Abstract
Studies investigating association of depression with overall survival (OS) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) yielded conflicting results. A nationwide cohort study, which included all adult patients [n = 7,170; depression group, 13.3% (N = 956); non-depression group, 86.7% (N = 6,214)] who received allo-HSCT from 2002 to 2018 in South Korea, analyzed risk of pre-transplant depression in OS of allo-HSCT. Subjects were followed from the day they received allo-HSCT, to occurrence of death, or last follow-up day (December 31, 2018). Median age at allo-HSCT for depression and non-depression groups were 50 and 45 (p < 0.0001), respectively. Two groups also differed in rate of females (depression group, 55.8%; non-depression group, 43.8%; p < 0.0001) and leukemia (depression group, 61.4%; non-depression group, 49.7%; p < 0.0001). After a median follow-up of 29.1 months, 5-year OS rate was 63.1%. Cox proportional-hazard regression evaluated an adjusted risk of post-transplant mortality related to depression: OS decreased sequentially from no depression (adjusted hazard ratio [aHR] = 1) to pre-transplant depression only (aHR = 1.167, CI: 1.007–1.352, p = 0.04), and to having both depression and anxiety disorder (aHR = 1.202, CI: 1.038–1.393, p = 0.014) groups. Pre-transplant anxiety (anxiety only) did not have significant influence in OS. Additional medical and psychiatric care might be necessary in patients who experienced depression, especially with anxiety, before allo-HSCT.
Subject terms: Neurology, Oncology, Risk factors, Signs and symptoms
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) provides curative therapy for patients with malignant and nonmalignant hematologic disorders1,2. However, allo-HSCT is a highly aggressive and demanding medical supports with a significant risk of mortality3. Besides primary hematologic disorder and disease relapse after transplantation, transplant-related mortality is an important obstacle influencing treatment outcome of allo-HSCT4. Thus, careful assessment of risk and accurate prediction of mortality are necessary before undertaking allo-HSCT5.
Depression is a common comorbidity of oncological and chronic hematologic diseases6. Studies showed that more than 20% of patients receiving allo-HSCT experience clinically significant depression before undergoing transplantation7. Besides its negative impact on the quality of life, depression is also associated with poor treatment survival outcome in patients receiving allo-HSCT8. Thus, most stem cell transplantation centers require patients to receive a comprehensive psychiatric evaluation as a part of the pre-transplant process9. Numerous prognostic scoring systems recognizes depression as an important risk factor of post-transplant mortality10. The Hematopoietic cell transplantation-specific comorbidity index (HCT-CI), one of the most important prognostic scoring systems in allo-HSCT, acknowledged that depression increases post-transplant mortality with hazard ratio of 1.811.
Studies investigating the impact of pre-transplant depression on overall survival (OS) of patients receiving allo-HSCT have yielded conflicting results12,13. A study comprising of 123 patients suggested that existing diagnosis of depression before allo-HSCT were not related to post-transplant OS14. In contrast, a more recent study showed that, among 190 patients receiving allo-HSCT, depression before transplantation predicted higher 1-year (hazard ratio [HR] = 2.59; p = 0.014) and 3-year mortality (HR = 2.04; 95% p = 0.041)15. Thus, studies containing larger cohorts are needed to confirm temporal association between pre-transplant depression and OS of allo-HSCT.
A recent large sized cohort (n = 7,433) study, using data from the Center for International Blood and Marrow Transplant Research (CIBMTR), showed that pre-transplant depression was associated with lower OS (HR = 1.13; 95% confidence interval [CI], 1.04–1.23; p = 0.004)16. However, the study neither used a validated measurement nor diagnostic assessment to define pre-transplant depression. Rather, the study merely used retrospective chart review of a single question reported in the CIBMTR database; was there “clinically significant depression requiring treatment?” Inevitably, the diagnosis of depression was prone to reporter bias. To overcome this bias, the study excluded transplant centers having more than 10 transplants without reports of both depressed and nondepressed patients to the registry. By doing so, selection bias became another important limitation. Co-occurrence of anxiety disorder and depression are very common in patients receiving allo-HSCT17. By neglecting possible overlapping impact of pre-transplant anxiety, it is still uncertain whether depression truly increased post-transplant mortality independent from the anxiety. Whether anxiety disorder causes additive risk in post-transplantation mortality risk among patients with depression history is another important issue needing investigation. Patients with leukemia are known to have a higher post-transplant mortality than patients with non-malignant hematologic disease18,19. Thus, subgroup analysis comprising more homogenous group, leukemia patients only, are needed to address detrimental effect of pre-transplant depression in OS of allo-HSCT. Lastly, CIBMTR is an US based registry, so studies containing a large sample size must also be repeated in diverse ethnicities to confirm impact of pre-transplant depression in outcomes of allo-HSCT.
To fill in this important gap, we conducted a nationwide longitudinal cohort study using the National Healthcare Insurance Service (NHIS) database containing data of majority of allo-HSCTs performed in South Korea (hereafter “Korea”). We first replicated previous findings by investigating association of pre-transplant depression in the long-term survival of allo-HSCT in Asian population (Koreans). Beyond replication, we extended previous work by analyzing independent risk of pre-transplant depression, separating possible overlapping effect of anxiety, in OS of allo-HSCT. Whether negative impact of pre-transplant depression in allo-HSCT is augmented by co-morbid anxiety was also evaluated. In addition, sub-group analysis of patients with leukemia was conducted to confirm risk of pre-transplant depression in allo-HSCT among higher risk group. Lastly, landmark analyses were performed at 100 days post-transplant to exclude patients at high risk of early mortality post-transplant and to evaluate the impact of prognostic variables on OS of patients still alive at these time points.
Results
Participant characteristics
Between January 2002 and December 2018, a total of 7,170 adult patients who received allo-HSCT were included in this study (Table 1). 13.3% (N = 956) were diagnosed with and visited hospital due to depression before transplantation whereas 86.7% (N = 6,214) did not. The mean duration of depression and anxiety before transplantations were 609.4 ± 569.3 days (median: 414.5 days) and 710.9 ± 587.5 days (median: 607 days) respectively. The median age at allo-HSCT was higher for depression group than non-depression group. Depression group had more subjects who were females, had leukemia, received peripheral blood as stem cell source, and had previous non-hematologic malignancies. Likewise, diagnosis of anxiety, hypertension, diabetes, dyslipidemia, chronic obstructive pulmonary disease, and cerebro- or cardiovascular disease were also higher in depression group than in non-depression group.
Table 1.
Basal demographical and clinical characteristics of adult patients who received allo-HSCT.
| Variables | Total cohort (N = 7,170) |
History of depression before allogeneic-HSCT |
||
|---|---|---|---|---|
| Yes (N = 956) | No (N = 6,214) | p-value | ||
| Median age at transplant, year (range) | 45 | 50 | 44 | < 0.0001 |
| Age ≤ median ( 45 years old) | 3,664 (51.1%) | 359 (37.6%) | 3,305 (53.2%) | |
| Age > median ( 45 years old) | 3,506 (48.9%) | 597 (62.4%) | 2,909 (46.8%) | |
| Gender | < 0.0001 | |||
| Male, no (%) | 3,917 (54.6%) | 423 (44.2%) | 3,494 (56.2%) | |
| Hematologic disease | < 0.0001 | |||
| Leukemia. no (%) | 3,676 (51.3%) | 587 (61.4%) | 3,089 (49.7%) | |
| Hodgkin lymphoma, no (%) | 23 (0.3%) | 6 (0.6%) | 17 (0.3%) | |
| Non-Hodgkin lymphoma, no (%) | 374 (5.2%) | 75 (7.8%) | 299 (4.8%) | |
| Multiple myeloma, no (%) | 144 (2.0%) | 31 (3.2%) | 113 (1.8%) | |
| MPN, no (%) | 68 ( 0.9%) | 11 (1.2%) | 57 (0.9%) | |
| MDS, no (%) | 663 (9.2%) | 68 (7.1%) | 595 (9.6%) | |
| Aplastic anemia, no (%) | 464 (6.4%) | 41 (4.3%) | 423 (6.8%) | |
| Unclassified, no (%) | 1758 (24.5%) | 137 (14.3%) | 1621 (26.1%) | |
| Stem cell source | < 0.0001 | |||
| Bone marrow, no (%) | 747 (10.4%) | 77 (8.1%) | 670 (10.8%) | |
| Peripheral blood, no (%) | 4,752 (66.3%) | 730 (76.4%) | 4,022 (64.7%) | |
| Unclassified, no (%) | 1671 (23.3%) | 149 (15.6%) | 1522 (24.5%) | |
| Anxiety | < 0.0001 | |||
| No, no (%) | 5,752 (80.2%) | 482 (50.4%) | 5,270 (84.8%) | |
| Yes, no (%) | 1,418 (19.8%) | 474 (49.6%) | 944 (15.2%) | |
| Previous non-hematologic malignancy | < 0.0001 | |||
| No, no (%) | 6,482 (90.4%) | 818 (85.6%) | 5,664 (91.1%) | |
| Yes, no (%) | 688 (9.6%) | 138 (14.4%) | 550 (8.9%) | |
| Hypertension | < 0.0001 | |||
| No, no (%) | 5,168 (72.1%) | 585 (61.2%) | 4,583 (73.8%) | |
| Yes, no (%) | 2002 (27.9%) | 371 (38.8%) | 1631 (26.2%) | |
| Diabetes | < 0.0001 | |||
| No, no (%) | 5,284 (73.7%) | 600 (62.8%) | 4,684 (75.4%) | |
| Yes, no (%) | 1886 (26.3%) | 356 (37.2%) | 1,530 (24.6%) | |
| Dyslipidemia | < 0.0001 | |||
| No, no (%) | 3,736 (52.1%) | 364 (38.1%) | 3,372 (54.3%) | |
| Yes, no (%) | 3,434 (47.9%) | 592 (61.9%) | 2,842 (45.7%) | |
| COPD | < 0.0001 | |||
| No, no (%) | 6,834 (95.3%) | 887 (92.8%) | 5,947 (95.7%) | |
| Yes, no (%) | 336 (4.7%) | 69 (7.2%) | 267 (4.3%) | |
| CVD | < 0.0001 | |||
| No, no (%) | 6,905 (96.3%) | 895 (93.6%) | 6,010 (96.7%) | |
| Yes, no (%) | 265 (3.7%) | 61 (6.4%) | 204 (3.3%) | |
COPD Chronic obstructive pulmonary disease; CVD Cerebro- or cardiovascular disease; HSCT Hematopoietic stem cell transplantation; MDS Myelodysplastic syndrome; MPN myeloproliferative neoplasm.
Survival outcome: total cohort (N = 7,170)
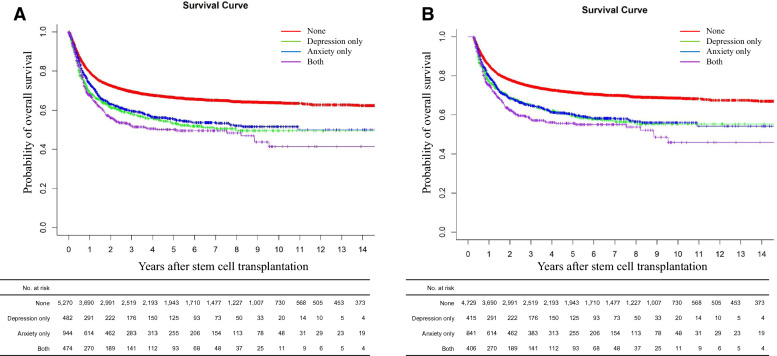
After a median follow-up of 29.1 months (range 0 to 207), the estimated overall survival (OS) rates at 5 years were 63.1% (supplementary Fig. 1). Supplementary Table 1 summarizes the unadjusted analyses comparing allo-HCT outcomes between depression and non-depression group. Patients with pre-transplant depression (depression only group) and anxiety (anxiety only group) had lower 5 year-OS than those who neither had depression nor anxiety (none group), and 5 year-OS was the lowest in patients having both depression and anxiety (both depression and anxiety group) (Fig. 1A).
Figure 1.
Overall survival outcome difference according to depression and or anxiety history; (A) in total cohort (B) in landmark cohort at the designated 100 days post-transplant.
The factors found to be significant in the univariable analysis were all included in the final multivariable analysis (Table 2). The post-transplantation OS decreased sequentially from none group (adjusted hazard ratio [aHR] = 1) to depression only group (aHR = 1.167, CI: 1.007–1.352, p = 0.04), and to both depression and anxiety group (aHR = 1.202, CI: 1.038–1.393, p = 0.014). However, anxiety only group did not have significant influence in OS.
Table 2.
Multivariable analysis of factors affecting overall survival of allo-HSCT in all patients (N = 7,170).
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Age | ||
| Age ≤ median (45 years old) | 1 | |
| Age > median (45 years old) | 1.249 (1.143–1.367) | < 0.0001 |
| Sex | ||
| Female | 1 | |
| Male | 1.151 (1.060–1.250) | < 0.0001 |
| Pre-transplantation depression | ||
| No without anxiety (none) | 1 | |
| Yes without anxiety (depression only) | 1.167 (1.007–1.352) | 0.040 |
| No with anxiety (anxiety only) | 1.038 (0.927–1.162) | 0.519 |
| Yes with anxiety (depression and anxiety) | 1.202 (1.038–1.393) | 0.014 |
| Hematologic disease | ||
| Aplastic anemia | 1 | |
| MDS/MPN | 2.083 (1.611–2.693) | < 0.0001 |
| Leukemia/all lymphomas/Multiple myeloma | 3.063 (2.435–3.853) | < 0.0001 |
| Stem cell source | ||
| Bone marrow | 1 | |
| Peripheral blood | 1.457 (1.268–1.675) | < 0.0001 |
| Previous other malignancy | ||
| No | 1 | |
| Yes | 1.188 (1.052–1.343) | 0.006 |
| Hypertension | ||
| No | 1 | |
| Yes | 1.260 (1.152–1.377) | < 0.0001 |
| Diabetes | ||
| No | 1 | |
| Yes | 1.080 (0.985–1.183) | 0.099 |
| Dyslipidemia | ||
| No | 1 | |
| Yes | 1.030 (0.985–1.123) | 0.509 |
| COPD | ||
| No | 1 | |
| Yes | 1.065 (0.893–1.270) | 0.486 |
| CVD | ||
| No | 1 | |
| Yes | 1.438 (1.206–1.715) | < 0.0001 |
COPD Chronic obstructive pulmonary disease; CVD Cerebro- or cardiovascular disease; HSCT Hematopoietic stem cell transplantation; MDS Myelodysplastic syndrome; MPN myeloproliferative neoplasm.
Survival outcome: landmark cohort (N = 6,391)
Among 7,170 adult patients who received allo-HSCT, 6,391 patients were alive at the designated 100 days landmark and were included in the landmark analysis (supplementary Fig. 2 for OS). In line with total cohort, depression only group and anxiety only group had lower 5 year-OS, with the both depression and anxiety group having the lowest 5 year-OS (Fig. 1B). The factors found to be significant in the univariable analysis (supplementary Table 2) were all included in the final multivariable analysis (Table 3). However, the multivariable analysis of the landmark cohort showed that the OS after transplantation was significantly lower only in both depression and anxiety group (aHR = 1.253, CI: 1.061–1.479, p < 0.001), and neither depression only nor anxiety only group showed statistical significance.
Table 3.
Multivariable analysis of factors affecting overall survival of allo-HSCT in landmark cohort (N = 6,391).
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Age | ||
| Age ≤ median (45 years old) | 1 | |
| Age > median (45 years old) | 1.265 (1.144–1.400) | < 0.0001 |
| Sex | ||
| Female | 1 | |
| Male | 1.196 (1.089–1.312) | < 0.0001 |
| Pre-transplantation depression | ||
| None | 1 | |
| Depression only | 1.155 (0.975–1.369) | 0.0947 |
| Anxiety only | 1.083 (0.955–1.229) | 0.2149 |
| Depression and anxiety | 1.253 (1.061–1.479) | < 0.001 |
| Hematologic disease | ||
| Aplastic anemia | 1 | |
| MDS/MPN | 3.006 (2.156–4.191) | < 0.0001 |
| Leukemia/all lymphomas/Multiple myeloma | 4.785 (3.530–6.487) | < 0.0001 |
| Stem cell source | ||
| Bone marrow | 1 | |
| Peripheral blood | 1.416 (1.215–1.651) | < 0.0001 |
| Previous other malignancy | ||
| No | 1 | |
| Yes | 1.109 (0.960–1.281) | 0.1597 |
| Hypertension | ||
| No | 1 | |
| Yes | 1.208 (1.091–1.338) | < 0.0001 |
| Diabetes | ||
| No | 1 | |
| Yes | 1.104 (0.995–1.225) | 0.0609 |
| Dyslipidemia | ||
| No | 1 | |
| Yes | 1.040 (0.943–1.148) | 0.4536 |
| COPD | ||
| No | 1 | |
| Yes | 1.009 (0.822–1.240) | 0.9288 |
| CVD | ||
| No | 1 | |
| Yes | 1.448 (1.180–1.778) | < 0.0001 |
COPD Chronic obstructive pulmonary disease; CVD Cerebro- or cardiovascular disease; HSCT Hematopoietic stem cell transplantation; MDS Myelodysplastic syndrome; MPN myeloproliferative neoplasm.
Subgroup analysis of patients with leukemia
In the multivariable analysis of total and landmark cohort, hematologic disease had the highest aHR (2.083–3.063 for total cohort and 3.006–4.785 for landmark cohort) (Tables 2, 3). In order to completely eliminate disease as a co-factor, we conducted sub-group analysis in patients with a more homogenous group, leukemia only. A total of 3,809 patients received allo-HSCT due to leukemia, and their baseline demographic and clinical data are summarized in Table 4, and OSs are shown in supplementary Fig. 3. Same trend was noted for univariable analyses (supplementary Table 3) and multivariable model (Table 5). Unadjusted analyses showed that depression only group, anxiety only group, and both depression and anxiety group all had lower 5 year-OS than none group (Fig. 2). Once again, the association was not detected for anxiety only group after adjusting for significant variables. In line with the total cohort, OS decreased sequentially from none group (aHR = 1) to depression only group (aHR = 1.220, CI: 1.031–1.444, p = 0.021), and to both depression and anxiety disorder group (aHR = 1.238, CI: 1.046–1.465, p = 0.0132).
Table 4.
Baseline demographical and clinical characteristics of adult patients who received allo-HSCT due to leukemia.
| Variables | Cohort (N = 3,809) | History of depression | p-value | |
|---|---|---|---|---|
| Yes (N = 613) | No (N = 3,196) | |||
| Median age at transplant, year (range) | 47 (18–74) | 49(18–72) | 46(18–74) | < 0.0001 |
| Under 47, no (%) | 1809 (52.2%) | 344 (56.1%) | 1,465 (45.8%) | |
| Over 47, no (%) | 2000 (47.8%) | 269 (43.9%) | 1731 (54.2%) | |
| Gender | ||||
| Male, no (%) | 2055 (54.0%) | 255 (41.6%) | 1,800 (56.3%) | < 0.0001 |
| Anxiety | ||||
| No | 2,909 (76.4%) | 305 (49.8%) | 2,604 (81.5%) | |
| Yes | 900 (23.6%) | 308 (50.2%) | 592 (18.5%) | |
| Stem cell source | 0.114 | |||
| Bone marrow, no (%) | 469 (12.3%) | 62 (10.1%) | 407 (12.7%) | |
| Peripheral blood, no (%) | 3,246 (85.2%) | 534 (87.1%) | 2,712 (84.9%) | |
| Unclassified, no (%) | 94 (2.5%) | 17 (2.8%) | 77 (2.4%) | |
| Previous non-hematologic malignancy | 0.010 | |||
| No, no (%) | 3,422 (89.8%) | 528 (86.1%) | 2,894 (90.6%) | |
| Yes, no (%) | 387 (10.2%) | 85 (13.9%) | 302 (9.4%) | |
| Hypertension | 0.005 | |||
| No, no (%) | 1,224 (32.1%) | 378 (61.7%) | 2,207 (69.1%) | |
| Yes, no (%) | 1,224 (32.1%) | 235 (38.3%) | 989 (30.9%) | |
| Diabetes | 0.002 | |||
| No, no (%) | 2,684 (70.5%) | 400 (65.3%) | 1,446 (45.2%) | |
| Yes, no (%) | 1,125 (29.5%) | 213 (34.7%) | 912 (28.5%) | |
| Dyslipidemia | < 0.0001 | |||
| No, no (%) | 1674 (43.9%) | 228 (37.2%) | 1,446 (45.2%) | |
| Yes, no (%) | 2,135 (56.1%) | 385 (62.8%) | 1,750 (54.8%) | |
| COPD | 0.022 | |||
| No, no (%) | 3,618 (95.0%) | 570 (93.0%) | 3,048 (95.4%) | |
| Yes, no (%) | 191 (5.0%) | 43 (7.0%) | 148 (4.6%) | |
| CVD | 0.079 | |||
| No, no (%) | 3,643 (95.6%) | 579 (94.5%) | 3,064 (95.9%) | |
| Yes, no (%) | 166 (4.4%) | 34 (5.5%) | 132 (4.1%) | |
COPD Chronic obstructive pulmonary disease; CVD Cerebro- or cardiovascular disease; HSCT Hematopoietic stem cell transplantation; MDS Myelodysplastic syndrome; MPN myeloproliferative neoplasm.
Table 5.
Multivariable analysis of factors affecting overall survival of patients receiving allogeneic hematopoietic stem cell transplantation due to leukemia (all leukemia, N = 3,809).
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Age | ||
| Age ≤ median (47 years old) | 1 | |
| Age > median (47 years old) | 1.185 (1.068–1.314) | < 0.0001 |
| Sex | ||
| Female | 1 | |
| Male | 1.144 (1.040–1.259) | 0.006 |
| Pre-transplantation depression | ||
| Neither depression nor anxiety (none) | 1 | |
| Depression without anxiety (depression only) | 1.220 (1.031–1.444) | 0.021 |
| Anxiety without depression (anxiety only) | 1.107 (1.031–1.259) | 0.1244 |
| Both depression and anxiety (depression and anxiety) | 1.238 (1.046–1.465) | 0.0132 |
| Stem cell source | ||
| Bone marrow | 1 | |
| Peripheral blood | 1.498 (1.285–1.746) | < 0.0001 |
| Previous other malignancy | ||
| No | 1 | |
| Yes | 1.161 (0.997–1.352) | 0.0545 |
| Hypertension | ||
| No | 1 | |
| Yes | 1.162 (1.046–1.291) | 0.005 |
| Diabetes | ||
| No | 1 | |
| Yes | 0.995 (0.893–1.109) | 0.9288 |
| Dyslipidemia | ||
| No | 1 | |
| Yes | 1.055 (0.954–1.167) | 0.2961 |
| Chronic obstructive pulmonary disease | ||
| No | 1 | |
| Yes | 1.033 (0.832–1.281) | 0.7704 |
| Cerebro- or cardiovascular disease | ||
| No | 1 | |
| Yes | 1.510 (1.224–1.861) | < 0.0001 |
Figure 2.

Overall survival outcome difference according to depression and or anxiety history in patients with leukemia.
Discussion
Our results showed that pre-transplant depression, independent from anxiety, decreased OS of adult patients receiving allo-HSCT. We also showed that co-occurrence of depression with anxiety disorder further decreased post-transplant OS, but pre-transplantation anxiety disorder did not independently increase mortality after allo-HSCT. In terms of sub-group analysis, same trend was found in patients who received allo-HSCT due to leukemia. The post-transplantation OS decreased sequentially from neither depression nor anxiety group (none; aHR = 1) to having depression only (aHR = 1.202), and then to having both depression and anxiety (aHR = 1.231). However, in the landmark analysis at 100 days post-transplantation, OS after transplantation was significantly lower only in patients having both depression and anxiety (aHR = 1.253).
Numerous studies suggested association of pre-transplantation depression with lower OS in allo-HSCT15,20–22. However, only one large sized longitudinal cohort study conducted in the US using CIBMTR data showed that depression history decreases the OS with aHR of 1.1316. To the best of our knowledge, this is the largest nationwide cohort study conducted in Asia, or outside US, showing the risk of pre-transplantation depression in OS of allo-HSCT. We replicated previous research and showed that pre-transplant depression decreases OS with aHR of 1.167, which was very similar to the HR reported by El-Jawahri et al. By carefully sub-classifying patients according to their anxiety disorder history, we were able to eliminate overlapping impact of pre-transplant anxiety from that of depression. Thus, our findings more specifically confirmed that pre-transplant depression independently decreases OS of allo-HSCT regardless of anxiety disorder. We also extended previous work by showing that OS decrement was more pronounced in patients who had both depression and anxiety before allo-HSCT (aHR = 1.202).
Interestingly, we did not detect significant association between anxiety disorder and OS of allo-HSCT. Anxiety is a frequent response to threat, so it is commonly found in patients having severe medical conditions23. Consequently, it is difficult to accurately distinguish normal anxiety or adjustment reaction from that of true morbid anxiety24. Thus, patients having anxiety reaction could have been over-diagnosed as having anxiety disorder in our cohort, which might have led to decreased significance in OS. In line with this hypothesis, patients in our cohort had twice more history of anxiety than depression. Previous study also showed that patients having pre-transplantation depression more concordantly experienced clinically significant depression even after transplantation, whereas patients having pre-transplant anxiety tended to experience anxiety symptoms less continuously17,25,26. Thus, depression might had longer impact than anxiety in OS of patients receiving allo-HSCT. In addition, landmark analyses performed at 100 days post-transplant, which excluded patients at high risk of early mortality post-transplant, showed that OS was significantly lower only in patients who had both depression and anxiety disorder. Thus, prospective studies which include more complete predictors of mortality in allo-HSCT are needed to confirm complicated relationship among depression, anxiety, and survival outcome after allo-HSCT.
Our results have additional strengths. First, by conducing a nationwide cohort study, we included all adult patients who received allo-HSCT from 2002 to 2018. We were able to prevent selection bias and minimize recruitment setting effect, so our results have higher generalizability. Second, a more stringent definition was used to determine pre-transplant depression. Rather than using a single questionnaire or retrospective chart review, all patients with pre-transplant depression visited hospital due to exact diagnosis of depression [ICD-10 code for major depressive disorder (F32 × or F33 ×)]. Thus, we further prevented selection and reporting bias. Lastly, by conducting a subgroup analysis comprising of patients with leukemia only, we eliminated underlying hematologic disease as a possible co-factor and confirmed the negative impact of depression, and additive effect of anxiety, in post-transplant mortality.
The pathophysiological basis of pre-transplantation depression having a negative impact in OS of patients receiving allo-HSCT is still obscure. Nevertheless, our findings could be predicted by association of depression with immune system dysregulations27. Studies showed that depression is often accompanied by inflammatory diseases including irritable bowel syndrome, type 2 diabetes, arthritis and autoimmune disorders, which can activate the peripheral and central inflammatory response28. Furthermore, other studies illustrated that depression and acute graft-versus-host-disease (GVHD) share common pro-inflammatory reactions: increment of inflammatory cytokines (i.e. tumor necrosis factor-alpha) and shifting toward T helper 1 response system29,30. Likewise, past study showed that pre-HCT depression increased the incidence of acute GVHD16. Poor treatment adherence due to depression is another important cause of higher mortality31. However, additional prospective studies focusing on biological mechanism are needed to define common pathway linking depression, and anxiety, with that of post-transplantation mortality in patients receiving allo-HSCT.
Several limitations should also be noted. First, occurrence of depression was based on clinical diagnosis with hospital visit records rather than using objective depressive scale measurements. As a result, we were unable to assess mortality difference depending on the depression severity. Since the depression severity is not known, some patients in the non-depression group may have had depression but have been undiagnosed. Second, we were also unable to investigate antidepressant effect, or treatment effect, in the risk of mortality. Likewise, depression episodes after transplantation were not considered. Thus, the effect of depression recurrence or chronicity in the post-transplant mortality were not studied. Third, whether time to diagnosis from depression and anxiety or duration of their illnesses influenced OS were not analyzed. Fourth, unbalanced baseline characteristics between depression group and non-depression group could harbor possible bias. Fifth, the cause of death, and relapse related and non-relapse related mortality according to depression were not investigated. In addition, other important clinical factors such as GVHD and infection episodes after transplantation, pre-transplantation conditioning, performance score, remission status at time of transplant, type of donor, smoking history, and alcohol status were not considered. Lastly, although Korea has a mandatory public health insurance system providing a comprehensive medical coverage to all residents of Korea, socioeconomic status could still have influenced the OS.
In conclusion, pre-transplant depression, independent from anxiety, decreased OS of adult patients receiving allo-HSCT. The mortality risk was higher in patients who had both depression and anxiety than patients having neither. Same trend was noted in patients who received allo-HSCT due to leukemia. Thus, our results suggest that patients having depression, especially both depression and anxiety, before allo-HSCT have a higher risk of post-transplantation mortality needing more intensive care.
Methods
Data source and study population
The Korean National Health Insurance Service (KNHIS) is a mandatory public health insurance system of Korea and offers comprehensive medical coverage to all residents of Korea. Data were acquired from the KNHIS database, which contains all medical claims for the population covered under the KNHIS. The database has been widely used in various epidemiological studies and is described in detail elsewhere32–34.
Adult patients (≥ 18 years) who underwent allo-HSCT between 2002 and 2018 were identified using KNHIS database procedure codes X5061 for bone marrow and X5063 for peripheral blood allo-HSCT. For those who received second allo-HSCT, we used first allo-HSCT data as their baseline values. As a result, nearly all allo-HSCTs performed in Korea during the study period were included in the analysis. Comorbidities including hypertension, diabetes mellitus, chronic obstructive pulmonary disease, cerebro- or cardiovascular disease, and anxiety disorder were also extracted using International Classification of Diseases, Tenth Revision (ICD-10) codes. This study was approved by the institutional review board of Seoul St. Mary’s Hospital, Seoul, Korea (KC19ZNSI0396). Consent from individual subjects were not needed because the study used publicly open, anonymous data.
Outcome variable and statistical analysis
The primary endpoint of this study was OS difference after allo-HSCT according to pre-transplant depression history. In order to utilize more conservative definition of depression, patients having ICD-10 code for major depressive disorder (F32 × or F33 ×) and visited hospital due to the same code within five years prior to the allo-HSCT was defined as having pre-transplant depression. Difference between the two groups (depression group and non-depression group) in baseline demographic and clinical characteristics were compared using student’s-T test for continuous variables and Chi-squared test for categorical variables.
To investigate independent risk of pre-transplant depression, and prevent overlapping impact of anxiety, we also identified patients who were diagnosed with and visited hospital due to anxiety disorder (F30 ×) before allo-HSCT. Thus, patients were subdivided in to four groups; no history of depression and anxiety disorder (none), depression history without anxiety (depression only), anxiety disorder without depression (anxiety only), and having both depression and anxiety disorder (both depression and anxiety).
Retrospective cohorts were followed from the day they received allo-HSCT to the occurrence of death or the last follow-up day (December 31, 2018), whichever came first. The OS rate represents the proportion of patients who were alive at the specified time after the date of transplantation and was associated with death due to any cause. Cox proportional-hazard regression evaluated risk of post-transplant mortality related to depression. All risk factors with p < 0.10 in univariable analysis were included in the single multivariable model. Thereafter, the factors found to be significant in this single multivariable model with depression variable were all included in the final multivariable model. For all statistical analysis, we used R statistical software (ver. 3.1.1, R Foundation for Statistical Computing, Vienna, Austria, 2012).
Ethical approval
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary information
Author contributions
S.-M.W., S.-S.P., S.H., and H.K.L. drafted the manuscript and contributed to project design, data collection, management, analysis and interpretation; S.-H.P. contributed to project design, data collection, statistical analysis, and management; N.-Y.K., D.W.K., H.-R.N., J.W.L. contributed to project design, data management and manuscript revision. All authors have read and approved the revised manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (NRF-2019R1C1C1011664).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sheng-Min Wang, Sung-Soo Park, Seunghoon Han and Hyun Kook Lim.
Contributor Information
Seunghoon Han, Email: waystolove@catholic.ac.kr.
Hyun Kook Lim, Email: drblues@catholic.ac.kr.
Supplementary information
is available for this paper at 10.1038/s41598-020-71208-2.
References
- 1.Mahmoud HK, et al. Allogeneic hematopoietic stem cell transplantation for non-malignant hematological disorders. J. Adv. Res. 2015;6:449–458. doi: 10.1016/j.jare.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wildes TM, Stirewalt DL, Medeiros B, Hurria A. Hematopoietic stem cell transplantation for hematologic malignancies in older adults: geriatric principles in the transplant clinic. J. Natl. Compr. Cancer Netw. 2014;12:128–136. doi: 10.6004/jnccn.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copelan EA, Chojecki A, Lazarus HM, Avalos BR. Allogeneic hematopoietic cell transplantation; the current renaissance. Blood Rev. 2019;34:34–44. doi: 10.1016/j.blre.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Bacigalupo A, et al. Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1238–1247. [PubMed] [Google Scholar]
- 5.Shouval R, et al. External validation and comparison of multiple prognostic scores in allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3:1881–1890. doi: 10.1182/bloodadvances.2019032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuelgasim KA, et al. Depression and anxiety in patients with hematological malignancies, prevalence, and associated factors. Saudi Med. J. 2016;37:877–881. doi: 10.15537/smj.2016.8.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amonoo HL, et al. Psychological considerations in hematopoietic stem cell transplantation. Psychosomatics. 2019;60:331–342. doi: 10.1016/j.psym.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrykowski MA, Brady MJ, Henslee-Downey PJ. Psychosocial factors predictive of survival after allogeneic bone marrow transplantation for leukemia. Psychosom. Med. 1994;56:432–439. doi: 10.1097/00006842-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Yalvac HD, Kotan Z, Tekgunduz E, Caykoylu A, Altuntas F. Could psychiatric assessment before hematopoietic stem cell transplantation predict the need for psychiatric consultation during transplantation period? Transfus. Apher. Sci. 2016;54:85–90. doi: 10.1016/j.transci.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Potdar R, et al. Prognostic scoring systems in allogeneic hematopoietic stem cell transplantation: where do we stand? Biol. Blood Marrow Transpl. 2017;23:1839–1846. doi: 10.1016/j.bbmt.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Sorror ML, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transpl. 2006;38:255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- 13.Akaho R, et al. Psychological factors and survival after bone marrow transplantation in patients with leukemia. Psychiatry Clin. Neurosci. 2003;57:91–96. doi: 10.1046/j.1440-1819.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- 14.Broers S, et al. Are pretransplant psychological variables related to survival after bone marrow transplantation? A prospective study of 123 consecutive patients. J. Psychosom. Res. 1998;45:341–351. doi: 10.1016/s0022-3999(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 15.Prieto JM, et al. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J. Clin. Oncol. 2005;23:6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 16.El-Jawahri A, et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer. 2017;123:1828–1838. doi: 10.1002/cncr.30546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo HJ, et al. Anxiety and depression of the patients with hematological malignancies during hospitalization for hematopoietic stem cell transplantation. Psychiatry Investig. 2019;16:751–758. doi: 10.30773/pi.2019.07.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann. Intern. Med. 2006;144:407–414. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 19.Thanarajasingam G, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2013;19:1713–1718. doi: 10.1016/j.bbmt.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loberiza FR, Jr, et al. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J. Clin. Oncol. 2002;20:2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- 21.Pillay B, Lee SJ, Katona L, Burney S, Avery S. Psychosocial factors associated with quality of life in allogeneic stem cell transplant patients prior to transplant. Psychooncology. 2014;23:642–649. doi: 10.1002/pon.3462. [DOI] [PubMed] [Google Scholar]
- 22.Socie G, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late effects working committee of the international bone marrow transplant registry. N. Engl. J. Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 23.Stark DP, House A. Anxiety in cancer patients. Br. J. Cancer. 2000;83:1261–1267. doi: 10.1054/bjoc.2000.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appart A, Lange AK, Sievert I, Bihain F, Tordeurs D. Adjustment disorder and DSM-5: a review. Encephale. 2017;43:41–46. doi: 10.1016/j.encep.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Fife BL, et al. Longitudinal study of adaptation to the stress of bone marrow transplantation. J. Clin. Oncol. 2000;18:1539–1549. doi: 10.1200/JCO.2000.18.7.1539. [DOI] [PubMed] [Google Scholar]
- 26.Pillay B, et al. A prospective study of the relationship between sense of coherence, depression, anxiety, and quality of life of haematopoietic stem cell transplant patients over time. Psychooncology. 2015;24:220–227. doi: 10.1002/pon.3633. [DOI] [PubMed] [Google Scholar]
- 27.Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30:1–16. doi: 10.1017/neu.2016.69. [DOI] [PubMed] [Google Scholar]
- 28.Leonard BE. The concept of depression as a dysfunction of the immune system. Curr. Immunol. Rev. 2010;6:205–212. doi: 10.2174/157339510791823835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang TL, Lee CT. T-helper 1/T-helper 2 cytokine imbalance and clinical phenotypes of acute-phase major depression. Psychiatry Clin. Neurosci. 2007;61:415–420. doi: 10.1111/j.1440-1819.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- 30.El-Jawahri A, Chen YB. Pleiotropic approach to graft-versus-host disease. J. Clin. Oncol. 2013;31:4462–4464. doi: 10.1200/JCO.2013.52.8182. [DOI] [PubMed] [Google Scholar]
- 31.Bortolato B, et al. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Song SO, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Im GJ, et al. Prevalence of severe-profound hearing loss in South Korea: a nationwide population-based study to analyse a 10-year trend (2006–2015) Sci. Rep. 2018;8:9940. doi: 10.1038/s41598-018-28279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon I, et al. Trends of the prevalence and incidence of hypertrophic cardiomyopathy in Korea: a nationwide population-based cohort study. PLoS ONE. 2020;15:e0227012. doi: 10.1371/journal.pone.0227012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.