Clinical Practice Points.
-
•
The coronavirus disease 2019 (COVID-19) pandemic has proved to have significant morbidity and mortality in high-risk patient populations, including those with cancer.
-
•
Chronic lymphocytic leukemia (CLL) is an indolent disease, and most patients with CLL undergo follow-up evaluations to monitor their status.
-
•
We have presented a case of a patient with CLL and severe COVID-19 who had presented with marked lymphocytosis within 2 to 3 days of the diagnosis.
-
•
An 80-year-old man with treatment-naive CLL was admitted to our hospital for COVID-19 pneumonia.
-
•
He was intubated because of hypoxic respiratory failure and developed multisystem organ failure requiring hemodialysis and vasopressor therapy.
-
•
His white blood cell count and absolute lymphocyte count had increased to more than threefold his baseline value during hospitalization.
-
•
To the best of our knowledge, the present report is the second case of “COVID-19–induced lymphocytosis” in patients with treatment-naive CLL with severe COVID-19 infection.
-
•
The ethology and prognosis of this new phenomenon is not clear.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has proved to have significant morbidity and mortality in many different patient populations. Patients with hematologic malignancies, especially those with lymphocytic leukemia, have a high risk of infection owing to the underlying immunodeficiency and inadequate immune response to infection. Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in adults in the United States.1 CLL is an indolent disease, and most patients with CLL will not benefit from immediate treatment and receive monitoring. At present, very limited data are available regarding the clinical course of patients with CLL who develop COVID-19.
We have described the case of a patient with CLL who had developed severe COVID-19 and had presented with marked lymphocytosis within 2 to 3 days of the COVID-19 diagnosis.
Case Report
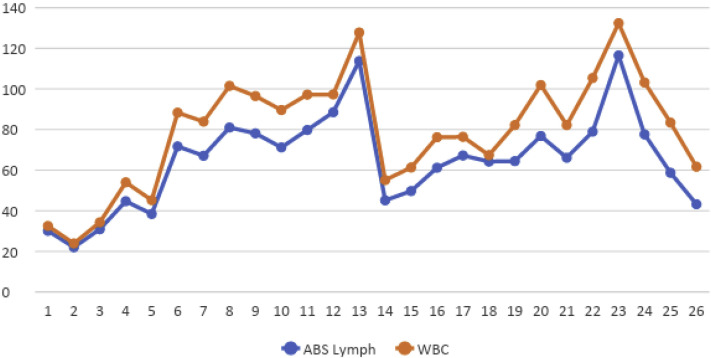
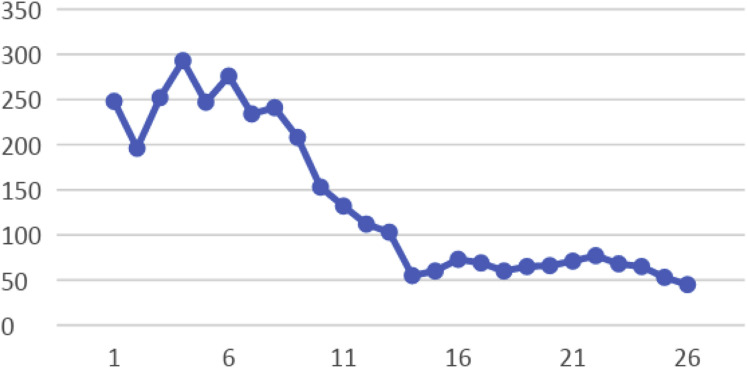
An 80-year-old man had presented to the emergency room with a 2-day history of productive cough and shortness of breath. His medical history included chronic hypertension, controlled asthma, gastroesophageal reflux disease, and stage 0 CLL. The CLL had originally been diagnosed in 2002, and the patient received active surveillance. He had never required treatment of his CLL. At 4 months before the present hospitalization, his CLL had been stable with a white blood cell (WBC) count of 43 × 103/μL. He was asymptomatic, with no evidence of lymphadenopathy seen on a computed tomography scan. In the May 2020 hospitalization, his oxygen saturation was 84% on room air and bilevel positive airway pressure was instituted. A chest radiograph showed diffuse bilateral opacities, and an arterial blood gas test showed that the patient was likely in acute hypoxic respiratory failure. The troponin, ferritin, and C-reactive protein levels were 243 pg/mL (reference range, 0-20 pg/mL), 761 ng/mL, and 251 mg/L (reference, > 3 mg/L, high risk), respectively. The D-dimer level was markedly elevated at 22,891 ng/mL (reference, < 500 ng/mL). The complete blood count showed a WBC count of 32.5 × 103/μL, absolute lymphocyte count (ALC) of 30.1 × 103/μL, hemoglobin of 12.3 g/dL, and platelet count of 248 × 103/μL. The COVID-19 test was positive using polymerase chain reaction. He was intubated on day 4 because of worsening hypoxic respiratory failure. In addition, he developed multisystem organ failure with acute kidney injury requiring hemodialysis and septic shock requiring vasopressor therapy. He received tocilizumab 800 mg on day 2, and remdesivir was initiated on day 3 (200 mg on day 3, followed by 100 mg daily for a total of 7 days). On day 8, his WBC count had increased to 101.5 × 103/μL with an ALC of 81.0 × 103/μL (Table 1 ). On day 13, the WBC had increased to 127.8 × 103/μL, with an ALC of 113.7 × 103/μL (Figures 1). Methylprednisolone was started at a dose of 100 mg every 8 hours on day 13 and tapered to 50 mg every 8 hours for days 19 to 24 and then to 40 mg every 12 hours for days 25 to 27. After the initiation of steroids, his WBC count had decreased to 55 × 103/μL but had gradually increased again, reaching a peak of 132.4 × 103/μL, with an ALC of 116 × 103/μL, on day 23. Changes in platelet count are shown in Figure 2. Immunoglobulin tests during this admission showed low normal IgA and IgG levels and decreased IgM levels (<20 mg/dL). Additional workup revealed elevated β2-microglobulin at 0.930 mg/dL (reference range, 0.097-0.184 mg/dL). Peripheral flow cytometry showed a monoclonal B-cell population with lambda light chain restriction comprising 81% of the total live cellular events). Fluorescence in situ hybridization showed deletion of 13q14 and IgVH (immunoglobulin variable region heavy chain) mutation positivity, which has been associated with a more favorable clinical course. However, after a long period of hospitalization, his family decided against resuscitation, and he died of COVID-19 on day 27 of admission. The patient’s wife (power of attorney and next of kin) provided consent to report the case of our patient.
Table 1.
Laboratory Values During Hospitalization
| Test | Day |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 6 | 7 | 9 | 12 | 13 | 14 | 15 | 16 | 20 | 23 | 24 | 26 | |
| ALC, × 103/μL | 30.1 | 30.9 | 44.7 | 71.7 | 67.1 | 78.1 | 88.54 | 113.74 | 45.1 | 49.7 | 61.2 | 76.8 | 116.51 | 77.6 | 43.2 |
| WBCC, × 103/μL | 32.5 | 34.3 | 54 | 88.3 | 83.9 | 96.5 | 97.3 | 127.8 | 55.1 | 61.3 | 76.2 | 101.9 | 132.4 | 103.2 | 61.7 |
Abbreviations: ALC = absolute leukocyte count; WBCC = white blood cell count.
Figure 1.
Graph Showing White Blood Cell (WBC) Count and Absolute (ABS) Lymphocyte (Lymph) Count Changes During Hospitalization
Figure 2.
Graph Showing Decreases in Platelet Count During Hospitalization
Discussion
Lymphopenia, in contrast to leukocytosis, is a common laboratory finding in patients with COVID-19. It will be more significant in those with severe disease and has been found to be a poor prognostic factor.2 , 3 In patients with severe COVID-19, Liu et al4 found an initial phase of a lymphocyte count decrease, followed by a gradual increase in the lymphocyte count from day 7 to 15 after disease onset. Elevation of the lymphocyte count in their study was toward resolution of the lymphopenia but without apparent leukocytosis. However, the course and severity of the lymphocyte count elevation in our patient did not follow the same pattern.
One hypothesis for our patient’s lymphocytosis was that it had resulted from the effects of the antiviral (remdesivir) and interleukin-6 receptor antagonist (tocilizumab) treatment on the lymphocyte count. In a recent randomized trial of the efficacy of remdesivir in 233 patients with severe COVID-19, no differences were found in the rate of a remdesivir-induced increased WBC count compared with placebo (7% in the remdesivir group vs. 8% in the placebo group).5 In addition, 68% of the patients in that trial had had COVID-19–related leukopenia before the administration of remdesivir.5
A prospective, single-arm study reported minimal improvement in lymphopenia in patients with severe COVID-19 after receipt of tocilizumab.6 However, compared with the non–tocilizumab groups in the SMACORE (SMAtteo COVID-19 REgistry) trial of patients with severe COVID-19 pneumonia, no significant difference was detected.7 The results from these studies have shown that the marked elevation of the lymphocyte count in our patient (>3 times his baseline) cannot be attributed to either tocilizumab or remdesivir. Liu et al4 reported a nonsignificant elevation in the lymphocyte count and a nonsignificant decrease in the neutrophil count of patients with COVID-19 after receiving methylprednisolone. The effect of methylprednisolone in our patient was in the opposite direction, and his lymphocyte count had decreased suddenly after the first dose of methylprednisolone but then gradually increased again.
A recent report from the University of Birmingham, United Kingdom, documented a three-to fourfold increase in the lymphocyte count of 4 patients with treatment-naive CLL with severe COVID-19 infection.8 The investigators described this phenomenon as “COVID-19–induced lymphocytosis.” All the patients in their study had received supportive care, including antibiotics. In the first patient in their study, the ALC had increased sixfold to 202 × 103/μL at day 3 but had declined to baseline at day 10, and the patient’s condition improved. Other patients had experienced a three-to fourfold increase in their ALC, and they died. In 1 of these patients, the ALC had increased from 2.8 × 103/μL at baseline to 8 × 103/μL. The underlying mechanism of this phenomenon has not yet been explained.
It is not clear whether a sudden increase in the lymphocyte count can be used as a prognostic factor in patients with treatment-naive CLL and acute COVID-19 infection. Also, not all patients with treatment-naive CLL will have similar outcomes. A report from a hospital in Barcelona described 4 patients with CLL and acute COVID-19 infection.9 Two of these patients had never received treatment of their CLL.9 Although no comparison with the baseline lymphocyte count was provided in their report, both patients had recovered from their acute COVID-19 infection.
Deletion of 13q is the only chromosomal abnormality in patients with CLL associated with a favorable prognosis and the longest median survival.10 Despite the early disease stage, treatment-naive status, absence of lymphadenopathy, favorable cytogenetics, and stable cell counts before infection with COVID-19, our patient’s lymphocyte counts had increased more than threefold, with the resulting poor outcome. To the best of our knowledge, the present report is the second description of the new phenomenon COVID-19–induced lymphocytosis (or COVID-19–induced hyperlymphocytosis). The elevated β2-macroglobulin in our patient had resulted from acute kidney injury and an increase in creatinine and should not be used as a disease severity index.
Regarding the continuation of CLL therapy, some experts have agreed to continue only Bruton tyrosine kinase (BTK) inhibitors and withhold other CLL therapies, even for patients with active COVID-19 infection.11 However, this is a totally different situation for patients with CLL who have not received treatment of CLL and have developed COVID-19–induced lymphocytosis. A case series reported the beneficial effect of BTK inhibitors in 6 patients receiving ibrutinib for Waldenström macroglobulinemia with a diagnosis of COVID-19.12 Five of the patients had had only mild symptoms and had not required hospitalization. One patient’s ibrutinib had been stopped during their admission, and, subsequently, the patient had developed dyspnea and oxygen desaturation. It was later resumed, followed by a dose escalation. The patient’s oxygen saturation then improved, and the patient recovered from acute COVID-19. The investigators suggested that BTK inhibitors can be protective against COVID-19–induced lung injury owing to their role in the reduction of proinflammatory cytokines in lung tissue. Another study also found similar beneficial effects in patients who had continued BTK inhibitors during hospitalization for COVID-19.13 Two phase II clinical trials are investigating the possible beneficial effect of BTK inhibitors (acalabrutinib and zanubrutinib) in patients with COVID-19 (ClinicalTrials.gov identifiers, NCT04346199 and NCT04382586). Considering a large population of patients with CLL under observation, it would not be surprising to encounter additional similar cases in the future. If these trials have promising results, one might consider initiating a BTK inhibitor in a patient with COVID-19–induced lymphocytosis/hyperlymphocytosis.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.American Cancer Society . American Cancer Society; Atlanta: 2020. Cancer Facts & Figures 2020.https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang K., Zuo P., Liu Y. Clinical and laboratory predictors of in-hospital mortality in patients with COVID-19: a cohort study in Wuhan, China [e-pub of print] https://doi.org/10.1093/cid/ciaa538 Clin Infect Dis. Accessed August 02, 2020. [DOI] [PMC free article] [PubMed]
- 3.Sun S., Cai X., Wang H. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Li S., Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [correction in Lancet 2020; 395:1694] Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciascia S., Aprà F., Baffa A. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 7.Colaneri M., Bogliolo L., Valsecchi P. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8:695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paneesha S., Pratt G., Parry H., Moss P. COVID-19 infection in therapy-naive patients with B-cell chronic lymphocytic leukemia. Leuk Res. 2020;93:106366. doi: 10.1016/j.leukres.2020.106366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann T., Delgado J., Montserrat E. CLL and COVID-19 at the Hospital Clinic of Barcelona: an interim report. Leukemia. 2020;34:1954–1956. doi: 10.1038/s41375-020-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Döhner H., Stilgenbauer S., Benner A. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 11.Koffman B., Mato A., Byrd J.C. Management of CLL patients early in the COVID-19 pandemic: an international survey of CLL experts. Am J Hematol. 2020;95:E199–E203. doi: 10.1002/ajh.25851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treon S.P., Castillo J.J., Skarbnik A.P. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thibaud S., Tremblay D., Bhalla S., Zimmerman B., Sigel K., Gabrilove J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br J Haematol. 2020;190:e73–e76. doi: 10.1111/bjh.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]