing agent. Colloids Surf. B Biointerfaces. 2009;68:55–60. doi: 10.1016/j.colsurfb.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Katsumoto Y., Fukuchi-Mizutani M., Fukui Y., Brugliera F., Holton T.A., Karan M., Nakamura N., Yonekura-Sakakibara K., Togami J., Pigeaire A., Tao G.Q. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007;48:1589–1600. doi: 10.1093/pcp/pcm131. [DOI] [PubMed] [Google Scholar]
- Kelley K.M., Behe B.K., Biernbaum J.A., Poff K.L. Consumer preference for edible flower color, container size, and price. Hortscience. 2001;36:801–804. [Google Scholar]
- Kelley K.M., Behe B.K., Biernbaum J.A., Poff K.L. Combinations of colors and species of containerized edible flowers: effect on consumer preferences. Hort. Sci. 2002;37:218–221. [Google Scholar]
- Khan M.M., Saadah N.H., Khan M.E., Harunsani M.H., Tan A.L., Cho M.H. Potentials of Costus woodsonii leaf extract in producing narrow band gap ZnO nanoparticles. Mater. Sci. Semicond. Process. 2019;91:194–200. [Google Scholar]
- Khara G., Padalia H., Moteriya P., Chanda S. Peltophorum pterocarpum flower-mediated synthesis, characterization, antimicrobial and cytotoxic activities of ZnO nanoparticles. Arab. J. Sci. Eng. 2018;43:3393–3401. [Google Scholar]
- Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaprabha M., Pattabi M. Melastoma malabathricum flower extract mediated rapid synthesis of spherical gold nanoparticles. Mater. Today Proce. 2019;9:133–141. [Google Scholar]
- Kumar P.S.M., Francis A.P., Devasena T. Biosynthesized and chemically synthesized titania nanoparticles: comparative analysis of antibacterial activity. J Environ. Nanotechnol. 2014;3:73–81. [Google Scholar]
- Lakshmanan G., Sathiyaseelan A., Kalaichelvan P.T., Murugesan K. Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Int. J. Mod Sci. 2018;4:61–68. [Google Scholar]
- Lee Y.J., Song K., Cha S.H., Cho S., Kim Y.S., Park Y. Sesquiterpenoids from Tussilago farfara flower bud extract for the eco-friendly synthesis of silver and gold nanoparticles possessing antibacterial and anticancer activities. Nanomaterials. 2019;9:819. doi: 10.3390/nano9060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.S., Wu S.S., Lin J.K. Determination of tea polyphenols and caffeine in tea flowers (Camellia sinensis) and their hydroxyl radical scavenging and nitric oxide suppressing effects. J. Agr. Food Chem. 2003;51:975–978. doi: 10.1021/jf020870v. [DOI] [PubMed] [Google Scholar]
- Mameneh R., Shafiei M., Aidy A., Karimi E., Badakhsh B., Abbasi N. Toxicity study of silver nanoparticles synthesized from aqueous flower extract of Scrophularia striata on MCF-7 human breast cancer cell line. Pharmacogn. Mag. 2019;15:66. [Google Scholar]
- Manisha D.R., Alwala J., Kudle K.R., Rudra M.P.P. Biosynthesis of silver nanoparticles using flower Extracts of catharanthus roseus and evaluation of its antibacterial efficacy. WJPPS. 2014;3:877–885. [Google Scholar]
- Manjari G., Saran S., Arun T., Devipriya S.P., Rao A.V.B. Facile Aglaia elaeagnoidea mediated synthesis of silver and gold nanoparticles: antioxidant and catalysis properties. J. Cluster Sci. 2017;28:2041–2056. [Google Scholar]
- Mapala K., Pattabi M. Mimosa pudica flower extract mediated green synthesis of Au-NPs. NanoWorld J. 2017;3:44–50. [Google Scholar]
- Marimuthu S., Rahuman A.A., Jayaseelan C., Kirthi A.V., Santhoshkumar T., Velayutham K., Bagavan A., Kamaraj C., Elango G., Iyappan M., Siva C., Karthik L., Rao K.V. Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac. J. Trop. Med. 2013;6:682–688. doi: 10.1016/S1995-7645(13)60118-2. [DOI] [PubMed] [Google Scholar]
- Mashwani Z.U., Khan M.A., Khan T., Nadhman A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016;234:132–141. doi: 10.1016/j.cis.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Mata R., Bhaskaran A., Sadras S.R. Green-synthesized gold nanoparticles from Plumeria alba flower extract to augment catalytic degradation of organic dyes and inhibit bacterial growth. Particuology. 2016;24:78–86. [Google Scholar]
- Mata R., Nakkala J.R., Sadras S.R. Catalytic and biological activities of green silver nanoparticles synthesized from Plumeria alba (frangipani) flower extract. Mater. Sci. Eng. C. 2015;51:216–225. doi: 10.1016/j.msec.2015.02.053. [DOI] [PubMed] [Google Scholar]
- Miranda M., Cuéllar A. Editorial Félix Varela; La Habana: 2001. Farmacognosia y productos naturales; p. 141. [Google Scholar]
- Mishra, V.K., Husen, A., Rahman, Q.I., Iqbal, M., Sohrab, S.S., Yassin, M.O., 2019. Plant-based fabrication of silver nanoparticles and their application, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 135–175.
- Mladenova B., Diankov S., Karsheva M., Stankov S., Hinkov I. Plant mediated synthesis of silver nanoparticles using extracts from Tilia cordata, Matricaria chamomilla, Calendula officinalis and Lavandula angustifolia flowers. J. Chem. Technol. Metall. 2018;53:623–630. [Google Scholar]
- Moteriya P., Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities Artif. Cells Nanomed. Biotechnol. 2017;45:1556–1567. doi: 10.1080/21691401.2016.1261871. [DOI] [PubMed] [Google Scholar]
- Nagaraj B., Divya T., Malakar B., Krishnamurthy N., Dinesh R., Negrila C.C., Predoi D. Phytosynthesis of gold nanoparticles using Caesalpinia pulcherrima (peacock flower) flower extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biostruct. 2012;7:899–905. [Google Scholar]
- Nakahara K., Roy M.K., Ono H., Maeda I., Ohnishi-Kameyama M., Yoshida M., Trakoontivakorn G. Prenylated flavanones isolated from flowers of Azadirachta indica (the neem tree) as antimutagenic constituents against heterocyclic amines. J. Agr. Food Chem. 2003;51:6456–6460. doi: 10.1021/jf034666z. [DOI] [PubMed] [Google Scholar]
- Nasseri M.A., Shahabi M., Allahresani A., Kazemnejadi M. Eco-friendly biosynthesis of silver nanoparticles using aqueous solution of Spartium junceum flower extract. Asian J. Green Chem. 2019;3:382–390. [Google Scholar]
- Nayan V., Onteru S.K., Singh D. Mangifera Indica flower extract mediated biogenic green gold nanoparticles: efficient nanocatalyst for reduction of 4-nitrophenol. Environ. Prog. Sustain Energy. 2018;37:283–294. [Google Scholar]
- Nørbæk R., Nielsen K., Kondo T. Anthocyanins from flowers of Cichorium intybus. Phytochemistry. 2002;60:357–359. doi: 10.1016/s0031-9422(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Ogunyemi S.O., Abdallah Y., Zhang M., Fouad H., Hong X., Ibrahim E., Masum M.I., Hossain A., Mo J., Li B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019;47:341–352. doi: 10.1080/21691401.2018.1557671. [DOI] [PubMed] [Google Scholar]
- Padalia H., Moteriya P., Chanda S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential Arab. J. Chem. 2015;8:732–741. [Google Scholar]
- Pandurangan P., Sahadeven M., Sunkar S., Dhana S.K.N.M. Comparative analysis of biochemical compounds of leaf, flower and fruit of C. guianensis and synthesis of silver nanoparticles. Pharmacogn J. 2018;10:315–323. [Google Scholar]
- Patil, M.P., Bayaraa, E., Subedi, P., Piad, L.L.A., Tarte, N.H., G.D., 2019. Biogenic synthesis, characterization of gold nanoparticles using Lonicera japonica and their anticancer activity on HeLa cells. J. Drug Deliv. Sci. Technol. 51, 83–90.
- Painuli, S., Semwal, P., Bachheti, A., Bachheti, R.K., Husen, A., 2020. Nanomaterials from non-wood forest products and their applications, in: Husen, A., Jawaid, M. (Eds.) Nanomaterials for Agriculture and Forestry Applications. Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp. 15–40.
- Preeti J., Ashish M., Swati S., Anita S.R. Alhagi maurorum flower extract mediated synthesis of gold nanoparticles. Asian J. Pharm. 2017;11:225–229. [Google Scholar]
- Pushkar B., Sevak P.I. Green synthesis of silver nanoparticles using Couroupita guianensis fruit pulp and its antibacterial Properties. World J. Pharm Res. 2018;5:1174–1187. [Google Scholar]
- Radini I.A., Hasan N., Malik M.A., Khan Z. Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J. Photochem. Photobiol. B. 2018;83:154–163. doi: 10.1016/j.jphotobiol.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Raghavan B.S., Kondath S., Anantanarayanan R., Rajaram R. Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochem. 2015;50:1966–1976. [Google Scholar]
- Rajakumar G., Gomathi T., Thiruvengadam T., Chung I.M. Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized Ag-NPs using from Millettia pinnata flower extract. Microb Pathog. 2017;103:123–128. doi: 10.1016/j.micpath.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Rao N.S., Rao M.V.B. Green synthesis, characterization and biological studies on silver nanoparticles from Caesalpinia bonduc stem bark extract. Der Pharma Chemica. 2016;8:14–19. [Google Scholar]
- Rauf M.A., Oves M., Rehman F.U., Khan A.R., Husain N. Bougainvillea flower extract mediated zinc oxide’s nanomaterials for antimicrobial and anticancer activity. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.108983. [DOI] [PubMed] [Google Scholar]
- Shaikh R., Syed I.Z., Bhende P. Green synthesis of silver nanoparticles using root extracts of Cassia toral L. and its antimicrobial activities. Asian J Green Chem. 2019;3:70–81. [Google Scholar]
- Sharma D., Sabela M.I., Kanchi S., Mdluli P.S., Singh G., Stenström A., Bisetty K. Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: synergistic antibacterial activity and molecular simulated facet specific adsorption studies. J. Photochem. Photobiol. B: Biol. 2016;162:199–207. doi: 10.1016/j.jphotobiol.2016.06.043. [DOI] [PubMed] [Google Scholar]
- Siddiqi K.S., Husen A., Rao R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnol. 2018;16:14. doi: 10.1186/s12951-018-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi, K.S., Rashid, M., Rahman, A., Tajuddin, Husen, A., 2019. Synthesis of silver nanoparticles using aqueous leaf extract of Diospyros montana Roxb. and their antimicrobial activity against some clinical isolates. BioNanoSci. 9,302–312.
- Siddiqi K.S., Rashid M., Rahman A., Tajuddin Husen A., Rehman S. Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea ongissima) and their antimicrobial activity. Biomat. Res. 2018;22:23. doi: 10.1186/s40824-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S., Husen, A., 2019. Role of nanomaterials in the mitigation of abiotic stress in plants, in: Husen, A., Iqbal, M. (Eds.) Nanomaterials and Plant Potential. Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham. pp. 441–471.
- Sivarajan, V.V. and Balachandran, I., 1994. Ayurvedic drugs and their plant sources. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, 570.
- Sone B.T., Manikandan E., Gurib-Fakim A., Maaza M. Single-phase α-Cr2O3 nanoparticles’ green synthesis using Callistemon viminalis’ red flower extract. Green Chem. Lett. Rev. 2016;9:85–90. [Google Scholar]
- Song J.Y., Jang H.K., Kim B.S. Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diopyros kaki) Korean J. Chem. Eng. 2008;25:808–811. [Google Scholar]
- Sriyatep T., Siridechakorn I., Maneerat W., Pansanit A., Ritthiwigrom T., Andersen R.J., Laphookhieo S. Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa. J. Nat. Prod. 2015;78:265–271. doi: 10.1021/np5008476. [DOI] [PubMed] [Google Scholar]
- Su S.K., Chen S.L., Lin X.Z., Hu F.L., Shao M. The determination of ingredient of tea (Camellia sinensis) pollen. Apicul China. 2000;51:3–5. [Google Scholar]
- Tanaka Y., Tsuda S., Kusumi T. Metabolic engineering to modify flower color. Plant Cell Physiol. 1998;39:1119–1126. [Google Scholar]
- Wang R., Wang W., Wang L., Liu R., Ding Y., Du L. Constituents of the flowers of Punica granatum. Fitoterapia. 2006;77:534–537. doi: 10.1016/j.fitote.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Thovhogi N., Diallo A., Gurib-Fakim A., Maaza M. Nanoparticles green synthesis by Hibiscus sabdariffa flower extract: main physical properties. J. Alloys Compd. 2015;647:392–396. [Google Scholar]
- Thovhogi N., Park E., Manikandan E., Maaza M., Gurib-Fakim A. Physical properties of CdO nanoparticles synthesized by green chemistry via Hibiscus Sabdariffa flower extract. J. Alloys Compd. 2016;655:314–320. [Google Scholar]
- Varadavenkatesan T., Selvaraj R., Vinayagam R. Dye degradation and antibacterial activity of green synthesized Ag-NPs using Ipomoea digitata Linn. flower extract. Int. J. Sci. Environ. Technol. 2018;140:35–45. [Google Scholar]
- Vidhu V.K., Philip D. Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flower. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;117:102–108. doi: 10.1016/j.saa.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Vinay C.H., Goudanavar P., Acharya A., Ahmed M.H., Kumar P.S.R. Development and characterization of orange peel extract based nanoparticles. Asian J. Pharm. Clin. Res. 2018;8:71–77. [Google Scholar]
- Wang B.C., He R., Li Z.M. The stability and antioxidant activity of anthocyanins from blueberry. Food Technol. Biotech. 2010;48:42. [Google Scholar]
- Yabuya T., Nakamura M., Iwashina T., Yamaguchi M., Takehara T. Anthocyanin-flavone copigmentation in bluish purple flowers of Japanese garden iris (Iris ensata Thunb) Euphytica. 1997;98:163–167. [Google Scholar]
- Yang Z., Tu Y., Susanne B., Dong F., Xu Y., Watanabe N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. LWT - Food Sci. Technol. 2009;42:1439–1443. [Google Scholar]