Abstract
Background
Prostate cancer (PCA) is a frequent cancer that mainly affects the men. Studying growth feature pathways modified during PCA development may facilitate researchers to expand embattled therapeutic strategies for prostate cancer. In present study, we examined the anticancer potentials of Ganoderma lucidum against the prostate cancer (PC-3) cells by inflection of JAK-1/STAT-3 signalling pathway.
Methods
The cytotoxicity of G. lucidum against the PC-3 cells was examined by MTT assay. The ROS production was monitored by using DCFH-DA staining. The apoptotic morphological alterations stimulatory potential of G. lucidum on PC-3 cells was inspected through the dual staining. The expression of Bcl-2, JAK-1, STAT3, Bax and CyclinD1 proteins were measured by western blotting. The caspase-3 and 9 functions were condensed by assay kit.
Results
Findings demonstrates the Ganoderma lucidum convince cytotoxicity, accretion of ROS, and apoptosis stimulation in PC-3 cells. In addition, signal transducer and activating transcription (STAT-3) is a successive oncogenic transcriptional factor that regularizes multiplication and apoptosis in cells. Discretion of STAT-3 transcription deliberated as original approach to hinder prostate cell growth. In present exploration, we ascertain that Ganoderma lucidum hold back STAT-3 translocation, in that way dropping the eminent expression of, BCL-2, cyclin-D1 and declined the Bax, caspase-3 and 9 expressions in PC-3 cells.
Conclusion
In the end our finding concluded that Ganoderma lucidum hinder prostate cell development and convinces apoptosis via hampering the translocation STAT-3.
Keywords: Ganoderma lucidum, PC-3 cells, Apoptosis, Prostate cancer, Cyclin-D1, STAT-3 pathway
1. Introduction
Prostate cancer is a frequent cancer that primarily affects the men, with a yearly estimate of 10,00,000 new cases and 3 million deaths globally (Sierra et al., 2016, Rawla, 2019). Basically all men with highly developed disease, who went in the course of androgen dispossession therapy, ultimately pass on because of progression of androgen self-governing metastatic prostate cancer (PCA) (Alpajaro et al., 2019, Ku et al., 2019). Elevated death rate from prostate cancer is connected with vital propagation of the prostate cancer that distributes to far-off organs with fondness to the tissues of bone (Lewis et al., 2019). Many research findings demonstrate and indicate that development of primary stage and metastatic stage of prostatic cancer is firmed via the diminution in the apoptotic potential of the cells (Jan and Chaudhry, 2019). The mitochondrial contribution in apoptotic events were authenticated by a numerous preceding reports recounting pro-apoptotic mitochondrial modifications, like reactive oxygen species (ROS) production, exhaustion of ATP and stimulation of permeability of mitochondrial (Wolf, 2019, Wacquier et al., 2019).
Most of the previous reports show that Bcl-2 and other apoptotic protein families are situated at the mitochondrial junctions or the inter-membrane space and normalize the apoptosis in the course of their possessions on mitochondrial permeability (Xu et al., 2019, Bhosale and Duchen, 2019). Reports on associations involving initiation of apoptosis in PC-3 cells, Bcl-2 and Bax associated protein expressions produce conflicting outcomes (Pilling and Hwang, 2019, Testa et al., 2019).
Signal transducer and activator of transcription-3 (STAT-3) is a transcription feature particularly stimulates in cancer cells and it was widely concerned as an idea for to treat the prostate cancer. Generally the prominent STAT3 expression requires activating via chemokines and interleukins. Enthused STAT-3 principally alarmed in multi impressive cellular sequence e.g. demarcation, propagation, cell endurance and apoptosis (Piperi et al., 2019, Ai et al., 2012). Ever since STAT-3 labeled as oncogenic protein, prominent STAT-3 expression is connected with further immoral action of tumor cells and substandard projection in several kinds of cancers like, prostrate, lung, brain, ovary, and breast (Schoppmann et al., 2012, Liu et al., 2012, Chen et al., 2013). Genetic modification stretch out underneath irregular STAT-3 signaling in cancer and agitated assortment of dogmatic mechanism in the JAK/STAT cascade that can root an insistent STAT-3 organization are frequently perceive in various tumors (Tan et al., 2019, Lee and Cheung, 2019, Pilati et al., 2011).
Impressions on the significance of STAT3 signaling and intending it with probable treatment for cancers in common have been the issue of modern evaluations (Kamran et al., 2013). On the other hand, the width and range of STAT3 networking regulation that constrain the development of PCA have not been tackled lately. Additionally, STAT-3 commencement results in enhanced appearance cyclin D1 a proliferative marker by altering the diverse gene’s expressions necessary for cancer cell adjustment, multiplication, for invasion and finally metastasis (Gurbuz et al., 2014). Thus, STAT-3 signaling pathway was implicated as central target for cancer treatment.
In our current exploration, we use Ganoderma lucidum as our candidate compound from polypore of fungi which is also possessing mostly triterpenes. Natural, synthetic triterpenoids and associated compound (CDDO-Me) can restrain commencement of the STAT-3 pathways (Yore et al., 2006, Liby et al., 2006). Ultimately apoptotic stimulation and all the way via inhibition of STAT-3 pathway will be an imperative approach for chemoprevention (Siddiquee and Turkson, 2008). Consequently, transforming the commotion of STAT-3 was deliberated as competent target using logical exciting small-molecule which restrains to convince apoptosis for cancer treatment.
Ganoderma lucidum, also called as, conventional Chinese mushroom which has many medicinal properties. Though a mixture of bioactive molecules recognized in this therapeutic mushroom, its anticancer special properties are primarily depicted with triterpene fractions (Yuen and Gohel, 2005). And it has been used for many ailments e.g. inflammation, immunological disorders and even treating cancer in East Asia region for many years (Pan et al., 2019). Ganoderma lucidum extract, enclosed triterpenes, was accounted to restrain development and metastatic prospective of breast cancer cells (MDA-231) by hindering the function of transcription factors like AP-1 and NF-kB, resultant in the decreased expression of cyclin D1 (Sliva, 2004, Slivova et al., 2005). Furthermore, recent reports that Ganoderma lucidum kills breast cancer cell line (SUM-149) which restrains cell viability and incursion, without distressing normal mammary epithelial cells, constructing it as a potential anti-cancer agent (Martinez-Montemayor et al., 2011).
With these entire basis, Ganoderma lucidum is measured a fascinating fungus, used in substitute medicine and established to have several allegation as a impending anticancer drug though, advance research required to be complete to enumerate it as tailored medicine, particularly in treating précised tumors like prostate cancer (Kao et al., 2016). So, we hunted to scrutinize the function of Ganoderma lucidum on cell growth by amending Jak-1/STAT-3 signalling pathway in prostate cancer (PC-3) cells.
2. Materials and methods
2.1. Chemicals
2, 7-diacetyl dichlorofluorescein (DCFH-DA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Ethidium Bromide, Acridine Orange were procured from Sigma chemical, USA. Dulbecco’s Modified Eagles Medium (DMEM), fetal bovine serum (FBS), phosphate buffered saline (PBS), trypsin-EDTA, streptomycin was acquired from Sigma, USA. Monoclonal antibodies, JAK-1, STAT-3, Bcl-2, Bax, cyclin-D1, β-actin and secondary antibodies were obtained from Santa Cruz, USA.
2.2. Cell culture
PC3 prostate cancer cells were collected from the American Type Culture Collection (ATCC, MD, USA). Cells were placed to culture in DMEM medium and preserved at 37 °C in moistened situations which contains 5% CO2 incubator.
2.3. Ganoderma lucidum
The Ganoderma lucidum was purchased from the local vendors and dried powdered G. lucidum was utilized to prepare the extract. 3 g of mushroom powder were amalgamated with 100 mL of dimethyl sulfoxide (DMSO) and positioned in shaker for 24hr at 37 °C. Then this suspension was filtered with Cartridge (3 M 740) and then located on the evaporator, for 15 min at 37 °C. Then the deposit was suspended in 150 mL of DMSO and stored at 4 °C. Ethanolic extract was equipped by utilizing the dehydrated Ganoderma lucidum (6gms) powder, in 6oml of ethanol (70% v/v) for 12hr at 37 °C, integrating with a mixer. Finally the suspension was filtered with No.1 Whatman filter paper; then the dig out was stored at −20 °C for further laboratory works.
2.4. MTT assay
The inhibitory actions of Ganoderma lucidum against the PC-3 cell growth was assessed by MTT assay. PC-3 cells were propagated in 96-wellplate at 6000–12,000 cells/well in an end volume of 100 µL with Dulbecco’s Modified Eagles Medium and then it was incubated for 24hr. Subsequently 100 µg of MTT solution was mixed to all wells then again incubated for 4hr at 37 °C. Followed by the MTT reagent was standing apart and 100 µL of DMSO was mixed to split up the purple formazan crystals. The plate was measured at 560 nm in an ELISA reader.
2.5. Measurement of intracellular ROS production
Ganoderma lucidum mediated ROS accretion in prostate cancer (PC-3) cells was noticed by using DCFH-DA staining. Initially the PC-3 cells were sowed (1X106cells/well) in 6-well plate; after collecting, cells were indulged with Ganoderma lucidum with different time period and then kept at a CO2 chamber for 24hr equally. Following appropriate incubation timing, DCFH-DA (1 mg/mL) was introduced to all the 6-wells and incubated for 37 °C in dark. The developed intensity was determined with Tecan multimode reader at 540 nm, respectively.
2.6. Acridine orange/ethidium (AO/EtBr) bromide staining
The apoptotic morphological alterations stimulatory potential of Ganoderma lucidum on PC-3 cells were inspected through the dual staining i.e. AO/EtBr technique. Briefly, 1 × 106 cells were loaded in 6-wellplate then supplemented with various dose incubation of Ganoderma lucidum. When the incubation is over, the supplemented cells were cleaned with chilled buffer solution then discolored with 12 µL of AO and 12 µL/mg EtBr at 37 °C for 45 min. Finally cells were examined beneath the fluorescent microscope.
2.7. Western blot analysis
The western blotting studies were done for, Bcl-2, JAK-1, STAT3, Bax and CyclinD1 protein expressions in Ganoderma lucidum supplemented PC-3. The outcomes were standardized to perpetuation the expression of marker β-actin. Later than protein inference, the indulged samples were estranged through SDS-PAGE and the proteins were relocated to PVDF membrane and indulged with appropriate primary and secondary antibodies (Santa Cruz Biotech, USA) to notice the target protein.
2.8. Caspase- 9 and 3 activity assay
The caspase-3 and 9 functions were condensed by Enzchek caspase assay kit. Abruptly, the PC-3 cells were sowed in 6-wellplate and supplemented with Ganoderma lucidum and kept at CO2 incubator for 24 hr respectively. Subsequently the cells were administered with caspase-3 and 9 reagents and again kept at CO2 incubator for 2 hr in dark. Finally, the absorbance was examined with the help of HT multi-mode microplate reader at 415 nm.
2.9. Statistical assessment
All investigations were aggrieved out in three self-determining tests and the results were portrayed as mean ± SD by using one-way analysis of variance (ANOVA). Values of P < 0.05 were regarded as significance.
3. Results
3.1. Ganoderma lucidum induces cytotoxicity in prostate cancer PC-3 cells
The cytotoxicity potential of Ganoderma lucidum against PC-3 cells was evaluated by MTT assay. In this test, we noticed that Ganoderma lucidum aggravated substantial cell death in a dose of drug in both 15 µg and 20 µg in time reliant mode, which was depicted in Fig. 1. But the optimal IC50 level of Ganoderma lucidum was found at 20 µg/mL for 24hr incubation. This result shows that Ganoderma lucidum provokes cytotoxicity in prostate cancer PC-3 cell lines (Fig. 1). Based on this result, 20 µg/mL was opted as optimal dose for additional investigations.
Fig. 1.
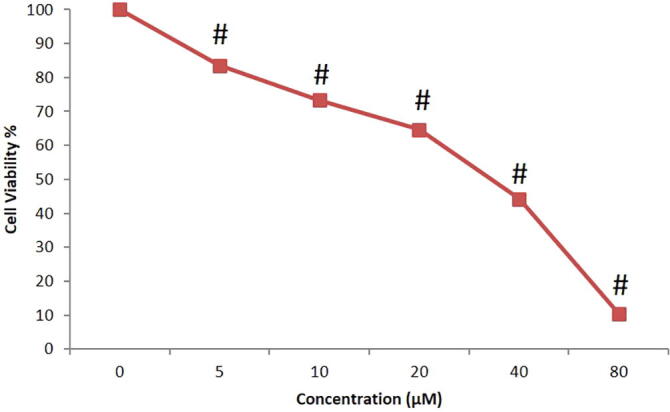
Cytotoxic effect of G. lucidum on PC-3 cells. Optimum IC50 value of G. lucidum was found to be 20 µg/mL for 24hr incubation. Hence, 20 µg/mL was opted as optimal dose for additional investigations.
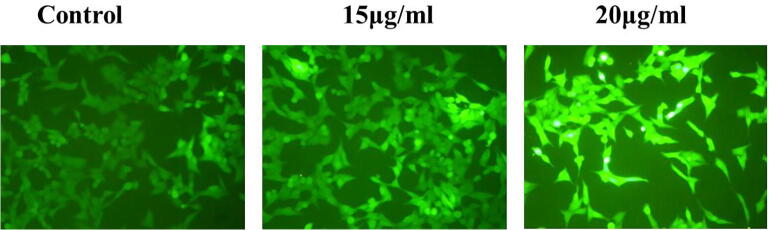
3.2. Ganoderma lucidum induces intracellular ROS in PC-3 cell lines
The status of intracellular ROS accretion was calculated by DCFH-DA dye. DCFH-DA passively transformed into DCFH that rejoin with ROS to develop the DCF (fluorescent). Augmented fabrications of ROS throw in cellular trauma and cell scrape. Ganoderma lucidum mediated intracellular ROS status was inspected via DCFH-DA fluorescent staining. This was shown in the Fig. 2 which denotes control PC-3 cell lines demonstrate diminish fluorescence intensity which specify zilch ROS. Ganoderma lucidum treated with 15 µg shows mild reactive oxygen species. In difference, treatment of 20 µg Ganoderma lucidum aggravates remarkable ROS production in PC-3 cell lines (Fig. 2).
Fig. 2.
Inside the cell ROS generation was precised by DCFH-DA staining. As illustrated in the Fig. 2, the control PC-3 cells demonstrate diminished fluorescence intensity that specifies zilch ROS. G. lucidum (15 & 20 µg) treated cells shows remarkable ROS production in PC-3 cell lines.
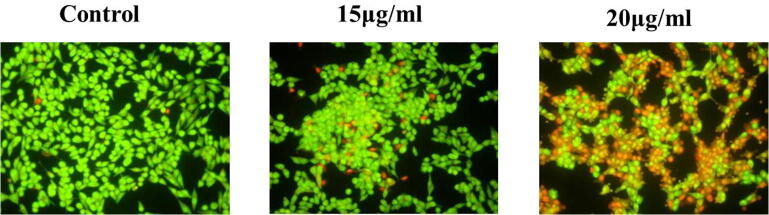
3.3. Ganoderma lucidum induced apoptotic studies for acridine orange/ethidium bromide in PC-3 cell lines
Furthermore, PC-3 cells apoptotic appearance were scrutinized by acridine orange (AO) and ethidium bromide (EtBr) to distinguish cells that formulate apoptotic and non-apoptotic by morphological assessment. Ganoderma lucidum interceded apoptosis and morphological alterations in PC-3 cell lines which were premeditated through the AO and EtBr which was depicted in Fig. 3 i.e., control PC-3 cells displayed no apoptotic cells, which examining by AO stained green cells. In distinction, supplementation of 20 µg Ganoderma lucidum for 24hr incubation persuades morphological alterations like, nuclear fragmentation and blurred membrane in PC-3 cell lines (Fig. 3).
Fig. 3.
Fluorescence images of apoptotic morphology by dual staining (AO/EtBr). The control PC-3 cells displayed no apoptotic cells, which examining by AO stained green cells. In contrast, the G. lucidum (15 & 20 µg) supplementation for 24 hr incubation persuades morphological alterations like, nuclear fragmentation and blurred membrane in PC-3 cell lines.
3.4. Ganoderma lucidum restrains JAK-1/STAT-3 translocation in PC-3 cells
Janus kinase-1 (JAK-1) is a preliminary factor for activating and translocation of STAT-3 which implicated in the cell growth and apoptosis. In present exploration, Fig. 4 shows that 20 µg of Ganoderma lucidum hold down the over expression of JAK-1 in PC-3 cells, which is in dissimilar to 15 µg Ganoderma lucidum treated PC-3 cells and control. At the same time 20 µg Ganoderma lucidum treated PC-3 cells showed elevated expression of Jak-1. Although previously mentioned Jak-1 is the starter for STAT-3 expression. In adding together, STAT-3 translocation was observed by total protein. In current study Fig. 4 shows that control PC-3 cells depicted the elevation in STAT-3 expression. In diverse, supplementation of 15 µg Ganoderma lucidum significantly diminished the expression of STAT-3 protein in PC-3 cells. Similarly, at last we noticed 24hr incubated 20 µg Ganoderma lucidum greatly hold back the translocation of STAT-3.
Fig. 4.
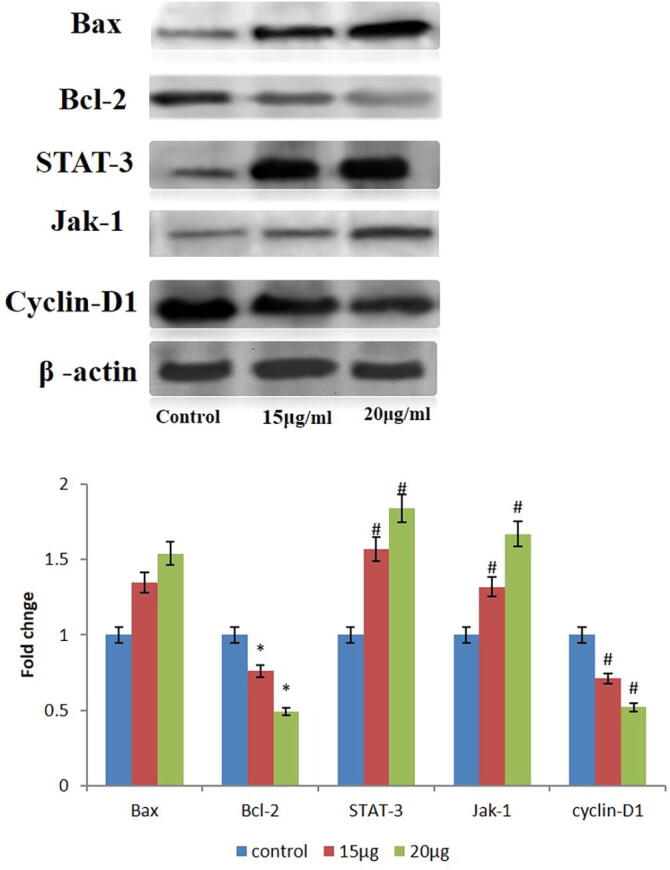
Western blotting analysis.Fig. 4 shows that the G. lucidum down-regulated the expression of JAK-1 and elevated the expression of Jak-1 in PC-3 cells. The G. lucidum significantly diminished the expression of STAT-3 protein and holds back the translocation of STAT-3 in PC-3 cells. The G. lucidum suppresses the cyclin-D1 expression and up lifted the Bax expression and declined the Bcl-2 expression in the PC-3 cells. β-actin was used as a internal control.
3.5. Ganoderma lucidum hold back proliferative marker in PC-3 cells
Cyclin-D1 is the notable proliferative indicator and the excessive expression of this indicator distressed in the PC-3 cell differentiation. Proliferative marker expressions were inspected through the western blotting and the result depicted in Fig. 4. Cyclin-D1 is a remarkable cell proliferative marker and excessive expression of this marker concerned in unrestricted multiplication in PC-3 cells. Expression of cyclin-D1 was depicted Fig. 4 which showed in current study, PC-3 normal cells are tremendously expressed in cyclin-D1. At the same instance 15 µg Ganoderma lucidum treated PC-3 cells showed some expression of proliferative marker. On the divergent, treatment of 20 µg Ganoderma lucidum suppresses the cyclin-D1 expression in PC-3 cells. This ultimately shows that Ganoderma lucidum hold back proliferative marker in PC-3 cells (Fig. 4).
3.6. Ganoderma lucidum stimulates apoptosis in PC-3 cells
In current study Ganoderma lucidum interrupted pro-apoptotic protein marker Bax and antiapoptotic protein marker Bcl-2 which was inspected through the western blotting studies. Ganoderma lucidum interfered with the pro-apoptotic protein i.e. Bax and anti-apoptotic protein i.e. Bcl-2 noticed by the elevated expression of Bax and diminished expressions of Bcl-2. The results were depicted in Fig. 4 which demonstrates that treatment of 15 µg of Ganoderma lucidum shows mild increase in Bax protein marker. Same results were obtained in Bcl-2 in which treatment of 15 µg of Ganoderma lucidum shows mild decrease in Bcl-2 protein marker. In different treatment of 20 µg Ganoderma lucidum influence apoptosis in PC-3 cells by investigating the up lifted the Bax expression and declined the Bcl-2 expression, which is in contrast to normal PC-3 cells (Fig. 4).
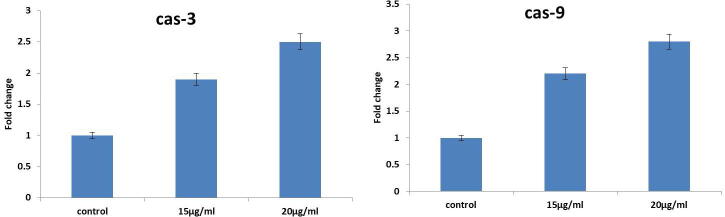
3.7. Ganoderma lucidum induces caspase expression in PC-3 cells
The expressions of caspase-3 and 9 were scrutinized by Enzchek caspase assay kit. Briefly, the PC-3 cells were loaded in 6-wellplate and administered with Ganoderma lucidum and incubated for 24hr uniformly at CO2 chamber. Afterward, cells were administered with caspase-3 and 9 assay reagents and then incubated for 2hr in dark. The graphical results displayed in Fig. 5 shows treatment of 15 µg of Ganoderma lucidum shows mild increased graphical expression (P < 0.05) of caspase 3 and 9 (Fig. 5). In different, supplementation of 20 µg Ganoderma lucidum trigger apoptosis in PC-3 cells via examining that elevated functions of caspase-9 and -3, which is in contrast to control PC-3 cells.
Fig. 5.
Caspase-3 and 9 expressions in PC-3 cells. The supplementation of 15 & 20 µg of G. lucidum triggers the apoptosis in PC-3 cells via augmenting the caspase-9 and -3 activities, which is in contrast to control PC-3 cells.
4. Discussion
In our time, cancers are pain staked as harmful diseases and foremost health vulnerability for humans around the world. Nevertheless, a division of patients with highly developed treatments like radiotherapy has been associated with high mortality (Haugen, 1999). In current study, we established the anti-tumor potentials of Ganoderma lucidum on PC-3 prostate cancer cells and partly described the causal molecular mechanism of STAT-3 molecule and its starter molecule Jak-1 which simultaneously described the causal of apoptotic properties. Ganoderma lucidum reserved transcriptional function of STAT-3 and by this means censored transcription of oncogenic STAT-3 target genes, leading to PC-3 cells growth inhibition. By executing PC-3 cells, we established that Ganoderma lucidum dose-dependently restrains multiplication and elevates apoptosis; depend on the status of STAT-3 and Jak-1pathway in these cells (Fig. 4). We demonstrated here that Ganoderma lucidum expressly inactivates STAT-3 signaling pathway and hold back the proliferative marker expression.
In current study, we affirmed for the first time, Ganoderma lucidum hold down prostate cancer cell multiplication by reducing STAT-3 translocation in PC-3 cells. Supplementation to the PC-3 cells with Ganoderma lucidum, we established the inhibition of proliferation which was examined by MTT assay. Concurrently ganoderma lucidum influence cell death in concentration depended manner (Fig. 1). IC50 dose of Ganoderma lucidum was noticed at 20 µg/mL for 24 hr incubation. Earlier, findings demonstrated that the inhibition dose and antioxidant potential of Ganoderma lucidum (Barbieri et al., 2017). Oxidative stress is a recklessness response connecting the commencement and elimination of ROS production that express the injure of macromolecules. Production of reactive oxygen species correlated to cell demise and cell endurance of tumor cells (Galadari et al., 2017).
In our finding, elevated reactive oxygen species levels were examined in Ganoderma lucidum treated prostate cancer cells in a time reliant mode (Fig. 2). The surprising renowned oxidative dependability in Ganoderma lucidum has been associated closely with its existence (Sun et al., 2017). In current study we examined Ganoderma lucidum able to work as a pro-oxidant and persuades ROS arbitrated oxidative stress in PC-3 cells. Cyclin-D1 is a significant proliferation indicator that involves in the multiplication of prostate cancer cells. In current investigation, supplementation of Ganoderma lucidum hold back the over expression of cyclin-D1 in PC-3 cells. A preceding investigation shows pro-oxidant reserve of growth of MCF-7 cells is linked with down regulated expression of cyclin D1 protein (Hongbo et al., 2002). STAT3 is a transcription factor activated via varied exciting optimistic genes like tyrosine kinases and interleukins that will pessimistically authorize the initiation of STAT3 that gives rise to transcriptional action in several proliferative indicators especially cyclin D1. In current research, we recognized first time that Ganoderma lucidum controls STAT3 commencement in PC-3 cells. Same outcomes were found in Jak-1 in which Ganoderma lucidum supplementation hold back the JAK-1 protein expression in the PC-3 cells.
A preceding result show that JAK-1 influence phosphorylation of STAT molecule (Guschin et al., 1995). Abundant researches have recognized that inconsistent initiation of STAT-3 signaling approach put in to neoplastic modification in a mixture of sarcoma and have endorsed STAT-3 as a competent target to treat the cancer (Denley et al., 2013). Cells have the potential to the degeneration the apoptotic array that was attentive as one of the noteworthy mechanism for the expansion of tumors (Hanahan and Weinberg, 2000). Bcl-2 is a feature of as uplifting molecules, which is considerably alarmed to hold down the cell apoptosis. As revealed before STAT-3 is a vital in transcriptional feature that normalize the expression of Bcl-2. Excited STAT-3 was translocated to nucleus that supports the elevated Bcl-2 expression. Consequently Bcl-2 has documented as a sturdy defeater of apoptosis (Yip and Reed, 2008). At this point, we recognized the Ganoderma lucidum interceded apoptotic factors and morphological modifications were identified by AO/EtBr. In current study, Ganoderma lucidum aggravates apoptotic features for example, blubbing or uneven membrane, fragment nucleus in PC-3 cells (Fig. 3). Moreover, we noticed that Ganoderma lucidum orientation persuades Bax, caspase-3 and9 which shows reduced or diminished expressions in the apoptotic pathway.
In addition, Ganoderma lucidum control Bcl-2 elevated expression in PC-3 cells. Earlier reports demonstrates that resveratrol have been trigger apoptosis in SKOV3 and A2780 cells via uplifting the Bax and Caspase-3 and 9 expressions through inflection of starter JAK and transcriptive STAT signaling cascade (Liu et al., 2018). In agreement with this current finding, our investigation also influences apoptosis via the guideline of STAT-3 cascade in PC-3 cells.
5. Conclusion
With the novel findings of this exploration, we conclude that Ganoderma lucidum aggravated cytotoxicity, ROS accretion and apoptosis in PC-3 cells by time reliant manner. Additionally, Ganoderma lucidum hold back STAT-3 translocation in this manner, aggravating the increased cyclin-D1, Bcl-2 expression and condensed Bax, caspase-9 and 3 expressions in PC-3 cells. Eventually Ganoderma lucidum hinder prostate cancer cell proliferation and provoke the apoptosis via reserving and translocation of STAT-3.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ai T., Wang Z., Zhang M., Zhang L., Wang N., Li W., Song L. Expression and prognostic relevance of stat3 and cyclin d1 in non-small cell lung cancer. Int. J. Biol. Markers. 2012;27:e132–e138. doi: 10.5301/JBM.2012.9146. [DOI] [PubMed] [Google Scholar]
- Alpajaro S.I.R., Harris J.A.K., Evans C. Non-metastatic castration resistant prostate cancer: a review of current and emerging medical therapies. Prostate Cancer Prostatic Dis. 2019;22(1):16–23. doi: 10.1038/s41391-018-0078-1. [DOI] [PubMed] [Google Scholar]
- Barbieri Antonio, Quagliariello Vincenzo, Del Vecchio Vitale, Falco Michela, Luciano Antonio, Amruthraj Nagoth Joseph, Nasti Guglielmo, Ottaiano Alessandro, Berretta Massimiliano, Vincenzo Iaffaioli Rosario, Arra Claudio. Anticancer and anti-inflammatory properties of Ganoderma lucidum extract effects on melanoma and triple-negative breast cancer treatment. Nutrients. 2017;9:210. doi: 10.3390/nu9030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale G., Duchen M.R. Investigating the mitochondrial permeability transition pore in disease phenotypes and drug screening. Curr. Protoc. Pharmacol. 2019;85(1):E59. doi: 10.1002/cpph.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang J., Wang X., Liu X., Li H., Lv Q., Zhu J., Wei B., Tang Y. STAT3, a poor survival predicator, is associated with lymph node metastasis from breast cancer. J. Breast Cancer. 2013;16:40–49. doi: 10.4048/jbc.2013.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denley S.M., Jamieson N.B., McCall P., Oien K.A., Morton J.P. Activation of the IL-6R/Jak/stat pathway is associated with a poor outcome in resected pancreatic ductal adenocarcinoma. J. Gastrointest. Surg. 2013;17:887–898. doi: 10.1007/s11605-013-2168-7. [DOI] [PubMed] [Google Scholar]
- Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Gurbuz V., Konac E., Varol N., Yilmaz A., Gurocak S. Effects of AG490 and S3I–201 on regulation of the JAK/STAT3 signaling pathway in relation to angiogenesis in TRAIL-resistant prostate cancer cells in vitro. Oncol. Lett. 2014;7:755–763. doi: 10.3892/ol.2014.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschin Dmitry, Rogers Neil, Briscoe James, Witthuhn Bruce, Watling Diane, Horn Friedemann, Pellegrini Sandra, Yasukawa Kiyoshi, Heinrich Peter, Stark George R., lhle James N., Kerr Ian M. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. The EMBO J. 1995;14(7):1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D., Weinberg, R.A., 2000. The hallmarks of cancer. Cell 7 100(1): 57–70. [DOI] [PubMed]
- Haugen B.R. Management of the patient with progressive radioiodine non-responsive disease. Semin. Surg. Oncol. 1999;16:34–41. doi: 10.1002/(sici)1098-2388(199901/02)16:1<34::aid-ssu7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hongbo H.U., Nam-Shik A.H.N., Xinlin Y.A.N.G., Yong-Soon L.E.E., Kyung-Sun K.A.N.G. Ganoderma Lucidum extract induces cell cycle arrest and apoptosis in Mcf-7 human breast cancer cell. Int. J. Cancer. 2002;102:250–253. doi: 10.1002/ijc.10707. [DOI] [PubMed] [Google Scholar]
- Jan R., Chaudhry G. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019;9(2):205–218. doi: 10.15171/apb.2019.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran M.Z., Patil P., Gude R.P. Role of STAT3 in cancer metastasis and translational advances. Biomed. Res. Int. 2013; 2013. doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C.H.J., Bishop K.S., Xu Y., Han D.Y., Murray P.M., Marlow G.J., Ferguson L.R. Identification of potential anticancer activities of novel ganoderma lucidum extracts using gene expression and pathway network analysis. Genom Insights. 2016;9:1–16. doi: 10.4137/GEI.S32477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku Y.K., Gleave M.E., Beltran H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 2019;16(11):645–654. doi: 10.1038/s41585-019-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Cheung S.T. STAT3: An emerging therapeutic target for hepatocellular carcinoma. Cancers (Basel). 2019;11(11):1646. doi: 10.3390/cancers11111646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.T., Kasper J.D., Bazil J.N., Frisbee J.C., Wiseman R.W. Quantification of mitochondrial oxidative phosphorylation in metabolic disease: application to type 2 diabetes. Int. J. Mol. Sci. 2019;20(21):5271. doi: 10.3390/ijms20215271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K., Voong N., Williams C.R. The synthetic triterpenoid CDDO-imidazole suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin. Cancer Res. 2006;12:4288–4293. doi: 10.1158/1078-0432.CCR-06-0215. [DOI] [PubMed] [Google Scholar]
- Liu X., He Z., Li C.H., Huang G., Ding C., Liu H. Correlation analysis of jak-stat pathway components on prognosis of patients with prostate cancer. Pathol. Oncol. Res. 2012;18:17–23. doi: 10.1007/s12253-011-9410-y. [DOI] [PubMed] [Google Scholar]
- Liu Yu, Tong Lin, Luo Yan, Li Xin, Chen Gaowen, Wang Yifeng. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell Biochem. 2018;1–11 doi: 10.1002/jcb.26822. [DOI] [PubMed] [Google Scholar]
- Martinez-Montemayor M.M., Acevedo R.R., Otero-Franqui E., Cubano L.A., Dharmawardhane S.F. Ganoderma lucidum (Reishi) inhibits cancer cell growth and expression of key molecules in inflammatory breast cancer. Nutr. Cancer. 2011;63:1085–1094. doi: 10.1080/01635581.2011.601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P., Huang Y., Oshima K., Yearsley M., Zhang J., Arnold M., Yu J., Wang L. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit. Rev. Food. Sci. Nutr. 2019;59(6):992–1007. doi: 10.1080/10408398.2018.1537237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilati C., Amessou M., Bihl M.P., Balabaud C., Nhieu J.T., Paradis V., Nault J., Izard T., Bioulac-Sage P., Couchy G. Somatic mutations activating stat3 in human inflammatory hepatocellular adenomas. J. Exp. Med. 2011;208:1359–1366. doi: 10.1084/jem.20110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling A.B., Hwang C. Targeting prosurvival BCL2 signaling through Akt blockade sensitizes castration-resistant prostate cancer cells to enzalutamide. Prostate. 2019;79(11):1347–1359. doi: 10.1002/pros.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperi C., Papavassiliou K.A., Papavassiliou A.G. Pivotal role of STAT3 in shaping glioblastoma immune microenvironment. Cells. 2019;8(11):1398. doi: 10.3390/cells8111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawla P. Epidemiology of prostate cancer. World J. Oncol. 2019;10(2):63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann S.F., Jesch B., Friedrich J., Jomrich G., Maroske F., Birner P. Phosphorylation of signal transducer and activator of transcription 3 (stat3) correlates with her-2 status, carbonic anhydrase 9 expression and prognosis in esophageal cancer. Clin. Exp. Metastasis. 2012;29:615–624. doi: 10.1007/s10585-012-9475-3. [DOI] [PubMed] [Google Scholar]
- Siddiquee Khandaker Al Zaid, Turkson James. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18(2):254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M.S., Soerjomataram I., Forman D. Prostate cancer burden in Central and South America. Cancer Epidemiol. 2016;44(1):S131–S140. doi: 10.1016/j.canep.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Sliva D. Cellular and physiological effects of Ganoderma lucidum (Reishi) Mini. Rev. Med. Chem. 2004;4:873–879. doi: 10.2174/1389557043403323. [DOI] [PubMed] [Google Scholar]
- Slivova V., Zaloga G., DeMichele S.J., Mukerji P., Huang Y.S. Greentea polyphenols modulate secretion of urokinase plasminogen activator (uPA) and inhibit invasive behavior of breast cancer cells. Nutr. Cancer. 2005;52:66–73. doi: 10.1207/s15327914nc5201_9. [DOI] [PubMed] [Google Scholar]
- Sun Xin-zhi, Liao Ying, Li Wei, Guo Li-mei. Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis. Neural Regeneration Res. 2017;12(6) doi: 10.4103/1673-5374.208590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M.S.Y., Sandanaraj E., Chong Y.K., Lim S.W., Koh L.W.H., Ng W.H., Tan N.S., Tan P., Ang B.T., Tang C. A STAT3-based gene signature stratifies glioma patients for targeted therapy. Nat. Commun. 2019;10:3601. doi: 10.1038/s41467-019-11614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa U., Castelli G., Pelosi E. Cellular and molecular mechanisms underlying prostate cancer development: therapeutic inmplications. Medicines (Basel). 2019;6(3):82. doi: 10.3390/medicines6030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacquier B., Combettes L., Dupont G. Cytoplasmic and mitochondrial calcium signaling: a two-way relationship. Cold Spring Harb. Perspect. Biol. 2019;11(10) doi: 10.1101/cshperspect.a035139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P. Tumor-specific induction of the intrinsic apoptotic pathway- a new therapeutic option for advanced prostate cancer? Front. Oncol. 2019;9:590. doi: 10.3389/fonc.2019.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., Lai, Y., Hua, Z., 2019. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 39(1): BBSR20180992. [DOI] [PMC free article] [PubMed]
- Yip, K.W., Reed, J.C., 2008. Bcl-2 family proteins and cancer. Oncogene 27(50): 6398–6406. [DOI] [PubMed]
- Yore M.M., Liby K.T., Honda T. The synthetic triterpenoid 1-[2-cyano3, 12-dioxooleana-1,9 (11)-dien-28-oyl)-dien-28-oyl] imidazole blocks nuclear factor-jB activation through direct inhibition of IjB kinase b. Mol. Cancer Ther. 2006;5:3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- Yuen J.W.M., Gohel M.D.I. Anticancer effects of Ganoderma lucidum: a review of scientific evidence. Nutr. Cancer. 2005;53:11–17. doi: 10.1207/s15327914nc5301_2. [DOI] [PubMed] [Google Scholar]