Abstract:
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is used to support patients with reversible cardiopulmonary insufficiency. Although it is a lifesaving technology, bleeding, inflammation, and thrombosis are well-described complications of ECMO. Adult porcine models of ECMO have been used to recapitulate the physiology and hemostatic consequences of ECMO cannulation in adults. However, these models lack the unique physiology and persistence of fetal forms of coagulation factors and fibrinogen as in human infants. We aimed to describe physiologic and coagulation parameters of piglets cannulated and supported with VA-ECMO. Four healthy piglets (5.7–6.4 kg) were cannulated via jugular vein and carotid artery by cutdown and supported for a maximum of 20 hours. Heparin was used with a goal activated clotting time of 180–220 seconds. Arterial blood gas (ABG) was performed hourly, and blood was transfused from an adult donor to maintain hematocrit (Hct) > 24%. Rotational thromboelastometry (ROTEM) was performed at seven time points. All animals achieved adequate flow with a patent circuit throughout the run (pre- and post-oxygenator pressure gradient <10 mmHg). There was slow but significant hemorrhage at cannulation, arterial line, and bladder catheter sites. All animals required the maximum blood transfusion volume available. All animals became anemic after exhaustion of blood for transfusion. ABG showed progressively declining Hct and adequate oxygenation. ROTEM demonstrated decreasing fibrin-only ROTEM (FIBTEM) clot firmness. Histology was overall unremarkable. Pediatric swine are an important model for the study of pediatric ECMO. We have demonstrated the feasibility of such a model while providing descriptions of physiologic, hematologic, and coagulation parameters throughout. Weak whole-blood clot firmness by ROTEM suggested defects in fibrinogen, and there was a clinical bleeding tendency in all animals studied. This model serves as an important means to study the complex derangements in hemostasis during ECMO.
Keywords: ECMO, animal model, transfusion, coagulopathy
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is a lifesaving temporary cardiopulmonary support modality for neonates and children with reversible heart and lung failure (1–3). The mortality of infants and children requiring ECMO support is high. In the most recent report of the International Extracorporeal Life Support Organization Registry reflecting contemporary clinical experience (2009–2016), overall survival of pediatric patients was 61% (4). Hemorrhagic and thrombotic complications are common and contribute considerably to mortality among pediatric patients on ECMO owing to anticoagulation, vascular instrumentation, and prematurity or other associated conditions (5). In one study, hemorrhage was the most common cause of anemia among children on ECMO, with 66% of patients experiencing an acute bleeding event requiring transfusion and over 80% of subjects receiving >40 mL/kg red blood cell (RBC) transfusion on at least 1 day throughout the study (6). Oxygenator or cannula thrombosis can cause devastating mechanical failure, and one prospective study estimated that the rate of thrombotic events (including stroke and limb-threatening ischemia) was 11.0/100 ECMO days (7). Intraventricular hemorrhage is a particularly devastating event that affects premature infants with immature intracerebral vasculature.
Given the complexity of the physiologic and hemostatic consequences associated with cannulation and extracorporeal circulation, investigating these effects in a large animal model that can reproduce human physiologic and hemostatic responses to ECMO and potential therapeutic interventions is essential. Such a model is even more critical when investigating pediatric ECMO. Infants have a small circulating blood volume compared with adults which limits the number blood draws possible for ex vivo clinical study. In addition, accruing infants on ECMO in prospective or randomized studies of interventions is logistically challenging. Our ability to extrapolate information from studies of adults on ECMO to the management of infants is limited by the unique physiology of neonates and their immature hemostatic system. There are significant differences between the infant and adult coagulation systems, with development occurring throughout the normal human life span (8,9). In particular, the fetal fibrinogen molecule is qualitatively different from that among maturing children and adults; clots from fetal and adult fibrin admixtures are structurally and chemically inferior to native clots (10). Accordingly, previously published adult animals used to model coagulation in ECMO with descriptions of coagulation potential are unlikely to adequately approximate ECMO’s effects on neonatal physiology and hemostasis (11,12).
The ideal model for infant ECMO is a large animal model with physiology and size similar to infants. This would mean recent transition from fetal to adult circulation, as well as an immature coagulum. There is precedent for using adult pigs to model the physiologic effects and hemostatic consequences of ECMO and cardiopulmonary bypass (13,14). Given that adult pig models have been previously characterized and found to be adequate for these applications, we aimed to describe the physiology and coagulation profiles of infant pigs (“piglets”) on ECMO, and then to provide comparisons with age-adjusted controls from human infants.
MATERIALS AND METHODS
All animal experimentation was approved and monitored by Duke University Institutional Animal Care and Use Committee. All animals were euthanized under deep anesthesia at the completion of experimentation using intravenous (IV) potassium chloride and an approved secondary technique to ensure humane euthanasia.
Donor Blood Collection
Two adult domestic farm pigs (Sus scrofa domesticus) from the same family group as the experimental piglets weighting 17 and 33 kg were used for whole blood banking. The whole blood was heparinized and maintained for ECMO circuit prime and transfusions throughout the ECMO run. In brief, in the morning of the experimental procedure, fasted adult pigs were anesthetized with ketamine, acepromazine, and isoflurane before endotracheal intubation. Anesthetized and mechanically ventilated adult pigs were then heparinized with 30 U/kg IV heparin and exsanguinated via carotid cannulation into 500-mL sterile collection bags containing 5,000 U of heparin. This whole heparinized blood was maintained at room temperature with an aseptic technique and used within 18 hours.
Experimental Animals and Monitoring
Four domestic farm piglets weighing 5.7–6.4 kg (mean: 6.1 kg) were fasted the morning of the procedure. After donor blood collection as mentioned earlier, piglets were anesthetized using inhalational anesthesia (.2–4% isoflurane), and an ear vein IV catheter was placed for propofol infusion (1.5–3 mg/kg/min). Piglets were endotracheally intubated and mechanically ventilated at 10–20 breaths/min. All piglets received 1.5 mg intramuscular enrofloxacin for prophylaxis before further instrumentation. Using local anesthesia with .25% bupivacaine, the right femoral artery was cannulated for monitoring, and a urinary bladder catheter was placed for decompression and monitoring. Following induction of anesthesia, vital signs were recorded every 15 minutes via the arterial line, two-lead electrocardiogram, and rectal temperature probe. End-tidal CO2, airway pressure, and SpO2 were also continuously monitored and recorded every 15 minutes.
ECMO Cannulation Procedure and Circuit Setup
All four piglets were cannulated via right supraclavicular cutdown to expose the right carotid artery and external jugular vein. Animals were heparinized with 50 U/kg heparin just before ligation and division of the carotid artery and insertion of cannulae. In all cases, 8-Fr (2.7 mm) venous and 8-Fr arterial cannulae with multiple side ports (Bio-Medicus Medtronic, Minneapolis, MN) were used for cannulation. Based on clinical experience with infants of similar weight, we anticipated using a 10-Fr venous cannula; however, the vein was only suitable in size for an 8-Fr cannula in all four cases (15). A target flow of 80 mL/kg/min was set for each animal, with the centrifugal pump speed as the dependent variable.
Simultaneously, ECMO circuits were first clear-primed with crystalloid and then whole heparinized donor blood immediately before beginning extracorporeal circulation (estimated circuit volume 425 mL). Although the (Hct) was not determined empirically because of sampling limitations, our experience in subsequent experiments with a similar priming strategy has resulted in a circuit Hct of 17–19%. This results in a prime that is slightly dilute compared with baseline Hct (mean of 26% in the current study). Commercially available adult membrane oxygenators (Quadrox-iD, Maquet Getinge Group, Wayne, NJ) were used with centrifugal pumps and uncoated polyvinyl chloride tubing as part of a commercially available perfusion pack (Custom Revolution Pump Base Pack and Tubing Pack, Sorin Group Liva Nova, London, United Kingdom). The circuit was connected to venous and arterial cannulae without entrainment of air.
All animals were maintained on VA-ECMO either for 20 hours or until humane euthanasia criteria were met. Pre- and post-membrane oxygenator pressures were interrogated throughout the run to evaluate for thrombosis. Flow rates and pump speed were monitored and recorded throughout. Oxygen flow (sweep gas) through the membrane oxygenator was titrated to maintain PaCO2 normality while allowing permissive hyperoxia based on hourly arterial blood gas (ABG). Species-specific normal values and ranges, which are nearly identical to those in humans, were used (16).
Laboratory Testing, Transfusion, and Anticoagulation Management
All piglets underwent whole blood rotational thromboelastometry (ROTEM) for analysis of coagulation at 0, 5, 30 minutes, 1, 4, 12, and 20 hours (or otherwise at euthanasia) following cannulation. ROTEM extrinsic pathway testing (EXTEM), intrinsic pathway testing (INTEM), and FIBTEM were used for all samples before heparinization, and heparinase-treated ROTEM (HEPTEM) was used in place of INTEM following heparinization to neutralize the effects of anticoagulation. A single ROTEM-delta analyzer was used for all samples, and all ROTEM STARTEM, EXTEM, INTEM, FIBTEM, and HEPTEM reagents were used according to the manufacturer’s instructions (Instrumentation Laboratory Werfen, Bedford, MA). Approximately 2.0 mL was taken for each ROTEM test time point to satisfy all three tests.
Other laboratory data collected included ABG at baseline and then hourly throughout the ECMO run. Hct, lactate, glucose, and electrolytes were measured with this sample as well and used to guide transfusion and fluid resuscitation as detailed in the following texts. All ABGs were performed on a single GEM Premier 3000 analyzer (Instrumentation Laboratory Werfen). The specimen volume was approximately .15 mL; any unused sample blood drawn in excess was re-transfused after submission of the specimen.
Anticoagulation was monitored at least hourly with activated clotting time (ACT) with a target of 180–220 seconds using glass bead/celite/kaolin tubes on a clinical point-of-care analyzer (MAX-ACT Tubes and Actalyte Mini II Analyzer, Helena Laboratories, Beaumont, TX). The specimen volume required was about .5 mL of whole blood for each ACT measurement. IV heparin was administered hourly based on the ACT. An hourly dose of 50 U/kg heparin was administered if the ACT was therapeutic. Otherwise, the dose was held (if ACT > 220 seconds) or a greater dose (100–150 U/kg if ACT ≪ 180 seconds) was administered, and then the ACT was rechecked 5 minutes later to ensure adequate response.
Depending on availability of donor blood, whole blood at room temperature was transfused in either 20 mL/kg (2/4 piglets) or 40 mL/kg (2/4 piglets) boluses with a goal Hct ≥ 24%.
Pathology
At the time of euthanasia, a necropsy was performed with gross examination of thoracic, abdominal, and intracranial organs. Kidney, lung, and brain specimens were fixed in neutral buffered formalin, and representative tissues were processed into paraffin for conventional histology, sectioned, stained with hematoxylin and eosin, and examined for microscopic morphologic changes.
Statistical Analysis
Descriptive statistics (mean and SD) were calculated for ROTEM values using all available data at each time point. Mean coagulation time (CT; EXTEM and INTEM/HEPTEM), clot formation time (CFT; EXTEM and INTEM/HEPTEM), maximum clot firmness (MCF; all tests), and alpha angle (EXTEM and INTEM/HEPTEM) were compared between baseline and each ensuing time point using unpaired t-tests with assumption of equal variance. The null hypothesis was rejected when p < .05. All statistics were calculated using JMP 13 (SAS Institute, Cary, NC). Graphical representations of ROTEM data including mean and SDs were generated using GraphPad Prism 8 (GraphPad Software, San Diego, CA).
Normal Age-Adjusted Values
For the purposes of comparison and discussion, historical normal control values were identified for human neonates from the developmental hemostasis literature. In particular, Oswald and colleagues’ study of ROTEM values from 51 children from birth through 3 months of age was used to compare with those values identified in the current study (17). The 0- to 3-month group was chosen based on the compatibility of both weight and age with the piglets in the current study.
RESULTS
Summary of Clinical Course
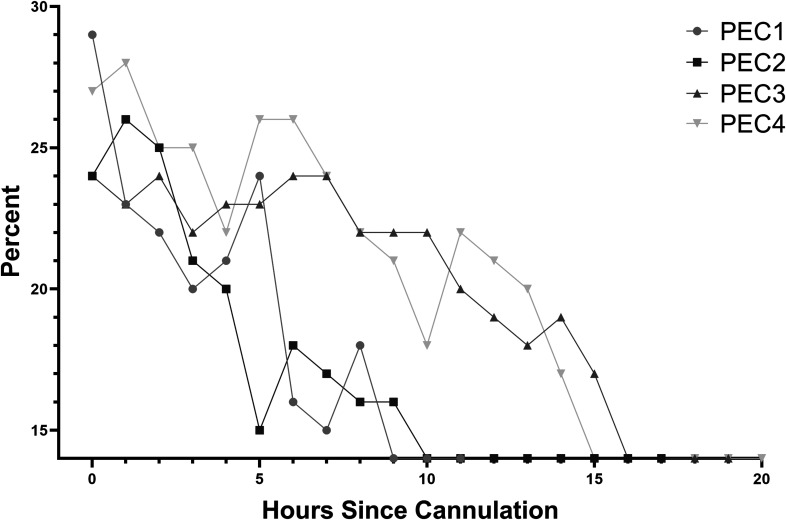
All animals had slow but significant hemorrhage from sites of cannulation and instrumentation that responded only partially to local hemostatic efforts, including pressure and hemostatic sutures (Figure 1). After exsanguination of the adult donor, a total of 450 mL of whole blood was available for transfusion (including circuit prime) for each of the first two animals, and 1 L was available for transfusion (including circuit prime) for each of the second two animals. Clinical and laboratory evidence of critical anemia with accompanying progressive hypotension was encountered in the first two animals after exhaustion of 450 mL whole blood transfusion and constituted criteria for humane euthanasia at 12 and 17 hours (Figure 2). The third and fourth experimental animals, which received 1 L total of transfusion each, also had laboratory evidence of anemia (Hct < 15%), but arterial pressure and oxygenation were sufficiently preserved, and the animals were euthanized at the conclusion of the study (20 hours). No major thrombotic events were noted clinically.
Figure 1.
Cannulation and instrumentation site bleeding was slow but persistent throughout ECMO run in all four animals and recurred, despite local hemostatic techniques.
Figure 2.
Hct (%) was monitored hourly throughout the run. Values <15% were not discernible by the GEM 3000 analyzer (Instrumentation Laboratory Werfen) and are indicated as such.
Coagulation and Hemostasis
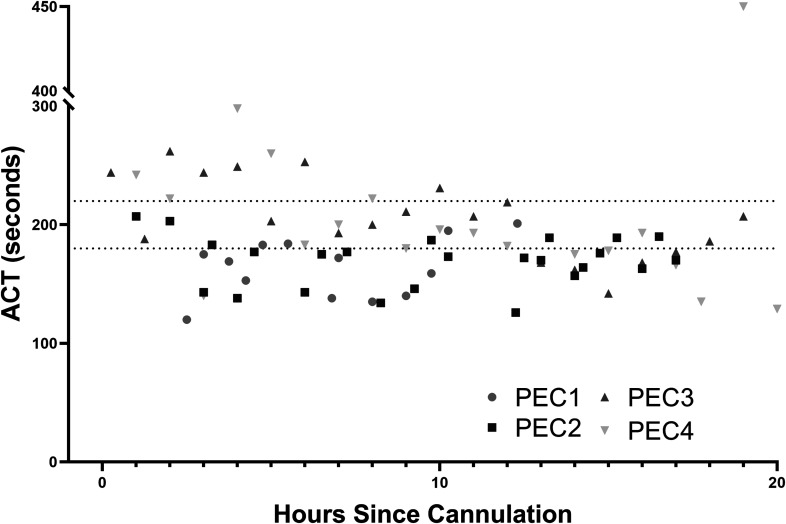
Using the scheme described earlier to achieve target ACT 180–220 seconds, the overall dose of heparin administered varied from 84 to 213 U/kg/h. After a short period of instability immediately following cannulation, ACT values were generally either therapeutic or subtherapeutic, despite the high dosing compared with human neonates (Figure 3).
Figure 3.
ACT (seconds) was monitored at least hourly throughout the run. The shaded box represents the target ACT (180–220 seconds).
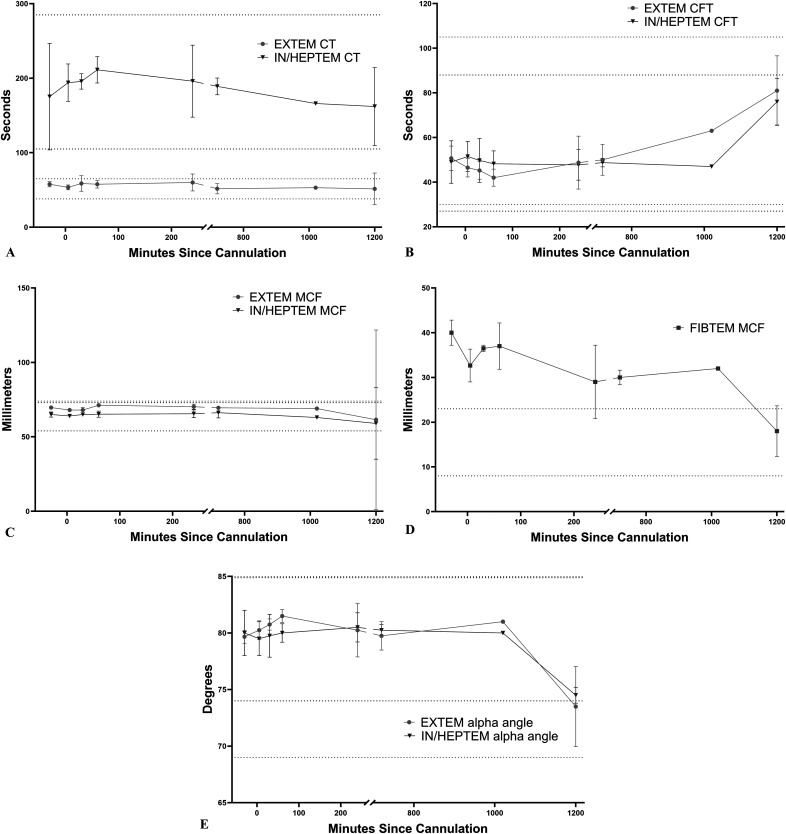
Baseline pre-ECMO, ROTEM, EXTEM, and INTEM were run and recorded for 3/4 animals because of a technical issue with the ROTEM analyzer at the beginning of one run. EXTEM and HEPTEM were run for all animals at terminal (12, 17, and 20 hours) and all intervening time points (5 and 30 minutes, 1, 4, and 12 hours). FIBTEM was run and recorded at select time points because of analyzer channel availability and necessity of fresh whole blood for the test (Figure 4). In brief, there were statistically significant decreases in EXTEM MCF over time, with the mean EXTEM clot firmness at 20 hours 61.5 mm compared with 70 mm at baseline (p = .024). INTEM/HEPTEM CFT was prolonged significantly at 20 hours compared with baseline as well, suggesting an acquired deficit in the intrinsic pathway of coagulation at later time points (76 seconds compared with 49 seconds, p = .036). The most striking change over time was the decrease in FIBTEM MCF at the 12- and 20-hour time points compared with baseline, in which the firmness decreased from a baseline mean of 40 to 30 mm at 12 hours and then finally 18 mm at 20 hours (p = .004 and .048, respectively). Finally, alpha angle was also significantly decreased throughout the run for both the extrinsic and intrinsic pathways (p < .05 at 20 hours for both and at 30 minutes and 1 hour for EXTEM).
Figure 4.
Mean ± SD of selected ROTEM variables from all animals available at each time point. (A) Mean EXTEM and HEPTEM CT, (B) mean EXTEM and HEPTEM CFT, (C) mean EXTEM and HEPTEM MCF, (D) mean FIBTEM MCF, and (E) mean EXTEM and HEPTEM alpha angle.
ECMO Settings
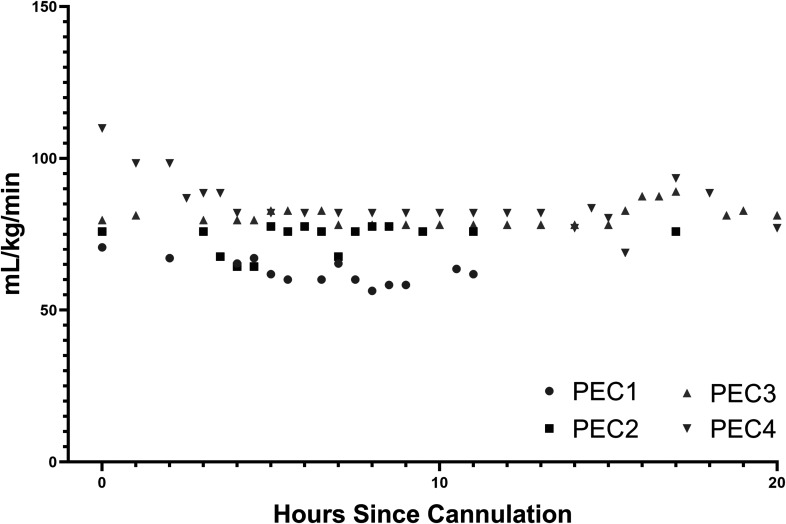
Sweep gas was titrated hourly to PaCO2 throughout the run and varied between 250 and 1,000 mL/min. Although there was considerable inter-animal variability, mean PaCO2 was generally near normal after an initial period of instability just after cannulation, whereas supranormal arterial O2 was tolerated throughout the run (Supplemental Figures 1–3). Flow rates were stable throughout each run without any change to pump speed, which was set at the start of the run to approximate a goal of 80 mL/kg/min flow (Figure 5). Flows were generally between 60 and 80 mL/kg/min (Figure 4). The pre- and post-oxygenator pressure gradient remained negligible for all animals throughout the run (≤10 mmHg at all time points for all animals). The mean pressure gradient was 1.9 ± 2.6 mmHg. The mean pre-membrane pressure was 132.6 ± 11.5 mmHg, and the mean post-membrane pressure was 130.7 ± 12.0 mmHg. Inspection of the oxygenator never revealed evidence of thrombosis.
Figure 5.
ECMO flow rate with constant pump speed setting was stable over the run.
Pathology
Necropsy was performed at the time of euthanasia of all four animals. Besides patchy violaceous areas of the lung with underlying collapse usually associated with atelectasis and a small amount of simple free abdominal fluid, there were no significant findings on examination of thoracoabdominal cavities nor viscera. Macroscopic examinations of bisected kidneys, lungs, and brain were further unrevealing. Microscopic evaluation of lung, kidney, and cerebral cortex specimens did not reveal any specific microhemorrhagic or thrombotic lesions. One animal had renal proximal tubular epithelial vacuolization.
DISCUSSION
Clinicians managing pediatric patients on ECMO manage a delicate balance between hemorrhage and thrombosis; and, unfortunately, both complications can occur simultaneously. The relative immaturity of the pediatric and especially neonatal hemostatic systems increases complexity and decreases endogenous physiologic coagulation reserves (18). Finally, the consequences of hemorrhage and thrombosis in this cohort are especially devastating, with an estimated 40% decrease in survival with clinically significant bleeding or thrombosis (19).
Using a combination of clinical findings and viscoelastic hemostatic testing (ROTEM), we report a partial description of the coagulation phenotype of piglets supported with VA-ECMO. Although piglets have been previously suggested as a model for neonatal or pediatric ECMO, only their physiology and immunology were characterized and found to be satisfactory in these studies, without specific analysis of hemostasis and coagulation (20–25). It has been established that infants and children on ECMO require very frequent RBC and platelet transfusions because of a combination of slow hemorrhage, frequent laboratory draws, and subclinical thrombosis with hemolysis in the context of a small circulating volume (6,26–28). In our model, all four experimental animals required ongoing RBC transfusions because of slowly progressive anemia throughout the run. Slow but persistent hemorrhage from sites of instrumentation due to heparinization and dys- or hypofibrinogenemia is analogous to a common human clinical dilemma of slow bleeding during ECMO, and transfusion requirements provide an actionable and easily quantifiable outcome associated with overall hemostasis.
The viscoelastic hemostatic tests ROTEM and closely related thromboelastography (TEG) are increasingly being used in the care of critically ill patients of all ages and etiologies. Their use has been proposed (although not yet rigorously validated) in the guidance of transfusion strategies for patients on ECMO, and an animal model may assist in development of these strategies (29,30). ROTEM and TEG are whole blood tests that provide information on the kinetics of clot formation and stability, as well as fibrinolysis. We have presented serially collected ROTEM data over the course of the run that implicate fibrinogen deficits as the primary acquired coagulopathy associated with ECMO beyond 12 hours. Interestingly, normal EXTEM and HEPTEM MCF suggest that platelet function is adequate and that the primary hemostatic lesion lies with fibrinogen. This may have been due to adequate platelet transfusions with whole donor blood. It is important to also qualify these findings, given that there are no established pediatric (piglet) reference ranges in pigs, and we extrapolated porcine normality from one study of ROTEM among swine (31). Therefore, the changes over the course of the run using the animals’ baseline values as their own controls may be more valuable and are presented as such.
One challenge in translation of this model was the relative hypercoagulability of pigs compared with humans, which has been well established in adults of both species based on quantitative and activity-based comparisons of plasma coagulation factors (32). However, the coagulation systems are otherwise similar, and the ACT allowed for monitoring of anticoagulation with values that translate directly into humans, with a lack of thrombotic events throughout the run as a testament to appropriate anticoagulation. By using a protocolized approach to anticoagulation based on serial ACT monitoring without setting maximum heparin dose administration, we were able to compensate for this species-specific hypercoagulability. It is worth noting, however, that heparin was administered hourly via bolus injection, rather than by constant infusion. This does represent a departure from clinical practice and was a limitation of pump equipment available during these pilot experiments; infusion via IV pump would better recapitulate clinical practice. The relatively high doses of heparin required for anticoagulation are a testament to these differences in biology, which appear to be present in the immature piglets used in the experiment. This phenomenon has been previously established in neonatal piglets (33). This apparent heparin resistance is likely because of normal, congenital antithrombin-III deficiency and increased volume of distribution compared with adult animals (34).
Although our intent in the current report was limited to description of some aspects of coagulation and physiology of piglets during VA-ECMO, future application may contribute to deeper understanding of the underlying mechanisms of neonatal coagulopathy associated with any extracorporeal circulation. There is currently particular interest in how consumption of plasma clotting factors may contribute to both coagulopathy and mortality, and markers of intravascular coagulation (i.e., fibrinogen split products, and D-dimer) represent an important area of future study with implications for clinical practice.
In summary, we have presented clinical and viscoelastic hemostatic testing data from four piglets during runs of VA-ECMO, along with comparisons to previously published findings among healthy human neonates. The description of this model may provide the basis for further studies on evolutionary developmental hemostasis, trials of novel anticoagulation strategies, and circuit or engineering improvements.
ACKNOWLEDGMENTS
This work was supported by an American Pediatric Surgical Association Research Fellowship (ETT). The authors would like to thank Mike Lowe, Ianthia Parker, and Christie Holmes for their expert technical assistance with all aspects of animal experimentation and collective wisdom. This work was funded with an American Pediatric Surgical Association (APSA) Foundation grant.
REFERENCES
- 1.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–8. [PubMed] [Google Scholar]
- 2.Bartlett RH, Roloff DW, Cornell RG, et al. Extracorporeal circulation in neonatal respiratory failure: A prospective randomized study. Pediatrics. 1985;76:479–87. [PubMed] [Google Scholar]
- 3.O’Rourke PP, Crone RK, Vacanti JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: A prospective randomized study. Pediatrics. 1989;84:957–63. [PubMed] [Google Scholar]
- 4.Barbaro RP, Paden ML, Guner YS, et al. Pediatric extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werho DK, Pasquali SK, Yu S, et al. Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: An analysis of the extracorporeal life support organization registry. Pediatr Crit Care Med. 2015;16:276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muszynski JA, Reeder RW, Hall MW, et al. RBC transfusion practice in pediatric extracorporeal membrane oxygenation support. Crit Care Med. 2018;46:e552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton HJ, Reeder R, Garcia-Filion P, et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017;196:762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrew M, Vegh P, Johnston M, et al. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 9.Mari D, Mannucci PM, Coppola R, et al. Hypercoagulability in centenarians: The paradox of successful aging. Blood. 1995;85:3144–9. [PubMed] [Google Scholar]
- 10.Brown AC, Hannan RT, Timmins LH, et al. Fibrin network changes in neonates after cardiopulmonary bypass. Anesthesiology. 2016;124:1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passmore MR, Fung YL, Simonova G, et al. Evidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation support. Crit Care. 2017;21:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. [DOI] [PubMed] [Google Scholar]
- 13.Chow E, Woodard JC, Farrar DJ. Rapid ventricular pacing in pigs: An experimental model of congestive heart failure. Am J Physiol. 1990;258(5 Pt 2):H1603–5. [DOI] [PubMed] [Google Scholar]
- 14.Schochl H, Solomon C, Schulz A, et al. Thromboelastometry (TEM) findings in disseminated intravascular coagulation in a pig model of endotoxinemia. Mol Med. 2011;17:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey C. Cannulation for neonatal and pediatric extracorporeal membrane oxygenation for cardiac support. Front Pediatr. 2018;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannon JP. Blood acid-base curve nomogram for immature domestic pigs. Am J Vet Res. 1983;44:2385–90. [PubMed] [Google Scholar]
- 17.Oswald E, Stalzer B, Heitz E, et al. Thromboelastometry (ROTEM) in children: Age-related reference ranges and correlations with standard coagulation tests. Br J Anaesth. 2010;105:827–35. [DOI] [PubMed] [Google Scholar]
- 18.Attard C, van der Straaten T, Karlaftis V, et al. Developmental hemostasis: Age-specific differences in the levels of hemostatic proteins. J Thromb Haemost. 2013;11:1850–4. [DOI] [PubMed] [Google Scholar]
- 19.Dalton HJ, Garcia-Filion P, Holubkov R, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med. 2015;16:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIlwain RB, Timpa JG, Kurundkar AR, et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest. 2010;90:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurundkar AR, Killingsworth CR, McIlwain RB, et al. Extracorporeal membrane oxygenation causes loss of intestinal epithelial barrier in the newborn piglet. Pediatr Res. 2010;68:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MohanKumar K, Killingsworth CR, McIlwain RB, et al. Intestinal epithelial apoptosis initiates gut mucosal injury during extracorporeal membrane oxygenation in the newborn piglet. Lab Invest. 2014;94:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golej J, Kahlbacher H, Schoffmann G, et al. The immediate haemodynamic response to the initiation of extracorporeal membrane oxygenation in a piglet model of infant hypoxic respiratory failure. Perfusion. 2002;17:421–6. [DOI] [PubMed] [Google Scholar]
- 24.Itoh H, Ichiba S, Ujike Y, et al. Effect of the pulsatile extracorporeal membrane oxygenation on hemodynamic energy and systemic microcirculation in a piglet model of acute cardiac failure. Artif Organs. 2016;40:19–26. [DOI] [PubMed] [Google Scholar]
- 25.Batts SG, Mu TS, Uyehara-Lock JH, et al. ECMO maintains cerebral blood flow during endotoxic shock in piglets. ASAIO J. 2016;62:732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henriquez-Henriquez M, Kattan J, Chang M, et al. Blood component usage during extracorporeal membrane oxygenation: Experience in 98 patients at a Latin-American tertiary hospital. Int J Artif Organs. 2014;37:233–40. [DOI] [PubMed] [Google Scholar]
- 27.Jackson HT, Oyetunji TA, Thomas A, et al. The impact of leukoreduced red blood cell transfusion on mortality of neonates undergoing extracorporeal membrane oxygenation. J Surg Res. 2014;192:6–11. [DOI] [PubMed] [Google Scholar]
- 28.Chevuru SC, Sola MC, Theriaque DW, et al. Multicenter analysis of platelet transfusion usage among neonates on extracorporeal membrane oxygenation. Pediatrics. 2002;109:e89. [DOI] [PubMed] [Google Scholar]
- 29.Faraoni D, Levy JH. Algorithm-based management of bleeding in patients with extracorporeal membrane oxygenation. Crit Care. 2013;17:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toulon P, Berruyer M, Brionne-Francois M, et al. Age dependency for coagulation parameters in paediatric populations. Results of a multicentre study aimed at defining the age-specific reference ranges. Thromb Haemost. 2016;116:9–16. [DOI] [PubMed] [Google Scholar]
- 31.Velik-Salchner C, Schnurer C, Fries D, et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thromb Res. 2006;117:597–602. [DOI] [PubMed] [Google Scholar]
- 32.Karges HE, Funk KA, Ronneberger H. Activity of coagulation and fibrinolysis parameters in animals. Arzneim Forsch. 1994;44:793–7. [PubMed] [Google Scholar]
- 33.Andrew M, Ofosu F, Schmidt B, et al. Heparin clearance and ex vivo recovery in newborn piglets and adult pigs. Thromb Res. 1988;52:517–27. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt B, Buchanan MR, Ofosu F, et al. Antithrombotic properties of heparin in a neonatal piglet model of thrombin-induced thrombosis. Thromb Haemost. 1988;60:289–92. [PubMed] [Google Scholar]