Abstract
The current Coronavirus Disease 2019 (COVID-19) pandemic has exerted an unprecedented impact across the globe. As a consequence of this overwhelming catastrophe, long-established prevailing medical and scientific paradigms have been disrupted. The response of the scientific community, medical journals, media, and some politicians has been far from ideal. The present manuscript discusses the failure of the scientific enterprise in its initiatives to address the COVID-19 outbreak as a consequence of the disarray attributable to haste and urgency. To enhance conveying our message, this manuscript is organized into 3 interrelated sections: 1) the accelerated pace of publications coupled with a dysfunctional review process; 2) failure of the clinical trial enterprise; 3) propagation of misleading information by the media. In response we propose a template comprising a focus on randomized controlled clinical trials, and an insistence on responsible journal publication, and enumeration of policies to deal with social media-propagated news. We conclude with a reconsideration of the appropriate role of academic medicine and journals.
Keywords: COVID-19, Media, Medical journals, Scientific community
Clinical Significance.
-
•
The COVID-19 outbreak has exposed major gaps within the scientific world.
-
•
The lack of coordination between countries and institutions, and the haste of publishing, has led to a massive output of observational studies.
-
•
International organizations and medical journals must be rendered accountable in the coordination and quality control of scientific output.
-
•
A fact-check system should be adopted by the media separating what is misleading or false from what is credible.
Alt-text: Unlabelled box
Introduction
As of the most recent October 2020 update, the current Coronavirus Disease 2019 (COVID-19) pandemic has exerted an unprecedented impact across the globe. More than 33 million cases and over 1 million deaths.1
The World Health Organization (WHO) has recommended “lockdown” to limit the spread of the virus and many countries have closed their borders, disrupting the global economy. Pressure has also increased on health care systems, attributable to a steep increase in patients requiring acute care and respiratory and circulatory support, and also by increasing the demand for the rapid testing of the safety and efficacy of novel treatments. As a consequence, there has been a disruption of the evidence-based scientific paradigms that are essential for maintaining public health. The short-term consequences of this disruption can readily be observed. For example, off-label use of medications without credible proof of benefit (eg, hydroxychloroquine), companies rushing to market vaccines without the requisite robust evidence of efficacy and safety, the downgrading of the role of disease-control agencies, and distrust of scientific expertise.2 While the long-term consequences are yet to be seen, a recent projection foreshadows a growing trend in antivaccination movements.3
In the context of this escalating chaos, how has the scientific community responded? What has been the role of medical journals? How has scientifically rigorous information been disseminated? What follows is an overview of how the response to the COVID-19 pandemic has disrupted the norms for reliable data acquisition, analysis, and dissemination. We then adopt a constructive tenor and propose a template comprising a focus on randomized controlled clinical trials, and an insistence on responsible journal publication policies to deal with social media-propagated news.
For ease of presentation we have organized this manuscript into 3 interrelated sections: 1) an accelerated pace of publication; 2) failure of the clinical trial enterprise; 3) media dysfunction and the propagation of misinformation. Importantly, we propose a template comprising a focus on randomized controlled clinical trials, and an insistence on responsible journal publication policies to deal with social media-propagated news, with reconsideration of the appropriate role of academic medical faculties and journals.
Accelerated Pace of Publications
The COVID-19 pandemic has resulted in a deluge of academic papers being published about the novel coronavirus in one of the greatest explosions of scientific literature ever observed. Professional organizations charged with grading scientific evidence are overwhelmed by the pace of publications. Researchers and clinicians have no time to keep up with the torrent of manuscripts coming out daily.4 Moreover, many of these publications have not been peer-reviewed and are widely available solely as preprint manuscripts, while others may have been peer-reviewed but lack quality and scientific rigor.5 , 6 Regardless of their quality and whether or not they have been peer-reviewed, many of these manuscripts are treated equally and shared widely on social media and are frequently picked up by numerous news outlets. This has led the preprint server “bioRxiv” to add a yellow banner warning on all new publications of coronavirus research saying: “A reminder: these are preliminary reports that have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behaviour, or be reported in news media as established information.” Consequently, it is appropriate to ask, why publish them?
In a technological era where a tweet or a Facebook feed may provoke more impact than a well-planned and diligently executed scientific project, we must rigorously question where the science stands currently, for the sake of transparency, rigor, and public trust. The quantity of manuscripts being published suggests that many of these may be observational or small trials with poor oversight, resulting in inappropriately hasty publication. But because good science requires discipline, caution, validation, and replication, the current rapid pace of publications may be contributing to more confusion among the public and to an overall decline in scientific trust.7 The failure to conduct large-scale hypothesis-driven randomized controlled trials, in concert with haste for fast-track publications, has added little to the treatment, and especially the prevention, of the novel coronavirus.
Failure of the Clinical Trial Enterprise
Randomized controlled trials (RCTs) are the gold standard test of whether or not a treatment is beneficial.8, 9, 10 The numerous biases and limitations of nonrandomized, observational studies have been thoroughly documented and are detailed in Table 1 . It must be emphasized, however, that underpowered and poorly conducted RCTs are equally prone to bias and spurious findings.11 Regretfully, observational, poor-quality randomized and good-quality randomized studies are allocated similar press coverage and dissemination.12 The growing access to large sets of data from thousands of patients and multiple data sources (ie, “big data”) has prompted many researchers to erroneously claim an accurate determination of treatment effects from nonrandomized studies.13 , 14 Although this approach may be used in unique settings wherein it is not feasible to perform an RCT, it should not be used to replace RCTs in settings where RCTs can (and should) be performed—as in the current coronavirus crisis.10 , 15, 16, 17, 18 Additionally, using “big data” in the absence of a prespecified power calculation, expected event rates, treatment effects, and follow-up time (ie, all prespecified parameters of RCTs) is fraught with errors (Table 1).19, 20, 21
Table 1.
Bias (Deviation from the Truth) of Observational Studies
| Bias | What Is It? | Example |
|---|---|---|
| Selection bias | The groups being studied are not comparable because they were not selected at random | Patients taking antihypertensive medication may have poorer outcome, not because of the medication but because they are sicker, that is, they represent a group of sicker people |
| Information bias | Incorrect determination of exposure, outcome, or both | Information about a treatment or an event is collected differently across patient populations, for example, hospital records vs phone calls vs face-to-face visits |
| Confounding | The association between the exposure and outcome is determined by another factor that can be measured or unmeasured | The association between a treatment for diabetes and outcome may be determined by the patients’ income or access to health care, for example, poorer people may not have insurance to cover their health expenses |
| Exaggeration of the effect | The magnitude of the effect seems greater on a relative scale than what it actually is on an absolute scale | In the absence of a prespecified sample size/events and expected treatment effect, an observational study may report an “important” relative effect even in the setting of a low event rate and small difference in events. For example, an outcome affecting 1.7% of the population on the “exposed/treated” and 1.3% on the “nonexposed/treated” may give an odds (or hazards) ratio around 1.3, which is usually translated by a “30% increase of event,” but the absolute difference is 0.4%, which should be translated into a 0.4% increase and not 30%. |
Given these limitations, the results from observational studies should be regarded as “hypothesis-generating” to be tested in randomized controlled trials; observational studies should not guide treatment decisions.
During the COVID-19 outbreak, many observational studies claiming treatment effects have been reported in top-tier medical journals with the inherent limitations described above (and in Table 1),22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 together with a few small-scale or open-label RCTs,36, 37, 38, 39, 40, 41, 42, 43 and no large-scale, international, well-powered, double-blinded RCT.
Observational studies can also jeopardize ongoing RCTs. For example, the recent publication (and subsequent retraction) of an observational study showing a possible deleterious effect of hydroxychloroquine and chloroquine for the treatment of COVID-1932 has prompted many ongoing trials to withhold the hydroxychloroquine arm. Because of the context, the decision of pausing the randomization to hydroxychloroquine might have been inevitable, simply because the patients started to express concern about participating in the trials.44 While intermediate analysis could be informative, much more informative is to complete the trials as planned.45 The recent retraction of manuscripts with data emanating from Surgisphere (Palatine, Ill)23 , 32 highlights the failure of adherence to good science practices (access to the original data source denied by Surgisphere) and of peer-reviewing and editorial oversight (not having vetted quality control of the database prior to their publication). The confusion caused by these episodes exposes researchers to the “double whammy” of 1) further disruption of RCT timely evidence generation, and 2) loss of confidence of the patients eligible for inclusion in the ongoing clinical trials. In a constructive vein, we have provided a framework to support and enhance the efforts of editors and reviewers in the handling of observational analyses, as shown in Table 2 .
Table 2.
Framework to Support Editors and Reviewers in the Handling of Observational Analyses
| Checklist | Comment |
|---|---|
|
Data coming from sources without proof of signed informed consent should not be taken seriously |
|
Data that do not meet ethical standards should not be considered for publication |
|
The people responsible for the data oversight and data-management must be identified and reachable if required |
|
The statistical methods must be very detailed, including on the handling of missing data and rationale of the adjustment technique used; the statistician(s) must be reachable and accountable. Whenever possible, the SAP should be published before the analysis is performed. |
|
The full dataset used for the analysis should be available for independent verification. For example, upon request from a journal |
|
It is strongly recommended a prior registry of the cohort along with its description (eg, in ClinicalTrials.gov) |
|
Observational studies should mimic the standards used for randomized trials |
Along with the multiple observational studies, some RCTs have also been published. Many of these RCTs have important methodological caveats that render their conclusions nondefinitive. A critical interpretation of these RCTs is presented in Table 3 .36, 37, 38, 39, 40, 41, 42, 43 Overall, our critique demonstrates a clear failure of the clinical trial enterprise, revealing a lack of coordination and international cooperation required to facilitate large, high-quality RCTs that could potentially respond to patients’ needs in a scientifically valid and timely manner.46
Table 3.
Critical Appraisal of the COVID-19 Randomized Controlled Trials
| Study | Patients | Treatments | Main Findings | Methodological Issues |
|---|---|---|---|---|
| A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-1936 Chinese Clinical Trial Register identifier, ChiCTR2000029308 |
199 adult patients hospitalized with confirmed SARS-CoV-2 infection | 1:1 assignment of lopinavir–ritonavir (400 mg and 100 mg, respectively) 2×/d plus standard care vs standard care alone for 14 d |
Lopinavir–ritonavir was not different from standard care in the time to clinical improvement. | The trial was not blinded, which could have influenced decision-making and the use of concomitant treatments. |
| Triple Combination of Interferon Beta-1b, Lopinavir–Ritonavir, and Ribavirin in the Treatment of Patients Admitted to Hospital with COVID-19: an Open-Label, Randomized, Phase 2 Trial37 ClinicalTrials.gov identifier, NCT04276688 |
127 adult patients hospitalized with confirmed SARS-CoV-2 infection | 2:1 assignment to a combination of lopinavir–ritonavir (400 mg and 100 mg, respectively) 2×/d, ribavirin 400 mg 2×/d, and three doses of 8 million IU of interferon beta-1b on alternate days (combination group) or lopinavir–ritonavir (400 mg and 100 mg, respectively) 2×/d (control group) for 14 d |
The combination group had a significantly shorter median time from start of study treatment to negative nasopharyngeal swab. | The trial was not blinded, which could have influenced decision-making and the use of concomitant treatments. |
| Remdesivir in Adults with Severe COVID 19: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial38 | 237 adult patients hospitalized with confirmed SARS-CoV-2 infection | 2:1 ratio assignment to intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2-10 in 1×/d) or the same volume of placebo infusions for 10 d |
Remdesivir was not associated with statistically significant clinical benefits. |
Exaggerated claims of treatment effect not supported by the data. |
| Remdesivir for the Treatment of Covid-19 – Preliminary Report39 Adaptive COVID-19 Treatment Trial (ACTT). ClinicalTrials.gov identifier, NCT04280705 |
1063 adult patients hospitalized with confirmed SARS-CoV-2 infection | 1:1 ratio assignment to intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in 1×/d) or the same volume of placebo infusions for 10 d |
Remdesivir reduced the time to recovery (median: 11 vs 15 d). | Treatment cross-over. Early trial stop. |
| Remdesivir for 5 or 10 Days in Patients with Severe Covid-1940 Study to evaluate the safety and antiviral activity of remdesivir (GS-5734) in participants with severe Coronavirus Disease (COVID-19). ClinicalTrials.gov identifier, NCT04292899 |
397 adult patients hospitalized with confirmed SARS-CoV-2 infection, oxygen saturation of 94% or less (in ambient air), and radiologic evidence of pneumonia | 1:1 ratio assignment to intravenous remdesivir (200 mg on day 1 followed by 100 mg on subsequent days) for either 5 or 10 d |
No difference between a 5-d course and a 10-d course of remdesivir was found. | Open label. No placebo control arm. |
| A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for COVID-1941 ClinicalTrials.gov identifier, NCT04308668 |
821 asymptomatic participants who had household or occupational exposure to someone with confirmed COVID-19 | Participants were randomly assigned to receive either placebo or hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 h, then 600 mg daily for 4 additional d) within 4 d after exposure | Hydroxychloroquine did not prevent illness compatible with COVID-19 or confirmed infection. | No consistent proof of exposure to SARS-CoV-2 or laboratory confirmation. Young patients with few comorbid conditions who are less likely to develop severe disease. |
| Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-1942 Chinese Clinical Trial identifier, ChiCTR2000029757 |
103 participants with laboratory-confirmed COVID-19 that was severe (respiratory distress or hypoxemia) or life-threatening (shock, organ failure, or requiring mechanical ventilation) | Convalescent plasma in addition to standard treatment vs standard treatment alone (control) | Convalescent plasma therapy did not result in a statistically significant improvement in time to clinical improvement within 28 d. | Open-label. Trial terminated early after 103 of a planned 200 patients were enrolled. Trial likely underpowered. |
| Study to evaluate the safety and antiviral activity of remdesivir (GS-5734) in participants with severe Coronavirus Disease. ClinicalTrials.gov identifier, NCT04292899 |
397 patients hospitalized with COVID-19 with oxygen saturation ≤94% while breathing ambient air, and radiologic evidence of pneumonia | Patients were randomly assigned in a 1:1 ratio to receive intravenous remdesivir for either 5 d or 10 d | No difference between a 5-d course and a 10-d course of remdesivir. | Open label. Lack of a randomized placebo control group. |
| Dexamethasone in Hospitalized Patients with Covid-19 – Preliminary Report43 Randomized Evaluation of COVID-19 Therapy (RECOVERY). ClinicalTrials.gov Identifier, NCT04381936 |
6425 patients hospitalized with COVID-19 | Oral or intravenous dexamethasone (6 mg once daily) for up to 10 d or usual care alone | The use of dexamethasone resulted in lower 28-d mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support. | Open-label. Heterogeneity of the treatment effect with benefit in patients who were receiving respiratory support and no benefit (with potential harm) in patients who did not require oxygen. |
COVID-19 = Coronavirus Disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
We propose that efficient coordination could have readily identified gaps in evidence, candidate hypotheses, and interventions to be tested, consequently engaging international stakeholders in a cooperative effort that could lead to high-grade clinical evidence. Moreover, COVID-19 represents a condition where well-designed RCTs should have been quick to deliver due to the availability of patients eligible to be enrolled and a high rate of critical outcomes that develop rapidly and early.47 , 48 Successful coordination could have been achieved by the WHO engaging complementarity among international institutions. Unfortunately, this ideal role has been denigrated and compromised due to the end of multilateralism, and also, admittedly, due to the many inefficiencies inherent in the processing of the WHO.49 , 50
This lack of coordination among public institutions, countries, and scientists has led to an increase in ostensible “expert opinions” that are not supported by solid scientific evidence.51 To deal with these issues, the International Coalition of Medicines Regulatory Authorities emphasized the need and commitment by global regulators to cooperate and align their approaches to clinical trial management and pharmacovigilance.52 To achieve this goal, a wider coordination of the media, medical academicians, and journals is urgently required.
Media and the Propagation of Misinformation
Evidence-based medicine has been readily adopted as the best standard of practice in most parts of the globe over the last decades. However, multiple digital sources that lack editorial oversight or peer review have led to a point where evidence-based, “traditional” medicine and fabricated facts are, regretfully, treated similarly.53 , 54 With billions of individuals online every day, health misinformation can spread at a very rapid pace.55 , 56 Many times, the spread of misinformation is supported by governments and public institutions, which may not only harm the public but may also enhance scientific mistrust.55 , 57
This rapid spread of viral misinformation renders it impossible for anyone to distinguish between scientifically valid facts and completely false claims that can jeopardize health and well-being, compromise public health measures, and ultimately, undermine society as a whole.55 , 58 , 59 Consequently, learning how to detect unsubstantiated medical news requires an important dose of critical thinking. The American Council on Science and Health has issued a list of “red flags” for facilitating the detection of the role of media and misinformation, taking into account the credibility of the journal, the use of exaggerated language, lack of appropriate methodology, and conclusions not supported by data.60 These steps may be helpful if one has a strong background in scientific methodology, which, unfortunately, most readers lack. Consequently, we need a strong engagement of social media platforms for early detection, scrutiny, and limiting the spread of the role of media and viral misinformation.61 Fortunately, the rapid development of publicly available datasets and innovative methods that can rapidly cross-check facts and track misinformation (eg, deep learning algorithms, natural language processing-assisted data mining, social network analysis), can facilitate the early detection of misinformation and be used for flagging users or groups that are contributing to the misinformation of the public.62 Although the scientific community generally still enjoys relatively high levels of public trust, disturbingly, 1 in 5 individuals express skepticism about scientists. These realities mandate that the medical community vet health misinformation on social media.54 , 63 Potential solutions to achieve this goal are delineated in Table 4 .
Table 4.
Potential Solutions to Increase the Quality of Randomized Trials and Information Diffusion
| Problem | Potential Solution |
|---|---|
| Observational and RCT findings are given the same consideration/weight by the media and many scientists |
|
| RCTs (to date) are underpowered for assessing mortality and have many methodological caveats |
|
| Exaggerated claims about efficacy are presented in the study conclusions and abstract |
|
| Spread of “fake news” |
|
DMSD = data monitoring and safety board; EMA = European Medicines Agency; “GRADE” = grading quality of evidence and strength of recommendations; NIH = National Institutes of Health; RCT = randomized controlled trial; WHO = World Health Organization.
GRADE Working Group (https://www.bmj.com/content/328/7454/1490).
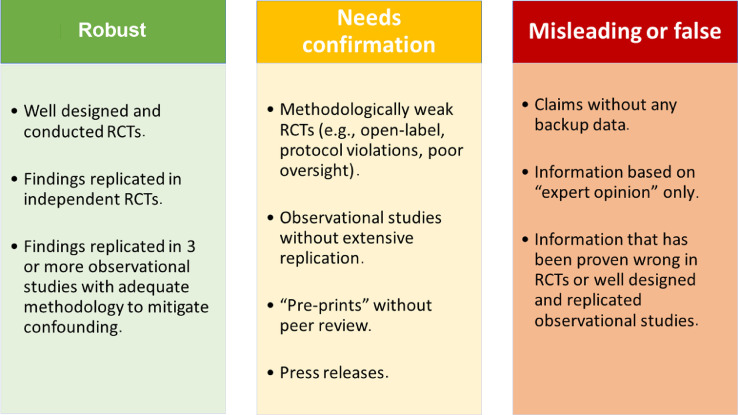
The current crisis has clearly revealed the worrisome lack of understanding by the general public, lay media, decision-makers, and politicians of the basics of the hierarchy of evidence. It is hoped that this crisis will contribute to an enhanced awareness about how medicine should transition from empirical-based practice to evidence-based practice. Education of the general public and political leaders about the simple but critical principles of clinical evidence generation is urgently required.64 To facilitate this goal, an easy-to-follow color grading scheme for presenting medical evidence by the media and general public is presented in the Figure .
Figure.
Color-code proposal for grading evidence on medical news in the media (including social media). The color-code fact check would appear as a bar or logo on top of the shared messages. RCT = randomized controlled trial.
A search for the best available evidence should constitute a mainstay of society, but to achieve this goal, a large-scale and global coordination is required, for which participation by medical faculty and journals is essential.
A Pivotal Role for Academic Medical Faculties and Journals Is Mandatory
In many countries, including Canada and the United States, medical faculties maintain close community relationships that provide communication platforms to counteract media-driven disinformation. Consequently, responsible journalism, along with the public, benefits from close and vibrant relationships with local medical faculties. This 2-way communication (local medical faculty to the public, and vice-versa) must be regarded as an essential responsibility for our academic medical institutions to counteract the impact of misinformation.
Scientific journals must also have active engagement in proactively counteracting misinformation. Despite often being perceived as inaccessible to the lay public, many scientific journals have more recently embraced the need to translate knowledge more broadly; for example, by adopting open-access “patient pages” or brief summaries of the major research findings, often in the form of a “central illustration.” Most journals now have digital media tools and promote their articles in social media. Furthermore, many journals work in close alliance with medical societies and patient associations. We believe these elements serve collectively to position medical journals as potentially powerful agents, capable of mitigating and counteracting medical misinformation.53 An increased involvement of the editors of medical journals, and the scientific community in general, is absolutely essential to help the public navigate a world rife with health-related misinformation.59 , 63
Medical journals could play a leading role in achieving global consensus and prompting action on matters of public interest. Similar to the publication of guidelines for the treatment of specific conditions, and reporting of observational studies or RCTs checklists, medical journals should provide guidance and help regulate medical misinformation by enabling real-time fact-checking prior to the dissemination of medical information in the media (Figure).
Another innovation that medical journals should consider is to publish “theme issues” of global health interest (eg, vaccination, climate change, or cardiovascular prevention). Such “theme issues” can then be readily disseminated widely in the media, utilizing several outlets in a coordinated manner.
A social media strategy of Twitter promotion may increase the online visibility of research papers and also increase the number of citations.65 Discussions are currently underway on whether Twitter should be used for continuous medical education.66 While a social media-based strategy can be utilized to promote good-quality research, it cannot and absolutely should not replace research itself. Moreover, the constant outpouring of information creates too many information inputs and options, and having too many options may be as bad as having none.67
A central issue that must be resolved is how medical journals will relate and coordinate with social media. Social media platforms are here to stay, but we must accept that they can be easily misused, prompting researchers to attempt to attain immediate recognition and feedback on Twitter as a substitute for engaging in years of rigorous research. Clearly, the value of a scientist should not be measured according to social media feedback, but rather by the quality and rigor of his/her research. To facilitate this evaluation, the scientific impact should be measured by long-term achievements rather than by short-term metrics. Instead of using metrics like Altmetric (London, UK), which aggregates short-term social media (eg, Twitter, Facebook, and other sources) outputs and displays, providing a composite score for each paper, medical journals should preferably deploy more long-term based metrics (eg, citation indexes and clinical utility) that value work product over time.55 , 68
Another important and glaring issue that we perceive as despicable, that disrupts reliable data acquisition, is “publication by press release.” Private companies, governments, and research institutes are convening news conferences to report “potential breakthroughs” that cannot be verified because the complete dataset on which the announcements are based have not been peer reviewed.69 , 70 A recent example is Moderna's (Cambridge, Mass) claim of “favorable” results from its vaccine trial, which was regrettably announced in the absence of supporting underlying data.69 Nonetheless, the announcement may have had its desired effect, that is, it successfully added billions of dollars to the value of the company in a single day.71
To summarize, medical journals must play a pivotal role in reducing the incentive for “publication by press release” by restricting publication of results that have been previously presented to the media in the absence of supporting robust data. As an acceptable alternative, we propose that medical journals can simultaneously provide “fast-track” peer review and publications with a simultaneous press release. Furthermore, scientific journal editors must exercise more oversight and stricter publication control, dedicating themselves to publishing only a limited number of articles per edition that are rigorous, data driven, and focused.
Our proposal for achieving a more central role for medical journals and editors in retaining transparency and quality of evidence is detailed in Table 5 .
Table 5.
The Central Role of Medical Journals and Editors for Retaining Transparency and Quality of Evidence
| Proposed Role of Medical Journals and Editors |
|---|
|
|
|
|
|
|
Conclusions
The COVID-19 outbreak has exposed major gaps and serious flaws, both within the scientific world and in how scientific information is disseminated in a technological world of “immediate information.” The lack of coordination between countries and institutions, in concert with the feverish haste of publishing, has regretfully led to a massive output of small studies, observational data, and poor-quality RCTs. We propose a wide array of discrete “next steps” to correct these deficiencies. International organizations such as the WHO, and medical journals must be rendered accountable and tasked to play a significant role in the coordination and quality control of scientific output. Simultaneously, a permanent and clearly identified fact-check system should be adopted by the media, clearly identifying to the reader what is misleading or false and what is likely true. To facilitate these goals, we have constructed a novel and user-friendly grading template for enhancing presentation of medical evidence by the media and general public.
Footnotes
Funding: None.
Conflict of Interest: None.
Authorship: All the authors contributed the content of the manuscript and approved its final version.
References
- 1.Johns Hopkins Coronavirus Resource Center. COVID-19 in the USA. Available at: https://coronavirus.jhu.edu/. Accessed August 16, 2020.
- 2.Larson HJ. Blocking information on COVID-19 can fuel the spread of misinformation. Nature. 2020;580(7803):306. doi: 10.1038/d41586-020-00920-w. [DOI] [PubMed] [Google Scholar]
- 3.Johnson NF, Velásquez N, Restrepo NJ. The online competition between pro- and anti-vaccination views. Nature. 2020;582(7811):230–233. doi: 10.1038/s41586-020-2281-1. [DOI] [PubMed] [Google Scholar]
- 4.Brainard J. Scientists are drowning in COVID-19 papers. Can new tools keep them afloat?Science. 2020. Available at: https://www.sciencemag.org/news/2020/05/scientists-are-drowning-covid-19-papers-can-new-tools-keep-them-afloat. Accessed September 20, 2020.
- 5.White RG, Hakim AJ, Salganik MJ. STrengthening the Reporting of Observational studies in Epidemiology for Respondent-Driven Sampling studies: "STROBE-RDS" statement. J Clin Epidemiol. 2015;68(12):1463–1471. doi: 10.1016/j.jclinepi.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma M, Scarr S, Kelland K. Speed science: the risks of swiftly spreading coronavirus research. Reuters. 2020. Available at:https://www.reuters.com/article/us-china-health-research-analysis/speed-science-the-risks-of-swiftly-spreading-coronavirus-research-idUSKBN20D21S. Accessed September 20, 2020.
- 7.Trogen B, Oshinsky D, Caplan A. Adverse consequences of rushing a SARS-CoV-2 vaccine: implications for public trust. JAMA. 2020;323(24):2460–2461. doi: 10.1001/jama.2020.8917. [DOI] [PubMed] [Google Scholar]
- 8.Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357(9253):373–380. doi: 10.1016/S0140-6736(00)03651-5. [DOI] [PubMed] [Google Scholar]
- 9.Barton S. Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. BMJ. 2000;321(7256):255–256. doi: 10.1136/bmj.321.7256.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rush CJ, Campbell RT, Jhund PS, Petrie MC, McMurray JJV. Association is not causation: treatment effects cannot be estimated from observational data in heart failure. Eur Heart J. 2018;39(37):3417–3438. doi: 10.1093/eurheartj/ehy407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackshaw A. Small studies: strengths and limitations. Eur Respir J. 2008;32(5):1141–1143. doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
- 12.Wang MT, Bolland MJ, Gamble G, Grey A. Media coverage, journal press releases and editorials associated with randomized and observational studies in high-impact medical journals: a cohort study. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guertin JR, Rahme E, Dormuth CR, LeLorier J. Head to head comparison of the propensity score and the high-dimensional propensity score matching methods. BMC Med Res Methodol. 2016;16:22. doi: 10.1186/s12874-016-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26(1):20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan RM, Chambers DA, Glasgow RE. Big data and large sample size: a cautionary note on the potential for bias. Clin Transl Sci. 2014;7(4):342–346. doi: 10.1111/cts.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freemantle N, Marston L, Walters K, Wood J, Reynolds MR, Petersen I. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ. 2013;347:f6409. doi: 10.1136/bmj.f6409. [DOI] [PubMed] [Google Scholar]
- 17.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359(9302):248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum PR. Discussing hidden bias in observational studies. Ann Intern Med. 1991;115(11):901–905. doi: 10.7326/0003-4819-115-11-901. [DOI] [PubMed] [Google Scholar]
- 19.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med. 2019;25(10):1601–1606. doi: 10.1038/s41591-019-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galea S, Hernán MA. Win-win: reconciling social epidemiology and causal inference. Am J Epidemiol. 2020;189(3):167–170. doi: 10.1093/aje/kwz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blagoev KB, Wilkerson J, Fojo T. Hazard ratios in cancer clinical trials–a primer. Nat Rev Clin Oncol. 2012;9(3):178–183. doi: 10.1038/nrclinonc.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geleris J, Sun Y, Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382(25):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson P, Griffin I, Tucker C. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg ES, Dufort EM, Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen C, Wang Z, Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elharrar X, Trigui Y, Dols AM. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grein J, Ohmagari N, Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartini C, Tresoldi M, Scarpellini P. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323(22):2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Abajo FJ, Rodríguez-Martín S, Lerma V. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395(10238):1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehra M, Desai S, Ruschitzka F, Patel A. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 May 22 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Zhu FC, Li YH, Guan XH. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas LE, Bonow RO, Pencina MJ. Understanding observational treatment comparisons in the setting of Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):988–990. doi: 10.1001/jamacardio.2020.1874. [DOI] [PubMed] [Google Scholar]
- 35.Fosbøl EL, Butt JH, Østergaard L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324(2):168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung IF, Lung KC, Tso EY. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Zhang D, Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beigel J, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med. 2020;383(10):994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 40.Goldman JD, Lye DCB, Hui DS. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015301. [e-pub ahead of print] May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boulware DR, Pullen MF, Bangdiwala AS. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Zhang W, Hu Y. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horby P, Lim WS, Emberson JR. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 Jul 17 doi: 10.1056/NEJMoa2015301. [e-pub ahead of print] [DOI] [Google Scholar]
- 44.Watson J. An open letter to Mehra et al and The Lancet. 2020. Available at: https://zenodo.org/record/3871094#.X2gTSS9h3AI. Accessed September 20, 2020.
- 45.Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020;173(4):287–296. doi: 10.7326/M20-2496. [DOI] [PubMed] [Google Scholar]
- 46.Alexander PE, Debono VB, Mammen MJ. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol. 2020;12:120–126. doi: 10.1016/j.jclinepi.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.North CM, Dougan ML, Sacks CA. Improving clinical trial enrollment – in the Covid-19 era and beyond. N Engl J Med. 2020 Jul 15 doi: 10.1056/NEJMp2019989. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Lane HC, Fauci AS. Research in the context of a pandemic. N Engl J Med. 2020 Jul 17 doi: 10.1056/NEJMe2024638. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peabody JW. An organizational analysis of the World Health Organization: narrowing the gap between promise and performance. Soc Sci Med. 1995;40(6):731–742. doi: 10.1016/0277-9536(94)00300-i. [DOI] [PubMed] [Google Scholar]
- 50.Bloom BR, Farmer PE, Rubin EJ. WHO's next – the United States and the World Health Organization. N Engl J Med. 2020;383(7):676–677. doi: 10.1056/NEJMe2024894. [DOI] [PubMed] [Google Scholar]
- 51.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.European Medicines Agency. Global regulators work towards alignment on policy approaches and regulatory flexibility during COVID-19 – update #3. Available at:https://www.ema.europa.eu/en/news/global-regulators-work-towards-alignment-policy-approaches-regulatory-flexibility-during-covid-19-2. Accessed August 16, 2020.
- 53.Armstrong PW, Naylor CD. Counteracting health misinformation: a role for medical journals? JAMA. 2019;321(19):1863–1864. doi: 10.1001/jama.2019.5168. [DOI] [PubMed] [Google Scholar]
- 54.Chou WS, Oh A, Klein WMP. Addressing health-related misinformation on social media. JAMA. 2018;320(23):2417–2418. doi: 10.1001/jama.2018.16865. [DOI] [PubMed] [Google Scholar]
- 55.Merchant RM, Asch DA. Protecting the value of medical science in the age of social media and "fake news". JAMA. 2018;320(23):2415–2416. doi: 10.1001/jama.2018.18416. [DOI] [PubMed] [Google Scholar]
- 56.Vosoughi S, Roy D, Aral S. The spread of true and false news online. Science. 2018;359(6380):1146–1151. doi: 10.1126/science.aap9559. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S. In India, Hindu pride boosts pseudoscience. Science. 2019;363(6428):679–680. doi: 10.1126/science.363.6428.679. [DOI] [PubMed] [Google Scholar]
- 58.Broniatowski DA, Jamison AM, Qi S. Weaponized health communication: twitter bots and Russian trolls amplify the vaccine debate. Am J Public Health. 2018;108(10):1378–1384. doi: 10.2105/AJPH.2018.304567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenzel RP. Medical education in the era of alternative facts. N Engl J Med. 2017;377(7):607–609. doi: 10.1056/NEJMp1706528. [DOI] [PubMed] [Google Scholar]
- 60.Berezow A. American Council on Science and Health. 2017. Infographic: the best and worst science news sites. Available at: https://www.acsh.org/news/2017/03/05/infographic-best-and-worst-science-news-sites-10948. Accessed September 20, 2020. [Google Scholar]
- 61.Roth Y, Achuthan A. Building rules in public: our approach to synthetic & manipulated media. Available at: https://blog.twitter.com/en_us/topics/company/2020/new-approach-to-synthetic-and-manipulated-media.html. Accessed August 16, 2020.
- 62.Fast SM, Kim L, Cohn EL, Mekaru SR, Brownstein JS, Markuzon N. Predicting social response to infectious disease outbreaks from internet-based news streams. Ann Oper Res. 2018;263(5):551–564. doi: 10.1007/s10479-017-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill JA, Agewall S, Baranchuk A. Medical misinformation: vet the message! Eur Heart J. 2019;40(5):404–405. doi: 10.1093/eurheartj/ehz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atkins D, Best D, Briss PA. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ladeiras-Lopes R, Clarke S, Vidal-Perez R, Alexander M, Luscher TF. Twitter promotion predicts citation rates of cardiovascular articles: a preliminary analysis from the ESC Journals Randomized Study. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa211. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Thamman R, Gulati M, Narang A, Utengen A, Mamas MA, Bhatt DL. Twitter-based learning for continuing medical education. Eur Heart J. 2020 Apr 27 doi: 10.1093/eurheartj/ehaa346. [e-pub ahead of print]? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thaler R, Sunstein C. Penguin Books; New York: 2008. Nudge: Improving Decisions about Health, Wealth, and Happiness. [Google Scholar]
- 68.van Eck NJ, Waltman L, van Raan AF, Klautz RJ, Peul WC. Citation analysis may severely underestimate the impact of clinical research as compared to basic research. PLoS One. 2013;8(4):e62395. doi: 10.1371/journal.pone.0062395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moderna. Moderna announces positive interim Phase 1 data for its mRNA vaccine (mRNA-1273) against novel coronavirus. Available at:https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-interim-phase-1-data-its-mrna-vaccine/. Accessed August 16, 2020.
- 70.Kirkpatrick DD. New York Times. April 27, 2020. In race for a coronavirus vaccine, an Oxford group leaps ahead. Available at: https://www.nytimes.com/2020/04/27/world/europe/coronavirus-vaccine-update-oxford.html. Accessed August 16, 2020. [Google Scholar]
- 71.Haseltine W. Washington Post. May 19, 2020. Rush to share good news on COVID-19 drugs is undermining science. Available at: https://www.washingtonpost.com/opinions/2020/05/19/rush-share-good-news-covid-19-drugs-is-undermining-science/. Accessed August 16, 2020. [Google Scholar]